Abstract
Learning potential and memory capacity are factors that strongly predict the level of rehabilitation and the long-term functional outcome in patients with schizophrenia. Unfortunately, however, the effects of antipsychotic drugs (i.e., the primary treatments for schizophrenia) on these components of cognition are unclear, particularly when they are administered chronically (i.e., a standard clinical practice). In this rodent study we evaluated the effects of different time periods (ranging from two weeks to six months) of oral treatment with the first generation antipsychotic chlorpromazine (10.0 mg/kg/day), or the second generation antipsychotic olanzapine (10.0 mg/kg/day) on the repeated acquisition of a water maze task (i.e., a method of assessing spatial learning potential in a repeated testing format). We assessed locomotor function (in an open field) and employed a radial arm maze (RAM) task to assess antipsychotic effects (5.0 and 10.0 mg/kg/day doses) on spatial working memory during the treatment period between fifteen days and two months. Finally, we conducted experiments using liquid chromatography/tandem mass spectrometry (LC-MS/MS) to evaluate the therapeutic relevance of our method of drug delivery (oral administration in drinking water). In the water maze experiments, both antipsychotics were associated with impairments in acquisition in the earlier test sessions that could eventually be overcome with repeated testing while olanzapine also impaired retention in probe trials. Both antipsychotics were also associated with impairments in delayed non-match-to-position trials in the RAM and some impairments of motor function (especially in the case of olanzapine) as indicated by slightly reduced swim speeds in the water maze and decreased activity in some components of the open field assessment. Finally, LC-MS/MS studies indicated that the method of antipsychotic administration generated clinically relevant plasma levels in the rat. These animal data indicate that chronic oral treatment with chlorpromazine or olanzapine can impair the performance of tasks designed to assess specific components of cognition that are affected in schizophrenia.
Keywords: schizophrenia, cognition, antipsychotic, water maze, radial arm maze, neuroleptic
Introduction
The class of pharmaceutical agents known as “antipsychotics” has been shown in most clinical trials to improve the positive behavioral symptoms (e.g., delusions, hallucinations) of schizophrenia. However, the negative symptoms of the illness (e.g., anhedonia, alogia, depression) are for the most part left untreated. Further, the older, conventional agents (also referred to as typical or first generation antipsychotics) are limited by adverse motor effects (e.g., Parkinsonian symptoms and tardive dyskinesia) whereas the newer agents (referred to as atypical or second generation antipsychotics) are limited by their financial burden as well as adverse metabolic effects that include abnormal weight gain, development of diabetes mellitus and hyperlipidemias (reviewed, Gardner et al., 2005; Miyamoto et al., 2005). An additional core feature of schizophrenia that is not adequately addressed by the available therapeutic interventions is the cognitive dysfunction, a significant predictor of long-term functional outcome (reviewed, Green et al., 2000). While second generation antipsychotics have been reported to have positive effects on cognitive function in schizophrenic patients (reviewed, Harvey et al., 2004), the cited studies were (for the most part) retrospective, open label in design, and/or of short duration. Furthermore, the magnitude of such improvements (when they were observed) has been questioned and there is growing sentiment among many researchers that meaningful cognitive improvements may not come from second-generation antipsychotics (Green, 2007). Finally, prospective clinical studies that would reflect the types of treatment periods encountered by most schizophrenic patients (i.e., multiple years) and designed specifically to identify antipsychotics that have optimal effects on cognition have not been conducted and may (in fact) be cost prohibitive.
A number of acute studies of antipsychotic drug effects on cognitive function in animal models have been published, however, only a relatively few chronic studies (which are more clinically relevant) have been conducted. The data from these experiments appear to suggest that representatives of both conventional and second generation antipsychotics can impair cognition. For example, haloperidol, clozapine, and risperidone, impaired acquisition in an eight arm radial maze task in rats while olanzapine had no effect (Rosengarten and Quartermain, 2002). Chronic haloperidol was also found to disrupt working memory in radial arm maze studies in rats in other laboratories (Levin et al., 1987; Levin 1997). Moreover, Didriksen and colleagues (Didriksen et al., 2006) found that while acute administration of clozapine and olanzapine impaired water maze performance in rats, in chronically treated animals, the impairments abated in the clozapine treated animals, but were exacerbated in the olanzapine-treated animals. Our laboratories have observed that several antipsychotics (when administered chronically) from both drug classes including haloperidol, olanzapine, ziprasidone, and risperidone can impair spatial learning in rats (Terry et al., 2003; Terry et al., 2006; Terry et al., 2007a; Terry et al., 2007b).
The purpose of the experiments described in this report was to further investigate such chronic (antipsychotic-related) effects on components of cognition that are known to be disrupted in schizophrenia. The effect of different time periods (ranging from two weeks to six months) of oral treatment with an archetypal conventional antipsychotic, chlorpromazine, or the commonly prescribed second generation antipsychotic olanzapine, on the repeated acquisition of a water maze (spatial learning) task was evaluated in rats. The water maze procedure was selected since it requires intact hippocampal function (which is often affected adversely in schizophrenic patients) as well as important components of human learning and memory such as information acquisition and encoding, consolidation, retention, and retrieval (McNamara and Skelton, 1993; McDonald and White, 1995). Antipsychotic effects on performance of a radial arm maze (RAM) procedure were also assessed during days 15–60 of treatment. Performance of the RAM test in rodents relies heavily on “spatial working memory” (Olton and Papas, 1979) which is commonly disrupted in schizophrenic patients across a variety of test procedures (Keefe et al., 1995; Park et al., 1999). We also conducted a series of experiments (open field, rotarod, grip strength) and data analyses (e.g., water maze swim speeds) to determine if the antipsychotics had significant effects on motor function that might influence task performance in the memory-related tests. Finally, a series of experiments using liquid chromatography/tandem mass spectrometry (LC-MS/MS) was conducted to evaluate the therapeutic relevance of our drug dosing approach.
Methods
Test Subjects
Male albino Wistar rats (Harlan Sprague-Dawley, Inc.) 2–3 months old were housed individually in a temperature controlled room (25°C), maintained on a 12-hour light/dark cycle. With the exception of the rats that were evaluated in the radial arm maze, all test subjects were allowed free access to food (Teklad Rodent Diet 8604 pellets, Harlan, Madison, WI). For the animals that were used in the radial arm maze task (see below), food intake was restricted to approximately 85% of ad libitum consumption beginning one week prior to testing. Additional food was given on weekends and holidays (if necessary) to maintain the weight of each rat at approximately 85% of its freely fed weight. Water was allowed ad libidum for the first week, but then replaced with solutions that contained antipsychotics for the remainder of the study (see below). Table 1 provides the details for the study cohorts, the numbers of animals tested per group, and the behavioral experiments conducted in each group. All procedures employed during this study were reviewed and approved by the Medical College of Georgia Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain or discomfort in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23 revised 1996). Significant efforts were also made to minimize the total number of animals used while maintaining statistically valid group numbers.
Table 1.
Rat Behavioral Testing Protocol
| Cohort | Group | N | Treatment | Days of Drug Exposure/Procedure Conducted | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8–14 | 15–60 | 22–28 | 39–45 | 50–53 | 83–90 | 174–180 | ||||
| 1 | A | 6 | VEHICLE | WM | WM | WM | Motor | WM | ||
| B | 6 | CPZ 10.0 mg/kg/day | WM | WM | WM | Motor | WM | |||
| C | 6 | OLZ 10.0 mg/kg/day | WM | WM | WM | Motor | WM | |||
| 2 | D | 6 | VEHICLE | WM | WM | WM | Motor | WM | WM | |
| E | 6 | CPZ 10.0 mg/kg/day | WM | WM | WM | Motor | WM | WM | ||
| F | 6 | OLZ 10.0 mg/kg/day | WM | WM | WM | Motor | WM | WM | ||
| 3 | G | 6 | VEHICLE | RAM | ||||||
| H | 6 | CPZ 5.0 mg/kg/day | RAM | |||||||
| I | 6 | OLZ 5.0 mg/kg/day | RAM | |||||||
| 4 | J | 6 | VEHICLE | RAM | ||||||
| K | 6 | CPZ 5.0 mg/kg/day | RAM | |||||||
| L | 6 | OLZ 5.0 mg/kg/day | RAM | |||||||
| 5 | M | 6 | VEHICLE | RAM | ||||||
| N | 6 | CPZ 10.0 mg/kg/day | RAM | |||||||
| O | 6 | OLZ 10.0 mg/kg/day | RAM | |||||||
| 6 | P | 6 | VEHICLE | RAM | ||||||
| Q | 6 | CPZ 10.0 mg/kg/day | RAM | |||||||
| R | 6 | OLZ 10.0 mg/kg/day | RAM | |||||||
CPZ = chlorpromazine; OLZ = olanzapine; WM =water maze; RAM= radial arm maze; Motor = motor function tests (open field, rotor-rod, and grip strength)
Drug Dosing for Chronic Antipsychotic Experiments
Oral antipsychotic dosing was based on several factors: 1) previous rodent studies in our laboratory in which time dependent behavioral and neurochemical effects were detected (Terry et al., 2002, 2003); 2) plasma drug levels were achieved that approximated those often associated with antipsychotic effects in humans Terry et al., 2005; 3) the doses selected (see below) were expected to achieve comparable and (therapeutically) relevant D2 receptor occupancy values in vivo (i.e., in the range 60–80%, see Kapur et al., 2003) based on the recent work of Barth and colleagues (Barth et al., 2006). Rats were thus treated with chlorpromazine (Sigma-Aldrich, St. Louis, MO), 5.0 or 10.0 mg/kg/day or olanzapine (A&A Pharmachem, Ottawa, Ontario Canada), 5.0 or 10.0 mg/kg/day orally in drinking water for time periods up to 180 days. The antipsychotics were dissolved in 0.1 M acetic acid and subsequently diluted (1:100) with deionized (ultrapure) water (Milli-Q® Biocel, water purification system Millipore Billerica, MA) for daily drug administration in drinking water. Drug dosing was based on the average daily fluid consumption and the weight of the animals.
Stability of the Chlorpromazine and Olanzapine as Concentrated Solutions and When Diluted in Rodent Drinking Water
In the initial portion of this study we conducted a series of experiments to ensure that the antipsychotic drugs evaluated were stable as concentrated solutions 0.1 M acetic acid and when diluted in tap water or deionized, at room temperature (i.e. to ensure that our method of administering the antipsychotics orally in drinking water was a valid approach). We performed the tap water assessment since it was used to deliver antipsychotic drugs in some of our earlier studies (e.g., Terry et al., 2003).
Preparation of Standard Solutions
Stock solutions of chlorpromazine and olanzapine were prepared in 0.1 M acetic acid at concentrations of 5.0 and 6.25 mg/ml, respectively and kept in glass bottles in a refrigerator at 4°C for up to 4 weeks. Dilutions of the concentrated solutions in tap water or deionized water (final concentrations of 20.0 µg/ml and 22.5 µg/ml for chlorpromazine and olanzapine, respectively) were also prepared and transferred into standard rodent drinking water bottles with rubber stoppers and then stored for up to 96 hours at room temperature.
Instrument Conditions for Drug Stability Study
Separations were carried out at ambient temperature on an Agilent Eclipse XBD C-8 column (4.6×150 mm, 5µm) with a Phenomenex Security Guard C-8 guard column (4.0mm×2.0mm). An Agilent 1100 series HPLC consisting of a degasser, quaternary pump, autosampler and variable wavelength ultraviolet detector was used (Palo Alto, CA, USA). The mobile phase consisted of a gradient of 30 mM ammonium acetate containing 0.05% (v/v) triethylamine, 0.025% (v/v), acetic acid, and acetonitrile. The mobile phase ratio began at 68% aqueous and changed linearly to 60% over 16 minutes. The aqueous percentage was then lowered to 20% for 6 minutes to flush the column and then reequilibrated for 8 minutes at the initial mobile phase conditions. The flow rate was 1.0 ml/min and the injection volume was 20 µL. Chlorpromazine was monitored at 245 nm and olanzapine was monitored at 255 nm. The retention time for chlorpromazine was 14.2 minutes and olanzapine was 5.5 minutes.
Plasma Antipsychotic Analysis
Plasma samples were obtained from rats administered the 10 mg/kg dose of olanzapine or chlorpromazine (i.e., in rats that were not behaviorally tested) using methods we have published previously for olanzapine and chlorpromazine (Zhang et al., 2007a; Zhang et al., 2007b). The 10 mg/kg dose of the antipsychotics was analyzed since this was the dose associated with behavioral effects in the present study (see below) as well as previous studies of olanzapine (Terry et al., 2002) and because we were interested in the effect of the long period of antipsychotic administration on plasma levels (and only the 10 mg/kg dose was evaluated over the 180 day treatment period). The two methods are summarized below.
Plasma Collection
Plasma samples were collected at days 45, 90, and 180 days of treatment (N=4–5). The subjects were anesthetized with isofluorane and 3.0 mL of blood was collected via cardiac puncture in sodium heparin. The blood was centrifuged for 15 min at 2500 × g at 4–5°C and the resulting plasma was frozen until analyzed.
Sample Preparation
To a 250 µl rat plasma sample, 25 µl of internal standard (80 ng/ml midazolam for chlorpromazine and 40 ng/ml for olanzapine) and 0.2 ml 0.5 M Na2HPO4 (pH = 10.7) were added. The samples were briefly mixed and extracted in 3 ml isopropyl ether for 10 min. After centrifugation at 2000 g for 10 min, the upper organic layer was removed and evaporated to dryness under reduced pressure in a vacuum centrifuge. To the residue, 200 µl of acetonitrile-methanol solutions (60:40) for chlorpromazine and 100 µl of methanol: 20 mM ammonium formate (pH = 3.9) (70:30) for olanzapine was added, sonicated, vortexed, and centrifuged at 16000 × g for 10 min. Ten µl for chlorpromazine or fifteen µl for olanzapine of the reconstitution solutions was injected for LC-MS/MS. Water samples, taken to test the stability of the compounds in the animal’s drinking water, were run without any sample pretreatment.
LC-MS/MS Procedure
Separations were carried out at 25°C on a Waters AtlantisTM dC-18 (2.1×30 mm, 3µm) with a Phenomenex Security Guard C-18 guard column (4.0mm×2.0mm). An Agilent 1100 series HPLC consisting of a degasser, binary pump, autosampler, and thermostated column compartment was used (Palo Alto, CA, USA). The mobile phase consisted of a gradient of 20 mM ammonium formate in water with pH adjusted to 4.25 using formic acid and acetonitrile for chlorpromazine and 5 mM ammonium formate buffer and acetonitrile for olanzapine. Two different gradient methods were used for chlorpromazine and olanzapine. For chlorpromazine, acetonitrile increased linearly from 15 to 70% over the first 5.0 min and to 77% over next 2.5 min. For olanzapine, the first minute is maintained at 15% acetonitrile and then increasing linearly from 15 to 50% acetonitrile over the next 4.0 min, and to 80% over the next 7 min. The flow rate for both of them was 0.3 ml/min. The mass spectrometer utilized for this work was a Waters Quattro Micro triple quadrupole mass spectrometer equipped with an electrospray ionization source. The capillary voltage was 3500 volts and the cone voltage was set at 32 volts for chlorpromazine and olanzapine. The collision energies were set at 20 eV for chlorpromazine and 23 eV for olanzapine and the collision cell pressure was 2.4 × 10−3 mbar. The collision gas was argon. The instrument was operated using multiple reaction monitoring (MRM) following the transitions from the protonated molecular ion to a diagnostic fragment ion for chlorpromazine (319→86), olanzapine (313 →256) and the internal standard midazolam (326 → 291).
Behavioral Experiments
All rats evaluated in behavioral tests were handled beginning the day after arrival, and received a minimum of two weeks of daily handling prior to the initiation of behavioral testing. Behavioral experiments were conducted in rooms equipped with white noise generators (San Diego Instruments, San Diego, CA) set to provide a constant background level of 70 dB and ambient lighting of approximately 25–30 Lux (lumen/m2). Test subjects were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before the beginning of experiments.
Water Maze Repealed Acquisition
At various times during drug treatment, test subjects (cohorts 1 and 2, see Table 1) were evaluated for performance of a water maze procedure we have published previously (Terry et al., 2007a). Sessions (which included hidden platform tests and probe trials) were conducted at the following time points of drug treatment: Session 1 = days 8–14; Session 2 = days 22–28; Session 3 days = 39–45; Session 4 = days 84–90; Session 5; days 174–180. For each session the hidden platform was moved to a new quadrant location in the pool.
Test Apparatus
Water maze experiments were performed in a circular pool (diameter: 180 cm, height: 76 cm) made of black plastic and filled to a depth of 35 cm of water (maintained at 25.0±1.0°C). The pool was located in a large room with a number of extra-maze visual cues including geometric images (squares, triangles, circles etc.) hung on the wall, and black curtains used to hide the experimenter (visually) and the resting test subjects. Swimming activity of each rat was monitored via a television camera mounted overhead, which relayed information including latency to find the platform, total distance traveled, time and distance spent in each quadrant etc. to a video tracking system (Noldus EthoVision® Pro 3.1)
Hidden Platform Task
For these experiments, an invisible (black) 10 cm × 10 cm square platform was submerged approximately 1.0 cm below the surface of the water and placed in the center of a quadrant (one-fourth of the total pool area defined via the tracking software). For each test session rats were given 2 trials per day for 6 consecutive days to locate and climb on to the hidden platform. A trial was initiated by placing the rat in the water directly facing the pool wall (i.e., nose approximately 2 cm from the wall) in one of the 4 quadrants. The daily order of entry into individual quadrants was pseudo-randomized such that all 4 quadrants were used once every two training days. For each trial, the rat was allowed to swim a maximum of 90 sec, in order to find the platform. When successful the rat was allowed a 30-sec rest period on the platform. If unsuccessful within the allotted time period, the rat was given a score of 90 sec and then physically placed on the platform and also allowed the 30-sec rest period. In either case the rat was given the next trial after an additional 1.5 min rest period (i.e., intertrial interval =2.0 min).
Probe Trials (Transfer Tests)
Twenty-four hours following the last hidden platform trial of each test session, probe trials (90 sec in duration) were conducted in which the platform was removed from the pool to measure spatial bias for the previous platform location. This was accomplished by measuring the number of crossings over the previous platform location, and provided a second estimate of the strength and accuracy of the memory of the previous platform location.
Visible Platform Task
After probe trials in session 5, a visible platform test was performed as a general estimate of visual acuity. To accomplish this task, a highly visible (white) cover fitted with a small white flag was attached to the platform (dimensions with cover attached = 12 cm × 12 cm) which raised the surface approximately 1.0 cm above the surface of the water. Each rat was gently lowered into the water in the quadrant diametrically opposite to the platform quadrant and given one or more trials with a 90 sec time limit to locate and climb on to the platform. When a rat was successful (on its own accord without assistance) it was then given a series of 4 additional trials (with a 1.0 min intertrial interval) and the latency (in sec) to locate the platform was recorded. The platform was moved on each trial to a different quadrant (the subject was always entered from the opposite quadrant) until the test was conducted once in all 4 quadrants.
Radial Arm Maze (RAM) Procedure
RAM testing was initiated on day 15 of antipsychotic or vehicle treatment (i.e., in cohorts 3–6, see Table 1) and continued until approximately two months had elapsed. These experiments were conducted in Med-Associates (MED-RAM-1R) computer-automated, 8-Arm Radial Mazes consisting of a central octagonal hub (arena) with automatic guillotine doors connected to aluminum arms radiating distally (45.7 cm long). IR-photo beam sensors were positioned at the entrance to each runway, and a food pellet receptacle and head entry detector was positioned at the end of each runway. The maze was positioned approximately 90 cm above the floor in a testing room with a number of extra-maze cues (composed of large geometrical shapes). This computer automated approach (used currently in our laboratory) is a modification of a previously published method by us (see Terry et al., 2001; Hernandez et al., 2003).
Habituation Phase
Test subjects were given one 15 min free exploration (habituation) session on the Friday prior to the Monday in which the Win-Shift portion of testing was conducted. This was done so that the animals became acquainted with the radial arm maze apparatus, as well as the handling procedures associated with it. Reinforcement food pellets were scattered randomly around the entire maze area during this session.
Acquisition (Win-Shift Training)
After the habituation phase, subjects were trained in a win-shift procedure. A trial began when the experimenter placed the test subject into the central octagonal arena. After a 30 sec delay, all guillotine doors raised allowing access to all of the 8 arms. When the animal broke a photobeam in the pellet receptacle at the end of each runway a reward pellet was delivered once. When the rat moved back into the central arena all doors closed for 5 seconds and then reopened. All reentries into an arm that previously delivered a reward were scored as working memory errors. All animals were trained in the win-shift task to meet a performance criterion of four consecutive days with ≤ 2 total errors. At this point, individual rats were moved to the Delayed non Match to Position task
Delayed non Match to Position (DNMTP)
DNMTP testing was similar to the acquisition mode except that two sessions were given in a trial block, with a predetermined delay imposed between the sessions. Testing began with an information (forced 4) session in which four of the eight arms are blocked, i.e. the animal could only investigate the four remaining open arms. This information session ended when all four arms were visited or when the trial timed out (15 min.). The animal remained in the testing room for the delay period. In the “free 8” (retention) session all eight arms were accessible; however, food reinforcement occurred only at the ends of the arms not visited in the previous information session. The test session continued until all four of the previously-blocked arms were visited, or until 15 min. elapsed. Any entry into an arm that had been visited in the information session (which then delivered no food reinforcement) or repeat visits to any arm during the second session was recorded as an error. Following the second (test) session in each trial block, the animal was returned to its home cage in the housing facility, until the next day’s information session. Animals were initially trained with 15 min delays in the DNMTP task to achieve a criterion of ≤ 1 error for four consecutive sessions during the free eight sessions. Subsequently, longer delays of 1, 3, and 6 hr were presented twice in a pseudorandom fashion along with 15 min delays.
Motor Function Tests
Tests of motor function were conducted in animal cohorts 1 and 2 during days 50–53 of drug exposure (see Table 1).
Open Field Activity
Rat open field activity monitors (43.2 × 43.2 cm, Med Associates St Albans, VT) were used for these experiments. The following parameters were recorded for the 5 min test session: horizontal activity (horizontal photobeam breaks or counts), number of stereotypical movements, and vertical activity (vertical photobeam breaks). Thus, spontaneous locomotor activity, olfactory activity (rearing and sniffing movements) and stereotypical movements were assessed. In light of previous reports indicating that some antipsychtoics (e.g., risperidone) have anxiolytic activity in rats (Nowakowska et al., 1999) we also recorded the time spent in the central and peripheral zones of the apparatus (defined areas represented approximately 75% and 25% of the total floor area, respectively). Drugs that possess anxiolytic activity tend to decrease the amount of time the rat spends in close proximity to the walls when placed into novel open field environments (thigmotaxis) and to increase exploratory activity in the center compartment (Treit and Fundytus, 1988).
Accelerating Rotarod
Motor coordination, balance, and motor learning were evaluated with an accelerating rotarod (Rotor-Rod System®, San Diego Instruments, San Diego, CA). Individual rats were assessed for their ability to maintain balance on a rotating bar that accelerated from 4 to 40 rpm over a 5.0 min period. The amount of time elapsed before each subject fell from the rod was recorded. Each test subject was given four trials per day for two consecutive days with an intertrial interval of 30 min.
Grip Strength
Forelimb grip strength was measured with a digital grip strength meter (Animal Grip Strength System®, San Diego Instruments, San Diego, CA) by holding the rat by the nape of the neck and by the base of the tail. The forelimbs were placed on the tension bar and the rat was pulled back gently until it released the bar. Each animal was assessed three times and mean grip strength (measured in kg of resistance) ± S.E.M. calculated.
Statistical Analyses
All statistical analyses were performed using JMP™ version 5 (Cary, NC) or SigmaStat 2.03 (SPSS Inc., Chicago, IL). One, two- or three-way analysis of variance (with repeated measures when indicated) was used for treatment comparisons. A Student Newman Keuls multiple comparison procedure was used to examine post hoc differences when indicated. Statistical significance was assessed using an alpha level of 0.05
Results
Stability of the Antipsychotics in Aqueous Solution
The stability of the concentrated antipsychotic solutions in 0.1M acetic acid immediately after preparation (time zero) and after storage at 4°C in a refrigerator for 1, 2, and 4 weeks is provided in Table 2A. The data indicate no significant degradation for up to 4 weeks. The stability of the diluted compounds in tap water or deionized water at room temperature at time zero (just after dilution) and at various time points up to 96 hrs after dilution is indicated in Table 2B. The data indicate no significant degradation for up to 96 hrs.
Table 2.
Stability of Chlorpromazine and Olanzapine
| A: Stability as Concentrated Solutions in 0.1 M acetic acid at 4°C | |||||||
|---|---|---|---|---|---|---|---|
| Chlorpromazine | Olanzapine | ||||||
| Time (weeks) | Concentration (mg/ml) | SD | R.S.D. (%) | Time (weeks) | Concentration (mg/ml) | SD | RSD. (%) |
| 0 | 20.5 | 0.34 | 1.66 | 0 | 12.3 | 0.20 | 1.60 |
| 1 | 19.7 | 0.22 | 1.14 | 1 | 12.4 | 0.05 | 0.41 |
| 2 | 19.8 | 0.60 | 3.05 | 2 | 12.1 | 0.05 | 0.38 |
| 4 | 19.6 | 0.01 | 6.54 | 4 | 12.2 | 0.13 | 1.09 |
| B: Stability of Dilutions in Rodent Drinking Water | |||||||
|---|---|---|---|---|---|---|---|
| Chlorpromazine (Tap water) | Chlorpromazine (distilled/deionized Water) | ||||||
| Time (hours) | Concentration (µg/ml) | SD | R.S.D. (%) | Time (hours) | Concentration (µg/ml) | SD | RSD. (%) |
| 0 | 87.4 | 2.33 | 2.67 | 0 | 87.6 | 1.14 | 1.30 |
| 6 | 87.1 | 2.75 | 3.15 | 6 | 86.2 | 0.53 | 0.61 |
| 24 | 88.1 | 1.53 | 1.74 | 24 | 87.6 | 0.76 | 0.86 |
| 48 | 87.2 | 1.89 | 2.17 | 48 | 87.8 | 0.88 | 1.01 |
| 72 | 85.6 | 2.22 | 2.60 | 72 | 86.0 | 1.17 | 1.36 |
| 96 | 81.8 | 2.47 | 3.02 | 96 | 81.7 | 0.26 | 0.32 |
| Olanzapine (Tap water) | Olanzapine(distilled/deionized Water) | ||||||
| 0 | 103.6 | 1.77 | 1.71 | 0 | 106.1 | 0.44 | 0.41 |
| 6 | 102.8 | 0.74 | 0.72 | 6 | 104.9 | 1.29 | 1.23 |
| 24 | 108.5 | 3.03 | 2.79 | 24 | 106.9 | 2.36 | 2.21 |
| 48 | 105.0 | 0.62 | 0.59 | 48 | 106.8 | 1.76 | 1.65 |
| 72 | 102.3 | 0.70 | 0.68 | 72 | 101.2 | 0.15 | 0.15 |
| 96 | 98.8 | 1.21 | 1.23 | 96 | 101.6 | 0.95 | 0.94 |
N=3 samples per determination; SD= Standard Deviation; RSD= relative standard deviation
Plasma Antipsychotic Levels
Plasma antipsychotic concentrations assessed after 45, 90, and 180 days of continuous oral administration of either chlorpromazine or olanzapine are provided in Table 3. As indicated, plasma drug levels were within the proposed therapeutic range for olanzapine at each of the 3 time points that were assessed. The plasma levels of chlorpromazine were slightly below the therapeutic range published by Curry et al., 1970 and Chetty et al., 1996, although other researchers have indicated that the optimal therapeutic range for chlorpromazine is unknown (Baldessarini et al., 1988).
Table 3.
Plasma Antipsychotic Levels
| Test Group | Dose/24 hr | Compound Measured | Plasma Concentration (ng/mL )± S.E.M. N=4–6 |
Estimated Human Therapeutic Plasma Range (ng/mL)* | ||
|---|---|---|---|---|---|---|
| 45 Days | 90 days | 180 days | ||||
| chlorpromazine | 10.0 mg/kg | chlorpromazine | 6.2 ± 2.1 | 15.4 ± 2.9 | 11.9 ± 2.8 | 25–350 (or unknown) |
| olanzapine | 10.0 mg/kg | olanzapine | 23.7 ± 8.9 | 31.0 ± 9.9 | 22.6 ± 7.5 | 9.0–80.0 |
Estimated human therapeutic plasma ranges listed are based on Curry et al., 1970; Baldessarini et al., 1988; Chetty et al., 1996; Perry et al., 2001; Rao et al., 2001; Gex-Fabry-Fabry et al., 2003.
Behavioral Experiments
Water Maze Testing
Hidden Platform Test
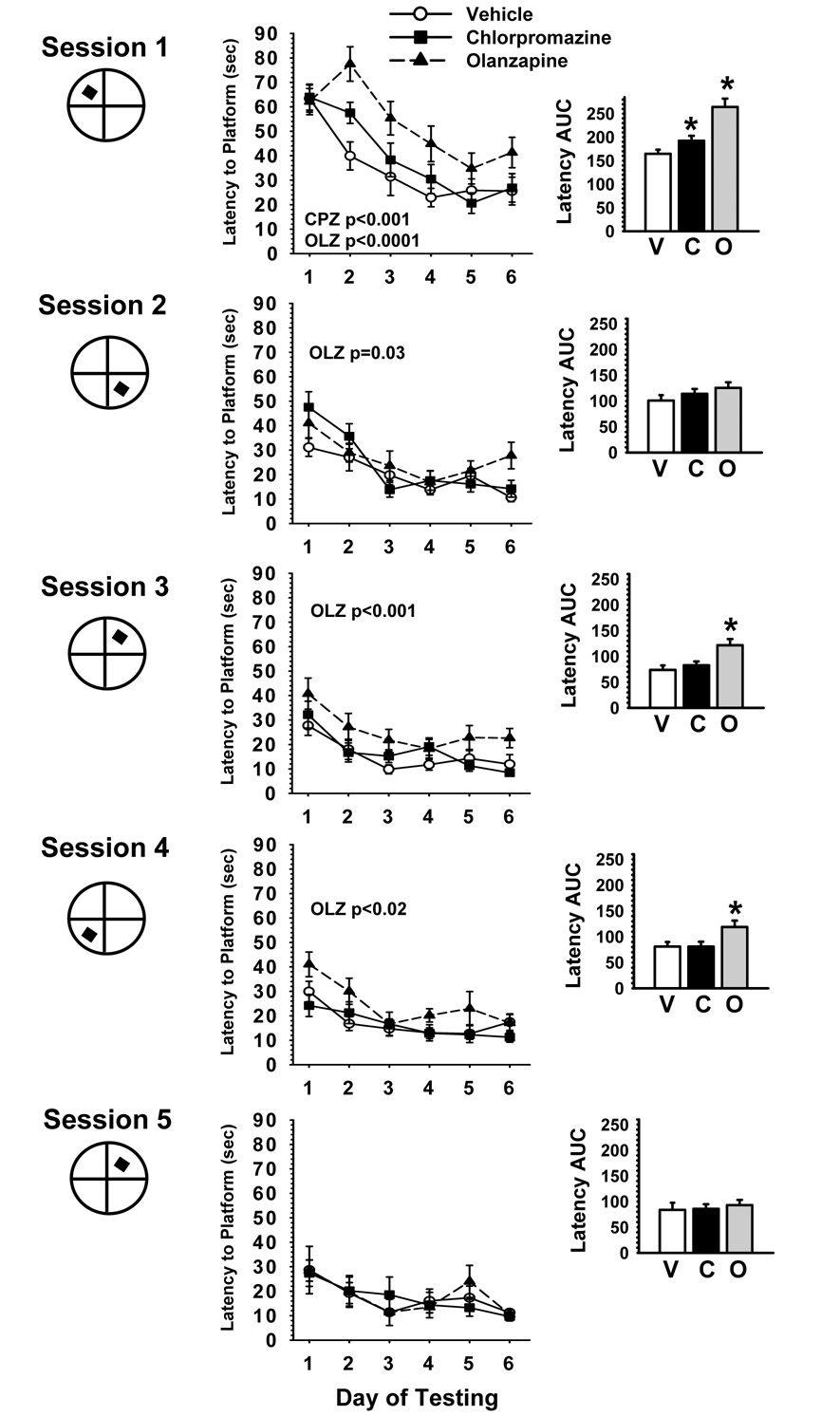
Fig 1 illustrates the efficiency of each experimental group to locate a hidden platform in the water maze task on 6 consecutive days during each of 5 different training sessions. The figures present both the acquisition curves (mean latencies ± SEM to locate the hidden platform) and the area under the curve (Fig 1 inset-AUC) for latencies. Under vehicle control conditions, rats progressively learned to locate the hidden platform with increasing levels of efficiency over the course of the 6 days during each testing session as well as over the course of the five different sessions. The latter observation was evident in both the decreasing slope of the acquisition curves over time as well as diminishing areas under the latency curves. In the treatment comparisons there were a number of significant differences detected (see Table 4). In the latency and AUC comparisons, the effects of treatment, session and the treatment×session interaction were all highly significant (all p values were <0.0001). Both olanzapine and chlorpromazine were associated with early impairments of acquisition that could be overcome by repeated testing. However, while impairments were observed only in session 1 in the case of chlorpromazine, in the case of olanzapine, impairments were observed during all sessions except session 5.
Fig. 1.
Antipsychotic effects on a water maze repeated acquisition procedure. S refers to the testing session, hidden platform tests were conducted during the first 6 days of each session. S1 = days 8–14; S2= days 22–28; S3 =days 39–45; S4 = days 84–90; S5= days 174–180 of drug treatment. For each session, the hidden platform was moved to a new quadrant location in the pool. Each point of the plotted curves represents the mean latency in seconds ± SEM for each testing day. Inset. Latency Area Under the Curve (AUC). Each bar represents the mean latency AUC ± SEM. VEH (or V) =Vehicle; CPZ (or C) =chlorpromazine 10.0mg/kg/day; OLZ (or O) =olanzapine 10.0mg/kg/day. Within each session, treatment effects (across the session) are noted when significant (p<0.05). Post hoc differences are indicated as follows: * = significantly (p<0.05) inferior performance when compared to vehicle control.
Table 4.
Statistical Results for Behavioral Analyses
| Variable | F-Value/Degrees of Freedom | P-value |
|---|---|---|
| Water Maze Latencies | ||
| Treatment | F2,36 = 13.97 | <0.0001 |
| Session | F4,912 = 58.95 | <0.0001 |
| Treatment × Session | F8,912 = 9.36 | <0.0001 |
| Day | F5,912 = 49.09 | <0.0001 |
| Treatment × Day | F10,912 = 0.61 | 0.805 |
| Session × Day | F20,912 = 2.22 | 0.002 |
| Treatment × Session × Day | F40,912 = 1.39 | 0.057 |
| Water Maze Latency (AUC) | ||
| Treatment | F2,36 = 13.50 | <0.001 |
| Session | F4,141 = 92.79 | <0.001 |
| Treatment × Session | F8,141 = 3.92 | <0.001 |
| Water Maze Probe Trials (Platform Area Crossings) | ||
| Treatment | F2,36 = 3.56 | 0.039 |
| Session | F4,143 = 1.87 | 0.118 |
| Treatment × Session | F8,143 = 0.43 | 0.905 |
| Water Maze Swim Speeds | ||
| Treatment | F2,36 = 5.64 | <0.007 |
| Session | F4,144 = 2.06 | 0.089 |
| Treatment × Session | F8,144 = 3.62 | <0.001 |
| Radial Arm Maze (Win-Shift Errors) | ||
| Treatment | F4,56 = 0.17 | 0.955 |
| Session | F9,504 = 43.11 | <0.001 |
| Treatment × Session | F36,504 = 0.69 | 0.912 |
| Radial Arm Maze Win-Shift (Trials to Criterion) | ||
| Treatment | F4,56 = 0.49 | 0.743 |
| Radial Arm Maze DNMTP (15 min Trials to Criterion | ||
| Treatment | F4,56 = 1.50 | 0.216 |
| Radial Arm Maze (DNMTP Variable Delay) | ||
| Treatment | F4,56 = 2.79 | 0.035 |
| Delay | F3,168 = 54.52 | <0.001 |
| Treatment × Delay | F12,168 = 1.65 | 0.084 |
| Open Field (Horizontal Activity) | ||
| Treatment | F2,36 = 1.11 | 0.339 |
| Open Field (Vertical Activity) | ||
| Treatment | F2,36 = 4.14 | 0.024 |
| Open Field (Stereotypical Movements) | ||
| Treatment | F2,36 = 3.76 | 0.033 |
| Open Field (Zone Time) | ||
| Treatment | F2,36 = 1.32 | 0.279 |
| Zone | F1,36 = 84.79 | <0.001 |
| Treatment × Zone | F2,36 = 1.57 | 0.222 |
| Grip Strength | ||
| Treatment | F2,36 = 0.371 | 0.693 |
| RotaRod | ||
| Treatment | F2,36 = 1.17 | 0.322 |
| Trial | F7,252 = 9.82 | <0.001 |
| Treatment × Trial | F14,252 = 1.22 | 0.259 |
DNMTP = Delayed Non-Match to Position
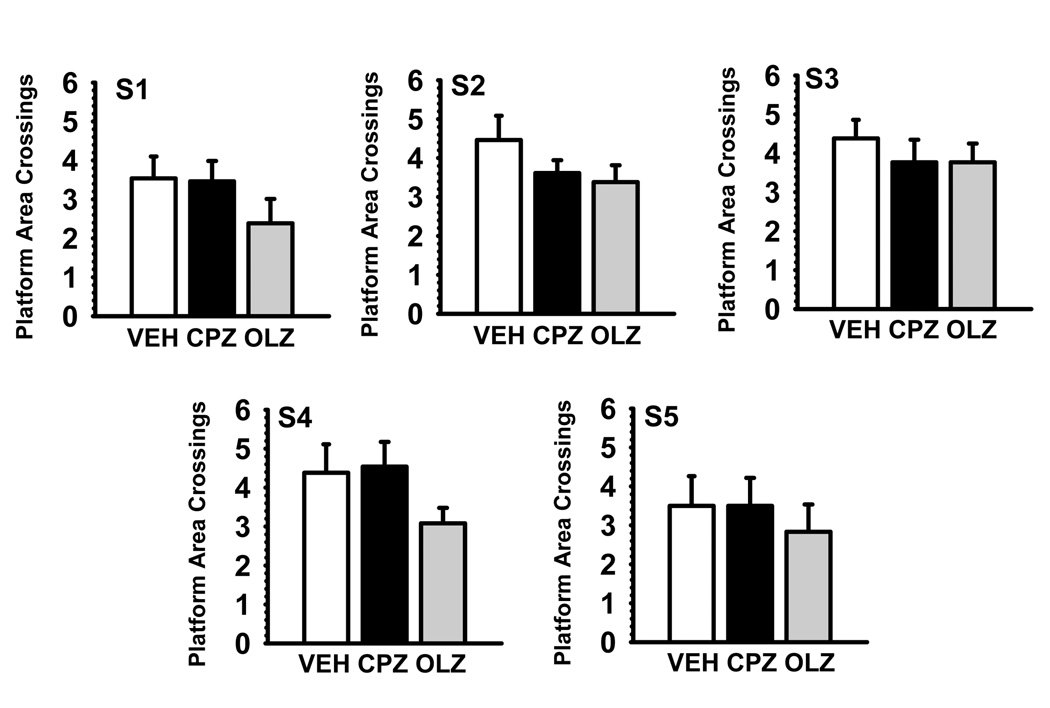
Probe Trials
Fig 2 illustrates the performance of probe trials by the various treatment groups at the end of sessions 1–5. There were significant treatment-related effects on performance as indicated by the number of crossings over the previous 10 cm × 10 cm target area (see Fig 2 and Table 4). Post hoc analysis indicated that olanzapine was associated with an overall impairment in performance (p=0.040) that was independent of the session. Chlorpromazine was not associated with significant effects on performance of probe trials
Fig. 2.
Antipsychotic effects on a water maze probe trial performance as assessed by the number of platform area crossings (mean ± S.E.M.). S refers to the testing session; the probe trial was conducted on the last day of each of the sessions. S1= days 8–14; S2= days 22–28; S3 =days 39–45; S4 = days 84–90; S5= days 174–180 of drug treatment. VEH= vehicle controls; CPZ= chlorpromazine 10.0mg/kg/day; OLZ =olanzapine 10.0mg/kg/day.
Visible Platform Test
After probe trials on session 5, visible platform tests (4 trials per session per group) were conducted to ensure that the test subjects did not exhibit crude deficits in visual acuity that might have confounded the water maze hidden platform and probe trial analyses. The latencies for the 3 groups to find the visible platform (i.e., the mean of the 4 trials per session) ranged from 5.2 to 7.7 sec. There were no significant treatment-related effects observed in this procedure (i.e., all p values were >0.05, data not shown).
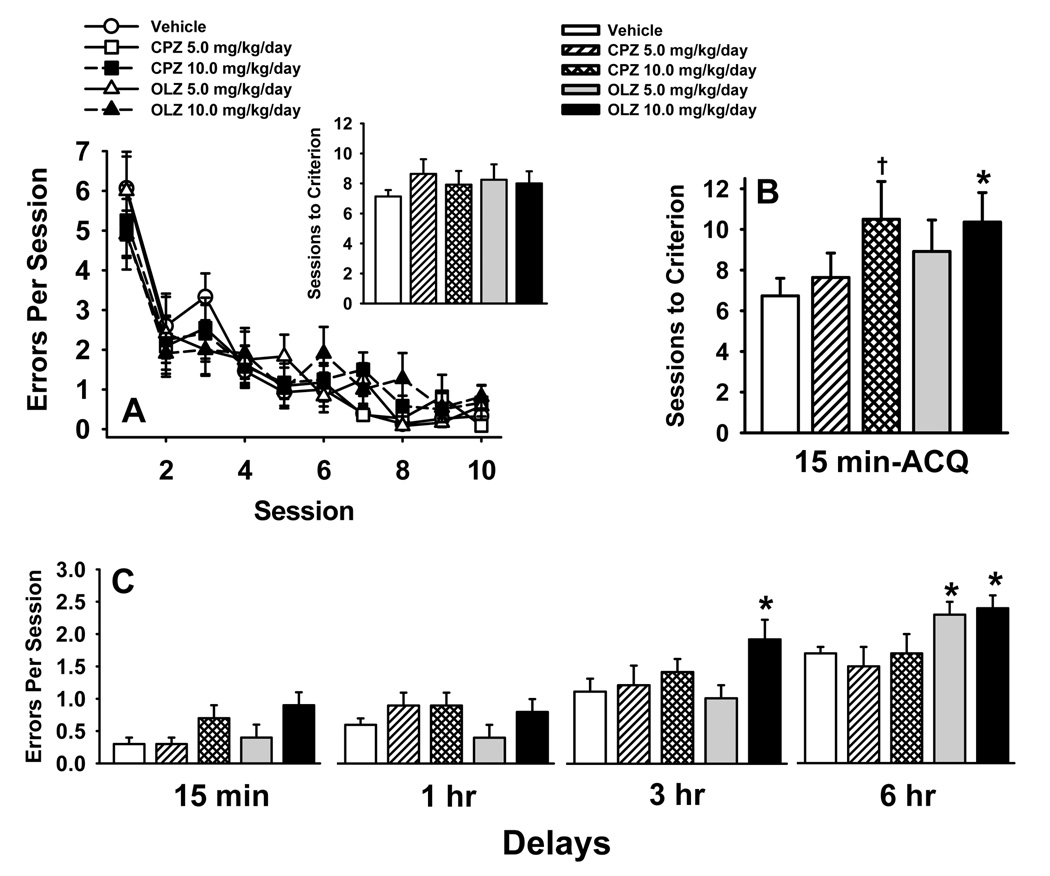
Radial Arm Maze
Fig 3A illustrates the effects of the antipsychotics on acquisition in an 8-arm radial maze over 10 consecutive days of testing (Win-Shift Task). There was a significant effect of the day of testing, (see Table 4) indicating that significant learning occurred over the 10 days of training), however, there were no significant antipsychotic-related differences in performance (i.e., the treatment and treatment × session interactions were not statistically significant) in the Win-Shift task. Likewise, there were no significance differences in the number of trials required to meet a pre-set training criterion of ≤ 2 errors for 4 consecutive sessions (Fig 3A inset). Fig 3B illustrates the effects of the antipsychotics on acquisition of a delayed non match to position (DNMTP) task at 15 minutes delays (number of trials to criterion). While the main effect for treatment was not statistically significant (see Table 4) there were clear trends toward inferior performances in the rats administered the higher doses of chlorpromazine and olanzapine. Specifically, pair-wise post hoc analyses indicated a p=0.06 and p=0.03 for differences in the number of trials required to meet the pre-set training criterion (≤ 1 error for 4 consecutive sessions) between groups administered the higher doses of chlorpromazine or olanzapine (respectively) and vehicle. Fig 3C illustrates the effects of the antipsychotics on performance of the DNMTP task where several delays were assessed. Depending on dose, olanzapine (but not chlorpromazine) was associated with significant impairments in accuracy at both the three and six hour delays.
Fig. 3.
Antipsychotic effects on radial arm maze performance conducted during days 15–60 of treatment. A. Win-shift acquisition over 10 consecutive days of testing as assessed by the number of errors per session (mean ± S.E.M.). Inset. Win-shift acquisition as assessed by the number of trials to reach a pre-determined criterion (mean ± S.E.M.) which was defined as four consecutive sessions with ≤ 2 total errors. B. Acquisition of a delayed non match to position (DNMTP) task at 15 minutes delays as assessed by the number of trials to criterion (mean ± S.E.M.) which was defined as ≤ 1 error for four consecutive sessions during the free eight sessions. C. Delay dependent performance of the DNMTP task as assessed by the number errors per session (mean ± S.E.M.). * = significantly (p<0.05) inferior performance when compared to vehicle control. † = nearly significant (p=0.06) performance deficit when compared to vehicle control.
Assessments of Motor Function
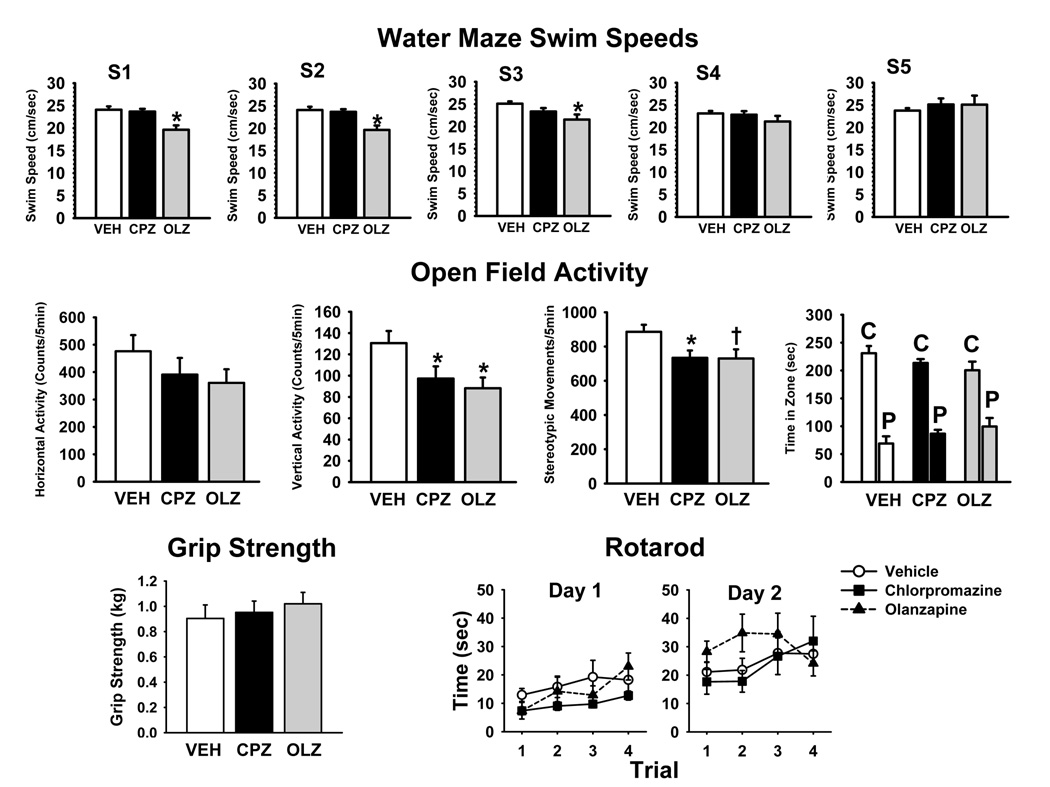
In these experiments we were interested in determining whether the antipsychotics had significant effects on motor function that might have influenced performance in the memory-related tests, particularly the water maze experiments. The results of these experiments are provided in Fig 4.
Fig. 4.
Antipsychotic effects on motor function conducted during days 50–53 of treatment. Top: Water maze swim speeds measured in cm/sec. S refers to the testing session; S1= days 8–14; S2= days 22–28; S3 =days 39–45; S4 = days 84–90; S5= days 174–180 of drug treatment. Middle: Open Field Activity, left to right: horizontal activity measured as the number of photobeam breaks/5 min; Vertical activity measured as the number of photobeam breaks/5 min; stereotypical movements (repetitive photobeam breaks/ 5 min); fear/anxiety related behavior measured as the time spent in the central (C) versus the peripheral (P) zone of the activity monitor. Bottom Left: Forelimb Grip strength measured in Kg of resistance. Bottom Right: Accelerating Rotarod performance expressed as time maintained on a rotating bar that accelerated from 4 to 40 rpm over a 5-min period. The bars or points represent the mean ± S.E.M. * = significantly different (p–0.05) from vehicle control. VEH= vehicle controls; CPZ= chlorpromazine 10.0mg/kg/day; OLZ =olanzapine 10.0mg/kg/day.
Water Maze Swim Speeds
Average swim speeds ± SEM at each of the water maze testing sessions are depicted in the top row of Fig 4. There were significant antipsychotic-related effects on swim speeds (i.e., both the treatment effect and the two factor interaction between treatment and session were statistically significant, see Table 4). Slightly (but significantly) slower swim speeds were observed in animals administered olanzapine (compared to vehicle controls) in the first three test sessions. There were no significant effects of chlorpromazine on swim speeds.
Open Field Activity
The middle row of Fig 4 illustrates the effects of the drug treatments on horizontal and vertical locomotor activity, stereotypical movements, as well as fear/anxiety-related behaviors (i.e., time spent in the peripheral versus central zone of the test apparatus). There were significant or nearly significant treatment-related decreases in vertical exploratory activity and stereotypical movements associated with both chlorpromazine and olanzapine. There were no significant treatment-related effects on horizontal exploratory activity or the time spent in the center versus peripheral zones of the open field apparatus.
Grip Strength
The effects of chlorpromazine and olanzapine on forelimb grip strength are illustrated at the lower left portion of Fig 4. There were no statistically significant treatment-relates effects on this measure of motor function (see Table 4).
Rotarod Performance
The effects of chlorpromazine and olanzapine on the performance of the rotarod task are illustrated at the bottom right of Fig 4. There was a significant trial effect indicating that the subjects performed better over the course of the two days of repeated testing. While there was a trend toward improved performance in the animals administered olanzapine, (particularly on day 2 of testing) there were no statistically significant treatment-related effects (see Table 4) on performance of the rotarod task.
Discussion
The results of this study can be summarized as follows: 1) both olanzapine and chlorpromazine produced impairments in the acquisition of a water maze spatial learning task that could eventually be overcome with repeated testing; 2) both olanzapine and chlorpromazine were associated with decrements in delayed non-match to position trials in the radial arm maze task, although the effects of olanzapine were more pronounced especially as the demands of the task increased (i.e., at longer delay intervals), 3) there was some evidence of impaired motor function associated with the antipsychotics (especially olanzapine) as indicated by slightly reduced swim speeds in the water maze and decreased activity in some components of the open field assessment, 4) the method used for administering antipsychotic drugs orally in drinking water is a valid approach as exemplified by the fact that the drugs were stable (i.e., diluted in tap water or deionized water) for at least 96 hours at room temperature, and that the plasma antipsychotic values generated in the rat generally approximated those generally considered therapeutic in humans.
In the behavioral studies, the repeated acquisition approach to water maze testing was chosen specifically for the purpose of evaluating the effects of olanzapine and chlorpromazine on learning/encoding and retrieval (repeatedly) over time. Impaired information encoding and retrieval capacity are commonly reported in schizophrenia (Gur, et al., 2000; Cairo et al., 2006). Moreover, learning potential (i.e., as determined in multiple administrations of neurocognitive tests) predicts work skill attainment in schizophrenia, a factor known to predict the rehabilitation outcome (Sergi et al., 2005). Accordingly, the data obtained in this study appear to suggest that both chlorpromazine and olanzapine can adversely affect information encoding and as a result, negatively affect learning potential.
As noted above, olanzapine was associated with slightly reduced swim speeds in the water maze in the earlier test sessions, and both olanzapine and chlorpromazine were associated with some reductions in vertical exploratory activity and stereotypical movements. Such observations raise the question of whether some type of psychomotor impairment may have contributed to poor water maze performance in the olanzapine-treated animals. However, the absence of antipsychotic-related effects in the visible platform tests in the water maze, on grip strength, rotarod performance, or horizontal activity (or time spent in the peripheral zones of the open field apparatus), argues against the premise that gross drug effects on locomotor activity, visual acuity, or anxiety levels could explain the observed deficits in water maze performance. Further support for this argument is evident in the fact that rats administered olanzapine performed less efficiently than vehicle controls in session 4 of water maze experiments even thought swims speeds were not different between the groups.
The radial arm maze experiments indicated that the rats in all of the treatment groups were quite efficient at learning the Win-Shift task over 10 days of repeated testing. Only when the demands of the task were increased, were treatment related impairments in performance apparent. Specifically, there was some evidence of impaired acquisition of the DNMTP task at a 15 minute delay associated with the higher doses of both chlorpromazine and olanzapine. In the subsequent DNMTP experiments, where several delays were evaluated, the most notable finding was that both doses of olanzapine clearly impaired performance during the most demanding test sessions (i.e., DNMTP with six hour delays).
As noted above in the Methods section, the oral antipsychotic dosing approach used in this study (i.e., drugs delivered in drinking water and dosed on a mg/kg/24 hour basis) was based on previous studies in our laboratory (e.g., Terry et al., 2005) where the plasma drug levels achieved approximated those generally considered therapeutic in humans. While there are no published pharmacokinetic and/or D2 occupancy studies using our specific method of drug administration in rats, based on the acute oral dosing study in rats by Barth et al., 2006 (and extrapolations from Fig 1 in their study) and the study of Kapur et al., 2003 (cited above) we expected our antipsychotic dosing approach to generate D2 occupancy levels roughly in the range of 60–80 for chlorpromazine and olanzapine. The olanzapine plasma levels detected in the present study (i.e., a range of 22.6–31.0 ng/ml) are well within the general range of 9.0 to 80.0 ng/ml that has been estimated as therapeutic in humans (see Chetty et al., 1996; Perry et al., 2001; Rao et al., 2001; Gex-Fabry-Fabry et al., 2003). In the case of chlorpromazine the data were less clear. Due to the wide variability in chlorpromazine concentrations observed in humans, the therapeutic range has often been described as unknown (Baldessarini et al., 1988), although a therapeutic window of 25–300 ng/ml (Curry et al., 1970) and 30–350 ng/ml (Chetty et al., 1996) has been suggested. Based on these studies, the levels detected in our study in rats (6.2–15.4) may be considered somewhat below the optimum therapeutic range. Accordingly, some caution should be exercised when interpreting the chlorpromazine-related behavioral results especially when making direct comparisons to the olanzapine-related findings.
There are similarities in the pharmacology of chlorpromazine and olanzapine that could be important to the behavioral findings in this study. Both antipsychotics serve as antagonists at dopamine D2 receptors in vitro (reviewed Seeman and Tallerico, 1998) and, interestingly, selective D2 antagonists (e.g., raclopride) have been observed to adversely affect spatial memory, and sustained attention (Von Huben et al., 2006). Both compounds also bind to all of the known muscarinic acetylcholine receptor subtypes with relatively high affinity (see Bolden et al., 1992 and Bymaster et al., 2003). Antagonists of muscarinic acetylcholine receptors are widely known to impair cognition in animals and humans across a variety of cognitive domains (see reviews, Decker and McGaugh, 1991; Bartus, 2000). The most notable difference in pharmacology between olanzapine and chlorpromazine (i.e., the feature which is thought to confer atypicality) is olanzapine’s high affinity (i.e., as an antagonist) for 5HT2A receptors (Schotte et al., 1996). However, 5HT2A antagonists have been associated with improvements in information processing, attention, and working memory in rodents and non-human primates (Varty et al., 1999; Winstanley et al., 2003; Terry et al., 2005b). Such observations would, therefore, not provide an explanation for impairments in behavioral performance observed with olanzapine in the present study. Finally, it should be emphasized that the cognitive effects of D2 and 5HT2A antagonists described above represent acute drug effects and it is unclear if such effects would persist during chronic treatment.
In conclusion, the results of this study in rats indicate that chronic oral treatment with clinically relevant doses of chlorpromazine or olanzapine can impair the performance of tasks designed to assess spatial learning and working memory. These data add to a growing body of animal evidence which suggests that both conventional and second generation antipsychotics can exert negative effects on specific components of cognition that are affected in schizophrenia (reviewed, Terry and Mahadik 2007). Given the importance of cognition to the functional outcome in schizophrenia, these studies emphasize the need for novel antipsychotics that are free of negative effects on cognition as well as adjunctive therapeutic agents that specifically target the cognitive deficits of schizophrenia and/or attenuate the negative effects of antipsychotics.
Acknowledgements
This work was supported by the National Institute of Mental Health (MH066233).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldessarini RJ, Cohen BM, Teicher MH. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch Gen Psychiatry. 1988;45:79–91. doi: 10.1001/archpsyc.1988.01800250095013. [DOI] [PubMed] [Google Scholar]
- Barth VN, Chernet E, Martin LJ, Need AB, Rash KS, Morin M, Phebus LA. Comparison of rat dopamine D2 receptor occupancy for a series of antipsychotic drugs measured using radiolabeled or nonlabeled raclopride tracer. Life Sci. 2006;78:3007–3012. doi: 10.1016/j.lfs.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J. Pharmacol. Exp. Ther. 1992;260:576–580. [PubMed] [Google Scholar]
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL. Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1125–1143. doi: 10.1016/j.pnpbp.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Cairo TA, Woodward TS, Ngan ET. Decreased encoding efficiency in schizophrenia. Biol Psychiatry. 2006;59:740–746. doi: 10.1016/j.biopsych.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Chetty M, Pillay VL, Moodley SV, Miller R. Response in chronic schizophrenia correlated with chlorpromazine, 7-OH-chlorpromazine and chlorpromazine sulfoxide levels. Eur Neuropsychopharmacol. 1996;6:85–91. doi: 10.1016/0924-977x(95)00047-s. [DOI] [PubMed] [Google Scholar]
- Curry SH, Marshall JH, Davis JM, Janowsky DS. Chlorpromazine plasma levels and effects. Arch Gen Psychiatry. 1970;22:289–296. doi: 10.1001/archpsyc.1970.01740280001001. [DOI] [PubMed] [Google Scholar]
- Decker MW, McGaugh JL. The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse. 1991;7:151–168. doi: 10.1002/syn.890070209. [DOI] [PubMed] [Google Scholar]
- Didriksen M, Kreilgaard M, Arnt J. Sertindole, in contrast to clozapine and olanzapine, does not disrupt water maze performance after acute or chronic treatment. Eur J Pharmacol. 2006;542:108–115. doi: 10.1016/j.ejphar.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005;172:1703–1711. doi: 10.1503/cmaj.1041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gex-Fabry M, Balant-Gorgia AE, Balant LP. Therapeutic drug monitoring of olanzapine: the combined effect of age, gender, smoking, and comedication. Ther Drug Monit. 2003;25:46–53. doi: 10.1097/00007691-200302000-00007. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognition, drug treatment, and functional outcome in schizophrenia: a tale of two transitions. Am J Psychiatry. 2007;164:992–994. doi: 10.1176/ajp.2007.164.7.992. [DOI] [PubMed] [Google Scholar]
- Gur RC, Moelter ST, Ragland JD. Learning and memory in schizophrenia. In: Sharma T, Harvey P, editors. Cognition in schizophrenia: Impairments, importance, and treatment strategies. Oxford, England: Oxford University Press; 2000. pp. 73–91. [Google Scholar]
- Harvey PD, Green MF, Keefe RS, Velligan DI. Cognitive functioning in schizophrenia: a consensus statement on its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry. 2004;65:361–672. [PubMed] [Google Scholar]
- Hernandez CM, Høifødt H, Terry AV., Jr Spontaneously hypertensive rats: Further evaluation of age-related memory performance and cholinergic marker expression. Journal of Psychiatry and Neuroscience. 2003;28:197–209. [PMC free article] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Roitman SE, Harvey PD, Blum CS, DuPre RL, Prieto DM, Davidson M, Davis KL. A pen-and-paper human analogue of a monkey prefrontal cortex activation task: spatial working memory in patients with schizophrenia. Schizophr Res. 1995;17(1):25–33. doi: 10.1016/0920-9964(95)00027-j. [DOI] [PubMed] [Google Scholar]
- Levin ED, Galen DM, Ellison GD. Chronic haloperidol effects on oral movements and radial-arm maze performance in rats. Pharmacol Biochem Behav. 1987;26:1–6. doi: 10.1016/0091-3057(87)90523-5. [DOI] [PubMed] [Google Scholar]
- Levin ED. Chronic haloperidol administration does not block acute nicotine-induced improvements in radial-arm maze performance in the rat. Pharmacol Biochem Behav. 1997;58:899–902. doi: 10.1016/s0091-3057(97)00052-x. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Hippocampal and nonhippocampal contributions to place learning in rats. Behav Neurosci. 1995;109:579–593. doi: 10.1037//0735-7044.109.4.579. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Nowakowska E, Chodera A, Kus K, Rybakowski J. Some behavioural effects of risperidone in rats: comparison with haloperidol. Eur Neuropsychopharmacol. 1999;9:421–426. doi: 10.1016/s0924-977x(99)00021-8. [DOI] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17(6):669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Park S, Puschel J, Sauter BH, Rentsch M, Hell D. Spatial working memory deficits and clinical symptoms in schizophrenia: a 4-month follow-up study. Biol Psychiatry. 1999;46(3):392–400. doi: 10.1016/s0006-3223(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Lund BC, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response: acute phase results of the North American Olanzapine Trial. J Clin Psychopharmacol. 2001;21:14–20. doi: 10.1097/00004714-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Rao ML, Hiemke C, Grasmäder K, Baumann P TDM Arbeitsgruppe Der AGNP. Olanzapine: pharmacology, pharmacokinetics and therapeutic drug monitoring. Fortschr Neurol Psychiatr. 2001;69:510–517. doi: 10.1055/s-2001-18381. [DOI] [PubMed] [Google Scholar]
- Rosengarten H, Quartermain D. The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three- and eighteen-month-old rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1047–1054. doi: 10.1016/s0278-5846(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3:123–134. doi: 10.1038/sj.mp.4000336. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Kern RS, Mintz J, Green MF. Learning potential and the prediction of work skill acquisition in schizophrenia. Schizophr Bull. 2005;31:67–72. doi: 10.1093/schbul/sbi007. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Hernandez CM, Buccafusco Dahl-salt sensitive and salt-resistant rats: examination of learning and memory performance, blood pressure, and the expression of central nicotinic-acetylcholine receptors. Neuroscience. 2001;103:351–363. doi: 10.1016/s0306-4522(00)00569-8. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Hill WD, Parikh V, Evans DR, Waller JL, Mahadik SP. Differential effects of chronic haloperidol and olanzapine exposure on brain cholinergic markers and spatial learning in rats. Psychopharmacology (Berl) 2002;164:360–368. doi: 10.1007/s00213-002-1230-z. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Hill WD, Parikh V, Waller JL, Evans DR, Mahadik SP. Differential effects of haloperidol, risperidone, and clozapine exposure on cholinergic markers and spatial learning performance in rats. Neuropsychopharmacology. 2003;28:300–309. doi: 10.1038/sj.npp.1300039. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Mahadik SP, Warsi S, Davis LW, Waller JL. Chronic exposure to typical or atypical antipsychotics in rodents: Temporal effects on central alpha 7 nicotinic acetylcholine receptors. Neuroscience. 2005a;136:519–529. doi: 10.1016/j.neuroscience.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Bartoszyk GD. Selective serotonin 5-HT2A receptor antagonist EMD 281014 improves delayed matching performance in young and aged rhesus monkeys. Psychopharmacology (Berl) 2005b;179:725–732. doi: 10.1007/s00213-004-2114-1. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Parikh V, Gearhart DA, Pillai A, Hohanadel E, Warner S, Nasrallah HA, Mahadik SP. Time dependent effects of haloperidol and ziprasidone on nerve growth factor, cholinergic neurons, and spatial learning in rats. J Pharmacol Exp Ther. 2006;318:709–724. doi: 10.1124/jpet.105.099218. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Warner SE, Zhang G, Bartlett MG, Middlemore ML, Beck WD, Jr, Mahadik SP, Waller JL. Oral haloperidol or risperidone treatment in rats: Temporal effects on nerve growth factor receptors, cholinergic neurons, and memory performance. Neuroscience. 2007a;146:1316–1332. doi: 10.1016/j.neuroscience.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Warner SE, Hohnadel EJ, Middlemore ML, Zhang G, Bartlett MG, Mahadik SP. Protracted Effects of Chronic Oral Haloperidol and Risperidone on Nerve Growth Factor, Cholinergic Neurons, and Spatial Reference Learning in Rats. Neuroscience. 2007b;150:413–424. doi: 10.1016/j.neuroscience.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Mahadik SP. Time Dependent Cognitive Deficits Associated with First and Second Generation Antipsychotics: Cholinergic Dysregulation as a Potential Mechanism. J Pharmacol Exp Ther. 2007;320:961–968. doi: 10.1124/jpet.106.106047. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Varty GB, Bakshi VP, Geyer MA. M100907, a serotonin 5-HT2A receptor antagonist and putative antipsychotic, blocks dizocilpine-induced prepulse inhibition deficits in Sprague-Dawley and Wistar rats. Neuropsychopharmacology. 1999;20:311–321. doi: 10.1016/S0893-133X(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Von Huben SN, Davis SA, Lay CC, Katner SN, Crean RD, Taffe MA. Differential contributions of dopaminergic D(1)- and D(2)-like receptors to cognitive function in rhesus monkeys. Psychopharmacology (Berl) 2006 Mar 15; doi: 10.1007/s00213-006-0347-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Zhang G, Terry AV, Bartlett MG. Liquid Chromatography / Tandem Mass Spectrometry for the Simultaneous Determination of Olanzipine, Risperidone, 9-Hydroxyrisperidone, Clozapine, Haloperidol and Ziprasidone in Rat Plasma.”. Rapid Communications in Mass Spectrometry. 2007a;21:920–928. doi: 10.1002/rcm.2914. [DOI] [PubMed] [Google Scholar]
- Zhang G, Terry AV, Bartlett MG. Sensitive Liquid Chromatography / Tandem Mass Spectrometry Method for the Determination of the Lipophilic Antipsychotic Drug Chlorpromazine in Rat Plasma and Brain Tissue. Journal of Chromatography B. 2007b;854:68–76. doi: 10.1016/j.jchromb.2007.03.045. [DOI] [PubMed] [Google Scholar]