Abstract
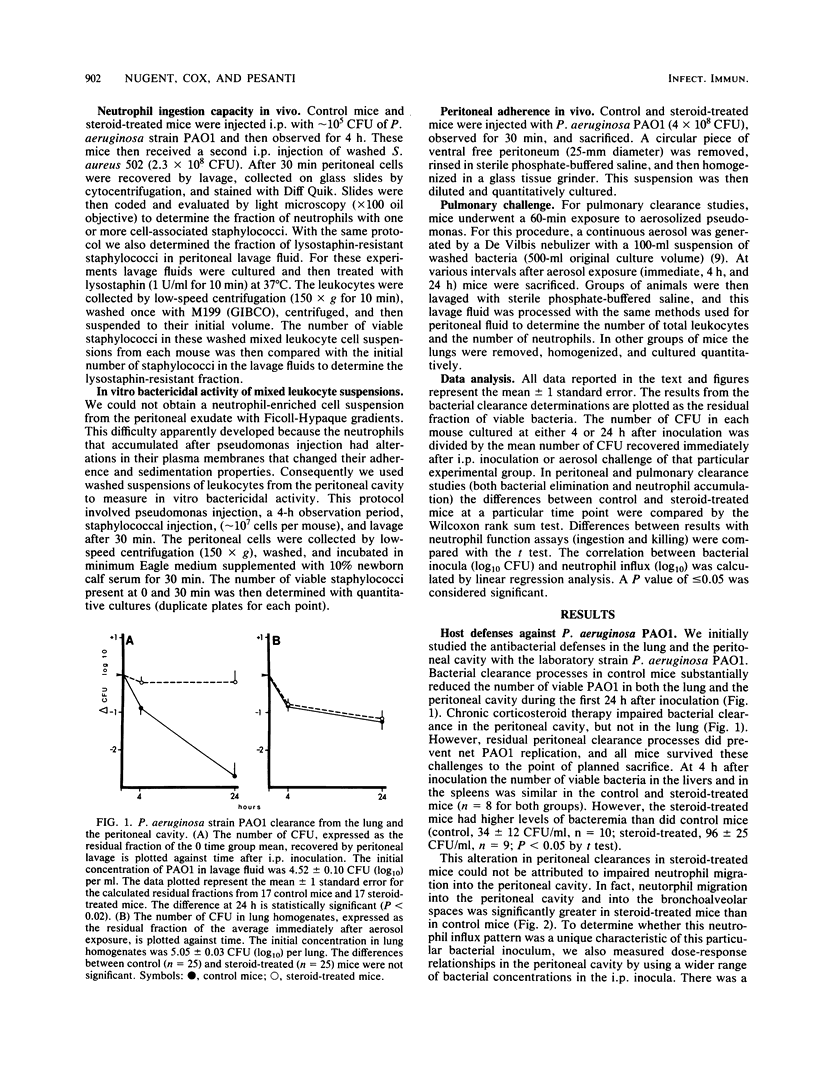
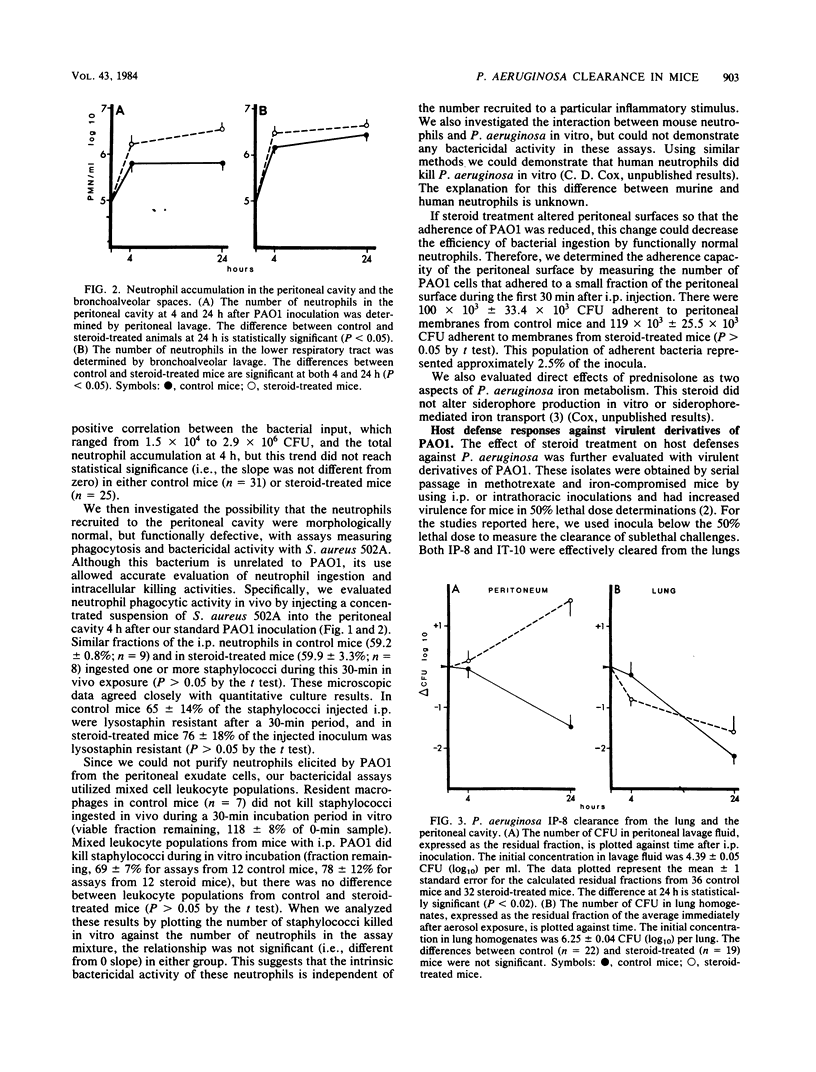
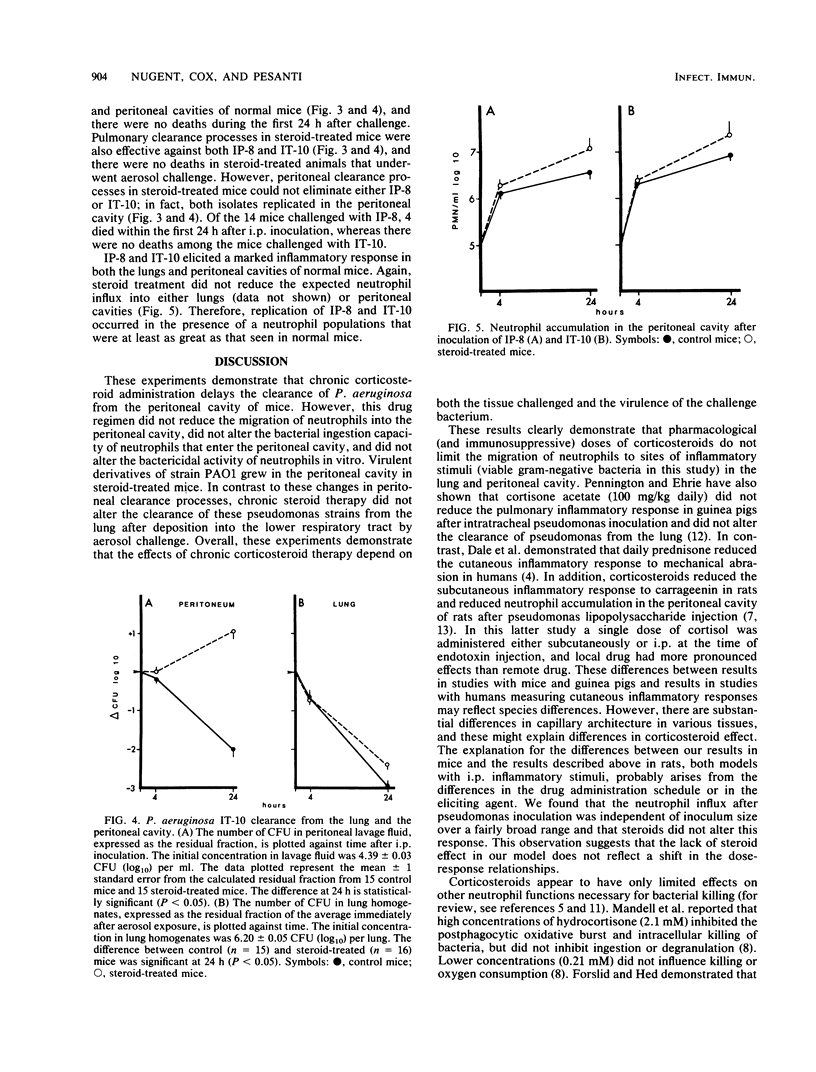
We used a murine model to determine whether chronic corticosteroid therapy has uniform effects on bacterial clearance processes in different tissues. After a 2-week regimen of oral prednisolone, Swiss Webster mice were challenged with Pseudomonas aeruginosa strain PAO1 or virulent derivatives of PAO1 (IP-8 and IT-10). Chronic corticosteroid therapy delayed the clearance of strain PAO1 from the peritoneal cavity. However, steroid treatment did not reduce the neutrophil influx, did not reduce the in vivo phagocytic capacity of neutrophils, and did not alter bactericidal activity of peritoneal exudate cells in vitro. Virulent isolates (IP-8 and IT-10) replicated in the peritoneal cavity in steroid-treated mice even though the neutrophil influx was similar to control mice. In contrast to the abnormal peritoneal clearance, all strains were rapidly cleared from the lungs of control and steroid-treated mice after aerosol challenge. Neutrophil influx into bronchoalveolar spaces was greater in steroid-treated mice than in control mice. All mice (control and steroid treated) survived these challenges, except for some steroid-treated mice infected intraperitoneally with IP-8. These results demonstrate that chronic steroid therapy alters bacterial clearance processes on the peritoneal surfaces to a greater extent than in the lower respiratory tract. The explanation for this altered clearance in the peritoneum is unclear, but cannot be explained by reductions in neutrophil migration to inflammatory stimuli. Therefore, the infectious risk associated with chronic corticosteroid therapy appears to depend on both the tissue and the virulence of the bacterial strain and may reflect alterations in clearance processes other than neutrophil migration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOGGS D. R., ATHENS J. W., CARTWRIGHT G. E., WINTROBE M. M. THE EFFECT OF ADRENAL GLUCOCORTICOSTEROIDS UPON THE CELLULAR COMPOSITION OF INFLAMMATORY EXUDATES. Am J Pathol. 1964 May;44:763–773. [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Effect of pyochelin on the virulence of Pseudomonas aeruginosa. Infect Immun. 1982 Apr;36(1):17–23. doi: 10.1128/iai.36.1.17-23.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Passage of Pseudomonas aeruginosa in compromised mice. Infect Immun. 1979 Oct;26(1):118–124. doi: 10.1128/iai.26.1.118-124.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale D. C., Fauci A. S., Wolff S. M. Alternate-day prednisone. Leukocyte kinetics and susceptibility to infections. N Engl J Med. 1974 Nov 28;291(22):1154–1158. doi: 10.1056/NEJM197411282912203. [DOI] [PubMed] [Google Scholar]
- FRUHMAN G. J. Adrenal steroids and neutrophil mobilization. Blood. 1962 Sep;20:355–363. [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C., Balow J. E. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976 Mar;84(3):304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- Forslid J., Hed J. In vitro effect of hydrocortisone on the attachment and ingestion phases of immunoglobulin G- and complement component 3b-mediated phagocytosis by human neutrophils. Infect Immun. 1982 Dec;38(3):811–816. doi: 10.1128/iai.38.3.811-816.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Rubin W., Hook E. W. The effect of an NADH oxidase inhibitor (hydrocortisone) on polymorphonuclear leukocyte bactericidal activity. J Clin Invest. 1970 Jul;49(7):1381–1388. doi: 10.1172/JCI106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent K. M., Pesanti E. L. Chronic glucocorticosteroid therapy impairs staphylococcal clearance from murine lungs. Infect Immun. 1982 Dec;38(3):1033–1036. doi: 10.1128/iai.38.3.1033-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent K. M., Pesanti E. L. Nonphagocytic clearance of Staphylococcus aureus from murine lungs. Infect Immun. 1982 Jun;36(3):1185–1191. doi: 10.1128/iai.36.3.1185-1191.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrillo J. E., Fauci A. S. Mechanisms of glucocorticoid action on immune processes. Annu Rev Pharmacol Toxicol. 1979;19:179–201. doi: 10.1146/annurev.pa.19.040179.001143. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Ehrie M. G. Pathogenesis of Pseudomonas aeruginosa pneumonia during immunosuppression. J Infect Dis. 1978 Jun;137(6):764–774. doi: 10.1093/infdis/137.6.764. [DOI] [PubMed] [Google Scholar]
- Perper R. J., Sanda M., Chinea G., Oronsky A. L. Leukocyte chemotaxis in vivo. II. Analysis of the selective inhibition of neutrophil or mononuclear cell accumulation. J Lab Clin Med. 1974 Sep;84(3):394–406. [PubMed] [Google Scholar]
- Rehm S. R., Gross G. N., Pierce A. K. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest. 1980 Aug;66(2):194–199. doi: 10.1172/JCI109844. [DOI] [PMC free article] [PubMed] [Google Scholar]



