Abstract
We present simple method to assess dental pain in the awake rat. Using a sensitive strain gauge we examined changes in bite strength and bite pattern in rats following dental injury. Rats with dental injury displayed a significant reduction in mean peak bite strength and an altered bite-cluster pattern. Both changes in the dental injury rats were reversed by an analgesic dose of morphine, and this could be reversed with naloxone. These changes were not observed in naive control animals. This simple method significantly improves our ability to evaluate dental pain syndromes.
Keywords: Dental Pain, Bite force, Bite pattern
1. INTRODUCTION
It is well established that patients with dental pain have a change in biting behavior [11]. For example, lesions involving the periodontal ligament (e.g. periapical or periodontal lesions) and pulp pathology (e.g. pulpitis) are usually accompanied by pain to tooth percussion [13]. Khan et al found teeth with acute periapical periodontitis or irreversible pulpitis to have a 77% reduction in mechanical pain thresholds, which was reversed with local anesthesia [5]. This pain has observable effects on the masticatory motor system. The pain from biting on diseased teeth induces the masseteric inhibitory reflex, leading to a jaw opening reflex (JOR) and a prolonged refractory period before another bite is made [9,14]. Indeed, the JOR is a well-used, validated measure of pain response in animals [8]. Similarly, following third molar extractions, patients also developed a reduced maximum bite force [11].
However, for an adequate understanding of the neurological mechanisms underlying dental pain, an animal model for dental pain is needed. Such a model would allow us to manipulate neural pathways for the study of those pathways and treatment options. Ideally, it would be representative of human dental pain, simple to perform, and have a consistent, graded response by the animal that can be measured quantitatively. Several animal models have been developed. Foong used an apparatus consisting of a cannula carefully implanted onto the rat’s incisor, and a protective neck funnel, to apply bradykinin into the pulp of an awake rat. It produced measurable changes in jaw movement pattern during biting. Morphine treatment reduced this biting activity, supporting a correlation between biting activity and induced pain [4]. Sunakawa et al similarly applied mustard oil, capsaicin, and bradykinin onto anaesthetized rats maxillary molars, and found increased electrical activity in the masseter muscle [18], again suggesting a functional link between dental pain and masticatory function. For this model, the rat may not be conscious. Byers and Chudler quantified dental pain-related behavioral changes using minimally-intrusive measurements. They demonstrated that rats displayed measurable weight loss, decreased exploration, and increased freezing behavior on the third day following Fluorogold implantation in the molars, [2]. And though not studying specifically dental pain, Ro quantified orofacial muscle pain in awake rats by measuring the reduction in bite force and bite pattern regularity following muscle inflammation [12].
We have previously shown that infraorbital nerve neuritis in the rat results in a reduced biteforce; the onset and duration were parallel to those seen in the sensory modalities that were tested (mechanohyperalgesia, allodynia) suggesting that biteforce reduction is an indicator of pain [1].
We sought to develop an animal model for measuring dental pain that is relatively simple to induce and measure in awake rats. Based on accumulated data, we hypothesized that nociceptive input from dental and orofacial structures also induces a decrease in bite force amplitude and an alteration bite pattern in the rat. The aim of this present study was to test that: the quantitative assessment of rat bite force and biting pattern as a measure of experimental dental pain.
2. MATERIALS AND METHODS
Protocol and regulations
Experiments were performed according to a protocol that was approved by the NIDCR Animal Care and Use Committee, and also in accordance with federal law, the regulations of the National Institute of Health, and the guidelines of the International Association for the Study of Pain [23].
Animals and Groups
Twenty-five Adult male Long Evans Rats (350–450g) were used. Sodium Pentobarbital (50 mg/kg i.p.) was used as a general anesthetic for all surgical procedures. Rats were randomly assigned to three following groups:
The first group consisted of 12 rats that underwent drilling of the maxillary left incisor on the mesial surface. The drill penetrated to the pulp, resulting in a pulpal exposure.
The second group had 6 rats that underwent superficial drilling of the maxillary left incisor. The drill passed through the dento-enamel junction, (DEJ), resulting in a dentinal exposure. If the pulp was inadvertently exposed, either directly or indirectly, the animal was excluded from the study and promptly euthanized.
A third group of 7 rats underwent general anesthesia with pentobarbital anesthesia alone.
No drilling was performed on this group, which served as a control.
All the rats were euthanized 48 hours following the procedure.
Testing apparatus and Equipment
Rats were placed in a Plexiglas restraining device that exposed the animal’s snout and allowed free movements of the jaw. Animals were allowed to spontaneously bite on a coated strain gauge. The apparatus (20mm × 5mm × 3mm, figure 1) was calibrated to measure the bite force using an oscilloscope. Bite force amplitudes were recorded in relation to time, forming a plot that displayed both bite strengths and patterns (figure 2). Regarding bite patterns, we looked specifically at the percentage of bites occurring in clusters. Bite clusters were defined as two bites or more occurring within less than 0.5 seconds from each other. The percentage of bites occurring in clusters was calculated for each animal. Also, for each rat, first eight bite amplitudes were measured prior to and following surgical or anesthetic manipulation. Measurements were taken for 10 minutes.
Figure 1.
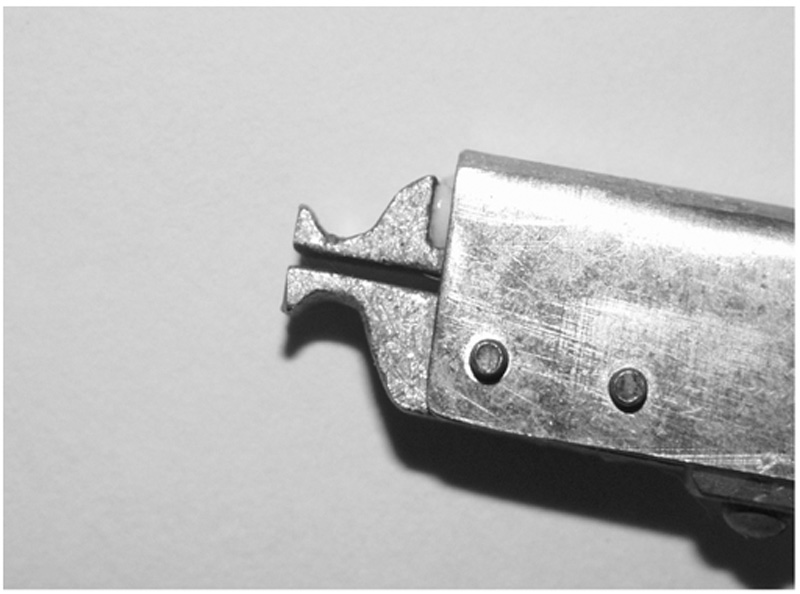
The bite gauge device; the design of the bite gauge helps the rat to bite on the concaved area.
Figure 2. Examples of bites as recorded on the monitor.
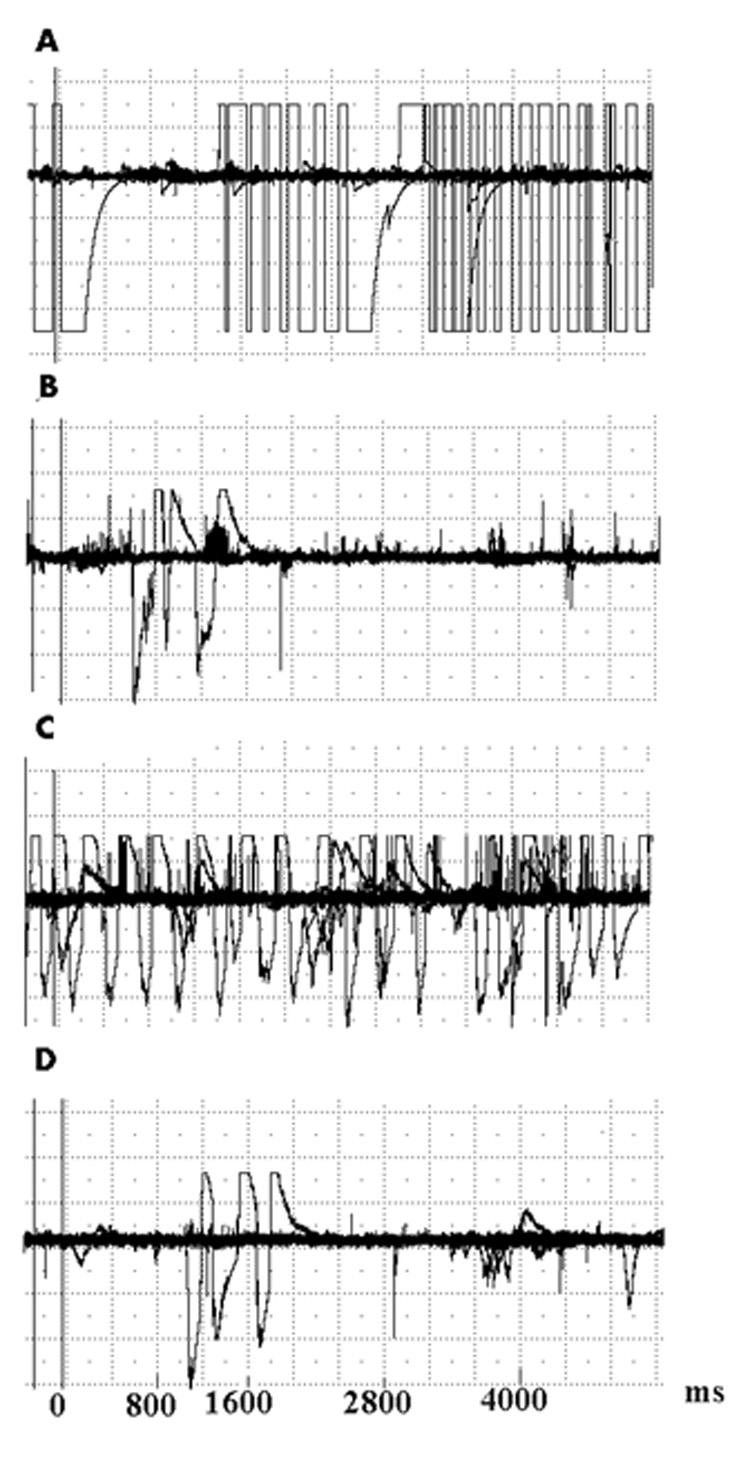
A. Base line recording, prior to treatment. In this case the baseline bites amplitude approached the upper level of the device capacity.
B. Twenty-four hours following pulp exposure, there is a reduction in bite force amplitudes and frequencies.
C. Following administration of 5mg/kg of morphine s.c., bite force amplitude and frequency are restored to near-baseline values.
D. Following administration of 1mg/kg naloxone i.p., bite force amplitude and frequency are again reduced.
Time Lines and measurements
All animals were tested for bite force amplitude and bite force pattern prior to any procedure. After baseline data was obtained, they were retested at 4 and 24 hours following the dental procedure.
Twenty-four hours post-surgery, morphine (5mg/kg s.q.) was administered to 5 of the 12 rats that had pulpal exposure. Bite force and bite pattern readings were again recorded one hour after the injection. Naloxone (1mg/kg i.p.) was subsequently administered, and bite readings were taken one hour later. The remaining rats in that group (n=7) were further tested for bite force amplitude and pattern 48 hours following the procedure. For all three groups, 48 hours after the dental procedure, three drilled incisors from each group were extracted under pentobarbital anesthesia. The teeth were decalcified and paraffin-embedded, and sections (5µm) were cut and stained with hematoxylin-eosin.
Statistical Methods
The alpha level for significance was set at 0.05. Data was tabulated and analyzed using Stat View 5 (SAS Inc, Cary, North Carolina). For bite force measurements changes from baseline were calculated and expressed as mean ± standard error of the mean. Changes in bite force amplitude and percentage of bites in cluster were tested within groups and comparisons between groups were made using a factorial analysis of variance (ANOVA) followed by a Student Newman Keuls (SNK) pair wise comparison. For bite patterns the percentage of bite measurements that appeared as clusters were calculated and data analyzed as above.
3. RESULTS
Baseline Results
No significant differences in the bite force amplitude and the bite force pattern were observed between the groups at baseline. The baseline bites amplitude approached the upper level of the device capacity.
Bite Force Amplitude (Fig. 3)
Figure 3.
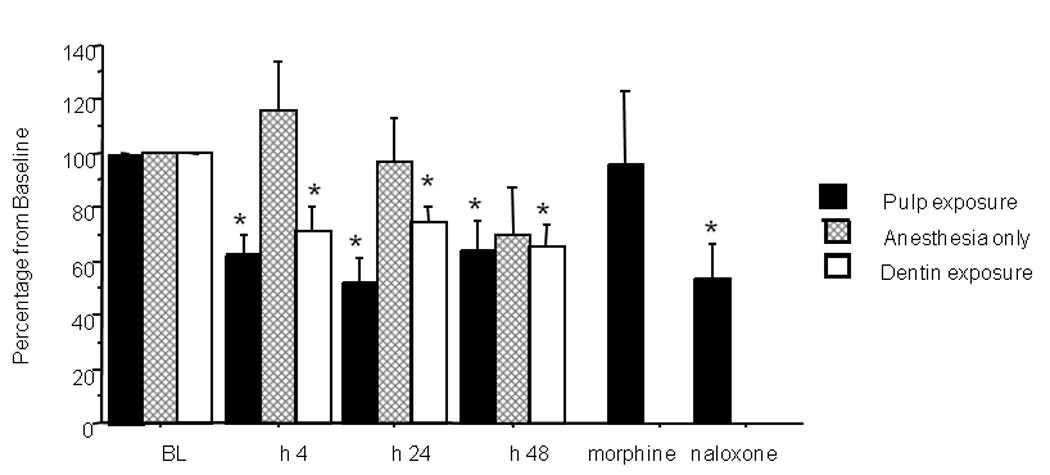
Effects of tooth manipulation on bite force amplitude (data expressed as percent change from baseline). A significant (*) reduction compared to baseline was observed at 4, 24 and 48 hours following pulp exposure (P= 0.001, 0.001 and, 0.015 respectively). Administration of 5 mg/kg s.c. morphine returned bite force amplitudes to baseline values, an effect that was significantly reversed by 1 mg/kg i.p. naloxone. Significant reductions were also observed in the superficial drilling (dentin exposure) group. The anesthesia-only group showed bite force changes that were not significantly different from baseline. Morphine was administered to 5 of the 12 rats that had pulpal exposure 24 houres following the pulp exposure, the remaining 7 rats were tested for the 48 h time point.
Four hours following pulp exposure, bite force amplitude was significantly reduced to 61.9 ± 8.2 % of baseline levels in the pulp exposure group (P = 0.001). Twenty-four hours post exposure, the bite force amplitude was 51.9 ± 9.4 % of baseline (P = 0.001). Forty-eight hours later the bite force amplitude was 63.5 ± 11.5 % of baseline (P = 0.015). One hour following morphine administration the bite force amplitude in the pulp-exposed group was 95.2 ± 28.6 % of baseline, which was not significantly different from the baseline value. Naloxone administration reversed the morphine effect and reduced the bite force amplitude to 53.6 ± 12.9% of baseline (P = 0.023).
In the dentin exposure (superficial drilling) group, bite force amplitude was reduced to 71.4 ±8.6 % of baseline (P=0.029) at 4 hours post-drilling. Twenty-four hours post drilling, bite force amplitude was 74.3 ± 5.6%t of baseline (P= 0.010). At the 48 hour time point, bite force was further reduced to 65.4 ± 8.5 % of baseline (P=0.015).
In the anesthesia-only group, there were no significant changes in bite force amplitude at the 4, 24 and 48-hour time points (115.7 ± 18.4%, 97.2 ± 15.7%, 70.4 ± 16.9% relative to baseline, respectively).
Between-group comparisons
There were significant differences in bite force amplitudes between groups at 4 and 24 hours following the procedure (P=0.0027, P=0.022 respectively ). Pair-wise comparison with a SNK revealed a significant difference in bite amplitudes between the pulpal exposure group and the anesthesia-only group. This was seen for both the 4 and 24 time-points. This was also observed between the pulpal exposure group and the dentin exposure group at the 24 hour time-point. The dentine exposure group bite force was not significantly reduced compared to the anesthesia-only group.
Bite Force Pattern (Fig. 4)
Figure 4.
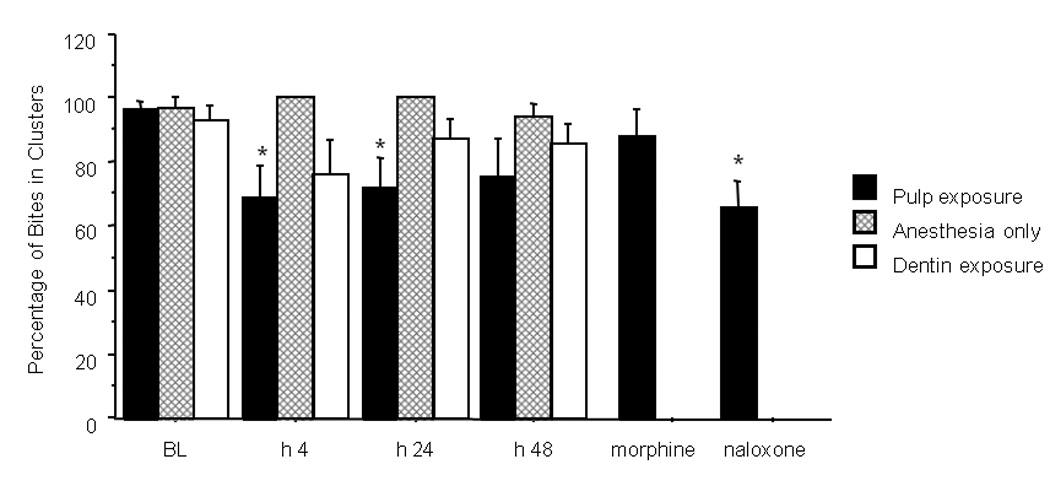
Changes in percent of bites occurring in clusters following dental drilling. Pulp exposure caused a significant (*) reduction at 4 and 24 hours post exposure (P= 0.043 and 0.046 respectively). Administration of 5 mg/kg s.c. morphine attenuated this effect to baseline values; this was significantly reversed by i.p. 1 mg/kg naloxone. In the superficially drilled group, a reduction (not significant ) was seen at 4 hours post-drilling that returned to normal by 24 hours post drilling. In the anesthesia only group no changes from baseline values were noted at all time point relative to baseline. Morphine was administered to 5 of the 12 rats that had pulpal exposure 24 houres following the pulp exposure, the remaining 7 rats were tested for the 48 h time point.
Prior to treatment, 96.3 ± 2.7 % of the bites in the pulp-exposed group were part of a cluster, 92.6 ± 4.9% in the superficial drilling group, and 96.9±3.1% in the anesthesia-only group.
At 4 hours post-drilling 68.9 ± 10.3% of the bites occurred in clusters (P=0.043) in the pulp-exposed group, 76.2± 10.4 in the superficially drilled group, and 100% in the anesthesia only group.
Twenty-four hours post-drilling 71.6 ± 9.8% of bites occurred in clusters in the pulp-exposed group (paired t test, compared to baseline P=0.046), 87.6± 5.6% in the superficial drill group, and 100% in the anesthesia only group were part of a cluster. Forty-eight hours post-drilling, 75.2 ± 12.5% of the bites occurred in clusters in the pulp-exposed group, 85.4± 6.1% in the superficial drill group and 100 % in the anesthesia only group.
Between-groups comparisons
There were significant differences between groups in the percentage of bites occurring in clusters 24 hours following the drilling (P=0.0139). Pair-wise comparison with SNK revealed a significant difference between the pulp exposure group and the anesthesia-only group, when comparing their changes from baseline to 24 hours post-drilling. In the dentin exposure group, the percentage of bites in clusters were not significantly reduced compared to the anesthesia-only group.
Histology
Light microscopic examination of hematoxylin-eosin stained sections taken from the manipulated teeth 48 hours following the procedure revealed numerous immune cells within the pulp tissue in the pulp-exposed teeth, including easily recognizable lymphocytes and granulocytes. In the superficially drilled teeth, immune cells were not detected inside the pulp tissue.
4. DISCUSSION
Our study quantified changes in both bite force (that is, the amplitude, or maximal bite force endurance) and bite pattern following dental injury as a possible measure of dental pain.
Bite force has been widely used as an acceptable test for masticatory system function in humans [3,10, 20]. A reduction in bite force amplitude has been demonstrated in patients with disorders of the masticatory system, such as osteoarthritis or internal derangements of the TMJ [16, 17]. This reduced bite force may return to normal levels following surgery [21]. Inflammation in masticatory muscles also can cause a reduction in bite force, which is again returned to normal with anti-inflammatory medications [12]. Dental pathologies such as poor periodontal and dental conditions may also result in a reduction in the bite force amplitude [22, 15].
A change in bite pattern following injury to the masticatory system has also been demonstrated, though less extensively. Thut et al [19] showed that damage to the TMJ can cause alterations in the biting patterns of the animal. These changes can cause wider intervals of feeding between animals for feeding after the damage to the joint and masticatory system has been afflicted. Experimental measures of pain in infra orbital neuritis correlates, with both a reduced bite force and an altered bite pattern [1].
However, even in the presence of such models, bite force magnitude and pattern have not been correlated to experimental dental pain in animal models. In the present study we were able to detect reduced bite force amplitude and altered biting patterns following dental damage. The results may suggest a simple method to quantify dental pain.
Drilling into the pulp chamber produced pulpal damage accompanied by pulpal inflammation, as shown by the histological presence of inflammatory cells within the pulp at 48 hours following the procedure. The nature of the procedure together with the histological picture suggests pulpitis, which in humans, is clinically associated with pain to tooth percussion [6,13]. A reflex withdrawal reaction to the pain evoked on biting, and the induction of massetric inhibitory periods [9], may explain the reduced bite force amplitude and increased duration between bites (altered biting pattern). The change in bite amplitude and pattern was more likely related to acute pain, rather than direct structural damage and a resulting physical inability to bite, because the readings returned to normal following morphine administration. Interestingly superficial drilling into the incisors also caused a reduction in bite force at all measured time points, but no had effect on the bite cluster pattern. We believe that the reduction in bite force amplitude in the dentin exposure group is due to dentinal sensitivity and not ongoing pain, and is supported by the rat’s ability to bite in clusters.
One of the major limitations of the study is the fact that in the baseline measurements the bites amplitude approached the maximum limit of the device. Although it did not change the proof of the concept that tooth manipulation (probably painful) reduces the bite amplitude, less robust changes could be masked by this device limitation. For future research a more sensitive with wider range device should be used.
Further research should assess the changes in biting patterns and amplitudes following dental treatments (root canal therapy, periodontal treatment or surgery) followed by analgesic drug trials. The ability to accurately quantify dental pain in the rat will contribute to our understanding of pain mechanisms in the trigeminal territory.
Figure 5.
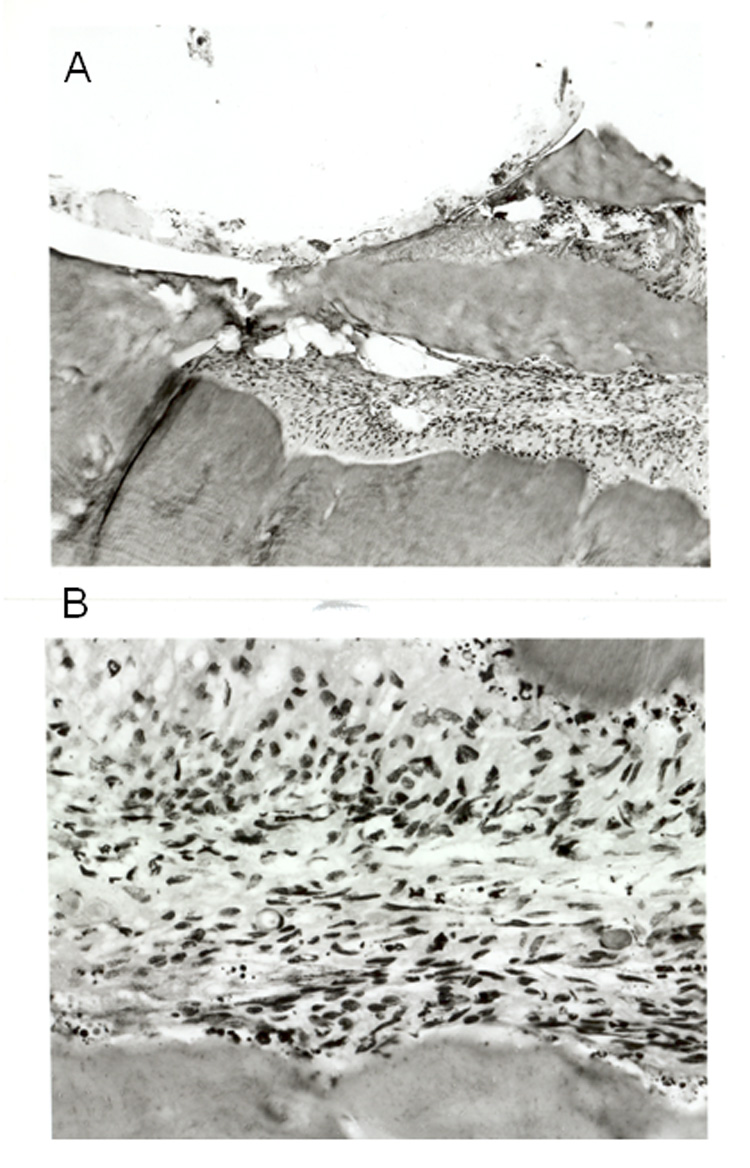
Light microscopic photographs of hematoxylin-eosin stained sections taken from the manipulated teeth 48 hours following the procedure. A. A section (x10) taken from the drilled area (upper part of the picture) B. A section (x 40) taken from the pulp, note the numerous immune cells within the tissue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Benoliel R, Wilensky A, Tal M, Eliav E. Application of a pro-inflammatory agent to the orbital portion of the rat infraorbital nerve induces changes indicative of ongoing trigeminal pain. Pain. 2002;99:567–578. doi: 10.1016/S0304-3959(02)00272-5. [DOI] [PubMed] [Google Scholar]
- 2.Chudler E, Byers M. Behavioural responses following tooth injury in rats. Arch Oral Biol. 2005;50:333–340. doi: 10.1016/j.archoralbio.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Coffey JP, Williams WN, Turner GE, Mahan PE, Lapointe LL, Cornell CE. Human bite force discrimination using specific maxillary and mandibular teeth. J Oral Rehabil. 1989;6:529–536. doi: 10.1111/j.1365-2842.1989.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 4.Foong FW, Satoh M, Takagi H. A newly devised reliable method for evaluating analgesic of drugs on trigeminal pain. J Pharmacol Methods. 1982;7:271–278. doi: 10.1016/0160-5402(82)90080-8. [DOI] [PubMed] [Google Scholar]
- 5.Khan AA, Owatz CB, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. Measure of mechanical allodynia and local anesthetic efficacy in patients with irreversible pulpitis and acute perioradicular periodontitis. J Endod. 2007;33:796–800. doi: 10.1016/j.joen.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Khan AA, McCreary B, Owatz CB, Schindler WG, Schwartz SA, Keiser K, Hargreaves K. The development of a diagnostic instrument for the measurement of mechanical allodynia. J Endod. 2007;33:663–667. doi: 10.1016/j.joen.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Lund JP, Stohler CS, Widmer CG. The relationship between pain and muscle activity in fibromyalgia and similar conditions. In: Vaeroy H, Mersky H, editors. Progress in Fibromyalgia and Myofascial Pain. Amsterdam: Elsevier; 1993. pp. 311–327. [Google Scholar]
- 8.Mason P, Strassman A, Maciewicz R. Is the jaw-opening reflex a valid model of pain? Brain Research. 1985;357:137–146. doi: 10.1016/0165-0173(85)90003-7. [DOI] [PubMed] [Google Scholar]
- 9.McGrath PA, Sharav Y, Dubner R, Gracely RH. Masseter inhibitory periods and sensations evoked by electrical tooth pulp stimulation. Pain. 1981;10:1–17. doi: 10.1016/0304-3959(81)90041-5. [DOI] [PubMed] [Google Scholar]
- 10.Osborn JW, Mao J. A thin bite-force transducer with three-dimensional capabilities reveals a consistent change in bite-force direction during human jaw-muscle endurance tests. Arch Oral Biol. 1993;38:139–144. doi: 10.1016/0003-9969(93)90198-u. [DOI] [PubMed] [Google Scholar]
- 11.Owatz CB, Khan AA, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. The incidence of mechanical allodynia in patients with irreversible pulpitis. J Endod. 2007;33:552–557. doi: 10.1016/j.joen.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Ro JY. Bite force measurement in awake rats: a behavioral model for persistent orofacial muscle pain and hyperalgesia. J Orofacial Pain. 2005;19:159–167. [PubMed] [Google Scholar]
- 13.Sharav Y. Orofacial pain. In: Wall PD, Melzack R, editors. Textbook of Pain. Fourth Edition. Hart Court publisher limited; 1999. pp. 711–738. [Google Scholar]
- 14.Sharav Y, McGrath P, Dubner R. Masseter inhibitory periods and sensations evoked by electrical tooth pulp stimulation in patients with oral facial pain and mandibular dysfunction. Arch Oral Biol. 1982;27:305–310. doi: 10.1016/0003-9969(82)90159-5. [DOI] [PubMed] [Google Scholar]
- 15.Shiau YY, Wang JS. The effects of dental condition on hand strength and maximum bite force. Cranio. 1993;11:48–54. doi: 10.1080/08869634.1993.11677940. [DOI] [PubMed] [Google Scholar]
- 16.Sinn DP, De Assis EA, Throckmorton GS. Mandibular excursions and maximum bite forces in patients with temporomandibular joint disorders. J Oral Maxillofac Surg. 1996;54:671–679. doi: 10.1016/s0278-2391(96)90678-3. [DOI] [PubMed] [Google Scholar]
- 17.Stegenga B, Broekhuijsen ML, De Bont LG, Van Willigen JD. Bite-force endurance in patients with temporomandibular joint osteoarthrosis and internal derangement. J Oral Rehabil. 1992;6:639–647. doi: 10.1111/j.1365-2842.1992.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 18.Sunakawa M, Chiang CY, Sessle BJ, Hu JW. Jaw electromyographic activity induced by application of algesic chemicals to the rat tooth pulp. Pain. 1999;80:493–501. doi: 10.1016/S0304-3959(98)00241-3. [DOI] [PubMed] [Google Scholar]
- 19.Thut PD, Hermanstyne TO, Flake NM, Gold MS. An operant conditioning model to assess changes in feeding behavior associated with temporomandibular joint inflammation in the rat. J Orofac Pain. 2007;21:7–18. [PubMed] [Google Scholar]
- 20.Waltimo A, Könönen M. A novel bite force recorder and maximal isometric bite force values for healthy young adults. Scand J Dent Res. 1993;101:1751–1755. doi: 10.1111/j.1600-0722.1993.tb01658.x. [DOI] [PubMed] [Google Scholar]
- 21.Widmark G, Haraldson T, Kahnberg KE. Joints problems reduces bite force but surgery elevates it almost back to normal Functional evaluation after TMJ surgery. J Oral Rehabil. 1995;8:589–593. doi: 10.1111/j.1365-2842.1995.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 22.Williams WN, Low SB, Cooper WR, Cornell CE. The effect of periodontal bone loss on bite force discrimination. J Periodontol. 1987;58:236–239. doi: 10.1902/jop.1987.58.4.236. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann M. Ethical guidelines for investigators of experimental pain in conscious animals. Pain. 1983;16:109–111. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


