Abstract
FXYD3 (Mat-8) proteins are regulators of Na,K-ATPase. In normal tissue, FXYD3 is mainly expressed in stomach and colon, but it is also overexpressed in cancer cells, suggesting a role in tumorogenesis. We show that FXYD3 silencing has no effect on cell proliferation but promotes cell apoptosis and prevents cell differentiation of human colon adenocarcinoma cells (Caco-2), which is reflected by a reduction in alkaline phosphatase and villin expression, a change in several other differentiation markers, and a decrease in transepithelial resistance. Inhibition of cell differentiation in FXYD3-deficient cells is accompanied by an increase in the apparent Na+ and K+ affinities of Na,K-ATPase, reflecting the absence of Na,K-pump regulation by FXYD3. In addition, we observe a decrease in the maximal Na,K-ATPase activity due to a decrease in its turnover number, which correlates with a change in Na,K-ATPase isozyme expression that is characteristic of cancer cells. Overall, our results suggest an important role of FXYD3 in cell differentiation of Caco-2 cells. One possibility is that FXYD3 silencing prevents proper regulation of Na,K-ATPase, which leads to perturbation of cellular Na+ and K+ homeostasis and changes in the expression of Na,K-ATPase isozymes, whose functional properties are incompatible with Caco-2 cell differentiation.
INTRODUCTION
Na,K-ATPase is an integral membrane protein that catalyzes an ATP-dependent transport of 3 Na+ out and 2 K+ into the cell. The Na+ and K+ gradients created by the Na,K-ATPase between the intra- and extracellular medium are crucial for the regulation of the cell volume and the membrane potential. The Na+ gradient also serves as the driving force for several facilitated membrane transport proteins, which import or export solutes and nutrients of vital importance for cellular homeostasis. Moreover, the maintenance of the cellular ion balance plays an important role in cell growth, differentiation, and cell death as well as in the activity of excitable tissues. Finally, Na,K-ATPase is abundantly and exclusively expressed in the basolateral membrane of epithelial cells, e.g., of the kidney and colon, and permits absorption of Na+ which contributes to the maintenance of the extracellular volume and blood pressure.
Structurally, Na,K-ATPase is an oligomeric protein consisting of a catalytic α subunit and two accessory subunits, a β subunit and a member of the FXYD protein family (Geering, 2008). The physiological relevance of this complex is highlighted by the recent elucidation of the crystal structure of renal Na,K-ATPase containing α, β, and γ subunits (Morth et al., 2007). The catalytic α subunit contains the binding sites for ATP and cardiotonic steroid inhibitors and transports the cations. Four α and three β isoforms have been identified. Their differential association results in diverse Na,K-ATPase isozymes, which are expressed and regulated in a tissue- and development-specific way and exhibit different functional properties (Blanco, 2005).
Recently, a family of small proteins called FXYD proteins was defined (Sweadner and Rael, 2000). In mammals, it contains seven members including phospholemman (FXYD1; Palmer et al., 1991), the Na,K-ATPase γ subunit (FXYD2; Mercer et al., 1993), mammary tumor marker 8, Mat-8 (FXYD3; Morrison et al., 1995), corticosteroid hormone-induced factor CHIF (FXYD4; Attali et al., 1995), protein “related to ion channel” Ric (FXYD5; Fu and Kamps, 1997), phosphohippolin (FXYD6; Yamaguchi et al., 2001), and FXYD7 (Béguin et al., 2002). In contrast to β subunits, which are indispensable for the structural and functional maturation of Na,K-ATPase (Geering, 2001), FXYD proteins are not necessary for Na,K-ATPase function. However, they were all found to be associated with Na,K-ATPase in a tissue-specific way and to modulate differentially Na,K-ATPase activity in line with the physiological needs of the tissues in which they are expressed (Geering, 2006, 2008).
In normal tissue, FXYD3 or Mat-8 is mainly expressed in colon and stomach where it is physically associated with Na,K-ATPase (Crambert et al., 2005). Interestingly, Mat-8 was also identified as a breast tumor protein, the expression of which is initiated by the oncogenes c-neu or v-Ha-ras but not by c-myc (Morrison and Leder, 1994) and which is up-regulated in prostate (Grzmil et al., 2004) and pancreatic (Kayed et al., 2006) cancer and down-regulated in colon and kidney cancer (Kayed et al., 2006). Grzmil et al. (2004) showed that small interfering RNA (siRNA)-mediated inhibition of FXYD3 expression promotes a decrease in prostate cancer cell line proliferation. Recently, we have identified two spliced variants of human FXYD3 in human colon adenocarcinoma cells (Caco-2 cells; Bibert et al., 2006). The short, prominent form and the long, minor form, which contains 26 additional amino acids in the juxtamembrane, cytoplasmic domain, are differentially expressed during Caco-2 cell differentiation and are associated with and can modify the transport properties of Na,K-ATPase in a distinct way after expression in Xenopus oocytes (Bibert et al., 2006). Together, these data suggest that FXYD3 may be implicated in the control of cell differentiation, proliferation, and/or apoptosis through regulation of Na,K-ATPase activity.
To better understand the physiological role of FXYD3 and the possible link with Na,K-ATPase regulation, we used Caco-2 cells to investigate the effect of FXYD3 silencing on cell proliferation, differentiation, and apoptosis and on Na,K-ATPase activity and expression. In particular, we show that knockdown of FXYD3 expression promotes a change in the functional properties of Na,K-ATPase and in Na,K-ATPase isozyme expression and impedes the differentiation process in Caco-2 cells.
MATERIALS AND METHODS
Lentiviral Production
Human FXYD3 directed shRNA lentiviral plasmids pLKO.1-puro were purchased from SIGMA. They consist of two constructs targeting different regions of the gene sequence common to the short and the long form of FXYD3. siRNA1: 183C AAA TGC AAG TTT GGC CAG AAG204 and siRNA2: 58GCC AAT GAC CTA GAA GAT AAA78.
Lentivectors were prepared by transient transfection of 293T cells using a calcium phosphate precipitation protocol. To generate lentivirus, for one 10-cm tissue culture dish, HEK293T cells were cotransfected with the different lentiviral transfer vector plasmids (15 μg) and with 5 μg of the pMD2G envelope plasmid (Plasmid Factory, Bielefeld, Germany) and 10 μg of the psPAX2 packaging construct (Plasmid Factory) in a total volume of 1 ml solution containing 125 mM CaCl2, 140 mM NaCl, 0.75 mM Na2HPO4, 5.5 mM glucose, and 25 mM HEPES, pH 7.03. The viral supernatants were harvested 72 h after transfection and filtered through a 0.45-μm filter. The viral supernatants were ultracentrifuged at 50,000 × g for 90 min, and the pellets were resuspended in culture medium.
Lentiviral Transduction of Caco-2 Cell Lines
Human colon adenocarcinoma cells, Caco-2 cells were maintained as previously described (Bibert et al., 2006). Nondifferentiated Caco-2 cells at 80% of confluency were seeded in six-well culture dishes, infected with viral supernatants, and incubated for 24 h at 37°C at 9% CO2. The supernatants were replaced with fresh culture medium containing 1 μg/ml puromycin (Sigma, St. Louis, MO) to select pLKO.1-puro plasmid-transfected cells. Single-cell clonal cultures were established in 96-well plates by limiting dilution, and transduced cells were selected for puromycin resistance for 1 mo by increasing concentrations of puromycin in cell culture medium (1–10 μg/ml). After selection, cells were lysed, and whole cell lysates were prepared for Western blot analysis as previously described (Bibert et al., 2006) at different time points after cell confluency.
Western Blot
Western blot analysis was performed as previously described (Bibert et al., 2006). The following primary antibodies were used: anti-human FXYD3 (1/500; Bibert et al., 2006), anti-Na,K-ATPase α subunits (1/20,000; Girardet et al., 1981), anti-Na,K-ATPase α1 subunit (1/5000), and anti-Na,K-ATPase α3 subunit (1/5000; Pressley, 1992) kindly provided by T. A. Pressley (Texas Tech University Health Sciences Center, Lubbock, TX), and anti-Na,K-ATPase β1 subunit (1/5000; Gonzalez-Martinez et al., 1994) kindly provided by P. Martin-Vasallo (Universidad de La Laguna, Tenerife, Spain).
Cell Proliferation
Cell numbers were assessed by the trypan blue dye-exclusion method. Cells were plated at a density of 3 × 104/well in six-well plates in duplicate. Caco-2 cell medium containing 10 μg/ml puromycin was used as growth medium. Cells were allowed to attach for 24 h and cultured during 15 d. Cells were trypsinized and mixed with an equal volume of trypan blue solution (Sigma). The stained/unstained cells were observed using an inverted microscope, and viable cells were counted at various time points in culture.
Apoptosis Analysis
Cells were seeded on slides, at a density of 3 × 104 and cultured for 3 d to obtain a layer of nonconfluent cells. Cells were fixed in phosphate-buffered saline (PBS) containing 3% paraformaldehyde for 15 min. After washing twice with PBS, the cells were stained with DAPI solution (1.5 μg/ml, Vector Laboratories, Burlingame, CA) for 5 min at room temperature in the dark. The stained nuclei were examined under a fluorescence microscope. Apoptotic cells were defined on the basis of nuclear morphology changes such as chromatin condensation and fragmentation. As a positive control, Caco-2 cells were incubated in medium containing 20 mM butyrate for 2 d.
Cell Differentiation
Caco-2 cells were grown on collagen-coated filters. The activity of the brush border enzyme alkaline phosphatase (AP) was used to assess differentiation of Caco-2 cells (Forstner et al., 1968). The assay was performed at different times after cell confluency. The cells were harvested with trypsin and washed twice with PBS. The cell pellet was homogenized, sonicated in 2 mM Tris, pH 7.1, containing 50 mM mannitol at 4°C, and centrifuged at 10,600 × g for 10 min. Cellular protein content was assayed by the method of Lowry (Lowry et al., 1951). AP activity was measured using p-nitrophenyl phosphate as substrate. Fifty microliters of cell supernatant was incubated with 50 μl of 1 mg/ml p-nitrophenyl phosphate (Merck, Dietikon, Switzerland) at pH 9.8 at 37°C for 10 min. The supernatant was transferred into a 96-well microplate, and the amount of p-nitrophenyl phosphate liberated was analyzed at 405 nm in a microplate fluorimeter using substrate without cells as a blank.
The expression of villin, which plays a mayor role in the initiation of brush border assembly (Friederich et al., 1990), was determined by Western blot analysis with a villin antibody (1:500; Serotec, Oxford, United Kingdom).
DIGE (Difference Gel Electrophoresis) Separation and Data Analysis
Cell lysates from wild-type or siRNA2-treated Caco-2 cells grown on collagen-coated filters for 21 d were prepared by sonication in lysis buffer consisting of 5 mM magnesium acetate, 7 M urea, 2 M thiourea, 4% (wt/vol) CHAPS, and 30 mM Tris, pH 8, followed by centrifugation at 13,000 × g. A 50-μg aliquot of each protein extract was labeled with 400 pmol of Cy3 and Cy5 DIGE Fluor minimal dyes following the standard protocol (Ettan DIGE User Manual, Amersham Biosciences, Piscataway, NJ; Tannu and Hemby, 2006). Equal amounts (25 μg) of each of the wild-type and siRNA2 cell lysates were mixed, designated as the “pooled internal standard” and labeled with CyDye DIGE Fluor Cy2 minimal dye. Two-dimensional (2D) electrophoresis was carried out essentially as described (Tannu and Hemby, 2006). Isoelectric focusing was performed on 18-cm Immobiline DryStrip gels pH 3–10 (nonlinear gradient; GE Healthcare, Uppsala, Sweden), whereas the second dimension was carried out on a 12.5% SDS-PAGE (26 × 20 cm). After migration, the gel was scanned at the characteristic dye wavelengths on a FX Promolecular imaging scanner (Bio-Rad, Hercules, CA). The resulting images were processed with ImageMaster Platinum 6.0 with DIGE module (GE Amersham Biosciences) with internal normalization using the internal standard. Only spots showing a fold change greater than 1.5 were considered as significant. A gel with identical samples but reverse dye labeling was run in the same batch, and only spots whose variation was concordant in the two gels were considered for analysis. Gels were stained after detection with Flamingo Pink (Bio-Rad), and spots were robotically excised for mass spectrometry analysis.
Protein Identification by MS
Gel spots were trypsinized as described (Shevchenko et al., 1996). Extracted peptides were evaporated to dryness and resuspended in 3 μl α-cyano-hydroxycinnamic acid matrix (5 mg/ml) for MALDI-MS-MS analysis on a 4700 Proteomics Analyzer (Applied Biosystems, Framingham, MA). Noninterpreted peptide tandem mass spectra were used to search the human subset of the Uniprot database (release 12.0, including Swiss-Prot release 54.0 and TrEMBL release 37.0) using Mascot 2.0 (http://www.matrixscience.com) with a mass tolerance of 50 ppm. Spots with ambiguous identification were further analyzed by LC-MS/MS on a hybrid linear trap LTQ-Orbitrap mass spectrometer (Thermo Fischer Scientific, Waltham, MA) interfaced to an Agilent 1100 nano HPLC system (Agilent Technologies, Wilmington, DE) equipped with a ZORBAX 300SB C18 (75 μm ID × 150 mm × 3.5 μm) capillary column (Agilent Technologies). Peptides were separated on a capillary column using a gradient from 5 to 85% acetonitrile in 0.1% formic acid, and multicharged precursors were chosen for tandem MS analysis. Tandem mass spectra were searched with Mascot as described above with a mass tolerance of 10 ppm. Proteins with a MASCOT score above 80 were considered automatically as valid, whereas all protein identifications with a score between 33 and 80 were manually validated. Generally, only proteins matched by at least two peptides were accepted.
Electrophysiological Measurements of Transepithelial Short Circuit Current (Isc)
Caco-2 cells were grown for 21 d on Snapwell filters coated with collagen and mounted in Ussing chambers in Na+- free buffer containing 116 mM potassium gluconate, 1.8 mM CaCl2, 1.6 mM MgCl2, 0.8 mM KH2PO4, 2 mM glucose, 0.4 mM glutamine, 25 mM HEPES, and 3 mM BaCl2, pH 7.4. All solutions were maintained at 37°C. After a stabilization period, the activity of the apical epithelial Na+ channel was blocked by 10 μM amiloride and the apical membrane was permeabilized to cations with 20 μg/ml amphotericin B (Calbiochem, La Jolla, CA) added to the apical medium. After stabilization of cell monolayer resistance, the Na,K-pump activity was activated by apical and basolateral addition of increasing concentrations of sodium gluconate (2, 5, 10, 20, 100, and 180 mM). At the end, 2 mM ouabain was added to the basolateral medium to inhibit Na,K-ATPase currents. Transepithelial voltage was recorded continuously and transepithelial resistance every 20 s, and Isc was calculated from these two parameters. The Hill equation was fitted to the data of the current induced by various concentrations of Na+ with a Hill coefficient of 3 and yielded estimation of the half-activation constant for Na+ (K1/2 Na+) and the maximal current.
Rubidium Uptake
Ouabain-sensitive uptake of 86Rb+ was measured on Caco-2 cells grown for 21 d on Transwell filters coated with collagen. All solutions were maintained at 37°C. First, cells were loaded with Na+ by incubation in a K+-free solution containing 130 mM NaCl, 25 mM NaHCO3−, 1 mM MgCl2, 0.9 mM NaHPO4, 20 mM NaHepes, pH 7.4, 1.8 mM CaCl2, and 2 mM BaCl2 for 1 h at 37°C. The cells were then incubated in solution containing different concentration of K+ (0, 0.5, 1, 5, and 10 mM) in the presence of 0.5 μCi/ml 86Rb+ in the basolateral medium, for 1 h at 37°C. Cells were washed six times, both on apical and basolateral sides with 100 mM ice-cold MgCl2. Filters dissolved in 2 ml of scintillation liquid were counted. Ouabain-sensitive 86Rb+ uptake was defined as the difference between values observed in the absence and presence of 100 μM ouabain. Nonspecific 86Rb uptake accounted for 10% of the total 86Rb uptake.
Na,K-ATPase Activity
Microsomes of Caco-2 cells, grown for 21 d on collagen-coated filters, were prepared by sonication (three times for 5 s) of cell pellets resuspended in a homogenization solution containing 30 mM dl-histidine, 5 mM EDTA, 320 mM sucrose, 2 mM PMSF, and 18 mM Tris base, pH 7.4, centrifugation at 200 × g for 10 min and centrifugation of the supernatant at 100,000 × g for 1 h. Microsomes resuspended in homogenization solution were frozen and thawed three times in liquid nitrogen before enzyme activity measurements. Final concentrations of constituents in the incubation medium were as follows: NaCl, 100 mM; KCl, 20 mM; MgCl, 1.8 mM; Tris-ATP (pH 7), 3 mM; EDTA, 3 mM; and Tris-HCl, 300 mM, pH 7.5. For determinations of K1/2Na+ and K1/2 K+ values, NaCl and KCl concentrations were varied, and choline chloride was used to compensate for variations in osmolarity. Fifty micrograms proteins were incubated in incubation medium for 15 min at 37°C. The enzyme reaction was stopped by addition of 30% trichloroacetic acid. After centrifugation, Pi (Pi) was assayed by the method of Fiske and Subbarow (1925). Each sample was incubated in duplicates in the absence or presence of 4 mM or 4.8 μM ouabain. Spontaneous ATP hydrolysis was assessed by a control sample, which contained no KCl, NaCl, microsomes, or ouabain. The Na,K-ATPase activity was expressed as μmoles Pi/mg protein/hour. Na,K-ATPase activity was calculated as the difference between activities in the absence or presence of ouabain. The maximal Na,K-ATPase activity and the apparent K+ affinity (K1/2K+) and Na+ affinity (K1/2Na+) were obtained by fitting the Hill equation to the data with a Hill coefficients of 1.6 (Jaisser et al., 1994) and 2.6, respectively.
[3H]Ouabain Binding
The total number of Na,K-ATPase expressed at the cell surface was determined on wild-type or siRNA-treated Caco-2 cells grown for 21 d on Transwell filters coated with collagen. Cells were apically and basolaterally washed three times and incubated during 1 h at 37°C, in the incubation buffer composed of 3 mM Na2HPO4, 10 mM glucose, 1.5 mM MgCl2, and 130 mM NaCl, pH 7.4. Cells were then incubated on the basolateral side with incubation buffer containing 0.3 μM [3H]ouabain (Perkin Elmer-Cetus, Norwalk, CT; 30 Ci/mmol) plus 0.7 μM unlabeled ouabain, for 10 min at 37°C. In preliminary experiments, we have determined that after 10 min, ouabain does not gain access to the apical medium. After incubation, cells were washed six times with cold PBS. Cells were solubilized and scrapped of filters after addition of 500 μl of 0.5 N NaOH and 1% SDS per filter and incubation at 4°C for 4 h. HCl at 0.5 N was then added, and cells were counted. Nonspecific ouabain binding was determined in the presence of 300 μM ouabain.
Quantitative Real-Time RT-PCR
The extraction and purification of total RNAs from Caco-2 cells treated or not with siRNA were carried out according to the manufacturer's specifications (RNeasy Mini Kit; Qiagen, Chatsworth, CA). Total isolated RNA (1 μg) was then reverse-transcribed using the MLV-Reverse Transcriptase (Promega, Madison, WI). Briefly, reaction was performed at 70°C for 5 min, followed by 40°C for 1 h and at 70°C for 15 min in a total volume of 20 μl.
Real-time RT-PCR was performed in a 20-μl reaction using specific primers (150 nM) for Na,K-ATPase β1, β2, β3 or α1, α2, α3 isoforms: 2 μl of the five-times–diluted RT reaction and the SYBR Green PCR MasterMix (Applied Biosystems, Rotkreuz, Switzerland). Amplification was carried out with one cycle of 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The reaction was performed in triplicate for each sample. The relative quantity of mRNA with respect to the standard (nondifferentiated wild-type cells) was calculated using standard equations (Applied Biosystems): Relative mRNA quantity (sample to standard) = 2(−ΔΔCT), where ΔΔCT = ΔCT (sample) − ΔCT (standard) and ΔCT = mean threshold cycle − mean CT (GAPDH). The expression of GAPDH was similar under all experimental conditions.
Immunocytochemistry
Nondifferentiated and differentiated Caco-2 cells were fixed for 30 min at room temperature in PBS containing 3% (wt/vol) paraformaldehyde, washed with PBS for two times 15 min at room temperature, and then permeabilized for 5 min at the same temperature in PBS containing 3% paraformaldehyde and 0.5% Triton X-100. After two washes with PBS at room temperature, cells were preincubated in PBS containing 3% BSA for 1 h at the same temperature. They were incubated for 1 h at room temperature with PBS containing 1% BSA, monoclonal antibodies against the Na,K-ATPase β3 subunit (Chiampanichayakul et al., 2006), kindly provided by W. Kasinrerk (Chiang Mai University, Chiang Mai, Thailand), and with polyclonal rabbit antibody against the Na,K-ATPase β1 subunit (Gonzalez-Martinez et al., 1994). After two washes with PBS, the primary antibodies were then detected with CY3-labeled goat anti-mouse IgG and with Alexa Fluor 488–labeled goat anti-rabbit IgG. After extensive washes with PBS, preparations were mounted using FluorSave Reagent. The immunostained cells were examined after an overnight incubation in the dark, at 4°C using fluorescence microscopy.
RESULTS
To investigate the putative role of FXYD3 in cell proliferation, apoptosis, and differentiation and to determine the link with the regulation of Na,K-ATPase, we used the RNA interference technique to down-regulate FXYD3 expression in human colon adenocarcinoma cells. Caco-2 cells are known to undergo spontaneous differentiation and acquire a mature polarized enterocytic phenotype when cultured in postconfluent cultures without any additional treatment (Pinto et al., 1983).
Down-Regulation of FXYD3 Expression by siRNAs
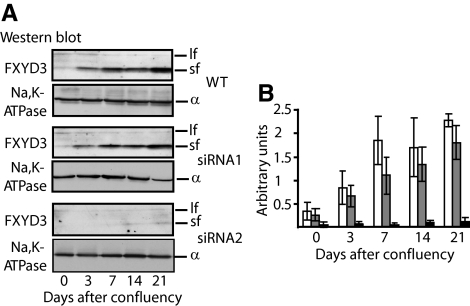
As previously shown (Bibert et al., 2006), in wild-type cells the expression of the short form of FXYD3 was markedly and gradually increased during Caco-2 cell differentiation, whereas the expression of the long form of FXYD3 decreased (Figure 1A). Two different FXYD3-specific siRNAs (see Material and Methods) were tested for their ability to silence FXYD3 expression in Caco-2 cells. These siRNAs did not allow discriminating between the long and the short form of FXYD3. Cells stably transduced with siRNA-containing lentiviruses were allowed to reach confluency, and the silencing effect of each siRNA was checked by monitoring the FXYD3 protein level by Western blot analysis at different time points after confluency for up to 3 wk. In cells treated with siRNA1, the expression pattern of FXYD3 variants was similar to that in wild-type cells, showing no inhibition of FXYD3 expression (Figure 1, A and B). siRNA1 was thus used as a control in further experiments. On the contrary, in siRNA2-treated cells, the expression of the prominent short form of FXYD3 was inhibited by more than 90% compared with wild-type cells at 21 d after cell confluency (Figure 1, A and B). The expression of the long form of FXYD3 was also inhibited but, because of its inherent low expression, the inhibition could not reliably be quantified. In contrast to FXYD3, the expression level of Na,K-ATPase α subunits remained constant during the 21 d of culture in siRNA2-treated cells (Figure 1A).
Figure 1.
Determination of the efficiency of FXYD3 gene silencing in human colon adenocarcinoma (Caco-2) cells. (A) Western blot analysis of FXYD3 expression in wild-type or siRNA-treated Caco-2 cells after different time points in culture after confluency. Fifty micrograms of proteins of cell lysates were subjected to SDS-PAGE and transferred onto nitrocellulose membranes, and FXYD3 was immunodetected. After stripping, the nitrocellulose membrane was probed with a Na,K-ATPase α subunit antibody. lf, FXYD3 long form; sf, FXYD3 short form. (B) Quantification of short forms of FXYD3 proteins in Caco-2 cells after different time points in culture after confluency. Shown are arbitrary units obtained by densitometric scanning of data shown in A normalized by the amount of Na,K-ATPase α subunits. Shown are means ± SEM of four independent experiments. □, wild-type Caco-2 cells; ▩, siRNA1-treated Caco-2 cells; ■, siRNA2-treated Caco-2 cells.
Effect of FXYD3 Silencing on Caco-2 Cell Proliferation, Apoptosis, and Differentiation
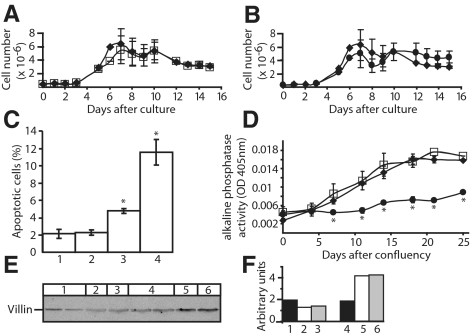
To assess the potential biological significance of FXYD3, we examined the effects of alterations in the level of FXYD3 expression on Caco-2 cell growth, differentiation, and cell death. FXYD3 siRNA-treatment did not affect overall cell morphology (data not shown). As shown in Figure 2, A and B, cell proliferation of wild-type Caco-2 cells ceased after 7 d in culture and the number of viable cells remained nearly constant up to 15 d in culture. Treatment of cells with siRNA1 (Figure 2A) or siRNA2 (Figure 2B) had no influence on cell proliferation.
Figure 2.
Effects of FXYD3 gene silencing on Caco-2 cell proliferation (A and B), apoptosis (C) and differentiation (D–F). (A and B) Cell proliferation was estimated by direct count of viable cells after different time points in culture. ♦, wild-type Caco-2 cells; □, siRNA1-treated Caco-2 cells; ●, and siRNA2-treated Caco-2 cells. Four independent experiments in duplicates were performed for wild-type, siRNA1-, and siRNA2-treated cells. Data are means ± SEM. (C) Condensed and fragmented nuclei were scored in nonconfluent cells stained with DAPI as described in Material and Methods. 1, wild-type Caco-2 cells; 2, siRNA1-treated Caco-2 cells; 3, siRNA2-treated Caco-2 cells; and 4, wild-type Caco-2 cells treated with 20 mM butyrate for 2 d. Approximately 1000 cells were scored for each condition. Shown are means ± SEM of four independent experiments. *p < 0.006 versus wild-type Caco-2 cells. (D–F) FXYD3 gene silencing prevents Caco-2 cell differentiation. (D) The activity of ALP was measured in cell lysates after different time points after cell confluency. Results were normalized to cellular protein content. Data are means ± SEM of three independent experiments in duplicates. *p < 0.05. ♦, wild-type Caco-2 cells; □, siRNA1-treated Caco-2 cells; and ●, siRNA2-treated Caco-2 cells. (E) Western blot analysis of villin expression in wild-type and siRNA-treated Caco-2 cells. Fifty micrograms of proteins of cell lysates were subjected to SDS-PAGE, transferred onto nitrocellulose membranes, and villin was immunodetected. 1, siRNA2-treated Caco-2 cells, at 80% confluency (two independent experiments); 2, wild-type Caco-2 cells, at 80% confluency; 3, siRNA1-treated Caco-2 cells, at 80% confluency; 4, siRNA2-treated Caco-2 cells; 21 d after confluency (two independent experiments); 5, wild-type Caco-2 cells, 21 d after confluency; and 6, siRNA1-treated Caco-2 cells, 21 d after confluency. (F) Quantification of villin expression. The relative amount of villin was obtained by densitometric scanning of data shown in E.
Compared with nonconfluent wild-type cells, the number of apoptotic cells did not change in siRNA1-treated cells but increased by about twofold in siRNA2-treated cells (Figure 2C). Treatment of wild-type cells with butyrate, known to increase apoptosis in Caco-2 cells (Hague et al., 1993), produced an approximately sixfold increase in apoptotic cells.
Finally, we asked whether Caco-2 cell differentiation was influenced by FXYD3 expression. Differentiation of Caco-2 cells is associated with increased activity of alkaline phosphatase (ALP), an enzyme of the brush border membrane (Forstner et al., 1968). As shown in Figure 2D, wild-type and siRNA1-treated cells showed a time-dependent increase in ALP activity during differentiation, whereas in siRNA2-treated cells, ALP activity remained constantly low during 25 d after confluency. Similar results were obtained for another differentiation marker, villin (Friederich et al., 1990), whose expression was increased in wild-type (Figure 2, E and F, compare lanes 2 and 5) and siRNA1-treated (compare lanes 3 and 6) cells during differentiation but not in siRNA2-treated cells 21 d after confluency (compare lanes 1 and 4). The specific effect of siRNA2 but not of siRNA1 confirms that cell differentiation is impeded as a direct consequence of FXYD3 silencing.
To further characterize the influence of FXYD3 silencing on cell differentiation, we analyzed total protein expression in siRNA2-treated and wild-type Caco-2 cells by difference 2D gel electrophoresis (2D-DIGE; Unlu et al., 1997). The relative abundance of a protein in the two samples was estimated as the ratio of Cy5 and Cy3 fluorescence after normalization. Our experimental data did not permit to analyze membrane proteins, but a total of 46 soluble proteins, which are differentially expressed in siRNA2-treated and wild-type cells by at least 1.5-fold were identified (Supplementary Table S1). Of these, 15 proteins were up-regulated and 31 proteins down-regulated in siRNA2-treated cells. At least eight of these proteins (Mazurek et al., 2000; Rosmann et al., 2002; Stierum et al., 2003; Balasubramani et al., 2006; Carpenter et al., 2006; Pshezhetsky et al., 2007; Santegoets et al., 2007; Willis et al., 2008), mainly involved in energy metabolism or signal transduction, have been reported to be involved in Caco-2 cell differentiation or in colorectal cancer (Table 1), and the observed changes in their abundance in siRNA2-treated cells reflect the poorly differentiated state of siRNA-treated cells.
Table 1.
Identification of changes in the abundance of proteins in siRNA2-treated Caco-2 cells
| Protein description | SWISS-PROT accession no. | General function | Observation | Ref |
|---|---|---|---|---|
| Up-regulation in cells treated with siRNA2 | ||||
| Isocitrate dehydrogenase | P48735 | Metabolism and mitochondria | Strong expression in primary colon tumor tissues | Mazurek et al. (2000) |
| Proliferation-associated protein 2 GA | P14555 | Signal transduction | Increased expression in colorectal tumors and in proliferative Caco-2 cells | Santegoets et al. (2007), Turck et al. (2004) |
| Heterogeneous nuclear ribonucleoprotein K | P61978 | Transcription factor and DNA binding | Overexpressed in colorectal cancer | Carpenter et al. (2006) |
| Lamin A | P02545 | Signal transduction/structural protein | Promoted tumour invasiveness in colorectal cancer | Willis et al. (2008) |
| Down-regulation in cells treated with siRNA2 | ||||
| Pro-Meprin A subunit alpha | Q16819 | Metabolism and mitochondria | Increased expression during Caco-2 cell differentiation | Rosmann et al. (2002) |
| Creatine kinase B | P12277 | Metabolism and mitochondria | Decreased expression level in colon tumors | Balasubramani et al. (2006) |
| Increased expression during Caco-2 cell differentiation | ||||
| Glutathione S-transferase | P09210 | Signal transduction | Increased expression of GST1 during Caco-2 cell differentiation | Stierum et al. (2003), Pshezhetsky et al. (2007) |
| Galectin-3 | P17931 | Signal transduction | Increased expression during Caco-2 cell differentiation | Pshezhetsky et al. (2007) |
Proteins are shown that have been reported to be involved in Caco-2 cell differentiation or in colorectal cancer.
Altogether, these results indicate that FXYD3 expression enhances Caco-2 cell differentiation and prevents cell apoptosis in nonconfluent cells, without playing a role in cell proliferation.
FXYD3 Silencing Modulates the Apparent K+ and Na+ Affinities of Caco-2 Cell Na,K-ATPase
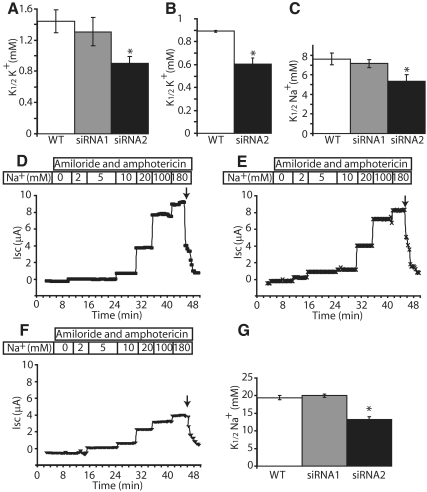
As previously described (Crambert et al., 2005; Bibert et al., 2006), coexpression of Na,K-ATPase with the short form of FXYD3 in Xenopus oocytes decreases its apparent K+ and Na+ affinities. The functional effects of FXYD3 silencing on the transport properties of Na,K-ATPase in Caco-2 cells were tested by Na,K-ATPase activity measurements (Figure 3, A and C), 86Rb uptake (Figure 3B), and short circuit current measurements (Figure 3, D–G). The absence of FXYD3 expression in siRNA2-treated cells produced a significant increase in the apparent affinities for extracellular K+ (Figure 3, A and B) and intracellular Na+ (Figure 3, C–G) of Na,K-ATPase. This effect was not observed in siRNA1-treated cells, which express FXYD3 proteins. Altogether these results indicate that the increase of Na+ and K+ affinities of Na,K-ATPase in siRNA2-treated cells is due to silencing of the short form of FXYD3, and they confirm the effect of FXYD3 on Na,K-ATPase observed in Xenopus oocytes. Short circuit measurements also showed that transepithelial electrical resistance (R) was reduced in siRNA2-treated cells (R = 177 ± 11.2 Ω · cm2, n = 3) compared with wild-type (R = 354 ± 11.2 Ω · cm2, n = 3) and siRNA1-treated (R = 336 ± 8.9 Ω · cm2, n = 3) cells (p < 0.003 vs. wild-type cells), which likely reflects the incomplete differentiation state of siRNA2-treated cells.
Figure 3.
FXYD3 gene silencing increases the apparent K+ (A and B) and Na+ (C–G) affinities of Na,K-ATPase. (A) Na,K-ATPase activity. Na,K-ATPase activity was assayed at varying K+ concentrations (0.5, 1, 2, and 10 mM) in microsomes from wild-type, siRNA1-, or siRNA2-treated Caco-2 cells, 21 d after confluency as described in Material and Methods. Na,K-ATPase represents the difference of the ATPase activity in the absence and the presence of 4 mM ouabain. Values of the apparent K+ affinities of Na,K-ATPase (K1/2K+) are means ± SEM of five to nine independent experiments. *p < 0.046 versus wild-type Caco-2 cells. (B) Ouabain-sensitive 86Rb uptake in Caco-2 cells. Twenty-one days after confluency, wild-type or siRNA2-treated Caco-2 cells were incubated for 1 h with 86Rb in the presence of various concentrations of K+ with or without ouabain, as described in Material and Methods. Na,K-ATPase-mediated 86Rb uptake was obtained as the difference between the uptake in the absence and presence of ouabain. K1/2K+ values are means ± SEM of three independent experiments. *p < 0.006 versus wild-type Caco-2 cells. (C) Na,K-ATPase activity. Na,K-ATPase activity was measured at varying Na+ concentrations (2.5, 5, 10, and 100 mM) in microsomes from wild-type, siRNA1-, or siRNA2-treated Caco-2 cells, 21 d after confluency as described in Material and Methods. Na,K-ATPase represents the difference of the ATPase activity in the absence and the presence of 4.8 μM ouabain. Values of the apparent affinities of Na,K-ATPase for Na+ (K1/2Na+) are means ± SEM of four to seven independent experiments. *p < 0.036 versus wild-type Caco-2 cells. (D–G) Examples of short-circuit current (Isc) traces in wild-type Caco-2 cells (D), siRNA1-treated (E), and siRNA2-treated Caco-2 cells (F), 21 d after confluency. Isc was measured as described in Material and Methods in the presence of amiloride and amphotericin B and different concentrations of sodium gluconate. Specific Na,K-ATPase activity was determined at the end of the experiment by inhibition with ouabain (arrow) and represented more than 90% of the total current. (G) Determination of K1/2Na+ values from experiments shown in D–F. Values of K1/2Na+ are means ± SEM of three independent experiments. *p < 0.0012 versus wild-type Caco-2 cells.
FXYD3 Silencing Influences Na,K-ATPase Turnover Number
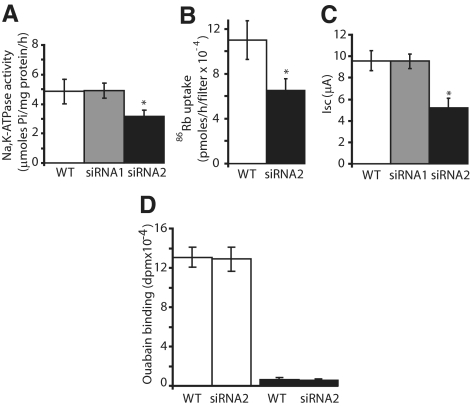
In Na,K-ATPase activity (Figure 4A), 86Rb flux (Figure 4B), and short circuit current (Figure 4C) measurements, we observed that the maximal pump activity was decreased in siRNA2- but not in siRNA1-treated cells compared with wild-type Caco-2 cells. To determine whether changes in maximal Na,K-pump activity is determined by the density of functional Na,K-ATPase at the cell surface or by the turnover number of Na,K-ATPase, we performed [3H]ouabain binding on intact cells grown on semipermeable filters to measure the density of functional Na,K-pumps at the cell surface (Figure 4D). Treatment of Caco-2 cells with siRNA2 did not influence the cell surface expression of functional Na,K-ATPase and as shown in Figures 1A and 5, B and C, the protein expression level of Na,K-ATPase was similar in wild-type and in siRNA2-treated cells. In consequence, it is likely that silencing of FXYD3 decreases the turnover number of Na,K-ATPase pumps, leading to the observed decrease in maximal activity.
Figure 4.
FXYD3 gene silencing decreases the maximal Na,K-pump activity but not the cell surface expression of functional Na,K-ATPase. Maximal Na,K-ATPase activity was assessed by (A) ATPase activity measurements (4–7 independent experiments, *p < 0.034 vs. wild-type cells), (B) ouabain-sensitive 86Rb+ uptake (six independent experiments, *p < 0.018 vs. wild-type cells) and (C) short-circuit current measurements (3–5 independent experiments, *p < 0.049 vs. wild-type cells), 21 d after confluency of Caco-2 cells as described in Material and Methods. (D) [3H]ouabain binding to intact Caco-2 cells, 21 d after confluency (□). Nonspecific binding was determined in the presence of a 1000-fold excess of unlabeled ouabain (■).
Figure 5.
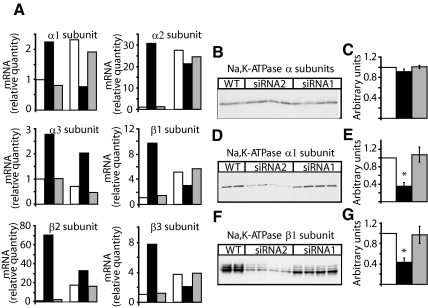
FXYD3 gene silencing changes Na,K-ATPase isozyme expression. (A) The abundance of Na,K-ATPase isozyme transcripts was quantified by real-time RT-PCR in Caco-2 cells at 80% confluency (three bars to the left) and at 21 d after confluency (three bars to the right). Two experiments with triplicate assays were performed. The target signal was normalized to the reference gene (GAPDH) and the signal for nondifferentiated wild-type cells was put to one. □, wild-type cells; ■, cells treated with siRNA2; and ▩, cells treated with siRNA1. (B–G) Expression of Na,K-ATPase isozymes in wild-type and in siRNA1- or siRNA2-treated Caco-2 cells, 21 d after confluency. Fifty micrograms of protein of cell lysates were subjected to SDS-PAGE and transferred onto nitrocellulose membranes, and the total Na,K-ATPase pool was immunodetected with an antibody recognizing all Na,K-ATPase α isoforms (B). After stripping, the nitrocellulose membrane was probed with a Na,K-ATPase α1 isoform-specific antibody (D) and with a β1 isoform-specific antibody (F). (C, E, and G) Densitometric scanning of data shown in B, D, and F. Results are normalized to the relative quantity of proteins in wild-type cells (□). Shown are means ± SEM of n = 4 for cells treated with siRNA1 (▩) or siRNA2 (■). *p < 0.018 versus wild-type Caco-2 cells. Similar results were obtained in five independent experiments.
FXYD3 Silencing Induces Changes in Na,K-ATPase Isozyme Expression
Because FXYD3 expressed in Xenopus oocytes has no effect on the maximal Na,K-pump activity (Crambert et al., 2005) and because it is known that different Na,K-ATPase isozymes exhibit different turnover numbers according to their α and β isozyme composition (Crambert et al., 2000), we studied whether the decrease in the turnover number of Na,K-ATPase observed in FXYD3-deficient Caco-2 cells may be due to regulation of Na,K-ATPase isozyme-related gene expression.
RT-PCR analysis shows that, in wild-type Caco-2 cells, all α (α1, α2) and β (β1, β2, and β3) isoform RNAs, with the exception of α3 isoform RNA, increased between 80% cell confluency and 21 d after confluency (Figure 5A, □). At the protein level, expression of α1 isoforms did not change, whereas that of α3 decreased and that of β1 isoforms increased (Supplemental Figure S1). A similar expression pattern of α1, α3, and β1 isoform RNA, and protein was observed in siRNA1-treated cells (Figure 5A, ▩, Supplemental Figure S1). In siRNA2-treated cells, the levels of all α and β isoform RNAs were higher than in wild-type cells at 80% confluency but decreased 21 d after confluency (Figure 5A, ■). Twenty-one days after confluency, both the RNA and protein levels of α1 (Figure 5, A, D, and E) and β1 (Figure 5, A, F, and G) isoforms were lower whereas those of α3 (Figure 5A, Supplemental Figure S1) were higher in siRNA2-treated cells than in wild-type or siRNA1-treated cells. In none of the selected cell clones, α2 and β2 isoforms could be detected in Western blots (data not shown). We had no β3 isoform-specific antibodies available to use in Western blots, but immunostaining of Caco-2 cells revealed that β3 isoforms were expressed at the cell surface both in wild-type and siRNA2-treated cells at 80% confluency and 21 d after confluency. In contrast, β1 isoforms were located intracellularly in wild-type and siRNA2-treated cells at 80% confluency and were expressed at the cell surface in wild-type but not in siRNA2-treated cells 21 d after confluency (Supplemental Figure S2).
DISCUSSION
In this study, we demonstrate that FXYD3 protein silencing has no effect on cell proliferation but promotes a small increase in apoptosis and in particular impedes differentiation of Caco-2 cells. These effects are accompanied by a change in Na,K-ATPase activity due to the lack of FXYD3 and a differential expression of Na,K-ATPase isoforms.
FXYD3 and Caco-2 Cell Differentiation
Cell differentiation is a complex process associated with exit from the cell cycle and entry into an alternate pathway permitting specialized cellular functions. Caco-2 cells are derived from a human colon adenocarcinoma but are able to polarize and form epithelia (Pinto et al., 1983). The transcriptional programs of proliferating nonpolarized and of polarized Caco-2 cells present striking similarities with those of human colon cancer and normal colon tissue, respectively (Saaf et al., 2007), making Caco-2 cells a suitable experimental model to determine the role of proteins on cell fate.
In a previous study, we have shown that Caco-2 cell differentiation is accompanied by a change in FXYD3 isoform expression (Bibert et al., 2006). Moreover, it was reported that FXYD3 is overexpressed in human tumors (Morrison and Leder, 1994; Kayed et al., 2006) and that FXYD3 silencing by siRNA promotes a decrease in prostate cancer cell line proliferation (Grzmil et al., 2004). These results suggest that FXYD3 is implicated in cell fate but the precise role of FXYD3 has not been established.
In this study, we used FXYD3 knockdown Caco-2 cells to assess the implication of FXYD3 in cell apoptosis, cell proliferation, and/or differentiation. Our siRNA approach did not allow discriminating between the distinct roles of the two FXYD3 isoforms, but our results clearly show that FXYD3 silencing impedes Caco-2 cell differentiation. This is reflected by lack of increased expression of differentiation markers such as alkaline phosphatase and villin, as well as by changes in other cellular proteins in line with their putative role in cell differentiation or carcinogenesis. Moreover, the observed decrease in transepithelial resistance may reflect impairment of cell polarization in FXYD3-lacking cells.
Silencing of FXYD3 expression also produced a small but significant increase in apoptosis, which, however, is not translated into a decreased cell proliferation. Indeed, cell proliferation is similar in wild-type and siRNA-treated cells at different time points in culture. Because apoptosis was determined in nonconfluent cells, it is possible that apoptotic susceptibility to FXYD3 silencing changes after cell confluency.
Our observation that FXYD3 may be implicated in cell differentiation but not in proliferation contrasts with results showing overexpression in breast tumors (Morrison and Leder, 1994) or suggesting a proliferative role of FXYD3 in prostate cancer cells (Grzmil et al., 2004). However, it is interesting that, in contrast to breast (Morrison and Leder, 1994) prostate (Grzmil et al., 2004), and pancreas (Kayed et al., 2006) tumors, FXYD3 was shown to be down-regulated in colon and kidney cancer compared with normal tissue (Kayed et al., 2006), which is compatible with our results that FXYD3 may not have a proliferating effect in Caco-2 cells. It is also conceivable that in cancer cells unable to differentiate, FXYD3 may promote cell proliferation, whereas in differentiation-competent cells such as Caco-2 cells, FXYD3 may have no effect on cell proliferation but temporally decreases cell apoptosis and promotes cell differentiation.
Functional Effects of FXYD3 Silencing on Na,K-ATPase Properties and Isozyme Expression in Caco-2 Cells
A general feature of FXYD proteins is their association with Na,K-ATPase and the modulation of its transport properties (Geering, 2006). Recently, we have shown that the common short form of mouse (Crambert et al., 2005) and human (Bibert et al., 2006) FXYD3 produces a decrease in both the apparent Na+ and K+ affinities of Na,K-ATPase after expression in Xenopus oocytes. In contrast, the long form of human FXYD3 decreases the apparent K+ affinity of Na,K-ATPase only at positive membrane potentials and slightly increases its apparent Na+ affinity (Bibert et al., 2006).
In this study, we show that inhibition of cell differentiation after FXYD3 silencing is accompanied by a change in kinetic properties of Na,K-ATPase, which reflects a reversal of the functional role of the short form of FXYD3 observed after expression in Xenopus oocytes (Bibert et al., 2006). Thus, a link exists between inhibition of cell differentiation and the knockdown of the expression of the prominent short form of FXYD3, which is normally up-regulated during cell differentiation (Bibert et al., 2006). We cannot totally exclude a possible impact of the long form of FXYD3 at early stages of the differentiation process, when this isoform is normally expressed. However, because this isoform represents a minor proportion of the FXYD3 population and is down-regulated during the differentiation process, it is likely that inhibition of cell differentiation, after knockdown of FXYD3 expression, is a reflection of the functional effect of the short form of FXYD3. Moreover, it is unlikely that compensatory mechanisms such as de novo expression of other members of the FXYD protein family could occur as described in some experimental systems in response to genotoxic effects (Arystarkhova et al., 2007). Indeed, none of the FXYD proteins produce, in in vitro systems, an increase in both the Na+ and K+ affinities of Na,K-ATPase (Geering, 2006), as observed in FXYD3-deficient cells.
Interestingly, we observe not only a change in Na+ and K+ affinities of Na,K-ATPase in FXYD3-deficient cells but also a significant decrease in total Na,K-ATPase activity, due to a decrease in its turnover number. This observation was unexpected because in Xenopus oocytes, FXYD3 has no effect on maximal Na,K-ATPase activity. We show that the decrease in the Na,K-ATPase turnover rate may be due to a change in Na,K-ATPase isozyme expression. Twenty-one days after confluency, we observe a decrease in α1 and an increase in α3 isoforms in FXYD3-deficient cells compared with wild-type cells. This could explain that the total number of Na,K-ATPase remains constant in wild-type and siRNA2-treated cells. A change from α1 to α3 isoform expression could also explain the lower turnover number of Na,K-ATPase in siRNA2-treated cells, consistent with the observation that human α3-β isozymes have a lower turnover number than α1-β isozymes after expression in Xenopus oocytes (Crambert et al., 2000). Our result showing a parallelism between inhibition of cell differentiation and changes in α1 and α3 isoform expression in FXYD3-deficient Caco-2 cells is consistent with the observation that α1 isoforms decrease and α3 isoforms increase in human colorectal cancers (Sakai et al., 2004) and highlight the role of molecular heterogeneity of Na,K-ATPase in cell fate.
In addition to the change of α isoform expression, we have observed a down-regulation of the β1 subunit expression in FXYD3 knockdown cells. This is consistent with an undifferentiated state of these cells because β1 subunits were shown to be involved in epithelial cell polarity and cell mobility and are expressed at highly reduced levels in poorly differentiated carcinoma cell lines (Rajasekaran et al., 2001), probably because of increased expression of the transcription factor Snail (Espineda et al., 2004).
It might be interesting to test whether reexpression of FXYD3 isoforms in FXYD3-deficient Caco-2 cells may reverse inhibition of cell differentiation. However, it might be predicted that this is not the case because lack of FXYD3 expression induces a complex change in the cellular program including not only a direct change in Na,K-ATPase activity but also a change in Na,K-ATPase isozyme expression.
Further studies are needed to definitively establish the mechanism by which lack of FXYD3 impedes cell differentiation of Caco-2 cells. However, because our results show that inhibition of cell differentiation is accompanied by specific changes in the functional properties of Na,K-ATPase, which reflect the lack of FXYD3, it is possible that improper regulation of Na,K-ATPase, leading to perturbation of the cellular ionic composition, may be a primary event in inhibition of cell differentiation. On the other hand, we cannot exclude that FXYD3 may have effects that are independent of Na,K-ATPase activity. It has indeed been reported that expression of FXYD3 in Xenopus oocytes induces a hyperpolarization-activated chloride conductance (Morrison et al., 1995). The importance of such effects of FXYD3 in cell differentiation remains to be demonstrated. Moreover, it remains to be shown whether the differential Na,K-ATPase isozyme expression in FXYD3-lacking Caco-2 cells may only be a reflection of the undifferentiated state of the cells or may be induced by perturbations of the cellular ionic composition produced by lack of FXYD3, and contribute to the decreased progression of cell differentiation in FXYD3-lacking cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank the team at the Protein Analysis Facility of the University of Lausanne for performing the proteomic analysis. Our thanks go also to T. A. Pressley, P. Martin-Vasallo, and W. Kasinrerk for the supply of antibodies. This study was supported by the Swiss National Science Foundation Grant 3100A0-107513 to K.G.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-10-0999) on December 24, 2008.
REFERENCES
- Arystarkhova E., Donnet C., Munoz-Matta A., Specht S. C., Sweadner K. J. Multiplicity of expression of FXYD proteins in mammalian cells: dynamic exchange of phospholemman and γ-subunit in response to stress. Am. J. Physiol. Cell Physiol. 2007;292:C1179–C1191. doi: 10.1152/ajpcell.00328.2006. [DOI] [PubMed] [Google Scholar]
- Attali B., Latter H., Rachamim N., Garty H. A corticosteroid-induced gene expressing an “IsK-like” K+ channel activity in Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 1995;92:6092–6096. doi: 10.1073/pnas.92.13.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani M., Day B. W., Schoen R. E., Getzenberg R. H. Altered expression and localization of creatine kinase B, heterogeneous nuclear ribonucleoprotein F, and high mobility group box 1 protein in the nuclear matrix associated with colon cancer. Cancer Res. 2006;66:763–769. doi: 10.1158/0008-5472.CAN-05-3771. [DOI] [PubMed] [Google Scholar]
- Béguin P., Crambert G., Monnet-Tschudi F., Uldry M., Horisberger J. -D., Garty H., Geering K. FXYD7 is a brain-specific regulator of Na,K-ATPase α1-β isozymes. EMBO J. 2002;21:3264–3273. doi: 10.1093/emboj/cdf330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibert S., Roy S., Schaer D., Felley-Bosco E., Geering K. Structural and functional properties of two human FXYD3 (Mat-8) isoforms. J. Biol. Chem. 2006;281:39142–39151. doi: 10.1074/jbc.M605221200. [DOI] [PubMed] [Google Scholar]
- Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin. Nephrol. 2005;25:292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Carpenter B., McKay M., Dundas S. R., Lawrie L. C., Telfer C., Murray G. I. Heterogeneous nuclear ribonucleoprotein K is over expressed, aberrantly localised and is associated with poor prognosis in colorectal cancer. Br. J. Cancer. 2006;95:921–927. doi: 10.1038/sj.bjc.6603349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiampanichayakul S., Khunkaewla P., Pata S., Kasinrerk W. Na, K ATPase β3 subunit (CD298): association with a subunit and expression on peripheral blood cells. Tissue Antigens. 2006;68:509–517. doi: 10.1111/j.1399-0039.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- Crambert G., Hasler U., Beggah A.T.Yu.C., Modyanov N. N., Horisberger J. D., Lelievre L., Geering K. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J. Biol. Chem. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- Crambert G., Li C., Claeys D., Geering K. FXYD3 (Mat-8), a new regulator of Na,K-ATPase. Mol. Biol. Cell. 2005;16:2363–2371. doi: 10.1091/mbc.E04-10-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espineda C. E., Chang J. H., Twiss J., Rajasekaran S. A., Rajasekaran A. K. Repression of Na,K-ATPase β1-subunit by the transcription factor snail in carcinoma. Mol. Biol. Cell. 2004;15:1364–1373. doi: 10.1091/mbc.E03-09-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske C. H., Subbarow Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925;66:375–400. [Google Scholar]
- Forstner G. G., Tanaka K., Isselbacher K. J. Lipid composition of the isolated rat intestinal microvillus membrane. Biochem. J. 1968;109:51–59. doi: 10.1042/bj1090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich E., Pringault E., Arpin M., Louvard D. From the structure to the function of villin, an actin-binding protein of the brush border. Bioessays. 1990;12:403–408. doi: 10.1002/bies.950120902. [DOI] [PubMed] [Google Scholar]
- Fu X., Kamps M. E2a-Pbx1 induces aberrant expression of tissue-specific and developmentally regulated genes when expressed in NIH 3T3 fibroblasts. Mol. Cell. Biol. 1997;17:1503–1512. doi: 10.1128/mcb.17.3.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering K. The functional role of β subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr. 2001;33:425–438. doi: 10.1023/a:1010623724749. [DOI] [PubMed] [Google Scholar]
- Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am. J. Physiol. Renal Physiol. 2006;290:F241–F250. doi: 10.1152/ajprenal.00126.2005. [DOI] [PubMed] [Google Scholar]
- Geering K. Functional roles of Na,K-ATPase subunits. Curr. Opin. Nephrol. Hypertens. 2008;17:526–532. doi: 10.1097/MNH.0b013e3283036cbf. [DOI] [PubMed] [Google Scholar]
- Girardet M., Geering K., Frantes J. M., Geser D., Rossier B. C., Kraehenbuhl J. -P., Bron C. Immunochemical evidence for a transmembrane orientation of both the (Na+,K+)-ATPase subunits. Biochem. J. 1981;20:6684–6691. doi: 10.1021/bi00526a025. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez L. M., Avila J., Marti E., Lecuona E., Martin-Vasallo P. Expression of the β-subunit isoforms of the Na,K-ATPase in rat embryo tissues, inner ear and choroid plexus. Biol. Cell. 1994;81:215–222. doi: 10.1016/0248-4900(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Grzmil M., Voigt S., Thelen P., Hemmerlein B., Helmke K., Burfeind P. Up-regulated expression of the MAT-8 gene in prostate cancer and its siRNA-mediated inhibition of expression induces a decrease in proliferation of human prostate carcinoma cells. Int. J. Oncol. 2004;24:97–105. [PubMed] [Google Scholar]
- Hague A., Manning A. M., Hanlon K. A., Huschtscha L. I., Hart D., Paraskeva C. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possible role of dietary fibre in the prevention of large-bowel cancer. Int. J. Cancer. 1993;55:498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- Jaisser F., Jaunin P., Geering K., Rossier B. C., Horisberger J. D. Modulation of the Na,K-pump function by β subunit isoforms. J. Gen. Physiol. 1994;103:605–623. doi: 10.1085/jgp.103.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed H., et al. FXYD3 is overexpressed in pancreatic ductal adenocarcinoma and influences pancreatic cancer cell growth. Int. J. Cancer. 2006;118:43–54. doi: 10.1002/ijc.21257. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mazurek S., Grimm H., Oehmke M., Weisse G., Teigelkamp S., Eigenbrodt E. Tumor M2-PK and glutaminolytic enzymes in the metabolic shift of tumor cells. Anticancer Res. 2000;20:5151–5154. [PubMed] [Google Scholar]
- Mercer R. W., Biemesderfer D., Bliss D. P., Collins J. H., Forbush B. Molecular cloning and immunological characterization of the γ-polypeptide, a small protein associated with the Na,K-ATPase. J. Cell Biol. 1993;121:579–586. doi: 10.1083/jcb.121.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison B. W., Leder P. neu and ras initiate murine mammary tumors that share genetic markers generally absent in c-myc and int-2-initiated tumors. Oncogene. 1994;9:3417–3426. [PubMed] [Google Scholar]
- Morrison B. W., Moorman J. R., Kowdley G. C., Kobayashi Y. M., Jones L. R., Leder P. Mat-8, a novel phospholemman-like protein expressed in human breast tumors, induces a chloride conductance in Xenopus oocytes. J. Biol. Chem. 1995;270:2176–2182. doi: 10.1074/jbc.270.5.2176. [DOI] [PubMed] [Google Scholar]
- Morth J. P., Pedersen B. P., Toustrup-Jensen M. S., Sorensen T.L.-M., Petersen J., Andersen J. P., Vilsen B., Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- Palmer C. J., Scott B. T., Jones L. R. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J. Biol. Chem. 1991;266:11126–11130. [PubMed] [Google Scholar]
- Pinto M., et al. Enterocyte-like differentiation and polarization of the human carcinoma cell line Caco-2 in culture. Biol. Cell. 1983;47:323–330. [Google Scholar]
- Pressley T. A. Phylogenetic conservation of isoform-specific regions within α- subunit of Na+-K+-ATPase. Am. J. Physiol. 1992;262:C743–C751. doi: 10.1152/ajpcell.1992.262.3.C743. [DOI] [PubMed] [Google Scholar]
- Pshezhetsky A. V., et al. Subcellular proteomics of cell differentiation: quantitative analysis of the plasma membrane proteome of Caco-2 cells. Proteomics. 2007;7:2201–2215. doi: 10.1002/pmic.200600956. [DOI] [PubMed] [Google Scholar]
- Rajasekaran S. A., Palmer L. G., Quan K., Harper J. F., Ball W. J., Jr., Bander N. H., Soler A. P., Rajasekaran A. K. Na,K-ATPase β-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol. Biol. Cell. 2001;12:279–295. doi: 10.1091/mbc.12.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmann S., Hahn D., Lottaz D., Kruse M. N., Stocker W., Sterchi E. E. Activation of human meprin-alpha in a cell culture model of colorectal cancer is triggered by the plasminogen-activating system. J. Biol. Chem. 2002;277:40650–40658. doi: 10.1074/jbc.M206203200. [DOI] [PubMed] [Google Scholar]
- Saaf A. M., Halbleib J. M., Chen X., Yuen S. T., Leung S. Y., Nelson W. J., Brown P. O. Parallels between global transcriptional programs of polarizing Caco-2 intestinal epithelial cells in vitro and gene expression programs in normal colon and colon cancer. Mol. Biol. Cell. 2007;18:4245–4260. doi: 10.1091/mbc.E07-04-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Suzuki T., Maeda M., Takahashi Y., Horikawa N., Minamimura T., Tsukada K., Takeguchi N. Up-regulation of Na+,K+-ATPase α3-isoform and down-regulation of the α1-isoform in human colorectal cancer. FEBS Lett. 2004;563:151–154. doi: 10.1016/S0014-5793(04)00292-3. [DOI] [PubMed] [Google Scholar]
- Santegoets S. J., et al. Identification and characterization of ErbB-3-binding protein-1 as a target for immunotherapy. J. Immunol. 2007;179:2005–2012. doi: 10.4049/jimmunol.179.3.2005. [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Stierum R., Gaspari M., Dommels Y., Ouatas T., Pluk H., Jespersen S., Vogels J., Verhoeckx K., Groten J., van Ommen B. Proteome analysis reveals novel proteins associated with proliferation and differentiation of the colorectal cancer cell line Caco-2. Biochim. Biophys. Acta. 2003;1650:73–91. doi: 10.1016/s1570-9639(03)00204-8. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J., Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics. 2000;68:41–56. doi: 10.1006/geno.2000.6274. [DOI] [PubMed] [Google Scholar]
- Tannu N. S., Hemby S. E. Two-dimensional fluorescence difference gel electrophoresis for comparative proteomics profiling. Nat. Protoc. 2006;1:1732–1742. doi: 10.1038/nprot.2006.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unlu M., Morgan M. E., Minden J. S. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18:2071–2077. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- Willis N. D., et al. Lamin A/C is a risk biomarker in colorectal cancer. PLoS ONE. 2008;3:e2988. doi: 10.1371/journal.pone.0002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi F., Yamaguchi K., Tai Y., Sugimoto K., Tokuda M. Molecular cloning and characterization of a novel phospholemman-like protein from rat hippocampus. Brain Res. Mol. Brain Res. 2001;86:189–192. doi: 10.1016/s0169-328x(00)00213-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.