Abstract
The physical proximity of the Golgi apparatus and the centrosome is a unique feature of mammalian cells whose functional significance is only poorly understood. Here, we demonstrate that the previously described regulation of centrosome organization and function by the Golgi protein, GM130, involves a Golgi-associated complex consisting of GM130, the Rho GTPase, Cdc42, and its guanine nucleotide exchange factor, Tuba. We identified Tuba as a novel GM130-interacting protein and showed that this association controls Tuba-mediated activation of Cdc42 at the Golgi apparatus. Blocking either Tuba or Cdc42 activity reproduced the GM130 depletion phenotype of aberrant, nonfunctional centrosomes. Expression of constitutively active Cdc42 bypassed the requirement for GM130 in centrosome regulation, indicating that Cdc42 functions downstream of GM130. Our studies demonstrate that Cdc42 has a novel role in controlling centrosome organization in unstimulated cells in addition to its known function as a regulator of centrosome reorientation in stimulated cells. This first description of a regulatory pathway between the Golgi apparatus and the interphase centrosome that complements the known role of Golgi proteins in controlling spindle formation during mitosis and may provide an explanation for the pericentriolar position of the mammalian Golgi apparatus during interphase.
INTRODUCTION
The physical proximity of the Golgi apparatus and the centrosome is a unique feature of mammalian cells, and there is emerging evidence that it is accompanied by a functional link between these organelles. A shared pool of proteins, such as CG-NAP and CAP350, are present at the Golgi apparatus and the centrosome but are not found elsewhere in the cell (Takahashi et al., 1999; Takatsuki et al., 2002; Hoppeler-Lebel et al., 2007). Moreover, specific proteins associated with Golgi membranes are required for centrosome-related processes and vice versa (Lin et al., 2000; Chang et al., 2005; Sutterlin et al., 2005; Hoppeler-Lebel et al., 2007). For example, the Golgi-localized proteins, GRASP65, Tankyrase, and Sac1, are essential for normal centrosome function during mitosis, and aberrant multipolar spindles are observed when these proteins are depleted (Chang et al., 2005; Sutterlin et al., 2005; Liu et al., 2008). In addition, we have shown that loss of the Golgi protein, GM130, results in aberrant, nonfunctional centrosomes characterized by multiple Centrin2-positive and γ-tubulin–negative foci that are unable to organize microtubules during interphase and mitosis (Kodani and Sutterlin, 2008). The mechanism by which GM130 controls centrosome organization and function has not been defined, and it is not clear whether it involves the known roles of GM130 in protein transport, the maintenance of Golgi organization or cell migration (Alvarez et al., 2001; Moyer et al., 2001; Preisinger et al., 2004; Puthenveedu et al., 2006; Marra et al., 2007).
In this report, we demonstrate that GM130-mediated regulation of centrosomal organization involves Cdc42. Cdc42 is a conserved member of the Rho family of small GTPases, which are known regulators of the actin cytoskeleton (Jaffe and Hall, 2005). Studies in yeast, flies, and mammalian cells have demonstrated that Cdc42 controls cell polarity in response to external stimuli (Etienne-Manneville, 2004). Specifically, Cdc42 regulates the activation of the Par polarity complex, which together with the noncanonical Wnt signal transduction pathway promotes centrosome reorientation and polarization of the actin and microtubule cytoskeletons at the leading edge of the cell (Etienne-Manneville and Hall, 2001; Schlessinger et al., 2007). Cdc42 is also involved in chromosome segregation and mitotic progression (Yasuda et al., 2004; Oceguera-Yanez et al., 2005). Interestingly, Cdc42 localizes to the Golgi apparatus, where it is proposed to function in protein transport and in the recruitment of regulators of the actin cytoskeleton (Wu et al., 2000; Luna et al., 2002; Matas et al., 2004).
Cdc42 is activated by a number of guanine nucleotide exchange factors (GEFs), including Tuba, a 180-kDa multidomain protein (Salazar et al., 2003; Sinha and Yang, 2008). In vitro analyses indicate that the GEF activity of Tuba is specific for Cdc42 (Salazar et al., 2003; Otani et al., 2006). Interestingly, in addition to its presence on the plasma membrane and in the cytosol, Tuba has been detected on the Golgi apparatus, but the significance of this specific localization is not known (Salazar et al., 2003).
In this study, we have investigated how GM130 is able to regulate centrosome organization and function from its position on the Golgi apparatus (Kodani and Sutterlin, 2008). Because GM130 is not detected on the centrosome, we searched for GM130-associated proteins that regulate centrosome organization and identified Cdc42 and its specific GEF, Tuba, as novel GM130-interacting proteins. Like GM130, Tuba and Cdc42 were found to be necessary for the maintenance of normal centrosome morphology and function. Furthermore, expression of constitutively active Cdc42 rescued the aberrant centrosome phenotype of GM130-deficient cells. Hence, GM130, Tuba, and Cdc42 constitute essential components of a novel Golgi-associated complex that regulates the normal morphology of the centrosome during interphase.
MATERIALS AND METHODS
Tissue Culture and Transfections
HeLa and U2-OS cells were cultured as previously described (Kodani and Sutterlin, 2008). hTER-RPE-1 were from ATCC (Manassas, VA) and were cultured in DMEM (GIBCO) supplemented with 10% FCS and L-glutamine (GIBCO).
Antibodies
Antibodies used in this study: anti-Tuba (Drs. P. De Camilli, Yale, and F. Gertler, MIT), anti-Centrin2 (Dr. J. Salisbury, Mayo Clinic), anti-GM130 (BD Biosciences, Sigma and Dr. A Linstedt, Carnegie Mellon University, Pittsburgh, PA), anti-FLAG, anti-α-tubulin, and anti-actin (Sigma), anti-Cdc42 and anti-β-PIX (Santa Cruz Biotechnology), anti-GFP (Roche and Sigma), and anti-γ-tubulin (Abcam). Secondary antibodies were from Molecular Probes (Eugene, OR) and Jackson ImmunoResearch Laboratories (West Grove, PA).
Molecular Biology
GM130 RNA interference (RNAi) was done as described (Kodani and Sutterlin, 2008). We depleted Tuba by targeting the sequence 5′-GAGCTTGAGGGAACATACAAGATTT-3′ in the human Tuba cDNA (base pairs 2581-2605) using Stealth siRNA duplexes (Invitrogen, Carlsbad, CA). A scrambled siRNA duplex was used as a negative control (Sutterlin et al., 2005).
Hemagglutinin (HA)-Tuba (Dr. P. De Camilli), green fluorescent protein (GFP)-tagged constitutively active (Cdc42 C12V) and dominant negative (Cdc42 T17N) Cdc42 (Dr. G. Egea, University of Barcelona, Spain), GST-CRIB-PAK (Dr. A. Putnam, UC Irvine). pFLAG-GM130 was generated by PCR amplification using GFP-GM130 (Dr. A. De Matteis, Mario Negri Sud, Italy) as a template, and ligation into the EcoRI and SalI sites of the pCMV-Tag2 vector (Stratagene, La Jolla, CA).
We used Oligofectamine (Invitrogen) for siRNA transfections and Fugene6 (Roche, Indianapolis, IN) or Lipofectamine with Plus Reagent (Invitrogen) for plasmid transfections.
Immunofluorescence
Immunofluorescence analysis was performed as described (Kodani and Sutterlin, 2008). Cells were imaged with a Zeiss Axiovert 200M microscope (Thornwood, NY) and analyzed with linear adjustments with the Zeiss Axiovision software.
In Vitro Transcription and Translation
Recombinant FLAG-GM130 and HA-Tuba were synthesized separately in the T3 or T7 TnT-coupled transcription/translation system (Promega, Madison, WI). Twenty microliters of each reaction were mixed and allowed to bind for 1 h at 30°C. We adjusted the reaction volume to 1 ml with lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, and protease inhibitors) and immunoprecipitated GM130 with a specific antibody and protein G-Sepharose (Amersham Biosciences, Piscataway, NJ). Immunocomplexes were washed, separated by SDS-PAGE, and analyzed by Western blotting.
Immunoprecipitation and Mass Spectrometry-based GM130 Interactor Screen
For the GM130 interactor screen, HeLa cells were harvested using a cell scraper and lysed on ice for 10 min in 50 mM Tris, pH 7.4, 150 mM NaCl, and 1% NP-40 supplemented with protease inhibitors. For each reaction, 100 mg of total lysate was incubated with 100 μg of FLAG or GM130 antibody at 4°C for 2 h and for 1 h with protein G-Sepharose (Amersham Biosciences). Immunocomplexes were washed, separated by SDS-PAGE, and visualized by silver staining. Bands were excised, digested, and extracted for LC MS/MS analysis as described (Wang and Huang, 2008). Protein identification was achieved through database searching using the development version of Protein Prospector (UCSF).
For normal coimmunoprecipitations, we used 2 mg of total cell lysates and proceeded as described above. To quantify the extent of coimmunoprecipitation, we determined the signal of immunoprecipitating or coimmunoprecipitating proteins from 2 mg of total lysate in comparison to 50 μg of total lysate loaded on the same gel.
Cdc42 Activity Assay
Recombinant glutathione S-transferase (GST) and GST-CRIB-PAK were generated in bacteria and used in Cdc42 activity assays (Benard et al., 1999). In brief, U2-OS cells were harvested using a cell scraper, lysed in buffer A (50 mM Tris, pH 7.4, 10 mM MgCl2, 200 mM NaCl, 1% NP-40, 5% glycerol, and protease inhibitors). 750 μg of cleared lysate was diluted into binding buffer (25 mM Tris, pH 7.4, 30 mM MgCl2, 40 mM NaCl, 0.5% NP-40, 1 mM DTT, and protease inhibitors) and incubated with 30 μg of GST or GST-CRIB-PAK immobilized on glutathione agarose beads (Amersham Biosciences) for 1 h at 4°C. Proteins on beads were washed with wash buffer (25 mM Tris, pH 7.4, 30 mM MgCl2, 40 mM NaCl, 1% NP-40, and protease inhibitors) separated by SDS-PAGE, and analyzed by Western blotting with a Cdc42-specific antibody. Band intensity was quantified using ImageJ (http://rsb.info.nih.gov/ij/).
RESULTS
Tuba, a Specific GEF for Cdc42, Binds Ddirectly to GM130
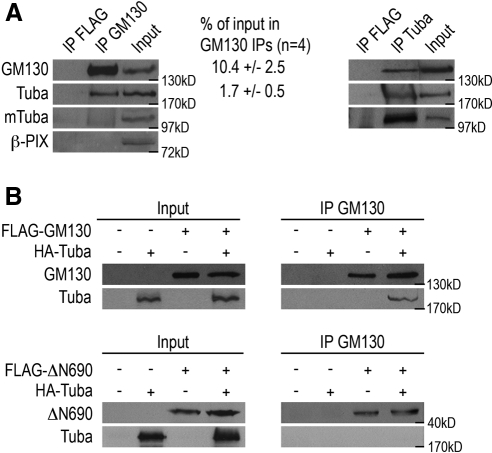
To determine the mechanism by which GM130 controls the organization of the centrosome, we searched for proteins that interact with GM130. Because none of the previously identified GM130 binding partners have obvious physical or functional links to the interphase centrosome, we focused our efforts on finding novel GM130-interacting proteins. We prepared total cell lysates from nonsynchronized HeLa cells using nondenaturing conditions and immunoprecipitated GM130 with a polyclonal rabbit antibody. As a negative control, we performed parallel experiments with a polyclonal rabbit antibody to the FLAG epitope. We separated the immunoprecipitates by SDS-PAGE and visualized coprecipitating proteins by silver staining. Proteins unique to the GM130 precipitates were excised from the gel and analyzed by mass spectrometry. We validated our experimental strategy by detecting the known GM130-binding proteins, GRASP65, YSK1, and Rab1b, by Western blot analysis (data not shown). With this approach, we identified Tuba, a 180-kDa multidomain protein with Cdc42-specific GEF activity, as a novel GM130-binding protein. The interaction between Tuba and GM130 was confirmed by reciprocal coimmunoprecipitations and was specific for Tuba, as other Cdc42-specific GEFs such as β-PIX did not associate with GM130 (Figure 1A). It is likely that GM130 binds to the N-terminal 775 amino acids of Tuba, as GM130 immunoprecipitates did not contain the smaller Tuba isoform, mTuba, which lacks this portion of the protein (Kovacs et al., 2006). Quantifications of these coimmunoprecipitation experiments revealed that GM130 immunoprecipitates contain 10.4% of cellular GM130 and 1.7% of cellular Tuba, suggesting that at least 16% of Tuba coimmunoprecipitates with GM130. However, this number is likely to be an underestimation of the actual percentage of Tuba that associates with GM130 at the Golgi, as coimmunoprecipitations can be inefficient.
Figure 1.
The Cdc42 GEF, Tuba, binds to GM130. (A) Total HeLa cell lysates were subjected to immunoprecipitations with polyclonal antibodies to GM130 (IP GM130), Tuba (IP Tuba), which detects Tuba and the Tuba isoform, mTuba, or to the FLAG epitope (FLAG IP), which served as a negative control. Immunoprecipitates were separated by SDS-PAGE and analyzed by Western blotting with antibodies to GM130, Tuba, and β-PIX. The input corresponds to 50 μg of total lysate, whereas each immunoprecipitation was performed from 2 mg of total lysate. The quantification of the amounts of GM130 and Tuba present in the GM130 immunoprecipitations is shown. (B) Recombinant HA-tagged Tuba, FLAG-tagged GM130, or FLAG-tagged GM130ΔN690 were synthesized in separate in vitro transcription and translation reactions. Samples were mixed to allow binding or kept separate as negative controls. Protein interactions were analyzed by coimmunoprecipitation experiments in which GM130 was immunoprecipitated from separate or mixed in vitro reactions using a GM130-specific antibody, followed by Western blot analysis with Tuba- and GM130-specific antibodies. The input material corresponds to 25% of each immunoprecipitation reaction.
We also confirmed the interaction between GM130 and Tuba in binding assays with in vitro–transcribed and –translated proteins in which we detected the association of recombinant HA-Tuba and full-length FLAG-GM130. We did not detect an interaction of Tuba and the truncated form of GM130, FLAG-GM130ΔN690, which lacks the first 690 amino acids and which was unable to complement the centrosome defect of GM130-depleted cells (Figure 1B; Kodani and Sutterlin, 2008). We conclude that Tuba is a novel GM130-interacting protein that is likely to bind to the N-terminal 690 amino acids of GM130.
GM130 Controls the Activity of Ccd42 in Unstimulated Cells
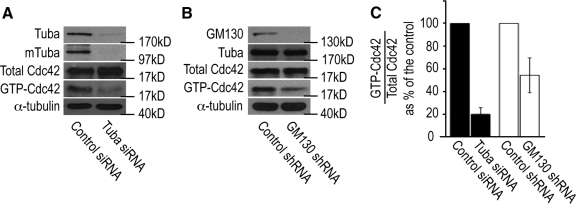
Because the GEF activity of Tuba is specific for Cdc42 (Salazar et al., 2003; Otani et al., 2006), we performed experiments to determine whether the GM130–Tuba interaction is necessary for the ability of Tuba to activate Cdc42. In preliminary studies, we first documented the effect of Tuba depletion on Cdc42 activity in U2-OS osteosarcoma cells by assaying the amount of activated Cdc42 in total cell extracts. RNAi-mediated depletion of Tuba and mTuba protein levels to ∼5% of control cells resulted in a decrease in activated Cdc42 by 80%, suggesting that Tuba functions as a key activator of Cdc42 activity in resting cells (Figure 2, A and C). We next depleted GM130 and examined if Tuba-mediated activation of Cdc42 was altered under these conditions. Extracts from GM130 knockdown cells had normal levels of Tuba, but contained only 50% of the level of activated Cdc42 measured in control extracts (Figure 2, B and C). Because depletion of GM130 had a smaller effect on Cdc42 activity than Tuba depletion, we propose that GM130 may regulate the activity of the subset of Cdc42 that is activated by the subset of Tuba that is associated with GM130 on the Golgi. We conclude from these experiments that GM130 has a novel function in the control of Cdc42 activity via Tuba. Our results are consistent with a model in which the interaction between GM130 and Tuba may promote the binding of Tuba to Cdc42 in order to activate Cdc42. Alternatively, GM130 binding to Tuba may stimulate the GEF activity of Tuba toward Cdc42.
Figure 2.
Tuba and GM130 regulate Cdc42 activation in unstimulated cells. (A) Cdc42 activity assays were performed by incubating total lysates from unstimulated control (control siRNA) and Tuba-depleted (Tuba siRNA) U2-OS cells with the recombinant CRIB domain of the Cdc42 effector, p21-activated kinase (GST-CRIB-PAK), which specifically associates with the activated, GTP-bound form of Cdc42 (Benard et al., 1999). The levels of overall cellular Cdc42 (total Cdc42) and activated Cdc42 (GTP-Cdc42) were determined by Western blotting with a Cdc42-specific antibody. α-Tubulin served as a loading control. (B) Cdc42 activity assays, as described in Figure 2A, were also performed in control and GM130-depleted cells. (C) A quantification of the ratio of activated GTP-Cdc42/total Cdc42 as percentage of the control is shown. Image J was used for these quantifications. These experiments were repeated three independent times, and a mean and SD were calculated.
GM130 Controls the Interaction between Tuba and Cdc42 at the Golgi Apparatus
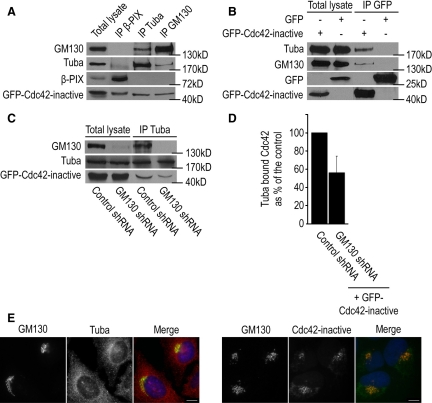
To further investigate the mechanism by which GM130 controls the activation of Cdc42, we examined whether GM130 and Cdc42 form a complex. We performed these studies in HeLa cells, because they contain higher levels of Tuba and GM130 than U2-OS cells (data not shown), and utilized a GFP-tagged dominant negative form of Cdc42 (Cdc42 N17) that binds more tightly to its GEF (Feig, 1999). We detected in reciprocal coimmunoprecipitation experiments that GM130, Tuba, and Cdc42 formed a trimeric complex that did not contain nonspecific components such as β-PIX or GFP (Figure 3, A and B). Intriguingly, Tuba and GM130 immunoprecipitates contained similar amounts of Cdc42, suggesting that dominant negative Cdc42 associated with GM130 and its GEF, Tuba, with comparable efficiency (Figure 3A).
Figure 3.
GM130, Tuba, and Cdc42 form a Golgi-associated complex. (A) HeLa cells were transfected with a GFP-tagged dominant negative form of Cdc42 (Cdc42-inactive = Cdc42 N17). Cell lysates were prepared and incubated with antibodies to β-PIX (IP β-PIX), Tuba (IP Tuba), or GM130 (IP GM130). The presence of coimmunoprecipitating proteins was determined by Western blotting with antibodies to GM130, Tuba, β-PIX, and GFP. The input corresponds to 2.5% of the total cell lysate that was used for each immunoprecipitation. (B) Cells expressing GFP-tagged dominant negative Cdc42 or GFP as control were subjected to immunoprecipitations with the GFP-specific antibody. Coimmunoprecipitating proteins were analyzed by Western blotting with antibodies to Tuba, GM130, and GFP. (C) Control or GM130-depleted cells expressing dominant negative Cdc42 (GFP-Cdc42-inactive) were subjected to immunoprecipitations with the Tuba-specific antibody (IP Tuba). The amount of coimmunoprecipitating GM130 and GFP-tagged Cdc42 were determined by Western blotting. Total lysate corresponds to 2.5% of the starting material for an immunoprecipitation. (D) Quantification of Tuba-bound Cdc42 in GM130-depleted cells as percentage of control levels (n = 3). (E) Left panel, U2-OS cells grown on coverslips were costained with antibodies to GM130, Tuba, and the DNA dye Hoechst. Right panel, U2-OS cells were transfected with GFP-tagged dominant negative Cdc42 (Cdc42 inactive). GM130 was visualized with an antibody to GM130, whereas the GFP signal revealed the localization of dominant negative Cdc42. Scale bar, 10 μm.
We next examined the role of GM130 in this complex by testing if loss of GM130 affected the ability of Tuba to associate with Cdc42. Coimmunoprecipitation experiments revealed that Cdc42 interacted less efficiently with Tuba in GM130-depleted cells than in control cells (Figure 3, C and D), suggesting that GM130 is required for the efficient interaction between specific subsets of Tuba and Cdc42.
To examine the subcellular localization of the GM130–Tuba–Cdc42 complex, we performed immunofluorescence studies on each of these proteins in U2-OS cells. GM130 was only found at the Golgi apparatus, as previously described (Nakamura et al., 1995). Tuba was observed on the plasma membrane and in the cytosol, but also at ER exit sites and in the pericentriolar region where it colocalized with GM130 (Figure 3E). Our results thus support the presence of a small pool of Tuba at the Golgi and is consistent with the previously reported detection of Tuba on the Golgi apparatus in frozen brain sections (Salazar et al., 2003). More precise localization studies were difficult because of adverse effects of Tuba overexpression on cell morphology and survival (data not shown). We detected dominant negative Cdc42 in the cytosol but primarily at the Golgi apparatus where it colocalized with GM130, confirming published results (Erickson et al., 1996; Luna et al., 2002).
Taken together, these results support a model in which GM130 regulates the activation of Golgi-localized Cdc42 by facilitating the interaction of Golgi-localized Tuba and Golgi-localized Cdc42. Thus, GM130 appears to function as a cofactor for Tuba that is required for the localized activation of Cdc42 at the Golgi complex.
Tuba-mediated Cdc42 Activation Controls the Organization and Function of the Centrosome during Interphase and Mitosis
To determine the significance of the physical and functional interactions of Tuba and Cdc42 with GM130, we investigated whether these proteins function downstream of GM130 in the regulation of the interphase centrosome. We blocked Tuba and Cdc42 function in separate experiments and assayed for the GM130 depletion phenotype of aberrant centrosome morphology and function during interphase and mitosis (Kodani and Sutterlin, 2008). Specifically, we examined centrosome organization by immunofluorescence with antibodies to Centrin2 and γ-tubulin, which are components of the centriole and the pericentriolar matrix, respectively. We also assayed centrosome function by staining for α-tubulin to visualize microtubule organization as a radial array during interphase and as a bipolar spindle during mitosis. We performed the interphase studies with U2-OS cells, but we were unable to use these cells for experiments in mitosis because their functional p53 precludes them from entering mitosis in the presence of defective centrosomes (data not shown; Mikule et al., 2007; Kodani and Sutterlin, 2008). Instead, we used HeLa cells, which are able to enter mitosis in the presence of abnormal centrosomes because they have a nonfunctional p53.
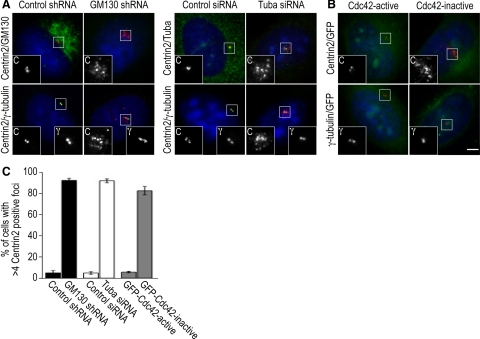
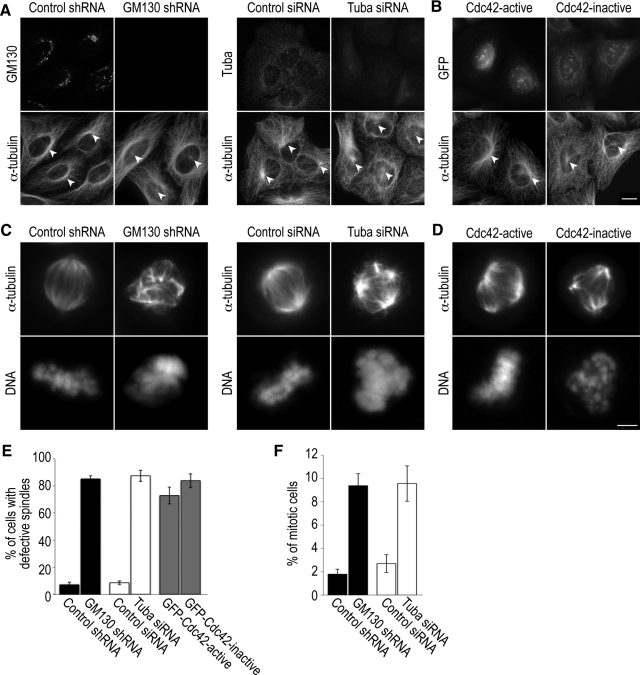
When we blocked Tuba function in U2-OS and HeLa cells by RNAi-mediated depletion, we observed centrosome defects during interphase and mitosis. Approximately 90% of Tuba-depleted cells contained abnormal interphase centrosomes, which were characterized by multiple Centrin2-positive and γ-tubulin–negative foci on top of the nucleus (Figure 4, A and C). We also detected disorganized microtubule bundles surrounding the nucleus, and only few microtubules were nucleated by the centrosome (Figure 5A). Similar effects of Tuba depletion on centrosome morphology were observed in retinal pigment epithelial cells (hTER-RPE-1) and interphase HeLa cells (data not shown). We also found that 90% of Tuba-depleted cells in mitosis contained aberrant spindles that were unable to align condensed chromosomes at the metaphase plate (Figure 5, C and E). This spindle defect of Tuba-depleted cells resulted in an increased mitotic index (Figure 5F) and eventually in cell death (data not shown), similar to what we have previously described for GM130-depleted cells (Kodani and Sutterlin, 2008).
Figure 4.
GM130, Tuba, and Cdc42 are required for normal centrosome organization and function during the cell cycle. (A) Control (control shRNA) or GM130-depleted (GM130 shRNA) U2-OS cells were were stained with antibodies to GM130 to confirm protein knockdown and to the centriolar marker, Centrin2 to visualize the organization and position of the centrosome (large image). These centrosomes were further characterized by costaining with Centrin2 (labeled C) and the pericentriolar marker protein, γ-tubulin (γ; small insets). Similar experiments were also performed for Tuba, which was depleted by siRNA. (B) Centrosome organization and position was examined in U2-OS cells in which Cdc42 activation was blocked by expressing GFP-tagged dominant negative Cdc42 (inactive). GFP-tagged constitutively active Cdc42 (active = Cdc42 V12) served as a negative control. The Scale bar, (A and B) 5 μm. (C) The percentage of cells with aberrant centrosomes, which were defined as centrosomes with >4 Centrin2-positive foci, were determined from three independent experiments, which were used to calculate the mean and SD.
Figure 5.
Inactivation of GM130, Tuba, and Cdc42 produces aberrant centrosomes that are unable to organize microtubules during interphase and mitosis. (A) Microtubule organization of control or GM130- and Tuba-depleted U2-OS cell was visualized with antibodies to α-tubulin. Loss of GM130 or Tuba was verified with specific antibodies to GM130 or Tuba, respectively. The microtubule organizing centers are marked with arrowheads. (B) Microtubule organization in U2-OS cells transfected with GFP-tagged constitutively active and dominant negative forms of Cdc42 as described for Figure 5A. The expression and Golgi localization of these constructs were verified by GFP fluorescence. The Scale bar, (A and B) 10 μm.(C) Mitotic HeLa cells, in which the functions of GM130 and Tuba were inactivated by RNAi-mediated protein depletion, were stained with an antibody to α-tubulin and the DNA dye Hoechst to visualize the organization of the mitotic spindle and the alignment of DNA, respectively. (D) Analysis of spindle organization in mitotic HeLa cells that expressed dominant negative (inactive) or constitutively active (active) Cdc42. Scale bar, (C and D) 5 μm. (E) The percentage of mitotic cells with aberrantly organized spindles is shown. These experiments were repeated three independent times and a mean and SD were calculated. (F) Quantifications of the percentage of cells in mitosis as determined by staining with a specific antibody to phospho-Histone H3 (n = 3).
Because depletion of GM130 and Tuba prevented the activation of Cdc42, we next tested if Cdc42 activity itself was required for the regulation of centrosome organization and function. We blocked Cdc42 function by expressing a GFP-tagged dominant negative form of Cdc42 (Cdc42 N17) in U2-OS cells, which produced centrosome abnormalities that mimicked those of GM130- and Tuba-depleted cells (Figures 4B and 5B). In control experiments, constitutively active Cdc42 (Cdc42 V12) had no effect on the interphase centrosome. In contrast, dominant negative and constitutively active Cdc42 produced similar nonfunctional aberrant spindles in mitotic HeLa cells (Figure 5, C and D). In light of our finding that blocking Cdc42 function affected the interphase centrosome, we postulate that spindle phenotypes due to altered Cdc42 activity can be produced by more than one mechanism. Spindle defects due to the expression of constitutively active Cdc42 have been described and attributed to altered interactions between spindle microtubules and kinetochores (Yasuda et al., 2004; Oceguera-Yanez et al., 2005). In contrast, the spindle abnormalities seen with dominant negative Cdc42 may result from the centrosome defects that we have detected in interphase (Figure 4B). In summary, blocking the function of either Tuba or Cdc42 primarily affects the organization of the interphase centrosome, producing a phenotype similar to depletion of GM130 (Figure 4, A and C; Kodani and Sutterlin, 2008). Thus, we have identified a novel function for Tuba and Cdc42 in the regulation of the interphase centrosome, which is in addition to the known role of Cdc42 in spindle formation.
Activated Cdc42 Bypasses the Requirement for GM130 in Centrosome Regulation
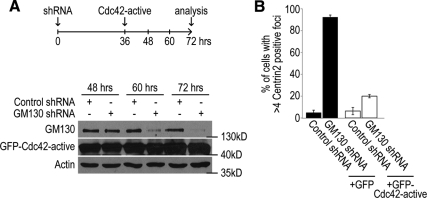
To directly determine if GM130 regulates centrosome organization and function via Cdc42, we examined whether constitutively active Cdc42 could restore centrosome morphology in the absence of GM130. We generated U2-OS cells that lacked GM130, due to RNAi-mediated depletion, and expressed GFP-tagged, constitutively active Cdc42 (Cdc42 V12) or GFP as a negative control (Figure 6A). We detected abnormal centrosomes in 90% of GM130-depleted cells, but only in 20% of cells that also expressed constitutively active Cdc42 (Figure 6B), demonstrating that constitutively active Cdc42 rescued GM130 knockdown-induced centrosome abnormalities. This finding indicates that Cdc42 functions downstream of GM130 and provides strong support for a model in which the GM130-Tuba complex regulates centrosome organization during interphase by activating a specific pool of Cdc42 at the Golgi apparatus.
Figure 6.
Constitutively active Cdc42 bypasses the requirement for GM130 in centrosome organization. (A) U2-OS cells were transfected with control (control shRNA) or GM130-specific short hairpin (GM130 shRNA) for 72 h and transfected for a second time with GFP or GFP-tagged constitutively active Cdc42 (Cdc42 active = Cdc42 V12). Because expression of constitutively active Cdc42 for more than 36 h caused cell death (data not shown), we performed the second transfection with a 36-h delay. Actin served as a loading control. (B) Control and GM130-depleted cells that contained either GFP (+GFP) or GFP-tagged constitutively active Cdc42 (+GFP-Cdc42 active) were stained with an antibody to Centrin2 to determine the organization of the centrosome 72 h after the initial transfection. The percentage of cells with >4 Centrin 2-positive foci is shown. These experiments were repeated three independent times and a mean and SD were calculated.
DISCUSSION
In this study, we describe a novel regulatory pathway by which the Golgi protein, GM130, controls the organization and function of the interphase centrosome. Components of this pathway are GM130, the Cdc42 GEF, Tuba, and Cdc42 itself, and we found that these proteins assemble into a complex at the Golgi apparatus. We demonstrate that GM130 controls Cdc42 activation by facilitating the interaction between Tuba and Cdc42 at the Golgi, which is necessary for the efficient activation of Golgi-localized Cdc42. GM130 therefore appears to have a previously undetected role in the regulation of Cdc42 activity. Like GM130, Tuba and Cdc42 functions were both required for the maintenance of centrosome organization and function during interphase. Finally, constitutively active Cdc42 was able to bypass the requirement for GM130 in centrosome regulation, placing Cdc42 downstream of GM130 in this regulatory pathway. These results are the first report that establishes Tuba and Cdc42, as essential components of a GM130-dependent complex at the Golgi that regulates the normal morphology of the centrosome during interphase.
Our results demonstrate that Tuba functions as an important regulator of Cdc42 activity in resting cells. Numerous GEFs are known to activate Cdc42 in response to specific external stimuli, such as scratch wounds or treatment with lysophosphatidic acid (Sinha and Yang, 2008). Tuba, however, appears to catalyze the activation of Cdc42 in the absence of an external stimulus and may function in maintaining basal levels of Cdc42 activity. Specifically, our results indicate that the GEF activity of Tuba is responsible for 80% of Cdc42 activity in unstimulated U2-OS cells. Our results are in contrast to a study in which depletion of Tuba in polarized CaCo-2 cells only produced a small reduction in Cdc42 activity (Otani et al., 2006). This discrepancy may be due to the use of a cell line in which Tuba expression was constitutively lowered, to specific differentiated cell phenotypes or to overall differences in growth conditions.
Our Cdc42 activity assays, binding and localization studies support a model in which GM130 promotes Tuba-dependent activation of Cdc42 by facilitating the interaction between these two proteins at the Golgi. We have detected only small amounts of Tuba at the Golgi by immunofluorescence (Figure 3E), which may be due to the fact that our antibody also recognizes Tuba at the plasma membrane and its smaller cytosolic isoform, mTuba, and its high background staining. However, our coimmunoprecipitations showed that at least 16% of Tuba associates with GM130 (Figure 1A), and it is likely that the actual amount of Tuba-bound GM130 is two- to threefold higher because coimmunoprecipitations can be inefficient. Such increased levels of GM130-bound Tuba at the Golgi would be consistent with the findings that loss of GM130 leads to a 40–50% reduction in the interaction between Tuba and Cdc42 and in Cdc42 activation. Thus, GM130 may have a critical function as a cofactor to control Tuba-dependent activation of Cdc42 at the Golgi. A role for GM130 in the recruitment of Tuba to the Golgi, which would also be consistent with our results, can be excluded as altering GM130 levels by RNAi or protein overexpression had no effect on Tuba levels at the Golgi (data not shown).
The novel role for Cdc42 in the regulation of centrosome organization that we described is consistent with published links between Cdc42 and the centrosome. For example, Cdc42 is known to control the reorientation of the centrosome during cell polarization in a process that requires the Par polarity complex (Etienne-Manneville and Hall, 2001; Schlessinger et al., 2007). Interestingly, the Par complex component, Par6α, a specific Cdc42 effector, localizes to the centrosome in neurons and controls centrosome organization and function (Solecki et al., 2004). Additionally, Cdc42 and its effector, PAK1, have been detected at the centrosome for possible roles in the activation of Aurora A, a centrosome-localized protein kinase that controls centrosome maturation at the G2/M transition (Ando et al., 2007). Our results are also consistent with spindle and chromosome segregation defects observed when Cdc42 activation was blocked (Yasuda et al., 2004; Oceguera-Yanez et al., 2005). However, as dominant negative Cdc42 had adverse effects on both the interphase centrosome and the mitotic spindle, we propose that the primary effect is on centrosome organization and function and that the spindle abnormalities are an indirect consequence of aberrant centrosomes.
Activated Cdc42 has established roles at the Golgi apparatus, but their involvement centrosome regulation is not known. centrosome. For example, Golgi-localized Cdc42 recruits the actin regulator, N-WASP, to the Golgi apparatus, which is required for protein transport and for the assembly of a local actin cytoskeleton (Luna et al., 2002). In addition, activated Cdc42 has been found to associate with the coatomer subunit, γ-COP, at the Golgi apparatus for a role in protein transport (Wu et al., 2000). Centrosome regulation appears to be a novel function for Golgi-localized Cdc42, but it is not clear whether the known Cdc42 effectors function in this regulatory pathway. In addition, it is not known whether Golgi-activated Cdc42 directs the organization of the centrosome from its position at the Golgi apparatus or whether its activation at the Golgi apparatus facilitates its subsequent relocation to the centrosome. Interestingly, the activity of Cdc42 at the Golgi apparatus is negatively controlled by ARHGAP10, a Golgi-localized Cdc42-specific GTPase-activating protein (Dubois et al., 2005). By demonstrating that GM130 and Tuba together control the activation of Golgi-localized Cdc42 activation, we have provided another example of Cdc42 regulation occurring at the Golgi apparatus.
In summary, we describe a novel pathway in which GM130, through association with Tuba at the Golgi apparatus, activates a subset of Cdc42, which in turn regulates centrosome organization in unstimulated cells. These findings provide strong support for a functional link between the Golgi apparatus and the centrosome to accompany the known physical juxtaposition. Thus, control of centrosome organization and function by the Golgi-associated GM130–Tuba–Cdc42 complex may provide an explanation for the close physical association of these organelles during interphase. Intriguingly, the temporary loss of proximity during mitosis, when mammalian Golgi membranes are fragmented and dispersed throughout the cytosol, adds the potential for yet another layer of regulation in mammalian cells.
ACKNOWLEDGMENTS
We thank Drs. Pietro De Camilli, Gustavo Egea, Adam Linstedt, Frank Gertler, Antonella de Matteis, Jeffrey Salisbury, and Andrew Putnam for their generous gifts of antibodies and DNA constructs. We are grateful to Drs. Ming Tan, Naomi Morrissette, Grant MacGregor, and Andrew Putnam for helpful comments on this manuscript. This work was supported by National Institutes of Health Grant GM74830 to L.H.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-08-0834) on December 24, 2008.
REFERENCES
- Alvarez C., Garcia-Mata R., Hauri H. P., Sztul E. The p115-interactive proteins GM130 and giantin participate in endoplasmic reticulum-Golgi traffic. J. Biol. Chem. 2001;276:2693–2700. doi: 10.1074/jbc.M007957200. [DOI] [PubMed] [Google Scholar]
- Ando Y., Yasuda S., Oceguera-Yanez F., Narumiya S. Inactivation of Rho GTPases with Clostridium difficile toxin B impairs centrosomal activation of Aurora-A in G2/M transition of HeLa cells. Mol. Biol. Cell. 2007;18:3752–3763. doi: 10.1091/mbc.E07-03-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard V., Bohl B. P., Bokoch G. M. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Chang P., Coughlin M., Mitchison T. J. Tankyrase-1 polymerization of poly(ADP-ribose) is required for spindle structure and function. Nat. Cell Biol. 2005;7:1133–1139. doi: 10.1038/ncb1322. [DOI] [PubMed] [Google Scholar]
- Dubois T., Paleotti O., Mironov A. A., Fraisier V., Stradal T. E., De Matteis M. A., Franco M., Chavrier P. Golgi-localized GAP for Cdc42 functions downstream of ARF1 to control Arp2/3 complex and F-actin dynamics. Nat. Cell Biol. 2005;7:353–364. doi: 10.1038/ncb1244. [DOI] [PubMed] [Google Scholar]
- Erickson J. W., Zhang C., Kahn R. A., Evans T., Cerione R. A. Mammalian Cdc42 is a brefeldin A-sensitive component of the Golgi apparatus. J. Biol. Chem. 1996;271:26850–26854. doi: 10.1074/jbc.271.43.26850. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Cdc42—the centre of polarity. J. Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Feig L. A. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol. 1999;1:E25–E27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- Hoppeler-Lebel A., Celati C., Bellett G., Mogensen M. M., Klein-Hitpass L., Bornens M., Tassin A. M. Centrosomal CAP350 protein stabilises microtubules associated with the Golgi complex. J. Cell Sci. 2007;120:3299–3308. doi: 10.1242/jcs.013102. [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Kodani A., Sutterlin C. The Golgi protein GM130 regulates centrosome morphology and function. Mol. Biol. Cell. 2008;19:745–753. doi: 10.1091/mbc.E07-08-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. M., Makar R. S., Gertler F. B. Tuba stimulates intracellular N-WASP-dependent actin assembly. J. Cell Sci. 2006;119:2715–2726. doi: 10.1242/jcs.03005. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Madsen M. L., Yarm F. R., Jang Y. J., Liu X., Erikson R. L. Peripheral Golgi protein GRASP65 is a target of mitotic polo-like kinase (Plk) and Cdc2. Proc. Natl. Acad. Sci. USA. 2000;97:12589–12594. doi: 10.1073/pnas.220423497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Boukhelifa M., Tribble E., Morin-Kensicki E., Uetrecht A., Bear J. E., Bankaitis V. A. The sac1 phosphoinositide phosphatase regulates Golgi membrane morphology and mitotic spindle organization in mammals. Mol. Biol. Cell. 2008;19:3080–3096. doi: 10.1091/mbc.E07-12-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna A., Matas O. B., Martinez-Menarguez J. A., Mato E., Duran J. M., Ballesta J., Way M., Egea G. Regulation of protein transport from the Golgi complex to the endoplasmic reticulum by CDC42 and N-WASP. Mol. Biol. Cell. 2002;13:866–879. doi: 10.1091/mbc.01-12-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra P., Salvatore L., Mironov A., Jr, Di Campli A., Di Tullio G., Trucco A., Beznoussenko G., Mironov A., De Matteis M. A. The biogenesis of the Golgi ribbon: the roles of membrane input from the ER and of GM130. Mol. Biol. Cell. 2007;18:1595–1608. doi: 10.1091/mbc.E06-10-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas O. B., Martinez-Menarguez J. A., Egea G. Association of Cdc42/N-WASP/Arp2/3 signaling pathway with Golgi membranes. Traffic. 2004;5:838–846. doi: 10.1111/j.1600-0854.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- Mikule K., Delaval B., Kaldis P., Jurcyzk A., Hergert P., Doxsey S. Loss of centrosome integrity induces p38–p53-p21-dependent G1-S arrest. Nat. Cell Biol. 2007;9:160–170. doi: 10.1038/ncb1529. [DOI] [PubMed] [Google Scholar]
- Moyer B. D., Allan B. B., Balch W. E. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic. 2001;2:268–276. doi: 10.1034/j.1600-0854.2001.1o007.x. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Rabouille C., Watson R., Nilsson T., Hui N., Slusarewicz P., Kreis T. E., Warren G. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oceguera-Yanez F., Kimura K., Yasuda S., Higashida C., Kitamura T., Hiraoka Y., Haraguchi T., Narumiya S. Ect2 and MgcRacGAP regulate the activation and function of Cdc42 in mitosis. J. Cell Biol. 2005;168:221–232. doi: 10.1083/jcb.200408085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T., Ichii T., Aono S., Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J. Cell Biol. 2006;175:135–146. doi: 10.1083/jcb.200605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisinger C., Short B., De Corte V., Bruyneel E., Haas A., Kopajtich R., Gettemans J., Barr F. A. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J. Cell Biol. 2004;164:1009–1020. doi: 10.1083/jcb.200310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu M. A., Bachert C., Puri S., Lanni F., Linstedt A. D. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat. Cell Biol. 2006;8:238–248. doi: 10.1038/ncb1366. [DOI] [PubMed] [Google Scholar]
- Salazar M. A., Kwiatkowski A. V., Pellegrini L., Cestra G., Butler M. H., Rossman K. L., Serna D. M., Sondek J., Gertler F. B., De Camilli P. Tuba, a novel protein containing bin/amphiphysin/Rvs and Dbl homology domains, links dynamin to regulation of the actin cytoskeleton. J. Biol. Chem. 2003;278:49031–49043. doi: 10.1074/jbc.M308104200. [DOI] [PubMed] [Google Scholar]
- Schlessinger K., McManus E. J., Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J. Cell Biol. 2007;178:355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Yang W. Cellular signaling for activation of Rho GTPase Cdc42. Cell Signal. 2008;20:1927–1934. doi: 10.1016/j.cellsig.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Solecki D. J., Model L., Gaetz J., Kapoor T. M., Hatten M. E. Par6alpha signaling controls glial-guided neuronal migration. Nat. Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- Sutterlin C., Polishchuk R., Pecot M., Malhotra V. The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol. Biol. Cell. 2005;16:3211–3222. doi: 10.1091/mbc.E04-12-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Shibata H., Shimakawa M., Miyamoto M., Mukai H., Ono Y. Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the golgi apparatus. J. Biol. Chem. 1999;274:17267–17274. doi: 10.1074/jbc.274.24.17267. [DOI] [PubMed] [Google Scholar]
- Takatsuki A., Nakamura M., Kono Y. Possible implication of Golgi-nucleating function for the centrosome. Biochem. Biophys. Res. Commun. 2002;291:494–500. doi: 10.1006/bbrc.2002.6433. [DOI] [PubMed] [Google Scholar]
- Wang X., Huang L. Identifying dynamic interactors of protein complexes by quantitative mass spectrometry. Mol. Cell Proteomics. 2008;7:46–57. doi: 10.1074/mcp.M700261-MCP200. [DOI] [PubMed] [Google Scholar]
- Wu W. J., Erickson J. W., Lin R., Cerione R. A. The gamma-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature. 2000;405:800–804. doi: 10.1038/35015585. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Oceguera-Yanez F., Kato T., Okamoto M., Yonemura S., Terada Y., Ishizaki T., Narumiya S. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature. 2004;428:767–771. doi: 10.1038/nature02452. [DOI] [PubMed] [Google Scholar]