Abstract
A hair cell’s tip links are thought to gate mechanoelectrical transduction channels. The susceptibility of tip links to acoustic trauma raises questions as to whether these fragile structures can be regenerated. We broke tip links with the calcium chelator 1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid and found that they can regenerate, albeit imperfectly, over several hours. The time course of tip-link regeneration suggests that this process may underlie recovery from temporary threshold shifts induced by noise exposure. Cycloheximide does not block tip-link regeneration, indicating that new protein synthesis is not required. The calcium ionophore ionomycin prevents regeneration, suggesting regeneration normally may be stimulated by the reduction in stereociliary Ca2+ when gating springs rupture and transduction channels close. Supporting the equivalence of tip links with gating springs, mechanoelectrical transduction returns over the same time period as tip links; strikingly, adaptation is substantially reduced, even 24 hr after breaking tip links.
Hair cells transduce displacements of their mechanically sensitive hair bundles into electrical signals (1, 2). A hair bundle consists of about 100 stereocilia, each of which contains hundreds of crosslinked actin filaments enshrouded by plasma membrane. Stereocilia are arrayed in rows of increasing height, producing a bundle that exhibits mirror symmetry along a central axis; this axis also corresponds to the bundle’s axis of highest displacement sensitivity. When a bundle is displaced along this axis, the tip of each shorter stereocilium slides along the side of its tallest neighbor. Such displacements stretch elastic mechanical elements called gating springs, which open transduction channels (3). Optimally poised to be gating springs, tip links are 5 × 150 nm extracellular filaments that stretch from the tip of a stereocilium to the side of its neighbor, parallel to the plane of mirror symmetry (4). Bundle displacements that stretch tip links also open transduction channels, and displacements that slacken tip links permit channels to close. Because 1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) eradicates tip links, simultaneously disrupting transduction by disengaging gating springs, tip links are thought to either be gating springs themselves or connect in series with the gating spring (5).
A hair cell’s transduction apparatus survives constant stimulation and occasionally experiences stimuli that can break tip links (6). If tip links indeed play an essential role in transduction, broken tip links should be replaced to maintain sensitivity to mechanical displacements. We intentionally broke tip links with BAPTA and found that they regenerate over a period of several hours. Regeneration is imperfect, as many links arise that connect stereociliary pairs not oriented along the plane of mirror symmetry. Although restoration of tip links does not require new protein synthesis, elevation of intracellular Ca2+ levels effectively prevents regeneration. As expected if tip links gate transduction channels, mechanoelectrical transduction returns over the same time course as tip links; strikingly, both adaptation of transduction and mechanical stability of the transduction apparatus are reduced substantially. These results illustrate the dynamic nature of the hair cell’s transduction apparatus.
MATERIALS AND METHODS
Tissue Preparation and Electron Microscopy.
The entire temporal bone containing the basilar papilla of newborn chickens was dissected and transferred to serum-free Medium 199 (GIBCO/BRL). After the epithelium of the basilar papilla was exposed by cutting away the tegmentum vasculosum, the tectorial membrane was detached from homogene cells on the superior margin of the papilla using an eyelash. Papillae were treated with 5 mM BAPTA (Molecular Probes) in 155 mM NaCl/6 mM KCl/3 mM d-glucose/5 mM Hepes at pH 7.2–7.4 for 15 min. Control and BAPTA-treated papillae were cultured in Medium 199 at 37°C for 0–24 hr. In early experiments, we noted that most bundles of BAPTA-treated papillae leaned toward the shortest stereocilia (the negative direction) after several days’ culture; we largely avoided this phenomenon by detaching the tectorial membrane.
Before fixation, tissues were treated with 50 μg/ml subtilisin (Sigma protease type XXIV) for 20 min to permit removal of the tectorial membrane. Papillae were fixed with 2% glutaraldehyde and 1% tannic acid for 2–4 hr on ice, and postfixed in 1% OsO4 for 1 min. Dehydration was carried out with an acetone series. The specimens were critical-point dried from liquid CO2, sputter-coated with gold-palladium, and examined in a scanning electron microscope (Amray, Bedford, MA). Measurements of tip links were derived from Polaroid pictures of hair bundles at about ×20,000; we examined 15–20 hair bundles at each time point, from six BAPTA-treated and six control basilar papillae. A total of 162 hair bundles were examined in detail. Hair bundles were chosen at low magnification, where tip links could not be seen, to prevent any bias. We attempted to include bundles from all regions of the basilar papilla; no systematic regional differences in tip-link regeneration were observed. A fraction of the hair bundles in cultured basilar papillae were splayed apart; BAPTA treatment increased this fraction, perhaps by dissociating lower crosslinks between stereocilia. We did not collect tip-link regeneration data from these bundles. Pictures were encoded and scored for the presence of tip links by four members of the laboratory in a blind fashion (5). Tip links were scored as present or absent between each pair of stereocilia that (i) were aligned along the bundle’s mirror symmetry axis and hence should possess a tip link, (ii) were in proper focus, (iii) were not in disarray, and (iv) were not obscured by debris. Data are presented as a percentage of stereociliary pairs parallel to the plane of mirror symmetry that have a tip link; means plus or minus the SEM are reported.
To block protein synthesis, papillae were incubated for 12 hr under similar conditions except that 40 μM cycloheximide (Sigma) was included in the culture medium. To show that cycloheximide blocked protein synthesis, we incubated basilar papillae in 0.1 mCi/ml (1 Ci = 37 GBq) [35S]methionine (Amersham) in methionine-free Medium 199 (GIBCO/BRL) for 12 hr in the presence or absence of cycloheximide. Incorporation of [35S]methionine into protein was measured in isolated hair bundles (unpublished work) and in basilar-papillae tissue remaining after bundle isolation. To raise Ca2+ in stereocilia during tip-link regeneration, we included 1 μM ionomycin (Calbiochem), diluted from a 5 mM stock in dimethyl sulfoxide, in the culture medium. Papillae were incubated for 12 hr under conditions identical to those indicated above. At least two BAPTA-treated and two control papillae were treated for 12 hr with 5 μg/ml actinomycin D, 10 μg/ml tunicamycin, 2 mM phenyl-N-acetyl-α-d-galactosaminide, 20 μM nocodazole, or 10 μM colchicine (each obtained from Sigma). The effectiveness of these agents was assessed qualitatively.
For transmission electron microscopy, tectorial membranes were removed with subtilisin treatment as above. Papillae were fixed for 1 hr with 2% formaldehyde, 2% glutaraldehyde, and 1% tannic acid, postfixed with 1% osmium tetroxide for 30 min, and dehydrated with an acetone series. Fixation, postfixation, and dehydration were all carried out on ice. The tissue was transferred to propylene oxide and then embedded in Eponate 12 resin (Ted Pella, Redding, CA). Ultramicrotome sections (50–100 nm) were stained with 3% uranyl acetate and 0.3% lead citrate and observed in a Zeiss transmission electron microscope.
Electrophysiological Recording.
Single basilar-papilla hair cells of newborn chicks were dissociated with gentle mechanical disruption and aspiration (7). Hair bundles were mechanically stimulated with a fire-polished patch electrode using a two-dimensional piezoelectric bimorph stimulator. The composition of the pipette solution was 95 mM CsCl/3 mM MgCl2/2.5 mM Na2ATP/1 mM EGTA/5 mM Hepes (pH 7.3). The bath solution contained 155 mM NaCl, 6 mM KCl, 2 mM MgCl2, 4 mM CaCl2, 3 mM d-glucose, and 5 mM Hepes at pH 7.2–7.4, oxygenated at room temperature (21–23°C). Transduction currents were recorded at a holding potential of −80 mV with the tight-seal whole-cell technique (8). Mechanical stimuli had a duration of 150 ms and were elicited by alternating from negative to positive displacements. Displacement amplitudes for the cells displayed ranged from −1.2 μm to 1.2 μm in intervals of 0.12 μm; transduction currents were elicited from other cells with a single displacement amplitude of 400 nm. Transduction currents were low-pass filtered at 1 kHz, digitized at 8 kHz, and later digitally filtered at 0.5 kHz. Traces shown are averages of three records.
RESULTS
Tip Links Regenerate After BAPTA Breakage.
To examine whether tip links regenerate, we treated the chicken’s hearing organ, the basilar papilla, with 5 mM BAPTA, cultured papillae in the presence of Ca2+ for 0–24 hr, and used scanning electron microscopy to assess the number of tip links present. Cultured control basilar papillae showed only a slight decrease in the number of tip links over a 24-hr period. As previously reported (5), BAPTA application nearly completely eradicated tip links (Fig. 1). Complete, reproducible elimination of tip links required BAPTA treatment for 10–15 min, rather than the few seconds reported for frog sacculus (5), perhaps because of the presence of the temporal bone and tectorial membrane. Over a time period of several hours, tip links reappeared; by 24 hr, the number of tip links on BAPTA-treated basilar papillae, in 64 ± 3% of the expected positions, nearly equaled the number on control papillae, which had tip links in 72 ± 2% of the expected positions.
Figure 1.

Tip links regenerate after BAPTA treatment. Tip links are defined by their electron-microscopic appearance along a hair bundle’s axis of mirror symmetry, the 1,00 lattice plane of Tilney and coworkers (9). (A) Scanning electron microscopic view of individual hair bundles before or after treatment with BAPTA. Bundles from basilar papillae cultured for 24 hr after BAPTA treatment have tip links in equal numbers to bundles from control papillae. (Bar = 1 μm.) (B) Compilation of data from 162 bundles. BAPTA-treatment data were fit with the equation {% of intact tip links = A + B[1 − exp(−t/τ)]n}, where A is the fraction of intact tip links after BAPTA treatment (4%) and B is the increase in tip links during regeneration (60%). With the time constant τ as the only unconstrained variable, values for τ were as follows: for n = 1, τ = 6.6 hr (r = 0.98); for n = 2, τ = 3.9 hr (r = 0.997); and for n = 3, τ = 3.1 hr (r = 0.998). The fit for n = 2 is plotted here. Control data were plotted with an exponential fit with a time constant of about 185 hr.
Because a thick metal coating applied during sample preparation for scanning electron microscopy confounds our ability to assess tip-link diameter and length, we examined hair cells in the basilar papilla using transmission electron microscopy. As reported previously, tip links in control papillae appeared to be 5–10 nm in diameter and 125–175 nm long (Fig. 2). BAPTA treatment eliminated tip links; their absence was most certain when both upper and lower osmiophilic densities were observed in the same section (Fig. 2). Morphologically similar tip links were found frequently in papillae that had been cultured for 12 hr after BAPTA treatment (Fig. 2). In these papillae, links with similar diameter and length were also seen at positions below those normally occupied by tip links (Fig. 2 Lower Left). These scanning and transmission electron microscopy experiments show that hair cells regenerate their tip links after they have been broken by BAPTA treatment.
Figure 2.

Ultrastructure of control and regenerated tip links. Basilar papillae were treated with BAPTA or a control solution, and then fixed and processed for transmission electron microscopy immediately or after a culture period of 12 hr. Tips links in control papillae appeared as previously described (for examples, see refs. 4, 5, 10, and 11); they occasionally appeared to be branched and were often associated with amorphous material, possibly a glycocalyx. If present, osmiophilic insertional densities at the tip of a stereocilium and on the side of its neighbor clearly marked tip-link ends (densities are most clear in the hair cell that was BAPTA-treated, but not cultured). Dimensions and features of regenerated tip links fell into ranges seen in control papillae. In no case did we see osmiophilic densities at ends of regenerated tip links that were as evident as those seen in control papillae. (Bar = 100 nm.)
Abnormal Links Between Stereocilia Appear During Regeneration.
BAPTA treatment increased the number of linkages connecting stereociliary tips to adjacent stereocilia off the axis of mirror symmetry (Fig. 3 A–C). Although tip links are defined morphologically as projecting only along the axis of mirror symmetry within the bundle (4), a few such angled tip links are found in control hair bundles and may also gate transduction channels (12). The ratio of angled tip links to normal tip links increases at 2 h, then declines to a level 2- to 3-fold over control (Fig. 3C). Angled tip links also increase in frequency in control basilar papilla cultures. We saw a large increase in the number of linkages between stereocilia that cannot be termed tip links or angled tip links (Fig. 3 A and D). These include links running horizontally from one tip to a neighboring stereocilium or links between stereocilia at lower levels. The ratio of “other” links relative to normal tip links is also elevated substantially at 2 hr after BAPTA treatment (Fig. 3E).
Figure 3.
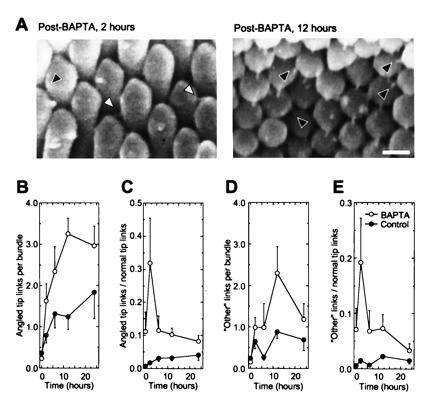
Angled tip links increase in frequency after tip-link regeneration. (A) Angled tip links (black arrowheads) and “other” links (white arrowheads). Angled tip links are linkages seen in scanning electron micrographs that have the same apparent diameter and length as normal tip links but connect a short stereocilium to a taller neighbor immediately adjacent to the closest stereocilium aligned along axis of mirror symmetry; because of hexagonal packing of stereocilia, these links are found at 60° to the axis of mirror symmetry (the 1,0 lattice plane of ref. 9). “Other” links also have similar apparent length and diameter but are found below normal tip link positions. “Other” links were relatively rare in normal bundles. (Bar = 250 nm.) (B–E) Absolute and relative numbers of angled tip links and “other” links during basilar-papilla culture. Two hours after BAPTA treatment, the relative numbers of angled tip links and “other” links increases sharply. Each observer counted angled tip links on the same 162 bundles as in Fig. 1B. For each bundle, the four observers’ estimates were averaged; at each time point, means ± standard errors derived from 15–20 bundles are reported.
New Protein Synthesis Is Not Required for Regeneration.
Sensitivity to neuraminidase (13) and susceptibility to proteolysis (14) suggests that tip links are composed of glycoproteins. To investigate whether new protein synthesis was required for tip-link regeneration, we incubated BAPTA-treated basilar papillae with 40 μM cycloheximide, a protein-translation inhibitor. Reappearance of tip links was not blocked, indicating that translated tip-link subunits are available for reconnection (Fig. 4). We confirmed that cycloheximide blocked protein synthesis in the basilar papilla; cycloheximide decreased incorporation of [35S]methionine into protein in whole basilar papilla or isolated hair bundles by nearly 50-fold (data not shown). Tip-link regeneration was not blocked by actinomycin D, a transcription inhibitor; tunicamycin and phenyl-N-acetyl-α-d-galactosaminide, inhibitors of N- and O-linked sugar processing; and nocodazole and colchicine, agents that disrupt microtubules (data not shown). These experiments suggest that tip links can be assembled with preexisting subunits and with minimal processing or transport.
Figure 4.
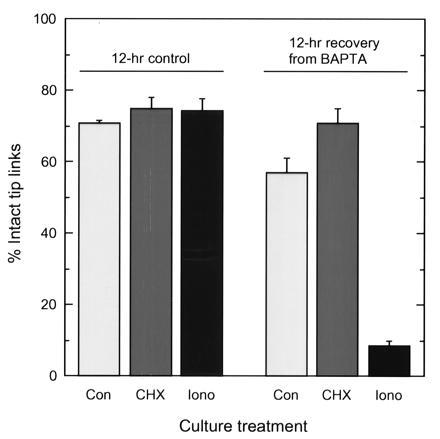
Effects of cycloheximide and ionomycin on tip-link regeneration. Basilar papillae were treated with BAPTA or a control solution and then incubated for 12 hr with no treatment (Con), 40 μM cycloheximide (CHX), or 1 μM ionomycin (Iono). For each experimental condition, means ± SEs derived from 14–22 bundles acquired from 6–12 papillae are reported.
Intracellular Calcium Ions Block Tip-Link Regeneration.
When tip links are broken, transduction channels close and the concentration of Ca2+ in stereocilia should fall (15, 16). Because regeneration of tip links may be stimulated by this decrease in Ca2+ concentration, we examined whether maintaining an elevated intracellular Ca2+ concentration blocks regeneration. We broke tip links with BAPTA and then cultured basilar papillae for 12 hr in the presence of 1 μM ionomycin, a Ca2+ ionophore (17). Ionomycin effectively blocked tip-link regeneration (Fig. 4). Ionomycin at this concentration had no effects on hair-cell survival or morphology, suggesting that the concentration of Ca2+ did not rise to an extreme level. Consistent with that conclusion, similar conditions raise intracellular Ca2+ to only 1–2 μM in cochlear hair cells (18).
Because closing of transduction channels should also hyperpolarize hair cells, we also tested whether depolarization blocks regeneration. Extracellular K+ concentrations of 18 or 50 mM, which should depolarize hair cells to about −55 mV or −25 mV, had no effect on tip-link regeneration.
Mechanoelectrical Transduction Returns During Tip-Link Regeneration.
If tip links are gating springs, regeneration of tip links should restore mechanoelectrical transduction (5). We therefore isolated hair cells from BAPTA-treated or control basilar papillae before or after 24 hr in culture and examined transduction using tight-seal, whole-cell recording. As previously reported (5, 19, 20), treatment with calcium chelators entirely abolished transduction (Fig. 5B). After a 24-hr recovery period in culture, however, transduction currents returned (Fig. 5D and Table 1). Although transduction-current amplitudes were similar in BAPTA-treated and control papillae cultured for 24 hr, transduction in hair cells from BAPTA-treated papillae often could only be elicited for a few large displacements before disappearing. In addition, adaptation was slowed after tip-link regeneration; the maximal adaptation rate for negative displacements was reduced by 2-fold and the rate constant for adaptation to positive stimuli was reduced by more than 5-fold. Slopes of displacement–response curves, characterized by the gating force Z (15) were also diminished in hair cells with regenerated tip links (Fig. 5E and Table 1).
Figure 5.

Mechanoelectrical transduction returns after BAPTA treatment. (A) Control hair cell. (Inset) Disruption of mechanoelectrical transduction (single displacements) by bath application of 5 mM BAPTA on an isolated hair cell. (B) Hair cell isolated 1 hr after 15-min treatment of the basilar papilla with 5 mM BAPTA. Transduction currents are absent. (C) Hair cell isolated from control basilar papilla cultured for 24 hr. Although transduction-current amplitudes and adaptation rates were unaffected by 24 hr culture, the extent of adaptation was diminished. (D) Hair cell isolated from BAPTA-treated basilar papilla, cultured for 24 hr. Transduction-current amplitudes resembled those from control cells, but rate constants for adaptation to positive displacements were diminished by more than 5-fold. (E) Instantaneous displacement-response relationships (for different cells than those in A–D).
Table 1.
Transduction and adaptation after BAPTA treatment
| I, transduction current* | S, slipping rate constant† | C, climbing rate‡ | Extent of adaptation§ | Z, gating force¶ | |
|---|---|---|---|---|---|
| Control | 45 ± 22 pA | 136 ± 50 s−1 | 13 ± 4 μm·s−1 | 0.73 ± 0.08 | 33 ± 4 fN |
| (1 hr) | (n=8) | (n = 5) | (n = 5) | (n = 5) | (n = 5) |
| BAPTA | 0 pA (n = 7) | — | — | — | — |
| (1 hr) | |||||
| Control | 38 ± 10 pA | 117 ± 12 s−1 | 14 ± 2 μm·s−1 | 0.45 ± 0.02 | 37 ± 9 fN |
| (24 hr) | (n = 8) | (n = 5) | (n = 5) | (n = 4) | (n = 5) |
| BAPTA | 30 ± 17 pA | 22 ± 3 s−1 | 7 ± 2 μm·s−1 | 0.41 ± 0.13 | 16 ± 1 fN |
| (24 hr) | (n = 9) | (n = 5) | (n = 5) | (n = 5) | (n = 6) |
In response to subsaturating, 400-nm displacement.
Adaptation rate constant for positive displacements; this is the maximum slope of displacement–adaptation rate curves (21). These rate constants have not been adjusted for the geometrical gain of the bundle, γ. Because stimulus amplitude affects adaptation rate, rate constants are reported (21).
Maximum adaptation rate for negative displacements >500 nm; because saturating negative displacements remove any external force applied to the adaptation motor, motors climb at a constant rate (21). These rates have not been adjusted for the geometrical gain of the bundle, γ.
Determined from displacement–extent curves (22); extent is distance of movement of the adaptation motor in response to a 150-msec stimulus, expressed as a fraction of stimulus amplitude.
Determined from two-state Boltzmann fit to displacement–open probability curve (15); the gating force (Z) indicates the displacement sensitivity of the trandsuction apparatus.
DISCUSSION
The reappearance of tip links coincident with the return of mechanoelectrical transduction provides independent support for the hypothesis that tip links directly gate transduction channels (4, 5). No reports have appeared previously on regeneration of tip links or return of transduction following Ca2+ chelator treatment, probably because a pronounced lag precedes tip-link reappearance and presumably recovery of transduction. That tip links regenerate perhaps is not surprising, however, as it seems improbable that these fragile structures could be formed once and then last for a lifetime.
Mechanism of Tip-Link Regeneration.
How are tip links regenerated? One possibility that seems unlikely is that BAPTA disconnects tip links only at one end, and the tip link’s free end rebinds to its receptor after the restoration of millimolar Ca2+ concentrations. Because a tip link is stretched ≈20 nm at rest (15) and the upper anchor of a tip link climbs an additional ≈75 nm after BAPTA treatment (23), the tip link’s free end would be too far from its receptor to reattach to its original anchor point. The increased appearance of angled tip links and other linkages below the stereociliary tips suggests instead that new tip links are strung between stereocilia. Because adjacent stereocilia are within ≈50 nm near stereociliary tips (Fig. 2; ref. 9), tip links may be formed where the stereocilia come closest together (Fig. 6). Although the structure of the tip link is unknown, several possible mechanisms of regeneration can be envisioned.
Figure 6.

Proposed model for tip-link regeneration. (A) After rupture of tip links, the concentration of Ca2+ drops in stereocilia, and tip-link regeneration is favored. In this diagram, two channels, one with an attached tip-link subunit, have been brought into the putative “assembly zone” by molecular motors. These motors have moved from the base of each stereocilium toward its tip, with the direction of movement indicated by arrows. The motor isozyme responsible for tip-link regeneration could be the same as that making up the mature adaptation-motor assembly, or it could be a distinct isozyme. If the two motors bring into close proximity a tip link and its receptor, such as a transduction channel, the tip link can bind to its receptor. The motors then move the transduction apparatus to its final resting position. The depiction of the tip link as being initially bound to the shorter stereocilium is arbitrary; in addition, although channels are shown at both tip-link ends, they may be randomly distributed between upper and lower ends (12). (B) After tip links have been regenerated, functional transduction channels maintain a modestly elevated Ca2+ concentration close to stereociliary tips (16). In our model, this elevated Ca2+ concentration prevents assembly of additional tip links. Over a time period that is presumably longer than 24 hr, the adaptation motor matures to give fast kinetics.
Tip links could be formed by Ca2+-dependent interaction of two extended subunits, each a transmembrane protein (12); alternatively, a tip link could be formed when an extracellular protein binds in a Ca2+-dependent manner to transmembrane receptors. In either model, if the appearance of a subunit present on each stereocilium where tip links can be reassembled follows an exponential relationship, and if the subunits interact rapidly, the connection of an intact tip link may be fit by a square of an exponential. Indeed, our data were fit well by eye by such a relationship (Fig. 1B). Considerably more data points, impractical with the laborious electron microscopy assay we used, will be required to distinguish this model from others that fit the data equally well.
Molecular motors, such as myosin isozymes (24, 25), could be used to carry out tip-link regeneration and assembly (26). Once a tip link is strung between two stereocilia, motors could attach to one or both ends and drag the nascent tip link up the stereocilium until it can move no further, either because a motor runs off its track at the end of the stereocilium or because increased tension in the tip link stalls the motor (Fig. 6).
Because elevation of intracellular Ca2+ blocks tip-link regeneration, the decline in free Ca2+ following tip-link loss and transduction-channel closing is a plausible signal to stimulate regeneration. But what keeps supernumerary tip links from forming when functional transduction channels are present? Open transduction channels permit a “tip blush” of elevated Ca2+ that extends down the stereocilium as far as several micrometers from the tip (16), and Ca2+ increases the climbing rate of the hair cell’s adaptation motor (21). If adaptation motors transport the transduction apparatus’ tip-link subunits, perhaps a tip-link subunit normally cannot connect to its receptor on the adjacent stereocilium, because Ca2+ entering through open channels increases the rate of motor movement near stereociliary tips. In the presence of functional channels, motors would move rapidly through the region of the hair bundle where the stereocilia are close enough together to permit tip-link attachment. When channels are instead closed, such as following BAPTA treatment, motors would linger in this assembly zone and tip links could attach. Alternatively, increased Ca2+ spreading down a stereocilium might arrest motors before they reach the “attachment zone,” reminiscent of the effects of Ca2+ on myosin-Iβ motility (27).
Important questions remain unanswered. For example, are tip links preassembled between stereociliary pairs or does regeneration require assembly? Interstereociliary linkages reside just below stereociliary tips; although at least some of these linkages resist calcium-chelator treatment (5, 10), nascent tip links may be found among them. In that case, extraordinarily large mechanical displacements might stretch these tip links and open transduction channels. In addition, how does the hair bundle recognize tip links formed off the primary axis and then remove them? In our assay, the dissected basilar papilla was removed from any normal mechanical cues, suggesting that individual hair cells have an intrinsic ability to recognize off-axis tip links. This mechanism may partially rely on mechanical cues, however, as the number of off-axis tip links in control basilar papillae grows during the culture period (Fig. 3 B and C). Finally, does the mechanism of tip-link regeneration differ from the mechanism of initial insertion during bundle development (11)? With the development of the tip-link regeneration assay, we can now investigate the mechanism of assembly of the transduction apparatus and address these and other questions.
Tip-Link Regeneration and Recovery from Temporary Threshold Shift.
Regeneration of tip links may underlie recovery from temporary threshold shift (TTS), where hearing acuity recovers from a loss of 40 dB or more (>100-fold) after exposure to loud sound (28). The cellular locus of damage during prolonged noise stimulation of amplitudes that elicit TTS is unknown; electron-microscopic investigations using methods that did not preserve tip links indicated that such noise has nearly undetectable effects on stereociliary structure (29). Although tip links apparently resist large static and dynamic stimuli (22), TTS-inducing stimuli nevertheless can break tip links (6, 30). Consistent with those observations, similar stimuli inactivate transduction channels (31) and decrease bundle stiffness (32), expected consequences of tip-link destruction. If TTS-inducing stimuli indeed break tip links, regeneration must occur in order for normal hearing to return. The exponential time course of recovery from TTS in humans, with a time constant of ≈7 hr (33), matches remarkably well the time course of tip-link regeneration seen in the chicken’s basilar papilla. Although our data are better fit by eye with a square or cubic exponential relationship (Fig. 1), a fit with a single exponential gives a time constant of 6.6 hr. Although proof will require careful examination of tip links before, during, and after TTS, we speculate that the recovery of normal hearing after sound exposure that induces TTS is ultimately limited by tip-link regeneration.
Acknowledgments
We are grateful to Paul Fuchs for teaching us hair-cell isolation from the chicken’s basilar papilla, as well as for critically reading an early version of the manuscript. We also appreciate constructive comments on the manuscript that were provided by Susan K. H. Gillespie, Fernan Jaramillo, and by members of the Gillespie laboratory. This work was supported by the National Institutes of Health and the Deafness Research Foundation. P.G.G. is a Pew Scholar in the Biomedical Sciences.
Footnotes
Abbreviations: BAPTA, 1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; TTS, temporary threshold shift.
References
- 1.Hudspeth A J. Nature (London) 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 2.Pickles J O, Corey D P. Trends Neurosci. 1992;15:254–259. doi: 10.1016/0166-2236(92)90066-h. [DOI] [PubMed] [Google Scholar]
- 3.Corey D P, Hudspeth A J. J Neurosci. 1983;3:962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickles J O, Comis S D, Osborne M P. Hear Res. 1984;15:103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- 5.Assad J A, Shepherd G M G, Corey D P. Neuron. 1991;7:985–994. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- 6.Pickles J O, Osborne M P, Comis S D. Hear Res. 1987;25:173–187. doi: 10.1016/0378-5955(87)90089-x. [DOI] [PubMed] [Google Scholar]
- 7.McNiven A I, Yuhas W A, Fuchs P A. Aud Neurosci. 1996;2:63–77. [Google Scholar]
- 8.Yamoah E N, Gillespie P G. Neuron. 1996;17:523–533. doi: 10.1016/s0896-6273(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 9.Tilney L G, Tilney M S, Cotanche D A. Hear Res. 1988;37:71–82. doi: 10.1016/0378-5955(88)90079-2. [DOI] [PubMed] [Google Scholar]
- 10.Neugebauer D-C, Thurm U. Cell Tissue Res. 1987;249:199–207. [Google Scholar]
- 11.Pickles J O, von Perger M, Rouse G W, Brix J. Hear Res. 1991;54:153–163. doi: 10.1016/0378-5955(91)90116-q. [DOI] [PubMed] [Google Scholar]
- 12.Denk W, Holt J R, Shepherd G M G, Corey D P. Neuron. 1995;15:1311–1331. doi: 10.1016/0896-6273(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 13.van Benthem P P G, de Groot J C M J, Albers F W J, Veldman J E, Huizing E H. Eur Arch Otorhinolaryngol. 1993;250:73–77. doi: 10.1007/BF00179301. [DOI] [PubMed] [Google Scholar]
- 14.Takumida M, Harada Y, Kanemi Y. ORL. 1993;55:77–83. doi: 10.1159/000276384. [DOI] [PubMed] [Google Scholar]
- 15.Hudspeth A J. In: Sensory Transduction. Corey D P, Roper S, editors. New York: Rockefeller Univ. Press; 1992. pp. 357–370. [Google Scholar]
- 16.Lumpkin E, Hudspeth A J. Proc Natl Acad Sci USA. 1995;92:10297–10301. doi: 10.1073/pnas.92.22.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C-m, Hermann T E. J Biol Chem. 1978;253:5892–5894. [PubMed] [Google Scholar]
- 18.Dulon D, Zajic G, Schacht J. J Neurosci. 1990;10:1388–1397. doi: 10.1523/JNEUROSCI.10-04-01388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sand O. J Comp Physiol A. 1975;102:27–42. [Google Scholar]
- 20.Crawford A C, Evans M G, Fettiplace R. J Physiol (London) 1991;434:369–398. doi: 10.1113/jphysiol.1991.sp018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assad J A, Corey D P. J Neurosci. 1992;12:3391–3309. doi: 10.1523/JNEUROSCI.12-09-03291.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepherd G M G, Corey D P. J Neurosci. 1994;14:6217–6229. doi: 10.1523/JNEUROSCI.14-10-06217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corey D P, Assad J A. In: Sensory Transduction. Corey D P, Roper S, editors. New York: Rockefeller Univ. Press; 1992. pp. 336–342. [Google Scholar]
- 24.Hudspeth A J, Gillespie P G. Neuron. 1994;12:1–9. doi: 10.1016/0896-6273(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie P G. Curr Opin Neurobiol. 1995;5:449–455. doi: 10.1016/0959-4388(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 26.Howard J, Hudspeth A J. In: Sensory Transduction. Hudspeth A J, MacLeish P R, Margolis F L, Wiesel T N, editors. Geneva: Fondation pour l’Etude du Système Nerveux Central et Périphérique; 1987. pp. 138–145. [Google Scholar]
- 27.Zhu T, Sata M, Ikebe M. Biochemistry. 1996;35:513–522. doi: 10.1021/bi952053c. [DOI] [PubMed] [Google Scholar]
- 28.Gulick W L, Gescheider G A, Frisina R D. Hearing: Physiological Acoustics, Neural Coding, and Psychoacoustics. New York: Oxford Univ. Press; 1989. [Google Scholar]
- 29.Liberman M C, Dodds L W. Hear Res. 1987;26:45–64. doi: 10.1016/0378-5955(87)90035-9. [DOI] [PubMed] [Google Scholar]
- 30.Takumida M, Fredelius L, Bagger-Sjöbäck D, Harada Y, Wersäll J. J Laryngol Otol. 1989;103:1125–1129. doi: 10.1017/s002221510011117x. [DOI] [PubMed] [Google Scholar]
- 31.Patuzzi R B, Yates G K, Johnstone B M. Hear Res. 1989;39:189–202. doi: 10.1016/0378-5955(89)90090-7. [DOI] [PubMed] [Google Scholar]
- 32.Saunders J C, Canlon B, Flock A. Hear Res. 1986;23:245–255. doi: 10.1016/0378-5955(86)90113-9. [DOI] [PubMed] [Google Scholar]
- 33.Mills J H, Gilbert R M, Adkins W Y. J Acoust Soc Am. 1979;65:1238–1248. doi: 10.1121/1.382791. [DOI] [PubMed] [Google Scholar]


