1. Introduction
Individuals with Mild Cognitive Impairment (MCI) include a heterogeneous group of subjects with cognitive disturbances, many of whom are at significantly increased risk for Alzheimer ’s Disease (AD). The clinical definition of MCI remains somewhat broad, although amnestic MCI presents with prominent memory impairment and is a significant risk factor for progression to AD (Petersen et al., 2001; Modrego et al., 2005). Patients often present to memory disorder clinics with memory complaints but may not yet meet specific neuropsychological criteria for amnestic MCI. The risk for progression to further cognitive impairment for this mild memory impaired group is unknown. Markers such as APOE 4 have been used to assess increased risk for conversion to AD but are not in general clinical practice at present. Volumetric MRI measures of medial temporal lobe structures have been studied and found to be very useful in predicting conversion from MCI to AD. Volumetric MRI studies have found more pronounced atrophy in the right vs. the left hippocampus among subjects at genetic risk for AD (Soininen et al., 1995; Lehtovirta et al., 2000; Tapiola, et al., 2006) and in age-associated memory impairment, a possible precursor to MCI (Soininen et al., 1994; Mega et al., 2002). These methods are labor intensive, however, and not suitable as a screening measure in the clinic. Magnetic Resonance Spectroscopy (MRS), which can directly evaluate specific metabolites in vivo, has identified localized decreases in N-acetylaspartate (NAA), a marker of neuronal integrity, and increases in myo-inositol (mI), a possible marker for glia but also a precursor for inositol lipid synthesis and a constituent of membrane lipids, among AD subjects (Huang et al., 2001; Valenzuela and Sachdev, 2001; Block et al., 2002; Chantal et al., 2002; Dixon et al., 2002; Firbank et al., 2002; Schuff et al., 2002) across different brain regions (Soininen et al., 1995; Lehtovirta et al., 2000; Tapiola et al., 2008). Few studies have evaluated the hippocampal regions of subjects with MCI and none have examined even earlier stages of cognitive impairment in the elderly where lateralized metabolic changes could be indicative of early neuronal damage. Bilateral hippocampal regions have not been systematically studied with 3T MRS in patients with mild cognitive disturbances presenting to a memory disorders clinic, a population that is less impaired than subjects with MCI, and may precede MCI. It would be crucial to identify subjects at the earliest stages of cognitive impairment in view of emerging pharmacotherapeutic approaches to delay disease onset or progression. We have used the term mild memory impairment (MMI) in this study since our subjects do not meet full criteria for amnestic MCI but do have specific memory complaints and manifest declines in memory related domains.
We hypothesize that there will be lateralized changes in NAA/Cr ratios in the hippocampal regions of subjects with MMI as compared to AD. In this preliminary investigation, NAA, mI and Cho to creatine ratios were determined using single voxel (MRS) at 3T in bilateral hippocampi and posterior cingulate gyrus in 31 subjects with MMI, probable AD, (PRAD), and elderly control subjects to assess it’s utility in identifying subjects with memory impairment who are at increased risk for conversion to AD.
2. Methods
2.1 Subjects
The study included 31 subjects, including 6 PRAD (3 males, 3 females), 8 MMI and (4 males, 4 females) and 17 controls (5 males, 12 females) Demographic information is presented in Table 1. There were no significant differences between the three groups in age or education. Subjects with either MMI or early PRAD were identified and enrolled through the Center for Comprehensive Care and Research in Memory Disorders (CCCRMD) at the University of Chicago. The healthy elderly controls were recruited from the Northwestern Alzheimer’s Disease Center (NADC). Written Institutional Review Board informed consent was obtained from all participants.
Table 1.
Demographics
| Controls | MMI | PRAD | |
|---|---|---|---|
| Number | 17 | 8 | 6 |
| Age | 70.6 ± 6 | 76.1 ± 8 | 73.8 ± 10.3 |
| Education | 12.9 ± 0.45 | 12.9 ± 0.35 | 12.7 ± 0.52 |
| MMSE | 29.2 ± 1.3 | 28.5 ± 1.7 | 22.8 ± 2.8* |
Means and Standard deviations
MMSE = Mini-Mental State Examination score.
P <0.01 Compared to controls and MMI
All subjects were right-handed. Additional inclusion criteria included: age greater than 59 and performance on the Mini-Mental Status Examination (MMSE) not less than 22. All six PRAD subjects met criteria defined by the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ARDA) Work Group (McKhann et al., 1984). Eight subjects met clinical criteria for mild memory impairment (MMI). The criteria used for this study included: memory complaint as primary presenting problem which was corroborated by family member; no functional impairment by self report and corroboration of functional independence by family member: neuropsychological testing showed evidence only of memory impairment that was less than 1.5 SD on the Hopkins Verbal Learning Test-Revised (HVLT-R) from the age appropriate mean.
All subjects received a complete physical and neurological exam with appropriate laboratory tests, including MRI. Consensus diagnosis of PRAD, MMI, or control status was made by the treatment team (geriatrician, neurologist, neuropsychologist, psychiatrist, nurse and social worker) at either the CCCRMD or the NADC. The standard battery of neuropsychological tests used for the MMI, PRAD and Control group included: MMSE, Boston Naming Test, The Hopkins Verbal Learning Test-R (HVLT-R), the Clock Drawing Test, Trail Making Test A and B, WRATT III, Brief Visual Memory Test--Revised (BVMT-R), Verbal Fluency, the Geriatric Depression Scale, the Wisconsin Card Sorting Test and Grooved Peg Board. The HVLT-R score was less than 1.5 SD below the age appropriate mean for all 8 MMI subjects, which does not meet criteria for MCI, amnestic type as defined by Petersen et al., 2001. Therefore the 8 subjects with MMI have very mild cognitive impairment, confined to memory, with no evidence of any other cognitive difficulties and no functional impairment. Exclusion criteria included other neurological disorders especially cerebrovascular disorder as determined by history or evidence on MRI, history of head trauma, cranial surgery, substance abuse, current psychiatric disorder, diabetes mellitus, claustrophobia and current treatment with acetylcholinesterase inhibitors. We excluded subjects with unstable medical illness, although stable medical illnesses (e.g. well-controlled hypertension, coronary artery disease, arthritis, GERD) were allowed.
Six of the 8 MMI subjects have been followed clinically in the CCCRMD. Two were lost to follow up. Five of the six subjects have converted to PRAD in three years, post evaluation.
2.2 Proton MRI and spectroscopy
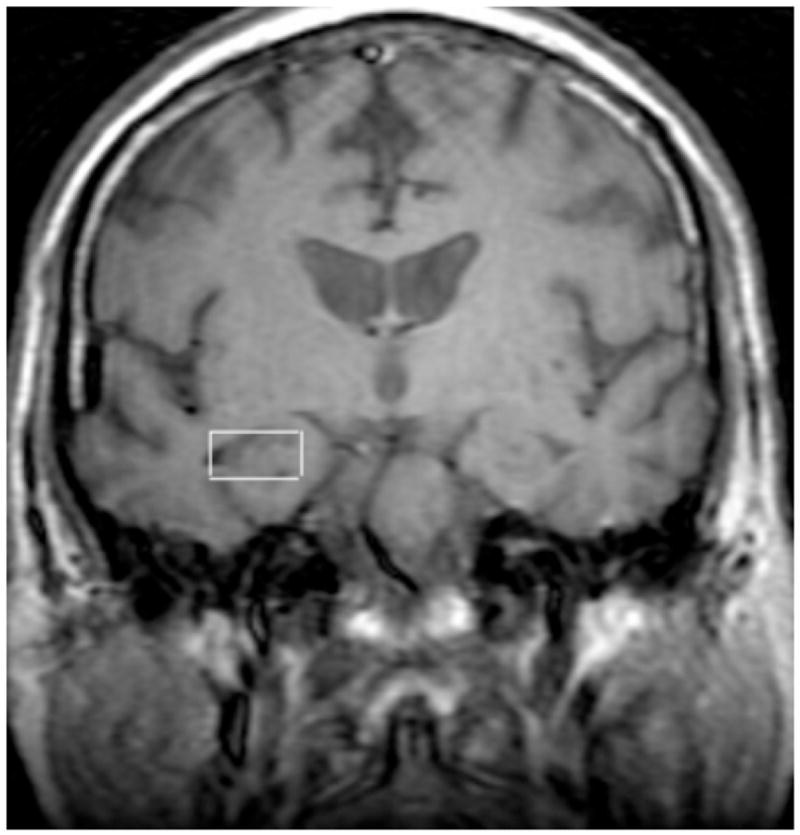
All 1H-MRS scans were performed at the Brain Research Imaging Center of the University of Chicago. The scanning technologist was blind to diagnosis. Voxels were placed in posterior cingulate gyrus and right and left hippocampi by the principal investigator (PI), who was blind to the diagnosis at the time of the scan. The PI reviewed all data from each subject and approved all subjects for the study prior to scanning, but on the day of the scan was blinded as to which subject was being studied, until after the completion of the scan, to minimize any bias in voxel placement. A 3.T whole body MR system (GE LX Signa Horizon VH/I, Milwaukee, WI, USA; quadrature transmit-receive head coil) was used to acquire images and single-voxel spectra. After a brief scout scan, a spin-echo T1-weighted coronal scan was performed consisting of 28 slices which were each 0.5mm mm thick with TR/TE = 700 ms/14 ms and a matrix of 256×128. A 2 cm × 2 cm × 2 cm voxel was placed in the cingulate gyrus (CG). The center of the hippocampal voxel was placed to bisect the hippocampal anterior-posterior axis, using the T1-coronal images as a guide. The same slice was used for both R and L hippocampal voxels, at the level of the interpeduncular cistern.
To resolve all metabolite peaks of interest, including mI, a single-voxel point-resolved spin echo (PRESS) sequence with TR/TE = 2000 ms/30 ms was chosen. Ratios of metabolite to Cr concentration were automatically calculated and reported by the software provided by the manufacturer(GE). The sequence utilized the scanner’s auto-shim ability and water suppression of 96% or higher. Line width was monitored to assure spectroscopic quality and was on average between 8 and 11 ppm. 128 acquisitions were taken for the CG voxel and 256 for H voxels. Scan times were approximately five minutes for the CG and nine minutes for each H region. Total scan time for each subject was approximately 45 minutes, which was well tolerated.
2.3 Statistical Analyses
Primary dependent measures for analyses included the specific MRS metabolite ratios (NAA/Cr, mI/Cr, and Cho/Cr) derived for the three separate regions of interest, including RH, LH and the posterior CG. Differences between the PRAD, MMI and elderly control groups were assessed with repeated measures analysis of variance with brain area (RH, LH and CG) as the within-subject factor. Each metabolite ratio (NAA/Cr, mI/Cr and Cho/Cr) was evaluated in a separate analysis. Statistical tests were executed with SPSS (Chicago, IL).
3. Results
Initially, the mental status scores were examined. The mental status scores demonstrated the anticipated differences corresponding to clinical status. MMSE for the PRAD group (22.83±2.8 differed significantly from both MMI (28.5±1.7; t(12)=4.74; P <0.001) and Controls (29.2±1.1); t(21)=7.95; P <0.001). This was consistent with findings for the HVLT-R from the neuropsychological assessment, which were available for MMI and PRAD groups. The PRAD group was significantly more impaired (−3.69±1.4) than the MMI group (−1.25±1.00) (t(12)=3.79; P=0.003). The mean HVLT-R score for the PRAD group was 3.7 SD below the age-appropriate mean. The average HVLT-R score for the MMI group was approximately1.2 SD below the age-appropriate mean, which is consistent with sub-clinical impairment and does not meet criteria for MCI.
The metabolite ratios for NAA, mI, and Cho were submitted to separate repeated measures analysis of variance, with brain area (CG, LH and RH) as the within-subject factor. A significant main effect for group was identified for NAA/Cr (F(2,15)= 15.6, p<0.001). Of the three studied brain areas, only reduced NAA/Cr in RH distinguished the MMI group from elderly controls (t (22)=−3.80; P=0.001). As shown in Table 2, mean values for NAA/Cr were generally reduced in the PRAD group across all studied brain areas (CG:t(20)=2.18; P=0.04. RH:t(19)=2.53; P=0.02 & LH:t(8)=2.45; P=0.044). Significant differences between the groups were not indicated in this sample for either Cho/Cr or for mI/Cr. See Table 2. and Figures 1A and B.
Table 2.
Metabolite ratios for MMI, PRAD and Controls
| NAA/Cr | mI/Cr | Cho/Cr | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CG | RH | LH | CG | RH | LH | CG | RH | LH | |
| CONT(N=17) | 1.43 (0.08) | 1.45 (0.22) | 1.46 (0.14) | 0.52 (0.06) | 0.84 (0.19) | 0.75 (0.16) | 0.61(0.10) | 1.0 (0.14) | 0.90 (0.14) |
|
| |||||||||
| MMI (N=8) | 1.40 (0.13) | 1.14 (0.12)** | 1.33 (0.13) | 0.50 (0.05) | 0.74 (0.14) | 0.81 (0.11) | 0.63 (0.06) | 0.91 (0.09) | 0.98 (0.13) |
|
| |||||||||
| PRAD(N=6) | 1.34 (0.10) | 1.18 (0.17)* | 1.21(0.17)* | 0.55 (0.08) | 0.80 (0.14) | 0.70 (0.05) | 0.62 (0.08) | 0.97 (0.12) | 0.92 (0.13) |
Means and Standard Deviations (SD)
CG= Cingulate Gyrus; RH = Right Hippocampus; LH= Left Hippocampus
NAA= N-acetyl aspartate; mI=myo-inositol; Cho= choline
P< 0.05
P= 0.001
Figure 1.
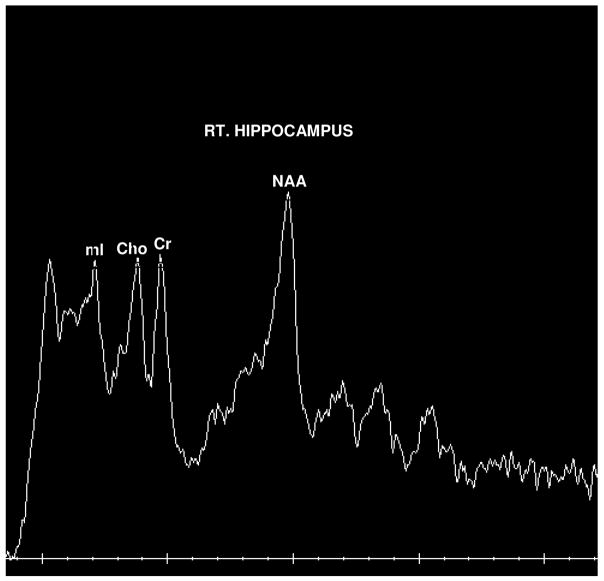
Figure 1A. Representative NMRS scan of a subject with Right Hippocampal voxel placement.
Figure 1B. Example of 1H-NMRS data obtained in a subject with MMI. NAA, N-acetyl aspartate; mI, myoinositol; Cr, creatine; Cho, choline.
4. Discussion
To our knowledge, this is the first study using MRS at 3T of bilateral hippocampal regions in subjects with mild memory impairment(MMI), not meeting criteria for amnestic MCI. Hippocampal NAA/Cr reductions in the MMI subjects in this study averaged approximately 15%; across right (21%) and left (9%) hemispheres. Bilateral hippocampal NAA/Cr reductions of approximately 18% were found in the PRAD subjects relative to controls. These findings are consistent with other MRS studies indicating average NAA/Cr ratios decreases in AD across studies of approximately 15% in the temporal lobes (Firbank et al., 2002). Of the three studied brain areas, NAA/Cr reductions were identified in the RH in subjects with MMI. While LH NAA/Cr measurements in the MMI subjects also trended lower than those of elderly controls, the difference was more pronounced for the RH. NAA/Cr were generally lower in bilateral hippocampal regions in subjects with PRAD. Of the three groups, the pattern of significantly lower RH than LH NAA/Cr was indicated only for the MMI subjects (paired t (6)=−4.2, p=0.006). The more prominent reduction in the RH suggests the possibility of early changes in this brain area.
Studies comparing lateralized hippocampal NAA/Cr levels in AD subjects have obtained inconsistent findings including reduced NAA for LH vs. RH (Dixon et al., 2002), for the RH vs. LH (Block et al., 2002) and no significant bilateral difference (Schuff et al., 2002; Chao et al., 2005). All have been done at 1.5T. None included subjects at very early stages of cognitive impairment. Our results are consistent with several volumetric MRI studies that have found more pronounced atrophy in the right vs. the left hippocampus among subjects at genetic risk for AD (Soininen et al., 1995; Lehtovirta et al., 2000; Tapiola et al., 2008), and in age-associated memory impairment, a possible precursor to MCI (Soininen et al., 1994; Mega et al., 2002). Volumetric measures of the entorhinal cortex (EC), particularly of the right EC, have been identified as predictive of conversion from MCI to AD (deToledo-Morrell et al., 2004). Previous studies have shown that medial temporal lobe atrophy on the right side predicted the conversion of MCI to dementia, corroborating our findings (Tapiola et al., 2008). Also, an fMRI study using a memory face-name recognition paradigm demonstrated greater hippocampal activation, especially right hippocampal and right frontal activation, in low performing normal older adults (Miller et al., 2008). The authors suggest that hyperactivation in the hippocampi and prefrontal cortices may serve as a compensatory mechanism in preserving memory performance in individuals who may already have early AD pathology.
We found no significant differences for other metabolite ratios examined. These negative findings are consistent with MRS studies examining mI/Cr in the hippocampi (Dixon et al., 2002; Ackl et al., 2005) and in the medial temporal lobe (Chantal et al., 2002). Ackl and colleagues (2005) have shown that MCI subjects have decreased NAA/Cr ratios in hippocampal regions but do not show mI/Cr changes in the hippocampus or parietal areas at this stage. mI/Cr ratio increases were seen only in subjects with AD. The authors conclude that the specificity of NAA/Cr decreases in the hippocampus correlates well with the neuropathological staging of AD (Braak and Braak, 1991). Findings for Cho have been inconsistent across studies (Valenzuela and Sachdev, 2001). It may be that larger samples are needed to detect subtle changes in these MRS measurements.
The MMI subjects in this study had MMSE exam scores that were not significantly different from the control group and whose HVLT-R scores did not meet established criteria for MCI, indicating that our subjects are at a very early stage of cognitive impairment. Three of our 8 MMI subjects were still employed at the time of the scan and all were functionally independent. Six of the 8 MMI subjects have been followed yearly at our center (one was lost to follow up and one moved away). Five have converted to PRAD (3 years post scan), further substantiating our finding that declines in R H NAA/Cr ratios measured at 3T in subjects with isolated memory complaints may be predictive of future cognitive decline.
This is a very small group of subjects which were studied cross-sectionally and the results need to be interpreted with caution. However, the subjects were evenly matched for sex in each of the memory impaired groups, as well as for education (all had 12 plus years). Their ApoE 4 status is not known and would be very informative. It is possible that the majority of our MMI group carried an ApoE4 allele. However, it has been shown that ApoE 4 status alone is not predictive of conversion to AD. (Tapiola et al., 2008). Larger, longitudinal studies in which subjects with memory complaints and neuropsychological data supporting mild, isolated memory changes, are followed across time will be necessary to substantiate the significance of RH NAA/Cr decreases measured at 3T as a predictor of conversion to dementia, with or without ApoE4. Higher field magnets are beginning to replace 1.5T clinical MRI scanners in many academic medical centers. The advantages of using the higher field strength for MRS include improved signal to noise ratio (SNR) and increased spectral resolution which are double relative to 1.5T. Also, functional imaging methods, such as fMRI, are usually performed at the higher field strengths and can be studied simultaneously with MRS to provide additional quantitative measures of susceptible brain areas for more in-depth analysis of neuronal mechanisms underlying disease processes
Our preliminary results suggest that RH NAA/Cr ratios measured at 3T may be a sensitive marker of future progression to dementia in a clinically defined elderly population with isolated, mild memory complaints.
Acknowledgments
This work was supported part by a K07 award (NIMH 10156) and the Dept of Psychiatry at the University of Chicago. We would like to gratefully acknowledge Robert Lyons, Yiping Du, PhD, and David Levin, MD, PhD, from the Brain Research Imaging Center and Amy Levin, RN from the Center for the Comprehensive Care and Research on Memory Disorders at the University of Chicago, the Northwestern Alzheimer’s Disease Center (NADC) (AG13854) and the NADC Clinical Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neuroscience Letters. 2005;384:23–8. doi: 10.1016/j.neulet.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Block W, Jessen F, Traber F, Flacke S, Manka C, Lamerichs R, Keller E, Heun R, Schild H. Regional N-acetylaspartate reduction in the hippocampus detected with fast proton magnetic resonance spectroscopic imaging in patients with Alzheimer disease. Archives of Neurology. 2002;59:828–34. doi: 10.1001/archneur.59.5.828. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathology. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Chantal S, Labelle M, Bouchard RW, Braun CM, Boulanger Y. Correlation of regional proton magnetic resonance spectroscopic metabolic changes with cognitive deficits in mild Alzheimer disease. Archives of Neurology. 2002;59:955–62. doi: 10.1001/archneur.59.6.955. [DOI] [PubMed] [Google Scholar]
- Chao LL, Schuff N, Kramer JH, Du AT, Capizzano AA, O’Neill J, Wolkowitz OM, Jagust WJ, Chui HC, Miller BL, Yaffe K, Weiner MW. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology. 2005;64:282–9. doi: 10.1212/01.WNL.0000149638.45635.FF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, Wuu J, Turner DA. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiology of Aging. 2004;25:1197–203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Dixon RM, Bradley KM, Budge MM, Styles P, Smith AD. Longitudinal quantitative proton magnetic resonance spectroscopy of the hippocampus in Alzheimer’s disease. Brain. 2002;125:2332–41. doi: 10.1093/brain/awf226. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Harrison RM, O’Brien JT. A comprehensive review of proton magnetic resonance spectroscopy studies in dementia and Parkinson’s disease. Dementia and Geriatric Cognitive Disorders. 2002;14:64–76. doi: 10.1159/000064927. [DOI] [PubMed] [Google Scholar]
- Huang W, Alexander GE, Chang L, Shetty HU, Krasuski JS, Rapoport SI, Schapiro MB. Brain metabolite concentration and dementia severity in Alzheimer’s disease: a (1)H MRS study. Neurology. 2001;57:626–32. doi: 10.1212/wnl.57.4.626. [DOI] [PubMed] [Google Scholar]
- Lehtovirta M, Laakso MP, Frisoni GB, Soininen H. How does the apolipoprotein E genotype modulate the brain in aging and in Alzheimer’s disease? A review of neuroimaging studies. Neurobiology of Aging. 2000;21:293–300. doi: 10.1016/s0197-4580(00)00120-2. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mega MS, Small GW, Xu ML, Felix J, Manese M, Tran NP, Dailey JI, Ercoli LM, Bookheimer SY, Toga AW. Hippocampal atrophy in persons with age-associated memory impairment: volumetry within a common space. Psychosomatic Medicine. 2002;64:487–92. doi: 10.1097/00006842-200205000-00013. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, Depeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrego PJ, Fayed N, Pina MA. Conversion from mild cognitive impairment to probable Alzheimer’s disease predicted by brain magnetic resonance spectroscopy. The American Journal of Psychiatry. 2005;162:667–75. doi: 10.1176/appi.ajp.162.4.667. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–42. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Schuff N, Capizzano AA, Du AT, Amend DL, O’Neill J, Norman D, Kramer J, Jagust W, Miller B, Wolkowitz OM, Yaffe K, Weiner MW. Selective reduction of N-acetylaspartate in medial temporal and parietal lobes in AD. Neurology. 2002;58:928–35. doi: 10.1212/wnl.58.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen H, Partanen K, Pitkanen A, Hallikainen M, Hanninen T, Helisalmi S, Mannermaa A, Ryynanen M, Koivisto K, Riekkinen P., Sr Decreased hippocampal volume asymmetry on MRIs in nondemented elderly subjects carrying the apolipoprotein E epsilon 4 allele. Neurology. 1995;45:391–2. doi: 10.1212/wnl.45.2.391. [DOI] [PubMed] [Google Scholar]
- Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikainen M, Koivisto K, Riekkinen PJ., Sr Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44:1660–8. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- Tapiola T, Pennanen C, Tapiola M, Tervo S, Kivipelto M, Hanninen T, Pihlajamaki M, Laakso MP, Hallikainen M, Hamalainen A, Vanhanen M, Helkala EL, Vanninen R, Nissinen A, Rossi R, Frisoni GB, Soininen H. MRI of hippocampus and entorhinal cortex in mild cognitive impairment: A follow-up study. Neurobiology of Aging. 2008;29:31–8. doi: 10.1016/j.neurobiolaging.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Magnetic resonance spectroscopy in AD. Neurology. 2001;56:592–8. doi: 10.1212/wnl.56.5.592. [DOI] [PubMed] [Google Scholar]