Abstract
The 22q11.2 deletion syndrome (22q11.2DS) is associated with very high rates of schizophrenia-like psychosis and cognitive deficits. Here we report the results of the first longitudinal study assessing brain development in individuals with 22q11.2DS. Twenty-nine children with 22q11.2DS and 29 age and gender matched controls were first assessed during childhood or early adolescence; Nineteen subjects with 22q11.2DS and 18 controls underwent follow-up during late adolescence-early adulthood. The 22q11.2DS subjects showed greater longitudinal increase in cranial and cerebellar white matter, superior temporal gyrus, and caudate nucleus volumes. They also had a more robust decrease in amygdala volume. Verbal IQ (VIQ) scores of the 22q11.2DS group that developed psychotic disorders declined significantly between assessments. Decline in VIQ in 22q11.2DS was associated with more robust reduction of left cortical grey matter volume. No volumetric differences were detected between psychotic and nonpsychotic subjects with 22q11.2DS. Brain maturation associated with verbal cognitive development in 22q11.2DS varies from that observed in healthy controls. Further longitudinal studies are likely to elucidate brain developmental trajectories in 22q11.2DS and their association to psychotic disorders and cognitive deficits in this population.
1. Introduction
The 22q11.2 deletion syndrome (22q11.2DS), also known as velocardiofacial and DiGeorge syndromes, is caused by a microdeletion in the long arm of chromosome 22 (Carlson et al., 1997). The exact incidence of the syndrome is not yet certain but is estimated to be at least 1 in 5,000 live births (Botto et al., 2003). Thus, 22q11.2DS is the most common microdeletion syndrome known in humans. The phenotypic expression of 22q11.2DS is extremely broad and includes physical anomalies, cognitive deficits, and psychiatric manifestations (Shprintzen, 2000).
Most subjects with 22q11.2DS have learning disabilities with full-scale IQ (FSIQ) scores ranging from normal range to moderate mental retardation with a mean FSIQ in the borderline range (mid-seventies) (Swillen et al., 1997). In addition to physical and cognitive deficits, individuals with 22q11.2DS have high rates of psychiatric disorders (Feinstein et al., 2002; Murphy et al., 1999). Most striking, up to one third of subjects with 22q11.2DS develop schizophrenia-like psychotic disorders by early adulthood. This makes 22q11.2DS the most common identifiable genetic risk factor for schizophrenia (Gothelf et al., 2005; Murphy et al., 1999).
Cross-sectional quantitative imaging studies indicate diffuse alterations in the brain structure of children with 22q11.2DS. Total brain volume is decreased by 8.5%-11% and there are extensive regional abnormalities in both gray matter (GM) and white matter (WM) volumes (Eliez et al., 2000; Kates et al., 2001; Simon et al., 2005). Frontal lobe grey and white matter volumes are relatively increased while posterior cortical regions including parietal and occipital grey and white matter volumes are reduced (Campbell et al., 2006; Eliez et al., 2000; Kates et al., 2001). In addition, there are aberrations of posterior fossa anatomy, most notably cerebellar grey and white matter volume reductions (Bish et al., 2006; Eliez et al., 2001). Abnormal volumes have also been reported for other brain regions, including enlarged volumes of ventricular cerebrospinal fluid (CSF), caudate nucleus, and amygdala (Campbell et al., 2006; Eliez et al., 2002; Eliez et al., 2001; Kates et al., 2006; Simon et al., 2005).
There are relatively fewer imaging studies of adults with 22q11.2DS (Chow et al., 2002; van Amelsvoort et al., 2004). These studies have compared brain anatomy of psychotic versus nonpsychotic 22q11.2DS individuals. In general, subjects with 22q11.2DS and psychosis demonstrate morphological abnormalities similar to those detected in schizophrenia. These abnormalities include reduced whole brain volume - particularly WM, smaller GM volume in frontal and temporal lobes, and increase in ventricular volume (Chow et al., 2002; van Amelsvoort et al., 2004).
The goal of the present study was to assess longitudinal neurodevelopment in individuals with 22q11.2DS during the critical period of adolescence. However, since this is the first comparative longitudinal study of brain development in 22q11.2DS, we could not test hypotheses based on prior information about neurodevelopmental trajectories in this disorder. Accordingly, we chose to focus on the development of candidate brain regions that were previously reported to be abnormal in cross-sectional studies of both 22q11.2DS (Campbell et al., 2006; Chow et al., 2002; Eliez et al., 2002; Eliez et al., 2001; Kates et al., 2006; Simon et al., 2005; van Amelsvoort et al., 2004) and schizophrenia (reviewed in (DeLisi et al., 2006; Shenton et al., 2001). Thus we analyzed change in volume of the cerebral lobes, superior temporal gyrus (STG), ventricular CSF, cerebellum, amygdala, hippocampus, and caudate nucleus.
In addition, we focused on identifying neuroanatomical markers for the evolution of psychotic disorders and for the decline in VIQ scores that were identified in a previous study of this cohort (Gothelf et al., 2005). We hypothesized that changes in the above mentioned candidate brain regions would be more robust in those 22q11.2DS individuals who developed psychotic disorders compared to those who remained nonpsychotic. Since language functions are predominantly related to the left hemisphere (Capozzoli, 1999; Gernsbacher and Kaschak, 2003), we also hypothesized that the decline in VIQ scores in subjects with 22q11.2DS would be associated with a more robust decline in left cerebral GM volume.
2. Methods
2.1 Participants
The time 1 (T1) sample, collected between 1998 and 2000, included 29 children with 22q11.2DS and 29 typically developing (TD) controls [nineteen 22q11.2DS subjects and no controls participated in a previous study by our group that reported on prefrontal imaging data (Gothelf et al., 2005)]. Prospective recruitment was performed through the Northern California Velocardiofacial Association and by advertising on our web site (cibsr.stanford.edu). The presence of the 22q11.2 microdeletion was confirmed in all subjects with 22q11.2DS by fluorescence in situ hybridization (FISH). Typically developing controls were recruited through advertisement within the local community. All controls were screened and were not included in the study if they had a history of major psychiatric disorder or neurological or cognitive impairment. At follow-up, time 2 (T2) evaluation of the initial sample was conducted between 2003 and 2005. Nineteen subjects with 22q11.2DS and 18 TD controls participated at T2 evaluation. The follow-up interval was 4.9 ± 0.7 for the 22q11.2DS group and 4.9 ± 0.9 years for the controls. The omissions from T2 evaluations in the 22q11.2DS group were due to inability to locate a participant after the family moved from Northern California (3 subjects), medical contraindications for scanning that emerged after the T1 study period (dental braces, 2 subjects, implantation of metal valve, 1 subject), and when participants and/or their parents choose not to participate in the T2 evaluation (4 subjects). The demographic and clinical characteristics of the T1 sample and the longitudinal subsample are presented in Table 1. All subjects were screened for substance abuse, at both time points, and none of them reported history of substance abuse of any kind.
Table 1.
Characteristics of baseline (T1) sample and of the longitudinal subsample
| T1 sample | Longitudinal subsample | |||||
|---|---|---|---|---|---|---|
| 22q11.2DS
(n = 29) |
Controls
(n = 29) |
P-value | 22q11.2DS
(n =19) |
Controls
(n = 18) |
P-value | |
| Age at T1 | 12.3 ± 4.0 | 12.7 ± 4.0 | 0.76 | 13.1 ± 4.0 | 13.4 ± 4.0 | 0.79 |
| Range | 6.3-19.7 | 5.8-20.7 | 6.9-19.7 | 6.7-20.7 | ||
|
| ||||||
| Age at T2 | N/A | N/A | 0.78 | 17.9 ± 3.8 | 18.3 ± 4.0 | 0.78 |
| Range | 12.0-24.1 | 10.2-26.4 | ||||
|
| ||||||
| Delta age (Follow-up interval) | N/A | N/A | 4.9 ± 0.7 | 4.9 ± 0.9 | 0.90 | |
| Range | 3.7-6.4 | 3.5-6.2 | ||||
|
| ||||||
| Males : Females | 20 : 9 | 20 : 9 | 1.0 | 11 : 8 | 10 : 8 | 1.0 |
|
| ||||||
| Ethnicity | 0.32 | 0.31 | ||||
| Caucasian | 24 | 22 | 16 | 14 | ||
| Mixed race | 5 | 7 | 3 | 4 | ||
|
| ||||||
| Handedness | 0.53 | 0.21 | ||||
| Right-handed | 25 | 27 | 16 | 18 | ||
| Left-handed | 3 | 2 | 2 | 0 | ||
| Mixed dominance | 1 | 0 | 1 | 0 | ||
|
| ||||||
| Parents education (yrs) | 16.4 ± 2.3 | 17.7 ± 2.5 | 0.15 | 16.4 ± 2.4 | 17.6 ± 2.3 | 0.19 |
|
| ||||||
| Full scale IQ at T1 | 72.8 ± 14.9 | 115.3 ± 12.7 | <0.001 | 74.5 ± 15.1 | 114.7 ± 11.5 | <0.001 |
±: standard deviation
The T1 sample and the longitudinal subsample were well matched across diagnostic groups in mean age, parents' years of education, male to female ratio, ethnicity, and handedness (Table 1). The 22q11.2DS group had a significant (P < .001) lower FSIQ than the TD control group.
By the time of the T2 scan, 10 participants with 22q11.2DS had received atypical antipsychotics (6 subjects, risperidone 3, quetiapine 2, olanzapine 1) or mood stabilizers (10 subjects, valproate 4, oxacarbazepine 3, gabapentin 3, lithium 1) for more than six months. All six subjects receiving antipsychotics had a psychotic disorder.
After providing a complete description of the study to the subjects and their parents, written informed consent was obtained at both time point assessments, according to protocols approved by the institutional review board of Stanford University, Stanford, California.
2.2 Cognitive and Psychiatric Measures
Cognitive and psychiatric assessments were conducted at both time points. For the cognitive assessment, the Wechsler Intelligence Scale for Children, 3rd edition (WISC III) (Wechsler, 1991) was used for subjects 17 years and younger and the Wechsler Adult Intelligence Scale, 3rd edition (WAIS III) was used for subjects older than 17 years (Wechsler, 1997).
For screening of psychotic disorders, the Screening Question portion of the Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version (K-SAD-PL) Parent version (Kaufman et al., 1997) was used. In addition, subjects above the age of 18 years were also evaluated with the Structured Clinical Interview for DSM–IV Diagnoses (SCID) (First et al., 1996).
2.3 MRI Protocol
Magnetic resonance images were acquired with the same GE 1.5 Tesla scanner (General Electric, Milwaukee, WI). Coronal images were acquired with a three-dimensional volumetric radiofrequency spoiled gradient echo pulse sequence using the following scan parameters: TR = 35 ms, TE = 6 ms, flip angle = 45°, NEX = 1, matrix size = 256 × 192, field of view = 24 cm2, slice thickness = 1.5 mm, 124 contiguous slices. MRI scans were imported into BrainImage (Stanford University, Stanford, CA) for semi-automated whole brain segmentation and quantification using previously described and validated methods that result in grey, white, CSF, and total tissue volume measurements for the whole brain, four cerebral lobes, cerebellum, and ventricles (Reiss et al., 1998).
Trained research assistants following detailed protocols delineated additional ROI volumes manually. The ROI variables included the volumes of the caudate nucleus (Eliez et al., 2002) hippocampus and amygdala (Kates et al., 1997), and the grey matter volume of the STG (Kesler et al., 2003). Raters were blind to subjects' diagnoses and achieved reliability of above 0.95 (intraclass correlation coefficient) for all ROIs.
2.4 Statistical Analyses
Data were first examined for normality to confirm the assumptions of the parametric statistics employed. Analyses of variance (ANOVA) and analyses of covariance (ANCOVA), adjusting for the effect of differences in total cranial tissue volume, were conducted to compare baseline (T1) brain volume differences between 22q11.2DS subjects and controls. For longitudinal analyses of brain development, we employed repeated measures ANOVAs with group (22q11.2DS vs. controls) as the between-subject factor and brain structure volumes at T1 and T2 as the within-subject factor. For each brain structure volume percent change per year was calculated using the following formula- (T2 volume-T1 volume)×100/(T1 volume × interval between scans in years). Repeated measures ANOVAs were used to evaluate symmetry of tissue volumes at T1 and the symmetry of tissue volume change. Within group (22q11.2DS subjects receiving or not receiving antipsycotics/mood stablizers) were compared using student's t tests. A two-tailed significance threshold of P < 0.05 was used. Associations between changes in VIQ scores and changes in cortical brain volumes were evaluated with spearman correlations.
3. Results
The summary of baseline (T1) and follow-up (T2) brain tissue volumes in 22q11.2DS and typically developing controls are presented in Table 2.
Table 2.
Summary of baseline (T1) and follow-up (T2) brain tissue volumes in children with 22q11.2DS and typically developing controls
| Baseline (T1) | Follow-up (T2) | |||||
|---|---|---|---|---|---|---|
| Brain Area | 22q11.2DS | Controls | P | 22q11.2DS | Controls | P |
| Total Tissue | ||||||
| Cranial | 1171.1 | 1316.9 | <0.001 | 1176.5 | 1282.0 | 0.07 |
| (144.8) | (98.3) | (135.9) | (105.5) | |||
| Frontal | 381.1 | 408.9 | <0.001 | 379.5 | 396.0 | 0.41 |
| (51.5) | (30.8) | (45.0) | (27.2) | |||
| Parietal | 250.8 | 288.2 | 0.04 | 249.5 | 275.9 | 0.18 |
| (30.5) | (29.9) | (30.6) | (29.9) | |||
| Temporal | 198.5 | 224.5 | 0.24 | 203.3 | 222.4 | 0.07 |
| (28.5) | (16.5) | (27.3) | (17.9) | |||
| Occipital | 113.6 | 137.2 | 0.01 | 112.8 | 131.8 | 0.88 |
| (21.3) | (13.0) | (19.4) | (18.1) | |||
| Cerebellum | 112.5 | 130.8 | <0.001 | 117.2 | 130.6 | 0.07 |
| (11.8) | (14.8) | (12.1) | (16.7) | |||
| Caudate | 8.4 | 8.6 | 0.39 | 8.7 | 8.5 | 0.001 |
| (1.3) | (0.9) | (1.4) | (0.9) | |||
| Amygdala | 4.2 | 4.1 | 0.13 | 3.8 | 4.1 | 0.01 |
| (0.7) | (0.7) | (0.5) | (0.5) | |||
| Hippocampus | 7.0 | 8.1 | 0.25 | 7.4 | 8.5 | 0.56 |
| (1.1) | (1.0) | (0.8) | (0.9) | |||
| Grey Matter | ||||||
| Cranial | 739.0 | 794.6 | <0.001 | 706.4 | 759.0 | 0.58 |
| (83.7) | (49.0) | (85.0) | (58.0) | |||
| Frontal | 229.3 | 245.1 | 0.04 | 221.9 | 234.4 | 0.17 |
| (31.6) | (16.5) | (26.2) | (14.2) | |||
| Parietal | 146.9 | 165.3 | 0.01 | 140.2 | 154.5 | 0.26 |
| (17.6) | (16.3) | (16.9) | (15.0) | |||
| Temporal | 143.1 | 157.3 | 0.23 | 143.2 | 154.7 | 0.30 |
| (19.3) | (10.6) | (20.0) | (11.7) | |||
| Occipital | 70.8 | 78.9 | 0.57 | 67.8 | 74.9 | 0.92 |
| (12.6) | (8.3) | (11.6) | (9.8) | |||
| STG | 25.2 | 28.4 | 0.44 | 24.6 | 23.9 | <0.0001 |
| (4.1) | (3.9) | (3.4) | (4.0) | |||
| White Matter | ||||||
| Cranial | 438.5 | 517.7 | <0.001 | 470.1 | 523.0 | 0.03 |
| (71.8) | (67.6) | (61.2) | (56.1) | |||
| Frontal | 154.8 | 163.8 | <0.001 | 157.6 | 161.6 | 0.83 |
| (25.2) | (18.7) | (21.8) | (16.4) | |||
| Parietal | 103.9 | 122.9 | 0.38 | 109.4 | 121.4 | 0.59 |
| (16.8) | (17.6) | (15.9) | (16.8) | |||
| Temporal | 55.3 | 67.2 | 0.19 | 60.2 | 67.8 | 0.21 |
| (11.5) | (8.9) | (9.5) | (8.7) | |||
| Occipital | 42.7 | 58.3 | <0.001 | 44.9 | 56.8 | 0.77 |
| (10.7) | (7.8) | (8.8) | (10.2) | |||
| Cerebellum | 25.9 | 43.7 | <0.001 | 38.7 | 47.6 | 0.002 |
| (10.5) | (10.7) | (8.7) | (10.1) | |||
| Ventricular | 16.3 | 12.2 | 0.005 | 18.3 | 14.2 | 0.25 |
| CSF | (8.9) | (7.0) | (8.7) | (7.9) | ||
3.1 Brain Tissue Volumes at Baseline
At T1, brain volume differences between 22q11.2DS and controls were similar to differences previously reported with subsets of patients from the current cohort (Eliez et al., 2002; Eliez et al., 2001; Eliez et al., 2000; Eliez et al., 2001). Total cranial tissue was decreased in 22q11.2DS compared to controls with a more robust reduction in WM (15.3%) than GM (7.0%). Also, in line with our previous reports, we found an increase in adjusted frontal lobe GM and WM and ventricular CSF volumes in the 22q11.2DS group and decreased adjusted parietal lobe GM volumes. There were no significant differences for STG, amygdala, hippocampus, and caudate nucleus adjusted volumes at T1. Differences between the 22q11.2DS cohort studied here that were not detected in our previous analyses with smaller number of subjects included decreased occipital lobe and cerebellar GM and WM volumes. Decrease in volumes in the occipital lobe and cerebellum were reported in other studies of children with 22q11.2DS (Campbell et al., 2006; Simon et al., 2005).
3.2 Brain Developmental Trajectories (Change From Time 1 to time 2)
There was a significant group difference in the developmental trajectory of cranial and cerebellar WM volumes as shown by significant time × group interaction on repeated-measures ANOVA for this variable in the 22q11.2 DS group relative to controls (Table 3 and Fig. 1A). At T1 cranial WM was reduced by 15.3% in 22q11.2DS compared to controls (P<0.001). Cranial WM volume at T2 remained significantly reduced by 10.1% in the 22q11.2S group compared to the control group (P=0.01).
Table 3.
Summary of significant differences from time 1 to time 2 in developmental brain trajectories between 22q11.2DS and controls
| Brain Area | % Difference
At Baseline1 |
% Change
22q11.2DS2 |
% Change
Controls2 |
F3 | P3 | Effect
Size4 |
|---|---|---|---|---|---|---|
| Amygdala | -9.1 | -1.71 | 0.23 | -6.5 | 0.01 | 0.16 |
| Caudate Nucleus | -4.6 | 0.83 | -0.32 | 3.6 | 0.001 | 0.27 |
| STG GM | 3.0 | -0.41 | -3.32 | 4.3 | <0.0001 | 0.38 |
| Cranial WM | 20.9 | 1.61 | 0.30 | 2.3 | 0.03 | 0.13 |
| Cerebellar WM | 48.0 | 19.1 | 0.97 | 3.5 | 0.002 | 0.25 |
STG GM: superior temporal gyrus grey matter; WM: white matter.
Percent difference between groups in structure volume at baseline.
Within group percent change per year from time 1 to time 2
Time x group interaction on repeated measures ANOVAs
Partial Eta2
Figure 1.
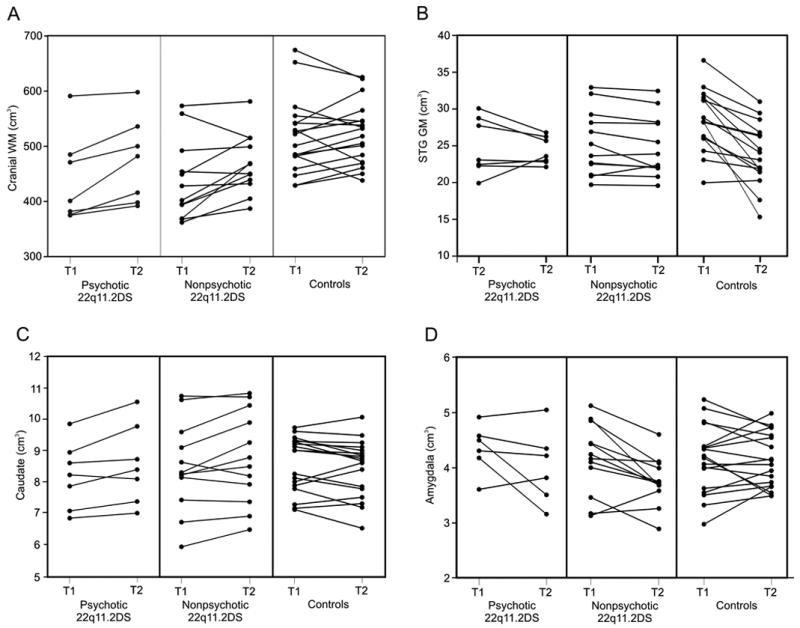
Brain volume changes from time 1 to time 2 in 22q11.2DS and controls.
There was also a significant group difference in the developmental trajectories of STG GM and caudate nucleus volume (Table 3 and Fig. 1B and 1C). Post hoc pairwise t tests indicated that STG volume nonsignificantly decreased in 22q11.2DS and significantly decreased in controls (t=5.7, P<0.0001) and caudate volume significantly increased in 22q11.2DS (t=-3.4, P=0.003) and nonsignificantly decreased in controls. The STG difference remained robust after excluding the lowest control outlier (t=4.6, p<0.0001).
Amygdala developmental trajectories were also different between groups, with 22q11.2DS showing an average significant decrease (t=3.6, p=0.002) and controls showing no change over time (Fig. 1D). No significant time × group interaction differences were detected between 22q11.2DS and controls for any of the cortical volumes. There was no significant gender or gender by group interaction effects in any of the time × group analyses. There was no time × group interaction on hippocampus volume. Repeated measures ANOVA indicated a R > L increase in hippocampal volume from T1 to T2 across both groups of subjects (F = 5.2, P < 0.05) but no side by group interaction was observed. No group differences in symmetry were observed for any of other analyses.
To examine the validity and specificity of our findings, we analyzed changes in overall cerebral GM and WM from T1 to T2 in our two groups. The cerebrum was chosen for analysis as tissue-specific developmental trajectories from childhood to young adulthood, are relatively well established for this region. Specifically, longitudinal neuroimaging studies have found an increase in WM and decrease in GM from mid-childhood to adulthood (Giedd et al., 1999; Giedd et al., 1999a; Rapoport et al., 1999; Sowell et al., 2003). Therefore, we expected to observe an increase in cerebral WM/GM ratio from T1 to T2 in the whole sample and in each of the groups separately (22q11.2DS and controls). As expected, the paired t-test cerebral WM/GM ratio significantly increased from T1 to T2 in the whole sample (t = -5.3, df=36, P < 0.001), in 22q11.2DS (t = -4.9, df=18, P < 0.001), and in controls (t = -2.8, df=17, P = 0.01).
3.3 Association between Development of Brain and Cognition in 22q11.2DS
In line with our previous publication (Gothelf et al., 2005), repeated measures ANOVA indicated a significant time by diagnosis interaction effect on VIQ (F = 12.44, P = 0.001). The interaction was driven by a significant decline in VIQ scores in the 22q11.2DS group from 80.1 ± 14.3 to 75.5 ± 15.4 and by an increase in VIQ scores in the controls (from 114.9 ± 8.8 to 118.5 ± 10.4).
To test the hypothesis that reduction in VIQ scores in 22q11.2DS is associated with decrease in left cerebral GM volume, we conducted correlation analyses. Within the 22q11.2DS group, there was a significant positive correlation between change in VIQ from T1 to T2 (ΔVIQ) and Δleft cerebral GM in the 22q11.2DS group (r = 0.59, P = 0.01, Fig. 2). Conversely, within the control group there was a nonsignificant negative correlation between ΔVIQ and Δleft cerebral GM (r = -0.39, P = 0.14). Fisher r-to-z transformation showed that the difference between groups' correlations was significant (z = 3.0, P < 0.005, Fig. 2). There was no significant correlation between ΔVIQ and Δright cerebral GM in the 22q11.2DS group (r = -0.12, P = 0.67). However, within the 22q11.2DS group the correlation between Δleft cerebral GM and ΔVIQ compared to the correlation of Δright cerebral GM with ΔVIQ differed significantly (z = 2.3, P = 0.02).
Figure 2.
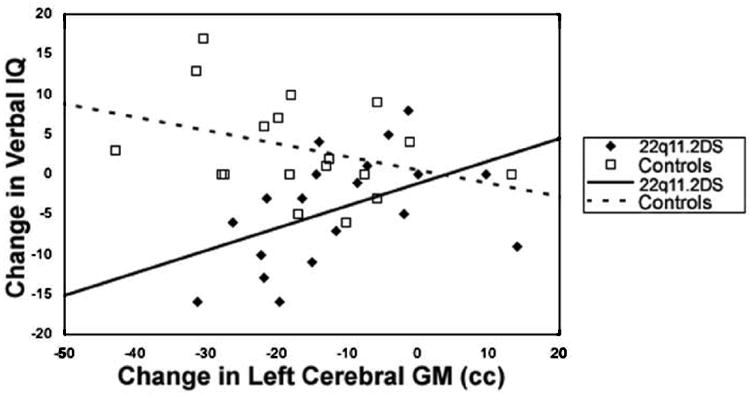
Correlation between change in left cerebral grey matter volume and change in verbal IQ scores in 22q11.2DS and controls.
3.4 Association Between Psychosis and Brain Development and Cognition in 22q11.2DS
Consistent with our previous publication (Gothelf et al., 2005), at T1, none of the 22q11.2DS subjects had a psychotic disorder; at T2, seven of the 19 participants (36.8%) met DSM-IV criteria for a psychotic disorder including schizophrenia (3 subjects), schizoaffective disorder (2 subjects), psychotic depression (1 subject) or schizophreniform disorder (1 subject). None of the control subjects had a psychotic disorder at either time point. There were no significant differences between the seven psychotic and 12 nonpsychotic 22q11.2DS subjects in the developmental trajectories of cortical lobes, cerebellar GM and WM, amygdala, hippocampus, and caudate nucleus volumes.
Repeated measures ANOVA revealed that within the 22q11.2DS group, decline in VIQ was more significant in the 22q11.2DS subjects who developed psychosis than in the 22q11.2DS subjects who did not develop psychosis (from 80.3± 15.1 at T1 to 78.6 ± 16.1 at T2 vs. and from 79.7 ± 14.1 to 69.3 ± 13.1, respectively, F = 8.8, P < 0.01).
3.5 Effect of antipsychotics and mood stabilizers on brain development
Though not unequivocally established, mood stabilizers and atypical antipsychotics may affect neurodevelopment, especially in the cerebral cortex and caudate nucleus (Gray et al., 2003; Lieberman et al., 2005; Massana et al., 2005). However, no significant differences in longitudinal development of cortical GM, cortical WM, caudate nucleus, amygdala or hippocampal volumes were found when the 10 22q11.2DS subjects who were exposed to mood stabilizers and/or atypical antipsychotics were compared with the other nine 22q11.2DS subjects (P's ≥ 0.38).
4. Discussion
This is the first longitudinal study to investigate brain development trajectories in children with 22q11.2DS as compared to a matched age and gender control group. Compared to controls, subjects with 22q11.2DS showed abnormal developmental trajectories in several brain regions during transition into late adolescence-young adulthood. Compared to controls, children with 22q11.2DS demonstrated a greater increase in total cranial and cerebellar WM, STG GM, and caudate nucleus volumes. They also exhibited a more robust decrease in amygdala volume. As hypothesized, the decline in VIQ in the 22q11.2DS group was associated with greater reduction in left cortical GM volume. No differences were found in the brain development trajectories of 22q11.2DS subjects with psychotic disorders compared to those without.
The results of our longitudinal analysis of brain development in 22q11.2DS should be interpreted in light of what is known about normal brain development. The changes observed in the study controls are in line with findings of longitudinal studies of typically developing children. Similar to longitudinal findings in healthy children and adolescents (Castellanos et al., 2002; Giedd et al., 1999; Lenroot and Giedd, 2006; Rapoport et al., 1999; Sowell et al., 2003), our control group showed an increase in cerebral WM/GM ratio, decrease in caudate volume, and no change in amygdala and hippocampus volumes.
It is of interest to compare the longitudinal brain changes detected in our sample of 22q11.2DS participants to results from other studies focusing on non-22q11.2DS subjects at high risk for schizophrenia or in the prodromal stage of the disease. Contrary to results obtained from high-risk or prodromal non-22q11.2DS subjects (Keshavan et al., 2005; Lawrie et al., 2002; Pantelis et al., 2003), we did not find accelerated decrease in cortical GM in adolescents with 22q11.2DS. The only cortical GM ROI that showed a longitudinal change significantly different from controls was STG GM, which in 22q11.2DS, in contrast to controls, did not show the normal significant decrease in volume. This is contrary to the accelerated decrease in STG volumes observed in schizophrenia (Kasai et al., 2003). Rather than reflecting a disease process related to schizophrenia, increased STG GM in 22q11.2DS may reflect, like increased caudate nucleus and cranial WM volumes, delayed or aberrant brain maturation in affected adolescents. Supporting this view, the STG GM reaches it maximal volume later than all other cortical regions after the age of 16 years (Lenroot and Giedd, 2006). The typical caudate development has been observed to increase in childhood and then begins to decrease in size during adolescence (Castellanos et al., 2002). Thus it seems that in 22q11.2DS adolescents pruning of the STG GM and caudate nucleus lags behind that of typically developing adolescents.
Several previous studies have demonstrated that WM abnormalities are a common feature of 22q11.2DS (Barnea-Goraly et al., 2003; Campbell et al., 2006; Eliez et al., 2000; Kates et al., 2001; Simon et al., 2005; van Amelsvoort et al., 2004) and also in children with idiopathic developmental delay (Fayed et al., 2006; Pujol et al., 2004). The present study shows that there is abnormal longitudinal development of WM in 22q11.2DS children. At baseline, children with 22q11.2DS showed significantly reduced total WM volume in comparison to controls. During adolescence, 22q11.2DS white matter growth was accelerated compared to that of controls. It is yet to be determined whether the accelerated growth of WM in adolescents with 22q11.2DS represents a functional ‘catch-up’ or whether it reflects aberrant brain maturation.
In the present study, amygdala volumes significantly decreased between T1 and T2 in 22q11.2DS, but remained constant in controls. The biological significance of more rapidly declining amygdala volumes in 22q11.2DS is presently unclear due to a paucity of longitudinal data on normal amygdala development during adolescence. A recent cross-sectional study of children with 22q11.2DS found proportionally larger amygdala volumes in 22q11.2DS (Kates et al., 2006). Similar to the Kates et al (2006) study, the adjusted mean T1 amygdala volume in our 22q11.2DS sample was 8% larger than controls. Although this difference was not significant (P = .13) this may be related to smaller sample size than the Kates study. Taken together, cross-sectional and longitudinal findings in 22q11.2DS indicate that aberrant amygdala development may be a dynamic process that begins with enlarged volume during childhood followed by accelerated decline in volume during adolescence.
The trajectory of change in the candidate brain regions selected for study did not distinguish between psychotic and nonpsychotic adolescents with 22q11.2DS. This could be related to the small number of psychotic patients (n=7) and does not rule out the possibility that more subtle anatomical differences exist between these subgroups. It is also possible that structural changes are associated with more specific endophenotypes of the schizophrenia-like disorder of 22q11.2DS, such as the decline in VIQ. Interestingly, there was a robust mean decline (10.3 points) in VIQ in the 22q11.2DS subgroup that developed psychotic disorder. The decline in VIQ observed in 22q11.2DS psychotic adolescents is in line with longitudinal studies of subjects with schizophrenia that demonstrated a decline in IQ, particularly in the language domain, appearing several years before disease onset (Cannon M 2002; Fuller R and others 2002).
As hypothesized, decline in verbal abilities of 22q11.2DS children significantly correlated with reduction in left cerebral GM volumes. In contrast, a negative correlation between change in VIQ and left cerebral GM volume was observed in controls. In a separate study, development of verbal skills in younger healthy children was found to be associated with left hemisphere cortical thinning (Sowell et al., 2004). Similarly, another study found that cortical thinning in early adolescence was associated with more robust cognitive development (Shaw et al., 2006). Cortical thinning likely represents normal pruning of GM neuropil associated with increased cognitive efficiency (Johnson and Munakata, 2005). Thus, our results suggest that in 22q11.2DS, loss of GM during adolescence is associated with a negative trajectory of VIQ, providing further evidence of abnormal brain maturational processes in this population.
4.1 Limitations
To the best of our knowledge, the study presented here represents the first longitudinal evaluation of overall brain development in 22q11.2DS. However, several limitations should be noted. A second control group consisting of individuals matched for IQ to the 22q11.2DS group would have allowed greater precision in determining the specificity of abnormal neurodevelopmental trajectories in 22q11.2DS. Our study focused on a relatively small group of children and adolescents with 22q11.2DS. Yet, the age range of the study subjects at baseline and follow-up varied. Therefore our study is underpowered to delineate age specific developmental trajectories. Another limitation of the study is the use of multiple statistical tests. To reduce the number of statistical tests and the chance findings, we focused in this work on developmental trajectories of key brain regions which were reported to be abnormal in 22q11.2DS and in schizophrenia.
All data collected from longitudinal imaging studies are subject to increased variance associated with software or hardware upgrades and this is an inherent methodological limitation of MRI studies in particular. Yet, we took special care that our T1 and T2 scans would be as consistent as possible by utilizing the same 1.5T GE scanner and pulse sequence throughout the duration of the study. Though software version upgrades did take place, we also routinely monitor for potential affects on data by performing pre- and post- upgrade scans.
It is also possible that the dynamic trajectory of cortical change of the two groups studied here are not linear. To address this question a third time point of evaluation is required. The relatively small sample size, and especially the low number of psychotic patients in our sample, was probably underpowered to detect more subtle abnormal neuroanatomical developmental trajectories in 22q11.2DS and in the 22q11.2DS psychotic subgroup. Future longitudinal studies with larger sample size will further elucidate brain development in 22q11.2DS and its relation to genes, cognitive development and psychopathology.
Acknowledgments
DG was supported by NARSAD Young Investigator Award and ALR was supported by NIH grants HD31715, MH50047, MH19908.
The authors would like to thank the subjects and their families for participating in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, Reiss AL. White matter tract alterations in fragile X syndrome: preliminary evidence from diffusion tensor imaging. Am J Med Genet B Neuropsychiatr Genet. 2003;118:81–8. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- Bish JP, Pendyal A, Ding L, Ferrante H, Nguyen V, McDonald-McGinn D, Zackai E, Simon TJ. Specific cerebellar reductions in children with chromosome 22q11.2 deletion syndrome. Neurosci Lett. 2006;399:245–8. doi: 10.1016/j.neulet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, Merritt RK, O'Leary LA, Wong LY, Elixson EM, Mahle WT, Campbell RM. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112:101–7. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, Ng V, van Amelsvoort T, Chitnis X, Cutter W, Murphy DG, Murphy KC. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129:1218–28. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- Capozzoli NJ. Why do we speak with the left hemisphere? Med Hypotheses. 1999;52:497–503. doi: 10.1054/mehy.1999.0004. [DOI] [PubMed] [Google Scholar]
- Carlson C, Sirotkin H, Pandita R, Goldberg R, McKie J, Wadey R, Patanjali SR, Weissman SM, Anyane-Yeboa K, Warburton D, Scambler P, Shprintzen R, Kucherlapati R, Morrow BE. Molecular definition of 22q11 deletions in 151 velo-cardio-facial syndrome patients. Am J Hum Genet. 1997;61:620–9. doi: 10.1086/515508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Jama. 2002;288:1740–8. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Chow EW, Zipursky RB, Mikulis DJ, Bassett AS. Structural brain abnormalities in patients with schizophrenia and 22q11 deletion syndrome. Biol Psychiatry. 2002;51:208–15. doi: 10.1016/s0006-3223(01)01246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, Szulc KU, Bertisch HC, Majcher M, Brown K. Understanding structural brain changes in schizophrenia. Dialogues Clin Neurosci. 2006;8:71–8. doi: 10.31887/DCNS.2006.8.1/ldelisi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliez S, Barnea-Goraly N, Schmitt JE, Liu Y, Reiss AL. Increased basal ganglia volumes in velo-cardio-facial syndrome (deletion 22q11.2) Biol Psychiatry. 2002;52:68–70. doi: 10.1016/s0006-3223(02)01361-6. [DOI] [PubMed] [Google Scholar]
- Eliez S, Blasey CM, Schmitt EJ, White CD, Hu D, Reiss AL. Velocardiofacial syndrome: are structural changes in the temporal and mesial temporal regions related to schizophrenia? Am J Psychiatry. 2001;158:447–53. doi: 10.1176/appi.ajp.158.3.447. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Reiss AL. Children and adolescents with velocardiofacial syndrome: a volumetric MRI study. Am J Psychiatry. 2000;157:409–15. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Wellis VG, Reiss AL. A quantitative MRI study of posterior fossa development in velocardiofacial syndrome. Biol Psychiatry. 2001;49:540–6. doi: 10.1016/s0006-3223(00)01005-2. [DOI] [PubMed] [Google Scholar]
- Fayed N, Morales H, Modrego PJ, Munoz-Mingarro J. White matter proton MR spectroscopy in children with isolated developmental delay: does it mean delayed myelination? Acad Radiol. 2006;13:229–35. doi: 10.1016/j.acra.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Feinstein C, Eliez S, Blasey C, Reiss AL. Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: usefulness as phenotypic indicators of schizophrenia risk. Biol Psychiatry. 2002;51:312–8. doi: 10.1016/s0006-3223(01)01231-8. [DOI] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J. User's Guide for the Structural Clinincal Interview for DSM-IV Axis I Disorders-Research Version. New York: Biometric Research; 1996. [Google Scholar]
- Gernsbacher MA, Kaschak MP. Neuroimaging studies of language production and comprehension. Annu Rev Psychol. 2003;54:91–114. doi: 10.1146/annurev.psych.54.101601.145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999a;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, Kwon H, Jin S, Jo B, Antonarakis SE, Morris MA, Reiss AL. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gray NA, Zhou R, Du J, Moore GJ, Manji HK. The use of mood stabilizers as plasticity enhancers in the treatment of neuropsychiatric disorders. J Clin Psychiatry. 2003;64 5:3–17. [PubMed] [Google Scholar]
- Johnson MH, Munakata Y. Processes of change in brain and cognitive development. Trends Cogn Sci. 2005;9:152–8. doi: 10.1016/j.tics.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:156–64. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Jabs EW, Rutberg J, Murphy AM, Grados M, Geraghty M, Kaufmann WE, Pearlson GD. Regional cortical white matter reductions in velocardiofacial syndrome: a volumetric MRI analysis. Biol Psychiatry. 2001;49:677–84. doi: 10.1016/s0006-3223(00)01002-7. [DOI] [PubMed] [Google Scholar]
- Kates WR, Miller AM, Abdulsabur N, Antshel KM, Conchelos J, Fremont W, Roizen N. Temporal lobe anatomy and psychiatric symptoms in velocardiofacial syndrome (22q11.2 deletion syndrome) J Am Acad Child Adolesc Psychiatry. 2006;45:587–95. doi: 10.1097/01.chi.0000205704.33077.4a. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Berger G, Zipursky RB, Wood SJ, Pantelis C. Neurobiology of early psychosis. Br J Psychiatry Suppl. 2005;48:s8–18. doi: 10.1192/bjp.187.48.s8. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Blasey CM, Brown WE, Yankowitz J, Zeng SM, Bender BG, Reiss AL. Effects of X-monosomy and X-linked imprinting on superior temporal gyrus morphology in Turner syndrome. Biol Psychiatry. 2003;54:636–46. doi: 10.1016/s0006-3223(03)00289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Miller P, Best JJ, Owens DG, Johnstone EC. Temporal lobe volume changes in people at high risk of schizophrenia with psychotic symptoms. Br J Psychiatry. 2002;181:138–43. doi: 10.1017/s0007125000161860. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–70. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Massana G, Salgado-Pineda P, Junque C, Perez M, Baeza I, Pons A, Massana J, Navarro V, Blanch J, Morer A, Mercader JM, Bernardo M. Volume changes in gray matter in first-episode neuroleptic-naive schizophrenic patients treated with risperidone. J Clin Psychopharmacol. 2005;25:111–7. doi: 10.1097/01.jcp.0000155818.29091.53. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–5. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–8. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pujol J, Lopez-Sala A, Sebastian-Galles N, Deus J, Cardoner N, Soriano-Mas C, Moreno A, Sans A. Delayed myelination in children with developmental delay detected by volumetric MRI. Neuroimage. 2004;22:897–903. doi: 10.1016/j.neuroimage.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, Nicolson R, Bedwell J, Lenane M, Zijdenbos A, Paus T, Evans A. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–54. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Hennessey JG, Rubin M, Beach L, Abrams MT, Warsofsky IS, Liu AM, Links JM. Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. J Comput Assist Tomogr. 1998;22:471–9. doi: 10.1097/00004728-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: a distinctive behavioral phenotype. Ment Retard Dev Disabil Res Rev. 2000;6:142–7. doi: 10.1002/1098-2779(2000)6:2<142::AID-MRDD9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neuroimage. 2005;25:169–80. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns JP. Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. J Med Genet. 1997;34:453–8. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amelsvoort T, Daly E, Henry J, Robertson D, Ng V, Owen M, Murphy KC, Murphy DG. Brain anatomy in adults with velocardiofacial syndrome with and without schizophrenia: preliminary results of a structural magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:1085–96. doi: 10.1001/archpsyc.61.11.1085. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children - Third Edition Manual. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Third Edition. Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]


