Abstract
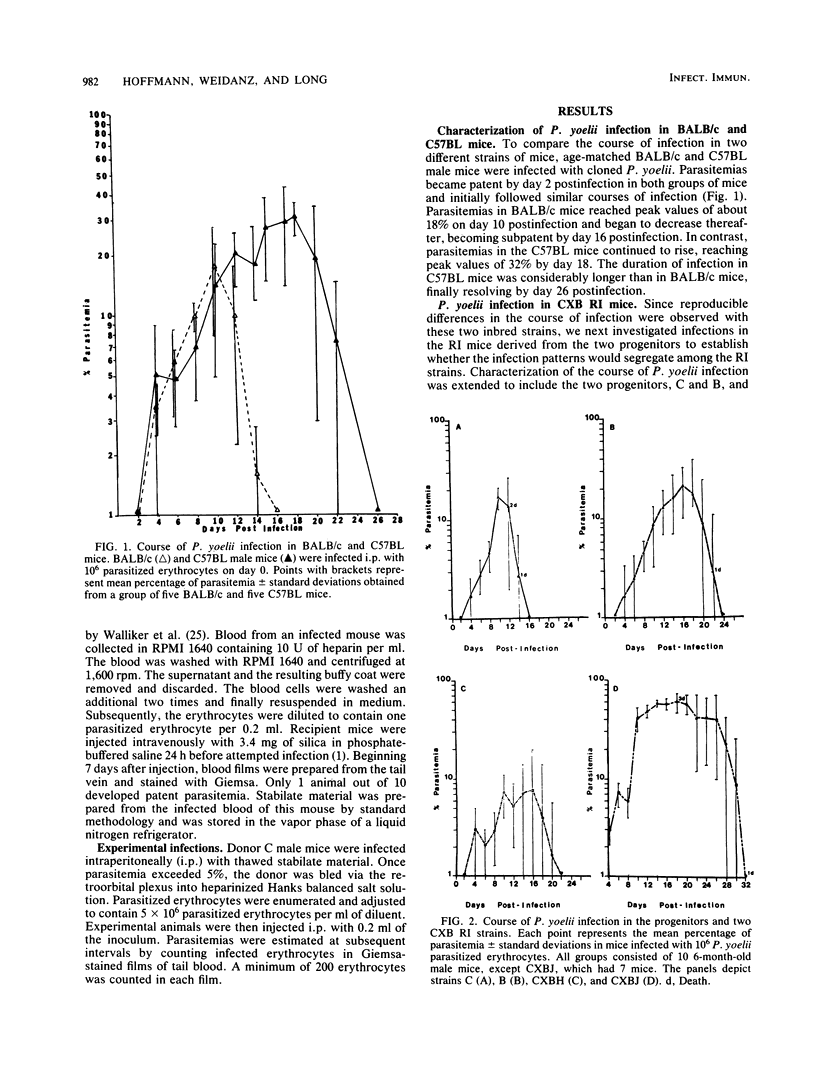
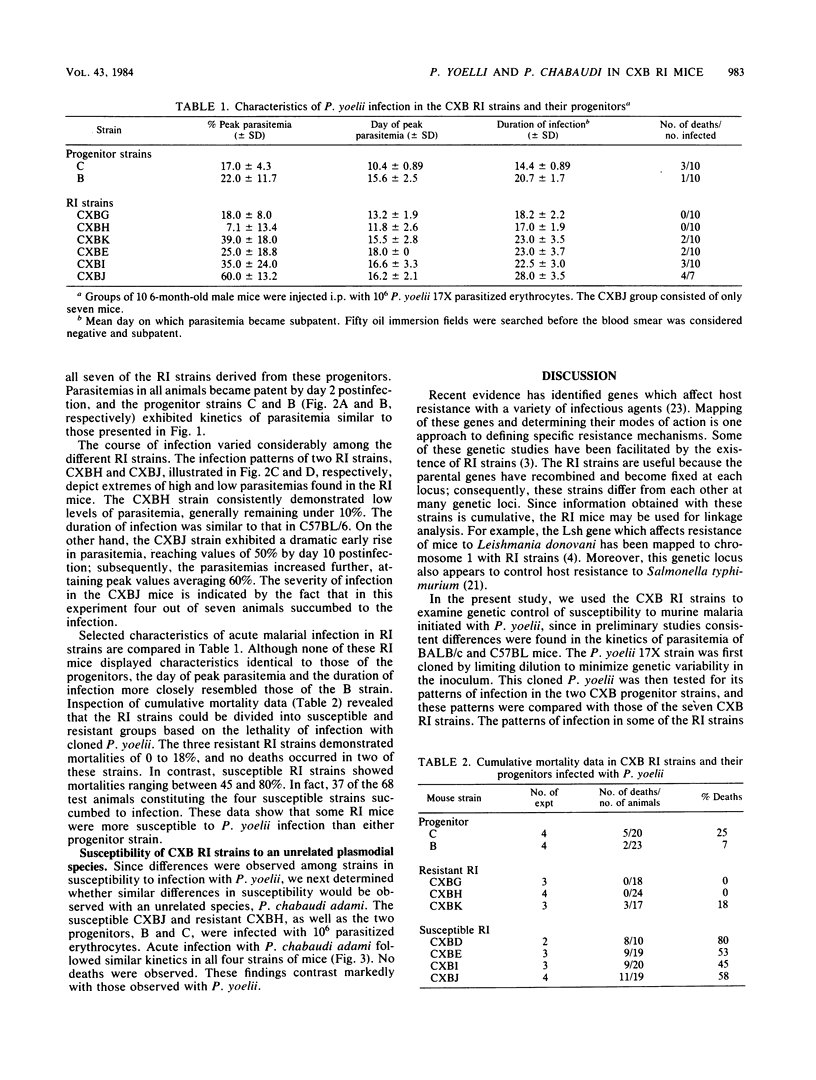
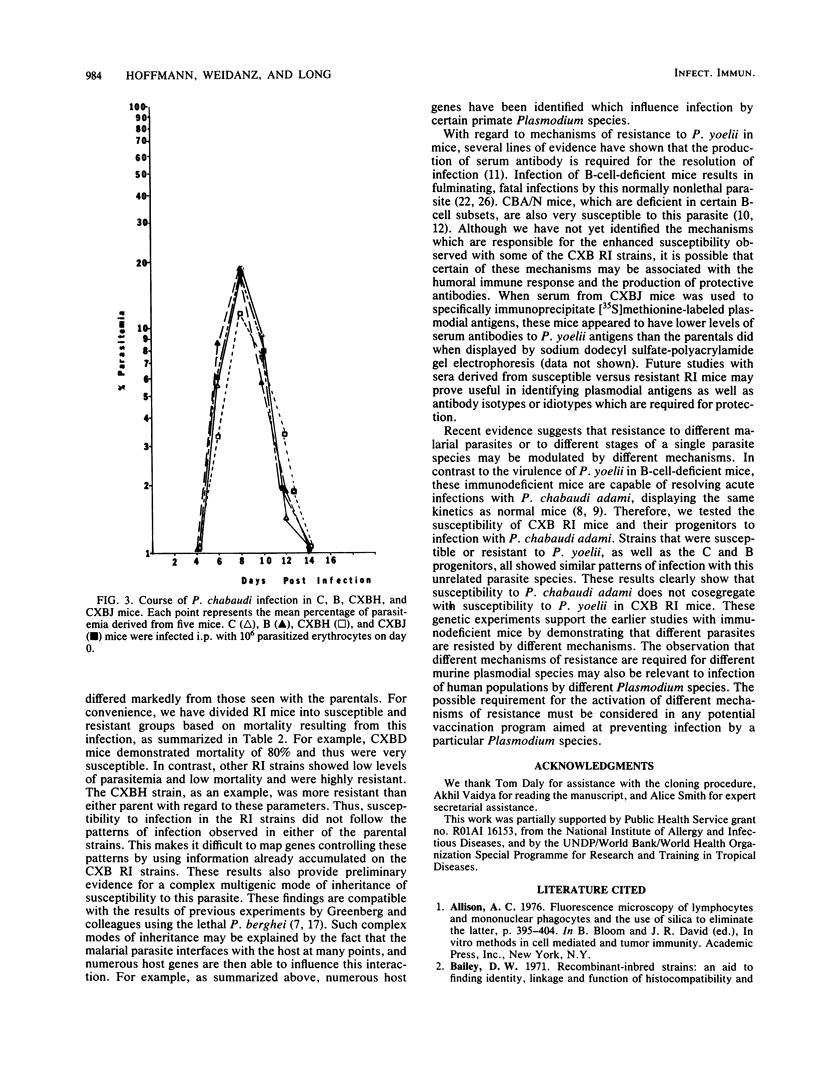
The genetic control of susceptibility to two species of murine malarial parasites was investigated with recombinant inbred (RI) mouse strains as a model system. Initially, the nonlethal Plasmodium yoelii 17X strain was cloned by limiting dilution to minimize parasite variability. This cloned P. yoelii was used to infect C57BL and BALB/c mice, strains which are the progenitors of the CXB RI strains. Since these two strains displayed consistent differences in the kinetics of parasitemia, the seven CXB RI strains were compared for their susceptibility to the same parasite. The RI strains varied considerably when infected with P. yoelii and could be divided into susceptible and resistant groups based on mortality observed with this normally mild infection. This suggests a complex, multigenic inheritance determining susceptibility to this parasite. However, when the susceptible and resistant CXB RI mice were infected with another, unrelated plasmodial species, Plasmodium chabaudi adami, all the mice showed identical patterns of disease. Since susceptibility to different murine plasmodia does not cosegregate in the CXB RI mice, different mechanisms of resistance may be required for different plasmodial species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey D. W. Recombinant-inbred strains. An aid to finding identity, linkage, and function of histocompatibility and other genes. Transplantation. 1971 Mar;11(3):325–327. doi: 10.1097/00007890-197103000-00013. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Eugui E. M., Allison A. C. Differences in susceptibility of various mouse strains to haemoprotozoan infections: possible correlation with natural killer activity. Parasite Immunol. 1980 Winter;2(4):277–292. doi: 10.1111/j.1365-3024.1980.tb00059.x. [DOI] [PubMed] [Google Scholar]
- GREENBERG J., KENDRICK L. P. Parasitemia and survival in mice infected with Plasmodium berghei; hybrids between Swiss (high parasitemia) and STR (low parasitemia) mice. J Parasitol. 1958 Oct;44(5):492–498. [PubMed] [Google Scholar]
- GREENBERG J., NADEL E. M., COATNEY G. R. Differences in survival of several inbred strains of mice and their hybrids infected with Plasmodium berghei. J Infect Dis. 1954 Jul-Aug;95(1):114–116. doi: 10.1093/infdis/95.1.114. [DOI] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Antibody-independent immunity to reinfection malaria in B-cell-deficient mice. Infect Immun. 1983 Sep;41(3):1197–1204. doi: 10.1128/iai.41.3.1197-1204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Finkelman F. D., Strickland G. T., Sayles P. C., Scher I. Defective resistance to Plasmodium yoelii in CBA/N mice. J Immunol. 1979 Jul;123(1):133–137. [PubMed] [Google Scholar]
- Jayawardena A. N., Janeway C. A., Jr, Kemp J. D. Experimental malaria in the CBA/N mouse. J Immunol. 1979 Dec;123(6):2532–2539. [PubMed] [Google Scholar]
- Kidson C., Lamont G., Saul A., Nurse G. T. Ovalocytic erythrocytes from Melanesians are resistant to invasion by malaria parasites in culture. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5829–5832. doi: 10.1073/pnas.78.9.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L. Genetics of red cells and susceptibility to malaria. Blood. 1979 Nov;54(5):961–976. [PubMed] [Google Scholar]
- Miller L. H., Haynes J. D., McAuliffe F. M., Shiroishi T., Durocher J. R., McGinniss M. H. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J Exp Med. 1977 Jul 1;146(1):277–281. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Mason S. J., Dvorak J. A., McGinniss M. H., Rothman I. K. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975 Aug 15;189(4202):561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- Nadel E M, Greenberg J, Jay G E, Coatney G R. Backcross Studies on the Genetics of Resistance to Malaria in Mice. Genetics. 1955 Sep;40(5):620–626. doi: 10.1093/genetics/40.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasvol G., Wainscoat J. S., Weatherall D. J. Erythrocytes deficiency in glycophorin resist invasion by the malarial parasite Plasmodium falciparum. Nature. 1982 May 6;297(5861):64–66. doi: 10.1038/297064a0. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Wilson R. J. The interaction of malaria parasites with red blood cells. Br Med Bull. 1982 May;38(2):133–140. doi: 10.1093/oxfordjournals.bmb.a071749. [DOI] [PubMed] [Google Scholar]
- Perkins M. Inhibitory effects of erythrocyte membrane proteins on the in vitro invasion of the human malarial parasite (Plasmodium falciparum) into its host cell. J Cell Biol. 1981 Sep;90(3):563–567. doi: 10.1083/jcb.90.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J. E., Blackwell J. M., O'Brien A. D., Bradley D. J., Glynn A. A. Are the Lsh and Ity disease resistance genes at one locus on mouse chromosome 1? Nature. 1982 Jun 10;297(5866):510–511. doi: 10.1038/297510a0. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Rank R. G., Weidanz W. P., Finerty J. F. Prevention of recrudescent malaria in nude mice by thymic grafting or by treatment with hyperimmune serum. Infect Immun. 1977 Jun;16(3):821–826. doi: 10.1128/iai.16.3.821-826.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Lyanga J. J., Skamene E. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun. 1982 Oct;38(1):80–88. doi: 10.1128/iai.38.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walliker D., Carter R., Morgan S. Genetic recombination in Plasmodium berghei. Parasitology. 1973 Apr;66(2):309–320. doi: 10.1017/s0031182000045248. [DOI] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]


