Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness and visual disability in patients aged over 60 years in Europe and North America.1 It is the third leading cause of blindness, behind cataract and glaucoma, causing 8.7% of all legal blindness across the world.2 AMD is a disease with progressive, painless loss of central vision associated with ageing. AMD is widely classified into ‘dry’ and ‘wet’ types. ‘Dry’ AMD accounts for 10% of patients with visual loss3 and can be further classified into early, intermediate and late stages characterized by the presence of hyper and/or hypo-pigmentation with drusen within the macula. Studies have shown that drusen size is an important risk factor for predicting risk of advanced AMD. Large drusen are defined as those (within 2 standard disc diameters of the centre of the macula) with (shortest) diameter greater than or equal to that of an average normal retinal vein at the disc margin, considered to be approximately one-twelfth disc diameter or approximately 125 μm, when the average disc diameter is taken as 1500 μm; intermediate drusen are those with a disc diameter greater than or equal to one-half that of large drusen (63 μm).4
Drusen are extracellular deposits that accumulate between the basal lamina of the retinal pigment epithelium (RPE) and the inner collagenous layer of Bruch's membrane in the human eye. They are typically associated with advancing age and are commonly observed in a variety of chorioretinal pathologies, including age-related macular degeneration. It is believed that local chronic inflammation through the activation of the alternative complement pathway with enucleation of drusen core. The consequent expansion affects the retinal pigment epithelium resulting in advancement from early to late stages.5 Late ‘dry’ AMD (geographical atrophy) and ‘wet’ AMD (choroidal neovascularization) are both classified as advanced AMD. Diagnosis and management of both these types of conditions differ.
Risk factors associated with AMD
Age-related macular degeneration is a complex multifactorial disease with increased age being the strongest risk factor associated with AMD.6 Other consistently associated risk factors include genetic markers7,8 and cigarette smoking.9 Women are more likely to develop AMD compared to men as are Caucasians compared to Afro-Carribeans.10 Research studies suggest a link between obesity and the progression of early and intermediate stage AMD to advanced AMD.11,12 Hypertension has also been linked to increased chances of developing AMD.13,14
Pathogenesis of AMD
Vision impairment in dry AMD occurs due to atrophic changes in the macular retinal pigment epithelium (RPE), together with degeneration of the photoreceptors resulting in central blurring of the affected eye.15 Drusen are insoluble lipid deposits that accumulate between the inner collagenous zone of Bruch's membrane and the retinal pigment epithelium, representing the hallmark of AMD.16 Cellular debris gets entrapped between Bruch's membrane and RPE provokes a local inflammatory response that activates complement and cytokine production. Proteins and lipids encapsulate this cellular debris due to this inflammatory process leading to drusen formation.17 The alternate complement pathway is thought to be associated with this inflammatory process. Possession of ‘at risk’ polymorphisms in the complement factor H genome is believed to associated with development of AMD.18,19
Drusen can be visualized by ophthalmoscopy with early AMD sufferers having either several small drusen or a few medium-sized drusen. At this stage vision may not be affected at all. Intermediate AMD sufferers have either many medium-sized drusen or one or more large drusen. There may be some blurring of vision at this stage. In addition to drusen, patients with advanced ‘dry’ AMD (late type) have a breakdown of photoreceptor cells and supporting tissue in the central retinal area therefore termed ‘geographical atrophy’. Vision can be affected in such patients, however as changes can take years to develop, these individuals learn to adapt very well and can remain asymptomatic until they develop severe visual disturbance.
‘Wet’ AMD, also known as neovascular AMD, is preceded by ‘dry’ AMD and is characterized by choroidal neovascularization (CNV). CNV is the formation of abnormal blood vessels, which grow from the choroid to develop in or under the retina. These blood vessels may bleed into the subretinal space resulting in oedema and damage.20 Vascular endothelial growth factor (VEGF) has been found to be a potent inducer of CNV.20,21 Neovascular AMD is further divided into classic, occult or mixed types, based on its appearance under fluorescein angiography. Patients with neovascular AMD usually report a sudden deterioration in vision that may be associated with distortion of the image.
How is AMD diagnosed?
Patients with early ‘dry’ AMD are usually asymptomatic which may progress to blurring of vision and loss of central vision. Patients with ‘wet’ AMD usually report a sudden deterioration in vision which may be associated with distortion of the image, due to the fluid and haemorrhage build-up in the subretinal space. This can be further tested with an Amsler grid. Patients report wavy, rather than straight lines, and missing lines on the grid. The Amsler grid can be used in monitoring progression in other retinal diseases affecting the macula that cause central scotomas and distortion. Visual acuity tested with a Snellen chart is markedly reduced.
If ‘wet’ AMD is suspected, fluorescein angiography and ocular coherence tomography (OCT) based on laser interoferometry is used to investigate and confirm this. As mentioned above, neovascular (wet) AMD can be divided into classic, occult or mixed types, based on its appearance under fluorescein angiography. On a fluorescein angiogram, classic CNV has a distinct border while occult CNV has diffuse and poorly-defined edges. The fluorescein dye fills the ‘classical’ CNV membranes in a well-defined lacy pattern while ‘occult’ lesions fill the membrane in a less well demarcated way. By identifying intraretinal, subretinal or sub-RPE fluid, OCT helps identify the type of neovascular AMD. CNV can have differing appearances on OCT.23 ‘Classic’ CNV may appear as a highly reflective fusiform thickening between the retina and the hyper-reflective external band that corresponds to the RPE/Bruch's membrane/choroid. Elevation of the RPE or irregularity of the external hyper-reflective band is more typical of ‘occult’ CNV. Interpretation of OCT images should, however, be correlated with both clinical examination and fluorescein angiographic findings. It is important to determine the type of CNV in order to tailor treatment (Figure 1).
Figure 1.
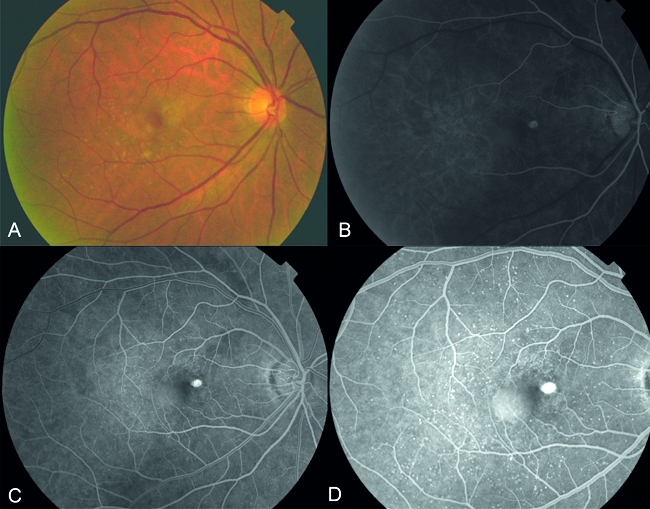
A, Fundus photograph showing focal changes in the right eye at the macula, with the presence of drusen and focal thickening of the retina; B–D showing early to late phases of fluorescein angiography demonstrating hyperfluorescence in the area of focal thickening confirming the presence of a true classic CNV membrane associated with an adjacent sub-retinal pigment epithelial detachment
Management
‘Dry’ AMD
Low vision aids are useful in treating early ‘dry’ type AMD but once early ‘dry’ AMD reaches the advanced stage, no form of treatment can prevent vision loss. Treatment and monitoring can delay and possibly prevent intermediate ‘dry’ AMD from progressing to advanced AMD. Age-Related Eye Disease Study (AREDS)22 found that taking a specific high-dose formulation of antioxidants and zinc significantly reduces the risk of advanced AMD and its associated vision loss. However use of antioxidants and zinc does not help in the primary prevention of AMD.24 Additionally the use of beta-carotene supplements is only recommended for those without any smoking history as current or former smokers on beta-carotene supplement have an increased risk of developing lung cancer.25 AREDS2, initiated in 2006, is a multicentre randomized trial designed to evaluate the effects of the oral supplementation of high dose dietary xanthophylls (lutein and zeaxanthin) that accumulate in macula and/or two omega-3 long-chain polyunsaturated fatty acids (LCPUFAs), docosahexaenoic acid and eicosapentaenoic acid for treatment of AMD and cataract. The primary objective of AREDS2 is to evaluate the effect of dietary xanthophylls (lutein/zeaxanthin) and/or omega-3 LCPUFAs (DHA and EPA) on progression to advanced AMD. This objective will be accomplished by collecting and assessing the data on approximately 4000 AREDS2 participants aged 50 to 85 years, who at the time of enrollment have either: (1) bilateral large drusen; or (2) large drusen in one eye and advanced AMD (neovascular AMD or central geographic atrophy) in the fellow eye. Enrolment has been completed in 2008 and follow-up will be between five and six years.26
‘Wet’ (neovascular) AMD
Recent advances in AMD have been related to the management of neovascular macular degeneration. Previously (before 2000) the only available treatments for neovascular macular degeneration were laser therapy and surgical procedures. Thermal laser therapy was used to photocoagulate the CNV that invariably resulted in destruction of the overlying retina while surgical techniques attempted to remove the CNV under the macula or translocate the macula to an area of the fovea without choroidal neovascularization. Results of both modes of treatment were suboptimal associated with poor visual outcomes and high recurrence rates.27,28
Since 2000, photodynamic therapy with verteporfin was being used to treat certain types of choroidal neovascular membrane. Verteporfin (injected intravenously) is a photosensitizer that is activated when exposed to low-intensity light of a specific wavelength. Activation of verteporfin induces the formation of free radicals that directly damage endothelial cells and induce secondary platelet adhesions, degranulation and thrombosis with subsequent occlusion of the abnormal vessels. This specifically destroys the choroidal neovascular membrane without damage to the overlying retina. Treatment indications were indicated specifically for actively leaking true classic (100%) and predominantly (>50%) classic membranes according to the TAP 1 and 2 studies.29,30 Severe adverse events associated with the procedure include visual disturbance and vision loss (2.6%), injection site events (13.1%), infusion-related back pain (2.4%) and photosensitivity reactions (2.4%).31,32
Results with this technique were marginally better compared to previous techniques. It moderately reduces further vision loss but only rarely improves visual acuity.29–32 Additionally the high recurrence rate of choroid neovascularization following the procedure compromises the success of the therapy.29–32
All the above-mentioned procedures were found to have poor outcomes with high recurrence rates. This lead to the rapid and widespread search for better modes of treatment. As mentioned earlier, vascular endothelial growth factor (VEGF), an angiogenic factor, has been found to be a potent inducer of choroidal neovascularization in macular degeneration.20,21 This led to the development of anti-VEGF antibody molecules that inhibit neovascularization and oedema. Several such anti-VEGF drugs are currently available for the treatment of neovascular macular degeneration.
Pegaptanib sodium (trade name: Macugen) is a highly selective anti-VEGF factor with angiogenic and antipermeability effects. It is injected into the eye, intravitreally, every 6 weeks for 2 years. It stabilizes vision in 70% of patients with neovascular AMD but only improves vision in 10% of patients.33 Bevacizumab (trade name: Avastin) is a full-length recombinant humanized monoclonal anti-VEGF antibody and binds all forms of VEGFA, unlikely pegaptanib that selectively binds to VEGF165. Bevacizumab was initially licensed for the use of metastatic colorectal cancer. However, it was successfully shown to reduce subretinal fluid and stabilize vision,34 and improve vision35 when administered intravitreally in patients with neovascular macular degeneration. This is an ‘off label’ therapy which has not been licenced by the FDA and therefore as there is no published randomized clinical trial on CNV treatment, its use in clinical practice is debatable.
Ranibizumab (trade name: Lucentis) is the antigen-binding fragment of an anti-VEGF antibody developed by the same company as bevacizumab specifically for intravitreal use. Ranibizumab is five to 10 times more potent than Bevacizumab.36 Two large prospective randomized controlled trials (MARINA and ANCHOR) investigating ranibizumab (Lucentis) showed >95% of treated eyes had stable or improved vision at 1 year compared to <65% in the control arms.37,38 Two-year results were similar. Additionally, the trials showed up to 40% of treated eyes experienced at least three lines of visual acuity improvement. At present the National Institute of Health and Clinical Excellence (NICE) has issued guidelines for treatment with ranibizumab only as pegaptanib is not recommended for the treatment of wet age-related macular degeneration. It is recommended as a possible treatment for people with wet AMD if all the following apply to their eye:
The best possible visual acuity after correction with glasses or contact lenses is between 6/12 and 6/96;
There is no permanent damage to the fovea (such as atrophy) the lesion size is less than or equal to 12 disc areas in greatest linear dimension;
There is evidence of recent presumed disease progression (blood vessel growth, as indicated by fluorescein angiography or recent visual acuity changes);
Treatment should be stopped if a person's vision gets worse and there are changes inside the eye which show that treatment isn't working.39
The Royal College of Ophthalmology has welcomed this guidance however has issued concerns that withdrawal of pegaptanib could compromise those patients allergic to ranibizumab (3%) and affect outpatient workload (monthly follow-up with Lucentis compared to six-weekly follow-up with pegaptanib).40
The risks for local ocular adverse events with injection therapies is low; patients should be consented for side-effects such as serious infection (endophthalmitis 0.9%), retinal tear and detachment, iritis and raised intra-ocular pressure.41
Anti-VEGF factors have helped preserve and even improve vision in patients with ‘wet’ type AMD. However this advance is not without its drawbacks. Anti-VEGF treatments need to be performed on a monthly basis, this increases clinic load and consequently clinic waiting times. The combined costs of these increases the overall cost of an already expensive treatment. One mode of tackling this issue is to introduce long-acting/slow-release agents/devices. The CABERNET and FOCUS studies have been initiated to investigate this.
In the CABERNET study,42 using vitrectomy, a targeted dose of strontium-90-beta radiation is directly delivered to the lesion causing vision loss (brachytherapy). The study will compare the results of beta-radiation therapy given in combination with two doses of the anti-VEGF drug, Ranibizumab (trade name: Lucentis), to the results of treatment with multiple doses of Ranibizumab alone. The FOCUS study,43 on the other hand, is investigating the safety and efficacy of Ranibizumab in combination with photodynamic therapy (PDT) with verteporfin compared to photodynamic therapy alone. Preliminary two-year results from the FOCUS study show that Ranibizumab in combination with PDT with verteporfin compared to PDT alone are better at improving visual loss. Both these studies are investigating the possibility of using lower doses of anti-VEGF to manage ‘wet’ AMD. The future management of AMD could very much be altered depending on the results of these studies.
Studying the physiological and pathological mechanisms as a whole is important for the evolution of other drugs in the field of ocular anti-angiogenesis therapy. Apart from VEGF, targeting other angiogenic pathways is important in the treatment of angiogenesis in neovascular AMD. Agents that have been identified on non-human models include protein kinase C inhibitors,44 matrix metalloproteinase inhibitors,45 squalamine46 and interferon alpha-2a.47 However further studies are required before their efficacy can be established humans.
Currently, available modes of treatment in AMD are invasive requiring injections into the vitreal space. Complications associated with this have been mentioned above. Long-term therapy for the treatment of this chronic condition will require the use of cheaper, non-invasive routes such as topical and oral preparations; and slow release preparations, e.g. implants, microspheres, nanoparticles48 to reduce complication risks of repeated injection and surgery. However, it is important to note that systemic side-effect profiles will need to be addressed for effective outcome measures.
Conclusion
AMD is the leading cause of visual loss in patients over 60 years of age. It is responsible for significant morbidity in the elderly population. There are two types of AMD: dry and wet, with further subtypes. Management of both types differ. Management of ‘dry’ AMD involves the use of low vision aids in addition to the use of vitamin supplements. Recent advances in the treatment of AMD are related to the ‘wet’ type. Use of anti-VEGF factors have helped preserve and even improve vision in patients with ‘wet’ type AMD. Current studies are investigating the use of modified combination anti-VEGF therapy regimes, perhaps the use of long-acting/slow-release agents/devices that will hopefully allow a reduction in frequency of injections. This would provide a long-term maintenance therapy that would be less invasive and could reduce costs and clinical workload, particularly as these drugs will have widespread applications in diseases such as diabetic retinopathy.49
Footnotes
DECLARATIONS —
Competing interests None declared
Funding None
Ethical approval Not applicable
Guarantor TA and NP
Contributorship TA wrote the preliminary draft of the article. NP reviewed and edited the contents of the article
Acknowledgements
None
References
- 1.Klein R, Klein BE, Jensen SC, Mares-Perlman JA, Cruickshanks KJ, Palta M. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056–65. doi: 10.1016/S0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 3.Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–2. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 4.Ferris FL, Davis MD, Clemons TE, et al. Age-Related Eye Disease Study (AREDS) Research Group. A simplified severity scale for age-related macular degeneration: AREDS Report No.18. Arch Ophthalmol. 2005;123:1570–4. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis PJ, Schultz DW, Hamon S, Ott J, Weleber RG, Klein ML. Haplotypes in the complement factor H (CFH) gene: associations with drusen and advanced age-related macular degeneration. PLoS ONE. 2007;2:e1197. doi: 10.1371/journal.pone.0001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gohel PS, Mandava N, Olson JL, Durairaj VD. Age-related macular degeneration: An update on management. Am J Med. 2008;121:279–81. doi: 10.1016/j.amjmed.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 8.Baird PN, Richardson AJ, Robman LD, et al. Apolipoprotein (APOE) gene is associated with progression of age-related macular degeneration (AMD) Hum Mutat. 2006;27:337–42. doi: 10.1002/humu.20288. [DOI] [PubMed] [Google Scholar]
- 9.Hornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: a review of association. Eye. 2005;19:935–44. doi: 10.1038/sj.eye.6701978. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 11.Seddon JM, Cote J, Davis N, et al. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003;121:785–92. doi: 10.1001/archopht.121.6.785. [DOI] [PubMed] [Google Scholar]
- 12.Schaumberg DA, Christen WG, Hunkinson SE, et al. Body mass index and the incidence of visually significant ARMD in men. Arch Ophthalmol. 2001;119:1259–65. doi: 10.1001/archopht.119.9.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wachter A, Sun Y, Dasch B, et al. Munster age and retina study (MARS): Association between risk factors for atherosclerosis and age-related macular degeneration. Ophthalmologe. 2004;101:50–3. doi: 10.1007/s00347-003-0868-1. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Tomany SC, et al. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam eye study. Ophthalmology. 2003;110:636–43. doi: 10.1016/S0161-6420(02)01448-3. [DOI] [PubMed] [Google Scholar]
- 15.Tezel TH, Bora NS, Kaplan HJ. Pathogenesis of age-related macular degeneration. Trends Mol Med. 2004;10:417–20. doi: 10.1016/j.molmed.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58:353–63. [PubMed] [Google Scholar]
- 17.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 18.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–89. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–24. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 20.Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005;40:352–68. doi: 10.1016/S0008-4182(05)80078-X. [DOI] [PubMed] [Google Scholar]
- 21.Amin R, Puklin JE, Frank RN. Growth factor localization ain choroidal neovascular membranes of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1994;35:3178–88. [PubMed] [Google Scholar]
- 22.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes EH, Khan J, Patel N, Kashani S, Chong NV. In vivo demonstration of the anatomic differences between classic and occult choroidal neovascularization using optical coherence tomography. Am J Ophthalmol. 2005;139:344–6. doi: 10.1016/j.ajo.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 24.Chong E, Wong T, Kreis A, Simpson J, Guymer R. Dietary antioxidants and primary prevention of age related macular degeneration: systematic review and meta-analysis. BMJ. 2007;335:755. doi: 10.1136/bmj.39350.500428.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans JR. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2006;2:CD000254. doi: 10.1002/14651858.CD000254.pub2. [DOI] [PubMed] [Google Scholar]
- 26. See http://www.areds2.org/
- 27.Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy: five-year results from randomized clinical trials. Arch Ophthalmol. 1991;109:1109–114. [PubMed] [Google Scholar]
- 28.Mruthyunjaya P, Stinnett SS, Toth CA. Change in visual function after macular translocation with 360 degrees ret inectomy for neovascular age-related macular degeneration. Ophthalmology. 2004;111:1715–24. doi: 10.1016/j.ophtha.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with vertiporfin: one-year results of 2 randomized clinical trials-TAP report 1. Arch Ophthalmol. 1999;117:1329–45. [PubMed] [Google Scholar]
- 30.Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-TAP report 2. Arch Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- 31.Azab M, Benchaboune M, Blinder KJ, et al. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: meta-analysis of 2-year safety results in three randomized clinical trials: Treatment Of Age-Related Macular Degeneration With Photodynamic Therapy and Verteporfin In Photodynamic Therapy Study Report no. 4. Retina. 2004;24:1–12. doi: 10.1097/00006982-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Wormald R, Evans J, Smeeth L, Henshaw K. Photodynamic therapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2007;3:CD002030. doi: 10.1002/14651858.CD002030.pub3. [DOI] [PubMed] [Google Scholar]
- 33.Gragoudas ES, Adamis AP, Cunningham ET, Jr, et al. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 34.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- 35.Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26:383–90. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- 36.Freeman WR, Falkenstein I. Avastin and new treatments for AMD: where are we? Retina. 2006;26:853–7. doi: 10.1097/01.iae.0000244722.35073.7c. [DOI] [PubMed] [Google Scholar]
- 37.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 38.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 39. See http://www.nice.org.uk/Guidance/TA155.
- 40. See http://www.rcophth.ac.uk/about/press/#NICEGuidanceWetAMD.
- 41.Jager RD, Aiello LP, Patel SC, Cunningham ET., Jr Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676–98. doi: 10.1097/00006982-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 42. See http://www.cabernetstudy.com/
- 43.Antoszyk AN, Tuomi L, Chung CY, Singh A FOCUS Study Group. Ranbizumab combined with verteporfin photodynamic therapy in neovascular age-related macular generation (FOCUS): Year 2 results. Am J Ophthalmol. 2008;145:862–74. doi: 10.1016/j.ajo.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 44.Saishin Y, Silva RL, Saishin Y, et al. Periocular injection of microspheres containing PKC412 inhibits choroidal neovascularization in a porcine model. Invest Ophthalmol Vis Sci. 2003;44:4989–93. doi: 10.1167/iovs.03-0600. [DOI] [PubMed] [Google Scholar]
- 45.Cheng L, Rivero ME, Garcia CR, et al. Evaluation of intraocular pharmacokinetics and toxicity of prinomastat (AG3340) in the rabbit. J Ocul Pharmacol Ther. 2001;17:295–304. doi: 10.1089/108076801750295326. [DOI] [PubMed] [Google Scholar]
- 46.Ciulla TA, Criswell MH, Danis RP, Williams JI, McLane MP, Holroyd KJ. Squalamine lactate reduces choroidal neovascularization in a laser-injury model in the rat. Retina. 2003;23:808–14. doi: 10.1097/00006982-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Miller JW, Stinson WG, Folkman J. Regression of experimental iris neovascularization with systemic alphainterferon. Ophthalmology. 1993;100:9–14. doi: 10.1016/s0161-6420(93)31712-4. [DOI] [PubMed] [Google Scholar]
- 48.Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2007;4:371–88. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- 49.Guidetti B, Azéma J, Malet-Martino M, Martino R. Delivery systems for the treatment of proliferative vitreoretinopathy: materials, devices and colloidal carriers. Curr Drug Deliv. 2008;5:7–19. doi: 10.2174/156720108783331050. [DOI] [PubMed] [Google Scholar]


