Abstract
The characteristics of tumor cell killing by an anti-cancer agent can determine the long-term effectiveness of the treatment. For example, if dying tumor cells release the immune modulator HMGB1 after treatment with anti-cancer drugs, they can activate a tumor-specific immune response that boosts the effectiveness of the initial treatment. Recent work from our group examined the mechanism of action of a targeted toxin called DT-EGF that selectively kills Epidermal Growth Factor Receptor-expressing tumor cells. We found that DT-EGF kills glioblastoma cells by a caspase-independent mechanism that involves high levels of autophagy, which inhibits cell death by blocking apoptosis. In contrast, DT-EGF kills epithelial tumor cells by caspase-dependent apoptosis and in these cells autophagy is not induced. These differences allowed us to discover that the different death mechanisms were associated with differences in the release of HMGB1 and that autophagy induction is required and sufficient to cause release of HMGB1 from the dying cells. These data identify a new function for autophagy during cell death and open up the possibility of manipulating autophagy during cancer treatment as a way to influence the immunogenicity of dying tumor cells.
Keywords: Autophagy, HMGB1, diphtheria toxin, glioblastoma, apoptosis
Many anti-cancer agents induce tumor cell autophagy (we use “autophagy” here to refer to macroautophagy) and there is general agreement that this autophagy is probably important. However, there is considerable disagreement about why. For example, in one recent edition of “Autophagy”, three addenda1-3 described tumor cell killing by agents that induce “autophagic cell death” (ACD), another addendum described a study where prostate cancer cells were protected from ACD,4 and two addenda discussed how drug-induced autophagy inhibits tumor cell death5,6. Therefore, autophagy is thought to promote tumor cell killing in four of these studies, and to inhibit killing in the other two. Adding to the confusion is the question of whether ACD in the sense of “death caused by autophagy” really exists7. Instead, what has been called ACD may actually be cell death with autophagy-i.e. autophagy happens while the cell dies by other means. The practical consequences of this confusion are important; for example one very basic unanswered question is whether we should aim to increase or decrease autophagy while using anti-cancer drugs8-10. An answer to this question is urgently needed because we are already trying to apply these ideas; clinical trials are recruiting patients to studies were autophagy is inhibited (e.g., with chloroquine) at the same time as treatment with anti-cancer drugs, while other studies combine drugs that increase autophagy (e.g., mTOR inhibitors) with other agents. The paper from our lab11 adds a new twist by identifying another, potentially important, characteristic of dying tumor cells that is regulated by autophagy.
The anti-cancer drug we have been studying is DT-EGF, a recombinant protein consisting of the Epidermal Growth Factor (EGF) fused to the catalytic domain of diphtheria toxin (DT). Targeted toxins take advantage of growth factor receptors to kill cancer cells via a “Trojan Horse” approach12. The targeted toxin binds to the cell surface receptor and is endocytosed along with it (hence the Trojan Horse analogy). The DT portion is subsequently released from the endosome and kills the cell by inhibiting protein synthesis. Interestingly, DT's mechanism of killing varies in different tumor cells; often caspase-dependent apoptosis is induced13 but sometimes we see caspase-independent death14.
With DT-EGF, we examined death mechanisms in different kinds of tumor cells. Epithelial cells activated caspases and died by apoptosis, however, in glioblastoma cell lines, tumor cell death occurred without caspase activation or any characteristics of apoptosis. Dying glioblastoma cells also did not show signs of membrane rupture and necrosis. However, in the glioblastoma cells (but not in the epithelial cells), DT-EGF caused high levels of autophagosome formation. Additionally using a GFP-mCherry-LC3 construct15,16 and a flux assay based on cleavage of an autophagy cargo protein17, we found that DT-EGF induces autophagy in glioblastoma cells. The obvious hypothesis was that DT-EGF induces ACD in glioblastoma cells. To test this hypothesis, we used dose response clonogenic assays, which we think are the best way to address this type of question18. If DT-EGF kills by activating ACD, more autophagy should, if anything, increase death, whereas inhibiting autophagy by siRNA knockdown of Atg genes should inhibit death. In fact, the opposite was found; increasing autophagy with trehalose reduced DT-EGF-induced death, while autophagy inhibition increased death. Therefore rather than death being due to ACD, autophagy protects the tumor cells against the drug. Furthermore, when we examined the dying cells where autophagy had been inhibited we found that DT-EGF could now activate caspases and cause apoptosis. Therefore, not only is this a case of “death with autophagy” rather than “autophagic cell death”7, the autophagy that is induced has a real effect on the drug action- it blocks DT-EGF's ability to induce apoptosis and reduces the amount of tumor cell killing.
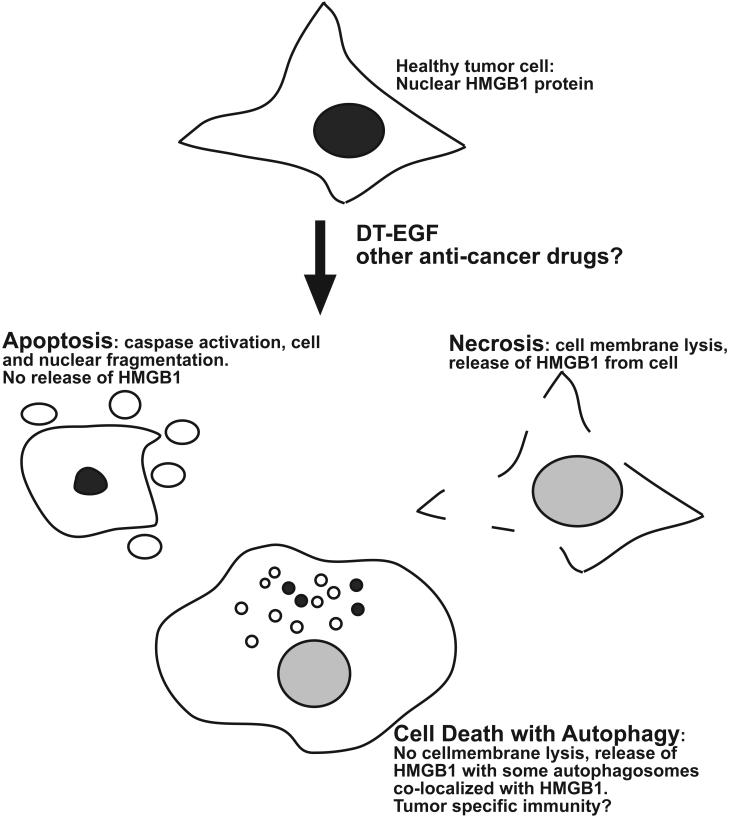
DT-EGF-treated glioblastoma cells that displayed high autophagy also released the nuclear protein HMGB1, which is one of the primary determinants of whether dying cells activate the immune system. Usually apoptotic cells do not release HMGB1 while necrotic cells do,19 and this, along with caspase-dependent oxidation of a cysteine residue on HMGB1,20 is why apoptosis is generally immunotolerant while necrosis is not. In glioblastoma cells treated with DT-EGF, HMGB1 release was abolished if autophagy was inhibited. Interestingly, release occurred without signs of necrosis. Therefore, our data indicate that autophagy allows selective release of HMGB1 rather than just induction of necrosis. We also asked if autophagy is sufficient to induce HMGB1 release. For these experiments, we used epithelial cells and found that while DT-EGF kills the cells by inducing apoptosis with little HMGB1 release, activation of autophagy before treatment with DT-EGF causes HMGB1 release. We further found that a subset of the autophagosomes co-labeled with the HMGB1 suggesting that autophagosomes themselves are involved in the selective release mechanism. These data show that autophagy is necessary and sufficient for HMGB1 release from tumor cells before their death (Figure 1).
Figure 1.
Autophagy-regulated release of HMGB1. Healthy tumor cells contain nuclear HMGB1 protein; when the cells are killed by DT-EGF, or perhaps other drugs, they can die by apoptosis, which involves caspase activation and cell fragmentation but little HMGB1 release. Or, they can die by necrosis, which involves abrupt lysis of the cell membrane and is associated with release of HMGB1. Alternatively, if autophagy is induced in the dying cells, the dying cells can selectively release HMGB1, through a mechanism that is associated with autophagosomes. Because HMGB1 release from dying tumor cells can lead to a tumor-specific immune response, autophagy manipulation may provide a way to improve cancer treatment by regulating the immunogenicity of dying tumor cells.
What might these findings mean? Recent work from the groups of Zitvogel and Kroemer21 showed that when dying tumor cells release HMGB1, this causes Toll-Like Receptor 4 (TLR4)-dependent activation of dendritic cells resulting in a tumor-specific immune response. If this happens, it leads to better treatment because tumor-specific T cells can kill cancer cells that avoided the initial drug treatment. This mechanism is important in real life because cancer patients who have a polymorphism that prevents HMGB1 activation of TLR4 obtain significantly less long-term benefit from chemotherapy. We propose that by manipulating autophagy during tumor treatment it may be possible to maximize these effects. If this idea is correct, it may allow us to enhance cancer treatment by harnessing the immune system to kill tumor cells that avoid being killed by chemotherapy22. In summary, our paper identified another function for autophagy that may have an impact upon the effectiveness of cancer therapy.
Acknowledgements
Supported by NIH grant CA11421.
Addendum to
Thorburn, J., Horita, H., Redzic, J. Hansen, K., Frankel, A.E., and Thorburn, A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death and Differentiation, in press, doi:10.1038/cdd.2008.143
References
- 1.García-Escudero V, Gargini R. Autophagy induction as an efficient strategy to eradicate tumors. Autophagy. 2008;4:923–5. doi: 10.4161/auto.6714. [DOI] [PubMed] [Google Scholar]
- 2.Lin MH, Liu SY, Liu YC. Autophagy induction by a natural ingredient of areca nut. Autophagy. 2008;4:967–8. doi: 10.4161/auto.6821. [DOI] [PubMed] [Google Scholar]
- 3.Turcotte S, Sutphin PD, Giaccia AJ. Targeted therapy for the loss of von Hippel-Lindau in renal cell carcinoma: a novel molecule that induces autophagic cell death. Autophagy. 2008;4:944–6. doi: 10.4161/auto.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roca H, Varsos ZS, Mizutani K, Pienta KJ. CCL2, survivin and autophagy: new links with implications in human cancer. Autophagy. 2008;4:969–71. doi: 10.4161/auto.6822. [DOI] [PubMed] [Google Scholar]
- 5.Hou W, Han J, Lu C, Goldstein LA, Rabinowich H. Enhancement of tumor-TRAIL susceptibility by modulation of autophagy. Autophagy. 2008;4:940–3. doi: 10.4161/auto.6769. [DOI] [PubMed] [Google Scholar]
- 6.Park MA, Zhang G, Norris J, Hylemon PB, Fisher PB, Grant S, Dent P. Regulation of autophagy by ceramide-CD95-PERK signaling. Autophagy. 2008;4:929–31. doi: 10.4161/auto.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008 doi: 10.1038/nrm2527. 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hippert MM, O'Toole PS, Thorburn A. Autophagy and cancer: good bad or both? Cancer Research. 2006;66:9349–51. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 9.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–9. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 10.Høyer-Hansen M, Jäättelä M. Autophagy: an emerging target for cancer therapy. Autophagy. 2008;4:574–80. doi: 10.4161/auto.5921. [DOI] [PubMed] [Google Scholar]
- 11.Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.143. 10.1038/cdd.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 13.Thorburn A, Thorburn J, Frankel AE. Induction of apoptosis by tumor cell-targeted toxins. Apoptosis. 2004;9:19–25. doi: 10.1023/B:APPT.0000012118.95548.88. [DOI] [PubMed] [Google Scholar]
- 14.Horita H, Frankel AE, Thorburn A. Acute myeloid leukemia-targeted toxins kill tumor cells by cell type-specific mechanisms and synergize with TRAIL to allow manipulation of the extent and mechanism of tumor cell death. Leukemia. 2008;22:652–5. doi: 10.1038/sj.leu.2404956. [DOI] [PubMed] [Google Scholar]
- 15.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–60. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 16.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 17.Mercer CA, Kaliappan A, Dennis PB. Macroautophagy-dependent, intralysosomal cleavage of a betaine homocysteine methyltransferase fusion protein requires stable multimerization. Autophagy. 2008;4:185–94. doi: 10.4161/auto.5275. [DOI] [PubMed] [Google Scholar]
- 18.Thorburn A. Studying autophagy's relationship to cell death. Autophagy. 2008;4:391–4. doi: 10.4161/auto.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 20.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 22.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]