Abstract
There is mounting evidence that neonatal animals exposed to nicotine in the prenatal period exhibit a variety of anatomic and functional abnormalities that adversely affect their respiratory and cardiovascular control systems, but how nicotine causes these developmental alterations is unknown. The principle that guides our work is that PNE impairs the ability of nicotinic acetylcholine receptors (nAChRs) to modulate the pre-synaptic release of both inhibitory (particularly GABA) and excitatory (glutamate) neurotransmitters, leading to marked alterations in the density and/or function of receptors on the (post-synaptic) membrane of respiratory neurons. Such changes could lead to impaired ventilatory responses to sensory afferent stimulation, and altered breathing patterns, including central apneic events. In this brief review we summarize the work that lead to the development of this hypothesis, and introduce some new data that support and extend it.
1. Introduction
Nicotine is a neuroteratogen that crosses the blood brain barrier and alters brain development in mammals (Slotkin, 1998). Neonatal mammals that are nicotine exposed in utero show abnormalities in central ventilatory control, such as reduced ventilatory output (Huang et al., 2004; St-John and Leiter, 1999), altered breathing pattern (Fewell et al., 2001; Hafstrom et al., 2002; Huang et al., 2004), increased apnea frequency (Fewell et al., 2001; Huang et al., 2004) and duration (Froen et al., 2002), delayed arousal in response to hypoxia (Hafstrom et al., 2000; Lewis and Bosque, 1995), decreased sensitivity to hypoxia (Bamford and Carroll, 1999; Bamford et al., 1996; Fewell et al., 2001, 2001; Froen et al., 2002; Hafstrom et al., 2005; St-John and Leiter, 1999) and diminished capacity for autoresuscitation following severe hypoxic exposure (Fewell and Smith, 1998; Froen et al., 2000), but we do not understand how prenatal nicotine exposure (PNE) causes these abnormalities. This is despite epidemiological findings showing that exposure to tobacco smoke is now the number one risk factor for the sudden infant death syndrome (SIDS), accounting for approximately one-third of all SIDS deaths (Anderson et al., 2005; Mitchell and Milerad, 2006). Here, we develop a working model that serves as a template for testing hypotheses that may lead to a deeper understanding of how PNE leads to the ventilatory abnormalities commonly observed in nicotine-exposed human neonates.
2. Prenatal nicotine exposure in animal models
The majority of the studies that we review here are based on animal models where PNE is achieved by implanting an osmotic minipump into pregnant dams on the 4th or 5th day of gestation. The pump delivers nicotine (usually as nicotine bitartrate, but see review by (Hafstrom et al., 2005) at doses that can be selected by the investigator. Typical doses are in the range of 6 mg/kg/day of nicotine bitartrate, which produces plasma levels of free nicotine of about 24 ng/ml (roughly 150 nM) in neonates and 18 ng/ml (111 nM) in pregnant dams (Chen et al., 2005). These levels are within the range (15–45 ng/ml) observed in the blood of pregnant women described as moderate smokers (Benowitz and Jacob, 1984), and in the amniotic fluid of smoke-exposed human fetuses (Luck et al., 1985). Since higher doses of nicotine are needed to elicit the same teratogenic effects in rat compared to man (Lichtensteiger et al., 1988; Slotkin, 1998), the dosing regimens typically used in rat models are reasonable, and provide a good model of the effects of nicotine exposure on fetal development and physiology in humans.
The osmotic minipump results in steady levels of plasma nicotine which has led some to suggest that continuous infusion is a poor model of nicotine exposure in human smokers, because smoke exposure is episodic. However, Benowitz and colleagues (Benowitz et al., 2002) have shown that smokers tend to maintain a constant blood level of nicotine throughout the day. In addition, humans routinely use transdermal nicotine patches, resulting in steady-state levels of the drug thus mimicking the actions of the osmotic minipump. Moreover, when nicotine is administered episodically by repeated daily injections, the physiological effects and steady-state blood levels are quite similar to those obtained with the infusion model (Slotkin, 1998, 2004).
Nicotine is one of over 3,000 chemicals found in tobacco smoke, so it is fair to ask if the nicotine infusion model is relevant for simulating the physiological effects of tobacco smoke. It has been demonstrated that exposure to tobacco smoke or nicotine infusion results in a similar up-regulation of primate nicotinic acetylcholine receptors (nAChRs) in the cortex and brainstem (Slotkin, 2004; Slotkin et al., 2002; Sugiyama et al., 1985), suggesting that nicotine is the major neuroteratogen in tobacco smoke. Moreover, daily exposure to cigarette smoke complicates interpretation of the data because it is very stressful to the pregnant dams, as indicated by marked increases in autonomic nervous system activity that is manifest as hypertension (Houdi et al., 1995), increased brainstem catecholamine release (Suemaru et al., 1992), hyperactivity (Suemaru et al., 1992) and gastric mucosal ulceration (Chow, 1997).
Another important issue is that most investigators use a 28-day osmotic pump, and since parturition in the rat occurs on gestational day 21, the pump continues to supply nicotine to the neonates through postnatal suckling. However, nicotine-induced alterations in brain development are identical in animals that are nicotine exposed both prenatally and postnatally, and animals that are nicotine-exposed prenatally, but removed from the dam following birth (Slotkin et al., 1993). Moreover, most women that smoke while pregnant will either continue to smoke after parturition, or may expose themselves and their infant to nicotine via transdermal patches or nicotine gum (DiFranza and Lew, 1995; Fingerhut et al., 1990). Thus, the continued postnatal nicotine exposure via suckling accurately simulates the effects of maternal smoking on brain development in human neonates. In conclusion, we believe that the nicotine infusion model closely simulates the adverse effects of maternal smoking on development, while at the same time avoiding some of the adverse effects associated with other methods of exposure.
3. Prenatal nicotine exposure and fast inhibitory neurotransmission
Recent studies show that PNE alters synaptic transmission in neurons that control the heart and respiratory muscles. To date, most of the studies have focused on the GABAA receptor, the glycine receptor and the nicotinic cholinergic receptors. There is substantial evidence showing that PNE leads to an up-regulation of pre-synaptic nAChRs on GABAergic (Covernton and Lester, 2002; Fisher et al., 1998; Neal et al., 2001; Neff et al., 2003; Zhu and Chiappinelli, 2002, 1999) and glycinergic (Lim et al., 2000) neurons. Excitation of these pre-synaptic receptors increases the release of GABA and glycine onto cerebellar, midbrain and brainstem neurons, with resultant changes in receptor density or function (Barazangi and Role, 2001; Luo, 2004; Magata et al., 2000; Meier et al., 1984; Slotkin et al., 2002; Zhu and Chiappinelli, 2002, 1999). Nonetheless, subsequent de-sensitization of these receptors leads to a diminished release of GABA and glycine (Aramakis et al., 2000; Covernton and Lester, 2002; Radcliffe et al., 1999). These observations, made in non-respiratory regions of the brain, suggest that the decreased release of GABA (and glycine) from GABAergic interneurons may lead to an increased expression and/or functional efficacy of GABAA receptors on the postsynaptic cell (see Section 5, below).
Recent observations demonstrating PNE-mediated changes in GABAergic control of cardiac parasympathetic neurons in the medulla of neonatal rats are consistent with the observations discussed above. Normally, the frequency of GABAergic and glycinergic synaptic events increases during the inspiratory phase in rodent inhibitory parasympathetic neurons in nucleus ambiguous (Neff et al., 2003), which leads to the rise in heart rate observed during inspiration in vivo. However, the respiratory-modulated increase in the density of GABAergic inhibitory post synaptic currents was blocked by antagonism of nicotinic acetylcholine receptors (specifically, the α4β2 subunit of the receptor), consistent with nicotinic modulation of GABAergic inputs to respiration-related neurons (see below). PNE was found to enhance the respiratory-modulated increase in GABAergic synaptic events in these cardioinhibitory neurons, suggesting that PNE may exaggerate the respiratory sinus arrhythmia (Neff et al., 2003).
Initial experiments from our own laboratory show that the slowing of respiratory rhythm by bath application of GABAA receptor agonists to the brainstem compartment of the brainstem-spinal cord preparation of neonatal rat was significantly greater in PNE animals compared to saline-exposed controls (Luo et al., 2004). These data showed that the response of GABAA receptors to an exogenous agonist was markedly enhanced, and could be due to an increased receptor density and/or an increase in the functional efficacy of the receptors on the postsynaptic membrane of respiratory neurons involved in the control of breathing frequency. More recent data show that PNE enhances glycinergic inhibition of the respiratory rhythm as well (Luo et al., 2007), suggesting an up-regulation or functional enhancement of glycine receptors. To further isolate the brainstem region responsible for these effects on the frequency of respiratory motor output, we microinjected muscimol or glycine into the preBotzinger complex, and transient apnea ensued (see Fig. 1). Importantly, the duration of the apneic period evoked by either glycine or muscimol was significantly greater in animals that were nicotine exposed in utero (Luo et al., 2007). These observations suggest that PNE enhances synaptic inhibition in brainstem neurons, including those involved in the central control of breathing frequency. These pharmacologic findings are consistent with recent immunocytochemical studies showing an up regulation of the alpha-3 subunit of the GABAA receptor in the preBotzinger complex of nicotine-exposed animals (Fregosi, 2008).
Figure 1.
This figure, taken from our recent study (Luo et al., 2007), shows that microinjection of glycine or muscimol into the preBotzinger complex causes transient apnea. Panel A shows representative recordings from saline-exposed and nicotine-exposed neonates, and demonstrates that the duration of the transient, drug-induced apnea is longer in the nicotine-exposed animals. Panel B provides average values for apnea duration induced with either drug, in saline-exposed and nicotine-exposed neonates. As discussed in the text, these effects of drug injection must be due to actions on GABAA receptors located on the post-synaptic membrane of neurons that control respiratory motor output.
4. Influence of PNE on respiratory-related nicotinic cholinergic neurotransmission
Nicotinic acetylcholine receptors (nAChRs) are common throughout the central and peripheral nervous systems, and they are all ligand-gated non-specific cation channels. In contrast to peripheral nAChRs (i.e., those in muscle) many of the nAChRs in the mammalian central nervous system are located on presynaptic terminals of GABAergic, glycinergic, glutamatergic, dopaminergic, catecholaminergic and serotonergic neurons (Barazangi and Role, 2001; Dajas-Bailador and Wonnacott, 2004; Hsieh et al., 2002; Vizi and Lendvai, 1999) (see Fig. 3). When acetylcholine (or nicotine) binds to presynaptic nAChRs the membrane potential increases, calcium channels open, and the presynaptic neuron releases neurotransmitter into the synaptic cleft. In this manner, neuronal nAChRs in the brain are well-positioned to modulate the pre-synaptic release of GABA, glycine, glutamate, serotonin, dopamine and norepinehprine (for review, see (Vizi and Lendvai, 1999; Wonnacott et al., 2005)), with complex effects on the postsynaptic neuron’s membrane potential. As a result of their modulatory role and widespread expression, a thorough understanding of neuronal nAChR function in the control of breathing has been elusive. In this section of the review, we focus on the role of nAChRs in respiratory-related neurotransmission in the brainstem with particular emphasis on the alterations in nicotinic cholinergic control that occur in response to PNE.
4.1. Central nAChRs and their role in the control of breathing
In general, central mammalian neurons express several pentameric combinations of alpha and beta nAChR subtypes made up of α2-α6 and β2-β4 subunits, and also homomeric nAChR subtypes composed of the α7-α9 subunits (Colquhoun and Patrick, 1997; Dani, 2001; Dani and Mayer, 1995; Le Novere and Changeux, 1995; Patrick et al., 1993). However, most of the nAChRs expressed in the mammalian brain are the heteromeric α4β2 subtype and homomeric α7 subtype, with the α4β2 subtype accounting for most (>90%) of the high-affinity binding sites in the brain (Brody et al., 2006; Flores et al., 1997). Similarly, in the ventrolateral medullary respiratory column, the majority of nAChRs expressed are α4β2 heteroreceptors and α7 homomeric receptors (Dehkordi et al., 2005; Quitadamo et al., 2005; Shao and Feldman, 2002). In vitro pharmacological and electrophysiological data show that the dominant nAChR subtype in preBötzinger complex inspiratory neurons is the α4β2 heteroreceptor (Hatori et al., 2006; Shao and Feldman, 2002). Genetic manipulations that mutate specific nAChR subunits also support a role for α4β2 nAChRs in breathing regulation. For example, both preBötzinger complex inspiratory neurons and hypoglossal motoneurons are hypersensitive to exogenous nicotine or acetylcholine in knock-in animals containing a point mutation in the M2 pore lining region of the α4 nAChR subunit (Shao et al., 2008). These data are consistent with earlier studies showing that the α4β2 heteroceptor could explain entirely the nicotine-induced increase in respiratory frequency recorded from hypoglossal motoneurons in a brainstem slice preparation (Shao and Feldman, 2002, 2001). Interestingly, two-day-old knockout mice lacking the β2 subunit of the nAChR had greater rates of pulmonary ventilation, a reduced ventilatory decline during acute exposure to hypoxia and a shorter arousal latency than wild-type littermates (Dauger et al., 2004). These data suggest that nicotinic receptors containing the β2 subunit normally reduce ventilatory output in freely behaving mice. However, the mutation of the β2 nAChR subunit in these animals is systemic, affecting not only all brainstem, spinal and ganglionic neurons, but also chemosensitive cells in the carotid body, so we do not know if the ventilatory effects were mediated centrally or peripherally. Moreover, based on the above discussion regarding presynaptic release of both excitatory and inhibitory neurotransmitters, it is clear that establishing the mechanisms behind phenotypic changes in freely behaving genetically altered mice is a daunting task.
Finally, although the consensus is that homomeric α7 nAChRs do not play a major role in the control of breathing, there is some evidence suggesting that this view may be overly simplistic. For example, recent studies using retrograde tracers and double receptor immunohistochemistry showed that α7 nAChRs are more common in the preBötzinger complex region than electrophysiological studies would suggest (Dehkordi et al., 2004). These investigators showed that 57% of neurokinin-1 receptor expressing neurons in the preBötzinger region also labeled for the α7 nAChR. Perhaps α7 nAChRs do not modulate drive to preBötzinger complex neurons directly, but instead modulate their discharge in complex ways that have yet to be resolved, including presynaptic modulation of neurotransmitter release.
4.2 Acute effects of nAChR stimulation by exogenous nicotine
The effects of exogenous nicotine applied to the brainstem or through the bloodstream have been studied in a variety of experimental preparations, and results confirm an overall excitation of central ventilatory output. Intravenous infusion of nicotine into anesthetized cats produces a three-fold increase in parasternal intercostal muscle activity as well as a two-fold increase in phrenic nerve activity, consistent with a global increase in respiratory drive (Kopczynska and Szereda-Przestaszewska, 1999). An increased respiratory drive following the administration of nicotine is also supported by experiments using more reduced preparations. For example, bath application of nicotine to brainstem slices enhances an excitatory, tetrodotoxin-insensitive inward current in hypoglossal motoneurons (Chamberlin et al., 2002; Robinson et al., 2002; Shao and Feldman, 2001). Similarly, nicotine increased the frequency of inspiratory bursts in brainstem/spinal cord or slice preparations from neonatal mice (Chatonnet et al., 2003) and rats (Robinson et al., 2002; Shao and Feldman, 2001, 2002). These effects of acute nicotine application were shown to be due to presynaptic mechanisms, because nicotine failed to change the current flow into voltage-clamped preBotzinger complex neurons studied during blockade of synaptic transmission with tetrodotoxin (Shao and Feldman, 2001). Thus, it is likely that the excitatory influence of nicotine on preBotzinger complex neuron depolarization (and hence the associated increase in the frequency of respiratory motor output) is due to the pre-synaptic release of glutamate from glutamatergic neurons, an effect that is likely mediated by the α4β2 nAChR subtype (see above, and Section 3.3, below).
4.3. Effects of prenatal nicotine exposure on the nicotinic control of breathing
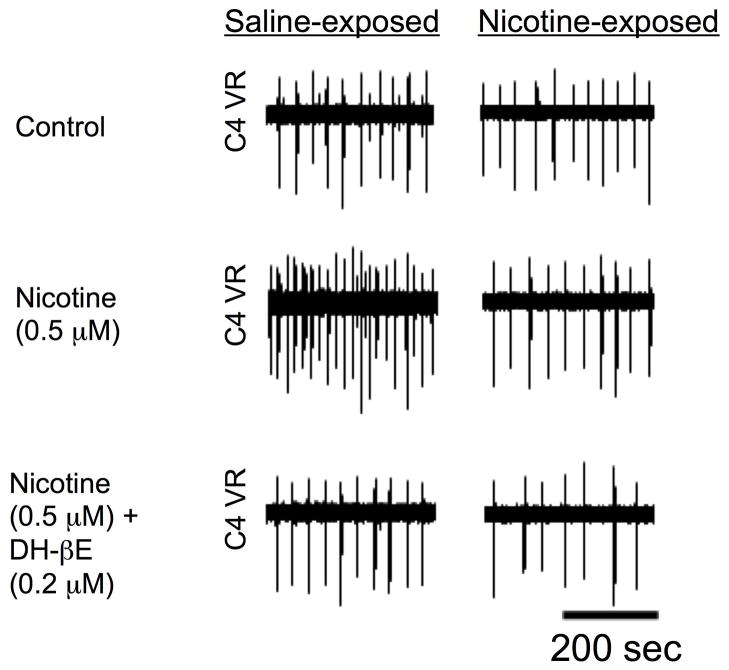
Data on the influence of chronic nicotine exposure on the density, structure and functional state of nAChRs in respiratory-related regions of the vertebrate brainstem is limited. This is despite many studies examining this topic in other brain regions and model expression systems (for review, see (Fregosi, 2008; Gentry and Lukas, 2002)). Here, we summarize new data that addresses two simple but important questions regarding the influence of PNE on nAChR-mediated control of central ventilatory output: 1) does PNE alter the respiratory motor response to exogenous nicotine? 2) which of the dominant nAChR subtypes (i.e., α4β2 and α7) is most influenced by PNE? With regard to the first question, we have shown (Fig. 2) that PNE greatly reduces the nicotine-induced increase in respiratory-related C4 ventral root nerve burst frequency in the en bloc brainstem spinal cord preparation ((Pilarski and Fregosi, 2008). Nicotine application increased the C4 ventral root nerve burst frequency by over 200% in saline-exposed control animals, but only about 150% percent in the nicotine-exposed neonates (we studied approximately 45 saline-exposed and 45 nicotine-exposed neonatal rats). These data suggest that PNE diminishes excitatory, nAChR-mediated neurotransmission in medullary regions that are involved in the control of breathing frequency, particularly the preBotzinger complex. As discussed above, nicotine likely exerts its effects by increasing the pre-synaptic release of glutamate, which brings the preBotzinger complex neuron membrane potential to firing threshold more rapidly, leading to the increase in the frequency of respiratory motor output (Shao and Feldman, 2001). Thus, our data strongly suggest that PNE is associated with a reduction in the pre-synaptic release of glutamate that normally occurs when nicotine binds to pre-synaptic nAChRs located on the terminals of glutamatergic neurons (explained in Section 5, below).
Figure 2.
Recordings from the C4 ventral nerve root (C4 VR) in a saline-exposed and a nicotine-exposed brainstem-spinal cord preparation from 2–3 day-old neonatal rat pups. Nicotine (0.5 uM nicotine bitartrate, equivalent to 175 nM free nicotine) was applied to the brainstem chamber of a split-bath configuration, leading to a sharp increase in C4 ventral nerve burst frequency in the saline-exposed, but not the nicotine-exposed, neonate. The increase in frequency with nicotine could be fully reversed with dihydro-b-erythroidine hydrobromide, which is an antagonist of the α4β2 receptor subtype, suggesting that this nAChR subtype is largely responsible for modulating respiratory frequency in this preparation. See text for details.
Additional experiments were designed to determine if α4β2 or α7 nAChR subtypes underlie the observed decrease in nicotine-mediated excitatory neurotransmission, since these are the dominant forms of the nAChR in the rat medulla. We found that the nicotine-induced excitation of respiratory-related motor output is mediated almost entirely by the α4β2 nAChR subtype in both control animals and animals exposed to nicotine in utero (Pilarski and Fregosi, 2008). Thus, as shown in Fig. 2 the increase in frequency of C4 ventral root nerve bursts evoked by bath application of 0.5 uM nicotine bitartrate (equivalent to about 175 nM free nicotine) was abolished when the nicotine was applied together with the α4β2 nAChR antagonist dihydro-beta-erythroidine. In contrast, blocking the α7 nAChR subtype had no discernible effect on the nicotine-mediated increases in C4 ventral root nerve burst frequency (Pilarski and Fregosi, 2008).
Taken together, these data suggest that the α4β2 nAChR subtype is the dominant receptor modulating nicotinic effects on breathing frequency, and that PNE induces a functional and/or molecular down-regulation of the α4β2 nAChR subtype. However, more detailed studies are needed to: 1) determine exactly how PNE alters α4β2 nAChRs (e.g., desensitization, change in receptor density, etc.); 2) to learn where the receptors are located (e.g., on presynaptic terminals or on the postsynaptic membrane); 3) to determine which population of brainstem respiratory neurons is most strongly modulated by cholinergic neurotransmission (e.g., the preBötzinger complex, the parafacial respiratory group, etc.); 4) to test whether acetylcholine exerts its effects by altering the presynaptic release of neurotransmitters.
5. Working model
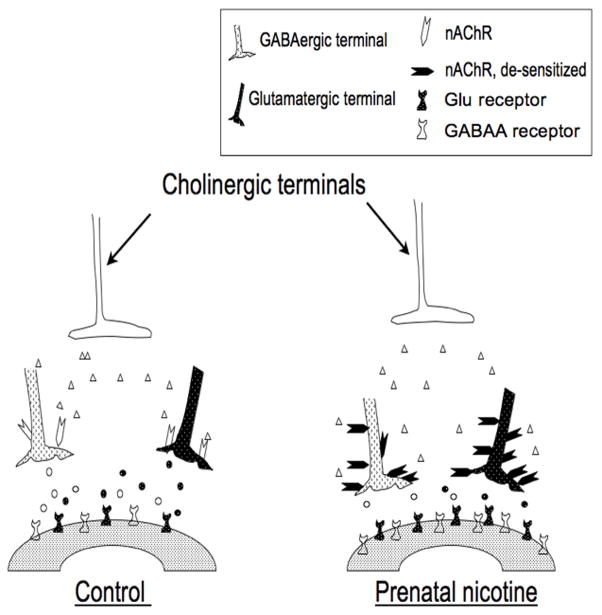
The observations discussed above are incorporated into a working model that was designed to generate testable hypotheses that will help us understand the complex functional changes in GABAergic and nicotine-mediated neurotransmission that are observed in nicotine-exposed neonatal rats (Fig. 3). Based on the above discussion, PNE is expected to induce an initial up-regulation of nAChRs primarily on the presynaptic terminals of GABAergic (and possibly glycinergic) and glutamatergic neurons (Barazangi and Role, 2001; Dajas-Bailador and Wonnacott, 2004; Hsieh et al., 2002; Vizi and Lendvai, 1999). These GABAergic and glutamatergic neurons are presynaptic to respiratory neurons in the pre-Botzinger complex region, and perhaps at other sites known to participate in ventilatory neurogenesis. Acetylcholine, released by cholinergic neurons that are found in abundance throughout the brain, binds to the nicotinic receptors, which mediate calcium influx, membrane depolarization and subsequent release of GABA and glutamate (Wonnacott, 1997). We hypothesize that the constant stimulation of the receptors by nicotine leads to their desensitization, followed by diminished presynaptic release of GABA (de Rover et al., 2004; Grilli et al., 2005; Mansvelder et al., 2002; Radcliffe et al., 1999) and glutamate (Aramakis and Metherate, 1998; Radcliffe et al., 1999). We also suggest that the prolonged reduction in GABA and glutamate release then leads to an up-regulation of GABAA and glutamate receptors on the post-synaptic neuron. Recent immunohistochemical data from our own laboratory shows that PNE increases the density of GABAA receptors in the preBotzinger complex (Fregosi, 2008). This apparent up-regulation of GABAA receptors likely explains why agonists applied to nicotine-exposed brains in vitro evokes enhanced functional responses (Luo et al., 2004; Luo et al., 2007). Although we do not know if glutamate receptors (nor which type of glutamate receptor) are also up-regulated in the preBotzinger complex and associated regions, there is evidence showing that chronic nicotine exposure up-regulates glutamate receptor subunits in the prefrontal cortex, the ventral tegmental area and the auditory forebrain of adult rats (Hsieh et al., 2002; Wang et al., 2007). As discussed above, the excitatory effects of nicotine on respiratory rhythm are the result of pre-synaptic glutamate release mediated by nicotine binding to nAChRs located on the pre-synaptic terminals of glutamatergic neurons (Shao and Feldman, 2001). The desensitization of nicotinic receptors would be expected to lead to a decreased release of glutamate, with subsequent up-regulation of glutamate receptors on the postsynaptic neuron. Thus, one would predict that nicotine-exposed neonates would have an exaggerated ventilatory response to exogenous glutamate, although this hypothesis has yet to be tested.
Figure 3.
Schematic diagram summarizing putative physiologic and anatomic changes that occur secondary to the influence of prenatal nicotine exposure on inhibitory synaptic transmission in brainstem respiratory neurons. Prenatal nicotine exposure results in an up-regulation of nicotinic acetylcholine receptors that are located presynaptically on both GABAergic and glutamatergic neurons. However, the nicotinic receptors are subsequently desensitized leading to a diminution of GABA and glutamate release. The reduction in GABA and glutamate release leads to an up-regulation of GABAA and glutamate receptors (probably both NMDA and AMPA subtypes) on the postsynaptic neuron. As a result, any endogenous stressor associated with an increase in GABA or glutamate release (e.g., hypoxia) would be associated with an exaggerated post-synaptic response (see text for detailed explanation). This model is an adaptation and extension of a similar one published by Luo et al. (Luo et al., 2007).
6. Conclusions
Since PNE alters both inhibitory and excitatory neurotransmission in the brain, understanding the quantitative relation between chronic-nicotine induced alterations in GABAergic and glutamatergic neurotransmission in respiratory neurons is essential. For example, if up-regulation of both inhibitory and excitatory pathways were identical, one could argue that there would be no net change in ventilatory output. However, the observed abnormalities in cardiorespiratory control consequent to PNE suggest that the balance is tilted towards net inhibition. This is consistent with direct evidence that chronic nicotine exposure induces greater changes in GABAergic than glutamatergic neurotransmission in mesolimbic reward regions of the rodent brain (Mansvelder et al., 2002). This idea is also consistent with recent data showing that behavioral deficits induced by other drugs, including ethanol, inhaled toxins and volatile anesthetics are the result of enhanced glycinergic and GABAergic neurotransmission and reduced glutamatergic transmission (Beckstead et al., 2000; Downie et al., 1996; Franks and Lieb, 1994; Lovinger, 1997; Mihic, 1999). We believe that this general model may also play a role in the ventilatory abnormalities commonly observed in neonatal animals and humans following prenatal nicotine exposure.
Acknowledgments
We wish to acknowledge the American Heart Association for supporting our research, and Ms. Katherine Promer for assistance with data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson ME, Johnson DC, Batal HA. Sudden Infant Death Syndrome and prenatal maternal smoking: rising attributed risk in the Back to Sleep era. BMC Med. 2005;3:4. doi: 10.1186/1741-7015-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramakis VB, Hsieh CY, Leslie FM, Metherate R. A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20:6106–16. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–95. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford OS, Carroll JL. Dynamic ventilatory responses in rats: normal development and effects of prenatal nicotine exposure. Respir Physiol. 1999;117:29–40. doi: 10.1016/s0034-5687(99)00054-7. [DOI] [PubMed] [Google Scholar]
- Bamford OS, Schuen JN, Carroll JL. Effect of nicotine exposure on postnatal ventilatory responses to hypoxia and hypercapnia. Respir Physiol. 1996;106:1–11. doi: 10.1016/0034-5687(96)00051-5. [DOI] [PubMed] [Google Scholar]
- Barazangi N, Role LW. Nicotine-induced enhancement of glutamatergic and GABAergic synaptic transmission in the mouse amygdala. J Neurophysiol. 2001;86:463–74. doi: 10.1152/jn.2001.86.1.463. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Weiner JL, Eger EI, 2nd, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57:1199–205. [PubMed] [Google Scholar]
- Benowitz NL, Hansson A, Jacob P., 3rd Cardiovascular effects of nasal and transdermal nicotine and cigarette smoking. Hypertension. 2002;39:1107–12. doi: 10.1161/01.hyp.0000018825.76673.ea. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–15. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Bocchiaro CM, Greene RW, Feldman JL. Nicotinic excitation of rat hypoglossal motoneurons. Neuroscience. 2002;115:861–70. doi: 10.1016/s0306-4522(02)00454-2. [DOI] [PubMed] [Google Scholar]
- Chatonnet F, Boudinot E, Chatonnet A, Taysse L, Daulon S, Champagnat J, Foutz AS. Respiratory survival mechanisms in acetylcholinesterase knockout mouse. Eur J Neurosci. 2003;18:1419–27. doi: 10.1046/j.1460-9568.2003.02867.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Parker SL, Matta SG, Sharp BM. Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. Eur J Neurosci. 2005;22:380–8. doi: 10.1111/j.1460-9568.2005.04229.x. [DOI] [PubMed] [Google Scholar]
- Chow J, Ma L, Zhu M, Cho CH. The potentiating actions of cigarette smoking on ethanol-induced gastric mucosal damage in rats. Gastroenterology. 1997;113:1188–1197. doi: 10.1053/gast.1997.v113.pm9322514. [DOI] [PubMed] [Google Scholar]
- Colquhoun LM, Patrick JW. Alpha3, beta2, and beta4 form heterotrimeric neuronal nicotinic acetylcholine receptors in Xenopus oocytes. J Neurochem. 1997;69:2355–62. [PubMed] [Google Scholar]
- Covernton PO, Lester RA. Prolonged stimulation of presynaptic nicotinic acetylcholine receptors in the rat interpeduncular nucleus has differential effects on transmitter release. Int J Dev Neurosci. 2002;20:247–58. doi: 10.1016/s0736-5748(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–24. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry. 2001;49:166–74. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- Dani JA, Mayer ML. Structure and function of glutamate and nicotinic acetylcholine receptors. Curr Opin Neurobiol. 1995;5:310–7. doi: 10.1016/0959-4388(95)80043-3. [DOI] [PubMed] [Google Scholar]
- Dauger S, Durand E, Cohen G, Lagercrantz H, Changeux JP, Gaultier C, Gallego J. Control of breathing in newborn mice lacking the beta-2 nAChR subunit. Acta Physiol Scand. 2004;182:205–12. doi: 10.1111/j.1365-201X.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- de Rover M, Mansvelder HD, Lodder JC, Wardeh G, Schoffelmeer AN, Brussaard AB. Long-lasting nicotinic modulation of GABAergic synaptic transmission in the rat nucleus accumbens associated with behavioural sensitization to amphetamine. Eur J Neurosci. 2004;19:2859–70. doi: 10.1111/j.0953-816X.2004.03370.x. [DOI] [PubMed] [Google Scholar]
- Dehkordi O, Haxhiu MA, Millis RM, Dennis GC, Kc P, Jafri A, Khajavi M, Trouth CO, Zaidi SI. Expression of alpha-7 nAChRs on spinal cord-brainstem neurons controlling inspiratory drive to the diaphragm. Respir Physiol Neurobiol. 2004;141:21–34. doi: 10.1016/j.resp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Dehkordi O, Millis RM, Dennis GC, Coleman BR, Johnson SM, Changizi L, Ovid Trouth C. Alpha-7 and alpha-4 nicotinic receptor subunit immunoreactivity in genioglossus muscle motoneurons. Respir Physiol Neurobiol. 2005;145:153–61. doi: 10.1016/j.resp.2004.09.003. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract. 1995;40:385–94. [PubMed] [Google Scholar]
- Downie DL, Hall AC, Lieb WR, Franks NP. Effects of inhalational general anaesthetics on native glycine receptors in rat medullary neurones and recombinant glycine receptors in Xenopus oocytes. Br J Pharmacol. 1996;118:493–502. doi: 10.1111/j.1476-5381.1996.tb15430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JE, Smith FG. Perinatal nicotine exposure impairs ability of newborn rats to autoresuscitate from apnea during hypoxia. J Appl Physiol. 1998;85:2066–74. doi: 10.1152/jappl.1998.85.6.2066. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, Ng VK. Prenatal exposure to nicotine impairs protective responses of rat pups to hypoxia in an age-dependent manner. Respir Physiol. 2001;127:61–73. doi: 10.1016/s0034-5687(01)00232-8. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, Ng VK. Threshold levels of maternal nicotine impairing protective responses of newborn rats to intermittent hypoxia. J Appl Physiol. 2001;90:1968–76. doi: 10.1152/jappl.2001.90.5.1968. [DOI] [PubMed] [Google Scholar]
- Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. Am J Public Health. 1990;80:541–4. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Pidoplichko VI, Dani JA. Nicotine modifies the activity of ventral tegmental area dopaminergic neurons and hippocampal GABAergic neurons. J Physiol Paris. 1998;92:209–13. doi: 10.1016/s0928-4257(98)80012-0. [DOI] [PubMed] [Google Scholar]
- Flores CM, Davila-Garcia MI, Ulrich YM, Kellar KJ. Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. J Neurochem. 1997;69:2216–9. doi: 10.1046/j.1471-4159.1997.69052216.x. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–14. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Fregosi R. Influence of prenatal nicotine exposure on development of neurotransmission in central respiratory neurons. In: Marcus CL, Carroll JL, Donnelly DF, Loughlin GM, editors. Sleep and Breathing in Children. 2. Informa Health care; New York: 2008. pp. 341–362. [Google Scholar]
- Froen JF, Akre H, Stray-Pedersen B, Saugstad OD. Adverse effects of nicotine and interleukin-1beta on autoresuscitation after apnea in piglets: implications for sudden infant death syndrome. Pediatrics. 2000;105:E52. doi: 10.1542/peds.105.4.e52. [DOI] [PubMed] [Google Scholar]
- Froen JF, Akre H, Stray-Pedersen B, Saugstad OD. Prolonged apneas and hypoxia mediated by nicotine and endotoxin in piglets. Biol Neonate. 2002;81:119–25. doi: 10.1159/000047196. [DOI] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord. 2002;1:359–85. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- Grilli M, Parodi M, Raiteri M, Marchi M. Chronic nicotine differentially affects the function of nicotinic receptor subtypes regulating neurotransmitter release. J Neurochem. 2005;93:1353–60. doi: 10.1111/j.1471-4159.2005.03126.x. [DOI] [PubMed] [Google Scholar]
- Hafstrom O, Milerad J, Asokan N, Poole SD, Sundell HW. Nicotine delays arousal during hypoxemia in lambs. Pediatr Res. 2000;47:646–52. doi: 10.1203/00006450-200005000-00015. [DOI] [PubMed] [Google Scholar]
- Hafstrom O, Milerad J, Sandberg KL, Sundell HW. Cardiorespiratory effects of nicotine exposure during development. Respir Physiol Neurobiol. 2005;149:325–41. doi: 10.1016/j.resp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hafstrom O, Milerad J, Sundell HW. Altered breathing pattern after prenatal nicotine exposure in the young lamb. Am J Respir Crit Care Med. 2002;166:92–7. doi: 10.1164/rccm.2107082. [DOI] [PubMed] [Google Scholar]
- Hatori E, Sakuraba S, Kashiwagi M, Kuribayashi J, Tsujita M, Hosokawa Y, Takeda J, Kuwana S. Association of nicotinic acetylcholine receptors with central respiratory control in isolated brainstem-spinal cord preparation of neonatal rats. Biol Res. 2006;39:321–30. doi: 10.4067/s0716-97602006000200014. [DOI] [PubMed] [Google Scholar]
- Houdi AA, Dowell RT, Diana JN. Cardiovascular responses to cigarette smoke exposure in restrained conscious rats. J Pharmacol Exp Ther. 1995;275:646–53. [PubMed] [Google Scholar]
- Hsieh CY, Leslie FM, Metherate R. Nicotine exposure during a postnatal critical period alters NR2A and NR2B mRNA expression in rat auditory forebrain. Brain Res Dev Brain Res. 2002;133:19–25. doi: 10.1016/s0165-3806(01)00314-5. [DOI] [PubMed] [Google Scholar]
- Huang YH, Brown AR, Costy-Bennett S, Luo Z, Fregosi RF. Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respir Physiol Neurobiol. 2004;143:1–8. doi: 10.1016/j.resp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kopczynska B, Szereda-Przestaszewska M. Response of respiratory muscles to intravenous nicotine challenge in anaesthetized cats. Respir Physiol. 1999;116:145–57. doi: 10.1016/s0034-5687(99)00049-3. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Changeux JP. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J Mol Evol. 1995;40:155–72. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- Lewis KW, Bosque EM. Deficient hypoxia awakening response in infants of smoking mothers: possible relationship to sudden infant death syndrome. J Pediatr. 1995;127:691–9. doi: 10.1016/s0022-3476(95)70155-9. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR. Prenatal adverse effects of nicotine on the developing brain. Prog Brain Res. 1988;73:137–57. doi: 10.1016/S0079-6123(08)60502-6. [DOI] [PubMed] [Google Scholar]
- Lim DK, Park SH, Choi WJ. Subacute nicotine exposure in cultured cerebellar cells increased the release and uptake of glutamate. Arch Pharm Res. 2000;23:488–94. doi: 10.1007/BF02976578. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Alcohols and neurotransmitter gated ion channels: past, present and future. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:267–82. doi: 10.1007/pl00005051. [DOI] [PubMed] [Google Scholar]
- Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8:384–95. doi: 10.1159/000457063. [DOI] [PubMed] [Google Scholar]
- Luo Z, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure increases the strength of GABA(A) receptor-mediated inhibition of respiratory rhythm in neonatal rats. J Physiol. 2004;561:387–93. doi: 10.1113/jphysiol.2004.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, McMullen NT, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure alters glycinergic and GABAergic control of respiratory frequency in the neonatal rat brainstem-spinal cord preparation. Respir Physiol Neurobiol. 2007;157:226–34. doi: 10.1016/j.resp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Luo Z, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure increases the strength of GABAA receptor-mediated inhibition of respiratory rhythm in neonates. J Physiol. 2004 doi: 10.1113/jphysiol.2004.062927. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magata Y, Kitano H, Shiozaki T, Iida Y, Nishizawa S, Saji H, Konishi J. Effect of chronic (−)-nicotine treatment on rat cerebral benzodiazepine receptors. Nucl Med Biol. 2000;27:57–60. doi: 10.1016/s0969-8051(99)00078-5. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–19. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Meier E, Drejer J, Schousboe A. GABA induces functionally active low-affinity GABA receptors on cultured cerebellar granule cells. J Neurochem. 1984;43:1737–44. doi: 10.1111/j.1471-4159.1984.tb06102.x. [DOI] [PubMed] [Google Scholar]
- Mihic SJ. Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int. 1999;35:115–23. doi: 10.1016/s0197-0186(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Milerad J. Smoking and the sudden infant death syndrome. Rev Environ Health. 2006;21:81–103. doi: 10.1515/reveh.2006.21.2.81. [DOI] [PubMed] [Google Scholar]
- Neal MJ, Cunningham JR, Matthews KL. Activation of nicotinic receptors on GABAergic amacrine cells in the rabbit retina indirectly stimulates dopamine release. Vis Neurosci. 2001;18:55–64. doi: 10.1017/s0952523801181058. [DOI] [PubMed] [Google Scholar]
- Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res. 2003;93:565–72. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- Patrick J, Sequela P, Vernino S, Amador M, Luetje C, Dani JA. Functional diversity of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1993;98:113–20. doi: 10.1016/s0079-6123(08)62387-0. [DOI] [PubMed] [Google Scholar]
- Pilarski J, Fregosi R. Influence of prenatal nicotine exposure on in vitro central respiratory-related cholinergic neurotransmission. Faseb J. 2008;22:954.10. [Google Scholar]
- Quitadamo C, Fabbretti E, Lamanauskas N, Nistri A. Activation and desensitization of neuronal nicotinic receptors modulate glutamatergic transmission on neonatal rat hypoglossal motoneurons. Eur J Neurosci. 2005;22:2723–34. doi: 10.1111/j.1460-9568.2005.04460.x. [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Fisher JL, Gray R, Dani JA. Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Ann N Y Acad Sci. 1999;868:591–610. doi: 10.1111/j.1749-6632.1999.tb11332.x. [DOI] [PubMed] [Google Scholar]
- Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, Funk GD. Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J Physiol. 2002;538:957–73. doi: 10.1113/jphysiol.2001.012705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Mechanisms underlying regulation of respiratory pattern by nicotine in preBotzinger complex. J Neurophysiol. 2001;85:2461–7. doi: 10.1152/jn.2001.85.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Pharmacology of nicotinic receptors in preBotzinger complex that mediate modulation of respiratory pattern. J Neurophysiol. 2002;88:1851–8. doi: 10.1152/jn.2002.88.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Tan W, Xiu J, Puskar N, Fonck C, Lester HA, Feldman JL. Alpha4* nicotinic receptors in preBotzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J Neurosci. 2008;28:519–28. doi: 10.1523/JNEUROSCI.3666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–45. [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–51. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Lappi SE, Seidler FJ. Impact of fetal nicotine exposure on development of rat brain regions: critical sensitive periods or effects of withdrawal? Brain Res Bull. 1993;31:319–28. doi: 10.1016/0361-9230(93)90224-y. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Auman JT, Qiao D, Seidler FJ. Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain. Brain Res Dev Brain Res. 2002;133:175–9. doi: 10.1016/s0165-3806(02)00281-x. [DOI] [PubMed] [Google Scholar]
- St-John WM, Leiter JC. Maternal nicotine depresses eupneic ventilation of neonatal rats. Neurosci Lett. 1999;267:206–8. doi: 10.1016/s0304-3940(99)00364-x. [DOI] [PubMed] [Google Scholar]
- Suemaru K, Oishi R, Gomita Y, Saeki K, Araki Y. Effect of long-term cigarette smoke exposure on locomotor activity and brain monoamine levels in rats. Pharmacol Biochem Behav. 1992;41:655–8. doi: 10.1016/0091-3057(92)90388-v. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Hagino N, Moore G, Lee JW. [3H]Nicotine binding sites in developing fetal brains in rats. Neurosci Res. 1985;2:387–92. doi: 10.1016/0168-0102(85)90048-3. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Lendvai B. Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res Brain Res Rev. 1999;30:219–35. doi: 10.1016/s0165-0173(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen H, Steketee JD, Sharp BM. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology. 2007;32:103–9. doi: 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–8. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–9. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Chiappinelli VA. Nicotine modulates evoked GABAergic transmission in the brain. J Neurophysiol. 1999;82:3041–5. doi: 10.1152/jn.1999.82.6.3041. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Chiappinelli VA. Nicotinic receptors mediate increased GABA release in brain through a tetrodotoxin-insensitive mechanism during prolonged exposure to nicotine. Neuroscience. 2002;115:137–44. doi: 10.1016/s0306-4522(02)00371-8. [DOI] [PubMed] [Google Scholar]