Abstract
GU-rich elements found in pre-mRNA and mRNA transcripts play diverse roles in the control of gene expression by regulating mRNA stability, translation and pre-mRNA processing. Regulatory GU-rich elements are highly conserved throughout evolution, and play major roles in development in diverse species from worms to mammals. The conservation of the GU-rich element allowed it to be identified as a sequence that was enriched in the 3′ UTR of human transcripts that exhibited rapid mRNA decay. This element functions, at least in part, as a molecular target for members of the CELF family of RNA-binding proteins, which recruit other components of the cellular posttranscriptional gene regulatory machinery to the transcript. Depending on the context, binding to GU-rich sequences by CELF proteins direct a variety of posttranscriptional regulatory events, including deadenylation, mRNA decay, translation or pre-mRNA processing. Thus, GU-rich elements and CELF proteins serve multiple functions in gene expression regulation and define an important evolutionarily conserved posttranscriptional regulatory network.
Keywords: conserved sequence elements, GU-rich element, CUGBP1, RNA-binding proteins, EDEN, EDEN-binding protein, CELF, mRNA decay, mRNA stability, deadenylation
In order to assure the precise utilization of genetic information, gene expression is regulated at the level of transcription as well as multiple post-transcriptional levels, including splicing, transport, localization, mRNA stability and translation.1-7 During evolution, the cellular machinery developed precise ways to discriminate one mRNA from another, so that each transcript can be stored, modified, translated or destroyed, depending on the need for the mRNA or encoded protein by the cell. Two major components of the machinery to discriminate between transcripts have been defined: cis-acting regulatory sequence elements and trans-acting factors. Cis-acting regulatory sequence elements are found in coding and regions in 3′ and 5′ untranslated regions of mRNAs. Transcript-specific sequence variation in any of those regions may lead to differences in mRNA fate. For most mRNAs, however, cis-regulatory motifs or trans-acting factors responsible for post-transcriptional RNA regulation have not been characterized.
Several studies demonstrated that specific sequences in the 3′ UTR or 5′ UTR of certain transcripts regulate mRNA stability and/or translation control, and thereby, play a key role in development, growth and cellular activation (reviewed in refs. 8-13). The best-characterized example of an mRNA sequence that controls post-transcriptional gene expression is the AU-rich element (ARE). The ARE was identified as a conserved sequence found in the 3′ UTR of numerous mammalian transcripts that exhibited rapid mRNA decay, including cytokine transcripts and proto-oncogene transcripts. ARE-containing mRNAs are regulated at multiple steps in addition to mRNA stability, including splicing,14 nucleo-cytoplasmic export15 and translation.16 Although considerable insight has been provided from studies that focus on the ARE, it should be noted that most transcripts that are regulated at posttranscriptional levels do not contain AREs or other known sequence or structural elements (reviewed in ref. 17). Thus, the mechanisms that orchestrate post-transcriptional gene regulation are poorly understood. In this “Point of View” article, we describe regulation of human mRNA decay by GU-rich elements (GREs) and CUG-binding protein 1 (CUGBP1) as an example of a posttranscriptional regulatory network that has been conserved throughout evolution.
CELF Proteins and GU-rich Sequences
The CELF (CUGBP and embryonically lethal abnormal vision-type RNA binding protein 3-like factors) protein family is an evolutionarily conserved family of RNA-binding proteins that plays essential roles in post-transcriptional gene regulation. Six members of the CELF family have been identified in humans and mice:18,19 CUGBP1 (CELF 1) and CUGBP2 (CELF 2) proteins are expressed ubiquitously and play vital roles in embryogenesis,20,21 whereas CELF proteins 3−6 are restricted to adult tissues and found almost exclusively in the nervous system.22-24 Human CUGBP1 and its homologs in chickens, Zebrafish, Xenopus, Drosophila and C. elegans have been known for many years to regulate gene expression at posttranscriptional levels and to control important developmental processes.25-29 CELF proteins from diverse species bind to RNA preferentially at GU-rich sequences25,30-37 and regulate posttranscriptional processes, including mRNA splicing,24,38-44 translation,39,45-54 deadenylation55-57 and mRNA degradation.35 In Xenopus embryos, the CELF protein, EDEN-BP binds to the GU-rich EDEN RNA element after fertilization and promotes the deadenylation and translational activation of target transcripts involved in development.25,30,58-61 The recent findings that the EDEN-BP homolog in humans, CUGBP1, binds to GU-rich RNA sequences32,34,35,62,63 and regulates transcript deadenylation64,65 and degradation35 suggests that CELF proteins and GU-rich sequences represent an evolutionarily conserved pathway for posttranscriptional gene regulation.
In the following sections, we review how genome-wide measurements of mRNA decay were used to identify the GU-rich element (GRE) as a conserved sequence that was enriched in short-lived human transcripts and functions to mediate human mRNA decay by binding to CUGBP1. We describe the functions of the GRE and CUGBP1 from an evolutionary point of view based on the previously characterized functions of CELF proteins and GU-rich sequences in diverse species. CELF proteins are important across evolution as posttranscriptional regulators, and as discussed here, the human CELF protein, CUGBP1, provides a prime example of how a genome-wide approach was used to elucidate the targets and function of a RNA-binding protein, thereby defining post-transcriptional RNA regulatory networks.
The GRE is a Regulator of Human mRNA Decay
In the pre-microarray era, only a small number of conserved sequences and structural elements in mRNA were defined as binding sites for specific RNA-binding proteins that regulate posttranscriptional mRNA expression. More recently, microarray-based studies that evaluated mRNA decay rates on a global basis in mammalian cells have provided valuable information about the role of post-transcriptional regulation of a wide variety of transcripts that have important physiological functions66-69 For example, although the ARE sequence was discovered before microarrays were developed, gene microarray technology allowed a more systematic and complete classification of ARE-containing genes. The construction of the ARED database,70-72 and examination of the mRNA decay rates of ARE-containing transcripts using microarray technology, enabled biologists to globally assess the physiological significance of ARE-mediated RNA regulation and to identify coordinate gene expression networks regulated by AREs.73 Current technology, including sequence motif discovery methods in conjunction with gene expression clustering, can be used to identify novel cis-regulatory elements in regulatory networks.
Our research group used oligonucleotide microarrays to measure mRNA decay on a global basis in resting and T cell receptor-activated primary human T cells after arresting transcription with actinomycin D.66 We identified hundreds of short-lived transcripts as well as hundreds of transcripts that exhibited stimulus-dependent changes in mRNA decay rates. Interestingly, most of the T cell transcripts that we found to be regulated at the level of mRNA decay did not contain AREs or other known sequence elements that regulate mRNA decay. We used computational methods to search for novel conserved putative cis-acting regulatory elements in transcripts that were regulated at the level of mRNA decay.35 Three computation-based sequence analysis algorithms were used to search for conserved sequence elements in the 3′ UTR of activation-induced transcripts that exhibited rapid mRNA decay: the Gibbs sampler algorithm,74 the MEME algorithm,75 and a clustering algorithm.35 The 11-mer sequence UGUUUGUUUGU, which we have termed a GU-rich element (GRE) was identified as conserved in the 3′ UTR of short-lived transcripts by all three methods, and therefore this sequence was analyzed in more detail. We compared the abundance of the GRE in the 3′ UTR in the set of transcripts that were short-lived to the set of transcripts that were long-lived, and we found that this sequence motif was conserved and highly enriched in the 3′ UTR of short-lived T cell transcripts. Based on these results, we hypothesized that the GRE could be a regulator of mRNA decay. To test this hypothesis, we inserted GRE-containing sequences from short-lived transcripts into the 3′ UTR of a beta-globin reporter transcript. We found that insertion of GRE-containing sequences but not mutated control sequences into the beta-globin 3′ UTR caused the otherwise stable beta-globin transcript to become unstable.35 Thus, we identified the GRE as a functional regulator of mRNA decay based on computational sequence analysis of short-lived transcripts that we identified using microarrays.
Human CUGBP1 is a Regulator of mRNA Decay
Our finding that GREs function as mediators of mRNA decay in human cells led us to search for proteins that bound to GRE sequences. Using a gel shift assay, we identified a protein present in cytoplasmic extracts from human T cells that bound specifically to GRE-containing ribo-oligonucleotides. We screened a small panel of antibodies against RNA-binding proteins known to bind to U-rich or GU-rich sequences and found that an antibody against CUG-binding protein 1 (CUGBP1, also called CELF1, BRUNOL2) specifically super-shifted this GRE-binding protein.35 Furthermore, we found that an antibody against CUGBP1 specifically co-immunoprecipitated beta-globin mRNA reporter transcripts that contained inserted GRE sequences but not mutated GRE sequences in cytoplasmic lysates from HeLa cells. Also, the anti-CUGBP1 antibody co-immunoprecipitated endogenous GRE-containing transcripts expressed in HeLa cells, including c-jun and jun B transcripts, presumably through their direct binding to CUGBP1. Thus, CUGBP1 appeared to be a specific GRE-binding protein that interacted with GRE-containing mRNA transcripts within human cells. This result is consistent with other results suggesting that CUGBP1 has preference for binding to GU-rich sequences.32,34,63
CUGBP1, a member of the CELF family of RNA-binding proteins, was first described as a protein that bound to the abnormally extended CUG mRNA repeats that occur in patients with type I myotonic dystrophy76,77 and has since been implicated as a regulator of alternative splicing,38-40 translation45,48 and dead-enylation.64,65 Because CUGBP1 regulates deadenylation,64,65 the first step in mammalian mRNA decay, we performed experiments to determine if CUGBP1 is a regulator of GRE-mediated mRNA decay. We performed siRNA-mediated knockdown of CUGBP1 in HeLa cells and found that GRE-containing beta-globin reporter transcripts, as well as endogenous GRE-containing transcripts, were stabilized following CUGBP1 knockdown.35 These results implicated CUGBP1 as a mediator of GRE-dependent mRNA decay.
Binding by CELF Proteins to GU-rich Sequences
CELF proteins regulate gene expression at posttranscriptional levels in diverse species by binding directly to RNA targets.25-29 It is not known whether CELF family member proteins are functionally redundant or if each CELF protein targets specific sub-populations of mRNA transcripts.50 Each CELF protein contains two N-terminal and one C-terminal RNA recognition motifs (RRMs), separated by a 160−230 residue divergent domain.41,78 The highly conserved RRMs, bind to RNA in a sequence-specific manner.67 A search for preferential RNA binding sites using systemic evolution of ligands exponential enrichment (SELEX) revealed that CUGBP1 bound preferentially to GU-rich RNA sequences.32 Binding by CUGBP1 to GREs was abrogated by mutation of G nucleotides to C both in vitro and in vivo.35,34 CUGBP2 (CELF2, also known as BRUNOL3, NAPOR and ETR-3) also preferentially bound to UGUU-rich sequences that were identified using SELEX.33 In the yeast three hybrid system for evaluating RNA-protein interactions, CUGBP1 bound preferentially to UG repeats rather that to CUG repeats.31 Another research group reported that CUGBP1 bound with high specificity to (UG)15 based on a surface plasma resonance (SPR) quantitative binding assay.63
Orthologues of CUGBP1 in other species also appear to have preferences for binding to GU-rich sequences. In Xenopus, the CUGBP1 ortholog, EDEN-BP (embryo deadenylation element binding protein), binds to the GU-rich EDEN element, which contains the sequence (UGUA)12, and functions as a deadenylation signal in Xenopus embryos after fertilization.25,30 RNA-protein immunoprecipitation of Xenopus extracts using an anti-EDEN-BP revealed that many EDEN-BP targets are GU-rich and are similar to human GREs.79 In Drosophila, the CUGBP1, orthologue, Bru-3, was found to bind specifically to (UG)15 repeats and also bound to the Xenopus GU-rich EDEN element.37 The Zebrafish protein Brul, a homologue of EDEN-BP with 81% identity was also shown to preferentially bind to GU-rich RNAs.80 CELF proteins, including CUGBP1, EDEN-BP and Bru-3 bind to RNA as dimers81,82 and may require GU-rich sequences of sufficient length to allow dimer formation. Surrounding sequences may be necessary for assembly of CELF proteins on RNA by allowing optimal secondary structure to facilitate the formation of RNA-protein complexes. Perhaps heterogeneity in the secondary structure of GU-rich sequences provides unique sites for RNA-protein and RNA-RNA interactions.83,84
Evolutionary Conservation of Deadenylation and Translation Regulation by GREs and CELF Proteins
Translation is a critical level of post-transcriptional control of gene expression that is regulated in response to environmental and developmental changes. In eukaryotic organisms, translation is regulated by deadenylation. For example, in Xenopus, maternal transcripts are stored in the cytoplasm of oocytes, and upon fertilization, the poly A tail is shortened or lengthened through specific regulatory mechanisms, activating or repressing the translation of these transcripts.1,85 For some dormant maternal transcripts, re-lengthening of the poly A tail mediated by the cytoplasmic polyadenylation element leads to translational activation,1 whereas for other transcripts, deadenylation promotes translational activation. Deadenylation is regulated by GU-rich sequences and CELF proteins across diverse species.56,57 For example, the EDEN element in Xenopus and the Bruno response element (BRE) in Drosophila are conserved GU or U/purine-rich regions that play an important role in mRNA deadenylation and regulation of translation during development.55 In Drosophila, oskar mRNA which encodes a regulator of posterior body morphogenesis, contains 3 U(G/A)U-rich BREs in its 3′ UTR that function as targets for the Bru-3 protein.26 Binding by Bru-3 to GU-rich sequences leads to translational repression.37 Regulation of translation through deadenylation in Xenopus of embryos is the best characterized model posttranscriptional gene regulation by GU-rich sequences and CELF proteins (see Fig. 1A). After fertilization of Xenopus oocytes, EDEN-BP bound to the EDEN element activates transcript deadenylation which subsequently leads to the translational repression of EDEN-containing transcripts,25,64 including transcripts that encode important cell cycle regulators.58-60 RNA-protein immunoprecipitation of Xenopus extracts using an anti-EDEN-BP antibody followed by microarray analysis of the precipitated transcripts identified 158 potential targets of EDEN-BP.79 Computational analysis using MEME of these EDEN-BP targets found GU-rich sequences, very similar to GREs, to be enriched.36 The finding that very similar GU-rich sequences were found to be targets of CELF proteins in humans35 and Xenopus36 strongly suggests that the interaction between CELF proteins and GREs in the posttranscriptional regulation of gene expression has been highly conserved throughout evolution.
Figure 1.
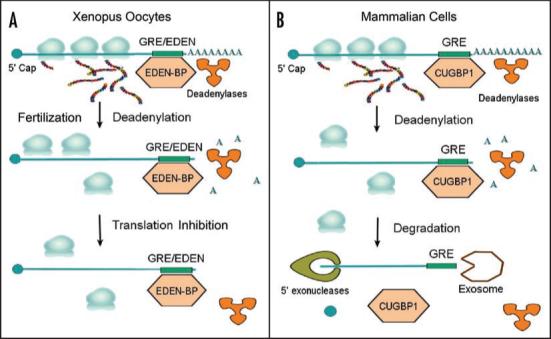
Evolutionary conservation of deadenylation by GU-rich sequences and CELF proteins. (A). In Xenopus, after fertilization, EDEN-BP bound to EDEN-containing mRNAs causes deadenylation and subsequent translational repression. (B). In mammalian cells, CUGBP1 binds to GREs within the 3′ UTR of specific transcripts and promotes their deadenylation and subsequent decay.
Deadenylation can activate or repress translation in lower organisms such as Xenopus; however, it is usually the first step leading to mRNA degradation in mammalian cells (see Fig. 1B). Although the consequences of deadenylation differ in different organisms, the mechanism of deadenylation appears to be evolutionarily conserved. For example, a GU-rich sequence from human c-jun mRNA could substitute for the EDEN element as a deadenylation signal in Xenopus extracts.62 Furthermore, human CUGBP1, which has 88% identity with EDEN-BP, was able to functionally substitute for EDEN-BP to mediate transcript deadenylation in Xenopus extracts,64 suggesting that the deadenylation function of GU-rich sequences and CELF proteins were conserved in diverse species.
Removal of the poly A tail is the rate-limiting step in the degradation of the majority of mammalian mRNAs. Our recent results demonstrated that GREs and CUGBP1 function as mediators of mRNA decay. Recently, CUGBP1 was shown to associate with poly A ribonuclease (PARN) and to stimulate poly A tail shortening in a cell-free assay using S100 extracts from human cells.65 It is not known if CUGBP1 activates other deadenylases in mammalian cells or how deadenylated transcripts are subsequently degraded. Since EDEN-BP, the CUGBP1 homolog in Xenopus, is a regulator of deadenylation, it is likely that CUGBP1 also regulates deadenylation in mammalian cells, leading to transcript degradation through unknown mechanisms.
In human cells, tethering of CUGBP1 to the 3′ UTR of mRNA through an interaction with the MS2 coat protein led to decreased steady state levels of reporter transcripts that contained a MS2 RNA-binding site, while reporter protein levels increased.50 These results suggest that CUGBP1 can activate translation in human cells. Other reports suggest that CELF proteins may play the opposite role in translational regulation. For example, CUGBP2, which has almost 100% identity in the consensus RNA binding domain with CUGBP1,86 bound to an AU-rich region of COX-2 mRNA and thereby facilitated transcript stabilization and translational shut-off.46 CUGBP1 has also been shown to function as an inhibitor of translation under conditions of stress, where it functions as a translational silencer in conjunction with T cell internal antigen 1.47 Upon UV-irradiation, CUGBP1 becomes activated by phosphorylation and then binds to a GC-rich sequence in the 5′ UTR of p21 mRNA in senescent fibroblasts, causing translational arrest.48 CUGBP1 has also been shown to differentially regulate the translation of alternative isoforms of CCAAT/enhancer-binding protein.45,51 Partial hepatectomy in the rat model activated CUGBP1 by phosphorylation and promoted formation of a complex between CUGBP1 and eIF2.52 This in turn, led to selective translation of the liver enriched inhibitory protein isoform of CCAAT/enhancer-binding protein.54 Thus, CELF proteins may function as activators or repressors of translation, depending on the context.
GREs and CELF Proteins as Regulators of Splicing
Alignment of the genomic regions adjacent to mammalian intron-exon splice sites, identified TG-rich motifs (TTCTG and TGTT) as conserved cis-elements found at splicing acceptor sites associated with alternative splicing.87,88 These C/UG-rich sequences can function as binding sites for CELF proteins which activate or repress the splicing of pre-mRNA targets, depending on the context.38-44,89 CELF-mediated regulation of alternative splicing is necessary for maintenance of normal muscle structure and function.42,90,91 In Zebrafish, ETR-3 and Brul promote muscle-specific splicing of alpha-actinin pre-mRNA via binding to GU/C-rich premRNA sequences.80 The Drosophila CELF protein Bruno92 and Caenorhabditis elegans CELF protein ETR1,27 also regulate alternative splicing, although the conserved binding sequence binding motif is U-rich rather than GU-rich.
Posttranscriptional gene networks regulated by GRes and CELF Proteins
Our microarray-based measurements of mRNA decay in primary human T cells demonstrate that only a small subset of transcripts exhibit rapid mRNA decay. The transcripts that are regulated at the level of mRNA decay, however, encode proteins that serve very important functions in the cell, including important regulators of T cell activation, apoptosis and cell growth, and the cell requires a mechanism to selectively recognize these transcripts. Specific RNA regulatory sequences and specific trans-acting proteins that recognize these sequences function to allow selective recognition of those transcripts that exhibit rapid mRNA decay. The coordinated regulation of multiple genes involved in a biological process that occurs through the interactions between conserved sequence elements and specific RNA-binding proteins has been termed post-transcriptional operons or regulons.10,93 Combinatorial associations of RNA-binding proteins with conserved sequence elements within mRNA creates a regulatory network (regulon) analogous to the regulation of transcription by the multiple transcription factors that bind to DNA in promoter regions. The best-characterized example of coordinate regulation of mRNA decay is ARE-mediated mRNA decay. The decay of a variety of cytokine and growth regulatory transcripts are coordinately regulated following immune cell activation by conserved AREs found in their 3′ untranslated regions.73,94 Thus, ARE-containing transcripts comprise a posttranscriptional regulatory network that is controlled by the expression and activity of specific ARE-binding proteins.
GREs and CUGBP1 define another example of a posttranscriptional regulatory network. We have found that numerous short-lived GRE-containing transcripts expressed in primary human T cells encode important regulators of cell cycle and apoptosis (See Fig. 2). Many of these short-lived GRE-containing transcripts were expressed transiently following T cell activation and then rapidly disappeared.66 The rapid decay of these transcripts allows the cell to turn off their expression very quickly, depending on the needs of the cell. For example, numerous regulators of cell cycle are turned on and off at precise times during cellular proliferation, and GRE-mediated mRNA decay may provide a mechanism to coordinate the timing of those events.
Figure 2.
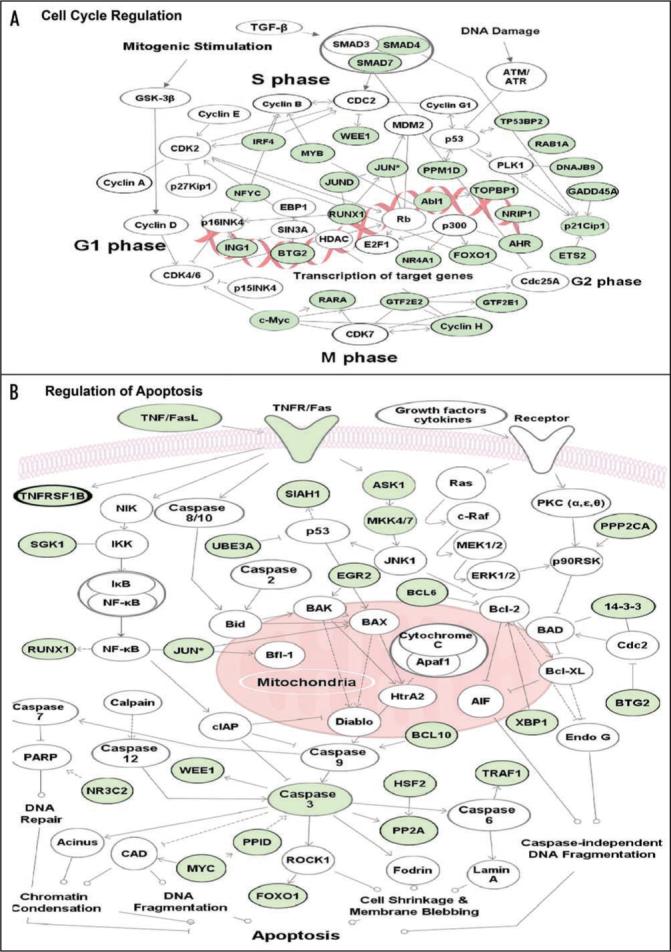
Posttranscriptional regulation of gene networks by GREs. The network diagrams show coordinate regulation of short-lived GRE-containing transcripts involved in (A) cell cycle and (B) apotosis pathways. The transcripts shown in green (on-line) or gray (print) are short-lived transcripts (half-life less than 90 minutes) expressed in primary human T cells66 that contain a consensus nonamer GRE sequence UUGUUUGUU, allowing one mismatch, in their 3′ UTR. Arrows represent direct (solid line) or indirect (dashed line) functional connections identified by Ingenuity Pathway Assistant software (Ingenuity Systems, CA).
Concluding remarks
Microarrays and gene expression clustering algorithms have been widely employed in the search for gene targets of transcription factors. The identification of posttranscriptional regulatory networks highlights the importance of posttranscriptional events in regulating gene expression. Understanding gene regulatory networks and the integration of transcriptional and posttranscriptional events are the next important tasks in bioinformatics and will require innovations in computational methods as well as experimental techniques. Novel computational approaches are needed to understand sequence/structural requirements and predict higher-order RNA structures for the recognition by RNA-protein complexes. It is also important to further investigate the biochemical interactions between CELF proteins and RNA as well as to identify the other components of CELF protein-containing complexes bound to RNA in order to discover unknown CELF partners that may be involved in the deadenylation, decay and/or translation regulation. Understanding the biochemistry of posttranscriptional regulation will lead to elucidation of posttranscriptional regulatory pathways and networks and lead to a better understanding of normal cellular function and disease states.
Acknowledgements
This work was supported by grant 1R01AI072068 from the National Institutes of Health, USA and by a Lymphoma Research Foundation Fellowship to IAV. We thank Yann Audic and Rebecca Hartley for helpful discussions regarding deadenylation in Xenopus.
Abbreviations
- ARE
AU-rich element
- CELF
CUGBP and embryonically lethal abnormal vision-type RNA binding protein 3-like factors
- CUGBP1
CUG-binding protein 1
- EDEN
embryo deadenylation element
- EDEN-BP
embryo deadenylation element-binding protein
- PARN
Poly A Ribonuclease
- RRM
RNA recognition motif
- SELEX
systemic evolution of ligands exponential enrichment
- 3′ UTR
3′ untranslated region
References
- 1.Richter JD. Cytoplasmic polyadenylation in development and beyond. Microbiol Mol Biol Rev. 1999;63:446–56. doi: 10.1128/mmbr.63.2.446-456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallikas O, Palin K, Sinjushina N, Rautiainen R, Partanen J, Ukkonen E, Taipale J. Genome-wide prediction of mammalian enhancers based on analysis of transcription-factor binding affinity. Cell. 2006;124:47–59. doi: 10.1016/j.cell.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 3.Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–43. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 4.Mata J, Marguerat S, Bahler J. Post-transcriptional control of gene expression: a genome-wide perspective. Trends Biochem Sci. 2005;30:506–14. doi: 10.1016/j.tibs.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Jansen RP. mRNA localization: message on the move. Nat Rev Mol Cell Biol. 2001;2:247–56. doi: 10.1038/35067016. [DOI] [PubMed] [Google Scholar]
- 6.Faustino NA, Cooper TA. Pre-mRNA splicing and human disease. Genes Dev. 2003;17:419–37. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- 7.Jans DA, Xiao CY, Lam MH. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays. 2000;22:532–44. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Brewer G. Messenger RNA decay during aging and development. Ageing Res Rev. 2002;1:607–25. doi: 10.1016/s1568-1637(02)00023-5. [DOI] [PubMed] [Google Scholar]
- 9.Mignone F, Gissi C, Liuni S, Pesole G. Untranslated regions of mRNAs. Genome Biol. 2002:3. doi: 10.1186/gb-2002-3-3-reviews0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keene JD. Biological clocks and the coordination theory of RNA operons and regulons. Cold Spring Harb Symp Quant Biol. 2007;72:157–65. doi: 10.1101/sqb.2007.72.013. [DOI] [PubMed] [Google Scholar]
- 11.Misquitta CM, Chen T, Grover AK. Control of protein expression through mRNA stability in calcium signalling. Cell Calcium. 2006;40:329–46. doi: 10.1016/j.ceca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Khabar KS. Rapid transit in the immune cells: the role of mRNA turnover regulation. J Leukoc Biol. 2007;81:1335–44. doi: 10.1189/jlb.0207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khabar KS, Young HA. Post-transcriptional control of the interferon system. Biochimie. 2007;89:761–9. doi: 10.1016/j.biochi.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izquierdo JM. Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. J Biol Chem. 2008 doi: 10.1074/jbc.M800017200. [DOI] [PubMed] [Google Scholar]
- 15.Jones H, Carver M, Pekala PH. HuR binds to a single site on the C/EBPbeta mRNA of 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2007;355:217–20. doi: 10.1016/j.bbrc.2007.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espel E. The role of the AU-rich elements of mRNAs in controlling translation. Semin Cell Dev Biol. 2005;16:59–67. doi: 10.1016/j.semcdb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Pesole G, Grillo G, Larizza A, Liuni S. The untranslated regions of eukaryotic mRNAs: structure, function, evolution and bioinformatic tools for their analysis. Brief Bioinform. 2000;1:236–49. doi: 10.1093/bib/1.3.236. [DOI] [PubMed] [Google Scholar]
- 18.Li D, Bachinski LL, Roberts R. Genomic organization and isoform-specific tissue expression of human NAPOR (CUGBP2) as a candidate gene for familial arrhythmogenic right ventricular dysplasia. Genomics. 2001;74:396–401. doi: 10.1006/geno.2001.6558. [DOI] [PubMed] [Google Scholar]
- 19.Good PJ, Chen Q, Warner SJ, Herring DC. A family of human RNA-binding proteins related to the Drosophila Bruno translational regulator. J Biol Chem. 2000;275:28583–92. doi: 10.1074/jbc.M003083200. [DOI] [PubMed] [Google Scholar]
- 20.Choi DK, Ito T, Tsukahara F, Hirai M, Sakaki Y. Developmentally-regulated expression of mNapor encoding an apoptosis-induced ELAV-type RNA binding protein. Gene. 1999;237:135–42. doi: 10.1016/s0378-1119(99)00312-1. [DOI] [PubMed] [Google Scholar]
- 21.Kress C, Gautier-Courteille C, Osborne HB, Babinet C, Paillard L. Inactivation of CUG-BP1/CELF1 causes growth, viability and spermatogenesis defects in mice. Mol Cell Biol. 2007;27:1146–57. doi: 10.1128/MCB.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meins M, Schlickum S, Wilhelm C, Missbach J, Yadav S, Glaser B, Grzmil M, Burfeind P, Laccone F. Identification and characterization of murine Brunol4, a new member of the elav/bruno family. Cytogenet Genome Res. 2002;97:254–60. doi: 10.1159/000066619. [DOI] [PubMed] [Google Scholar]
- 23.Loria PM, Duke A, Rand JB, Hobert O. Two neuronal, nuclear-localized RNA binding proteins involved in synaptic transmission. Curr Biol. 2003;13:1317–23. doi: 10.1016/s0960-9822(03)00532-3. [DOI] [PubMed] [Google Scholar]
- 24.Ladd AN, Nguyen NH, Malhotra K, Cooper TA. CELF6, a member of the CELF family of RNA-binding proteins, regulates muscle-specific splicing enhancer-dependent alternative splicing. J Biol Chem. 2004;279:17756–64. doi: 10.1074/jbc.M310687200. [DOI] [PubMed] [Google Scholar]
- 25.Paillard L, Omilli F, Legagneux V, Bassez T, Maniey D, Osborne HB. EDEN and EDEN-BP, a cis element and an associated factor that mediate sequence-specific mRNA deadenylation in Xenopus embryos. EMBO J. 1998;17:278–87. doi: 10.1093/emboj/17.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim-Ha J, Kerr K, Macdonald PM. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 1995;81:403–12. doi: 10.1016/0092-8674(95)90393-3. [DOI] [PubMed] [Google Scholar]
- 27.Milne CA, Hodgkin J. ETR-1, a homologue of a protein linked to myotonic dystrophy, is essential for muscle development in Caenorhabditis elegans. Curr Biol. 1999;9:1243–6. doi: 10.1016/s0960-9822(99)80504-1. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto Y, Suzuki H, Kageyama Y, Yasuda K, Inoue K. Bruno-like protein is localized to zebrafish germ plasm during the early cleavage stages. Gene Expr Patterns. 2006;6:201–5. doi: 10.1016/j.modgep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Brimacombe KR, Ladd AN. Cloning and embryonic expression patterns of the chicken CELF family. Dev Dyn. 2007;236:2216–24. doi: 10.1002/dvdy.21209. [DOI] [PubMed] [Google Scholar]
- 30.Paillard L, Osborne HB. East of EDEN was a poly(A) tail. Biol Cell. 2003;95:211–9. doi: 10.1016/s0248-4900(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi N, Sasagawa N, Suzuki K, Ishiura S. The CUG-binding protein binds specifically to UG dinucleotide repeats in a yeast three-hybrid system. Biochem Biophys Res Commun. 2000;277:518–23. doi: 10.1006/bbrc.2000.3694. [DOI] [PubMed] [Google Scholar]
- 32.Marquis J, Paillard L, Audic Y, Cosson B, Danos O, Le Bec C, Osborne HB. CUG-BP1/CELF1 requires UGU-rich sequences for high-affinity binding. Biochem J. 2006;400:291–301. doi: 10.1042/BJ20060490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faustino NA, Cooper TA. Identification of putative new splicing targets for ETR-3 using sequences identified by systematic evolution of ligands by exponential enrichment. Mol Cell Biol. 2005;25:879–87. doi: 10.1128/MCB.25.3.879-887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goraczniak R, Gunderson SI. The regulatory element in the 3'-untranslated region of human papillomavirus 16 inhibits expression by binding CUG-binding protein 1. J Biol Chem. 2008;283:2286–96. doi: 10.1074/jbc.M708789200. [DOI] [PubMed] [Google Scholar]
- 35.Vlasova IA, Tahoe NM, Fan D, Larsson O, Rattenbacher B, Sternjohn JR, Vasdewani J, Karypis G, Reilly CS, Bitterman PB, Bohjanen PR. Conserved GU-rich elements mediate mRNA decay by binding to CUG-binding protein 1. Mol Cell. 2008;29:263–70. doi: 10.1016/j.molcel.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graindorge A, Le Tonqueze O, Thuret R, Pollet N, Osborne HB, Audic Y. Identification of CUG-BP1/EDEN-BP target mRNAs in Xenopus tropicalis. Nucleic Acids Res. 2008;36:1861–70. doi: 10.1093/nar/gkn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delaunay J, Le Mee G, Ezzeddine N, Labesse G, Terzian C, Capri M, Ait-Ahmed O. The Drosophila Bruno paralogue Bru-3 specifically binds the EDEN translational repression element. Nucleic Acids Res. 2004;32:3070–82. doi: 10.1093/nar/gkh627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philips AV, Timchenko LT, Cooper TA. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280:737–41. doi: 10.1126/science.280.5364.737. [DOI] [PubMed] [Google Scholar]
- 39.Savkur RS, Philips AV, Cooper TA. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet. 2001;29:40–7. doi: 10.1038/ng704. [DOI] [PubMed] [Google Scholar]
- 40.Charlet BN, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell. 2002;10:45–53. doi: 10.1016/s1097-2765(02)00572-5. [DOI] [PubMed] [Google Scholar]
- 41.Ladd AN, Charlet N, Cooper TA. The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Mol Cell Biol. 2001;21:1285–96. doi: 10.1128/MCB.21.4.1285-1296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ladd AN, Stenberg MG, Swanson MS, Cooper TA. Dynamic balance between activation and repression regulates pre-mRNA alternative splicing during heart development. Dev Dyn. 2005;233:783–93. doi: 10.1002/dvdy.20382. [DOI] [PubMed] [Google Scholar]
- 43.Gromak N, Matlin AJ, Cooper TA, Smith CW. Antagonistic regulation of alpha-actinin alternative splicing by CELF proteins and polypyrimidine tract binding protein. RNA. 2003;9:443–56. doi: 10.1261/rna.2191903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mankodi A, Takahashi MP, Jiang H, Beck CL, Bowers WJ, Moxley RT, Cannon SC, Thornton CA. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell. 2002;10:35–44. doi: 10.1016/s1097-2765(02)00563-4. [DOI] [PubMed] [Google Scholar]
- 45.Timchenko NA, Welm AL, Lu X, Timchenko LT. CUG repeat binding protein (CUGBP1) interacts with the 5' region of C/EBPbeta mRNA and regulates translation of C/EBPbeta isoforms. Nucleic Acids Res. 1999;27:4517–25. doi: 10.1093/nar/27.22.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukhopadhyay D, Jung J, Murmu N, Houchen CW, Dieckgraefe BK, Anant S. CUGBP2 plays a critical role in apoptosis of breast cancer cells in response to genotoxic injury. Ann N Y Acad Sci. 2003;1010:504–9. doi: 10.1196/annals.1299.093. [DOI] [PubMed] [Google Scholar]
- 47.Fujimura K, Kano F, Murata M. Dual localization of the RNA binding protein CUGBP-1 to stress granule and perinucleolar compartment. Exp Cell Res. 2008;314:543–53. doi: 10.1016/j.yexcr.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 48.Iakova P, Wang GL, Timchenko L, Michalak M, Pereira-Smith OM, Smith JR, Timchenko NA. Competition of CUGBP1 and calreticulin for the regulation of p21 translation determines cell fate. Embo J. 2004;23:406–17. doi: 10.1038/sj.emboj.7600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 2005;33:7138–50. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barreau C, Watrin T, Beverley Osborne H, Paillard L. Protein expression is increased by a class III AU-rich element and tethered CUG-BP1. Biochem Biophys Res Commun. 2006;347:723–30. doi: 10.1016/j.bbrc.2006.06.177. [DOI] [PubMed] [Google Scholar]
- 51.Bae EJ, Kim SG. Enhanced CCAAT/enhancer-binding protein beta-liver-enriched inhibitory protein production by Oltipraz, which accompanies CUG repeat-binding protein-1 (CUGBP1) RNA-binding protein activation, leads to inhibition of preadipocyte differentiation. Mol Pharmacol. 2005;68:660–9. doi: 10.1124/mol.105.012997. [DOI] [PubMed] [Google Scholar]
- 52.Timchenko NA, Wang GL, Timchenko LT. RNA CUG-binding protein 1 increases translation of 20-kDa isoform of CCAAT/enhancer-binding protein beta by interacting with the alpha and beta subunits of eukaryotic initiation translation factor 2. J Biol Chem. 2005;280:20549–57. doi: 10.1074/jbc.M409563200. [DOI] [PubMed] [Google Scholar]
- 53.Wang GL, Salisbury E, Shi X, Timchenko L, Medrano EE, Timchenko NA. HDAC1 cooperates with C/EBPalpha in the inhibition of liver proliferation in old mice. J Biol Chem. 2008 doi: 10.1074/jbc.M803544200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang GL, Salisbury E, Shi X, Timchenko L, Medrano EE, Timchenko NA. HDAC1 promotes liver proliferation in young mice via interactions with C/EBP beta. J Biol Chem. 2008 doi: 10.1074/jbc.M803545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Audic Y, Omilli F, Osborne HB. Embryo deadenylation element-dependent deadenylation is enhanced by a cis element containing AUU repeats. Mol Cell Biol. 1998;18:6879–84. doi: 10.1128/mcb.18.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ezzeddine N, Paillard L, Capri M, Maniey D, Bassez T, Ait-Ahmed O, Osborne HB. EDEN-dependent translational repression of maternal mRNAs is conserved between Xenopus and Drosophila. Proc Natl Acad Sci USA. 2002;99:257–62. doi: 10.1073/pnas.012555499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osborne HB, Gautier-Courteille C, Graindorge A, Barreau C, Audic Y, Thuret R, Pollet N, Paillard L. Post-transcriptional regulation in Xenopus embryos: role and targets of EDEN-BP. Biochem Soc Trans. 2005;33:1541–3. doi: 10.1042/BST0331541. [DOI] [PubMed] [Google Scholar]
- 58.Mendez R, Richter JD. Translational control by CPEB: a means to the end. Nat Rev Mol Cell Biol. 2001;2:521–9. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- 59.Brittle AL, Ohkura H. Centrosome maturation: Aurora lights the way to the poles. Curr Biol. 2005;15:880–2. doi: 10.1016/j.cub.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt A, Rauh NR, Nigg EA, Mayer TU. Cytostatic factor: an activity that puts the cell cycle on hold. J Cell Sci. 2006;119:1213–8. doi: 10.1242/jcs.02919. [DOI] [PubMed] [Google Scholar]
- 61.Paris J, Philippe M. Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev Biol. 1990;140:221–4. doi: 10.1016/0012-1606(90)90070-y. [DOI] [PubMed] [Google Scholar]
- 62.Paillard L, Legagneux V, Maniey D, Osborne HB. c-Jun ARE targets mRNA deadenylation by an EDEN-BP (embryo deadenylation element-binding protein)-dependent pathway. J Biol Chem. 2002;277:3232–5. doi: 10.1074/jbc.M109362200. [DOI] [PubMed] [Google Scholar]
- 63.Mori D, Sasagawa N, Kino Y, Ishiura S. Quantitative analysis of CUG-BP1 binding to RNA repeats. J Biochem. 2008;143:377–83. doi: 10.1093/jb/mvm230. [DOI] [PubMed] [Google Scholar]
- 64.Paillard L, Legagneux V, Beverley Osborne H. A functional deadenylation assay identifies human CUG-BP as a deadenylation factor. Biol Cell. 2003;95:107–13. doi: 10.1016/s0248-4900(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 65.Moraes KC, Wilusz CJ, Wilusz J. CUG-BP binds to RNA substrates and recruits PARN deadenylase. RNA. 2006;12:1084–91. doi: 10.1261/rna.59606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raghavan A, Ogilvie RL, Reilly C, Abelson ML, Raghavan S, Vasdewani J, Krathwohl M, Bohjanen PR. Genome-wide analysis of mRNA decay in resting and activated primary human T lymphocytes. Nucleic Acids Res. 2002;30:5529–38. doi: 10.1093/nar/gkf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lam LT, Pickeral OK, Peng AC, Rosenwald A, Hurt EM, Giltnane JM, Averett LM, Zhao H, Davis RE, Sathyamoorthy M, Wahl LM, Harris ED, Mikovits JA, Monks AP, Hollingshead MG, Sausville EA, Staudt LM. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2001:2. doi: 10.1186/gb-2001-2-10-research0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frevel MA, Bakheet T, Silva AM, Hissong JG, Khabar KS, Williams BR. p38 Mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol Cell Biol. 2003;23:425–36. doi: 10.1128/MCB.23.2.425-436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang E, van Nimwegen E, Zavolan M, Rajewsky N, Schroeder M, Magnasco M, Darnell JE., Jr Decay rates of human mRNAs: correlation with functional characteristics and sequence attributes. Genome Res. 2003;13:1863–72. doi: 10.1101/gr.1272403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bakheet T, Frevel M, Williams BR, Greer W, Khabar KS. ARED: human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 2001;29:246–54. doi: 10.1093/nar/29.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bakheet T, Williams BR, Khabar KS. ARED 3.0: the large and diverse AU-rich transcriptome. Nucleic Acids Res. 2006;34:111–4. doi: 10.1093/nar/gkj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halees AS, El-Badrawi R, Khabar KS. ARED Organism: expansion of ARED reveals AU-rich element cluster variations between human and mouse. Nucleic Acids Res. 2008;36:137–40. doi: 10.1093/nar/gkm959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raghavan A, Dhalla M, Bakheet T, Ogilvie RL, Vlasova IA, Khabar KS, Williams BR, Bohjanen PR. Patterns of coordinate downregulation of ARE-containing transcripts following immune cell activation. Genomics. 2004;84:1002–13. doi: 10.1016/j.ygeno.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 74.Neuwald AF, Liu JS, Lawrence CE. Gibbs motif sampling: detection of bacterial outer membrane protein repeats. Protein Sci. 1995;4:1618–32. doi: 10.1002/pro.5560040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grundy WN, Bailey TL, Elkan CP, Baker ME. Meta-MEME: motif-based hidden Markov models of protein families. Comput Appl Biosci. 1997;13:397–406. doi: 10.1093/bioinformatics/13.4.397. [DOI] [PubMed] [Google Scholar]
- 76.Timchenko LT, Miller JW, Timchenko NA, DeVore DR, Datar KV, Lin L, Roberts R, Caskey CT, Swanson MS. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–14. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Timchenko LT, Timchenko NA, Caskey CT, Roberts R. Novel proteins with binding specificity for DNA CTG repeats and RNA CUG repeats: implications for myotonic dystrophy. Hum Mol Genet. 1996;5:115–21. doi: 10.1093/hmg/5.1.115. [DOI] [PubMed] [Google Scholar]
- 78.Han J, Cooper TA. Identification of CELF splicing activation and repression domains in vivo. Nucleic Acids Res. 2005;33:2769–80. doi: 10.1093/nar/gki561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Graindorge A, Thuret R, Pollet N, Osborne HB, Audic Y. Identification of post-transcriptionally regulated Xenopus tropicalis maternal mRNAs by microarray. Nucleic Acids Res. 2006;34:986–95. doi: 10.1093/nar/gkj492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suzuki H, Jin Y, Otani H, Yasuda K, Inoue K. Regulation of alternative splicing of alpha-actinin transcript by Bruno-like proteins. Genes Cells. 2002;7:133–41. doi: 10.1046/j.1356-9597.2001.00506.x. [DOI] [PubMed] [Google Scholar]
- 81.Bonnet-Corven S, Audic Y, Omilli F, Osborne HB. An analysis of the sequence requirements of EDEN-BP for specific RNA binding. Nucleic Acids Res. 2002;30:4667–74. doi: 10.1093/nar/gkf586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salisbury E, Sakai K, Schoser B, Huichalaf C, Schneider-Gold C, Nguyen H, Wang GL, Albrecht JH, Timchenko LT. Ectopic expression of cyclin D3 corrects differentiation of DM1 myoblasts through activation of RNA CUG-binding protein, CUGBP1. Exp Cell Res. 2008;314:2266–78. doi: 10.1016/j.yexcr.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu C, Alwine JC. Secondary structure as a functional feature in the downstream region of mammalian polyadenylation signals. Mol Cell Biol. 2004;24:2789–96. doi: 10.1128/MCB.24.7.2789-2796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mooers BH, Logue JS, Berglund JA. The structural basis of myotonic dystrophy from the crystal structure of CUG repeats. Proc Natl Acad Sci USA. 2005;102:16626–31. doi: 10.1073/pnas.0505873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jackson RJ, Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990;62:15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- 86.Barreau C, Paillard L, Mereau A, Osborne HB. Mammalian CELF/Bruno-like RNA-binding proteins: molecular characteristics and biological functions. Biochimie. 2006;88:515–25. doi: 10.1016/j.biochi.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 87.Voelker RB, Berglund JA. A comprehensive computational characterization of conserved mammalian intronic sequences reveals conserved motifs associated with constitutive and alternative splicing. Genome Res. 2007;17:1023–33. doi: 10.1101/gr.6017807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ladd AN, Cooper TA. Finding signals that regulate alternative splicing in the post-genomic era. Genome Biol. 2002:3. doi: 10.1186/gb-2002-3-11-reviews0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ladd AN, Cooper TA. Multiple domains control the subcellular localization and activity of ETR-3, a regulator of nuclear and cytoplasmic RNA processing events. J Cell Sci. 2004;117:3519–29. doi: 10.1242/jcs.01194. [DOI] [PubMed] [Google Scholar]
- 90.Ladd AN, Taffet G, Hartley C, Kearney DL, Cooper TA. Cardiac tissue-specific repression of CELF activity disrupts alternative splicing and causes cardiomyopathy. Mol Cell Biol. 2005;25:6267–78. doi: 10.1128/MCB.25.14.6267-6278.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ho TH, Bundman D, Armstrong DL, Cooper TA. Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum Mol Genet. 2005;14:1539–47. doi: 10.1093/hmg/ddi162. [DOI] [PubMed] [Google Scholar]
- 92.de Haro M, Al-Ramahi I, De Gouyon B, Ukani L, Rosa A, Faustino NA, Ashizawa T, Cooper TA, Botas J. MBNL1 and CUGBP1 modify expanded CUG-induced toxicity in a Drosophila model of myotonic dystrophy type 1. Hum Mol Genet. 2006;15:2138–45. doi: 10.1093/hmg/ddl137. [DOI] [PubMed] [Google Scholar]
- 93.Keene JD, Tenenbaum SA. Eukaryotic mRNPs may represent posttranscriptional operons. Mol Cell. 2002;9:1161–7. doi: 10.1016/s1097-2765(02)00559-2. [DOI] [PubMed] [Google Scholar]
- 94.Raghavan A, Bohjanen PR. Microarray-based analyses of mRNA decay in the regulation of mammalian gene expression. Brief Funct Genomic Proteomic. 2004;3:112–24. doi: 10.1093/bfgp/3.2.112. [DOI] [PubMed] [Google Scholar]


