Abstract
Serotonin (5-hydroxytryptamine; 5-HT) is a neurotransmitter produced by a small number of neurons in the midbrain, pons and medulla. These neurons project widely throughout the neuraxis, where they release 5-HT and co-localized neuropeptides such as substance P (SP) and thyrotropin-releasing hormone (TRH). Each of these chemicals produce effects largely through G protein-coupled receptors, second messenger systems and subsequent neuromodulatory effects on target neurons. Emerging evidence suggests that 5-HT has additional modes of action during development and in adult mammals, including trophic effects (neurogenesis, cell differentiation, proliferation, migration and maturation) and influences on synaptic plasticity. Here, we discuss some of the neuromodulatory and trophic roles of 5-HT in general and in the context of respiratory control, as well as the regulation of release of modulatory neurotransmitters from 5-HT neurons. Future directions of study are also discussed.
1. Introduction
Serotonin (5-Hydroxytryptamine, 5-HT) is one of the oldest bioactive molecules in nature, where it is found in diverse species from the psychoactive seeds of mucuna pruriens to venoms in toads and spiders (Collier, 1958). 5-HT plays an important role in nature as a signaling molecule, acting as a neurotransmitter in all species of the animal kingdom. In the mammalian CNS, the relatively small number of neurons that produce 5-HT are located along and near the midline of the brainstem. However, these few neurons project to target regions throughout the entire neuraxis. The effects of 5-HT (and co-released neuropeptides such as SP and TRH) depend upon the complement of receptors (15 different 5-HT receptor subtypes cloned, of which some are post-transcriptionally modified), the second messenger systems they are coupled to, and the developmental stage in which they are expressed. 5-HT neurons are some of the first neurons to emerge in the developing hindbrain, where they may play a morphogenetic and organizational role in neuronal circuits.
With diverse projections and numerous pre- and post-synaptic receptor subtypes, it is not surprising that the 5-HT system has been proposed to contribute to numerous brain functions and pathology, including but not limited to neurogenesis, synaptic plasticity, brain homeostasis, sleep and circadian rhythms, appetite, pain, thermoregulation, breathing, micturition, addiction, migraine, depression, fear and anxiety, aggression and rage, learning and memory, obsessive compulsive disorder, schizophrenia, Prader-Willi syndrome, autism and sudden infant death syndrome (SIDS). Understanding the anatomy, as well as the classical and novel mechanisms by which 5-HT operates within the CNS is of great importance in our understanding the role of 5-HT in these processes.
2.5-HT as a neuromodulator
5-HT belongs to a class of neurochemicals regarded as neuromodulators. Neuromodulation has a variety of definitions, including “the ability of neurons to alter their electrical properties in response to intracellular biochemical changes resulting from synaptic or hormonal stimulation” (Kaczmarek & Levitan, 1987). Neuromodulators are also widely considered to be substances that alter the response of target neurons to traditional neurotransmitters, but without directly causing depolarization or hyperpolarization. The effects of many neuromodulators are dependent upon activation of intracellular signaling cascades by G protein-coupled receptors, which through changes in cAMP production, intracellular calcium levels, phosphorylation and other biochemical changes alter ion channel properties. These effects include changes in spike frequency adaptation via modulation of SK channels (Klein et al., 1982), modification of A-current (Kaczmarek & Strumwasser, 1984), activation of the hyperpolarization-activated cation current Ih (Pape & McCormick, 1989), changes in the delayed rectifier K+ current IK(r) (Benson & Levitan, 1983), the non-inactivating leak K+, or M-current (Brown & Adams, 1980; Wang et al., 1998), and conversion of tonically firing neurons to intrinsic bursters (Dekin et al., 1985). Neuromodulators, and 5-HT in particular, can also influence glutamate and GABA receptor currents by phosphorylation and/or altering receptor trafficking via G-protein coupled second messenger pathways (Feng et al., 2001; Bocchiaro & Feldman, 2004).
Most neurotransmitters can act as both neuromodulators and classical neurotransmitters depending on the receptors present on the target neuron. For example, glutamate and GABA can act either as fast synaptic neurotransmitters via ionotropic receptors (e.g. GABAA, NMDA and non-NMDA) or as neuromodulators via G protein-coupled receptors (e.g. GABAB and metabotropic glutamate). 5-HT can also act as either a fast neurotransmitter or a neuromodulator, depending on the receptor that it activates. However, since six of the seven major 5-HT receptor subtypes (5-HT1–2, 4–7) are G protein-coupled receptors (Bockaert et al., 2006), 5-HT usually acts as a neuromodulator. 5-HT3 receptors are the only 5-HT receptors that are ligand-gated ion channels (Maricq et al., 1991; van Hooft & Yakel, 2003), and have a low affinity for 5-HT but fast activation (milliseconds). In contrast, the metabotropic 5-HT receptors have high affinity and slow activation (seconds).
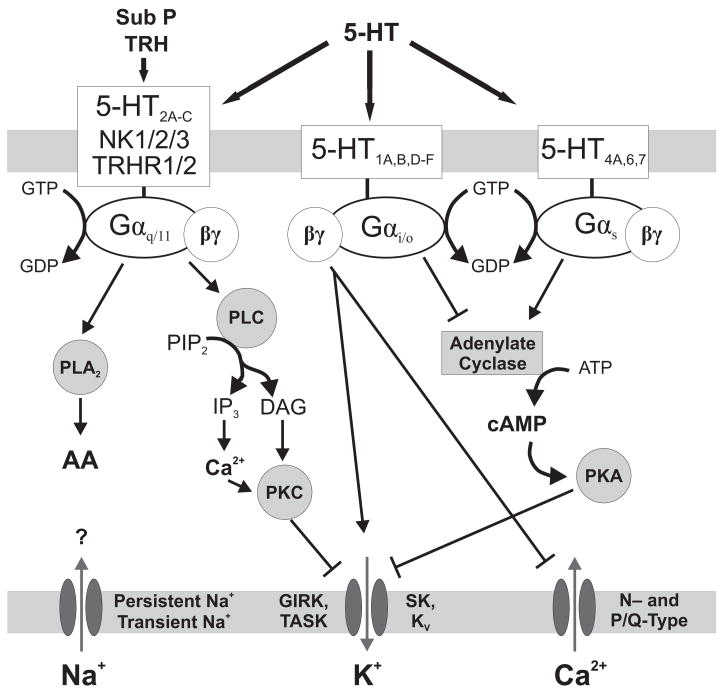
The specific G proteins through which different metabotropic 5-HT receptors interact lead to a diversity of pre- and post-synaptic effects (Figure 1). Generally, 5-HT1A,B,D–F, 5-HT4B and 5-HT5 receptors couple with Gαi/o proteins, 5-HT2A–C receptors couple with Gαq proteins, and 5-HT4A,6,7 receptors couple with GS proteins (Richter et al., 2003; Bockaert et al., 2006). Activation of each of these G proteins leads to the initiation of a different intracellular signaling cascade, thus providing a range of different effects depending upon which 5-HT receptors are expressed. Much of the work on signal transduction by 5-HT receptors in general, and specifically in respiratory neurons, has been reviewed previously in detail (Bayliss et al., 1997b; Rekling et al., 2000; Raymond et al., 2001; Feldman et al., 2005; Bockaert et al., 2006).
Figure 1. General neuromodulatory effector pathways of 5-HT, SP and TRH receptors.
Shown are some of the known (arrow = activation, blunt line = inhibition) and postulated (question marks) pathways and effectors through which 5-HT, SP and TRH receptor activation affect membrane excitability. Abbreviations: AA (arachidonic acid), DAG (diacylglycerol), GIRK (G-protein-gated inwardly rectifying potassium channel), IP3 (inositol trisphosphate), KV (voltage-gated potassium channel), PIP2 (phosphatidylinositol bisphosphate), PK (protein kinase), PL (phospholipase), SK (small conductance calcium-activated potassium channel), TASK (TWIK-related acid-sensitive potassium channel)
5-HT receptors are expressed both pre- and post-synaptically, and can also be localized extra-synaptically. In many cases 5-HT is released at sites lacking the specialized membrane characteristics that define classical synapses. In addition to axon terminals within target fields, 5-HT neurons often have additional release sites in the form of en passant varicosities (Liposits et al., 1987; Maley et al., 1990). While the apparent density of en passant varicosities varies in target regions, these release sites allow 5-HT to have paracrine effects, reaching multiple local (and more distant) neuronal (and non-neuronal) cells. The distribution of en passant varicosities versus classical synapses varies in different brain regions, where en passant varicosities represent 20% in the superior colliculus (Dori et al., 1998), and as much as 97% in the sensorimotor cortex (DeFelipe & Jones, 1988).
3. The 5-HT system and breathing: Projections and receptors
Neurons in all of the nuclei that govern respiratory control in the pons and medulla are innervated by 5-HT neurons (Steinbusch, 1981; Holtman, Jr. et al., 1984; Connelly et al., 1989; Voss et al., 1990). The origin of the projections to respiratory nuclei largely arise in the medullary raphé nuclei and ventrolateral medulla (parapyramidal region) (Holtman, Jr. et al., 1984; Connelly et al., 1989; Thor & Helke, 1989; Smith et al., 1989; Manaker & Tischler, 1993). 5-HT-immunoreactive (5-HT-ir) fibers are found in the nucleus of the solitary tract (NTS), nucleus ambiguus, retrotrapezoid nucleus (RTN), pre-Bötzinger complex (pre-BötC), and hypoglossal and phrenic motor nuclei (Fuxe, 1965; Holtman, Jr. et al., 1984; Holtman, Jr., 1988; Zhan et al., 1989; Pilowsky et al., 1990; Voss et al., 1990; Holtman, Jr. et al., 1990b; Jacobs & Azmitia, 1992), as illustrated in Figure 2A. 5-HT-ir projections within respiratory nuclei are also often immunoreactive for the neuropeptides SP and TRH, and these synaptic terminals primarily also originate from the medullary raphé nuclei and parapyramidal region (Holtman, Jr. et al., 1984; Holtman, Jr. et al., 1990a; Hokfelt et al., 2000). Thus, it is clear that medullary 5-HT neurons project to and are positioned to modulate respiratory neurons at multiple sites, including at both the pre-motor and motor neuron levels.
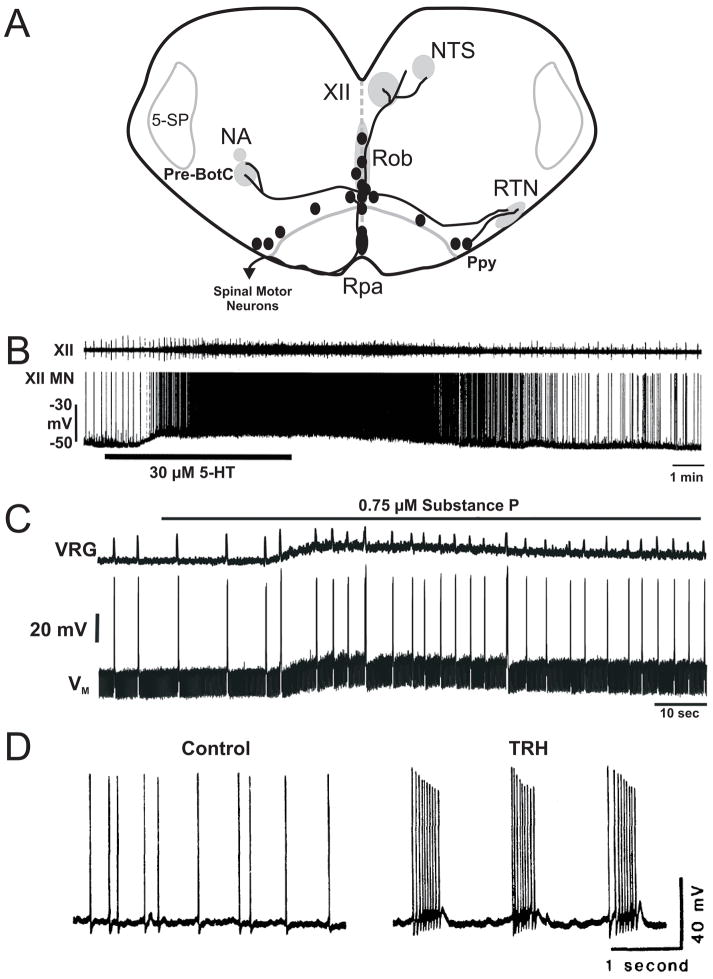
Figure 2. 5-HT neuron projections and neurotransmitter effects on respiratory output.
A) Illustration of cell body location of 5-HT neurons (ovals), and representative projections (bold lines) to the nucleus of the solitary tract (NTS), hypoglossal motor nucleus (XII), nucleus ambiguus (NA), pre-Botzinger complex (pre-BotC), and the retrotrapezoid nucleus (RTN) from the raphé obscurus (Rob), raphé pallidus (Rpa) and parapyramidal (Ppy) regions. Also shown is the spinal trigeminal nucleus (5-SP). B) Activity recorded from a hypoglossal nerve rootlet (top) and motor neuron (bottom) in a brain slice before, during and after bath application of 5-HT. C) Integrated ventral respiratory group (VRG) activity and membrane potential of a rhythmic VRG neuron (VM) activity before and during bath application of SP. D) Bath application of TRH during a recording from the respiratory portion of the NTS converts this tonically-firing neuron (left) into an intrinsic bursting pacemaker (right). Data in B), C) and D) were adapted from Schwarzacher et al., 2002, Pena and Ramirez, 2004, and Dekin et al., 1985.
The neuromodulatory effects of 5-HT, SP and TRH on breathing depend upon the complement of pre-and post-synaptic 5-HT receptors on neurons involved in respiratory control. Information regarding 5-HT receptor expression is incomplete, but it is clear that there are many different subtypes of 5-HT receptors expressed within respiratory nuclei (see the Allen Brain Atlas (Lein et al., 2007)). With regards to the control of breathing, some 5-HT receptors (5-HT1, 5-HT2, and recently 5-HT4 and 5-HT7 receptors) have been studied more extensively than others, and these will be the focus of this review.
5-HT1 Receptors
5-HT1A receptors are expressed post-synaptically, particularly in limbic regions (Miquel et al., 1994; Lanfumey & Hamon, 2004). However, the highest levels of expression of 5-HT1A receptors are on the somatic and dendritic membranes of 5-HT neurons themselves - often referred to as 5-HT1A somatodendritic autoreceptors (Sotelo et al., 1990; Aghajanian & Sanders-Bush, 2002). On both 5-HT and non-5-HT neurons, 5-HT1A receptors are usually coupled with Gαi/Gαo proteins, which inhibit adenylate cyclase and cyclic AMP generation (Figure 1). Activation of these receptors generally causes inhibition of neuronal firing, for example through activation of G protein-gated inwardly rectifying K+ (GIRK) channels (Andrade et al., 1986; Innis et al., 1988), and inhibition of N- and P/Q-type Ca2+ channels (Bayliss et al., 1995; Bayliss et al., 1997a). 5-HT1B receptors are primarily localized to axon terminals of serotonergic, dopaminergic, GABAergic and glutamatergic neurons (Bockaert et al., 2006), couple to Gαi/Gαo (Bouhelal et al., 1988), and activate K+ channels and inhibit Ca2+ channels (Raymond et al., 2001). 5-HT1A and 5-HT1B receptors are expressed in the raphé nuclei, NTS and hypoglossal motor nuclei (Manaker & Verderame, 1990; Okabe et al., 1997). These properties and synaptic localization of 5-HT1A and 5-HT1B receptors are of critical importance in determining the effects of 5-HT on 5-HT neurons themselves and on excitability of respiratory neurons (see below). They are also important in interpreting data from experiments applying agonists and antagonists of these receptors, since these drugs can simultaneously interact with post-synaptic receptors on non-serotonergic neurons and inhibitory autoreceptors on serotonergic neurons, and these two sites of action can sometimes lead to opposite effects on respiratory output.
5-HT2 Receptors
5-HT2A, 5-HT2B, and 5-HT2C receptors usually couple with Gαq proteins (Figure 1), which are positively coupled to phospholipase C (PLC). PLC activation generates inositol trisphosphate (IP3) and diacylglycerol (DAG). The former increases cytosolic Ca2+ via release from intracellular stores and the latter activates protein kinase C (PKC) (de Chaffoy de et al., 1985; Conn et al., 1986). These effects generally lead to increased neuronal excitability through a variety of mechanisms, including an increase in AMPA-mediated excitatory post-synaptic potentials (Aghajanian & Marek, 1999), and enhancement of persistent sodium current (Pena & Ramirez, 2002). There is also evidence for 5-HT2 receptor-mediated activation of phospholipase A2 independent of the PLC pathway, generating arachidonic acid (Felder et al., 1990). 5-HT2 receptors play a critical role in respiratory rhythm generation (Pena & Ramirez, 2002; Gunther et al., 2006; Tryba et al., 2006), and modulation of respiratory motor neurons (Brandes et al., 2006).
5-HT4 and 5-HT7 Receptors
Functional information regarding 5-HT4 and 5-HT7 family receptors in respiratory control is just beginning to emerge (Manzke et al., 2003; Richter et al., 2003; Manzke et al., 2008). 5-HT4/7 receptors are usually coupled to Gαs proteins, which activate adenylate cyclase (Figure 1: (Bockaert et al., 1990)). Cyclic AMP production leads to protein kinase A (PKA) activation, which can directly inhibit voltage-gated K+ channels, Ca2+-activated K+ channels, and in some cases GABAA currents (Bockaert et al., 2006). These effects can combine to increase excitability, and are possible mechanisms by which 5-HT4A receptor activation reverses respiratory depression following fentanyl administration (Manzke et al., 2003). Both 5-HT4A and 5-HT7 receptors are expressed in the pre-BötC (Manzke et al., 2008). 5-HT7 receptors have also received attention due to the moderate affinity for 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT: (Richter et al., 2003)), a drug previously thought to be a specific agonist for 5-HT1A receptors.
Tachykinins and TRH Receptors
The tachykinins (SP, neurokinin A and neurokinin B) and TRH (pyroglutamic acid-histidine-prolineamide) act via the G protein-coupled neurokinin receptors (NK1, NK2 and NK3) and TRH-R1 and TRH-R2 (Khawaja & Rogers, 1996; Sun et al., 2003). Like 5-HT2 family receptors, neurokinin and TRH receptors are generally coupled to Gαq proteins, PLC and increases in intracellular Ca2+. For example, neurokinin receptor activation in inferior mesenteric ganglion cells leads to depolarization via inhibition of inwardly rectifying K+ channels (Minota et al., 1981). In some cases NK receptors can also positively or negatively couple with adenylate cyclase (Khawaja & Rogers, 1996). NK1 receptors are important in the identification and function of the pre-BötC (Gray et al., 2001), and both TRH and NK1 (as well as 5-HT) receptor activation strongly stimulates RTN neurons (Mulkey et al., 2007).
4. Neuromodulation of respiratory output
Over the years different investigators have made a variety of conclusions about the effect of 5-HT on respiratory output, from net stimulatory, to net inhibitory, to biphasic, to “stabilizing.” However, we believe that the bulk of the evidence now supports the conclusion that when 5-HT neurons increase their firing rate under physiological conditions in vivo this leads to a net stimulatory effect on respiratory output (Richerson, 2004). This occurs via neuromodulation by 5-HT, SP and TRH acting at multiple sites within the respiratory network, including neurons that generate the respiratory rhythm, others that act as or integrate input from chemoreceptors, and still others that are part of the motor output pathways.
4.1 Respiratory rhythm generation
In vitro experiments have helped to define the mechanisms of the effects of 5-HT neurons on some of the core rhythm generating elements of the respiratory network, particularly by studying rhythmic respiratory output generated by slices of the medulla (Smith et al., 1991). In this neonatal preparation, bath or pre-BötC-specific application of 5-HT, or focal application of AMPA into the raphé, increases the frequency of hypoglossal motor output (Figure 2B: (Al-Zubaidy et al., 1996; Schwarzacher et al., 2002; Ptak et al., 2006)). The effect of 5-HT on hypoglossal nerve output can be mimicked by the 5-HT2 agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), and blocked by the 5-HT2A/C antagonist ketanserin (Al-Zubaidy et al., 1996; Pena & Ramirez, 2002). Hypoglossal nerve root and/or ventral respiratory group activity has a range of patterns in vitro, including eupnea-like, sigh-like and gasp-like output (Lieske et al., 2000), where eupnea-like (Pena & Ramirez, 2002; Ptak et al., 2006), and gasp-like (Tryba et al., 2006) output is dependent upon 5-HT2A receptors. The 5-HT2A-mediated effects on respiratory rhythm are thought to occur through PLC and PKC activation and subsequent modulation of transient and persistent Na+ currents (Pena & Ramirez, 2002).
In contrast, Gunther and colleagues report that hypoglossal nerve output from a similar slice preparation is blocked by a 5-HT2B receptor antagonist, but not ketanserin (Gunther et al., 2006). Additionally, earlier work by Al-Zubaidy et al. showed that respiratory rhythm in a similar preparation persisted after bath application of methysergide (Al-Zubaidy et al., 1996).
The reasons for the contradictory effects with ketanserin and methysergide are unclear, but may be due to differences in the experimental approach used in different labs (e.g. age, slice thickness and/or rostrocaudal level). Alternatively, there may be non-selective effects of these drugs, since methysergide is an antagonist at some 5-HT receptors and a partial agonist at others, whereas ketanserin is a 5-HT2 receptor antagonist and also has high affinity for α1-adrenoreceptors (Korstanje et al., 1986). Another possible explanation is that respiratory rhythm generation is less dependent on 5-HT in preparations containing more of the respiratory network. For example, in the in situ perfused brain preparation both eupnea and gasping have been reported to be unaffected by ketanserin or methysergide (St-John & Leiter, 2007; Toppin et al., 2007). Similarly, baseline ventilation is normal in adult mice in which serotonin neurons have been genetically deleted (see below), although there is a lower breathing frequency (Hodges et al., 2008). These differences in dependence on 5-HT may be due to the ability of other neurotransmitter systems (e.g. noradrenergic) to maintain normal respiratory activity in the absence of 5-HT (St-John & Leiter, 2007). This is similar to mechanisms of cortical arousal, where 5-HT, norepinephrine, histamine and acetylcholine are all capable of converting thalamocortical rhythms from a sleeping to a waking pattern (Pape & McCormick, 1989; McCormick & Pape, 1990; McCormick, 1992; Steriade et al., 1993). Although 5-HT contributes to cortical arousal, loss of 5-HT does not cause loss of consciousness as long as the other neurotransmitter systems are active. Thus, as with cortical arousal, it is clear that 5-HT has a general stimulatory effect on the respiratory rhythm, but other neuromodulators may substitute for 5-HT in its absence.
SP and TRH, which are co-localized in some 5-HT neurons, also strongly stimulate respiratory output. For example, SP applied to the rhythmically-active rostral medullary slice increases respiratory frequency (Figure 2C) and regularity, while blockade of endogenous NK-1 receptor activity with spantide or SSR140333 decreases frequency and regularity (Gray et al., 1999; Telgkamp et al., 2002; Pena & Ramirez, 2004; Ptak et al., 2006). Capsaicin-induced SP and glutamate depletion also blocks respiratory rhythm generation in brainstem slices (Morgado-Valle & Feldman, 2004). Moreover, the respiratory rhythm in preprotachykinin-A null mice, as well as rats with lesions targeting NK-1 receptor-expressing pre-BötC neurons, is highly variable and irregular (Gray et al., 2001; Telgkamp et al., 2002). Both SP and TRH increase respiratory output in the brainstem-spinal cord preparation (Murakoshi et al., 1985; Lindsay & Feldman, 1993; Greer et al., 1996), and in vivo (Yamamoto et al., 1981; Hedner et al., 1983; Holtman, Jr. et al., 1986; Chen et al., 1990). In addition, tonically firing neurons in the respiratory region of the NTS are converted to intrinsically bursting pacemaker neurons by application of TRH (Figure 2D: (Dekin et al., 1985). These and other data (Richerson, 2004) support the concept that 5-HT neurons directly enhance respiratory output via release of 5-HT and co-localized SP and TRH onto neurons that generate the respiratory rhythm.
4.2 The retrotrapezoid nucleus
The RTN is located in the rostral VLM (Smith et al., 1989), has reciprocal connections with the pre-BötC (Connelly et al., 1989; Ellenberger & Feldman, 1990), receives afferent projections from peripheral chemoreceptors (Mulkey et al., 2004), is intermixed with the parafacial respiratory group (Onimaru & Homma, 2003; Guyenet et al., 2005), and contributes tonic drive to breathe (Nattie, 2006). Projections from the raphé nuclei to both the dorsal cap and marginal layer of the RTN are rich in 5-HT, suggesting a functional relationship (Cream et al., 2002; Mulkey et al., 2007). Consistent with this, TRH injections into the ventrolateral medulla stimulate ventilation under anesthesia (Cream et al., 1997) and in awake rats (Cream et al., 1999).
Similar to 5-HT neurons (see below), glutamatergic, Phox2b-expressing RTN neurons respond to changes in CO2/pH in the presence of glutamate and GABA receptor blockers (Mulkey et al., 2007), and they have been proposed to be central chemoreceptors. Recent studies have revealed an intriguing relationship between 5-HT neurons and RTN neurons, where the effects on the hypercapnic ventilatory response of simultaneous inhibition within the raphé and RTN are not additive but synergistic (Li et al., 2006). Consistent with this, individual RTN neurons are strongly stimulated by exogenous 5-HT, SP and TRH, and the effects of 5-HT are mostly if not completely 5-HT2A-dependent (Mulkey et al., 2007). These data provide support for the conclusion that RTN neurons are strongly stimulated by 5-HT neurons via release of 5-HT, and co-localized SP and TRH. Given that 5-HT neurons are pH-sensitive (Wang et al., 2001; Richerson, 2004), one might suspect that the pH sensitivity of RTN neurons is mediated by synaptic input from raphé neurons. This possibility was tested using voltage clamp experiments. The authors found that the current induced by changes in pH had a different reversal potential than that induced by exogenous 5-HT (Mulkey et al., 2007), and from this they concluded that the chemosensitivity of RTN neurons is intrinsic. However, these experiments are not a direct test of this possibility. First, involvement of SP and TRH was not excluded. Second, the only way to directly prove that the pH response is not mediated in part by raphé input is to either physically separate the two groups of neurons, or to demonstrate that changes in firing rate induced in RTN neurons by changes in pH are not reduced or prevented by antagonists of 5-HT, SP and TRH receptors. Indeed, since there are many 5-HT neurons present in the rostral medulla (some of which increase their firing rate in response to acidosis) (Wang et al., 2001; Richerson, 2004), it remains possible that some or all of the response of RTN neurons to pH is indirectly mediated by an increase in release of 5-HT, SP and TRH from 5-HT neurons.
Clearly, more studies are needed to delineate anatomic and functional relationships between 5-HT and RTN neurons in order to understand the partnership among these two sets of putative chemoreceptors. Since RTN neurons also receive inputs from peripheral chemoreceptors, they may be a key site for neuromodulation by 5-HT neurons. Whether or not the response of RTN neurons to acidosis is intrinsic, they may play an important role as a relay center integrating information from other chemoreceptors.
4.3 Motor Neuron Excitability
Motor neurons are the final integration point in motor behavior, and as such receive diverse synaptic inputs from many neurons, including 5-HT neurons. The effects of 5-HT on cranial and spinal motor neurons have recently been reviewed in detail (Rekling et al., 2000). The predominant effect of 5-HT is to enhance motor neuron excitability, by a variety of mechanisms including inhibition of leak K+ currents, activation of Ih and enhancement of L-type Ca2+ currents (Talley et al., 1997; Rekling et al., 2000; Talley et al., 2000). The most extensively studied respiratory motor neurons are those in the hypoglossal (XII) nucleus, which express 5-HT1B, 5-HT2A, 5-HT2C, 5-HT3, and 5-HT7 receptors (Okabe et al., 1997). Hypoglossal motor neurons (HMs) also transiently express 5-HT1A receptors during development, and in neonates their activation causes depolarization, inhibition of the after hyperpolarization (AHP), and an increase in firing rate, without affecting input resistance (Bayliss et al., 1995; Talley et al., 1997). The inhibition of the AHP in this case is due to inhibition of N- and P/Q-type calcium channels, secondarily decreasing Ca2+-dependent K+ conductance. In juvenile HMs, the mechanisms of 5-HT-induced depolarization are different, where there is a decrease in input resistance with no change in the AHP (Talley et al., 1997). In adults, both endogenous and exogenous 5-HT directly depolarizes HMs via 5-HT2A receptors, likely through inhibition of leak K+ channels (Fenik & Veasey, 2003; Brandes et al., 2006). Interestingly, second messengers coupled to NK1 and TRH receptors also converge on and inhibit leak K+ channels (Talley et al., 2000), so that 5-HT, SP and TRH all lead to stimulation of HMs.
In the in vitro brainstem spinal cord preparation from neonatal rats, phrenic motor neurons are depolarized by 5-HT via (postsynaptic) 5-HT2 receptors, while inspiratory synaptic drive to these neurons is inhibited via activation of (likely pre-synaptic) 5-HT1B receptors (Lindsay & Feldman, 1993; Di Pasquale E. et al., 1997). Similarly, excitatory post-synaptic currents (EPSCs) evoked in hypoglossal motor neurons (by stimulation of afferents that were not identified as respiratory) are inhibited by 5-HT1B receptor activation in rat brain slices, and this was proposed to be due to pre-synaptic inhibition of glutamatergic synaptic input (Singer et al., 1996). Taken together, these data suggest that there is a combination of postsynaptic stimulation of respiratory motor neurons, pre-synaptic inhibition of excitatory synaptic inputs, and enhancement of rhythmic drive from the central pattern generator. Despite the decreased synaptic efficacy, the net effect still appears to be enhancement of respiratory output (Lindsay & Feldman, 1993). Experiments performed in vivo support the conclusion that the net effect of 5-HT neurons on motor output is stimulatory (Fenik & Veasey, 2003; Brandes et al., 2006). Unilateral injection of the 5-HT2A receptor antagonist MDL 100,907 into the XII nucleus of rats in vivo decreases XII nerve output by > 60%, and blocks the excitatory effects of 5-HT application (Fenik & Veasey, 2003). Brandes and colleagues found similar effects in decerebrate dogs in vivo, where iontophoretic application of ketanserin decreased, and exogenous 5-HT increased the inspiratory activity of individual hypoglossal motor neurons (Brandes et al., 2006).
Enhancement of motor neuron output is also characteristic of the role of 5-HT in long-term facilitation (LTF) and some other forms of respiratory plasticity. LTF is a prolonged enhancement of respiratory motor output that can be induced by intermittent hypoxia. The increase in motor output is dependent upon activation of 5-HT2 receptors, which acts in part by increased BDNF production and enhanced efficacy of glutamatergic neurotransmission from descending respiratory drive. The mechanisms and role of 5-HT in LTF is the subject of several reviews (Baker-Herman & Mitchell, 2002; Feldman et al., 2003; Baker-Herman et al., 2004).
In summary, the predominant effect of 5-HT on respiratory motor neurons is excitation. The receptors and downstream mechanisms involved differ with age, experimental protocols and the specific motor neuron pools.
5. Integrating pre- and post-synaptic effects of 5-HT neurons
Different authors have come to different conclusions regarding the net effects of 5-HT on ventilation. This is due in part to early studies using systemic p-chlorophenylalanine (PCPA) to deplete serotonin. This causes hyperventilation (Olson, Jr. et al., 1979; Mitchell et al., 1983), which was interpreted by the authors as indicating that 5-HT normally inhibits breathing. However, in one of these same studies (Olson, Jr. et al., 1979) and in others (Mueller et al., 1984), 5-HT neuron-specific lesions using 5,7-dihydroxytryptamine (5,7-DHT) causes hypoventilation, which leads to the opposite conclusion, i.e. that 5-HT neurons stimulate breathing. It is now known that PCPA can lead to results that are erroneous (Jouvet, 1999). This is due in part because this agent, in addition to depletion of central 5-HT (Koe & Weissman, 1966), can alter or deplete peripheral 5-HT and other neurotransmitter systems (Reader & Gauthier, 1984; Dailly et al., 2006). Remarkably, when PCPA is given at a dose sufficient to reduce brain 5-HT by 90%, there is no change in the post-synaptic response to stimulation of ascending 5-HT fibers to hippocampal neurons (Chaput et al., 1990). This maintenance of normal serotonergic synaptic transmission appears to be due to a decrease in inhibition of autoreceptors on 5-HT terminals, leading to enhanced efficacy of 5-HT release in response to pre-synaptic action potentials. In addition, PCPA would not deplete co-localized neuropeptides, and in fact could cause a compensatory increase in release of TRH and SP. Thus, experiments using serotonin depletion are not an effective means to determine the role of 5-HT neurons in ventilatory control. This problem is avoided when using 5,7-DHT, which prevents release of 5-HT as well as the co-transmitters SP and TRH (Olson, Jr. et al., 1979; Mueller et al., 1984), potentially explaining why this approach leads to different conclusions.
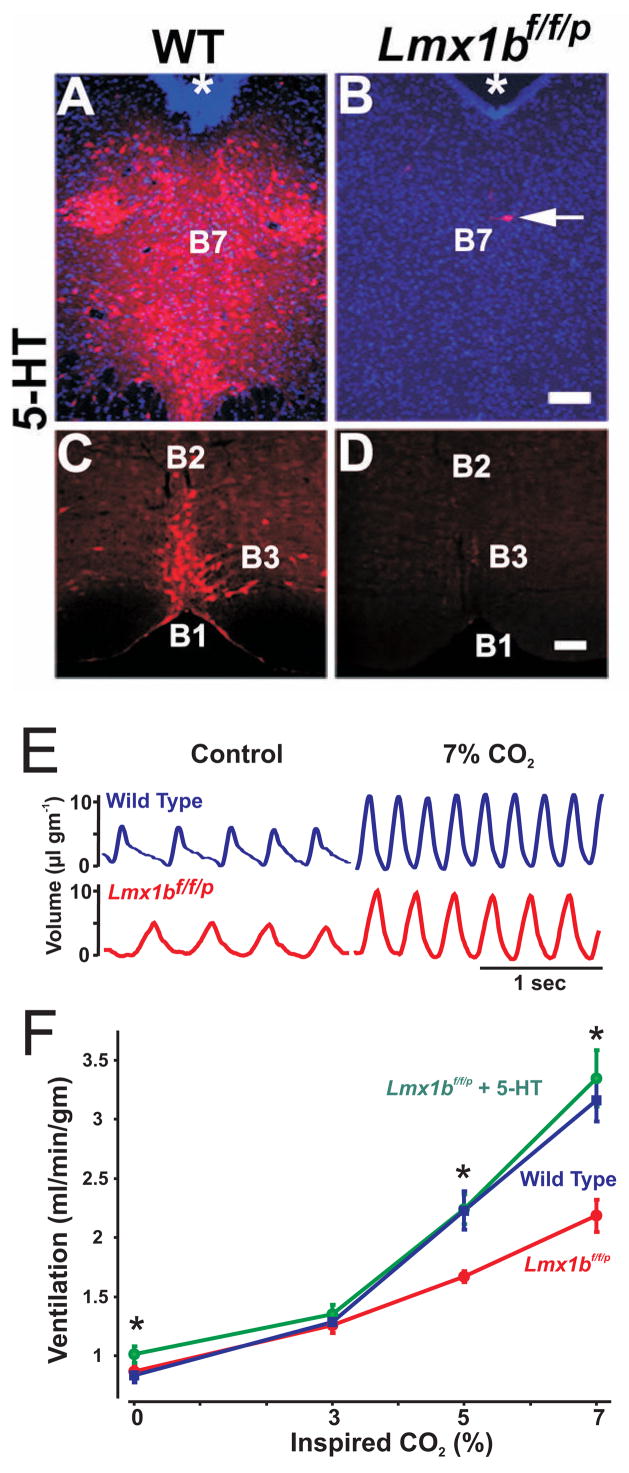
While 5-HT can inhibit some respiratory neurons (Lalley et al., 1995), and it is possible that 5-HT neurons inhibit respiratory output under some conditions (Richerson, 2004), the effects of 5-HT, SP and TRH at multiple sites within the respiratory network suggests a general facilitory role. The neuromodulatory effects of 5-HT, SP and TRH on respiratory rhythm generation, non-5-HT chemosensitive neurons, and respiratory motor neurons likely combine to enhance ventilation, as suggested previously (Richerson, 2004; Hodges et al., 2008). Recent data from a genetically modified mouse support this conclusion. Lmx1bf/f/p mice, in which the transcription factor Lmx1b is genetically deleted in Pet1-expressing (5-HT) neurons, exhibit a near-complete absence of 5-HT neurons as adults (Figure 3A-D: (Zhao et al., 2006)). As a result, 5-HT and 5-HIAA levels in the brain and spinal cord are severely reduced, without affecting peripheral 5-HT or other monoamines such as dopamine and noradrenaline. As with 5,7-DHT, the absence of 5-HT neurons in Lmx1bf/f/p mice also leads to loss of co-localized SP and TRH, but this genetic approach has an advantage over 5,7-DHT, because the latter is only able to eliminate a minority of 5-HT neurons. Minute ventilation in adult Lmx1bf/f/p mice is relatively normal, although on average they have a lower breathing frequency at rest (Figure 3E: (Hodges et al., 2008)). When 5-HT is administered intracerebroventricularly there is stimulation of ventilation (Figure 3E–F), indicating that the net central effect of 5-HT on the respiratory network is stimulatory in the absence of pre-synaptic, somatodendritic autoreceptor inhibition of 5-HT, TRH and SP release.
Figure 3. Exogenous 5-HT stimulates breathing in mice lacking central 5-HT neurons.
Immunocytochemical staining for 5-HT in the dorsal raphé and caudal raphé of wild type (A & B) and Lmx1bf/f/p (knockout) mice (C & D). E) Plethysmography tracings from WT (blue), Lmx1bf/f/p (red) and Lmx1bf/f/p mice with intracerebroventricular (ICV) 5-HT (green) at baseline (control) and breathing 7% CO2. F) ICV 5-HT stimulates ventilation at rest and while breathing 5 and 7% CO2. A-D is adapted from Zhao et al., 2006, and E & F from Hodges et al., 2008.
6. Intrinsic properties of 5-HT neurons and regulation of neurotransmitter release
In addition to understanding the mechanisms that underlie the downstream modulatory effects of 5-HT, TRH and SP, we must also identify the conditions that alter 5-HT neuron activity and thus neurotransmitter release. 5-HT neurons have a characteristic, pacemaker-like tonic pattern of firing, with action potentials that arise from slow ramp depolarizations and are followed by a prominent after hyperpolarization (Aghajanian & Sanders-Bush, 2002). The firing rate of 5-HT neurons is remarkably unaffected in vivo by a wide range of behavioral, physiological and environmental conditions (Jacobs & Fornal, 2008). In fact, increasing ambient temperature, pyrogen-induced fever, tonic or phasic painful stimuli, acute or chronic changes in blood pressure, heart rate or norepinepherine levels, significant blood loss (hemorrhagic shock) or other forms of “stress” are all unable to alter the firing rate of electrophysiologically identified 5-HT neurons (Auerbach et al., 1985; Fornal et al., 1987; Martin-Cora et al., 2005). Extracellular 5-HT levels have also been found to be relatively constant in the forebrain of rats during other “stressful” activities (pain, forced swim, exposure to natural enemy) supporting the conclusion that 5-HT neuron activity is relatively constant in vivo (Jacobs & Azmitia, 1992; Rueter & Jacobs, 1996). The relatively constant firing of 5-HT under most conditions would thus be expected to provide tonic stimulation of respiratory output.
There are conditions that do cause a change in firing rate of some 5-HT neurons. These include sleep state (Jacobs & Fornal, 1991), environmental cooling (Martin-Cora et al., 2000), repetitive motor activities (e.g. walking and chewing: (Fornal et al., 1996)) and CO2 inhalation (Veasey et al., 1995; Veasey et al., 1997). The firing rates of 5-HT neurons are highest during wakefulness, decrease during slow-wave sleep, and reach a minimum during REM sleep (Jacobs & Fornal, 1991). This sleep state dependence is consistent for all raphé nuclei, though 5-HT neurons in the medulla do not reduce their firing rate during sleep as much as 5-HT neurons in the midbrain (Jacobs & Azmitia, 1992). Most 5-HT neurons in the medulla are also activated during repetitive motor activity, increasing 5-HT neuron firing rates 2–5 fold during feeding and walking (Veasey et al., 1995). Additionally, as many as 50% of raphé pallidus/obscurus 5-HT neurons increase activity during environmental cooling (Martin-Cora et al., 2000), and 5-HT neuron activity also increases with hypothalamic cooling (Nason, Jr. & Mason, 2006). Likewise, a subpopulation (22%) of both medullary and midbrain 5-HT neurons increase their firing rate during CO2 inhalation in conscious cats (Veasey et al., 1995; Veasey et al., 1997). This response is an intrinsic property of some medullary and midbrain 5-HT neurons, because hypercapnic acidosis induces large changes in their firing rate in the absence of fast synaptic transmission in brainstem slices and after physical isolation in culture (Wang et al., 2001; Severson et al., 2003).
Thus, one would predict that changes in sleep state, locomotion, environmental temperature or hypercapnic acidosis would alter 5-HT levels in target regions. Indeed, hippocampal 5-HT levels correlate well with changes in sleep state as predicted, with extracellular 5-HT levels greater during wakefulness than non-REM and REM sleep (Penalva et al., 2003). Additionally, 5-HT synthesis and turnover are elevated in the thoracic spinal cord after environmental cooling (Passerin & Henley, 1994), and extracellular 5-HT levels increase in the hypoglossal motor nucleus during hypercapnic acidosis (Kanamaru & Homma, 2007).
The relatively constant firing of 5-HT neurons during wakefulness would be expected to contribute to a tonic drive to breathe, and the reduction in 5-HT neuron population activity likely contributes to the decrease in ventilation that normally occurs during sleep. Likewise, an increase in 5-HT neuron firing during environmental cooling and in response to inhalation of CO2 may contribute to the increase in ventilation that occurs under these conditions, as suggested by recent experiments (Hodges et al., 2008). The increase in 5-HT neuron activity with locomotion may also contribute to the increase in ventilation with exercise.
7. Trophic Effects of 5-HT
It has been postulated that, due to the relatively early emergence of 5-HT neurons in embryogenesis, 5-HT may contribute to CNS development (Lauder & Bloom, 1974; Lauder & Bloom, 1975). Indeed, there are data suggesting “non-traditional” roles for 5-HT in cell proliferation, migration and differentiation, as well as synaptogenesis, neurogenesis, and cortical network organization (Gould, 1999; Buznikov et al., 2001; Santarelli et al., 2003; Janusonis et al., 2004; Vitalis et al., 2007). 5-HT has also been proposed to promote maintenance of brain homeostasis via trophic and metabolic effects on both neuronal and non-neuronal cells (Azmitia, 2007). Many of these effects also appear to be regulated by G proteins and other second messenger pathways that activate gene transcription and protein translation.
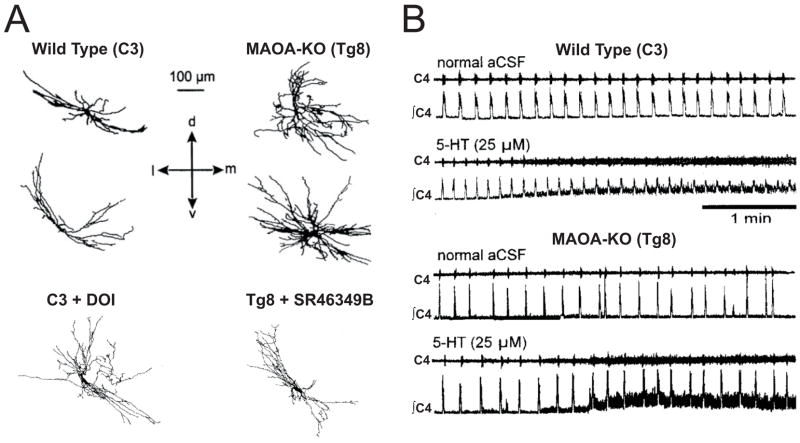
5-HT can exert morphogenetic, or trophic effects on neurons within the developing respiratory network. Mice with deletion of the gene encoding monoamine oxidase A (MAOA), characterized by extremely high levels of 5-HT in the brain during development, display altered thalamocortical and spinal neuronal morphologies (Cases et al., 1996; Bou-Flores et al., 2000; Vitalis et al., 2007) Phrenic motor neurons from MAOA-KO mice have dense arborizations, with greater numbers of dendritic spines and varicosities (Figure 4A: (Bou-Flores et al., 2000)). The altered phrenic motor neuron morphology observed in MAOA-KO mice can be reversed by prenatal treatment with the 5-HT2A antagonist SR46349B, and mimicked by prenatal DOI application in wild type mice. These morphologic alterations have apparent functional significance, as phrenic nerve output from neonatal en bloc preparations of MAOA-KO mice shows greater variability in respiratory cycle duration, which is reversed with prenatal treatment with PCPA or SR46349B. In addition, wild type mice display a 25% increase in frequency of phrenic output in response to exogenous 5-HT, whereas there is no effect in neonatal MAOA-KO mice (Figure 4B). Finally, adult MAOA-KO mice also exhibit an increased breathing frequency and decreased tidal volume, blunted responses to lung inflation and hypoxia, and altered morphology of intercostal (but not phrenic) motor neurons (Burnet et al., 2001). While it is unclear if these functional effects of excess 5-HT arise at the level of the phrenic motor neurons or elsewhere in the respiratory network, these findings show a trophic role for 5-HT in the development of the respiratory network (Hilaire & Duron, 1999), and further suggest that some of these developmental effects persist into adulthood.
Figure 4. Trophic effects of 5-HT on cortical and brainstem networks in monoamine oxidase A knockout (MAOA-KO) mice.
A) Camera lucida drawings of biocytin-stained phrenic motor neurons from wild type (C3) and MAOA-KO (Tg8) mice. Note that DOI in the wild type increases, whereas SR46349B in MAOA-KO mice decreases, dendritic morphology. B) Phrenic nerve recordings (raw and integrated data) from brainstem-spinal cord preparations. Frequency responses to 5-HT in MAOA-KO mice are completely blunted, likely due to extremely high levels of endogenous 5-HT. A & B were adapted from Bou-Flores et al., 2000.
Conversely, there are other experiments in which 5-HT is reduced or absent that suggest that decreased 5-HT does not have an equal and opposite effect on development. Pet-1 null mice for example, which lack ~70% of all 5-HT neurons show normal gross anatomy of most brain structures (Hendricks et al., 2003). Similarly, our analysis of Lmx1bf/f/p mice with near-complete absence of central 5-HT neurons also reveals normal gross neuroanatomy (Hodges et al., 2008). Consistent with this are the observations of only subtle and transient changes in cortical development after chronic prenatal treatment with PCPA (Vitalis et al., 2007). In contrast, PCPA has been found to influence spinal cord development and motor output during early post-natal life (Pflieger et al., 2002), and destruction of spinal projecting 5-HT fibers with 5,7-DHT or blocking 5-HT1A receptors with NAN-190 can inhibit the development of mature motor behaviors in tadpole larvae (Sillar et al., 1995). Thus, it is clear that 5-HT can influence development of the respiratory network and spinal cord function, but more experiments are needed to determine the relative importance of trophic effects of 5-HT (and other transmitters) on the developing and adult respiratory network.
8. Defects of the 5-HT system: relevance to SIDS
SIDS has long been associated with defects in respiratory control and thermoregulation during sleep (Shannon et al., 1977; Hunt et al., 1981; Dunne & Matthews, 1988), and recent advances point to multiple defects in the 5-HT system in SIDS victims (Panigrahy et al., 2000; Paterson et al., 2006). These defects include a decrease in 5-H T1A receptor binding and serotonin transporter density, as well as an increase in the number of granular (possibly immature) 5-HT neurons (Paterson et al., 2006). Thus, determining the physiologic roles of 5-HT neurons would help advance our understanding of the pathophysiology of SIDS.
The contributions of 5-HT neurons to breathing, thermoregulation and sleep are becoming clearer (Richerson, 2004; Madden & Morrison, 2006; Toth et al., 2006; Hoffman et al., 2007; Hodges et al., 2008). Based on the observations of severe reductions in the hypercapnic ventilatory response and thermoregulatory failure in a cold environment in Lmx1bf/f/p mice, 5-HT neurons may act to coordinate metabolic, ventilatory and thermoregulatory demands, particularly when faced with an exogenous stressor (Hinrichsen et al., 1998; Hodges et al., 2008). This concept, when combined with the non-traditional trophic roles of 5-HT during development discussed in this review, suggests a link between abnormalities of the 5-HT system and developmental dysregulation of breathing, body temperature and sleep. Dysfunction of the 5-HT system may contribute to and/or cause SIDS by leading to disruption in the coordination of respiratory output with changes in sleep state, CO2/pH levels and body temperature (Richerson, 2004; Hodges et al., 2008).
9. Summary, open questions and future directions
5-HT neurons project to multiple respiratory nuclei and release 5-HT, SP and TRH - each of which activate G protein-coupled receptors and act to modulate neuronal excitability via second messenger systems. Our current understanding of pre- and post-synaptic receptor expression, and the specific second messenger cascades each receptor activates provides some clarity to the sometimes seemingly opposing effects of 5-HT in breathing. Pre-synaptic activation of 5-HT receptors directly inhibits the release of 5-HT and other neurotransmitters, while post-synaptic 5-HT, SP and TRH receptor activation is generally stimulatory. Thus, the net effect of an increase in firing of serotonin neurons appears to be excitatory at the pre-motor and motor neuron levels in the respiratory network, and a decrease in stimulation of the respiratory network by 5-HT neurons may lead to hypoventilation (e.g. during sleep).
Decades of research investigating the role of 5-HT neurons in respiratory control have provided us with a variety of data using multiple approaches, but left us with a significant number of questions. For example, our current knowledge of pre- and post-synaptic 5-HT, NK-1 and TRH receptor distribution within specific respiratory nuclei is limited, and extending our knowledge would enhance our interpretation of how 5-HT neurons interact with other respiratory neurons. We must also determine if the function of the 5-HT system changes during development, and if so what impact this has on the control systems that 5-HT neurons regulate. Additionally, further investigation is required to determine other possible conditions/interactions (in addition to sleep state, CO2/pH and environmental temperature) that alter the activity of 5-HT neurons and/or the release of these neuromodulators. Finally, furthering our understanding of the function of the 5-HT system will ultimately shed light on human diseases linked to 5-HT system dysfunction, and may lead to new modes of prevention and treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Sanders-Bush E. Serotonin. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmocology: The Fifth Generation of Progress. Lippincott Williams and Wilkins; 2002. pp. 15–34. [Google Scholar]
- Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflugers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- Andrade R, Malenka RC, Nicoll RA. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Auerbach S, Fornal C, Jacobs BL. Response of serotonin-containing neurons in nucleus raphe magnus to morphine, noxious stimuli, and periaqueductal gray stimulation in freely moving cats. Exp Neurol. 1985;88:609–628. doi: 10.1016/0014-4886(85)90075-5. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Serotonin and brain: evolution, neuroplasticity, and homeostasis. Int Rev Neurobiol. 2007;77:31–56. doi: 10.1016/S0074-7742(06)77002-7. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: inhibition of N- and P/Q-type calcium channels and the after hyperpolarization. J Neurophysiol. 1997a;77:1362–1374. doi: 10.1152/jn.1997.77.3.1362. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Umemiya M, Berger AJ. Inhibition of N- and P-type calcium currents and the after-hyperpolarization in rat motoneurones by serotonin. J Physiol. 1995;485 (Pt 3):635–647. doi: 10.1113/jphysiol.1995.sp020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Talley EM, Berger AJ. Neuromodulation of hypoglossal motoneurons: cellular and developmental mechanisms. Respir Physiol. 1997b;110:139–150. doi: 10.1016/s0034-5687(97)00079-0. [DOI] [PubMed] [Google Scholar]
- Benson JA, Levitan IB. Serotonin increases an anomalously rectifying K+ current in the Aplysia neuron R15. Proc Natl Acad Sci U S A. 1983;80:3522–3525. doi: 10.1073/pnas.80.11.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Sebben M, Dumuis A. Pharmacological characterization of 5-hydroxytryptamine4(5-HT4) receptors positively coupled to adenylate cyclase in adult guinea pig hippocampal membranes: effect of substituted benzamide derivatives. Mol Pharmacol. 1990;37:408–411. [PubMed] [Google Scholar]
- Bou-Flores C, Lajard AM, Monteau R, De ME, Seif I, Lanoir J, Hilaire G. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of a serotonin excess. J Neurosci. 2000;20:4646–4656. doi: 10.1523/JNEUROSCI.20-12-04646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhelal R, Smounya L, Bockaert J. 5-HT1B receptors are negatively coupled with adenylate cyclase in rat substantia nigra. Eur J Pharmacol. 1988;151:189–196. doi: 10.1016/0014-2999(88)90799-6. [DOI] [PubMed] [Google Scholar]
- Brandes IF, Zuperku EJ, Stucke AG, Jakovcevic D, Hopp FA, Stuth EA. Serotonergic modulation of inspiratory hypoglossal motoneurons in decerebrate dogs. J Neurophysiol. 2006;95:3449–3459. doi: 10.1152/jn.00823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Burnet H, Bevengut M, Chakri F, Bou-Flores C, Coulon P, Gaytan S, Pasaro R, Hilaire G. Altered respiratory activity and respiratory regulations in adult monoamine oxidase A-deficient mice. J Neurosci. 2001;21:5212–5221. doi: 10.1523/JNEUROSCI.21-14-05212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buznikov GA, Lambert HW, Lauder JM. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305:177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De ME, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Chaput Y, Lesieur P, de MC. Effects of short-term serotonin depletion on the efficacy of serotonin neurotransmission: electrophysiological studies in the rat central nervous system. Synapse. 1990;6:328–337. doi: 10.1002/syn.890060404. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hedner J, Hedner T. Substance P in the ventrolateral medulla oblongata regulates ventilatory responses. J Appl Physiol. 1990;68:2631–2639. doi: 10.1152/jappl.1990.68.6.2631. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E, Hoffman BJ, Hartig PR. A unique serotonin receptor in choroid plexus is linked to phosphatidylinositol turnover. Proc Natl Acad Sci U S A. 1986;83:4086–4088. doi: 10.1073/pnas.83.11.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett. 1989;105:34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Cream C, Li A, Nattie E. The retrotrapezoid nucleus (RTN): local cytoarchitecture and afferent connections. Respir Physiol Neurobiol. 2002;130:121–137. doi: 10.1016/s0034-5687(01)00338-3. [DOI] [PubMed] [Google Scholar]
- Cream C, Nattie E, Li A. TRH microdialysis into the RTN of the conscious rat increases breathing, metabolism, and temperature. J Appl Physiol. 1999;87:673–682. doi: 10.1152/jappl.1999.87.2.673. [DOI] [PubMed] [Google Scholar]
- Cream CL, Li A, Nattie EE. RTN TRH causes prolonged respiratory stimulation. J Appl Physiol. 1997;83:792–799. doi: 10.1152/jappl.1997.83.3.792. [DOI] [PubMed] [Google Scholar]
- Dailly E, Chenu F, Petit-Demouliere B, Bourin M. Specificity and efficacy of noradrenaline, serotonin depletion in discrete brain areas of Swiss mice by neurotoxins. J Neurosci Methods. 2006;150:111–115. doi: 10.1016/j.jneumeth.2005.06.008. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de CD, Leysen JE, De CF, Van BH, Janssen PA. Evidence that phospholipid turnover is the signal transducing system coupled to serotonin-S2 receptor sites. J Biol Chem. 1985;260:7603–7608. [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. A light and electron microscopic study of serotonin-immunoreactive fibers and terminals in the monkey sensory-motor cortex. Exp Brain Res. 1988;71:171–182. doi: 10.1007/BF00247532. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229:67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Lindsay A, Feldman J, Monteau R, Hilaire G. Serotonergic inhibition of phrenic motoneuron activity: an in vitro study in neonatal rat. Neurosci Lett. 1997;230:29–32. doi: 10.1016/s0304-3940(97)00469-2. [DOI] [PubMed] [Google Scholar]
- Dori IE, Dinopoulos A, Parnavelas JG. The development of the synaptic organization of the serotonergic system differs in brain areas with different functions. Exp Neurol. 1998;154:113–125. doi: 10.1006/exnr.1998.6937. [DOI] [PubMed] [Google Scholar]
- Dunne KP, Matthews TG. Hypothermia and sudden infant death syndrome. Arch Dis Child. 1988;63:438–440. doi: 10.1136/adc.63.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci U S A. 1990;87:2187–2191. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Neverova NV, Saywell SA. Modulation of hypoglossal motoneuron excitability by intracellular signal transduction cascades. Respir Physiol Neurobiol. 2005;147:131–143. doi: 10.1016/j.resp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Litto WJ, Morilak DA, Jacobs BL. Single-unit responses of serotonergic dorsal raphe nucleus neurons to environmental heating and pyrogen administration in freely moving cats. Exp Neurol. 1987;98:388–403. doi: 10.1016/0014-4886(87)90250-0. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Metzler CW, Marrosu F, Ribiero-do-Valle LE, Jacobs BL. A subgroup of dorsal raphe serotonergic neurons in the cat is strongly activated during oral-buccal movements. Brain Res. 1996;716:123–133. doi: 10.1016/0006-8993(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Fuxe K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965;(SUPPL) [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, al-Zubaidy Z, Carter JE. Thyrotropin-releasing hormone stimulates perinatal rat respiration in vitro. Am J Physiol. 1996;271:R1160–R1164. doi: 10.1152/ajpregu.1996.271.5.R1160. [DOI] [PubMed] [Google Scholar]
- Gunther S, Maroteaux L, Schwarzacher SW. Endogenous 5-HT2B receptor activation regulates neonatal respiratory activity in vitro. J Neurobiol. 2006;66:949–961. doi: 10.1002/neu.20253. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner J, Hedner T, Wessberg P, Lundberg D, Jonason J. Effects of TRH and TRH analogues on the central regulation of breathing in the rat. Acta Physiol Scand. 1983;117:427–437. doi: 10.1111/j.1748-1716.1983.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev. 1999;79:325–360. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF, Maskrey M, Mortola JP. Ventilatory and metabolic responses to cold and hypoxia in conscious rats with discrete hypothalamic lesions. Respir Physiol. 1998;111:247–256. doi: 10.1016/s0034-5687(98)00002-4. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall G, Harris MB, McEvoy S, Richerson D, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, Brown JW, Sirlin EA, Benoit AM, Gill WH, Harris MB, Darnall RA. Activation of 5-HT1A receptors in the paragigantocellularis lateralis decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol. 2007 doi: 10.1152/ajpregu.00816.2006. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Arvidsson U, Cullheim S, Millhorn D, Nicholas AP, Pieribone V, Seroogy K, Ulfhake B. Multiple messengers in descending serotonin neurons: localization and functional implications. J Chem Neuroanat. 2000;18:75–86. doi: 10.1016/s0891-0618(99)00037-x. [DOI] [PubMed] [Google Scholar]
- Holtman JR., Jr Immunohistochemical localization of serotonin- and substance P-containing fibers around respiratory muscle motoneurons in the nucleus ambiguus of the cat. Neuroscience. 1988;26:169–178. doi: 10.1016/0306-4522(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Buller AL, Hamosh P, Gillis RA. Central respiratory stimulation produced by thyrotropin-releasing hormone in the cat. Peptides. 1986;7:207–212. doi: 10.1016/0196-9781(86)90214-7. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Marion LJ, Speck DF. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neuroscience. 1990a;37:541–552. doi: 10.1016/0306-4522(90)90422-z. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Norman WP, Skirboll L, Dretchen KL, Cuello C, Visser TJ, Hokfelt T, Gillis RA. Evidence for 5-hydroxytryptamine, substance P, and thyrotropin-releasing hormone in neurons innervating the phrenic motor nucleus. J Neurosci. 1984;4:1064–1071. doi: 10.1523/JNEUROSCI.04-04-01064.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman JR, Jr, Vascik DS, Maley BE. Ultrastructural evidence for serotonin-immunoreactive terminals contacting phrenic motoneurons in the cat. Exp Neurol. 1990b;109:269–272. doi: 10.1016/s0014-4886(05)80016-0. [DOI] [PubMed] [Google Scholar]
- Hunt CE, McCulloch K, Brouillette RT. Diminished hypoxic ventilatory responses in near-miss sudden infant death syndrome. J Appl Physiol. 1981;50:1313–1317. doi: 10.1152/jappl.1981.50.6.1313. [DOI] [PubMed] [Google Scholar]
- Innis RB, Nestler EJ, Aghajanian GK. Evidence for G protein mediation of serotonin- and GABAB-induced hyperpolarization of rat dorsal raphe neurons. Brain Res. 1988;459:27–36. doi: 10.1016/0006-8993(88)90282-x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of brain serotonergic neurons in the behaving animal. Pharmacol Rev. 1991;43:563–578. [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Brain serotonergic neuronal activity in behaving cats. In: Monti JM, Prandi-Perumal SR, Jacobs BL, Nutt DJ, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Birkhauser; Verlag/Switzerland: 2008. pp. 185–204. [Google Scholar]
- Janusonis S, Gluncic V, Rakic P. Early serotonergic projections to Cajal-Retzius cells: relevance for cortical development. J Neurosci. 2004;24:1652–1659. doi: 10.1523/JNEUROSCI.4651-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet M. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21:24S–27S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK, Levitan IB. Neuromodulation: The Biochemical Control of Neuronal Excitability. Oxford University Press; New York: 1987. [Google Scholar]
- Kaczmarek LK, Strumwasser F. A voltage-clamp analysis of currents underlying cyclic AMP-induced membrane modulation in isolated peptidergic neurons of Aplysia. J Neurophysiol. 1984;52:340–349. doi: 10.1152/jn.1984.52.2.340. [DOI] [PubMed] [Google Scholar]
- Kanamaru M, Homma I. Compensatory airway dilation and additive ventilatory augmentation mediated by dorsomedial medullary 5-hydroxytryptamine 2 receptor activity and hypercapnia. Am J Physiol Regul Integr Comp Physiol. 2007;293:R854–R860. doi: 10.1152/ajpregu.00829.2006. [DOI] [PubMed] [Google Scholar]
- Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int J Biochem Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- Klein M, Camardo J, Kandel ER. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982;79:5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe BK, Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966;154:499–516. [PubMed] [Google Scholar]
- Korstanje C, Sprenkels R, Doods HN, Hugtenburg JG, Boddeke E, Batink HD, Thoolen MJ, Van Zwieten PA. Characterization of flufylline, fluprofylline, ritanserin, butanserin and R 56413 with respect to in-vivo alpha 1-, alpha 2- and 5-HT2-receptor antagonism and in-vitro affinity for alpha 1-, alpha 2- and 5-HT2-receptors: comparison with ketanserin. J Pharm Pharmacol. 1986;38:374–379. doi: 10.1111/j.2042-7158.1986.tb04590.x. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Schwarzacher SW, Richter DW. 5-HT2 receptor-controlled modulation of medullary respiratory neurones in the cat. J Physiol. 1995;487 (Pt 3):653–661. doi: 10.1113/jphysiol.1995.sp020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfumey L, Hamon M. 5-HT1 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:1–10. doi: 10.2174/1568007043482570. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol. 1974;155:469–481. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra of the rat. II. Synaptogenesis. J Comp Neurol. 1975;163:251–264. doi: 10.1002/cne.901630302. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol. 2006;577:307–318. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liposits Z, Phelix C, Paull WK. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86:541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley BE, Engle MG, Humphreys S, Vascik DA, Howes KA, Newton BW, Elde RP. Monoamine synaptic structure and localization in the central nervous system. J Electron Microsc Tech. 1990;15:20–33. doi: 10.1002/jemt.1060150104. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- Manaker S, Verderame HM. Organization of serotonin 1A and 1B receptors in the nucleus of the solitary tract. J Comp Neurol. 1990;301:535–553. doi: 10.1002/cne.903010405. [DOI] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Manzke T, Preusse S, Hulsmann S, Richter DW. Developmental changes of serotonin 4(a) receptor expression in the rat pre-Botzinger complex. J Comp Neurol. 2008;506:775–790. doi: 10.1002/cne.21581. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Jacobs BL. Single-unit responses of serotonergic medullary raphe neurons to cardiovascular challenges in freely moving cats. Eur J Neurosci. 2005;22:3195–3204. doi: 10.1111/j.1460-9568.2005.04519.x. [DOI] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–309. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minota S, Dun NJ, Karczmar AG. Substance P-induced depolarization in sympathetic neurons: not simple K-inactivation. Brain Res. 1981;216:224–228. doi: 10.1016/0006-8993(81)91294-4. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Kia HK, Boni C, Doucet E, Daval G, Matthiessen L, Hamon M, Verge D. Postnatal development and localization of 5-HT1A receptor mRNA in rat forebrain and cerebellum. Brain Res Dev Brain Res. 1994;80:149–157. doi: 10.1016/0165-3806(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Smith CA, Vidruk EH, Jameson LC, Dempsey JA. Effects of p-chlorophenylalanine on ventilatory control in goats. J Appl Physiol. 1983;54:277–283. doi: 10.1152/jappl.1983.54.1.277. [DOI] [PubMed] [Google Scholar]
- Morgado-Valle C, Feldman JL. Depletion of substance P and glutamate by capsaicin blocks respiratory rhythm in neonatal rat in vitro. J Physiol. 2004;555:783–792. doi: 10.1113/jphysiol.2003.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller RA, Towle AC, Breese GR. Supersensitivity to the respiratory stimulatory effect of TRH in 5,7-dihydroxytryptamine-treated rats. Brain Res. 1984;298:370–373. doi: 10.1016/0006-8993(84)91440-9. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Murakoshi T, Suzue T, Tamai S. A pharmacological study on respiratory rhythm in the isolated brainstem-spinal cord preparation of the newborn rat. Br J Pharmacol. 1985;86:95–104. doi: 10.1111/j.1476-5381.1985.tb09439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason MW, Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci. 2006;26:1190–1198. doi: 10.1523/JNEUROSCI.4707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE. The retrotrapezoid nucleus and the ‘drive’ to breathe. J Physiol. 2006;572:311. doi: 10.1113/jphysiol.2006.107284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Mackiewicz M, Kubin L. Serotonin receptor mRNA expression in the hypoglossal motor nucleus. Respir Physiol. 1997;110:151–160. doi: 10.1016/s0034-5687(97)00080-7. [DOI] [PubMed] [Google Scholar]
- Olson EB, Jr, Dempsey JA, McCrimmon DR. Serotonin and the control of ventilation in awake rats. J Clin Invest. 1979;64:689–693. doi: 10.1172/JCI109510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59:377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Passerin AM, Henley WN. Activation of spinal cord serotonergic neurons accompanies cold-induced sympathoexcitation. Can J Physiol Pharmacol. 1994;72:884–892. doi: 10.1139/y94-125. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penalva RG, Lancel M, Flachskamm C, Reul JM, Holsboer F, Linthorst AC. Effect of sleep and sleep deprivation on serotonergic neurotransmission in the hippocampus: a combined in vivo microdialysis/EEG study in rats. Eur J Neurosci. 2003;17:1896–1906. doi: 10.1046/j.1460-9568.2003.02612.x. [DOI] [PubMed] [Google Scholar]
- Pflieger JF, Clarac F, Vinay L. Postural modifications and neuronal excitability changes induced by a short-term serotonin depletion during neonatal development in the rat. J Neurosci. 2002;22:5108–5117. doi: 10.1523/JNEUROSCI.22-12-05108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky PM, De CD, Llewellyn-Smith I, Lipski J, Voss MD. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci. 1990;10:1091–1098. doi: 10.1523/JNEUROSCI.10-04-01091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Zhang R, Milescu LS, Richerson GB, Smith JC. Modulation of neuronal activity in the pre-Botzinger complex by medullary raphe neurons. Society for Neuroscience Abstracts. 2006;32 Ref Type: Abstract. [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Reader TA, Gauthier P. Catecholamines and serotonin in the rat central nervous system after 6-OHDA, 5-7-DHT and p-CPA. J Neural Transm. 1984;59:207–227. doi: 10.1007/BF01250009. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res. 1996;739:57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Gunther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115:1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Shannon DC, Kelly DH, O’Connell K. Abnormal regulation of ventilation in infants at risk for sudden-infant-death syndrome. N Engl J Med. 1977;297:747–750. doi: 10.1056/NEJM197710062971403. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Woolston AM, Wedderburn JF. Involvement of brainstem serotonergic interneurons in the development of a vertebrate spinal locomotor circuit. Proc Biol Sci. 1995;259:65–70. doi: 10.1098/rspb.1995.0010. [DOI] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Cholley B, El MS, Gozlan H, Hamon M. Direct Immunohistochemical Evidence of the Existence of 5-HT1A Autoreceptors on Serotoninergic Neurons in the Midbrain Raphe Nuclei. Eur J Neurosci. 1990;2:1144–1154. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of {alpha}-1 adrenergic receptors and serotonin 5HT2 receptors. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.00599.2007. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Sun Y, Lu X, Gershengorn MC. Thyrotropin-releasing hormone receptors -- similarities and differences. J Mol Endocrinol. 2003;30:87–97. doi: 10.1677/jme.0.0300087. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Sadr NN, Bayliss DA. Postnatal development of serotonergic innervation, 5-HT1A receptor expression, and 5-HT responses in rat motoneurons. J Neurosci. 1997;17:4473–4485. doi: 10.1523/JNEUROSCI.17-11-04473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol. 2002;88:206–213. doi: 10.1152/jn.2002.88.1.206. [DOI] [PubMed] [Google Scholar]
- Thor KB, Helke CJ. Serotonin and substance P colocalization in medullary projections to the nucleus tractus solitarius: dual-colour immunohistochemistry combined with retrograde tracing. J Chem Neuroanat. 1989;2:139–148. [PubMed] [Google Scholar]
- Toppin VA, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol. 2007;103:220–227. doi: 10.1152/japplphysiol.00071.2007. [DOI] [PubMed] [Google Scholar]
- Toth IE, Toth DE, Boldogkoi Z, Hornyak A, Palkovits M, Blessing WW. Serotonin-synthesizing neurons in the rostral medullary raphe/parapyramidal region transneuronally labelled after injection of pseudorabies virus into the rat tail. Neurochem Res. 2006;31:277–286. doi: 10.1007/s11064-005-9018-2. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooft JA, Yakel JL. 5-HT3 receptors in the CNS: 3B or not 3B? Trends Pharmacol Sci. 2003;24:157–160. doi: 10.1016/S0165-6147(03)00051-8. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience. 1997;79:161–169. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. Eur J Neurosci. 2007;26:331–344. doi: 10.1111/j.1460-9568.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- Voss MD, De CD, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990;295:208–218. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Lagercrantz H, von EC. Effects of substance P and TRH on ventilation and pattern of breathing in newborn rabbits. Acta Physiol Scand. 1981;113:541–543. doi: 10.1111/j.1748-1716.1981.tb06935.x. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Ellenberger HH, Feldman JL. Monoaminergic and GABAergic terminations in phrenic nucleus of rat identified by immunohistochemical labeling. Neuroscience. 1989;31:105–113. doi: 10.1016/0306-4522(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]