Abstract
We discuss the time course of postnatal development of selected neurotransmitter receptors in motoneurons that innervate respiratory pump and accessory respiratory muscles, with emphasis on other than classic respiratory signals as important regulatory factors. Functions of those brainstem motoneurons that innervate the pharynx and larynx change more dramatically during early postnatal development than those of spinal respiratory motoneurons. Possibly in relation to this difference, the time course of postnatal expression of distinct receptors for serotonin differ between the hypoglossal (XII) and phrenic motoneurons. In rats, distinct developmental patterns include a decline or increase that extends over the first 3−4 postnatal weeks, a rapid increase during the first two weeks, or a transient decline on postnatal days 11−14. The latter period coincides with major changes in many transmitters in brainstem respiratory regions that may be related to a brain-wide reconfiguration of sensorymotor processing resulting from eye and ear opening and beginning of a switch from suckling to mature forms of food seeking and processing. Such rapid neurochemical changes may impart increased vulnerability on the respiratory system. We also consider rapid eye movement sleep as a state during which some brain functions may revert to conditions typical of perinatal period. In addition to normal developmental processes, changes in the expression or function of neurotransmitter receptors may occur in respiratory motoneurons in response to injury, perinatal stress, or disease conditions that increase the load on respiratory muscles or alter the normal levels and patterns of oxygen delivery.
Keywords: motor development, norepinephrine, respiratory motoneurons, serotonin, sleep, sudden infant death syndrome
1. Introduction
Motoneurons are a common end-point of the central neural integration of information about the internal and external environments. Therefore, observations of changes in their biophysical, biochemical and morphological properties, as well as their activity, offers an invaluable insight into brain functions. In this brief review, we present and discuss selected data about postnatal developmental changes in motoneurons that control the respiratory pump and accessory respiratory muscles. Unless indicated otherwise, we focus on the results from rats, which have been most commonly used as a model system in this field. Postnatal development of respiratory behavior and responses to classic respiratory challenges, such as hypoxia, hypercapnia or altered conditions in the lungs and airways, are covered in other contributions to this Special Issue and in another special Special Issue that was entirely devoted to the development of respiratory control (Respir. Physiol. Neurobiol. 149:1−353, 2005). Here, we choose to give more emphasis to non-respiratory contexts of postnatal developmental changes that may impact the development of respiratory motoneurons because the subject is under-represented in respiratory literature.
2. Developmental requirements on motoneurons that innervate respiratory pump and accessory respiratory muscles in rats
An important distinction needs to be made. In contrast to the diaphragm-innervating phrenic motoneurons whose main function is to providing adequate pumping action, the motoneurons that innervate accessory respiratory muscles subserve multiple non-respiratory but also vital functions. Indeed, the respiratory functions of the latter are often secondary and/or limited to special conditions or disease states. The trigeminal, facial, hypoglossal (XII) and vagal motoneurons that innervate about 27 different muscles of the upper airway play key roles in digestive behaviors and phonation in addition to their important airway-protective functions throughout the life span and their role in the maintenance of airway patency that they may need to fulfill occasionally or in individuals with special conditions. The motoneurons that innervate intercostal and abdominal muscles enhance the respiratory pumping action controlled by phrenic motoneurons, but they also have major tonic and phasic roles in postural control, locomotion and waste discharge. Also important to note in the context of motoneuronal development is that different motoneuronal groups and parts of pontomedullary respiratory network emerge during embryonic development from distinct hindbrain segments (rombomeres) (Carroll, 2003; Borday et al., 2006; Chatonnet et al., 2006). Different rombomeric origins may determine the time course of normal development of different motoneuronal pools in addition to the gradually changing functional requirements during the postnatal period.
Rats belong to altricial species. They are born able to generate adequate rhythmic ventilation and suckling and exhibit huddling behavior, but they are blind, deaf, unable to maintain body temperature, digest food other than mother's milk, or eliminate waste without maternal assistance (Henning, 1981; Westneat and Hall, 1992). Their sleep-wake pattern is also grossly underdeveloped, with the state of an undifferentiated “active sleep” interrupted by short bouts of “quiet sleep” occupying nearly 90% of their life after birth (Frank and Heller, 1997; Seelke et al., 2005). Neonatal rats breathe, suckle and sniff both during sleep and when awake (Seelke and Blumberg, 2004). These three competing behaviors significantly share the same motor outputs and are apparently well synchronized and coordinated at birth. Due to technical limitations, the activity of the diaphragm and orofacial muscles has not been studied in rats in vivo right after birth. More information is available from neonatal lambs, but the recordings are limited to the diaphragm and laryngeal muscles (Praud and Reix, 2005). Given that lambs belong to precocial species (more mature at birth than rats), the observations from neonatal lambs may pertain to a relatively more advanced period of development than in rats, and may also be specific to ruminating species.
3. Postnatal milestones that may impact the development of respiratory motoneurons in rats
The first three weeks of postnatal life of the rat are marked by profound changes in the pup's ability to interacts with the environment, changes that also dramatically increase the demands on the central integration of many behaviors. Figure 1 shows the time course of major non-respiratory developments during the first 30 postnatal days of a laboratory rat (cf. Henning, 1981; Westneat and Hall, 1992; Frank and Heller, 1997; Christensson and Garwicz, 2005; Landers and Philip, 2006). This time course can be related to the progress of neural development in other species, including humans, using a regression analysis based on nearly 100 changes in the morphology of various subcortical structures (Clancy et al., 2001). According to this analysis, gestational day 10 in rats (peak of cranial motor nuclei development) corresponds to the gestational day 35 in human fetus, and postnatal day 14 (eye opening) in rats corresponds to gestational day 133 according to the model, or day 81 empirically, in humans. However, application of this scale to the development of vagal visceral afferents and their central connections within the nucleus of the solitary tract suggests a more rapid development of viscerosensory pathways in rats when compared to humans, embryonic (E) days E15−19 vs. E63−105 (Zhang and Ashwell, 2001; Cheng et al., 2006).
Fig. 1.
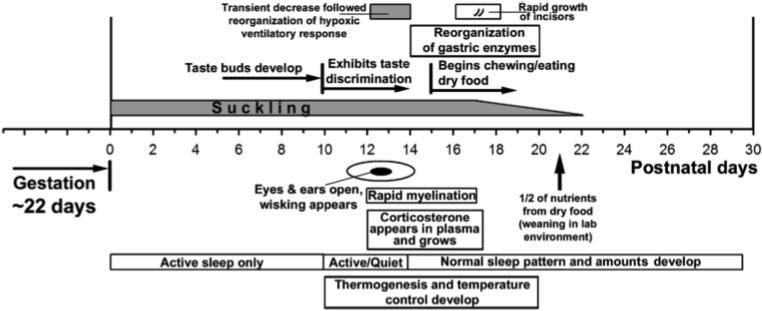
Time course of major developmental events during the first 30 postnatal days in rats. Of particular note is the period between the postnatal days 11 and 14 because it is characterized by a host of major developmental changes, such as rapid myelination in the brain, appearance of corticosterone in plasma and a dramatic sensory enrichment related to eye and ear opening. These events precede the processes of metabolic transition into consumption of food other than mother's milk and occur superimposed on parallel development of thermoregulation and maturation of sleep behavior. Together, these changes impose major new demands on sensorymotor processing and greatly increase the complexity of motor behaviors. The period between days 11 and 14 is also characterized by a significant reconfiguration of the ventilatory response to hypoxia.
It is apparent from Fig. 1 that, in rats, the period between the 11th and 14th postnatal day is particularly rich in events that transform the pup from being fully dependent on the maternal environment to one that can see, hear, control its own temperature, and seek food other than maternal milk. During this period, there is a major neurochemical reorganization in pontomedullary respiratory regions, including the medullary respiratory motor and viscerosensory nuclei (Wong-Riley and Liu, 2005; Yoshioka et al., 2006). At the systemic level, the same period is associated with a transient blunting of hypoxic ventilatory response followed by a reconfiguration of its respiratory rate and volume components (Waters and Gozal, 2003; Liu et al., 2006).
4. Development of aminergic control of respiratory-modulated motoneurons
Serotonin (5-HT) and norepinephrine have been identified as major modulators of motoneuronal activity in relation to rhythmic motor behaviors (Jacobs and Fornal, 1995; Pena and Ramirez, 2002; Viemari and Ramirez, 2006), sleep-wake states (Kubin et al., 1998; Fenik et al., 2005; Chan et al., 2006), control of pain and transmission in reflex pathways (Stafford & Jacobs, 1990; Clark and Proudfit, 1993; Van Bockstaele and Aston-Jones, 1995), central CO2 chemosensitivity (Bradley et al., 2002; Feldman et al., 2003; Wang and Richerson, 2007; Guyenet et al., 2008), and long-term changes in respiratory motor output resulting from stress or injury (Ling et al., 2001; Zhou et al., 2001; Feldman et al., 2003; Neverova et al., 2007). Data indicate that, in rats, serotonergic neurons and their projections to the motor regions are nearly mature at birth, whereas the projections to sensory regions, including those that control the transmission of pain, are not fully developed (Jacobs and Azmitia, 1992; Talley et al., 1997; Tanaka et al., 2006). Brainstem noradrenergic neurons and their projections are also present at birth, but tyrosine hydroxylase activity in the pontomedullary catecholaminergic groups exhibits distinct postnatal peaks, one on postnatal (P) day 3 and another on P21. In addition, there is a peak involving only locus coeruleus on P14 (Roux et al., 2003). The developmental patterns of 5-HT and adrenergic receptors are complex and differ among the brain regions and receptor types. The lack of a clear temporal relationship between 5-HT cell and axon terminal development and receptor development indicates that receptor changes are only to a limited extent determined by their endogenous ligand.
Studies of the developmental time course of 5-HT and related receptors in respiratory motoneurons begin to provide insights into the underlying mechanisms and developmentally changing roles of these receptors. Figure 2 summarizes the limited developmental data available for 5-HT receptors in the hypoglossal (XII) nucleus, as representative for orofacial motoneurons, and in upper cervical (spinal) motoneurons. We also include the data relative to thyrotropin-releasing hormone (TRH) receptor binding in the XII nucleus (Bayliss et al., 1997) because serotoninergic terminals are a major source of this hormone (Johnson et al., 1993).
Fig. 2.
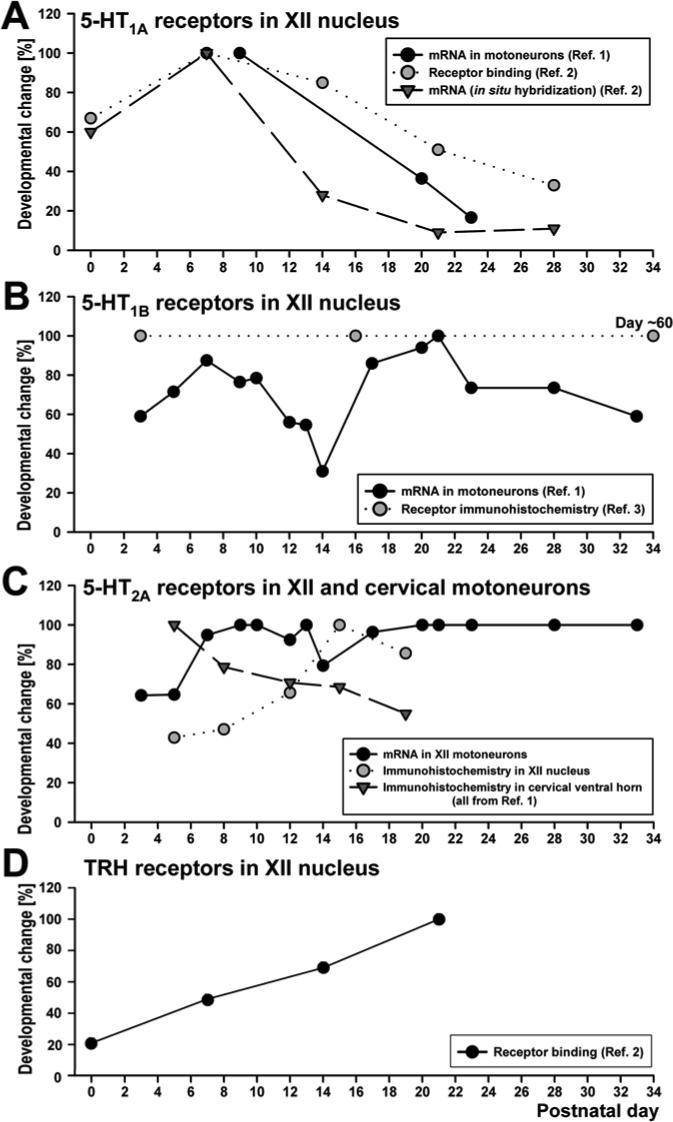
Time course of postnatal changes in expression of major serotoninergic and other related receptors in orofacial and spinal motoneurons. The diagrams summarize data for selected orofacial and spinal motoneurons. Since the results from different sources used different scales and units of measure, we normalized published results relative to the highest value in each study. The data used to compile the diagram were derived from: Reference (Ref.) #1 - proportion of XII motoneurons expressing 5-HT1A, 5-HT1B and 5-HT2A receptor mRNA, and 5-HT2A receptor immunohistochemistry in XII and upper cervical motoneurons (Volgin et al., 2003); Ref. #2 − 5-HT1A receptor mRNA and 5-HT1A and thyrotropin releasing hormone receptor binding in the XII nucleus (Bayliss et al., 1997); Ref. 3 − 5-HT1B receptor immunohistochemistry (Berger and Huynh, 2002). Of general note here are three points: (1) the time course of expression differs among distinct receptor subtypes for the same neurotransmitter even within a single motoneuronal pool (e.g., 5-HT1A, 5-HT1B and 5-HT2A receptors in XII motoneurons); (2) the same receptor can follow different developmental time courses in orofacial (XII) and spinal motoneurons (e.g., 5-HT2A receptors in the XII nucleus and cervical ventral horn); and (3) some receptors can exhibit very large and biphasic changes, at least at the mRNA level, within just a few days (e.g., 5-HT1B receptor mRNA in XII motoneurons).
The different 5-HT receptors considered here have different postnatal patterns and, at least at the mRNA level, can exhibit very rapid changes over just a few days (Fig. 2). Some receptor types have a different developmental time course in orofacial (XII) and spinal motoneurons (5-HT2A), whereas others (5-HT1A) have similar developmental patterns at both levels (Talley et al., 1997). Similar to 5-HT1A receptors, a declining pattern also exist for inhibitory adrenergic α2A receptors in XII motoneurons (Winzer-Serhan et al., 1997).
By far the most complex changes shown in Fig. 2 are those in the expression of 5-HT1B receptors in XII motoneurons. These receptors are frequently located on presynaptic terminals and modulate release of other transmitters (Boschert et al., 1994). However, their expression in motoneurons suggests that they also mediate postsynaptic effects (Berger and Huynh, 2002; Volgin et al., 2003). To date, postsynaptic effects appear to be weak in XII motoneurons (Bouryi and Lewis, 2003). An alternative possibility is that 5-HT1B receptors expressed in motoneurons presynaptically control transmitter release at the motor plate level (D'Agostino et al., 2006). Broadly supportive of this are the findings that 5-HT1B receptor expression declines in the brain in response to physical training (Chennaoui et al., 2000). There is also evidence that 5-HT1B receptors carry trophic and other signals during embryonic and postnatal development both in the brain and peripheral nervous system (Nebigil et al., 2001). The coincidence of the rapid decline and then increase in 5-HT1B receptor mRNA expression in XII motoneurons (Fig. 2B) with the period of a particularly active reconfiguration of many sensory, motor and homeostatic functions (Fig. 1) would be consistent with an important role of 5-HT1B receptors in the guidance of developmental processes. The corresponding changes of 5-HT1B receptor protein expression in the XII nucleus remain to be studied because the data currently available were not collected frequently enough to address the question (Berger and Huynh, 2002).
The excitatory 5-HT2A receptors are expressed at high levels in spinal motoneurons right after birth, but in XII and ambiguus motoneurons their expression gradually increases through at least P12 (Volgin et al., 2003; 2004). Recent data suggest that the postnatal time course of 5-HT2A receptor development is similar in identified phrenic motoneurons and other motoneurons of the upper cervical spinal cord (Bou-Flores and Hilaire, 2000; Volgin et al., 2003). As expected, the increase in receptor protein lags behind the receptor mRNA expression. The mature pattern of receptor expression in distal dendrites is established only after XII motoneurons complete the first phase of a major developmental change in their dendritic morphology, which progresses from P0 to P15 (Cameron and Núñez-Abades, 2000). The developmental time course of excitation of XII motoneurons mediated by α1-adrenergic receptors in mice (Funk et al., 1994) is similar to the time course of 5-HT2A receptor expression in these motoneurons in rats (Volgin et al., 2003). Interestingly, phrenic motoneurons do not exhibit bi-phasic morphological changes during postnatal development as those described for XII motoneurons (Lindsay et al., 1991; Núñez-Abades et al., 1994); this may be associated with their more mature status at birth, as suggested by relatively late expression of 5-HT2A receptors in XII motoneurons (Lindsay and Feldman, 1993; Bayliss et al., 1997). The steady or increasing patterns of 5-HT2A receptor expression in spinal and orofacial motoneurons contrast with a decrementing pattern for 5-HT1A receptors and the complex patterns observed for 5-HT1B receptors. Both 5-HT1A and 5-HT1B receptors are principally inhibitory, whereas 5-HT2A receptors are excitatory. However, it appears that 5-HT1A receptors may also mediate excitatory effects during an early postnatal period, and 5-HT2A receptors may mediate suppression of respiratory motor output under some experimental conditions (Monteau et al., 1990; Berger et al., 1992). Thus, the postnatal patterns of 5-HT receptor expression in motoneurons are not always related to the excitatory or inhibitory nature of the effects they typically evoke.
The more advanced during the early postnatal period development of phrenic and other spinal motoneurons than that of orofacial motoneurons appears to correlate with better developed motoneuronal 5-HT2A receptors in spinal than in XII or ambiguus motoneurons (Lindsay et al., 1991; Núñez-Abades et al., 1994; Volgin et al., 2003; 2004). These receptors may mediate uncoupling of gap junctions (Rorig and Sutor, 1996). The postnatal increase of 5-HT2A receptor expression in XII motoneurons (Volgin et al., 2003) coincides with the disappearance of electrotonic coupling, which occurs in these motoneurons around P10 but, in phrenic motoneurons, 5-HT2A receptors appears significantly earlier than the disappearance of electrotonic coupling (reviewed by Greer and Funk, 2005).
There is a good agreement between the magnitude of the excitatory effects of TRH and the time course of development of TRH receptors in XII motoneurons (Funk et al., 1994; Bayliss et al., 1997). However, unlike 5-HT receptors that change most dramatically during the first two postnatal weeks, TRH receptor levels increase gradually for over three weeks. This time course may correspond to a distinct developmental process that occurs in motoneurons from birth to the end of the weaning period (Fig. 1). It may be related to peripheral metabolic changes (Henning, 1981), as well as maturation of the hypothalamic regulation of both sleep and metabolism. For example, expression of mRNA for the type 2 receptors for the hypothalamic excitatory peptides, orexins, in XII motoneurons reaches a peak around P21 (Volgin et al., 2002). The serotonergic and peptidergic innervation of the locus coeruleus, a noradrenergic nucleus strongly involved in processing of emotionally relevant sensory information and regulation of cognitive functions and sleep, also develops gradually over a period of several postnatal weeks (Niwa et al., 1992).
5. Signals and factors that may shape development of aminergic control of respiratory motoneurons
The genetically pre-programmed development of the respiratory system can be disrupted pre- and postnatally by a host of factors and environmental conditions. As mentioned earlier, 5-HT may regulate receptor expression during development (reviewed by Sodhi and Sanders-Bush, 2004). The evidence for this in the respiratory system is derived from studies in transgenic mice that produce and release excessive amount of 5-HT during the late embryonic and postnatal periods (Bou-Flores and Hilaire, 2000), studies of the response of phrenic motoneurons to spinal cord injury (Mitchell et al., 1992; McCrimmon et al., 1994; Zhou et al., 2001; Zimmer et al., 2007), or studies of animals subjected to acute or chronic intermittent hypoxia (Fuller et al., 2001; Baker-Herman and Mitchell, 2002).
Stress and stress-related hormones can powerfully alter the time course of neural development. Rats born to mothers subjected to acute or recurrent environmental stress exhibit delayed development of spontaneous and reflexly-evoked motor activity (Patin et al., 2004).Prenatal exposure to stressors can lead to long-lasting effects through re-programming of the hypothalamo-pituitary-adrenal (HPA) axis (Weinstock, 2001; Welberg and Seckl, 2001; Matthews, 2002). Maternal stress causes multiple endocrine and cardiovascular reactions in the mother's organism, and the resulting signals such as ACTH, glucocorticoids, catecholamines and opioids can reach the fetal brain. Activation of the fetal HPA axis also may result from fetal hypoxia caused by catecholamine-induced placental vasoconstriction (Matthews, 2002). Glucocorticoids are a major stress-related programming signal for the fetal HPA axis and brain development (reviewed by Seckl, 2004). They impact brain development by regulating the expression of neuronal adhesion molecules and neurotrophic factors (Rodriguez et al., 1998; Hansson et al., 2000). Manipulations of glucocorticoid levels early in development can permanently alter brain structure and functions (Fuxe et al., 1996; Muneoka et al., 1997; Matthews, 2002). Whereas normal rat pups have extremely low corticosterone levels until P12 (Fig. 1), maternal stress can increase plasma corticosterone in both the mother and fetus (Takahashi et al., 1998; Koehl et al. 1999).
In rats, prenatal exposure to glucocorticoids can cause long-term changes in brainstem monoaminergic systems. Activity of tryptophan hydroxylase is particularly strongly regulated by glucocorticoids (Whitaker-Azmitia, 1993). Prenatal exposure to dexamethasone caused postnatal increase of 5-HT levels in the brainstem lasting as long as 3−14 weeks and long-lasting increases in [3H]paroxetine binding in the brainstem indicative of elevated levels and/or activity of 5-HT transporter (Slotkin et al., 1996; Muneoka et al., 1997). In contrast, following similar exposures, there was only an early and transient increase in brainstem norepinephrine turnover that was limited to the pre-weaning period and no subsequent changes in brainstem norepinephrine or dopamine contents, or receptor binding, were detected (Slotkin et al., 1992; Muneoka et al., 1997). Thus, the 5-HT system is a specific target of glucocorticoids.
Elevated levels of 5-HT resulting from prenatal exposure to stress or glucocorticoids may alter postnatal expression of 5-HT receptors in motoneurons. Prenatal stress results in a decreased 5-HT1A receptor ligand- and immuno-binding in some, but not all, brain regions in adults (Meerlo et al., 2001; Van den Hove et al., 2006). In monoamine oxidase-A knockout mice that have elevated brain levels of 5-HT, density of functional 5-HT1A receptors is decreased in the raphe nuclei and their brainstem targets (Lanoir et al., 2006).
Interestingly, based on the absence of thermoregulation and other features of rapid eye movement (REM) sleep, it has been proposed that numerous organismal controls during this state revert to those during fetal or early postnatal life (Zepelin et al., 2005). Others have observed that physiology of REM sleep, essentially a brainstem-controlled state, is determined by ontogeny of the hypothalamus (Parmeggiani, 1982), thus suggesting that a functional disconnection of the brainstem from the latter may facilitate occurrence of neural phenomena characteristic of an immature brain. The level of brain maturity at birth is related to the amounts of REM sleep in adults of various species (Jouvet-Mounier et al., 1970). Of note in the context of this review is that central 5-HT and noradrenergic neurons cease firing during REM sleep. This causes a temporary withdrawal of the aminergic control of many brain functions, including breathing, and sets the brainstem in a state neurochemically similar to that when aminergic systems are not yet fully developed. Coincidentally, REM sleep is associated with the appearance of strong phasic activity in the muscles of the tongue in rats (Megirian et al., 1978; Lu et al., 2005) and in lingual and laryngeal muscles in humans (Chokroverty 1980; Kuna et al., 1991). The phasic activity that gradually emerges during the progression of REM sleep is often rhythmic but faster than diaphragmatic activity (Lu and Kubin, 2007) (Fig. 3A2). If the state of REM sleep is in some regards reminiscent of the conditions during perinatal period, such fast rhythms present in orofacial motoneurons in an altricial species like the rat may correspond to suckling-like activity. It is plausible that interference from non-respiratory rhythm generators is one cause of the respiratory rhythm irregularity that characterizes REM sleep. This interpretation is supported by recent in vitro studies of the interaction between respiratory and putative suckling rhythms in orofacial motoneurons (Koizumi et al., 2007). Further studies are needed to take advantage of the potential similarities between the respiratory control during REM sleep and in perinatal period.
Fig. 3.
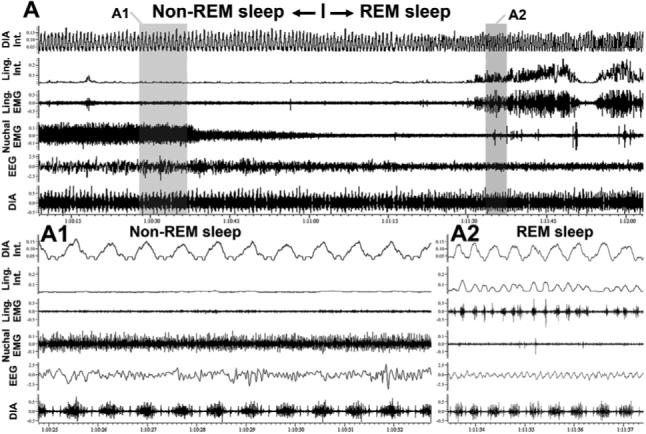
Muscles of the tongue exhibit non-respiratory, rhythmic activity during REM sleep. The top panel shows a continuous, about 2 min-long, record centered around a transition from non-REM to REM sleep in an adult rat. The signals are: diaphragmatic (DIA) and lingual (Ling.) EMGs and their integrated (Int.) versions, nuchal EMG and cortical EEG. The bottom panels show the highlighted segments of the top record on an expanded time scale. A1: there is no activity in the lingual EMG during non-REM sleep. A2: soon after the onset of REM sleep, lingual muscles begin exhibiting rhythmic activity with a rhythm much faster than the respiratory rate, as indicated by the recording from the diaphragm (unpublished data from Lu and Kubin, 2007). We relate the consistent appearance of fast rhythmic activity in lingual muscles during REM sleep to the possibility that, at least in an altricial species like the rat, such a rhythm may represent suckling-like activity.
6. Conclusions
Periods of potential vulnerability and critical periods have been identified in the development of the respiratory system in rats. The distinction between the two is that critical periods are those during which an insult or intervention may lead to long-term, often irreversible, changes in subsequent development (Carroll, 2003). In contrast, vulnerable periods are those during which rapid development occurs in a manner that carries a potential for inadequate response to environmental challenges. By this standard, the period around 11−14 postnatal days in the rat represents a period of vulnerability (Volgin et al., 2003; Liu and Wong-Riley 2005; 2006; Liu et al., 2006), but it remains to be determined whether it also is a “critical period” for some aspects of respiratory regulation. In humans, the rapid brain growth spurt period starts in the third trimester of pregnancy and lasts up to three years after birth. During this extended period, insults that alter transmitter release or efficiency of synaptic transmission (e.g., alcohol, anesthetics and other modulators of excitatory and inhibitory transmission) may trigger abnormal apoptotic events (Ikonomidou et al., 2001). Data suggest that a transient vulnerability resulting from a subtle mismatch in the development of brainstem serotoninergic and other neurochemical systems important for the control of both breathing and sleep increases the risk of the sudden infant death syndrome (Ozawa and Okado, 2002; Paterson et al., 2006).
As briefly discussed in this review, respiratory motoneuron performance changes as a result of genetically-programmed developmental processes and may be altered by injury to afferent pathways, unbalance in release of central transmitters, rapidly changing demands, and in response to various stressors. Changes in function of motoneuronal receptors represent an important, but only a partial, aspect of these processes. As receptors change developmentally or in response to challenges, their coupling to second messenger systems may also change (e.g., Lucaites et al., 1996; Nebigil et al., 2001). This has not been investigated in respiratory motoneurons, but developmentally changing respiratory effects mediated by 5-HT1A and 5-HT2A receptors suggest that this occurs (see Volgin et al., 2003 for discussion).
Conditions such as motor training elicit changes in aminergic and other afferent pathways to motoneurons (Cote et al., 2003), and in motoneuronal expression of inhibitory receptors (Tillakaratne et al., 2002). Overall, however, there is little information about feedback effects of altered load on muscles onto central control of motoneurons. The question is relevant for respiratory disorders caused by degenerative muscular diseases and the obstructive sleep apnea syndrome where anatomically compromised upper airway requires increased activation of upper airway motoneurons (Suratt et al., 1988; Mezzanotte et al., 1992; Hendricks et al., 1993; Katz and White 2004). The impact of altered load on upper airway and other respiratory muscles on receptors in, and afferent pathways to, their motoneurons is an attractive area for future studies.
Acknowledgments
Our studies were supported by grants HL-47600 and HL-60287.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J. Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Talley EM, Berger AJ. Neuromodulation of hypoglossal motoneurons: cellular and developmental mechanisms. Respir. Physiol. 1997;110:139–150. doi: 10.1016/s0034-5687(97)00079-0. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci. Lett. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Huynh P. Activation of 5HT1B receptors inhibits glycinergic synaptic inputs to mammalian motoneurons during postnatal development. Brain. Res. 2002;956:380–384. doi: 10.1016/s0006-8993(02)03464-9. [DOI] [PubMed] [Google Scholar]
- Borday C, Coutinho A, Germon I, Champagnat J, Fortin G. Pre-/post-otic rhombomeric interactions control the emergence of a fetal-like respiratory rhythm in the mouse embryo. J. Neurobiol. 2006;66:1285–1301. doi: 10.1002/neu.20271. [DOI] [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Hilaire G. 5-Hydroxytryptamine2A and 5-hydroxytryptamine1B receptors are differently affected by the monoamine oxidase A-deficiency in the Tg8 transgenic mouse. Neurosci. Lett. 2000;296:141–144. doi: 10.1016/s0304-3940(00)01653-0. [DOI] [PubMed] [Google Scholar]
- Bouryi VA, Lewis DI. The modulation by 5-HT of glutamatergic inputs from the raphe pallidus to rat hypoglossal motoneurons in vitro. J. Physiol. (Lond.) 2003;553:1019–1031. doi: 10.1113/jphysiol.2003.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat. Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Núñez-Abades PA. Physiological changes accompanying anatomical remodeling of mammalian motoneurons during postnatal development. Brain Res. Bull. 2000;53:523–527. doi: 10.1016/s0361-9230(00)00385-3. [DOI] [PubMed] [Google Scholar]
- Carroll JL. Plasticity in respiratory motor control. Invited review: developmental plasticity in respiratory control. J. Appl. Physiol. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am. J. Respir. Crit. Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- Chatonnet F, Borday C, Wrobel L, Thoby-Brisson M, Fortin G, McLean H, Champagnat J. Ontogeny of central rhythm generation in chicks and rodents. Respir. Physiol. Neurobiol. 2006;154:37–46. doi: 10.1016/j.resp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Cheng G, Zhu H, Zhou X, Qu J, Ashwell KW, Paxinos G. Development of the human nucleus of the solitary tract: a cyto- and chemoarchitectural study. Auton. Neurosci. Bas. Clin. 2006;128:76–95. doi: 10.1016/j.autneu.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Chennaoui M, Grimaldi B, Fillion MP, Bonnin A, Drogou C, Fillion G, Guezennec CY. Effects of physical training on functional activity of 5-HT1B receptors in rat central nervous system: role of 5-HT-moduline. Naunyn-Schmiedebergs Arch. Pharmacol. 2000;361:600–604. doi: 10.1007/s002100000242. [DOI] [PubMed] [Google Scholar]
- Chokroverty S. Phasic tongue movements in human rapid-eye-movement sleep. Neurology. 1980;30:665–668. doi: 10.1212/wnl.30.6.665. [DOI] [PubMed] [Google Scholar]
- Christensson M, Garwicz M. Time course of postnatal motor development in ferrets: ontogenetic and comparative perspectives. Behav. Brain Res. 2005;158:231–242. doi: 10.1016/j.bbr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. The projections of noradrenergic neurons in the A5 catecholamine cell group to the spinal cord in the rat: anatomical evidence that A5 neurons modulate nociception. Brain Res. 1993;616:200–210. doi: 10.1016/0006-8993(93)90210-e. [DOI] [PubMed] [Google Scholar]
- Cote MP, Menard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J. Neurosci. 2003;23:2789–2796. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino G, Condino AM, Gallinari P, Franceschetti GP, Tonini M. Characterization of prejunctional serotonin receptors modulating [3H]acetylcholine release in the human detrusor. J. Pharmacol. Exp. Ther. 2006;316:129–135. doi: 10.1124/jpet.105.092551. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Ann. Rev. Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am. J. Respir. Crit. Care Med. 2005;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. Am. J. Physiol. 1997;272:R1792–1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J. Appl. Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J. Neurophysiol. 1994;72:2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Diaz R, Cintra A, Bhatnagar M, Tinner B, Gustafsson J, Ogren S, Agnati L. On the role of glucocorticoid receptors in brain plasticity. Cell. Mol. Neurobiol. 1996;16:239–258. doi: 10.1007/BF02088179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Funk GD. Perinatal development of respiratory motoneurons. Respir. Physiol. Neurobiol. 2005;149:43–61. doi: 10.1016/j.resp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, Mulkey DK, Stornetta RL, Moreira TS, Takakura AT. The retrotrapezoid nucleus and central chemoreception. Adv. Exp. Med. Biol. 2008;605:327–332. doi: 10.1007/978-0-387-73693-8_57. [DOI] [PubMed] [Google Scholar]
- Hansson A, Cintra A, Belluardo N, Sommer W, Bhatnagar M, Bader M, Ganten D, Fuxe K. Gluco- and mineralocorticoid receptor-mediated regulation of neurotrophic factor gene expression in the dorsal hippocampus and the neocortex of the rat. Eur. J. Neurosci. 2000;12:2918–2934. doi: 10.1046/j.1460-9568.2000.00185.x. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Petrof BJ, Panckeri K, Pack AI. Upper airway dilating muscle hyperactivity during non-rapid eye movement sleep in English bulldogs. Am. Rev. Respir. Dis. 1993;148:185–194. doi: 10.1164/ajrccm/148.1.185. [DOI] [PubMed] [Google Scholar]
- Henning SJ. Postnatal development: coordination of feeding, digestion, and metabolism. Am. J. Physiol. 1981;241:G199–214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, Tenkova T, Dikranian K, Olney JW. Neurotransmitters and apoptosis in the developing brain. Biochem. Pharmacol. 2001;62:401–405. doi: 10.1016/s0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and behavior. A general hypothesis. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 461–469. [Google Scholar]
- Johnson H, Ulfhake B, Dagerlind A, Bennett GW, Fone KC. The serotoninergic bulbospinal system and brainstem-spinal cord content of serotonin-, TRH, and substance P-like immunoreactivity in the aged rat with special reference to the spinal cord motor nucleus. Synapse. 1993;15:63–89. doi: 10.1002/syn.890150108. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the state of sleep in rat, cat, and guinea pig during the first postnatal month. Dev. Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2004;170:553–560. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudéry M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J. Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Koizumi H, Nomura K, Ishihama K, Yamanishi T, Enomoto A, Kogo M. Inhibition of trigeminal respiratory activity by suckling. J. Dent. Res. 2007;86:1073–1077. doi: 10.1177/154405910708601110. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol. Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Insalaco G, Villeponteaux RD. Arytenoideus muscle activity in normal adult humans during wakefulness and sleep. J. Appl. Physiol. 1991;70:1655–1664. doi: 10.1152/jappl.1991.70.4.1655. [DOI] [PubMed] [Google Scholar]
- Landers M, Philip ZH. Development of rodent whisking: trigeminal input and central pattern generation. Somatosens. Mot. Res. 2006;23:1–10. doi: 10.1080/08990220600700768. [DOI] [PubMed] [Google Scholar]
- Lanoir J, Hilaire G, Seif I. Reduced density of functional 5-HT1A receptors in the brain, medulla and spinal cord of monoamine oxidase-A knockout mouse neonates. J. Comp. Neurol. 2006;495:607–623. doi: 10.1002/cne.20916. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J. Physiol. (Lond.) 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AD, Greer JJ, Feldman JL. Phrenic motoneuron morphology in the neonatal rat. J. Comp. Neurol. 1991;308:169–179. doi: 10.1002/cne.903080204. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr., Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J. Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J. Physiol. (Lond.) 2006;577:957–970. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J. Appl. Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits α1, α2, and α3 in brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Lu JW, Kubin L. Sleep-wake patterns of genioglossal EMG at the base and tip of the tongue in the rat. Sleep. 2007;30(Suppl):A206. [Google Scholar]
- Lu JW, Mann GL, Ross RJ, Morrison AR, Kubin L. Differential effect of sleep-wake states on lingual and dorsal neck muscle activity in rats. Respir. Physiol. Neurobiol. 2005;147:191–203. doi: 10.1016/j.resp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lucaites VL, Nelson DL, Wainscott DB, Baez M. Receptor subtype and density determine the coupling repertoire of the 5-HT2 receptor subfamily. Life Sci. 1996;59:1081–1095. doi: 10.1016/0024-3205(96)00423-7. [DOI] [PubMed] [Google Scholar]
- Matthews S. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocr. Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Dekin MS, Mitchell GS. Glutamate, GABA, and serotonin in ventilatory control. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. Dekker; New York: 1994. pp. 151–218. [Google Scholar]
- Megirian D, Cespuglio R, Jouvet M. Rhythmical activity of the rats's tongue in sleep and wakefulness. Electroencephalogr. Clin. Neurophysiol. 1978;44:8–13. doi: 10.1016/0013-4694(78)90100-1. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Horvath K, Luiten P, Angelucci L, Catalani A, Koolhaas J. Increased maternal corticosterone levels in rats: effects on brain 5-HT1A receptors and behavioral coping with stress in adult offspring. Behav Neurosci. 2001;115:1111–1117. [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J. Clin. Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Sloan HE, Jiang C, Miletic V, Hyashi F, Lipski J. 5-hydroxytryptophan (5-HTP) augments spontaneous and evoked phrenic motoneuron discharge in spinalized rats. Neurosci. Lett. 1992;141:75–78. doi: 10.1016/0304-3940(92)90338-8. [DOI] [PubMed] [Google Scholar]
- Monteau R, Morin D, Hennequin S, Hilaire G. Differential effects of serotonin on respiratory activity of hypoglossal and cervical motoneurons: an in vitro study on the newborn rat. Neurosci. Lett. 1990;111:127–132. doi: 10.1016/0304-3940(90)90356-e. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Mikuni M, Ogawa T, Kitera K, Kamei K, Takigawa M, Takahashi K. Prenatal dexamethasone exposure alters brain monoamine metabolism and adrenocortical response in rat offspring. Am. J. Physiol. 1997;273:R1669–1675. doi: 10.1152/ajpregu.1997.273.5.R1669. [DOI] [PubMed] [Google Scholar]
- Nebigil CG, Etienne N, Schaerlinger B, Hickel P, Launay JM, Maroteaux L. Developmentally regulated serotonin 5-HT2B receptors. Int. J. Dev. Neurosci. 2001;19:365–372. doi: 10.1016/s0736-5748(01)00022-3. [DOI] [PubMed] [Google Scholar]
- Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of α1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J. Neurosci. 2007;27:4435–4442. doi: 10.1523/JNEUROSCI.2803-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Kawano H, Masuko S, Takeichi M. Postnatal changes in development of serotonin-, neuropeptide Y-, Leu-enkephalin- and substance P- terminals in the rat locus coeruleus; a quantitative immunohistochemical study. J. Hirnforsch. 1992;33:133–140. [PubMed] [Google Scholar]
- Núñez-Abades PA, He F, Barrionuevo G, Cameron WE. Morphology of developing rat genioglossal motoneurons studied in vitro: changes in length, branching pattern, and spatial distribution of dendrites. J. Comp. Neurol. 1994;339:401–420. doi: 10.1002/cne.903390308. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- Parmeggiani PL. Regulation of physiological functions during sleep in mammals. Experientia. 1982;38:1405–1408. doi: 10.1007/BF01955751. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Bellivieau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in the Sudden Infant Death Syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Patin V, Vincent A, Lordi B, Caston J. Does prenatal stress affect the motoric development of rat pups? Dev. Brain Res. 2004;149:85–92. doi: 10.1016/j.devbrainres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J. Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praud JP, Reix P. Upper airways and neonatal respiration. Respir. Physiol. Neurobiol. 2005;149:131–141. doi: 10.1016/j.resp.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Montaron M, Petry K, Aurousseau C, Marinelli M, Premier S, Rougon G, Le Moal M, Abrous D. Complex regulation of the expression of the polysialylated form of the neuronal cell adhesion molecule by glucocorticoids in the rat hippocampus. Eur. J. Neurosci. 1998;10:2994–3006. doi: 10.1046/j.1460-9568.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- Rorig B, Sutor B. Serotonin regulates gap junction coupling in the developing rat somatosensory cortex. Eur. J. Neurosci. 1996;8:1685–1695. doi: 10.1111/j.1460-9568.1996.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Roux JC, Mamet J, Perrin D, Peyronnet J, Royer C, Cottet-Emard JM, Pequignot JM, Dalmaz Y. Neurochemical development of the brainstem catecholaminergic cell groups in rat. J. Neur. Transm. 2003;110:51–65. doi: 10.1007/s00702-002-0767-7. [DOI] [PubMed] [Google Scholar]
- Seckl J. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 2004;151(Suppl 3):U49–62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Blumberg MS. Sniffing in infant rats during sleep and wakefulness. Behav. Neurosci. 2004;118:267–273. doi: 10.1037/0735-7044.118.2.267. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Karlsson KA, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. Eur. J. Neurosci. 2005;22:911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T, Barnes G, McCook E, Seidler F. Programming of brainstem serotonin transporter development by prenatal glucocorticoids. Dev. Brain. Res. 1996;93:155–161. doi: 10.1016/0165-3806(96)00027-2. [DOI] [PubMed] [Google Scholar]
- Slotkin T, Lappi S, McCook E, Tayyeb M, Eylers J, Seidler F. Glucocorticoids and the development of neuronal function: effects of prenatal dexamethasone exposure on central noradrenergic activity. Biol. Neon. 1992;61:326–336. doi: 10.1159/000243761. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int. Rev. Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- Stafford IL, Jacobs BL. Noradrenergic modulation of the masseteric reflex in behaving cats. I. Pharmacological studies. J. Neurosci. 1990;10:91–98. doi: 10.1523/JNEUROSCI.10-01-00091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am. Rev. Respir. Dis. 1988;137:889–894. doi: 10.1164/ajrccm/137.4.889. [DOI] [PubMed] [Google Scholar]
- Takahashi L, Turner J, Kalin N. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology. 1998;23:571–581. doi: 10.1016/s0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Talley EM, Sadr NN, Bayliss DA. Postnatal development of serotonergic innervation, 5-HT1A receptor expression, and 5-HT responses in rat motoneurons. J. Neurosci. 1997;17:4473–4485. doi: 10.1523/JNEUROSCI.17-11-04473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Amamiya S, Miura N, Araki A, Ohinata J, Fujieda K. Postnatal development of brainstem serotonin-containing neurons projecting to lumbar spinal cord in rats. Brain Dev. 2006;28:586–591. doi: 10.1016/j.braindev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, de Leon RD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J. Neurosci. 2002;22:3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Aston-Jones G. Integration of the ventral medulla and coordination of sympathetic, pain and arousal functions. Clin. Exp. Hyperten. 1995;17:153–165. doi: 10.3109/10641969509087062. [DOI] [PubMed] [Google Scholar]
- Van den Hove D, Lauder J, Scheepens A, Prickaerts J, Blanco C, Steinbusch H. Prenatal stress in the rat alters 5-HT1A receptor binding in the ventral hippocampus. Brain Res. 2006;1090:29–34. doi: 10.1016/j.brainres.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J. Neurophysiol. 2006;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Mackiewicz M, Kubin L. α1B receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. J. Chem. Neuroanat. 2001;22:157–166. doi: 10.1016/s0891-0618(01)00124-7. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. NeuroReport. 2002;13:433–436. doi: 10.1097/00001756-200203250-00014. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2A and 2C receptors in brainstem motoneurons. Eur. J. Neurosci. 2003;17:1179–1188. doi: 10.1046/j.1460-9568.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Fenik VB, Fay R, Okabe S, Davies RO, Kubin L. Serotonergic receptors and effects in hypoglossal and laryngeal motoneurons semi-quantitative studies in neonatal and adult rats. Adv. Exp. Med. Biol. 2004;551:183–188. doi: 10.1007/0-387-27023-x_28. [DOI] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Changes in glucose do not alter baseline firing rate or chemosensitivity of serotonin neurons cultured from the medullary raphe. Respir. Physiol. Neurobiol. 2007;157:235–241. doi: 10.1016/j.resp.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Waters KA, Gozal D. Responses to hypoxia during early development. Respir. Physiol. Neurobiol. 2003;136:115–129. doi: 10.1016/s1569-9048(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Progr. Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Welberg L, Seckl J. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Westneat MW, Hall WG. Ontogeny of feeding motor patterns in infant rats: an electromyographic analysis of suckling and chewing. Behav. Neurosci. 1992;106:539–554. doi: 10.1037//0735-7044.106.3.539. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. The role of serotonin and serotonin receptors in development of the mammalian nervous system. In: McLaughlin PJ, Zagon IS, editors. Receptors in the Developing Nervous System. Chapman and Hall; London: 1993. pp. 43–53. [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of α2 adrenoceptors during rat brain development - I. α2A messenger RNA expression. Neuroscience. 1997;76:241–260. doi: 10.1016/s0306-4522(96)00368-5. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir. Physiol. Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Tashiro Y, Inoue K, Kawai Y. Postnatal development of GABAergic axon terminals in the rat nucleus of tractus solitarius. Brain Res. 2006;1107:111–120. doi: 10.1016/j.brainres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zepelin H, Siegel JM, Tobler I. Mammalian sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier-Saunders; Philadelphia: 2005. pp. 91–100. [Google Scholar]
- Zhang LL, Ashwell KW. The development of cranial nerve and visceral afferents to the nucleus of the solitary tract in the rat. Anat. Embryol. 2001;204:135–51. doi: 10.1007/s004290100185. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG. Serotonin2 receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J. Appl. Physiol. 2001;91:2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J. Spin. Cord Med. 2007;30:319–330. doi: 10.1080/10790268.2007.11753947. [DOI] [PMC free article] [PubMed] [Google Scholar]


