Abstract
Background
In tertiary referral patients, there is association between altered sleep patterns, functional bowel disorders, and altered gut motor function. Body mass index (BMI) is also associated with GI symptoms including diarrhea, and with sleep disturbances. Our hypothesis is that sleep disturbances are associated with gastrointestinal symptoms, and this is not explained by BMI.
Methods
A 48-item validated questionnaire was mailed to 6,939 community participants in Olmsted County, MN. The survey included gastrointestinal symptoms, sleep disturbance, daily lifestyle, and quality of life (QOL). Independent contributions of sleep disturbance to individual symptoms were assessed using logistic regression adjusting for age, gender, lifestyle and mental health status. The association of an overall sleep score with an overall symptom score was examined and the ability of both scores to predict SF-12 physical and mental functioning scores assessed in multiple linear regression models.
Results
Among 3228 respondents, 874 (27%) reported trouble staying asleep. There was a significant correlation of overall sleep scores with overall GI symptom scores (partial r =0.28, p<0.001). Waking up once nightly at least 4 times a month was significantly associated with pain, nausea, dysphagia, diarrhea, loose stools, urgency and a feeling of anal blockage. Trouble falling asleep was significantly associated with rectal urgency. Associations were independent of gender, age, lifestyle factors and BMI. Overall sleep scores and GI symptom scores were both significant independent predictors of impaired QOL.
Conclusion
In the community, reporting poor sleep is associated with upper and lower GI symptoms, but this is independent of BMI.
Introduction
Approximately one-half of the adult American population has been reported to suffer from some degree of sleep disturbance [1]. Data from tertiary referral patients suggest that up to 70% of patients with irritable bowel syndrome (IBS) and functional dyspepsia, conditions also highly prevalent in the general population [2], report having sleep disturbances, irrespective of anxiety and depression scores [3]. During sleep or disturbed sleep, several functional changes have been reported to occur in the gastrointestinal tract including increased transient lower esophageal sphincter relaxations (TLESRs) aggravating gastroesophageal reflux [4], motor abnormalities of the upper GI tract [5] and small bowel [6]. Visceral hypersensitivity has also been associated with reporting poor sleep [7]. In 10 patients with IBS compared to controls, Orr and colleagues reported significantly longer rapid eye movement (REM) sleep phase, and suggested that this finding could be a proxy for autonomic dysfunction in IBS [8]. However, a similarly sized case-control study failed to show any differences in REM sleep [9].
A recent population-based study of 2,700 individuals from our center showed that subjects self-reporting poor nighttime sleep were more likely than people without sleep disturbance to fulfill symptom criteria for IBS, even after accounting for age, gender and somatization score [10]; the same association was not present in dyspepsia [10]. However, potential confounders such as body mass index (BMI) and exercise levels could not be considered in that study, and to our knowledge no other population-based data are available. Several population-based studies have observed that BMI is associated with both upper and some lower gastrointestinal symptoms although the underlying mechanisms remain poorly understood [11-13]. High BMI is associated with sleep disturbances including obstructive sleep apnea as well as a shorter sleep duration [14, 15], raising the possibility that obesity accounts for the development of GI symptoms in those experiencing sleep disturbances.
Our hypothesis is that, in the general population, sleep disturbance is associated with gastrointestinal symptoms, but this is not explained by BMI. The aims of the present study were to determine whether there is an association between self-reported sleep disturbances and frequent upper and lower gastrointestinal symptoms in community participants, after controlling for potential confounding factors such as BMI and exercise levels.
Methods
Sampling frame
We aimed to survey a community sample of non-institutionalized adults aged 18 and older in Olmsted County. Addresses and telephone numbers of directory-listed households were purchased from an outside contractor that maintained the residential address list for the U.S. Postal Service (USPS). From this list, random sampling of 7,000 residents for participation was performed. The USPS list used comprises all U.S. households in every ZIP code. The general address file is updated every month. The list of addresses for Olmsted county area residents has one telephone number corresponding to each address. This allowed subjects who failed to respond to the new questionnaire to be contacted by phone; this was repeated twice. Once reached by phone, subjects were asked by a trained operator the same question items in identical order and format as in the mailed questionnaire. Of the 7000 residents identified, 61 were ineligible (see ineligibility criteria in the following section). The remaining 6939 subjects received the questionnaire, and in addition were randomized to either receive or not to receive a Health Insurance Portability and Accountability Act (HIPAA) document. The effect of including the HIPAA form on response to questionnaires has been published elsewhere [16].
Survey
A first mailing of a 48-item questionnaire was performed between November 2005 and February 2006. Institutionalized subjects, or those who could not be located by mail, subjects who had moved from the Olmsted County from the last contact with the Mayo Clinic, and subjects who had denied authorization for use of their medical records for research were considered ineligible and excluded. The Mayo Clinic Survey Research Center performed data retrieval and built the database.
Structure of the questionnaire
The questionnaire included 16 questions on upper and lower gastrointestinal symptoms. These were based on the previously validated Talley Bowel Disease Questionnaire [17], but used a 5-point Likert scale to further characterize the severity of symptoms. For the purpose of the primary analysis of gastrointestinal symptoms, we dichotomized most of the Likert symptom response scales using a cutoff ≥ 4 (Often or Very Often) indicating a moderate to severe impact. Thus for example, a positive endorsement of constipation was defined as often or very often having less than 3 bowel movements per week, and diarrhea as more than 3 bowel movements per day. In order to keep the questionnaire brief, and to prevent the responders from excessively focusing on specific symptoms, visual displays of stool forms (such as components of the Bristol scale form) were not included. Vomiting was dichotomized as being troubled by any in the last 3 months, and nausea at ≥3 (Sometimes, Often, Very Often). This difference in the threshold for dichotomizing vomiting and nausea was necessary as both these symptoms are known to be less common than the remaining set of complaints [10]. Overall, gastrointestinal symptom scores were also computed by averaging the Likert scale (1-5) scores over all 16 symptoms, just the lower gastrointestinal symptoms, and also just the upper gastrointestinal symptoms.
Sleep disturbance was characterized by means of the following questions, that have been validated as part of the Insomnia Severity Index [18] and assess sleep problems over the course of the previous month:
Have you had trouble falling asleep?
Did you wake up several times in the night?
Have you had trouble staying asleep?
Have you woken up after your usual amount of sleep feeling tired and worn out?
The responses were on an ordinal 6-point scale based on the number of days of sleep disturbance, from 1 (Not at all), 2 (1 to 3 days), 3 (4 to 7 days), 4 (8 to 14 days), 5 (15 to 21 days), and 6 (22 to 31 days in the past month). For the primary analyses, the sleep disturbance symptoms were dichotomized with subjects considered to indicate an important sleep disturbance if they reported having the disturbance at least 8 times a month (≥ 4 on the 1-6 scale). An aggregate sleep score was also computed using the mean of the individual scores over the 4 sleep disturbance questions. The sleep score was assessed as a continuous scale variable.
Self-report of physical activity levels was also included, and categorized levels of activity, as high, moderate and light, according to their report from a monthly to daily basis [19]. Specifically, physical activity was measured as follows [19]:
Aside from any work you do at home or at a job, do you do anything regularly, that is, on a daily basis that helps you keep physically fit? (choice Yes/No),
How often in your free time, do you take part in moderate physical activity (such as bowling, golf, light sports or physical exercise, gardening, taking long walks)? (choices more than 4 times a week, 2-4 times a week, about once a week, a few times a month, a few times a year, rarely or never),
How often, in your free time, do you take part in vigorous physical activity (such as jogging, racket sports, swimming, aerobics, strenuous sports)? (frequency choices as in the previous question).
The two questions eliciting self-report of exercise levels [19]: “moderate”(e.g. walking, gardening) and “vigorous” (e.g. swimming, jogging, aerobics), both on 6-point Likert frequency scales, rarely to daily) were used to compute a quantitative physical activity level score. A greater score was indicative of higher physical activity levels.
Body Mass Index (BMI) was calculated from self-reported height and weight. Data from the Third National Health and Nutrition Examination Survey (NHANES III) showed that self-report of body weight and height results in correct classification of BMI [20]. The BMI categorizations were based on the WHO classification of obesity [21].
Alcohol consumption (past 30 days), and current smoking status (every day, some days, not at all), diagnosis of diabetes mellitus, education and ethnicity were asked using single items covering these issues. In addition, the questions from the SF-12 short questionnaire for emotional and physical functioning were included in the survey instrument [22]. A mental health status score was computed by averaging the responses from two of the SF-12 questions (one that asked about having a lot of energy and one about feeling downhearted and blue). An additional question assessed respondents regarding any history of gastrointestinal disease in their immediate family.
Approximately within one month of the end of survey mailings, the subjects who failed to respond to the new questionnaire were contacted by phone, with 2 repeated attempts, and were asked by a trained operator the same question items in identical order and format as in the mailed questionnaire.
Statistical analysis
The associations between disturbed sleep and individual upper and lower gastrointestinal symptoms were studied using multiple logistic regression. Age, gender, alcohol consumption, cigarette smoking, physical activity score and the mental health score, were used as co-variates in the final regression models. Odds ratios (ORs) and their 95% confidence intervals (CI) for the reporting of a moderate to severe impact based on the dichotomized responses (e.g. Often or Very Often) for each individual symptom were estimated from the logistic regression model coefficients corresponding to each of the dichotomized sleep disturbance characteristics.
The sleep score (computed as the mean of the 4 sleep disturbance item responses) was used in a multiple linear regression analysis to assess the associations between the overall sleep score and the (mean) GI symptom score. Other co-variates in the regression model were age, BMI, the mental health score, the physical activity score, current smoking status, and recent (30 days) alcohol use.
Using multiple regression, the sleep and GI symptom scores were used as predictors of the SF-12 quality of life domains (physical function and separately, the mental function scores). Other co-variates were age, gender, BMI, diabetes mellitus, physical activity score, current smoking status and recent alcohol use.
Results
Responders' demographics and sleep disturbance
We analyzed data on 3228 responders (46% of the total sample); 629 subjects (19.4% of the responders) were contacted and provided their information by telephone interview. Overall, 1958 participants (60.2%) reported some degree of sleep disturbance and for 874 participants (27%), sleep disturbance occurred at least 4 times a month. In Table 1 the distributions of several subject characteristics and measured responses are summarized overall and by average sleep score category (average sleep score >=4, corresponding to >= 8 days per month on the Likert scale for each of the individual sleep disturbance questions).
Table 1. Demographics and summary data for gastrointestinal symptoms, physical exercise and SF-12 scores.
Data are presented as mean (SEM)
| Overall (N=3228) | Normal sleep reported (n=2686) | Abnormal sleep reported (n=542) | |
|---|---|---|---|
| Age | 52.7±0.3 | 53±0.3 | 51.5±0.7 |
| Gender (% female) | 49 | 47 | 60 |
| BMI (kg/m2) | 27.5±0.1 | 27.4±0.1 | 28.3±0.3 |
| GI symptom score | 1.53±0.01 | 1.46±0.01 | 1.87±0.03 |
| Lower GI Score | 1.54±0.01 | 1.48±0.01 | 1.84±0.03 |
| Upper GI Score | 1.52±0.01 | 1.44±0.01 | 1.90±0.03 |
| Physical exercise score | 9.7±0.1 | 9.9±0.1 | 8.7±0.2 |
| SF-12 physical score | 50.1±0.2 | 50.8±0.2 | 46.9±0.5 |
| SF-12 mental score | 52±0.2 | 53.3±0.1 | 45.8±0.5 |
Associations of sleep disturbance with GI symptoms
Trouble with waking up tired or worn out at least 8 days a month increased the odds for reporting 6 of the 9 upper and 5 of the 7 lower abdominal symptoms (figures 1 and 2). The associations found were of similar strength for upper and lower abdominal symptoms (Table 2).
Figure 1. The odds for moderate to severe impact on specific upper GI symptoms in subjects reporting waking up tired/worn out at least 8 times a month relative to less than 8 times per month.
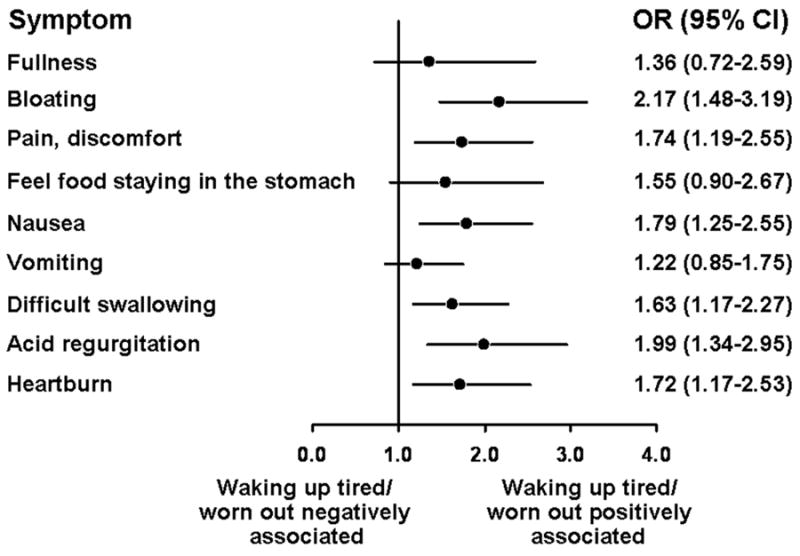
Odds Ratios (OR, and 95% confidence intervals, CI) are adjusted for age, gender, smoking status, alcohol use, physical activity score and SF-12 mental health status score.
Figure 2. The odds for moderate to severe impact on specific lower GI symptoms in subjects reporting waking up tired/worn out at least 8 times a month relative to less than 8 times per month.

Odds Ratios (OR, and 95% confidence intervals, CI) are adjusted for age, gender, smoking status, alcohol use, physical activity score and SF-12 mental health status score.
Table 2. The odds for moderate to severe impact on specific GI symptoms with frequent (≥8 days/month) trouble falling asleep, trouble staying asleep, nighttime awakenings, relative to less frequent (<8 days/month) sleep disturbance characteristics.
| GI Symptom | Trouble Falling Asleep | Wake >1 per night | Trouble staying Asleep |
|---|---|---|---|
| Fullness | 1.36 (0.72, 2.60) | 0.48 (0.21, 1.07) | 2.92 (1.29, 6.64) |
| Bloating | 0.81 (0.54, 1.24) | 1.11 (0.71, 1.74) | 1.43 (0.91, 2.26) |
| Pain | 1.29 (0.87, 1.93) | 2.06 (1.35, 3.16) | 1.02 (0.66, 1.57) |
| Food staying in the stomach | 1.62 (0.94, 2.79) | 1.19 (0.64, 2.23) | 1.37 (0.73, 2.57) |
| Nausea | 1.40 (0.96, 2.03) | 1.82 (1.22, 2.71) | 0.94 (0.62, 1.41) |
| Vomiting | 1.26 (0.85, 1.88) | 1.28 (0.85, 1.93) | 1.17 (0.76, 1.80) |
| Dysphagia | 1.23 (0.86, 1.77) | 1.49 (1.04, 2.14) | 1.10 (0.75, 1.61) |
| Acid regurgitation | 1.11 (0.72, 1.69) | 1.45 (0.92, 2.28) | 1.12 (0.71, 1.79) |
| Heartburn | 1.20 (0.78, 1.85) | 1.35 (0.88, 2.07) | 0.89 (0.56, 1.40) |
| Diarrhea | 1.20 (0.79, 1.84) | 2.17 (1.43, 3.29) | 0.86 (0.56, 1.33) |
| Urgency | 1.55 (1.00, 2.40) | 2.23 (1.43, 3.49) | 0.85 (0.54, 1.35) |
| Loose stools | 1.11 (0.73, 1.69) | 2.29 (1.48, 3.54) | 0.99 (0.64, 1.55) |
| Constipation | 0.88 (0.49, 1.57) | 1.62 (0.91, 2.90) | 1.06 (0.59, 1.93) |
| Blockage at evacuation | 0.61 (0.35, 1.08) | 1.88 (1.01, 3.49) | 1.63 (0.90, 2.96) |
| Hard stools | 0.71 (0.45, 1.13) | 1.43 (0.90, 2.27) | 1.48 (0.92, 2.37) |
| Fecal incontinence | 1.10 (0.63, 1.93) | 1.41 (0.85, 2.34) | 0.91 (0.52, 1.59) |
Odds ratios (95% C.I.) adjusted for age, gender, smoking status, alcohol use and SF-12 mental health status score.
GI symptoms with presence reported as often or very often (>3 on Likert scale) were considered moderate to severe impact.
There was a significant correlation of overall sleep scores with overall GI symptom scores (partial r =0.28, p< 0.001, adjusted for age, gender, mental health status score, physical activity score, current smoking status and alcohol use). A total of 178 subjects had a sleep score of 5-6 (trouble sleeping ≥ 15 days /month), which reflects severe sleep disturbance in virtually all domains, and these subjects reported a mean (95% Confidence Interval) GI symptom score of 1.97 (95% CI 1.88-2.06). This implies reporting at least occasional occurrence of all the symptoms listed, or frequent/very frequent occurrence of some of the symptoms. There was a consistent, stepwise increase of mean symptom scores with each increase of sleep scores (Figure 3).
Figure 3. Univariate association between sleep symptom score and gastrointestinal symptom score.
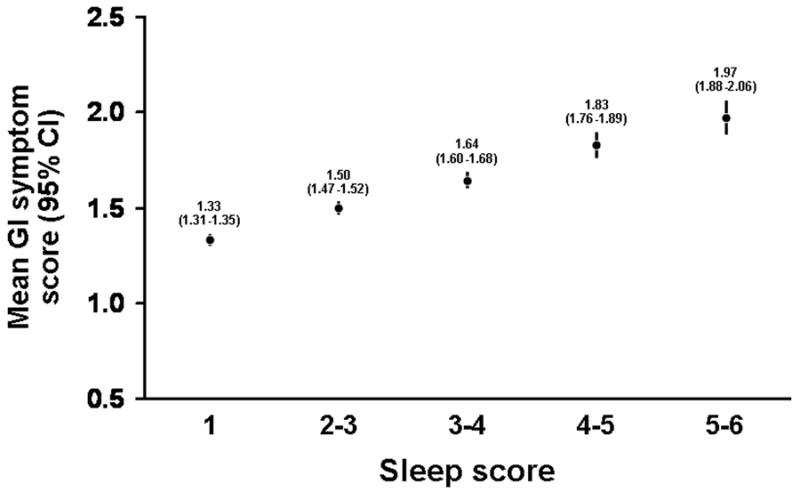
Note the step-wise increase in the mean gastrointestinal (GI) symptoms aggregate score with increased sleep scores. An increased sleep score implies more frequent or more severe sleep disturbance.
Waking up once nightly at least 4 times a month was associated with pain, nausea, dysphagia, diarrhea, loose stools, urgency and a feeling of anal blockage (Table 2). The association between trouble falling asleep and GI symptoms was less common, but was significant for a sense of urgency. These associations were all adjusted for age, gender, smoking status, alcohol use, physical activity score and the SF-12 mental health score.
Associations of sleep disturbance and GI symptoms with quality of life
Age, BMI, diabetes mellitus, physical activity score, sleep disturbance score and GI symptom score were all significant predictors in the model for the SF-12 Physical Functioning Score (Table 3). Similar results were observed for the SF-12 Mental Functioning Scale (Table 4). In particular, both the GI symptom score and the sleep disturbance were independent predictors of impaired functioning and accounted for the majority of the variation explained by the model for the SF-12 mental functioning score.
Table 3. Predictors for Physical Functioning SF-12 Score.
| Predictor Variable | p-value | Partial R-square (%) |
|---|---|---|
| Age | <.001 | 13.4 |
| Gender (1=M, 0=F) | 0.14 | <1.0 |
| BMI | <.001 | 2.7 |
| Diabetes (1=Yes, 0=No) | <.001 | <1.0 |
| Physical activity Score | <.001 | 4.3 |
| Current Smoker (1=Yes, 0=No) | 0.29 | <1.0 |
| Alcohol Use (1=Yes, 0=No) | 0.003 | <1.0 |
| Sleep Score | <.001 | 1.1 |
| GI Symptom Score | <.001 | 4.4 |
Multiple linear regression analysis was used to predict the SF-12 Physical Functioning Score.
The overall adjusted R-square is 33%.
Table 4. Predictors for Mental Functioning SF-12 Score.
| Predictor Variable | p-value | Partial R-square (%) |
|---|---|---|
| Age | <.001 | 3.8 |
| Gender (1=M, 0=F) | 0.52 | <1.0 |
| BMI | <0.001 | <1.0 |
| Diabetes (1=Yes, 0=No) | 0.003 | <1.0 |
| Physical activity Score | 0.012 | <1.0 |
| Current Smoker (1=Yes, 0=No) | 0.02 | <1.0 |
| Alcohol Use (1=Yes, 0=No) | 0.34 | <1.0 |
| Sleep Score | <0.001 | 7.9 |
| GI Symptom Score | <0.001 | 3.9 |
Multiple linear regression analysis was used to predict the SF-12 Mental Functioning Score.
The overall adjusted R-square is 22%.
Discussion
The present population-based study showed that reporting poor sleep is associated with increased odds for multiple upper and lower GI symptoms, including upper abdominal pain and discomfort, nausea, difficulty swallowing, reflux symptoms, diarrhea and loose stools, and constipation. The associations found were independent of the effects of BMI, and multiple demographic and lifestyle co-variates.
The associations of poor sleep with upper and lower GI symptoms have been the object of a few prior physiological and epidemiological studies. In a small series of tertiary IBS patients, Goldsmith and colleagues described the association between symptom severity on the day of assessment and poor sleep during the prior night [23]. A large tertiary care center study of 505 patients found 41% of IBS and 50% of functional dyspepsia patients reported sleep disturbances; these directly correlated with abdominal symptoms, with over one-half of the patients being awoken by their GI symptoms. However, the background prevalence of sleep disturbance was high, and the tertiary nature of the patient population renders such observations of interest but not necessarily generalizable.
Among upper GI complaints, gastroesophageal reflux symptoms have been linked to sleep. In a large cohort of patients, reporting of poor sleep, a high BMI, snoring, daytime sleepiness and consumption of carbonated drinks were independent predictors of heartburn during sleep [24]. Potential contributors to the origin of nighttime reflux include a longer esophageal acid contact time [25], lower pressure in the lower esophageal sphincter, increased nocturnal gastric acid secretion, lack of conscious perception of acid reflux, reduced salivation, and infrequent clearance by swallowing. While an increased BMI may contribute to the symptoms of nocturnal reflux and heartburn as reported in the Sleep Heart Health Study, a multicenter, longitudinal, cohort study of the cardiovascular consequences of sleep-disordered breathing [24], our results suggest that BMI alone does not explain the link of poorer sleep with reflux symptoms in the community.
Less work has been done on the link between gastroduodenal symptoms and sleep disturbances. In functional dyspepsia, manometric studies demonstrated a reduced number of nocturnal migrating motor complexes in the duodenum compared to controls during sleep, with some correlation with symptoms [5]. It remains unclear whether these manometric findings have specific clinical relevance. In a nation-wide study involving 10,000 general practitioners, and 43,446 patients, nocturnal awakening as well as male gender, smoking, overlapping reflux symptoms and a history of peptic ulcer were more frequent in dyspeptic patients with predominant pain, but not in patients with predominant discomfort [26].
Sleep disturbance has been most studied in patients with lower abdominal symptoms. Patients with IBS may exhibit a longer duration of the REM sleep phase [8]. As REM sleep is associated with increased colonic propagating and non-propagating motility [27], a longer REM phase could theoretically predispose to development of symptoms by induction of persistent motor activity. However, this hypothesis has not been confirmed by others. Heitkemper et al. studied 82 women affected by IBS and 35 controls using daily diaries, and found poor night time sleep consistently preceded a day with worse GI symptoms [28]. On the other hand, the same group [29] and others [30] also performed polysomnographic studies but failed to show a relationship between abnormal sleep studies, self-reported worse sleep and IBS symptoms. Attempts to describe abnormal motility patterns of the small intestine and colon during sleep in IBS were initially considered promising as periodic activity in the gut was shown to be modulated by the presence or absence of sleep [31]. However, synchronous polysomnography and recording of upper small bowel motility in six healthy subjects and six patients with IBS showed that, during sleep, there was no difference in the patterns of intestinal motility, the REM latency or number of REM episodes between the two groups [32]. Subsequent studies also showed that motility patterns while asleep were similar in both normal and symptomatic subjects [6]. In healthy individuals [33] and in subjects with daytime slow transit [34], colonic motor function is quiescent during sleep, and resumes promptly after awakening. It seems unlikely therefore that sleep induces motor abnormalities to account for the disparity in symptoms in those with sleep disturbances. The data are more consistent with the concept that such patients may develop abnormal gut function during the day after a poor night sleep. The effect of such sleep deprivation on intestinal sensitivity has not been thoroughly evaluated. However, in a small study of dyspeptics with sleep disturbances, amitriptyline improved patients' symptoms although the authors concluded that the benefit was not related to changes in measures of arousal from sleep [35].
Thus far, only one population-based study has been conduct to explore these associations between sleep and GI symptoms. In 2,269 participants from Olmsted County, Vege and colleagues retrospective analyzed data from questionnaires that aimed to describe GI symptom prevalence; they found that individuals with sleep disturbances had a higher prevalence of IBS but not dyspepsia, compared to individuals reporting normal sleep [10]. The main limitations of that study were its retrospective nature and the use of a single non-validated question to define sleep disturbance. However, they identified very similar symptom associations with comparable estimates to the present results, even though the present study adjusted not only for age and gender but also BMI, mental and physical well-being, and other lifestyle co-variates. Hence, the prior results support the validity of the present findings and strengthen our conclusions. A novel aspect of the present study compared to the study of Vege et al. is the demonstration that these associations are independent of BMI and physical exercise, that were not included as potential co-variates in the prior study [10]. As BMI is a major determinant of sleep apnea and has been linked traditionally to upper and lower GI symptoms, the independence of the associations from self-reported BMI categories suggests this is not a major confounder. The associations found were of modest size in the present study; however, the estimates are also more precise, as shown by the narrower confidence intervals, due to a larger sample size than in the study of Vege et al. [10]. Another additional insight from our current study is the apparently linear relationship between severity of disturbed sleep and severity of the GI symptoms, as shown in figure 3. The results also indicate that GI symptom and sleep scores are both significant factors in the impairment of quality of life seen in people with GI symptoms in the community. This represents an important observation, further expanding prior observations reporting an association of poor sleep with lower mental quality of life in 770 tertiary referred patients with IBS [36].
Our study has some limitations. First, the response rate to the survey was not optimal, although the response rate obtained (46%) is typical of other survey studies conducted in the same population during the same period. We have reported elsewhere the impact of HIPAA forms on the response rates to such population-based studies [16]. Moreover, the sample obtained was demographically very similar to the Olmsted County population; we have previously reported a comparison of the age, gender, race and education level of respondents to the survey with the Olmsted County population, and identified only a 5-10% under-representation of non-whites and people with lower levels of formal education, with no other important differences [34]. Secondly, the cross-sectional design and the nature of any epidemiological investigation are inadequate to establish a cause-effect relationship. Thus, we cannot definitely conclude that poor sleep would lead to symptoms or vice versa. As physiological studies of sleep function and motility have shown contrasting results, an alternative hypothesis is that sleep disturbance might be related only to increased symptom reporting, but not to the pathogenesis of symptoms. Nevertheless, the bottom-line message is that sleep disturbance is associated with increased gastrointestinal symptoms and that BMI is not a significant factor in this association, in contrast to the experience obtained in the literature focusing on reflux symptoms [24]. Also, we could not assess the potential effect of therapeutic agents commonly used for insomnia, which may modulate both the arousal level and gut sensori-motor function. It should be noted that defining IBS (and its bowel function subtypes) and functional dyspepsia in this study may have been limited by the use of a short survey. Finally, the extrapolation of the findings to the entire U.S. population needs to be done with caution, as Olmsted County residents differ significantly from the U.S, population in the number of years of formal education, and being mostly middle class and predominantly white [37].
In summary, we have shown that moderate sleep disturbance is a prevalent condition, with 27% of the general population reporting sleep disturbance at least 4 times a month. In our sample, reporting poor sleep was associated with reporting symptoms that are consistent with the symptom complexes of dyspepsia, reflux and IBS. The association was modest in size but the CIs are narrow, reflecting the large sample. The overall association was independent of age, gender, BMI, mental health status and lifestyle factors. The present study provides epidemiological information that may serve to generate testable hypotheses. Thus, mechanistic studies in large samples remain imperative to establish whether functional GI disorders and sleep disorders share an underlying “organic” basis, such as central nervous system or autonomic dysfunction, or if one influence the other through an overall impact on lifestyle, behavioral changes or symptom reporting attitudes.
Supplementary Material
Acknowledgments
This study was supported in part by DK 54681 from the National Institutes of Health to MC and NJT
References
- 1.N.S. Foundation, editor. 2003 Sleep in America Poll. Washington DC: 2003. [Google Scholar]
- 2.Cremonini F, Talley NJ. Irritable bowel syndrome: epidemiology, natural history, health-care seeking and emerging risk factors. Gastroenterol Clin North Am. 2005;34:189–204. doi: 10.1016/j.gtc.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Fass R, Fullerton S, Tung S, Mayer EA. Sleep disturbance in clinic patients with functional bowel disorders. Am J Gastroenterol. 2000;95(5):1195–2000. doi: 10.1111/j.1572-0241.2000.02009.x. [DOI] [PubMed] [Google Scholar]
- 4.Kahrilas P, Dodds WJ, Dent J, Haeberle B, Hogan WJ, Arndorfer RC. Effect of sleep, spontaneous gastroesophageal reflux, and a meal on upper esophageal sphincter pressure in normal human volunteers. Gastroenterology. 1987;92(2):466–71. doi: 10.1016/0016-5085(87)90143-0. [DOI] [PubMed] [Google Scholar]
- 5.David D, Mertz H, Fefer L, Sytnik B, Raeen H, Niazi N, Kodner A, Mayer EA. Sleep and duodenal motor activity in patients with severe non-ulcer dyspepsia. Gut. 1994;35(7):916–25. doi: 10.1136/gut.35.7.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellow JE, Gill RC, Wingate DL. Prolonged ambulant recordings of small bowel motility demonstrate abnormalities in the irritable bowel syndrome. Gastroenterology. 1990;98(5 Pt 1):1208–18. doi: 10.1016/0016-5085(90)90335-x. [DOI] [PubMed] [Google Scholar]
- 7.Orr WC, Chen CY. Sleep and the gastrointestinal tract. Neurol Clin. 1997;23(4):1007–24. doi: 10.1016/j.ncl.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Orr WC, Crowell MD, Lin BK, Harnish MJ, Chen JD. Sleep and gastric function in irritable bowel syndrome: derailing the brain-gut axis. Gut. 1997;41(3):390–3. doi: 10.1136/gut.41.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorard DA, Vesselinova-Jenkins CK, Libby GW, Farthing MJ. Migrating motor complex and sleep in health and irritable bowel syndrome. Dig Dis Sci. 1995;40(11):2383–9. doi: 10.1007/BF02063242. [DOI] [PubMed] [Google Scholar]
- 10.Vege SS, Locke GR, 3rd, Weaver AL, Farmer SA, Melton LJ, 3rd, Talley NJ. Functional gastrointestinal disorders among people with sleep disturbances: a population-based study. Mayo Clin Proc. 2004;79:1501–6. doi: 10.4065/79.12.1501. [DOI] [PubMed] [Google Scholar]
- 11.Delgado-Aros S, Locke GR, III, Camilleri MC, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., III Obesity is associated with increased risk of gastrointestinal symptoms: A population-based study. Am J Gastroenterol. 2004;99:1801–6. doi: 10.1111/j.1572-0241.2004.30887.x. [DOI] [PubMed] [Google Scholar]
- 12.Talley N, Howell S, Poulton R. Obesity and chronic gastrointestinal tract symptoms in young adults: a birth cohort study. Am J Gastroenterol. 2004;99:1807–14. doi: 10.1111/j.1572-0241.2004.30388.x. [DOI] [PubMed] [Google Scholar]
- 13.Talley NJ, Quan C, Jones MP, Horowitz M. The association of upper and lower gastrointestinal tract symptoms with body mass index in an Australian cohort. Neurogastro Motil. 2004;16:413–9. doi: 10.1111/j.1365-2982.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 14.Kohatsu ND, Tsai R, Young T, Vangilder R, Burmeister LF, Stromquist AM, Merchant JA. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006;166(16):1701–5. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 15.Sperber AD, Tarasiuk A. Disrupted sleep in patients with IBS--a wake-up call for further research? Nat Clin Pract Gastroenterol Hepatol. 2007;4(8):412–3. doi: 10.1038/ncpgasthep0847. [DOI] [PubMed] [Google Scholar]
- 16.Beebe TJ, Talley NJ, Camilleri M, Jenkins SM, Anderson KJ, Locke GR., 3rd The HIPAA authorization form and effects on survey response rates, nonresponse bias, and data quality: a randomized community study. Med Care. 2007;45(10):959–65. doi: 10.1097/MLR.0b013e31805468b0. [DOI] [PubMed] [Google Scholar]
- 17.Talley N, Phillips S, Wiltgen C, Zinsmeister A, Melton LJ. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–79. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 18.Morin CMEJ. Sleep disorders: Evaluation and diagnosis. In: Turners SM, H M, editors. Adult Psychopathology and Diagnosis. New York: Wiley; 1997. pp. 483–507. [Google Scholar]
- 19.Moore DB, Folsom AR, Mink PJ, Hong CP, Anderson KE, Kushi LH. Physical activity and incidence of postmenopausal breast cancer. Epidemiology. 2000;11:292–96. doi: 10.1097/00001648-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self-reported height, weight, and body mass index: findings from the Third National Health and Nutrition Examination Survey, 1988-1994. J Am Diet Assoc. 2001;101:28–34. doi: 10.1016/S0002-8223(01)00008-6. quiz 35-6. [DOI] [PubMed] [Google Scholar]
- 21.Organization, W. H. Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity. Geneva: 1997. [PubMed] [Google Scholar]
- 22.Koloski NA, Talley NJ, Boyce PM. The impact of functional gastrointestinal disorders on quality of life. Am J Gastroenterol. 2000;95:67–71. doi: 10.1111/j.1572-0241.2000.01735.x. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith G, Levin JS. Effect of sleep quality on irritable bowel syndrome. Dig Dis Sci. 1993;38(10):1809–14. doi: 10.1007/BF01296103. [DOI] [PubMed] [Google Scholar]
- 24.Fass R, Quan SF, O'Connor GT, Ervin A, Iber C. Predictors of heartburn during sleep in a large prospective cohort study. Chest. 2005;127(5):1658–66. doi: 10.1378/chest.127.5.1658. [DOI] [PubMed] [Google Scholar]
- 25.Fass R, Achem SR, Harding S, Mittal RK, Quigley E. Review article: supra-oesophageal manifestations of gastro-oesophageal reflux disease and the role of night-time gastro-oesophageal reflux. Aliment Pharmacol Ther. 2004;20 9:26–38. doi: 10.1111/j.1365-2036.2004.02253.x. [DOI] [PubMed] [Google Scholar]
- 26.Maconi G, Tosetti C, Stanghellini V, Porro GB, Corinaldesi R. Dyspeptic symptoms in primary care. An observational study in general practice. European Journal of Gastroenterology & Hepatology. 2002;14:985–990. doi: 10.1097/00042737-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa Y, Cook IJ, Panagopoulos V, McEvoy RD, Sharp DJ, Simula M. Relationship between sleep patterns and human colonic motor patterns. Gastroenterology. 1994;107(5):1372–81. doi: 10.1016/0016-5085(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 28.Jarrett M, Heitkemper M, Cain KC, Burr RL, Hertig V. Sleep disturbance influences gastrointestinal symptoms in women with irritable bowel syndrome. Dig Dis Sci. 2000;45(5):952–9. doi: 10.1023/a:1005581226265. [DOI] [PubMed] [Google Scholar]
- 29.Heitkemper M, Jarrett M, Burr R, Cain KC, Landis C, Lentz M, Poppe A. Subjective and objective sleep indices in women with irritable bowel syndrome. Neurogastroenterol Motil. 2005;17(4):523–30. doi: 10.1111/j.1365-2982.2005.00700.x. [DOI] [PubMed] [Google Scholar]
- 30.Elsenbruch S, Harnish MJ, Orr WC. Subjective and objective sleep quality in irritable bowel syndrome. Am J Gatsroenterol. 1999;94(9):2447–52. doi: 10.1111/j.1572-0241.1999.01374.x. [DOI] [PubMed] [Google Scholar]
- 31.Kumar D, Idzikowski C, Wingate DL, Soffer EE, Thompson P, Siderfin C. Relationship between enteric migrating motor complex and the sleep cycle. Am J Physiol. 1990;259(6 Pt 1):G983–90. doi: 10.1152/ajpgi.1990.259.6.G983. [DOI] [PubMed] [Google Scholar]
- 32.Kumar D, Thompson PD, Wingate DL, Vesselinova-Jenkins CK, Libby G. Abnormal REM sleep in the irritable bowel syndrome. Gastroenterology. 1992;103(1):12–7. doi: 10.1016/0016-5085(92)91089-m. [DOI] [PubMed] [Google Scholar]
- 33.Narducci F, Bassotti G, Gaburri M, Morelli A. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut. 1987;28(1):17–25. doi: 10.1136/gut.28.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bassotti G, Germani U, Fiorella S, Roselli P, Brunori P, Whitehead WE. Intact colonic motor response to sudden awakening from sleep in patients with chronic idiopathic (slow-transit) constipation. Dis Colon Rectum. 1998;41(12):1550–5. doi: 10.1007/BF02237305. discussion 1555-6. [DOI] [PubMed] [Google Scholar]
- 35.Mertz H, Fass R, Kodner A, Yan-Go F, Fullerton S, Mayer EA. Effect of amitriptyline on symptoms, sleep, and visceral perception in patients with functional dyspepsia. Am J Gastroenterol. 1998;93:160–65. doi: 10.1111/j.1572-0241.1998.00160.x. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel BM, Gralnek IM, Bolus R, Chang C, Dulai GS, Mayer EA, Naliboff B. Clinical determinants of health-related quality of life in patients with irritable bowel syndrome. Arch Intern Med. 2004;164:1773–80. doi: 10.1001/archinte.164.16.1773. [DOI] [PubMed] [Google Scholar]
- 37.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


