Abstract
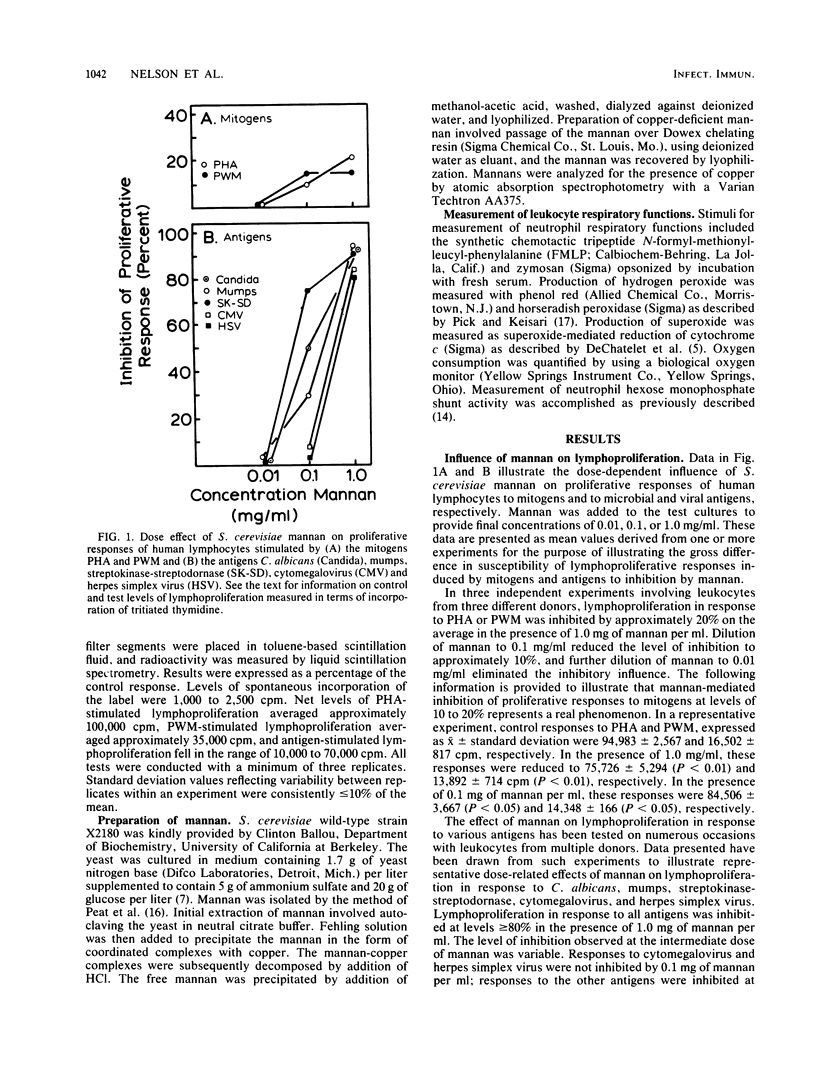
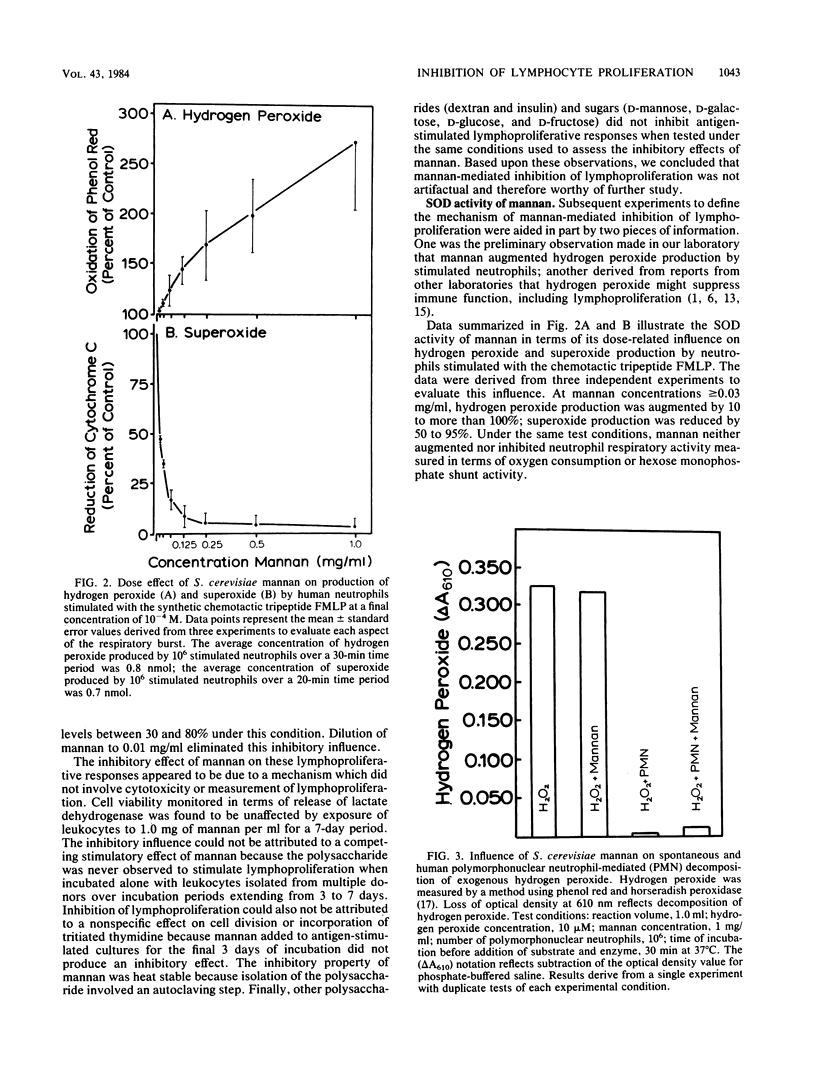
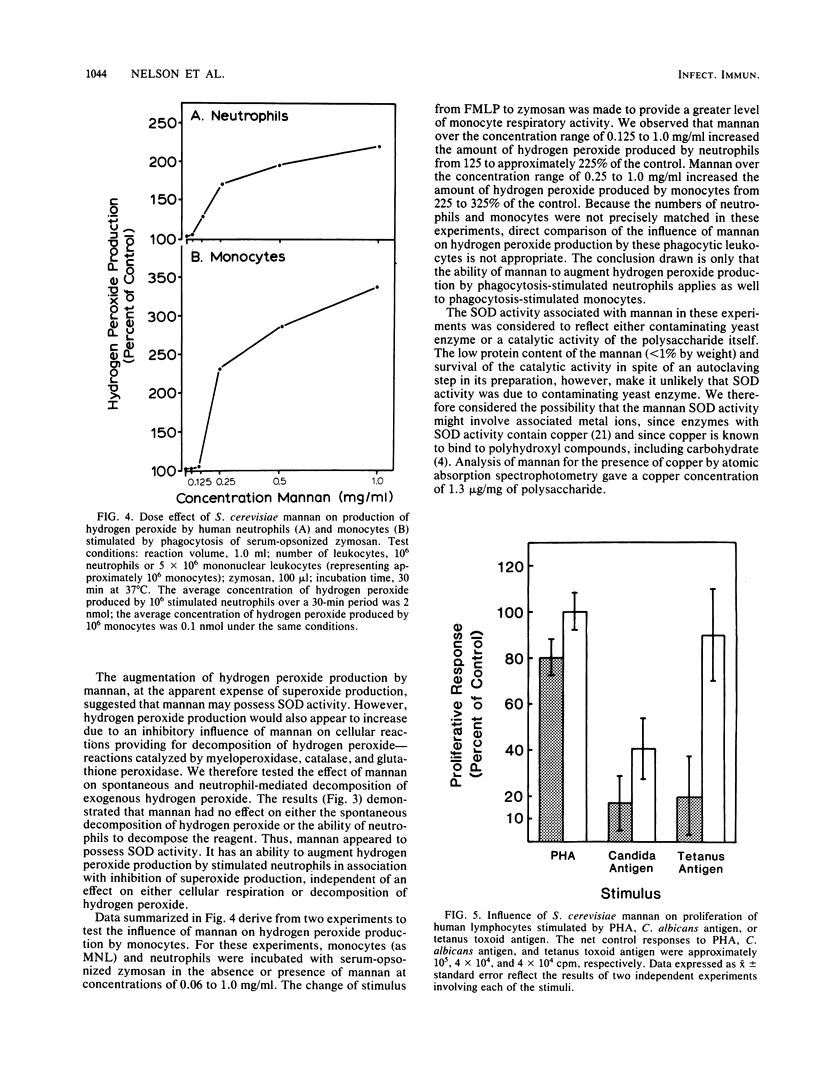
The literature on chronic mucocutaneous candidiasis contains multiple reports which suggest that loss of cell-mediated immunity in this disease may be related in part to the presence of an inhibitory factor(s) present in patient plasma. One such inhibitory factor has been suggested to be mannan polysaccharide released from the cell wall of the pathogen. The present report describes results of experiments to consider mechanisms by which yeast mannan influences proliferative responses of human lymphocytes. Mannan for these experiments was isolated from Saccharomyces cerevisiae. We observed that mannan-mediated inhibition of proliferative responses to a battery of stimuli (phytohemagglutinin, pokeweed mitogen, and Candida, mumps, streptococcus, cytomegalovirus, and herpes simplex virus antigens) was related in part to an effect of copper associated with the mannan and possibly to the superoxide dismutase activity of the mannan-copper complex. Mannan made deficient in copper by use of a copper-chelating resin appeared to inhibit only lymphoproliferation stimulated by the Candida antigen. These results suggest that inhibitory effects of yeast mannans on lymphoproliferative responses may involve at least two mechanisms, one related to hydrogen peroxide production augmented by mannan-copper complexes and another related to still unknown effects independent of the metal ligand. We propose that our results represent a significant novel observation which may be useful in understanding mechanisms of immunoinhibitory effects of C. albicans mannan.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune T. M., Pierce C. W. Conversion of soluble immune response suppressor to macrophage-derived suppressor factor by peroxide. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5099–5103. doi: 10.1073/pnas.78.8.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- Deshazo R. D., Ewel C., Londono S., Metzger Z., Hoffeld J. T., Oppenheim J. J. Evidence for the involvement of monocyte-derived toxic oxygen metabolites in the lymphocyte dysfunction of Hodgkin's disease. Clin Exp Immunol. 1981 Nov;46(2):313–320. [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. A rapid one-step procedure for purification of mononuclear and polymorphonuclear leukocytes from human blood using a modification of the Hypaque-Ficoll technique. J Immunol Methods. 1978;24(3-4):389–393. doi: 10.1016/0022-1759(78)90143-6. [DOI] [PubMed] [Google Scholar]
- Fischer A., Ballet J. J., Griscelli C. Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharidic antigens present in the serum of patients with chronic mucocutaneous candidiasis. J Clin Invest. 1978 Nov;62(5):1005–1013. doi: 10.1172/JCI109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrz R. C., Christianson W. R., Linner K. M., Groth K. E., Balfour H. H., Jr Cytomegalovirus vaccine. Specific humoral and cellular immune responses in human volunteers. Arch Intern Med. 1980 Jul;140(7):936–939. doi: 10.1001/archinte.140.7.936. [DOI] [PubMed] [Google Scholar]
- Kocourek J., Ballou C. E. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969 Dec;100(3):1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E. Modulation of human antibody production in vitro by D-penicillamine and CuSO4: inhibition of helper T cell function. J Rheumatol Suppl. 1981 Jan-Feb;7:69–73. [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., Gracyk J. M., Simmons R. L. Esterase dependence of human neutrophil functions: lysozyme secretion, spontaneous migration, and continuity of the respiratory burst. J Infect Dis. 1981 Jan;143(1):94–100. doi: 10.1093/infdis/143.1.94. [DOI] [PubMed] [Google Scholar]
- Nishida Y., Tanimoto K., Akaoka I. Effect of free radicals on lymphocyte response to mitogens and rosette formation. Clin Immunol Immunopathol. 1981 Jun;19(3):319–324. doi: 10.1016/0090-1229(81)90074-x. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Valdimarsson H., Higgs J. M., Wells R. S., Yamamura M., Hobbs J. R., Holt P. J. Immune abnormalities associated with chronic mucocutaneous candidiasis. Cell Immunol. 1973 Mar;6(3):348–361. doi: 10.1016/0008-8749(73)90035-x. [DOI] [PubMed] [Google Scholar]
- Weser U., Barth G., Djerassi C., Hartmann H. J., Krauss P., Voelcker G., Voelter W., Voetsch W. A study on purified apo-erythrocuprein. Biochim Biophys Acta. 1972 Aug 31;278(1):28–44. doi: 10.1016/0005-2795(72)90104-3. [DOI] [PubMed] [Google Scholar]


