Abstract
Despite its vital importance to life, respiration is not mature at birth in mammals, but rather, it undergoes a great deal of growth, refinement, and adjustments postnatally. Many adjustments do not follow smooth paths, but assume abrupt changes during certain postnatal periods that may render the animal less capable of responding to respiratory stressors. The present review focuses on neurochemical and physiological correlates of a critical period of respiratory development in the rat. In addition to an imbalanced expression of reduced excitatory and enhanced inhibitory neurotransmitters, a switch in the expressions of GABAA receptor subunits from α3 to α1 occurs around postnatal day (P)12 in the Pre-Bötzinger nucleus and the ventrolateral subnucleus of the solitary tract nucleus. Possible subunit switches in a number of other neurotransmitter receptors are discussed. These neurochemical changes are paralleled by ventilatory adjustments at the end of the second postnatal week. At P13 and under normoxia, respiratory frequency reaches its peak before assuming a gradual fall, and both tidal volume and minute ventilation exhibit a significant rise prior to a plateau or a gradual decline until P21. The response to acute hypoxia is markedly reduced between P12 and P16, being lowest at P13. Thus, the end of the second postnatal week can be considered as a critical period of respiratory development, during which multiple neurochemical and physiological adjustments and switches are orchestrated at the same time, rendering the system extremely dynamic but, at the same time, vulnerable to externally imposed perturbations and insults. The critical period embodies a time of multi-system, multifaceted growth and adjustments. It is a plastic, transitional period that is also a part of the normal development of the respiratory system.
Keywords: Brain stem, development, neurochemical, receptor subunit switch, respiration, ventilation
1. Introduction
Respiration is a key function vital for the survival of all living cells and for the organism. In mammals, automatic inspiratory and expiratory functions are mediated by a network of nuclei residing in the brain stem and the spinal cord, and these, in turn, regulate the activity of pump and airway respiratory muscles via the phrenic nerves and specific cranial and intercostal nerves. The orchestration of coordinated activities among all of these components is indeed complex and yet almost completely autonomous, without significant conscious control by the individual. Such orchestration appears to occur virtually instantaneously at birth, when the infant mammal expands its lungs and adapts to atmospheric breathing. However, not all components are fully mature at birth. A period of refinement has been shown to take place during early postnatal development.
A hint of instability during postnatal development comes from observations of human infants who died from Sudden Infant Death Syndrome (SIDS). Most of these infants did not exhibit any overt, life-threatening respiratory pathology or abnormality before the catastrophic event, and the highest incidence is not at birth, but rather, between the second and the fourth months after birth (Moon et al., 2007). The culmination of available evidence led Filiano and Kinney to propose the Triple Risk Model in 1994 (Filiano and Kinney, 1994). This model states that SIDS occurs, and only occurs, when (a) a vulnerable infant encounters (b) an external stressor or stressors during (c) a critical period of postnatal development.
Is there a critical period of postnatal development during which a vulnerable infant may succumb to external stressor/s? Most studies on rodents indicate that the first two postnatal weeks are critical for the postnatal development of respiratory control. The exact timing reported, however, varies among studies because typically only a few selected time points were examined. The general pattern of neurochemical development in various brain stem respiratory nuclei has been reviewed previously (Wong-Riley and Liu, 2005). The present review concentrates on neurotransmitter receptor subunit switches during development and on postnatal changes in ventilation during normoxia and acute hypoxia. The overall objective is to gain a better understanding of neurochemical and physiological bases of a sensitive or critical period in the development of the respiratory system in rats.
2. Neurochemical development of brain stem respiratory nuclei
Neurotransmitters and receptors are expected to undergo postnatal changes in their expressions as synapses get established, pruned, and mature. What is surprising, however, is that the developmental trends of some of the major neurochemicals do not follow smooth paths. Rather, they take brief and abrupt turns within a narrow time window. In this remarkable window (around postnatal day P12 in the rat), multiple neurochemicals exhibit changes at the same time. These include a sudden decrease in the immunohistochemical expressions of the excitatory neurotransmitter glutamate and its receptors (N-methyl-D-aspartate or NMDA), a marked increase in the immunohistochemical expressions of the inhibitory neurotransmitter GABA and the inhibitory transmitter receptors (GABAB and glycine), and an abrupt reduction in the activity of an energy-generating enzyme, cytochrome oxidase, all in individual neurons of several brain stem respiratory nuclei (Liu and Wong-Riley, 2002; Liu and Wong-Riley, 2003; Liu and Wong-Riley, 2005)(reviewed in (Wong-Riley and Liu, 2005)). Notably, the AMPA (αamino-3-hydroxy-5-methylisoxazole-4-propionic acid) glutamate receptor subunit 2 (GluR2), which decreases the permeability of receptors to Ca2+ and decreases neuronal excitation (Geiger et al., 1995), also shows an abrupt rise in its expression at P12 (Liu and Wong-Riley, 2002). This is in line with a general shift toward decreased neuronal excitability at this time. If the critical period of sensitivity is “a time in which processes undergoing rapid maturational changes are particularly vulnerable” (Slotkin et al., 1986), then the sudden, dichotomous, and imbalanced expressions of inhibitory and excitatory neurochemicals would signify such a critical period.
3. Receptor subunit switches in postnatal development
The action of any neurotransmitter is not governed solely by its inherent properties, but by the type of receptors that mediate the postsynaptic response. This fact was made clear more than 70 years ago, when Otto Loewi (in 1921) discovered that acetylcholine slowed the heart (Loewi, 1956), and Henry Dale (in 1936) found that the same chemical mediated skeletal muscular contraction (Dale et al., 1936). The receptor types were found to be muscarinic and nicotinic, respectively (Purves, 1976). Since then, the number of receptor subtypes for various neurotransmitters has mushroomed. Glutamate receptors hold a commanding lead, with NMDA and non-NMDA receptors as the two major subtypes, and AMPA, kainate, and metabotropic receptors under the non-NMDA umbrella (Keinanen et al., 1990; Nakanishi, 1992). GABA receptors encompass A, B, and C categories, each with its own kinetic and pharmacological properties (Johnston, 1996). In recent years, however, attention has been paid to the subunit composition of each receptor type and subtype. Subunit composition not only determines the physiological and pharmacological properties of the receptor, but it also undergoes developmental changes by switching from one major subunit type to another (see below). These changes often occur without any external cue, are likely to be genetically determined, and coincide temporally with other neurochemical, physiological, and maturational adjustments during a sensitive or critical period of postnatal development.
In the respiratory system, GABAA receptor α subunit switch is the one that is best studied (see below). AMPA receptor subunit 2 (GluR2) also undergoes distinct postnatal changes. This review will examine such findings as well as switches in other neurotransmitter receptors elsewhere in the brain as an incentive for future investigations in the respiratory system.
3.1 GABAA receptor subunit switch in development
Gamma-aminobutyric acid (GABA) is a major inhibitory neurotransmitter in the central nervous system. It acts mainly on GABAA receptors to mediate fast transmission and on GABAB receptors for slow transmission. The native GABAA receptor is a heteropentameric protein, most often consisting of two α (α 1–6), two β (β1–3) and a fifth subunit, which can be either a third β, a γ (γ1–3), δ, σ (σ 1–2), or an ε subunit (Hevers and Luddens, 1998; Farrar et al., 1999; Klausberger et al., 2001; Baumann et al., 2002). The structure and assembly of these different subunits determine the functional characteristic of GABAA receptors (Hevers and Luddens, 1998), which undergo significant postnatal changes in kinetics and functional properties, such as from slow to fast kinetics and from depolarizing to hyperpolarizing transmission (Cherubini et al., 1991; Hollrigel and Soltesz, 1997; Kapur and Macdonald, 1999; Hutcheon et al., 2000; Taketo and Yoshioka, 2000; Banks et al., 2002; Bosman et al., 2002). These changes are postulated to be the outcome of, at least in part, changes in GABAA receptor subunit composition (Hornung and Fritschy, 1996; Banks et al., 2002), especially(Bosman of et al., 2002) the α as well as a change in intracellular chloride concentration due to a shift from a predominance of Na+-K+-2Cl− cotransporter to that of K+-coupled Cl− transporter (Plotkin et al., 1997; Lee et al., 2005). The majority of GABAA receptor subunit changes reported thus far involve a developmental decrease in α2 or α3 expression and an (Laurie et al., increase 1992; Fritschy et al., 1994; Hornung and Fritschy, 1996; Okada et al., 2000; Bosman et al., 2002; Heinen et al., 2004). The proposal is that α2 (or α3) is correlated with depolarizing GABA transmission in early life (Hornung and Fritschy, 1996), whereas the onset of α1 subunit expression in the maturing brain signifies a new receptor subtype involved in synaptic inhibition (Fritschy et al., 1994). Although direct information is not yet available about the relationship between subunit composition and postsynaptic potentials mediated by GABAA receptors, the fact that α2 and/or α3 subunit is expressed predominantly in neurons with excitatory GABAA receptors supports that relationship (Cherubini et al., 1990; Mercuri et al., 1991; Michelson and Wong, 1991; Reichling et al., 1994). Furthermore, the GABA-induced increase in intracellular calcium observed in immature rat cortical neurons disappears when GABAA receptor α2 subunit is replaced by α1 subunit (Lin et al., 1994).
The time course of transition from depolarizing to hyperpolarizing GABA transmission and the timing of the GABAA receptor subunit switch most likely vary among different species, different brain regions, and different neuronal types. In the rat hippocampus, approximately 85–93% of CA3 neurons exhibit spontaneous giant depolarizing potentials mediated by GABA between P0 and P8. By P11–12, only 25% of neurons show that characteristic, and after P12 they are no longer present (Ben-Ari et al., 1989), indicating that GABA transmission has switched to exclusive hyperpolarization. The reversal potential of synaptic and GABAA receptor-mediated responses undergoes a developmental shift from −55 mV at P0–2 to −74 mV at P13–15, as the effect of GABA transitions from excitation to inhibition some time around the second postnatal week in the rat hippocampus (Isaev et al., 2007; Tyzio et al., 2007). GABAA receptor-mediated currents shift from slower rise and decay kinetics to faster, more mature ones around the end of the second postnatal week in dentate granule cells (Hollrigel and Soltesz, 1997). In the murine respiratory system, the reversal potential of GABAA receptors reportedly mediates the current switch from depolarizing to hyperpolarizing within the first postnatal week (Ritter and Zhang, 2000). However, depolarizing GABA transmission is reportedly not found in respiratory neurons of P0–P2 rats in vitro (Shao and Feldman, 1997; Brockhaus and Ballanyi, 1998). Future in vivo studies may shed more light on this issue. A switch from α2- to α1-subunit occurs during the first postnatal week in the mesencephalon and hypothalamus, and during the second and third weeks in the thalamus, pallidum, and medulla (Fritschy et al., 1994). A switch from α3- to α1-subunit starts around P11 in the visual cortex of rats, i.e., before eye opening, and it is not prevented by dark rearing, indicating that it is not triggered by visual input (Heinen et al., 2004), but rather, is genetically pre-programmed.
Possible switches in GABAA receptor subunits in the brain stem respiratory nuclei were not examined until recently, when the expressions of α1, α2, and α3 were followed at a closely timed sequence from P0 to P21 in the rat (Liu and Wong-Riley, 2004; Liu and Wong-Riley, 2006). In the pre-Bötzinger nucleus (PBC), a postulated center of respiratory rhythmogenesis (Smith et al., 2000), α3 is expressed at relatively high levels at P0 and declines with age, whereas α1 is expressed at relatively low levels at birth but increases with age. Significantly, the two developmental trends intersect close to P12 (Fig. 1A). The expression of α2 subunit remains relatively constant during development (Liu and Wong-Riley, 2004). Similar trends are observed in the ventrolateral subnucleus of the solitary tract nucleus (NTSVL), which receives input from the carotid body (Finley and Katz, 1992) and relays slowly-adapting pulmonary stretch receptor information via its pump cells (which are mainly GABAergic) to other regions of the NTS (such as the commissural nucleus) and to the ventrolateral medulla and pons (Miyazaki et al., 1999; Otake et al., 2001; Kubin et al., 2006). Whether these trends exist in the commissural and other subnuclei of NTS is unknown at this time. Subunit α1 expression gradually increases from birth, peaks at P12, and remains high thereafter. Subunit α3 expression is prominent during the first postnatal week, but is significantly reduced from P11 to P12 and remains low until P21 (Fig. 1B) (Liu and Wong-Riley, 2006). Thus, for both PBC and NTSVL, the switch from α 3 to α 1 subunit dominance occurs around P12. Such switches may contribute to alterations in the kinetics of postsynaptic potentials mediated by GABAA receptors, rather than to the reversal potential of GABAA receptor mediated current, in affecting the function of GABA transmission. In this regard, α3 subunit is thought to be responsible for slow decay time and slow kinetics at early stages of visual cortical development, whereas α1 subunit is for fast decay time and fast kinetics in the adult (Bosman et al., 2002). The same analogy is applied to the murine superior colliculus (a switch form α3 to α1) (Juttner et al., 2001) and the rat thalamus (a switch from α2 to α1) (Okada et al., 2000). Thus, GABAA α subunit switching may prove to be a common theme in neuronal development, but the precise timing of the switch may vary among regions. In the non-respiratory cuneate nucleus, for example, the apparent switch occurs approximately two days earlier than those in the PBC and NTSVL (Fig. 1C) (Liu and Wong-Riley, 2006).
Fig.1.
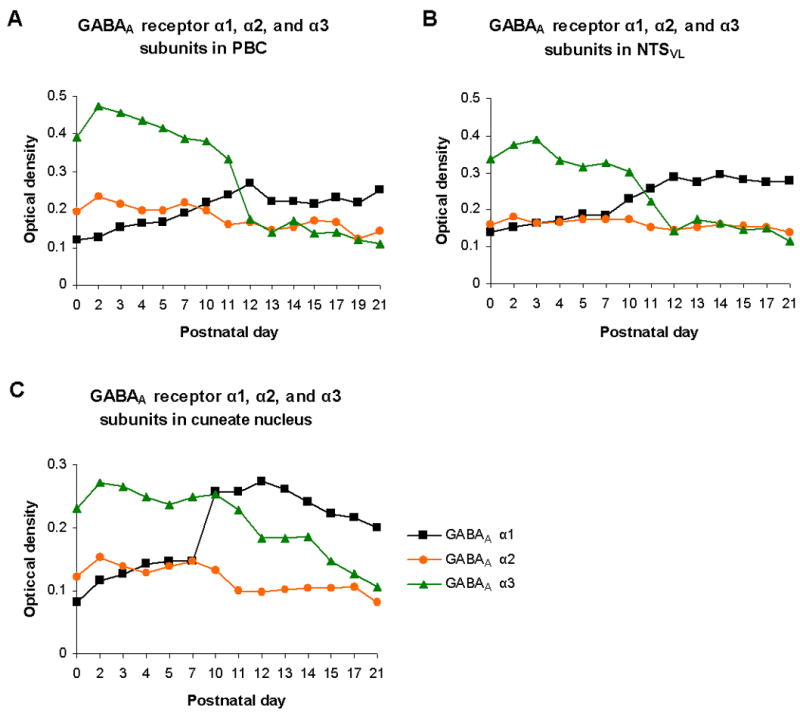
Composite graphs based on data of previous reports (Liu and Wong-Riley, 2004; Liu and Wong-Riley, 2006) for the developmental expressions of GABAA receptor subunits α1, α2, and α3 in the Pre-Bötzinger complex PBC (A), the ventrolateral subnucleus of the nucleus tractus solitarius NTSVL (B), and the non-respiratory cuneate nucleus (C). Note that in all three nuclear groups, the expression of subunit α1 increases with age, whereas that of subunit α3 decreases with age. The expression of subunit α2 remains relatively low and constant during development. Subunit α1 reaches its peak expression at P12 in both PBC and NTSVL, but earlier at P10 in the cuneate nucleus. The developmental trends of α1 and α3 intersect close to P12 in the PBC and NTSVL, but at P10 in the cuneate nucleus.
A delicate balance between excitation and inhibition maintains the normal functioning of the central nervous system. Small changes in GABA-mediated inhibition can profoundly alter neuronal excitability (Mody et al., 1994). Switching of α3 and α1 subunits in GABAA receptors around P12 in the PBC and NTSVL may result in a transient imbalance between excitation and inhibition, i.e., increased inhibitory drive and decreased excitatory drive. The temporal correlation between GABAA α subunit witching and an imbalance in the expressions ofs inhibitory and excitatory neurochemicals in various brain stem respiratory nuclei around P12 (Liu and Wong-Riley, 2002) underscores a state of dynamic synaptic adjustments during this critical period. The second postnatal week is also a time when GABAergic terminals reportedly undergo reorganization in the NTS, thereby contributing to possible local adjustment in the cardiorespiratory network (Yoshioka et al., 2006). The PBC likewise undergoes other developmental changes, such as the disappearance of most bulbospinal projections when transitioning from the neonate to the adult, although the exact timing of such a change has not been defined (Ellenberger, 1999).
3.2 Possible subunit switches in other neurotransmitter receptor types during development
3.2.1 Glycine receptors
Besides GABAA α subunits, receptor subunits of undergo developmental switches as well. Similar to GABA, glycine receptors also mediate depolarization during development, are heteropentameric (α1–4, 1β) -gated anionligand channels, and complete their subunit switches by P20 in the rat CNS (Lynch, 2004; Kirsch, 2006). In the rat hypoglossal motoneurons, glycinergic postsynaptic potentials transition from depolarizing and prolonged in the neonate to hyperpolarizing and fast during the first two postnatal weeks (Singer et al., 1998). This is accompanied by a switch from α2- to α1-subunit mRNA expression, an increase in the miniature inhibitory postsynaptic currents, and a faster kinetic of evoked inhibitory postsynaptic response (Singer et al., 1998; Singer and Berger, 1999). A detailed analysis of the time course of glycine receptors subunit switch in brain stem respiratory nuclei awaits future investigation.
3.2.1 NMDA receptors
Experience-dependent synaptic plasticity has been postulated in the visual cortex and elsewhere in the brain to be associated with a change in the complement of postsynaptic glutamate receptors, specifically the NMDA receptors, thereby altering the functional properties of these receptors (Quinlan et al., 1999). During the second postnatal week of cerebellar development, there is evidence for a switch in the dominance of subunit composition of the NMDA receptors from NR2B to NR2A and NR2C; at the same time, the decay time of NMDA receptor-mediated excitatory postsynaptic current is significantly reduced (Sasner and Buonanno, 1996; Rumbaugh and Vicini, 1999; Cathala et al., 2000). The switch from NR2B to NR2A is also found in murine somatosensory cortex and thalamus during the second postnatal week of development (Liu et al., 2004). Similar switches are also reported in the forebrain and midbrain during development (van Zundert et al., 2004; Kubota and Kitajima, 2008), with the implication that it potentiates rapid and stable growth of immature synapses and regulates synaptic plasticity. Virtually nothing is known about possible switches in NMDA receptor subunits in the brain stem respiratory network.
3.2.2 AMPA receptors
In the respiratory system, the excitatory neurotransmitter glutamate acts mainly at non-NMDA receptors, such as AMPA and kainate, although metabotropic receptors may also be involved (Liu et al., 1990; Pierrefiche et al., 1994; Dogas et al., 1995). In the brain stem and elsewhere, evidence is emerging that subunit composition undergoes developmental changes.
In the cerebral cortex, pyramidal neurons reportedly undergo a switch in Ca2+ permeability of their glutamate AMPA receptors through an alteration of subunit composition (Kumar et al., 2002). AMPA receptors are known to have 4 major subunits, GluR1–4, all except GluR2 are permeable to Ca2+ (Brorson et al., 1999). AMPA receptors with GluR2 are found to increase with age in the rat neocortex, indicating a developmental switch in AMPA receptor subunit composition from ones dominated by Ca2+–permeable subunits to those that incorporate more of the Ca2+–impermeable GluR2 subunits (Pellegrini-Giampietro et al., 1992; Kumar et al., 2002). This radically changes their current-voltage relationship, their response to external or internal polyamines, and their susceptibility to cell death. It also has strong implications for plasticity. Remarkably, GluR2 expression during postnatal development in six brain stem nuclei involved in respiratory control (pre-Bötzinger complex, ventrolateral subnucleus of nucleus tractus solitarius, nucleus ambiguus, hypoglossal nucleus, dorsal motor nucleus of the vagus, and medial accessory olivary nucleus) exhibits a significant increase at P12, in stark contrast to a concurrent but notable decrease in the expressions of glutamate and NMDA receptors (Liu and Wong-Riley, 2002; Liu and Wong-Riley, 2005). Such a rise in GluR2 at P12 in brain stem respiratory nuclei is consistent with the increase described above in the neocortex. However, it remains to be determined if the other AMPA receptor subunits also undergo developmental adjustments, and if AMPA receptors in brain stem respiratory nuclei alter their physiological properties during postnatal development.
3.3 Serotonin receptors
The actions of serotonin (5-hydroxytryptamine or 5-HT) differ among systems, nuclear groups, age, and the type of receptors, which exist in multiple forms (Barnes and Sharp, 1999; Taylor et al., 2005). Transient expression of 5-HT1A receptors has been reported in the hypoglossal and other somatic motoneurons during development (Talley and Bayliss, 2000). Likewise, age-dependent changes in the expressions of 5-HT1B, 5-HT2A, and 5-HT2C have been noted in postnatal dissociated hypoglossal, facial, and cervical spinal motoneurons (Volgin et al., 2003). For example, 5-HT1B mRNA expression is transiently reduced at P14 in rat hypoglossal motoneurons, and dendritic labeling of 5-HT2A in hypoglossal motoneurons does not appear until after P12 (Volgin et al., 2003).
In the hippocampus of rats, serotonin receptors linked to phosphoinositide hydrolysis undergo a subunit switch from 5-HT2A to 5-HT2C between first and third weeks of life (Ike et al., 1995). Comparable switches have not been reported for brain stem respiratory nuclei. However, the fact that the effect of 5-HT evolves from inhibitory to excitatory in the hypoglossal motoneurons through development strongly implies that changes in receptor subunit expression may be an underlying mechanism (Volgin et al., 2003).
In short, neurotransmitter receptor subunit or subtype switches may play a significant role in transitioning from a neonatal to a more mature form of synaptic transmission during the critical period of respiratory network development. Such changes are likely to be genetically programmed, but can, nonetheless, be influenced by external perturbations. Subunit switches in receptors of different neurotransmitters may be developmentally staggered or temporally coincidental. Future studies will determine if switching converges around the end of the second postnatal week in the respiratory network of rats.
4. Physiological correlates of a critical period of respiratory development
Are there physiological correlates to the significant neurochemical changes occurring toward the end of the second postnatal week in rats? What is the normal developmental trend in ventilatory response during the first three postnatal weeks? What are the features that stand out during development and when do they take place? Is there any indication that the ventilatory response to hypoxia is different around the time of the presumed critical period? These questions are discussed next in this review.
4.1 Developmental changes in respiratory response to normoxia
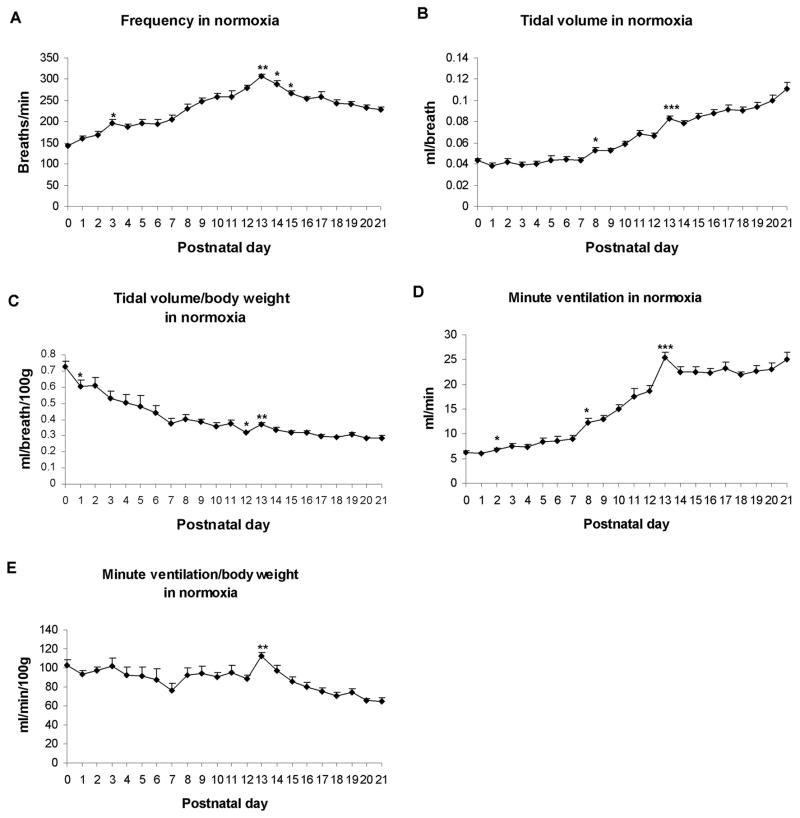
The normal development of respiration in normoxia has been examined mostly in the context of hypoxia or hypercapnia, and typically only a few selected time points were chosen (Mortola et al., 1986; Eden and Hanson, 1987; Cameron et al., 2000; Stunden et al., 2001). When a detailed, daily study was carried out for the first three postnatal weeks in rats (Liu et al., 2006), striking features were revealed (Fig. 2). Respiratory frequencies undergo a steady increase with age, peaking at postnatal day (P) 13 and decline thereafter until P21 (Fig. 2A). Absolute tidal volume (VT) before adjustment to body weight assumes a relative plateau during the first postnatal week, followed by a steady rise until P21, with statistically significant peaks at P8 and P13 (Fig. 2B). When VT is normalized to body weight, it exhibits a steady decrease with age, with a small but significant fall at P1 and P12, a significant increase at P13, followed by a relative plateau until P21 (Fig. 2C). The V̇E response without adjustment to body weight shares a trend similar to that of VT (Fig. 2D). When it is normalized to body weight, V̇E assumes a fluctuating pattern from P0 through P12, with a slight dip at P7 and P12 but a significant increase at P13, followed by a gradual decline until P21 (Fig. 2E).
Fig. 2.
Developmental trends of frequency (f) (A), tidal volume (VT) (B), and minute ventilation (V̇E) (D) during the first 3 postnatal weeks. In normoxia, all three trends increase significantly during the 2nd postnatal week, peaking at P13 (P < 0.001); after which, f values fall steadily until P21, whereas VT and V̇E values initially fall at P14 but rise again until P21. When VT is normalized to body weight (C), its trend decreases with age, with a significant reduction from P0 to P1, and from P11 to P12 (P < 0.05 for both). There is a significant increase at P13 (P < 0.01) followed by a relative plateau until a small rise at P21. The developmental trend of V̇E normalized to body weight (E) exhibits minor fluctuations from P0 to P12. There is a significant rise in V̇E at P13 (P < 0.01), followed by a gradual decrease thereafter. ANOVA within each parameter reveals significant differences among ages (P < 0.01). Statistical comparisons (Tukey's Studentized range test) between successive age groups: *P < 0.05; **P < 0.01; ***P < 0.001 (significance between one age group and its adjacent younger age group). (Modified from Liu and Wong-Riley, 2006).
The newborn rat has a greater surface area-to-body mass ratio that requires a higher metabolic rate per unit body weight than older animals to compensate for greater heat loss (Mortola, 1984). Thus, during normoxia, neonatal rat pups have relatively high VT and V̇E values when adjusted to body weight. During the first postnatal week, the absolute tidal volume in response to normoxia changes very little, while the VT adjusted to body weight exhibits a steady decline with age. During the second week, both frequency and absolute VT sharply escalate, resulting in an increase in V̇E that peaks at P13. This corresponds to the development of the lungs, in which the rapid outgrowth of secondary septa is largely completed by the end of the 2nd postnatal week and the surface area for gas exchange is increasing (Burri, 1974; Burri et al., 1974; Blanco, 1995). When adjusted to body weight, VT still shows a small but significant increase at P13, while V̇E reaches its highest value on that day, most likely reflecting the highest peak attained for respiratory frequency at P13. From P14 onward and during the 3rd postnatal week, frequency values steadily decline, while those of absolute VT increase, denoting the attainment of more mature, deeper, and slower breaths. At the same time, there is an enormous thinning of the alveolar septa in the lung (Burri, 1974), indicating a tremendous increase in the efficiency of gas exchange. Thus, the alveolar ventilation is actually increasing even though the minute ventilation stays relatively constant. The developmental pattern of frequency, with a rise in the first 2 weeks and a fall in the 3rd postnatal week is also evident in Huang et al.’s data (Huang et al., 2004), which include only 6 time points.
The reason for increased baseline ventilation at and around P13 is not entirely clear at this time. Many factors are expected to be involved, such as maturational process of neurotransmission, imbalance of excitatory versus inhibitory synapses, conversion of neonatal to mature forms of receptor subunit composition, hypothalamic and other supra-brain stem influences, maturational process of the gas exchange mechanism in the lung, adjustment of body temperature, and maturational process of locomotion and sleep. The end of the second postnatal week is a period of multiple developmental changes that may impinge upon respiratory behavior of the animal. However, even though the baseline ventilation is increased, the animals are less capable of responding to hypoxia (see below).
4.2 The hypoxic ventilatory response undergoes postnatal developmental changes
The response to hypoxia, or hypoxic ventilatory response (HVR), undergoes developmental regulation via a variety of mechanisms (Berquin et al., 2000; Waters and Gozal, 2003; Simakajornboon and Kuptanon, 2005). Mammalian HVR to acute hypoxia is known to be biphasic, consisting of an initial increase in ventilation followed by a later ventilatory suppression that is termed hypoxic ventilatory depression (HVD) or hypoxic ventilatory roll-off (Mortola, 1984; Easton et al., 1986; Eden and Hanson, 1987; Vizek et al., 1987; Gershan et al., 1994; Powell et al., 1998; Mortola, 1999; Moss, 2002). HVR is a complex process in which several excitatory, inhibitory, and modulatory components are involved. The early HVR may be mediated by glutamate and its N-methyl-D-aspartate (NMDA) receptors (Kazemi and Hoop, 1991; Ohtake et al., 1998; Richter et al., 1999) via carotid body-nucleus tractus solitarius (NTS) pathway and perhaps other central pathways (Mizusawa et al., 1994; Ohtake et al., 2000). It also involves the activation of protein kinase C, neuronal nitric oxide synthase, and tyrosine kinase, and such activation increases with age and NMDA receptor expression (reviewed in (Simakajornboon and Kuptanon, 2005)). HVD is postulated to be attributable to the central depressant effect of hypoxia (Vizek et al., 1987), but peripheral (arterial chemoreceptor) mechanism may not be excluded (reviewed in (Bissonnette, 2000)). One possibility is that HVD may act by depressing synaptic pathways activated by arterial chemoreceptors (Powell et al., 1998). Several neurotransmitters and neuromodulators, such as GABA (Kneussl et al., 1986; Kazemi and Hoop, 1991; Richter et al., 1999), serotonin (Di Pasquale et al., 1992; Richter et al., 1999), adenosine (Neylon and Marshall, 1991; Elnazir et al., 1996; Richter et al., 1999), and platelet-derived growth factor (PDGF-β) (Gozal et al., 2000; Simakajornboon and Kuptanon, 2005)) may play important roles in HVD. In addition, hypoxic hypometabolism (Mortola, 1999) may contribute to HVD, and nitric oxide has been implicated in both excitatory and inhibitory components of the HVR (Gozal et al., 1997). Gasping, which is activated in severe hypoxia or apnea, is reportedly mediated by 5-HT2A receptors (Tryba et al., 2006).
HVD differs between the neonate and the adult. In neonatal mammals exposed to sustained hypoxia, the minute ventilation (V̇E) gradually decreases to levels near or below those observed in normoxia (baseline) (Eden and Hanson, 1987; Mortola and Rezzonico, 1988; Fung et al., 1996; Cohen et al., 1997; Martin et al., 1998), whereas in adult mammals, HVD displays a milder ventilatory decline to levels that remain higher than the baseline (Easton et al., 1986; Vizek et al., 1987; Gershan et al., 1994; Maxova and Vizek, 2001). Is there a critical period during postnatal development when the animal is less responsive to hypoxia? The answer to this question requires a more closely-spaced temporal study.
A comprehensive, daily monitoring of ventilation in normoxia and hypoxia during the first three postnatal weeks of rats was conducted recently (Liu et al., 2006). The response to acute hypoxia (10% O2 + 90% N2 for 5 minutes every fifth day for each of the animals tested) is indeed biphasic, especially from P3 onward, with ventilation in the first 30 seconds to 1 minute being higher than the rest of the 5-min period. Significantly, this biphasic response undergoes developmental changes during the first 3 postnatal weeks. From P0 to P11, the ratio of V̇E in hypoxia to that in normoxia throughout the 5-min period is > 1 (Fig. 3C), indicating that the system is able to respond adequately to hypoxia. However, between P12 to P16, much of the late phase of HVR is < 1, suggesting that the depressive effect of hypoxia is much more prominent at this time, despite the fact that ventilation during normoxia is quite high (Liu et al., 2006). This reduction is due mainly to decreased frequency response, but on P13, when HVR is at its lowest, the decrease is caused by a distinct suppression of both frequency and VT (Fig. 3A, B, and C). P13 is the only time during development when the VT(Hypoxia):VT(Normoxia) ratio falls below 1. The marked reduction in HVR at the end of the second postnatal week is consistent with a predominance of inhibitory neurotransmitter expression, a switch in GABAA receptor subunits from a neonatal to an adult form, as well as heightened expression of GluR2 receptors occurring at the same time in multiple brain stem respiratory nuclei (discussed above and reviewed in (Wong-Riley and Liu, 2005)), all of which point to a stronger inhibitory suppression that may dampen the hypoxic ventilatory response. This can be tested in the future by recordings of respiratory nuclei, including the phrenic motor nerve nuclei, in intact animals exposed to hypoxia at the end of the second postnatal week versus those at other ages. Between P17 and P21, HVR returns to a level above the baseline, indicating that, once again, the system regains a better capability to cope with hypoxia. This renewed ability is due primarily to increasing tidal volume (Fig. 3B), despite decreasing frequency of respiration, with age (Fig. 3A). Thus, a deep and slow breathing pattern in response to hypoxia is characteristic of a more mature response, as it improves the efficiency of V̇E by maximizing alveolar ventilation (Frappell and Mortola, 1994).
Fig. 3.
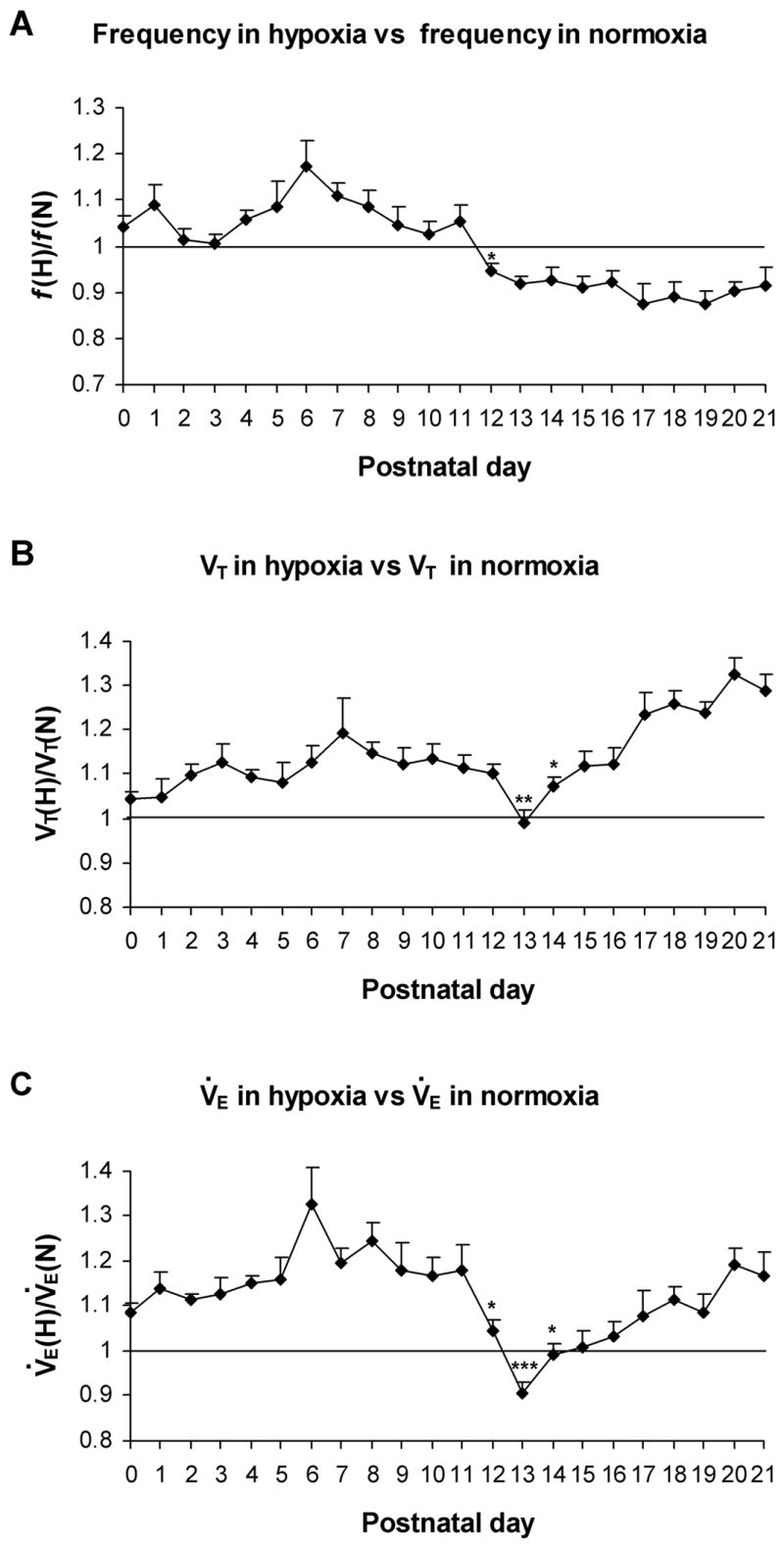
Developmental changes in the mean values of frequency (f) (A), tidal volume (VT) (B), and minute ventilation (V̇E) (C) averaged over 5-min of hypoxic ventilatory response (in 10% O2 + 90% N2) versus those in normoxia. These values are all above 1 from P0 to P12, with a peak at P6 (for f and V̇E) or P7 (for VT). At P12, the f and V̇E ratios drop precipitously (P < 0.05 for both), and at P13, the ratios for VT and V̇E are significantly lower than those at P12 (P < 0.05 – 0.001), with the VT and V̇E ratios reaching their lowest on that day, and V̇E ratio is significantly below 1. After P13, VT and V̇E ratios both increase with age (especially at P14, P < 0.05) while f ratios remain below 1 through P21. ANOVA of each parameter indicates significant differences among ages (P < 0.01). Statistical comparisons (Tukey's Studentized range test) between successive age groups: *P < 0.05; **P < 0.01; ***P <0.001. (Modified from Liu and Wong-Riley, 2006).
The period around P13 (P12–P15) stands out as developmentally distinct in the rat (Liu et al., 2006). Both frequency and V̇E have reached their highest peaks at P13 under normoxia, and HVR is lowest at P13, with values at P12–P15 being lower than the rest of the 3 postnatal weeks. The validity of these findings is strengthened by the relatively large number of animals (between 12 to14 animals from 7 litters per day) examined each day of the first 3 postnatal weeks. Thus, the end of the 2nd postnatal week is distinctly marked as a time of substantially reduced ability of the rat pups to respond to hypoxia.
Another developmental perturbation occurs at P3, the only time during the 1st postnatal week when the breathing frequency in response to hypoxia falls below baseline (Liu et al., 2006). However, HVR (V̇E) may be marginally adequate at this time because tidal volume is above the baseline level. Remarkably, significant alterations in neurochemical expressions (decrease in glutamate and NMDA receptors, and increase in GABA, GABAB, glycine and GluR2) and a reduction in cytochrome oxidase activity take place at P3–4 in eight brain stem nuclei involved in respiratory control (Liu and Wong-Riley, 2001; Liu and Wong-Riley, 2002). These changes are comparable, though less drastic, than those around P12.
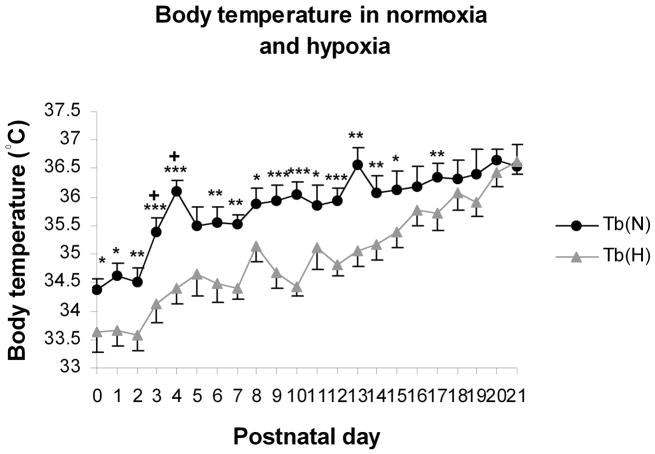
4.3 The body temperature undergoes postnatal developmental changes
The increase in body temperature (taken rectally) with age does not follow a steady path, but rather, it has a peak at P4 and another striking one at P13 (Liu et al., 2006) (Fig. 4). This may be correlated with a significant increase in respiratory frequency at P3 and an overall peak frequency at P13 (Fig. 2A). Increased core temperature especially at P13 may reflect a change in hypothalamic regulation, which may increase thyroid hormone levels and raise the basal metabolic rate, or it may reflect an uncoupling of electron transport and oxidative phosphorylation, generating heat (Dussault and Labrie, 1975; Lanks et al., 1986). If the heat generation is at the expense of ATP production, it could result in decreased energy supply for neuronal activity. Exposure to hypoxia lowers the body temperature by 1–2°C during the first 2 postnatal weeks (Liu et al., 2006). This may be due to hypoxia-induced decreases in metabolic rate, especially the component of oxygen consumption (V̇O2) related to thermogenesis (Mortola and Frappell, 2000). By the third postnatal week, with the acquisition of a thick coat of fur, increased body mass, and reduced frequency of respiration, the animals are then able to maintain their body temperature fairly well during 5 minutes of hypoxia (Liu et al., 2006).
Fig.4.
Changes in body temperature (Tb) and body weight with age. (A) Under normoxic conditions, Tb is lowest at P0 – P2, increases significantly between P3 and P4, then gradually increases until another peak at P13, followed by a small decline at P14 and a gradual increase with age until P21. After 5-min of exposure to hypoxia, Tb falls 1–2°C from P0-15, but close to the baseline (Tb in normoxia) thereafter, and the two trends intersect at P21. Statistical comparisons (Student’s t test) between Tb in normoxia and hypoxia: *P < 0.05; **P < 0.01; ***P < 0.001. (Modified from Liu and Wong-Riley, 2006).
4.4 Possible switches in physiological response during development
Postnatal changes in the expressions of neurotransmitters, receptors, and metabolic enzymes appear to be correlated temporally with changes in physiological response, both in normoxia and hypoxia, as discussed above. By the same token, postnatal switches in the expression of neurochemicals, such as neurotransmitter receptor subunit switches, may be paralleled by comparable “switches” in physiological responses during specific period or periods of postnatal development. A few possible switches to consider include: GABAA receptor and chloride cotransporter-mediated switch from depolarizing to hyperpolarizing transmission (discussed above), a change in phrenic nerve output from irregular to regular (Zhou et al., 1996), a switch from a triphasic to a monophasic gasping response (Gozal and Torres, 2001), a switch in the hypoxic response from mainly a central to both central and peripheral chemoresponsive mechanisms (Saetta and Mortola, 1987) in conjunction with significant maturational changes in carotid body chemoreceptors postnatally (Donnelly, 2005), and a rearrangement of the sympathetic and vagal control of the heart rate (Mills, 1978). The precise timing of these switches needs to be investigated further, as most studies focused on a few selected postnatal days, and profound changes can occur in a relatively short time span, sometimes even in a single day (see above). In the case of hypoxic ventilatory response in the rat, the switch from being influenced by both frequency and VT to that mainly by VT occurs around P13 (Liu et al., 2006) (Fig. 3).
5. Significance of the end of the second postnatal week in rats: a critical period of development
The overriding insight gained from recent studies is that the end of the 2nd postnatal week is a highly plastic and narrow window of respiratory development. This time window may be regarded as the “critical period” described previously as a period “devoted to structural and/or functional shaping of the neural system subserving respiratory control” (Carroll, 2003).
Neurochemically, there is an imbalance between excitatory and inhibitory systems and a switch in GABAA receptor subunit expression (reviewed in (Wong-Riley and Liu, 2005) and above), and physiologically, the response to hypoxia is at its weakest but is stabilizing thereafter (Liu et al., 2006). The response to hypercapnia does not become mature until after the second postnatal week (Davis et al., 2006). The hypothalamo-pituitary-adrenal axis function is close to maturing and alveolarization of the lung is reaching completion (Giulian et al., 1974; Walker et al., 1986; Schmiedl et al., 2007). It is a time of dynamic adjustments by the respiratory network to transition from the neonatal to the adult form of ventilatory control, and it involves an intricate orchestration of multiple players at the molecular, cellular, organellar, and whole body levels. This window coincides with other bodily changes, such as the opening of eyelids, the opening of the external auditory canal, the onset of non-REM sleep, the onset of power-law distribution of wake bout distribution, the thickening of fur, a switch from polyneuronal to mononeuronal innervation of muscle fibers, the pruning of synapses onto Purkinje cells of the cerebellum, and a change from crawling to walking (Jouvet-Mounier et al., 1970; Crepel et al., 1976; Hoath, 1986; Petrosini et al., 1990; Blumberg et al., 2005). Whether one or more of these changes influence respiratory behavior is not fully understood. Other neurochemical and hormonal changes may also contribute to dynamic homeostatic regulation at this time. It is a period of growth, pruning, adjustment, adaptation, switches in various mechanisms, and an interplay between genetic and environmental factors. The end of the second postnatal week in rats is the most dynamic period, but it can also be a most vulnerable period, when the individual is uniquely susceptible to external perturbations. If such a critical period exists in humans (weeks or months in humans instead of days in rats), and if respiratory insults are introduced at this time to a vulnerable infant, especially during sleep when respiratory control system is further suppressed (Olson and Simon, 1996; Moss, 2002), it is possible that catastrophic events, such as Sudden Infant Death Syndrome, may result.
In conclusion, it cannot be overemphasized that sudden and major developmental adjustments are normal events that, by themselves, are not threatening to life. The fact that they have survived evolution indicates that a combination of external stressors and other vulnerable attributes (such as nonlethal organic defects or severe prenatal exposure to nicotine) during the critical period is still a relatively rare event, and the respiratory system, by and large, is able to survive the period of turmoil relatively unscathed to continue on to a path of normal maturity.
Acknowledgments
This review is dedicated to the fond memory of a dear colleague and friend, Dr. Ralph Franciosi, who worked tirelessly as the former Director of Pediatric Pathology at the Medical College of Wisconsin, and who persuaded one of us (MW-R) to embark on respiratory research almost a decade ago. Supported by the Children’s Hospital and Health System Foundation, Milwaukee, WI and by NIH Grant HD048954.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks MI, Hardie JB, Pearce RA. Development of GABA(A) receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol. 2002;88:3097–3107. doi: 10.1152/jn.00026.2002. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin P, Cayetanot F, Gros F, Larnicol N. Postnatal changes in Fos-like immunoreactivity evoked by hypoxia in the rat brainstem and hypothalamus. Brain Res. 2000;877:149–159. doi: 10.1016/s0006-8993(00)02632-9. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1391–1400. doi: 10.1152/ajpregu.2000.278.6.R1391. [DOI] [PubMed] [Google Scholar]
- Blanco LN. Mechanisms for the generation of gas-exchange surface area in rat lung. Am J Physiol. 1995;269:L698–708. doi: 10.1152/ajplung.1995.269.5.L698. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AM, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proc Natl Acad Sci U S A. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LW, Rosahl TW, Brussaard AB. Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. J Physiol. 2002;545:169–181. doi: 10.1113/jphysiol.2002.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Brorson JR, Zhang Z, Vandenberghe W. Ca(2+) permeation of AMPA receptors in cerebellar neurons expressing glu receptor 2. J Neurosci. 1999;19:9149–9159. doi: 10.1523/JNEUROSCI.19-21-09149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri PH. The postnatal growth of the rat lung. 3. Morphology. Anat Rec. 1974;180:77–98. doi: 10.1002/ar.1091800109. [DOI] [PubMed] [Google Scholar]
- Burri PH, Dbaly J, Weibel ER. The postnatal growth of the rat lung. I. Morphometry. Anat Rec. 1974;178:711–730. doi: 10.1002/ar.1091780405. [DOI] [PubMed] [Google Scholar]
- Cameron YL, Merazzi D, Mortola JP. Variability of the breathing pattern in newborn rats: effects of ambient temperature in normoxia or hypoxia. Pediatr Res. 2000;47:813–818. doi: 10.1203/00006450-200006000-00022. [DOI] [PubMed] [Google Scholar]
- Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- Cathala L, Misra C, Cull-Candy S. Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci. 2000;20:5899–5905. doi: 10.1523/JNEUROSCI.20-16-05899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Rovira C, Gaiarsa JL, Corradetti R, Ben Ari Y. GABA mediated excitation in immature rat CA3 hippocampal neurons. Int J Dev Neurosci. 1990;8:481–490. doi: 10.1016/0736-5748(90)90080-l. [DOI] [PubMed] [Google Scholar]
- Cohen G, Malcolm G, Henderson-Smart D. Ventilatory response of the newborn infant to mild hypoxia. Pediatr Pulmonol. 1997;24:163–172. doi: 10.1002/(sici)1099-0496(199709)24:3<163::aid-ppul1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976;7:567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Dale HH, Feldberg W, Vogt M. Release of acetylcholine at voluntary motor nerve endings. J Physiol. 1936;86:353–380. doi: 10.1113/jphysiol.1936.sp003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol. 2006;101:1097–1103. doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci Lett. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Dogas Z, Stuth EA, Hopp FA, McCrimmon DR, Zuperku EJ. NMDA receptor-mediated transmission of carotid body chemoreceptor input to expiratory bulbospinal neurones in dogs. J Physiol. 1995;487( Pt 3):639–651. doi: 10.1113/jphysiol.1995.sp020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF. Development of carotid body/petrosal ganglion response to hypoxia. Respir Physiol Neurobiol. 2005;149:191–199. doi: 10.1016/j.resp.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Dussault JH, Labrie F. Development of the hypothalamic-pituitary-thyroid axis in the neonatal rat. Endocrinology. 1975;97:1321–1324. doi: 10.1210/endo-97-5-1321. [DOI] [PubMed] [Google Scholar]
- Easton PA, Slykerman LJ, Anthonisen NR. Ventilatory response to sustained hypoxia in normal adults. J Appl Physiol. 1986;61:906–911. doi: 10.1152/jappl.1986.61.3.906. [DOI] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol. 1987;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH. Nucleus ambiguus and bulbospinal ventral respiratory group neurons in the neonatal rat. Brain Res Bull. 1999;50:1–13. doi: 10.1016/s0361-9230(99)00078-7. [DOI] [PubMed] [Google Scholar]
- Elnazir B, Marshall JM, Kumar P. Postnatal development of the pattern of respiratory and cardiovascular response to systemic hypoxia in the piglet: the roles of adenosine. J Physiol. 1996;492( Pt 2):573–585. doi: 10.1113/jphysiol.1996.sp021330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J Biol Chem. 1999;274:10100–10104. doi: 10.1074/jbc.274.15.10100. [DOI] [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Frappell PB, Mortola JP. Hamsters vs. rats: metabolic and ventilatory response to development in chronic hypoxia. J Appl Physiol. 1994;77:2748–2752. doi: 10.1152/jappl.1994.77.6.2748. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung ML, Wang W, Darnall RA, St John WM. Characterization of ventilatory responses to hypoxia in neonatal rats. Respir Physiol. 1996;103:57–66. doi: 10.1016/0034-5687(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Gershan WM, Forster HV, Lowry TF, Korducki MJ, Forster AL, Forster MA, Ohtake PJ, Aaron EA, Garber AK. Effect of metabolic rate on ventilatory roll-off during hypoxia. J Appl Physiol. 1994;76:2310–2314. doi: 10.1152/jappl.1994.76.6.2310. [DOI] [PubMed] [Google Scholar]
- Giulian D, McEwen BS, Pohorecky LA. Altered development of the rat brain serotonergic system after disruptive neonatal experience. Proc Natl Acad Sci U S A. 1974;71:4106–4110. doi: 10.1073/pnas.71.10.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Torres JE, Gozal YM, Nuckton TJ, Hornby PJ. Nitric oxide modulates ventilatory responses to hypoxia in the developing rat. Am J Respir Crit Care Med. 1997;155:1755–1762. doi: 10.1164/ajrccm.155.5.9154888. [DOI] [PubMed] [Google Scholar]
- Gozal D, Simakajornboon N, Czapla MA, Xue YD, Gozal E, Vlasic V, Lasky JA, Liu JY. Brainstem activation of platelet-derived growth factor-beta receptor modulates the late phase of the hypoxic ventilatory response. J Neurochem. 2000;74:310–319. doi: 10.1046/j.1471-4159.2000.0740310.x. [DOI] [PubMed] [Google Scholar]
- Gozal D, Torres JE. Brainstem nitric oxide tissue levels correlate with anoxia-induced gasping activity in the developing rat. Biol Neonate. 2001;79:122–130. doi: 10.1159/000047078. [DOI] [PubMed] [Google Scholar]
- Heinen K, Bosman LW, Spijker S, van Pelt J, Smit AB, Voorn P, Baker RE, Brussaard AB. GABAA receptor maturation in relation to eye opening in the rat visual cortex. Neuroscience. 2004;124:161–171. doi: 10.1016/j.neuroscience.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hoath SB. Treatment of the neonatal rat with epidermal growth factor: differences in time and organ response. Pediatr Res. 1986;20:468–472. doi: 10.1203/00006450-198605000-00017. [DOI] [PubMed] [Google Scholar]
- Hollrigel GS, Soltesz I. Slow kinetics of miniature IPSCs during early postnatal development in granule cells of the dentate gyrus. J Neurosci. 1997;17:5119–5128. doi: 10.1523/JNEUROSCI.17-13-05119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung JP, Fritschy JM. Developmental profile of GABAA-receptors in the marmoset monkey: expression of distinct subtypes in pre- and postnatal brain. J Comp Neurol. 1996;367:413–430. doi: 10.1002/(SICI)1096-9861(19960408)367:3<413::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Huang YH, Brown AR, Costy-Bennett S, Luo Z, Fregosi RF. Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respir Physiol Neurobiol. 2004;143:1–8. doi: 10.1016/j.resp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Morley P, Poulter MO. Developmental change in GABAA receptor desensitization kinetics and its role in synapse function in rat cortical neurons. J Physiol 522 Pt. 2000;1:3–17. doi: 10.1111/j.1469-7793.2000.t01-5-00003.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike J, Canton H, Sanders-Bush E. Developmental switch in the hippocampal serotonin receptor linked to phosphoinositide hydrolysis. Brain Res. 1995;678:49–54. doi: 10.1016/0006-8993(95)00143-e. [DOI] [PubMed] [Google Scholar]
- Isaev D, Isaeva E, Khazipov R, Holmes GL. Shunting and hyperpolarizing GABAergic inhibition in the high-potassium model of ictogenesis in the developing rat hippocampus. Hippocampus. 2007;17:210–219. doi: 10.1002/hipo.20259. [DOI] [PubMed] [Google Scholar]
- Johnston GA. GABAc receptors: relatively simple transmitter -gated ion channels? Trends Pharmacol Sci. 1996;17:319–323. [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Juttner R, Meier J, Grantyn R. Slow IPSC kinetics, low levels of alpha1 subunit expression and paired-pulse depression are distinct properties of neonatal inhibitory GABAergic synaptic connections in the mouse superior colliculus. Eur J Neurosci. 2001;13:2088–2098. doi: 10.1046/j.0953-816x.2001.01587.x. [DOI] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Postnatal development of hippocampal dentate granule cell gamma-aminobutyric acidA receptor pharmacological properties. Mol Pharmacol. 1999;55:444–452. [PubMed] [Google Scholar]
- Kazemi H, Hoop B. Glutamic acid and gamma-aminobutyric acid neurotransmitters in central control of breathing. J Appl Physiol. 1991;70:1–7. doi: 10.1152/jappl.1991.70.1.1. [DOI] [PubMed] [Google Scholar]
- Keinanen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science. 1990;249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kirsch J. Glycinergic transmission. Cell Tissue Res. 2006;326:535–540. doi: 10.1007/s00441-006-0261-x. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Sarto I, Ehya N, Fuchs K, Furtmuller R, Mayer B, Huck S, Sieghart W. Alternate use of distinct intersubunit contacts controls GABAA receptor assembly and stoichiometry. J Neurosci. 2001;21:9124–9133. doi: 10.1523/JNEUROSCI.21-23-09124.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussl MP, Pappagianopoulos P, Hoop B, Kazemi H. Reversible depression of ventilation and cardiovascular function by ventriculocisternal perfusion with gamma-aminobutyric acid in dogs. Am Rev Respir Dis. 1986;133:1024–1028. doi: 10.1164/arrd.1986.133.6.1024. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Kitajima T. A model for synaptic development regulated by NMDA receptor subunit expression. J Comput Neurosci. 2008;24:1–20. doi: 10.1007/s10827-007-0036-8. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanks KW, Hitti IF, Chin NW. Substrate utilization for lactate and energy production by heat-shocked L929 cells. J Cell Physiol. 1986;127:451–456. doi: 10.1002/jcp.1041270315. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Chen CX, Liu YJ, Aizenman E, Kandler K. KCC2 expression in immature rat cortical neurons is sufficient to switch the polarity of GABA responses. Eur J Neurosci. 2005;21:2593–2599. doi: 10.1111/j.1460-9568.2005.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MH, Takahashi MP, Takahashi Y, Tsumoto T. Intracellular calcium increase induced by GABA in visual cortex of fetal and neonatal rats and its disappearance with development. Neurosci Res. 1994;20:85–94. doi: 10.1016/0168-0102(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol. 2006;577:957–970. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Botzinger complex. J Appl Physiol. 2002;92:923–934. doi: 10.1152/japplphysiol.00977.2001. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in cytochrome oxidase expressions in brain stem nuclei of rats: implications for sensitive periods. J Appl Physiol. 2003;95:2285–2291. doi: 10.1152/japplphysiol.00638.2003. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in the rat pre-Botzinger complex. J Appl Physiol. 2004;96:1825–1831. doi: 10.1152/japplphysiol.01264.2003. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Developmental changes in the expression of GABA(A) receptor subunits alpha1, alpha2, and alpha3 in brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Wong-Riley MT. Developmental study of cytochrome oxidase activity in the brain stem respiratory nuclei of postnatal rats. J Appl Physiol. 2001;90:685–694. doi: 10.1152/jappl.2001.90.2.685. [DOI] [PubMed] [Google Scholar]
- Loewi O. On the intraneural state of acetylcholine. Experientia. 1956;12:331–333. doi: 10.1007/BF02165331. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Martin RJ, DiFiore JM, Jana L, Davis RL, Miller MJ, Coles SK, Dick TE. Persistence of the biphasic ventilatory response to hypoxia in preterm infants. J Pediatr. 1998;132:960–964. doi: 10.1016/s0022-3476(98)70391-9. [DOI] [PubMed] [Google Scholar]
- Maxova H, Vizek M. Biphasic ventilatory response to hypoxia in unanesthetized rats. Physiol Res. 2001;50:91–96. [PubMed] [Google Scholar]
- Mercuri NB, Calabresi P, Stefani A, Stratta F, Bernardi G. GABA depolarizes neurons in the rat striatum: an in vivo study. Synapse. 1991;8:38–40. doi: 10.1002/syn.890080106. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Wong RK. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991;253:1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Mills E. Time course for development of vagal inhibition of the heart in neonatal rats. Life Sci. 1978;23:2717–2720. doi: 10.1016/0024-3205(78)90651-3. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Tanaka I, Ezure K. Excitatory and inhibitory synaptic inputs shape the discharge pattern of pump neurons of the nucleus tractus solitarii in the rat. Exp Brain Res. 1999;129:191–200. doi: 10.1007/s002210050889. [DOI] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol. 1994;478( Pt 1):55–66. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Moon RY, Horne RS, Hauck FR. Sudden infant death syndrome. Lancet. 2007;370:1578–1587. doi: 10.1016/S0140-6736(07)61662-6. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Breathing pattern in newborns. J Appl Physiol. 1984;56:1533–1540. doi: 10.1152/jappl.1984.56.6.1533. [DOI] [PubMed] [Google Scholar]
- Mortola JP. How newborn mammals cope with hypoxia. Respir Physiol. 1999;116:95–103. doi: 10.1016/s0034-5687(99)00038-9. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Frappell PB. Ventilatory responses to changes in temperature in mammals and other vertebrates. Annu Rev Physiol. 2000;62:847–874. doi: 10.1146/annurev.physiol.62.1.847. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Morgan CA, Virgona V. Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol. 1986;61:1329–1336. doi: 10.1152/jappl.1986.61.4.1329. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Rezzonico R. Metabolic and ventilatory rates in newborn kittens during acute hypoxia. Respir Physiol. 1988;73:55–67. doi: 10.1016/0034-5687(88)90127-2. [DOI] [PubMed] [Google Scholar]
- Moss IR. Maturation of respiratory control in the behaving mammal. Respir Physiol Neurobiol. 2002;132:131–144. doi: 10.1016/s1569-9048(02)00070-8. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Neylon M, Marshall JM. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. J Physiol. 1991;440:529–545. doi: 10.1113/jphysiol.1991.sp018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake PJ, Simakajornboon N, Fehniger MD, Xue YD, Gozal D. N-Methyl-D-aspartate receptor expression in the nucleus tractus solitarii and maturation of hypoxic ventilatory response in the rat. Am J Respir Crit Care Med. 2000;162:1140–1147. doi: 10.1164/ajrccm.162.3.9903094. [DOI] [PubMed] [Google Scholar]
- Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D. NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol. 1998;84:853–861. doi: 10.1152/jappl.1998.84.3.853. [DOI] [PubMed] [Google Scholar]
- Okada M, Onodera K, Van Renterghem C, Sieghart W, Takahashi T. Functional correlation of GABA(A) receptor alpha subunits expression with the properties of IPSCs in the developing thalamus. J Neurosci. 2000;20:2202–2208. doi: 10.1523/JNEUROSCI.20-06-02202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EJ, Simon PM. Sleep-wake cycles and the management of respiratory failure. Curr Opin Pulm Med. 1996;2:500–506. [PubMed] [Google Scholar]
- Otake K, Nakamura Y, Tanaka I, Ezure K. Morphology of pulmonary rapidly adapting receptor relay neurons in the rat. J Comp Neurol. 2001;430:458–470. doi: 10.1002/1096-9861(20010219)430:4<458::aid-cne1043>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Bennett MV, Zukin RS. Are Ca(2+)-permeable kainate/AMPA receptors more abundant in immature brain? Neurosci Lett. 1992;144:65–69. doi: 10.1016/0304-3940(92)90717-l. [DOI] [PubMed] [Google Scholar]
- Petrosini L, Molinari M, Gremoli T. Hemicerebellectomy and motor behaviour in rats. I. Development of motor function after neonatal lesion. Exp Brain Res. 1990;82:472–482. doi: 10.1007/BF00228789. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Foutz AS, Champagnat J, Denavit-Saubie M. NMDA and non-NMDA receptors may play distinct roles in timing mechanisms and transmission in the feline respiratory network. J Physiol. 1994;474:509–523. doi: 10.1113/jphysiol.1994.sp020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Purves RD. Function of muscarinic and nicotinic acetylcholine receptors. Nature. 1976;261:149–151. doi: 10.1038/261149a0. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Kyrozis A, Wang J, MacDermott AB. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol. 1994;476:411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514( Pt 2):567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. Eur J Neurosci. 2000;12:2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetta M, Mortola JP. Interaction of hypoxic and hypercapnic stimuli on breathing pattern in the newborn rat. J Appl Physiol. 1987;62:506–512. doi: 10.1152/jappl.1987.62.2.506. [DOI] [PubMed] [Google Scholar]
- Sasner M, Buonanno A. Distinct N-methyl-D-aspartate receptor 2B subunit gene sequences confer neural and developmental specific expression. J Biol Chem. 1996;271:21316–21322. doi: 10.1074/jbc.271.35.21316. [DOI] [PubMed] [Google Scholar]
- Schmiedl A, Vieten G, Muhlfeld C, Bernhard W. Distribution of intracellular and secreted surfactant during postnatal rat lung development. Pediatr Pulmonol. 2007;42:548–562. doi: 10.1002/ppul.20623. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Simakajornboon N, Kuptanon T. Maturational changes in neuromodulation of central pathways underlying hypoxic ventilatory response. Respir Physiol Neurobiol. 2005;149:273–286. doi: 10.1016/j.resp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Singer JH, Berger AJ. Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons. J Neurophysiol. 1999;81:1608–1616. doi: 10.1152/jn.1999.81.4.1608. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J Neurophysiol. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cowdery TS, Orband L, Pachman S, Whitmore WL. Effects of neonatal hypoxia on brain development in the rat: immediate and long-term biochemical alterations in discrete regions. Brain Res. 1986;374:63–74. doi: 10.1016/0006-8993(86)90395-1. [DOI] [PubMed] [Google Scholar]
- Smith JC, Butera RJ, Koshiya N, Del Negro C, Wilson CG, Johnson SM. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir Physiol. 2000;122:131–147. doi: 10.1016/s0034-5687(00)00155-9. [DOI] [PubMed] [Google Scholar]
- Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol. 2001;127:135–155. doi: 10.1016/s0034-5687(01)00242-0. [DOI] [PubMed] [Google Scholar]
- Taketo M, Yoshioka T. Developmental change of GABA(A) receptor-mediated current in rat hippocampus. Neuroscience. 2000;96:507–514. doi: 10.1016/s0306-4522(99)00574-6. [DOI] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Postnatal development of 5-HT(1A) receptor expression in rat somatic motoneurons. Brain Res Dev Brain Res. 2000;122:1–10. doi: 10.1016/s0165-3806(00)00036-5. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Holmes GL, Ben-Ari Y, Khazipov R. Timing of the developmental switch in GABA(A) mediated signaling from excitation to inhibition in CA3 rat hippocampus using gramicidin perforated patch and extracellular recordings. Epilepsia. 2007;48(Suppl 5):96–105. doi: 10.1111/j.1528-1167.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- van Zundert B, Yoshii A, Constantine-Paton M. Receptor compartmentalization and trafficking at glutamate synapses: a developmental proposal. Trends Neurosci. 2004;27:428–437. doi: 10.1016/j.tins.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Vizek M, Pickett CK, Weil JV. Biphasic ventilatory response of adult cats to sustained hypoxia has central origin. J Appl Physiol. 1987;63:1658–1664. doi: 10.1152/jappl.1987.63.4.1658. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2 A and 2C receptors in brainstem motoneurons. Eur J Neurosci. 2003;17:1179–1188. doi: 10.1046/j.1460-9568.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- Waters KA, Gozal D. Responses to hypoxia during early development. Respir Physiol Neurobiol. 2003;136:115–129. doi: 10.1016/s1569-9048(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Tashiro Y, Inoue K, Kawai Y. Postnatal development of GABAergic axon terminals in the rat nucleus of tractus solitarius. Brain Res. 2006;1107:111–120. doi: 10.1016/j.brainres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zhou D, Huang Q, Fung ML, Li A, Darnall RA, Nattie EE, St John WM. Phrenic response to hypercapnia in the unanesthetized, decerbrate, newborn rat. Respir Physiol. 1996;104:11–22. doi: 10.1016/0034-5687(95)00098-4. [DOI] [PubMed] [Google Scholar]