Abstract
Purpose
To determine the electro-clinical significance and histopathological correlates of cortical gamma-aminobutyric acidA (GABAA) receptor abnormalities detected in and remote from human neocortical epileptic foci.
Methods
Cortical areas with decreased [11C]flumazenil (FMZ) binding were objectively identified on PET images and correlated to intracranial EEG findings, clinical seizure variables, histology findings, and surgical outcome in 20 patients (mean age: 9.9 years) with intractable partial epilepsy of neocortical origin and non-localizing MRI.
Results
Focal decrease of cortical FMZ binding was detected in the lobe of seizure onset in 17 (85%) patients. Eleven patients (55%) had 17 remote cortical areas with decreased FMZ binding outside the lobe of seizure onset. Thirteen of those 16 (81%) of the 17 remote cortical regions that were covered by subdural EEG were around cortex showing rapid seizure spread on intracranial EEG. Remote FMZ PET abnormalities were associated with high seizure frequency and, when resected, showed gliosis in all six cases where material was available. Higher number of unresected cortical regions with decreased FMZ binding was associated with poorer surgical outcome.
Conclusions
Focal decreases of cortical GABAA receptor binding on PET may include cortical regions remote from the primary focus, particularly in patients with high seizure frequency, and these regions are commonly involved in rapid seizure propagation. Although these regions may not always need to be resected to achieve seizure freedom, a careful evaluation of cortex with decreased GABAA receptor binding prior to resection using intracranial EEG may facilitate optimal surgical outcome in patients with intractable neocortical epilepsy.
Keywords: neocortical epilepsy, positron emission tomography, GABAA receptors, [11C]flumazenil, intracranial EEG, surgery outcome
INTRODUCTION
The major inhibitory neurotransmitter in the human brain, γ-aminobutyric acid (GABA), plays a key role in regulating central nervous system excitability and susceptibility to seizures (Sivilotti & Nistri, 1991; Lambert & Grover, 1995), and its involvement in the pathophysiology of human epilepsy is widely accepted (Treiman, 2001). Recent advances in neuroimaging permit the in vivo measurement of GABAergic function in human epileptic brain. For example, proton magnetic resonance spectroscopy studies have shown decreased GABA concentration in the brain of some patients with partial seizures, a finding that predicts poor seizure control (Petroff et al., 1996). In vivo postsynaptic changes of the GABAA receptor complex can be imaged using positron emission tomography (PET) with 11C-flumazenil (FMZ), a ligand that binds to α subunits of the GABAA receptor complex (Pritchett et al., 1989; Doble, 1999).
PET studies consistently have demonstrated focal cortical changes (usually decreases, but occasionally increases) of FMZ binding in patients with epilepsy of both medial temporal and neocortical origin, including patients with normal conventional MRI (Savic et al., 1995; Richardson et al., 1998; Ryvlin et al., 1998; Muzik et al., 2000a; Koepp et al., 2000; Juhász et al., 2001; Hammers et al., 2003). However, detailed comparisons of FMZ PET abnormalities with intracranial EEG findings have been scarce. A few studies suggest that in some cases, cortex showing decreased FMZ binding corresponds to cortical seizure onset zones (Ryvlin et al., 1998; Arnold et al., 2000; Muzik et al., 2000a). However, decreased cortical FMZ binding has been also reported outside the lobe of seizure onset, in remote cortical regions, often without apparent corresponding scalp EEG changes (Savic et al., 1996; Juhász et al., 2000; Koepp et al., 2000; Hammers et al., 2003). Presence of such multifocal FMZ PET abnormalities may limit the clinical usefulness of this imaging modality for presurgical evaluation, unless a more detailed understanding of clinical, electrophysiological and neuropathological correlates of such abnormalities can be achieved. Therefore, in the present study we analyzed the significance of focal cortical decreases of GABAA receptor binding using PET in 20 children with non-localizing MRI who underwent resective epilepsy surgery following intracranial EEG monitoring with subdural electrodes because of intractable neocortical epilepsy. The goal of the study was to determine the clinical (seizure variables, surgical outcome), electrophysiological (intracranial EEG) and histopathological correlates (where available) of cortical decreases of GABAA receptor binding detected in and outside the lobe of the primary epileptic focus.
SUBJECTS
Patients with epilepsy
Twenty young patients (12 girls, age: 2.3 - 20 years, mean age: 9.9 years) were selected from a group of patients who underwent epilepsy surgery at the Children’s Hospital of Michigan in Detroit (Table 1). Inclusion criteria: (1) Medically refractory epilepsy consisting of partial seizures; (2) Seizures of neocortical origin, excluding the medial surfaces and insula, as defined by scalp and intracranial EEG studies; (3) No obvious lesion on MRI; (4) No previous surgery. These patients were selected from a cohort of 54 consecutive young patients with non-localizing MRI who underwent presurgical evaluation and subsequent epilepsy surgery at the Children’s Hospital of Michigan between 1998 and 2006. Thirty-four of the 54 patients were excluded from the study because of one or more of the following reasons: (1) No FMZ PET available (parents refused any research PET scan or another research PET scan, with alpha[11C]methyl-L-tryptophan, was done preoperatively); (2) Infantile or epileptic spasms without partial seizures; (3) Patients less than 2 years of age; (4) Seizure onset in the medial temporal or deep insular region; this region could not be analyzed the same way as neocortical regions using the analytic method described below. In the 20 patients included in the study, presurgical evaluation included MRI, scalp and chronic intracranial (subdural) EEG monitoring, as well as glucose and FMZ PET. All patients were on mono- or polytherapy with various antiepileptic drugs, but none were on benzodiazepines or vigabatrin, which might interfere with interfere with FMZ binding on PET (Verhoeff et al., 1999; Juhász et al., 2001). Seizure frequency measures for the 1-year period before surgery were obtained from seizure diaries and medical charts. In all patients, the majority of seizures were complex partial, either without or with occasional secondary generalization. Seizure frequency was calculated and characterized by a single value. The date and time of the last clinical seizure before PET were recorded at the time of the PET scan, and the time since the last seizure was calculated. Postoperative seizure outcome (class I-IV) was determined according to the criteria of Engel et al. (1993) at least one year after the surgery.
Table 1.
Clinical data, EEG, FDG and [11C]flumazenil (FMZ) PET, as well as histology findings of the 20 patients
| No./Sex | Age (years) | Age at sz onset (years) | Seizure frequency (per week) | Scalp EEG ictal focus | Intracranial EEG focus onset / spread | Decreased FDG uptake | Decreased FMZ binding in onset region | Decr. FMZ binding remote from onset (i.cr. EEG correlate) | Histology in onset region | Histology in lobe of remote region | Surgical outcome class |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/M | 3.6 | 1.2 | 210 | L hem. | L TPO / - | L TPOF | L TPO | L F(spk) | FCD | GM gliosis | I |
| 2/M | 4.7 | 1.0 | 350 | L TPO | L OT / F, P | L TPOF | L OT | L F(spr),P(spr) | WM gliosis | WM gliosis | I |
| 3/M | 5.4 | 2.0 | 70 | L T | L T / F, P | L FTP | L T | LF(spr),P(spr) | WM gliosis | WM gliosis | I |
| 4/F | 7.2 | 6.2 | 105 | R T | R T / - | R T | R T | R F (spk) | GM/WM gliosis | WM gliosis | I |
| 5/F | 7.5 | 2.0 | 21 | L FP | L P / T | L P, T, O | L P | L F(-), T(spr) | FCD w. balloon cells | n.a. | III |
| 6/M | 11.7 | 11.0 | 14 | R FT | R T / F | R T | R T | R F (spr) | FCD | n.a. | I |
| 7/F | 12.7 | 3.0 | 21 | L FT | L F / T | L FT, O | L F | L T(spr) | GM/WM gliosis | n.a. | I |
| 8/M | 14.1 | 5.0 | 14 | R FT | R P / F, T | R T, P | R P | R F(spr), T(spr) | GM/WM gliosis | GM/WM gliosis | II |
| 9/F | 14.8 | 12.0 | 7 | R anterior | R F / T, P | R F, T, P | R F | R T(spr), P(nc) | GM/WM gliosis | GM/WM gliosis | IV |
| 10/M | 14.9 | 9.0 | 4 | R TP | R P / F, T | none | R P | R F(spr) | FCD w. balloon cells | n.a. | I |
| 11/M | 15.9 | 8.0 | 14 | R>L F | R F / P, T | R F, T, P | R F | R P(spr), T(spr) | WM gliosis | n.a. | III |
| 12/F | 2.3 | 0.8 | 3 | R>L hem. | R F / - | R F | R F | none | FCD, GM/WM gliosis | - | III |
| 13/F | 2.5 | 2.1 | 70 | L FTP | L F / FP | L F, T | L F | none | GM/WM gliosis | - | I |
| 14/F | 3.3 | 0.8 | 1 | R FT | R FP, T / - | none | R T | none | WM gliosis | - | III |
| 15/M | 7.0 | 3.0 | 7 | L F | L FT / F | L FT, O | L FT | none | GM/WM gliosis | - | I |
| 16/F | 13.9 | 11.0 | 7 | R T | R PTO / - | R O | R O | none | GM/WM gliosis | - | I |
| 17/F | 20.0 | 0.3 | 3 | L TF | L T, P / TP | L F, T, P | L T, P | none | FCD, GM/WM gliosis | - | I |
| 18/F | 10.4 | 7 | 70 | R FP | R F / - | R F, T | none | none | WM gliosis | - | I |
| 19/F | 12.3 | 5 | 7 | L FT | L F / - | L F, T | none | none | WM gliosis | - | I |
| 20/F | 13.0 | 0.3 | 7 | L FT | L T, P / F, T | L F, T, P | none | none | GM/WM gliosis | - | I |
Normal controls
Since healthy children cannot be studied by PET due to ethical reasons, 15 healthy adults (8 men, age 22-49 years, mean 31.2 years) underwent FMZ PET scanning using the same protocol applied for the pediatric epilepsy group. All healthy subjects had a normal high-resolution MRI scan, and none of them were taking drugs affecting the central nervous system. PET scans of the control group were only used to assist in establishing the threshold for the minimal extent of cortical abnormalities considered to be abnormal, while absolute values of GABAA receptor binding, which undergo developmental changes (Chugani et al., 2001), were not used in this study. The study was approved by the Institutional Review Board Committee at Wayne State University, and informed consent was obtained from all normal controls and the guardians of all children with epilepsy.
METHODS
EEG evaluation
All patients underwent prolonged video-EEG monitoring initially with scalp/sphenoidal electrodes, and subsequently with subdural electrodes in order to identify more precisely the area of seizure onset (visually, as described previously [Asano et al., 2004]), early seizure spread (defined as areas involved in seizure activity <10 s after onset of the seizure), and frequent interictal spiking (>10 spikes per minute). Electrodes showing ictal onset and seizure spread were identified by the consensus of two board certified electroencephalographers (E.A., A.S.) based on the analysis of at least three habitual seizures.
MRI procedure
All patients underwent preoperative MRI scans performed on a GE 1.5 Tesla Signa 5.7 unit (GE Medical Systems, Milwaukee, WI) located at the Children’s Hospital of Michigan. The protocol included axial and coronal T2-weighted, coronal fluid-attenuated inversion recovery (FLAIR) and T1-weighted volumetric spoiled gradient echo (SPGR) sequences. The SPGR sequence generates 124 contiguous 1.5 mm sections of the entire head in the coronal plane using a 35/5/1 (TR/TE/NEX) pulse sequence, flip angle of 35 degrees, matrix size of 256 × 128, and field of view of 240mm × 240mm. All preoperative MRI scans were evaluated for possible focal lesions by a certified neuroradiologist who was blinded to other localizing information, and patients with a lesion, other than non-specific abnormalities with no localizing value (e.g., mild, non-specific white matter abnormalities), were not included in this study. All healthy subjects had a normal SPGR MRI.
PET Scanning Protocol
All PET studies were performed using a CTI/Siemens EXACT/HR whole body positron tomograph located at Children’s Hospital of Michigan, Detroit. This scanner has a 15 cm field of view and generates 47 image planes with a slice thickness of 3.125 mm. The reconstructed image in-plane resolution obtained is 6.5 ± 0.3 mm at full-width-at-half-maximum (FWHM) and 7.0 ± 0.5 mm in the axial direction. Methods of image acquisition for FMZ PET have been described previously (Juhász et al., 1999). The EEG was monitored throughout all PET examinations, and all scans were verified to be interictal. Summed images representing tracer activity concentration between 10-20 minutes after injection of FMZ (14.8 MBq/kg) were used to display GABAA receptor binding in brain. This procedure is especially advantageous in children because it does not require collection of arterial blood samples. It has been shown previously that abnormalities defined in FMZ activity images based on this time frame and using a threshold of 10% for defining small cortical areas with abnormal asymmetry are essentially equivalent to those obtained from corresponding parametric volume of distribution images (Niimura et al., 1999). All patients also underwent PET scanning with 2-deoxy-2[18F]fluoro-D-glucose (FDG), which was used clinically for presurgical evaluation.
Image processing
Definition of location and extent of cortical PET abnormalities
Focal decreases of cortical FMZ binding ipsilateral to the presumed epileptic focus were identified prospectively, i.e., before subdural grid placement, using an objective method based on a semi-automated software package applied to all supratentorial planes of the PET image volume (Muzik et al., 1998). This procedure allows the definition of abnormal cortical areas of FMZ binding based on an asymmetry index derived from contralateral homotopic cortical areas according to a predefined cutoff threshold. Our previous studies indicated that a 10% asymmetry cutoff threshold yields the best combination between sensitivity and specificity for detection of the seizure onset area on subdural EEG with FMZ PET scans (Muzik et al., 2000a). Therefore, we used a 10% asymmetry cutoff threshold to mark areas of decreased FMZ binding by setting them to the absolute maximum value found in the whole data volume. First, all PET images were loaded into MPITool, an interactive software that allows display and manual realignment and co-registration of various image datasets (Pietrzyk et al., 1990). All PET images were displayed in three orthogonal (axial, coronal and sagittal) cuts and realigned, using several landmarks (e.g., top and bottom slices of the brain, subcortical structures, insular region, etc.), to make sure that all images are approximately symmetric and each axial plane displays homotopic cortical regions. Using the realigned axial PET image planes, the cortical mantle was divided to 60 sectors for each hemisphere in each axial plane including cerebral cortex, except the top and bottom planes, that were affected by partial volume effects. Sectors exceeding the cutoff threshold were marked, provided that at least two adjacent sectors in at least two adjacent planes exceeded the threshold. Thus, the smallest possible marked cortical region encompassed 2×2 sectors in two consecutive axial image planes corresponding to approximately 0.3% of the entire cortex in one hemisphere (and 0.15-0.3% in image planes close to the top or the bottom of the brain, where the 60 segments where distributed in a shorter cortical mantle). A new “marked” image file was created that included all 47 planes of the original frame. FDG PET scans were analyzed using the same procedure, and localizing findings of this analysis are given in Table 1. In an additional step, the minimal extent of cortical areas that could be considered abnormal on FMZ PET was further refined based on analysis of PET scans of normal control subjects (see below).
Comparison of surface distribution of intracranial EEG and PET abnormalities
The SPGR MR and marked PET images of the children with epilepsy were co-registered using MPITool and further processed using the 3D-Tool software package (von Stockhausen et al., 1997, 1998; Muzik et al., 2001). The brain was automatically segmented from MRI data using morphological operations, and 3D surface views were created. Color-coded functional data obtained from the marked PET image volumes were projected onto the brain surface using reverse gradient fusion (Stokking et al., 1994). The exact surface location of subdural electrodes was determined and visualized on the 3D reconstructed brain surface by utilizing digitalized X-ray images acquired with the subdural electrode arrays in place (von Stockhausen et al., 1997; Muzik et al., 2001). The main advantage of this method is that skull X-rays can be obtained easily at the bedside and, thus, this procedure does not require disconnection of EEG electrodes, patient transportation, or sedation of the children. During the skull X-ray procedure, three metallic fiducial markers were placed at three standard, anatomically well defined locations (lowermost point the earlobe and lateral point of the parpebral fissure ipsilateral to the grid, and the superior attachment point of the earlobe to the scalp contralateral to the grid) on the patients’ head by the first author and a digital X-ray image was acquired. The fiducial markers were directly identified on the X-ray as well as on the corresponding 3D reconstructed MRI image volume, using the 3D-Tool software package. To identify virtual marker locations in the MRI image volume (which contained no external markers), the skin of the head was surface rendered showing details of the face, ears and scalp. The user then clicked on the three standard locations on the 3D skin surface image to define coordinates of three virtual markers. An iterative algorithm minimized the differences between the two sets of coordinate triplets by adjusting the three euler-angles and the image zoom. As a result, a cortical surface view was created allowing the location of electrodes to be directly defined on the MRI 3D brain surface, and compared to the locations of marked PET abnormalities. Intraoperative images showing the exposed brain surface before and after placement of electrodes were also used to verify the accuracy of the electrode positions on the 3D brain surface; slight inaccuracies were corrected manually in 3D-Tool, using brain surface landmarks.
Surgical planning
Findings of video-EEG monitoring, MRI, neuropsychological evaluation, and FDG PET scans were discussed at a surgical planning meeting, and these data were used to determine the location and extent of intracranial electrode placement. The epilepsy surgery team was aware of the FMZ PET findings (visual assessment). However, based on the findings of scalp video-EEG monitoring and FDG PET, subdural grid coverage typically extended well beyond cortical areas showing decreased FMZ binding: in patients with abnormal FMZ PET, the total number of electrodes ranged between 52 and 120 (mean: 86), while electrodes overlying cortex with decreased FMZ binding ranged between 4 and 62 (mean: 16). In patients with a suspected temporal lobe focus, electrode coverage always included both lateral and inferior temporal cortex (with one or more electrode strips slipped under the temporal lobe). Anterior-posterior X-ray images were used to determine whether the subtemporal strip(s) extended to the medial temporal region. In addition, subdural electrodes were also used for functional cortical mapping to identify the sensory-motor and language cortex.
The resections were performed based on the ictal and interictal intracranial EEG data as well as findings from functional mapping. Areas involved in seizure onset, early spread, and areas showing frequent interictal spiking were resected, unless they overlapped with eloquent (sensorimotor, language) cortex. Inclusion of such cortical areas in the resection was individually determined. No cortical areas were resected solely based on the PET results.
Histology
Surgical specimens were collected and labeled according to lobar/sub-lobar location. Thus, specimens collected from the lobe and region (such as, “posterior frontal”, etc.) of seizure onset and also remote regions showing abnormal FMZ binding on PET were identified. This method did not allow us to study histology changes in cortex underlying specific electrodes; thus, in some cases with small PET abnormalities, histology results may have included adjacent cortical regions. Surgical specimens were stained with hematoxilin and eosin, cresyl-violet, Luxol fast-blue, and glial fibrillary acidic protein antibody (GFAP). Further stains (such as periodic acid Schiff, Bielschowsky, Bodian, Congo red, or various immunostains) were applied as needed to clarify the nature of the pathology.
Study design and statistical analysis
Definition of the minimal extent of cortex with abnormal FMZ binding based on PET image analysis in normal control subjects
To assess the extent and lobar location of ‘physiologically’ asymmetric FMZ binding in normal subjects at the 10% asymmetry threshold, FMZ PET scans of all healthy subjects were processed using the analysis protocol used in the patient group. Both right and left hemispheric cortical decreases were evaluated separately. The location and number of marked cortical sectors marked were recorded. The extent of cortical decreases was calculated (as a percentage) by dividing the number of marked cortical sectors in each hemisphere by the total number of total sectors assessed in the hemisphere (typically 60 sectors in 20 consecutive axial planes, i.e., 1,200 cortical sectors per hemisphere). Cortical asymmetries exceeded the 10% asymmetry threshold in at least one small cortical region in at least one hemisphere in 13 subjects. The extent of these marked areas, however, was very small: 0.5% of the hemispheric surface (range: 0 - 1.0%). There was no difference between left and right hemispheres in the mean extent of areas marked. Small areas of asymmetric FMZ binding occurred in various cortical regions, scattered in all four lobes, without showing clustering in any particular region. Based on these results, in the patient group, cortical decreases of FMZ binding were considered to be abnormal only when their extent exceeded 1% of the hemispheric surface when using the 10% asymmetry threshold; thus, marked cortical regions smaller than 1% of the surface were not used in data analysis.
Assessment of children with epilepsy
FMZ PET findings were evaluated by their lobar location, as compared to location of the area(s) of seizure onset on intracranial EEG, as follows:
(1) Perifocal FMZ PET abnormalities
i.e., decreased cortical FMZ binding only in the lobe of seizure onset. Perifocal FMZ abnormality was identified when one or more subdural electrodes showing seizure onset was overlying marked cortex with decreased FMZ binding on the 3D surface images showing PET and EEG abnormalities.
(2) Remote FMZ PET abnormalities
cortical areas showing decreased FMZ binding outside the lobe(s) of seizure onset and not continuous with a perifocal FMZ PET abnormality; or inside the lobe of seizure onset, but non-continuous with the perifocal FMZ abnormality and at least 2 electrode distance (2 cm) from it. We defined remote FMZ abnormalities as detecting regions of early seizure spread or frequent interictal spiking if at least one subdural electrode overlying the FMZ PET abnormality manifested these electrophysiological criteria or when such an electrode was not more than one electrode distance (1 cm) from a marked cortical region with decreased FMZ binding.
Clinical seizure variables (age, age at seizure onset, duration of epilepsy, seizure frequency, time since last clinical seizure) among patient subgroups based on the FMZ PET findings were compared using the Mann Whitney U test. Correlation between surgical outcome and the number of unresected lobes containing cortex with decreased FMZ binding was calculated using the Spearman’s rank correlation test. P<0.05 was considered to be significant.
RESULTS
Perifocal FMZ PET abnormalities in children with epilepsy
Of the 20 patients, 17 (85%) had localizing FMZ PET, i.e., focal cortical decrease of FMZ binding in the epileptic hemisphere, including two patients (#10 and #14) with non-localizing FDG PET. In each of these 17 patients, at least one of the detected abnormalities was located in the lobe of seizure onset as defined by intracranial EEG (perifocal FMZ abnormalities; see Table 1). In two of these 17 patients (#14 and #16) with unilateral multilobar seizure onset, FMZ PET was abnormal in only one of three affected lobes, thus missing parts of the extensive seizure onset zone in adjacent lobes. In the remaining 15 patients with localizing PET, decreased FMZ binding was found in all lobes involved in seizure onset, and the marked areas were at least partially overlapping with areas showing ictal onset (Figure 1).
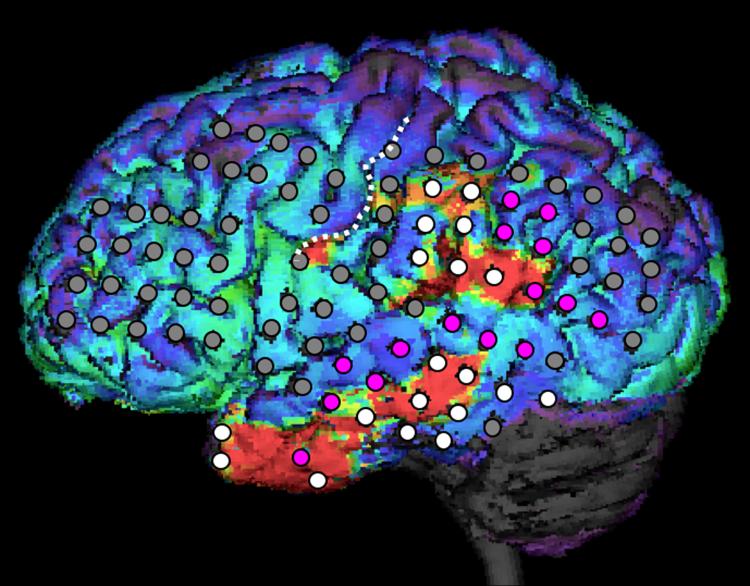
Figure 1.
Perifocal decrease of FMZ binding (red areas represent >10% decrease) displayed on the 3D MRI surface of a 20-year old patient (#17) with seizure onset (white electrodes of the subdural electrode arrays) in the left temporal and parietal cortex. Note that most of the cortex with seizure onset showed decreased FMZ binding. Electrodes in purple represent areas with rapid seizure spread. No remote areas of decreased FMZ binding were seen; the lateral frontal cortex has been also evaluated by intracranial EEG, using a total of 33 subdural electrodes (see locations on the figure) to ensure that anterior temporal seizure onset on scalp EEG did not involve the frontal region and also because the frontal lobe showed hypometabolism on glucose PET. However, neither subdural EEG nor flumazenil PET showed abnormality in the frontal cortex. The white dotted line indicates the left central sulcus.
FMZ PET abnormalities remote from the primary epileptic focus
Eleven patients showed a total of 17 cortical areas with remote FMZ PET abnormalities (Table 1). Sixteen of these regions were covered by subdural electrodes and 13 (81%) were involved in rapid seizure spread (Figure 2), whereas additional two areas (in patients #1 and #4) showed frequent interictal spiking without ictal involvement (Figure 3). Out of these 15 remote regions, which were abnormal on both FMZ PET and intracranial EEG, 8 were not specifically detected by scalp EEG (in patients #2-5 and #9-11) and 4 showed normal FDG uptake on PET in the affected lobe (in patients #4, 6, 8, 10; Table 1).
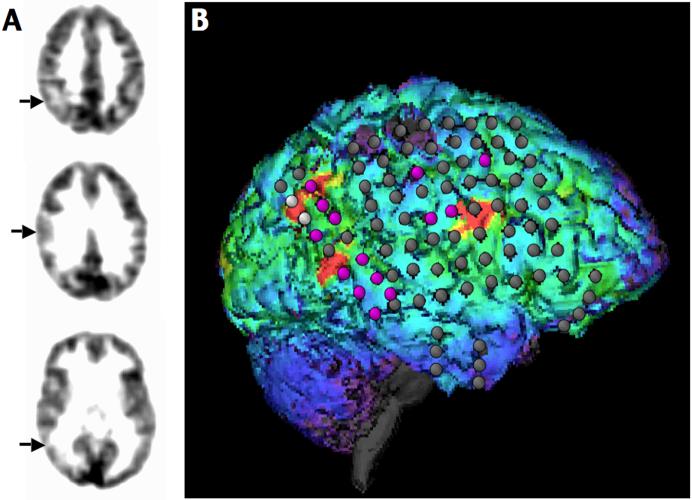
Figure 2.
Multiple cortical FMZ PET abnormalities vs. intracranial EEG findings in a 14-year old boy (patient #8) with daily complex partial seizures. FMZ PET detected three separate areas of decreased FMZ binding (right posterior parietal, posterior frontal, and lateral temporal cortex; see arrows on transaxial slices (A) and red “marked” areas on the 3D surface display (B). The parietal and temporal regions were resected, but the frontal lobe was left intact. The patient was seizure-free for a year, and then rare seizures occurred.
Figure 3.
A. Decreased FMZ binding in the right inferior temporal and frontal cortex in a 7 year-old girl (patient #4) with very frequent (10-20/day) complex partial seizures. B. Intracranial EEG showed seizure onset (electrodes in white) from the right inferior temporal cortex and frequent interictal spiking without ictal involvement in the right lateral temporal and frontal regions (spike frequency: orange electrodes: >20/min; yellow electrodes: 10-20/min). The highest average spike frequency (45/min) occurred in a single frontal electrode (shown in red), overlying the frontal area with decreased FMZ binding. Right temporal and frontal resection resulted in seizure-free outcome. Histology showed gliosis in both the temporal and frontal specimens.
Correlation between FMZ PET abnormalities and clinical variables
The onset of seizures varied between 0.3 and 12 years of age (mean: 4.9 years), duration of epilepsy was between 0.4 and 19.7 years (mean: 5.4 years), seizure frequency varied between 1 seizure per week and 50 seizures per day (see Table 1), while time of last clinical seizure before PET could be reliably determined in 15 patients and varied between 6 hours and 2 months. In those children with localizing FMZ PET (N=17), presence of remote FMZ PET abnormalities (N=11) was associated with higher seizure frequency (median: 3 per day), as compared to those with only perifocal abnormalities of FMZ binding (median: 5 per week, p=0.023); other clinical variables (age: p=0.23; duration of epilepsy: p=0.48; age at seizure onset: p=0.13; time of last seizure: p=0.15) were not different between these two subgroups. The difference in seizure frequency between the two subgroups remained significant when the three FMZ PET-negative patients were also included in the group with no remote FMZ abnormalities (p=0.029).
Surgical histology findings and postoperative outcome
Histology of the resected specimens from the lobe of seizure onset showed evidence of focal cortical dysplasia in 6 cases (30%; all showed decreased FMZ binding), and non-specific gliosis in the other 14 patients. Histology from remote regions including cortex with decreased FMZ binding was available in 6 patients and all showed gliosis (Table 1; Figure 4).
Figure 4.
White matter gliosis in resected tissue samples of a 7-year old girl (patient #4) demonstrated by glial fibrillary acid protein (GFAP) staining. This patient had seizure onset in the inferior temporal cortex as well as frequent interictal spiking in the superior frontal cortex, and both of these regions showed decreased GABAA receptor binding on PET (see Figure 3). Both the inferior temporal (A) and superior frontal (B) resected tissues showed an increase of the number of stained astrocytes, consistent with gliosis. Gliosis was patchy in the frontal specimen, and some regions showed no gliosis (C).
Fourteen patients (70%) were seizure-free, 1 had more than 90% reduction of seizure frequency (Class II) after a 1-year seizure-free period, 4 patients showed worthwhile improvement (Class III), and 1 patient showed no significant improvement of seizure frequency (Class IV) (Table 1). In this latter patient (#9), the EEG-defined seizure onset zone with decreased FMZ binding was incompletely resected because it involved the motor cortex. Of the 4 patients with Class III outcome, either resection of the seizure onset zone was incomplete (N=2, patients #12 and #14) because it involved the motor cortex, or two remote cortical regions with FMZ PET abnormalities were not removed (patients # 5 and #11). Overall, in the 17 patients with localizing FMZ PET, the number of non-resected cortical regions with decreased FMZ binding correlated with surgical outcome class (Spearman’s rho = 0.79, p<0.001).
DISCUSSION
The present study demonstrates that, in a substantial proportion patients with neocortical epilepsy, focal decreases of cortical FMZ binding are not confined to the lobe of seizure onset but also include remote cortical regions. Such multiple cortical regions with decreased GABAA receptor binding may participate in an epileptic network (Spencer, 2002) that includes cortical areas of seizure onset and rapid seizure propagation to remote connected areas detected by intracranial EEG. Presence of such remote PET abnormalities was also associated with high clinical seizure frequency. Although the number of subjects was limited, our results suggest that detection of such cortical regions can be facilitated by FMZ PET, thus allowing better coverage by subdural EEG electrodes. This is indeed highly relevant clinically as removal of both perifocal and remote cortex, some of which may be missed by scalp EEG alone or FDG PET, may optimize surgical outcome. This needs to be confirmed in a larger cohort of patients with longer postoperative follow-up.
Detection of the epileptic focus by FMZ PET
Diagnostic usefulness and sensitivity of FMZ PET is dependent on several factors, including image acquisition parameters, methods of quantification and approaches of detecting focal abnormalities (Niimura et al., 1999; Hammers et al., 2008). Several previous studies showed decreased FMZ binding in the region of seizure onset in patients with neocortical epilepsy (Savic et al., 1995; Richardson et al., 1998; Ryvlin et al., 1998; Arnold et al., 2000; Muzik et al., 2000a; Juhász et al., 2001; Hammers et al., 2003); however, these studies applied various analytic methods to different patient populations. Therefore, their conclusions regarding the sensitivity and clinical utility of FMZ PET are difficult to compare. In the present study, we included only children with non-localizing MRI but whose scalp EEG and seizure semiology successfully lateralized the seizure focus so that they were considered to be surgical candidates. Once the side of the presumed epileptic focus was established, an asymmetry-based analytic method was applied to objectively identify cortical decreases of FMZ binding beyond the normal or physiological range. Asymmetry measurements are indeed very sensitive if the assumption of a relatively normal non-epileptic hemisphere is valid (van Bogaert et al., 2000). This assumption is reasonable based on studies in adult patients with temporal lobe epilepsy, which found no differences in FMZ binding between the non-epileptic hemisphere and normal control subjects (Chugani et al., 2001; Henry et al., 1993). Also in a previous study using a voxel-based approach to assess the whole brain (Hammers et al., 2003) only two patients (out of 16 with focal decreases of FMZ binding) showed decreased cortical FMZ binding in bilateral apparently homotopic regions on the lateral cortical surface. An advantage of the asymmetry-based method in pediatric studies is that it allows analysis of PET images regardless of the patient’s age, while the spatial normalization procedure required for voxel-based approaches is unreliable in children below 6 years of age (Muzik et al., 2000b). On the other hand, the asymmetry method used in our laboratory is not designed to assess medial/inferior temporal or medial hemispheric as well as deep insular cortex abnormalities. Such brain regions were always addressed by subdural grid electrodes if these areas were suspected to be potentially epileptogenic based on clinical and electrographic data or visual analysis of PET images. Application of new image analysis approaches, such as the recently described approach with landmark-constrained conformal surface mapping (Muzik et al., 2007), may be able to circumvent some of the limitations of the current image methods in pediatric image analysis.
A potential difficulty of the interpretation of multiple FMZ PET abnormalities is that PET itself cannot differentiate between perifocal and remote abnormalities. Therefore, careful comparison of PET and electro-clinical data remains essential to determine which of the FMZ PET abnormalities are most likely located in the region of the primary epileptic focus and which one (if any) may represent remote abnormalities. Still, since cortical areas with decreased FMZ binding are typically less extensive than areas of glucose hypometabolism on PET (Savic et al., 1995; Muzik et al., 2000a; Juhász et al., 2000), it is usually feasible to place subdural electrodes over cortical areas showing an abnormality on FMZ PET. Since remote FMZ PET abnormalities appear to be common sites of rapid seizure propagation or, sometimes, frequent interictal spiking, coverage of both perifocal and remote areas appears to be desirable even if they cannot be reliably differentiated presurgically.
Potential mechanisms and significance of decreased FMZ binding remote from the primary focus
The occurrence of GABAA receptor binding abnormalities remote from the primary seizure focus in patients with very frequent seizures raises the possibility that at least some of these remote cortical FMZ binding abnormalities may be related to repeated seizures recruiting remote cortical regions into an epileptic network. Savic et al. (1996) have reported on a small group of patients with temporal lobe epilepsy, where GABAA receptor binding abnormalities in remote projection areas were no longer apparent after successful temporal lobectomy. Both of these findings would be consistent with the model of secondary epileptogenesis proposed by Morrell (1985, 1989) who postulated that frequent seizures can facilitate development of secondary foci remote from the primary focus. Based on this model, it is conceivable that remote areas connected to the primary epileptic focus via well established pathways are readily involved in seizure propagation and may eventually undergo changes leading to impaired GABAergic function and increased excitability. This could be further addressed in a longitudinal study.
Multiple cortical regions with decreased GABAA receptor binding could also be due to multifocal cortical dysplasia; however, in our patients only gliosis was found in remote cortical regions when resected. Can gliosis be the result of repeated seizures? Seizure-induced gliosis in rats following status epilepticus has been described using high-field MRI, proton spectroscopic imaging (1H-MRSI), as well as histology (Tokumitsu et al., 1997; Pirttila et al., 2001; Pitkanen et al., 2002). Similarly, seizure-induced neuronal loss and gliotic changes in the affected hemisphere have been described in humans who died following focal status epilepticus (Mori et al., 1992; Fukijawa et al., 2000; Men et al., 2000). Progressive development of seizure-induced neuronal loss and gliosis in the hippocampus is also a postulated mechanism leading to severe hippocampal sclerosis in both animal models of limbic epilepsy (Cavazos et al., 1994; Kotloski et al., 2002) and in patients with chronic temporal lobe epilepsy (Duncan, 2002; Fuerst et al., 2003). However, there is less evidence for similar reactive gliotic changes in the neocortex as a result of repeated seizures without status epilepticus. While small regions with subtle neuronal loss and gliosis may not be detected by structural MRI (Bronen et al., 1997; Cukiert et al., 2001), 1H-MRSI studies have demonstrated evidence of neuronal injury outside the primary epileptic region in patients with both temporal and extratemporal lobe epilepsy (Stanley et al., 1998; Li et al., 2000; Vermathen et al., 2003). Also, a recent study combining diffusion tensor imaging with voxel-based morphometry demonstrated extratemporal atrophy of remote projection areas in patients with temporal lobe epilepsy (Halford et al., 2007). Reactive gliosis and seizure-induced astrocytic reorganization, with a 10-fold increase in overlap of astrocytic processes, have been recently demonstrated in epileptic mice, suggesting that morphological changes of astrocytes may be required for establishment of recurrent excitatory pathways (Oberheim et al., 2008).
As an alternative explanation, multifocal cortical abnormalities, including gliosis and functional changes in GABAA receptors, may represent an underlying pathology associated with an increased susceptibility to seizures; more widespread pathology could result in more severe epilepsy. Prospective, longitudinal studies of GABAA-receptor imaging started at the early course of epilepsy may determine which of these mechanisms are responsible for multifocal cortical abnormalities of GABAA receptor binding.
The location and extent of FMZ binding abnormalities do not necessarily match with the exact location of cortex with early seizure spread; rather, partial overlap and involvement of cortex adjacent to the FMZ abnormality was common. Extension of ictal epileptiform acivity to areas surrounding cortex with decreased cortical FMZ binding has been reported in some previous studies (Ryvlin et al., 1998; Muzik et al., 2000a). A potential reason for this partial mismatch between ictal epileptiform activity (either onset or rapid spread) and decreased FMZ binding could be that decreased FMZ binding indicates a small structural abnormality (dysplasia or gliosis) not visualized on MRI, and surrounding cortical areas may be hyperexcitable. Such perilesional hyperexcitability has been reported in animal models of neocortical epilepsy (Mittmann et al., 1994; Neumann-Haefelin et al., 1995; Jacobs et al., 1999). Since no histology was obtained separately from cortex adjacent to areas with decreased FMZ binding in the present study, this remains speculative. It is possible that even these adjacent areas would show mild abnormalities on histology. Epileptogenicity of cortical areas normal on MRI has been demonstrated in patients with cortical dysplasia (Tassi et al., 2001). Nevertheless, involvement of remote cortical regions with decreased FMZ binding in rapid seizure spread indicates that most of these FMZ binding abnormalities occur in areas readily accessible to propagating seizures, therefore, they are potential sites for secondary epileptic foci. As discussed above, the extent of cortex with decreased FMZ binding makes it feasible to address such regions by intracranial EEG monitoring in most cases.
Non-localizing FMZ PET
No focal cortical decreases of FMZ binding could be detected in the epileptic hemisphere in 3 of our 20 patients, although gliosis was found in the resected epileptogenic cortex in all these cases. It is possible that the extent and/or severity of GABA receptor changes in these patients were small and undetectable by PET. Another possibility is that some of the patients had only focal increases (but not decreases) of cortical FMZ binding ipsilateral to the seizure focus, although no focal cortical increases were apparent by visual inspection. Such cases have been reported in a previous study in approximately 1/3 of MRI-negative adult patients (Hammers et al., 2003). Many of those increases were multifocal often involving white matter regions and apparently not specific for the presumed seizure focus in most cases. Such increases may represent neuronal migrational abnormalities but would be difficult to objectively detect on PET in young children due to the lack of a normal control group and without the benefit of a voxel-based method, which is difficult in a young cohort of patients including very young children (Muzik et al., 2000b). Therefore, in this study, we have focused on cortical decreases, which were detected in a higher rate of patients than reported in other studies, possibly due to a different age range, different patient selection, different analytic approach or a combination of these factors. Future studies could address the intracranial EEG correlates of increased cortical FMZ binding in similar patient groups.
In conclusion, the present study shows that in addition to perifocal GABAA receptor binding abnormalities, decreased FMZ binding remote from the seizure onset zone can occur, particularly involving cortex showing rapid seizure propagation. Findings from FMZ PET can facilitate enhanced coverage of potentially epileptogenic areas by intracranial electrodes in patients with intractable epilepsy and non-localizing MRI. From the surgical point of view, ipsilateral cortical abnormalities appear to be particularly relevant since such areas, if unresected, may account for surgical failure in at least some cases. This concept might be further tested by long-term follow-up of those patients in whom the cortex with decreased FMZ binding was not resected. The subsequent development of a seizure focus in these regions would support this hypothesis.
Acknowledgements
The authors thank Thomas Mangner, PhD and Pulak Chakraborty, PhD for the reliable radiosynthesis of [11C]flumazenil, as well as Galina Rabkin, CNMT, Angie Wigeluk, CNMT, and Mei-li Lee, MS, for their expert technical assistance in performing the PET studies. They also thank William Kupsky, MD, from the Department of Pathology for performing careful histopathologic examination on the resected tissues, and Jagdish Shah, M.D., for performing clinical EEG assessment for some of the patients. The study was supported by an NIH grant (NS-34488, to H.T. Chugani). We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The definitive version of this publication is available at www.blackwell-synergy.com.
Abbreviations
- FDG
2-deoxy-2[18F]fluoro-D-glucose
- sz
seizure
- i.cr.
intracranial
- F
female
- M
male
- L
left
- R
right
- P
parietal
- F
frontal
- T
temporal
- O
occipital; lobes with no or incomplete resection of cortex with EEG or FMZ PET involvement are underlined and italicized
- hem.
hemisphere
- spr
early seizure spread
- spk
frequent interictal spiking (>10 spikes per min)
- nc
cortex with PET abnormality was not covered by subdural electrodes
- (-)
no epileptiform abnormality in lobe showing PET abnormality
- FCD
focal cortical dysplasia
- GM
gray matter
- WM
white matter
- n.a.
not available (tissue)
Footnotes
None of the authors has any conflict of interest to disclose.
REFERENCES
- Arnold S, Berthele A, Drzezga A, Tölle TR, Weis S, Werhahn KJ, Henkel A, Yousry TA, Winkler PA, Bartenstein P, Noachtar S. Reduction of benzodiazepine receptor binding is related to the seizure onset zone in extratemporal focal cortical dysplasia. Epilepsia. 2000;41:818–824. doi: 10.1111/j.1528-1157.2000.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Asano E, Muzik O, Shah A, Juhász C, Chugani DC, Kagawa K, Benedek K, Sood S, Gotman J, Chugani HT. Quantitative visualization of ictal subdural EEG changes in children with neocortical focal seizures. Clin Neurophysiol. 2004;115:2718–2727. doi: 10.1016/j.clinph.2004.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronen RA, Fulbright RK, King D, Kim JH, Spencer SS, Spencer DD, Lange RC. Qualitative MR imaging of refractory temporal lobe epilepsy requiring surgery: correlation with pathology and seizure outcome after surgery. AJR Am J Roentgenol. 1997;169:875–882. doi: 10.2214/ajr.169.3.9275915. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106–21. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Juhász C, Janisse JJ, Ager J, Chugani HT. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol. 2001;49:618–26. [PubMed] [Google Scholar]
- Cukiert A, Buratini JA, Machado E, Sousa A, Vieira JO, Argentoni M, Forster C, Baldauf C. Results of surgery in patients with refractory extratemporal epilepsy with normal or nonlocalizing magnetic resonance findings investigated with subdural grids. Epilepsia. 2001;42:889–894. doi: 10.1046/j.1528-1157.2001.00201.x. 2001. [DOI] [PubMed] [Google Scholar]
- Doble A. New insights into the mechanism of action of hypnotics. J Psychopharmacol. 1999;13:S11–20. doi: 10.1177/026988119901304S03. [DOI] [PubMed] [Google Scholar]
- Duncan JS. Seizure-induced neuronal injury: human data. Neurology. 2002;59(Suppl 5):S15–20. doi: 10.1212/wnl.59.9_suppl_5.s15. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical treatment of the epilepsies. Raven Press; New York: 1993. pp. 609–621. [Google Scholar]
- Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol. 2003;53:413–6. doi: 10.1002/ana.10509. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG, Itabashi HH, Wu A, Shinmei SS. Status epilepticus-induced neuronal loss in humans without systemic complications or epilepsy. Epilepsia. 2000;41:981–91. doi: 10.1111/j.1528-1157.2000.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Halford JJ, Roberts DR, Rumboldt Z, Rorden C, Bonilha L. Hippocampal deafferentation and extra-temporal atrophy in temporal lobe epilepsy. American Epilepsy Society; Philadelphia, PA: 2007. Program No. 2.084. Abstract Viewer. Abstract. [Google Scholar]
- Hammers A, Koepp MJ, Richardson MP, Hurlemann R, Brooks DJ, Duncan JS. Grey and white matter flumazenil binding in neocortical epilepsy with normal MRI. A PET study of 44 patients. Brain. 2003;126:1300–1318. doi: 10.1093/brain/awg138. [DOI] [PubMed] [Google Scholar]
- Hammers A, Panagoda P, Heckemann RA, Kelsch W, Turkheimer FE, Brooks DJ, Duncan JS, Koepp MJ. [11C]Flumazenil PET in temporal lobe epilepsy: do we need an arterial input function or kinetic modeling? J Cereb Blood Flow Metab. 2008;28:207–216. doi: 10.1038/sj.jcbfm.9600515. [DOI] [PubMed] [Google Scholar]
- Henry TR, Frey KA, Sackellares JC, Gilman S, Koeppe RA, Brunberg JA, Ross DA, Berent S, Young AB, Kuhl DE. In vivo cerebral metabolism and central benzodiazepine-receptor binding in temporal lobe epilepsy. Neurology. 1993;43:1998–2006. doi: 10.1212/wnl.43.10.1998. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Kharazia VN, Prince DA. Mechanisms underlying epileptogenesis in cortical malformations. Epilepsy Res. 1999;36:165–188. doi: 10.1016/s0920-1211(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Juhász C, Nagy F, Watson C, da Silva EA, Muzik O, Chugani DC, Shah J, Chugani HT. Glucose and [11C]flumazenil PET abnormalities of thalamic nuclei in temporal lobe epilepsy. Neurology. 1999;53:2037–2045. doi: 10.1212/wnl.53.9.2037. [DOI] [PubMed] [Google Scholar]
- Juhász C, Chugani DC, Muzik O, Watson C, Shah J, Shah A, Chugani HT. Electroclinical correlates of flumazenil and fluorodeoxyglucose PET abnormalities in lesional epilepsy. Neurology. 2000;55:825–836. doi: 10.1212/wnl.55.6.825. [DOI] [PubMed] [Google Scholar]
- Juhász C, Chugani DC, Muzik O, Shah A, Shah J, Watson C, Canady A, Chugani HT. Relationship of flumazenil and glucose PET abnormalities to neocortical epilepsy surgery outcome. Neurology. 2001;56:1650–1658. doi: 10.1212/wnl.56.12.1650. [DOI] [PubMed] [Google Scholar]
- Juhász C, Muzik O, Chugani DC, Shen C, Janisse J, Chugani HT. Prolonged vigabatrin treatment modifies developmental changes of GABA(A)-receptor binding in young children with epilepsy. Epilepsia. 2001;42:1320–1326. doi: 10.1046/j.1528-1157.2001.05401.x. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Hammers A, Labbe C, Woermann FG, Brooks DJ, Duncan JS. 11C-flumazenil PET in patients with refractory temporal lobe epilepsy and normal MRI. Neurology. 2000;54:332–339. doi: 10.1212/wnl.54.2.332. [DOI] [PubMed] [Google Scholar]
- Kotloski R, Lynch M, Lauersdorf S, Sutula T. Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits. Prog Brain Res. 2002;135:95–110. doi: 10.1016/S0079-6123(02)35010-6. [DOI] [PubMed] [Google Scholar]
- Lambert N, Grover L. The mechanism of biphasic GABA responses. Science. 1995;269:928–929. doi: 10.1126/science.7638614. [DOI] [PubMed] [Google Scholar]
- Li LM, Cendes F, Andermann F, Dubeau F, Arnold DL. Spatial extent of neuronal metabolic dysfunction measured by proton MR spectroscopic imaging in patients with localization-related epilepsy. Epilepsia. 2000;41:666–674. doi: 10.1111/j.1528-1157.2000.tb00226.x. [DOI] [PubMed] [Google Scholar]
- Men S, Lee DH, Barron JR, Munoz DG. Selective neuronal necrosis associated with status epilepticus: MR findings. AJNR Am J Neuroradiol. 2000;21:1837–40. [PMC free article] [PubMed] [Google Scholar]
- Mittmann T, Luhmann HJ, Schmidt-Kastner R, Eysel UT, Weigel H, Heinemann U. Lesion-induced transient suppression of inhibitory function in rat neocortex in vitro. Neuroscience. 1994;60:891–906. doi: 10.1016/0306-4522(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Mori H, Mizutani T, Yoshimura M, Yamanouchi H, Shimada H. Unilateral brain damage after prolonged hemiconvulsions in the elderly associated with theophylline administration. J Neurol Neurosurg Psychiatry. 1992;55:466–469. doi: 10.1136/jnnp.55.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell F. Secondary epileptogenesis in man. Arch Neurol. 1985;42:318–35. doi: 10.1001/archneur.1985.04060040028009. [DOI] [PubMed] [Google Scholar]
- Morrell F. Varieties of human secondary epileptogenesis. J Clin Neurophysiol. 1989;6:227–75. doi: 10.1097/00004691-198907000-00002. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Shen C, da Silva EA, Shah J, Shah A, Canady A, Watson C, Chugani HT. Objective method for localization of cortical asymmetries using positron emission tomography to aid surgical resection of epileptic foci. Comput Aided Surgery. 1998;3:74–82. doi: 10.1002/(SICI)1097-0150(1998)3:2<74::AID-IGS4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Muzik O, da Silva EA, Juhász C, Chugani DC, Shah J, Nagy F, Canady A, von Stockhausen HM, Herholz K, Gates J, Frost M, Ritter F, Watson C, Chugani HT. Intracranial EEG vs. flumazenil and glucose PET in children with extratemporal lobe epilepsy. Neurology. 2000a;54:171–179. doi: 10.1212/wnl.54.1.171. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhász C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000b;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Shen C, Juhász C, von Stockhausen HM, Chugani HT. Assessment of the performance of FDG and FMZ PET imaging against the gold standard of invasive EEG monitoring for the detection of extratemporal lobe epileptic foci in children. In: Gjedde A, Hansen SB, Knudsen GM, Paulson OB, editors. Physiological imaging of the brain with PET. Academic Press; San Diego: 2001. pp. 381–387. [Google Scholar]
- Neumann-Haefelin T, Hagemann G, Witte OW. Cellular correlates of neuronal hyperexcitability in the vicinity of photochemically induced cortical infarcts in rats in vitro. Neurosci Lett. 1995;193:101–104. doi: 10.1016/0304-3940(95)11677-o. [DOI] [PubMed] [Google Scholar]
- Niimura K, Muzik O, Chugani DC, Shen C, Chugani HT. [11C]flumazenil PET: activity images versus parametric images for the detection of neocortical epileptic foci. J Nucl Med. 1999;40:1985–1991. [PubMed] [Google Scholar]
- Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff OA, Rothman DL, Behar KL, Mattson RH. Low brain GABA level is associated with poor seizure control. Ann Neurol. 1996;40:908–911. doi: 10.1002/ana.410400613. [DOI] [PubMed] [Google Scholar]
- Pietrzyk U, Herholz K, Heiss WD. Three-dimensional alignment of functional and morphological tomograms. J Comput Assist Tomogr. 1990;14:51–59. [PubMed] [Google Scholar]
- Pirttila TR, Pitkanen A, Tuunanen J, Kauppinen RA. Ex vivo MR microimaging of neuronal damage after kainate-induced status epilepticus in rat: correlation with quantitative histology. Magn Reson Med. 2001;46:946–954. doi: 10.1002/mrm.1281. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Nissinen J, Nairismagi J, Lukasiuk K, Gröhn OH, Miettinen R, Kauppinen R. Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Prog Brain Res. 2002;135:67–83. doi: 10.1016/S0079-6123(02)35008-8. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Luddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Koepp MJ, Brooks DJ, Duncan JS. 11C-flumazenil PET in neocortical epilepsy. Neurology. 1998;51:485–492. doi: 10.1212/wnl.51.2.485. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Bouvard S, Le Bars D, De Lamérie G, Grégoire MC, Kahane P, Froment JC, Mauguière F. Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. A prospective study in 100 patients. Brain. 1998;121:2067–2081. doi: 10.1093/brain/121.11.2067. [DOI] [PubMed] [Google Scholar]
- Savic I, Thorell JO, Roland P. [11C]flumazenil positron emission tomography visualizes frontal epileptogenic regions. Epilepsia. 1995;36:1225–1232. doi: 10.1111/j.1528-1157.1995.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Savic I, Svanborg E, Thorell JO. Cortical benzodiazepine receptor changes are related to frequency of partial seizures: a positron emission tomography study. Epilepsia. 1996;37:236–44. doi: 10.1111/j.1528-1157.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Stanley JA, Cendes F, Dubeau F, Andermann F, Arnold DL. Proton magnetic resonance spectroscopic imaging in patients with extratemporal epilepsy. Epilepsia. 1998;39:267–273. doi: 10.1111/j.1528-1157.1998.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Stokking R, Zuiderveld H, Hulshoff-Pol H, Viergever M. Integrated visualization of SPECT and MR images for frontal lobe damaged regions. In: Robb E, editor. Visualization in Biomedical Computing. Vol. 2359. SPIE Press Bellingham; WA: 1994. pp. 282–290. [Google Scholar]
- Tassi L, Pasquier B, Minotti L, Garbelli R, Kahane P, Benabid AL, Battaglia G, Munari C, Spreafico R. Cortical dysplasia: electroclinical, imaging, and neuropathologic study of 13 patients. Epilepsia. 2001;42:1112–1123. doi: 10.1046/j.1528-1157.2001.00501.x. [DOI] [PubMed] [Google Scholar]
- Tokumitsu T, Mancuso A, Weinstein PR, Weiner MW, Naruse S, Maudsley AA. Metabolic and pathological effects of temporal lobe epilepsy in rat brain detected by proton spectroscopy and imaging. Brain Res. 1997;744:57–67. doi: 10.1016/s0006-8993(96)01071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl 3):8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- Van Bogaert P, Massager N, Tugendhaft P, Wikler D, Damhaut P, Levivier M, Brotchi J, Goldman S. Statistical parametric mapping of regional glucose metabolism in mesial temporal lobe epilepsy. NeuroImage. 2000;12:129–138. doi: 10.1006/nimg.2000.0606. [DOI] [PubMed] [Google Scholar]
- Verhoeff NP, Petroff OA, Hyder F, Zoghbi SS, Fujita M, Rajeevan N, Rothman DL, Seibyl JP, Mattson RH, Innis RB. Effects of vigabatrin on the GABAergic system as determined by [123I]iomazenil SPECT and GABA MRS. Epilepsia. 1999;40:1433–8. doi: 10.1111/j.1528-1157.1999.tb02016.x. [DOI] [PubMed] [Google Scholar]
- Vermathen P, Laxer KD, Schuff N, Matson GB, Weiner MW. Evidence of neuronal injury outside the medial temporal lobe in temporal lobe epilepsy: N-acetylaspartate concentration reductions detected with multisection proton MR spectroscopic imaging--initial experience. Radiology. 2003;226:195–202. doi: 10.1148/radiol.2261011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stockhausen HM, Thiel A, Herholz K, Pietrzyk U. A convenient method for topographical localization of intracranial electrodes with MRI and a conventional radiograph. Neuroimage. 1997;5:S514. Abstract. [Google Scholar]
- von Stockhausen H, Pietrzyk U, Herholz K. “3D-Tool” - A software for visualization and analysis of coregistered multimodality volume datasets of individual subjects. Neuroimage. 1998;7:S799. Abstract. [Google Scholar]