Abstract
The complement system is a crucial part of innate and adaptive immunity which exerts a significant evolutionary pressure on pathogens. It has selected for those pathogens, mainly micro-organisms but also parasites, that have evolved countermeasures. The characterization of how pathogens evade complement attack is a rapidly developing field of current research. In recent years, multiple complement evasion strategies have been characterized. In this review, we focus on complement escape mechanisms expressed by hematophagous parasites, a heterogeneous group of metazoan parasites that share the property of ingesting the whole blood of their host. Complement inhibition is crucial for parasite survival within the host tissue or to facilitate blood feeding. Finally, complement inhibition by hematophagous parasites may also contribute to their success as pathogen vectors.
Keywords: complement system, immune evasion, hematophagous parasites, Darwinian evolution
Introduction
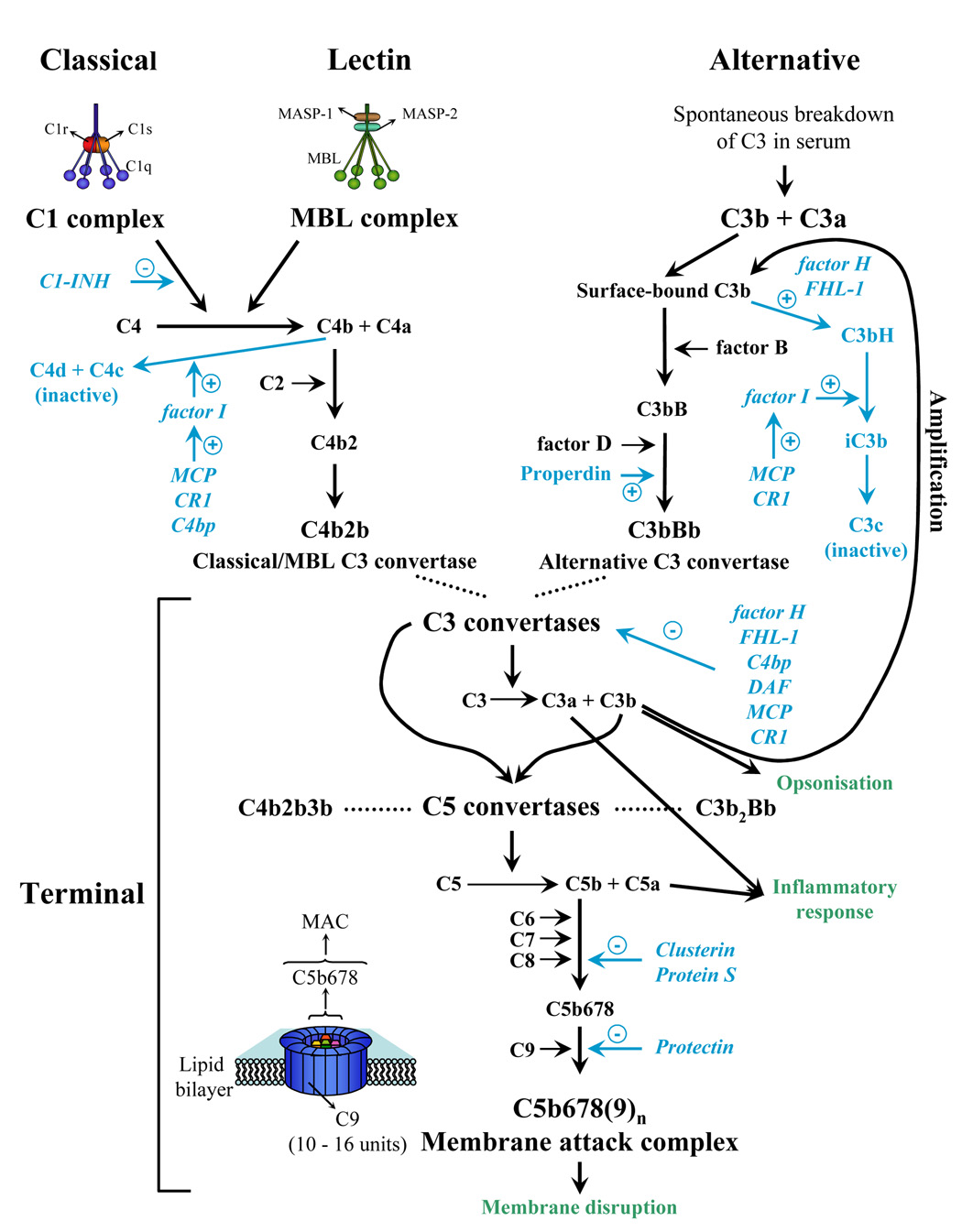
The complement system of vertebrates is a crucial part of innate and adaptive immunity [1]. It acts in a cascade manner through three activation pathways known as classical, lectin and alternative (Fig. 1). The classical and the lectin pathways are activated by the recognition proteins C1q and mannose-binding lectin, respectively. These proteins bind to charge clusters and neutral sugars on targets, respectively. In contrast, activation of the alternative pathway is a default process that proceeds unless down-regulated by regulators of complement activation (RCAs). Complement activation results in production of inflammatory anaphylatoxins, deposition of opsonic C3 fragments on surfaces and assembly of the membrane attack complex which disrupts cellular lipid bilayers [2].
FIG. 1.
Diagrammatic representation of the activation pathways of the complement cascade. The complement system is a central host defence system of innate immunity and is activated immediately upon contact of a microbe with host body fluids. The activated complement system is highly toxic and can have devastating effects. Complement is activated by three pathways: the classical, the lectin and the alternative pathway. The classical pathway is initiated by antigen-antibody complexes. Upon complex formation, additional zymogens like C4 are activated and consequently form additional binding sites for other components of this cascade and for proteases. Upon cleavage e.g. by a serine protease, active enzyme complexes are generated, which form the classical pathway convertase C4b2b. The lectin pathway is initiated when the mannan binding lectin (MBL) binds to carbohydrates which are accessible on the surface of pathogens. After binding, MBL is activated by MBL associated serine proteases (MASPs) and consequently activates C4 and leads to C1 independent formation of the classical pathway convertase C4b2b. The alternative pathway is initiated by a spontaneous conformational change of the central complement component C3b which exposed its active thioester and by binding the additional component factor B. This complex C3bB is activated by the serine protease factor D in an active C3bBb complex which displays enzymatic activity. This C3 convertase initiates a powerful amplification reaction and generates more C3b molecules. All three pathways of complement activation merge at the level of C3 and form two different types of enzymes that cleave C3, i.e. C3 convertases (C3bBb and C4b2b). These act on C3 and generate the active component C3b and the anaphylactic and antimicrobial component C3a. C3b can act as an opsonin to enhance phagocytosis of a pathogen or particle by phagocytic cells which are equipped with specific C3 receptors (Complement Receptors CR1 to CR4). In addition, C3 formation can lead to the generation of a C5 convertase (C3b2Bb or C4b2b3b) which cleaves C5 to form C5b as well as the anaphylactic and antimicrobial component C5a. C5b initiates the terminal pathway, by activating sequentially and non enzymatically the terminal complement components C6, C7, C8 and C9, resulting in formation of the membrane attack complex (MAC, also called the terminal complement complex). The end product of the MAC pathway is a pore, which is composed of multiple assembled C9 proteins. These insert into the membrane of the target and cause target/cell lysis and membrane disruption. The activated complement system generates highly toxic effects on target cells or surfaces. The complement system has an intrinsic control activity which ensures that activation proceeds on foreign particles, such as microbes, but is actively controlled and blocked on self cells, i.e. host cell surfaces. Multiple regulators control the action of the complement cascade and these are shown in blue. Regulators show a variable distribution: some are present in fluid phase, e.g. factor H, FHL-1 (factor H-like protein 1) and C4bp (C4 binding protein); and others are inserted into the membrane of the host cells, e.g. MCP/CD46 (membrane cofactor protein), CR1/CD35 (complement receptor 1) and DAF/CD55 (decay accelerating factor). C1-INH, the C1 inhibitor, is present in plasma in relative high concentrations and binds covalently to active C1s and C1r. The terminal pathway is controlled by three inhibitors: clusterin and protein S bind to soluble C5-7 complexes and prevent integration into the membrane, protectin/CD59 inhibits binding of C9 to the complex and thus polymerization. Several regulators like CR1, DAF and MCP act in the classical and also in the alternative pathway. CR1 and DAF favour dissociation of the C3bBb, as well as the C4b2b complexes and similarly MCP acts as cofactor for degradation of both C3b and C4b. The symbols (+) and (−) associated with each regulator illustrate its positive or negative effect on the indicated reaction, respectively. (*) Schistosome ntigenic turnover applies to all three activation pathways.
Complement activation on host cells is prevented by several RCAs expressed as trans-membrane or soluble proteins (Fig. 1), whose activities are predominantly restricted to complement of the same species, a phenomenon called homologous restriction [3, 4]. RCA proteins down-regulate complement activity at several steps in the complement cascade (Fig. 1) [5]. RCA genes form a gene family, most members of which are clustered in the genome [6, 7]. RCA proteins share a common structure comprised of several short consensus repeats (SCR), termed complement control protein repeats (CCPRs), which are functional units. Each SCR contains four cysteines forming disulfide bonds conferring a pearl shape to the SCR domain [8].
The host complement system exerts significant evolutionary pressure on pathogens. Pathogens, including parasites, have acquired mechanisms to protect themselves from deleterious effects of complement [9, 10]. The characterization of how pathogens evade complement attack is a rapidly expanding field of research. In recent years, multiple complement evasion strategies have been characterized. Growing lists of studies are supporting the description of pathogen proteins which are essential for complement escape as novel virulence factors. The functional characterization of their roles at the host-pathogen interface is an important emerging field that not only contributes to our knowledge in fundamental biology but also identifies new targets to develop original anti-pathogen strategies and provides original approaches for the development of anti-inflammatory pharmalogical molecules.
Pathogens avoid complement attack or complement mediated inflammation by evading recognition by complement activators (antibodies and MBL) and/or by expressing complement inhibitors. The later are either host RCA recruited on the pathogen surface [11– 14] or pathogen encoded RCA expressed as secreted or membrane associated products [15].
Recent reviews have covered the multiple complement and immune evasion strategies used by the wide range of pathogens [10, 16–22]. In this review, we focus on complement escape by hematophagous parasites. Hematophagous parasites are metazoan that ingest and digest the whole blood of their host using specialized organs. With the exception of this common feature, hematophagous parasites form an extremely heterogeneous group that includes helminths and arthropods, endo- and ectoparasites, parasites that take a blood meal that lasts from a few seconds to a few days. This review is structured in three main parts. The first and second sections will address the complement escape mechanisms described for schistosomes and ticks, respectively. In the third part, we will overview calreticulin homologues expressed as anticomplement molecule in the saliva of some hematophagous parasites. The complement escape mechanisms described in this review are illustrated in Fig. 2 and are listed in Table 1.
FIG. 2.
Diagrammatic representation of reported subversion mechanisms of the complement cascade by hematophagous parasites. Hematophagous parasites known to inhibit complement activation are presented in red. The name of the molecule responsible for the inhibition (if known) is indicated in brackets. Interrogation mark means that the molecule has not been identified yet. The symbol (−) indicates a negative effect on the indicated reaction.
Table 1.
Complement subversion mechanisms developed by hematophagous parasites
| Parasite | Mechanism (molecule involved) | Reference | |
|---|---|---|---|
| Trematodes | Schistosomes | Antigen turnover | [24] |
| Low immunogenicity of the membranocalyx | [24] | ||
| Binding to Fc domain of host immunoglobulins (Paramyosin) | [33] | ||
| Binding to C2 (CRIT) | [29] | ||
| Binding to C3 (C3bp) | [31] | ||
| Acquisition of host RCAs (DAF, Cryy) | [32, 34] | ||
| CD59/Protectin-like protein (SCIP-1) | [30] | ||
| Double membrane to resist to MAC lysis | [24] | ||
| Nematodes | Necator americanus | Binding to C1q (Calreticulin) | [67] |
| Haemonchus contortus | Binding to C1q (Calreticulin) | [66] | |
| Insects | Triatomines | ||
| Panstrongylus megistus, Triatoma brasiliensis and Rhodnius prolixus | Inhibition of the classical pathway | [77] | |
| Phlebotomines | |||
| Lutzomyia longipalpis and Lutzomyia migonei | Inhibition of the classical pathway | [77] | |
| Lutzomyia longipalpis | Inhibition of the alternative pathway | [77] | |
| Arachnids | Ixodes scapularis | Prevent deposition of C3b (Isac) | [47] |
| Binding to properdin (Salp20) | [55] | ||
| Ixodes ricinus | Prevent deposition of C3b (IRACs and IxACs) | [53, 54] | |
| Binding to properdin (IRACs and IxACs) | [54] | ||
| Ornithodoros moubata | Binding to C5 (OmCI) | [43] |
Schistosomes and complement
Schistosomes are parasitic worms that cause a chronic, often debilitating, disease called schistosomiasis that afflicts over 200 million people worldwide. Schistosomes have complex life cycles; they infect freshwater snails where they undergo asexual replication to produce larval forms called cercariae. Cercariae infect vertebrate hosts by penetrating the skin where they transform into schistosomula that are highly adapted to life inside their vertebrate hosts. Transformation involves the loss of the cercarial outer membrane with its fibrillar covering - the glycocalyx, which is known to be a potent activator of complement [23]. Glycocalyx shedding coincides with the synthesis of a new and unique surface that is composed of two closely apposed lipid bilayers in the form of a normal plasma membrane covered by a membrane-like secretion called the membranocalyx [24]. This novel surface seems refractory to the action of immune effectors, and schistosomula, as they become enveloped in this new covering, become rapidly resistant to immunological (including complement-mediated) attack [25, 26]. These parasites migrate through the peripheral tissue, invade a local blood vessel and migrate through the lungs, the heart, and finally to the blood vessels of the liver where they mature. Here, male and female parasites meet, mate and migrate to suitable, species-specific egg-laying sites.
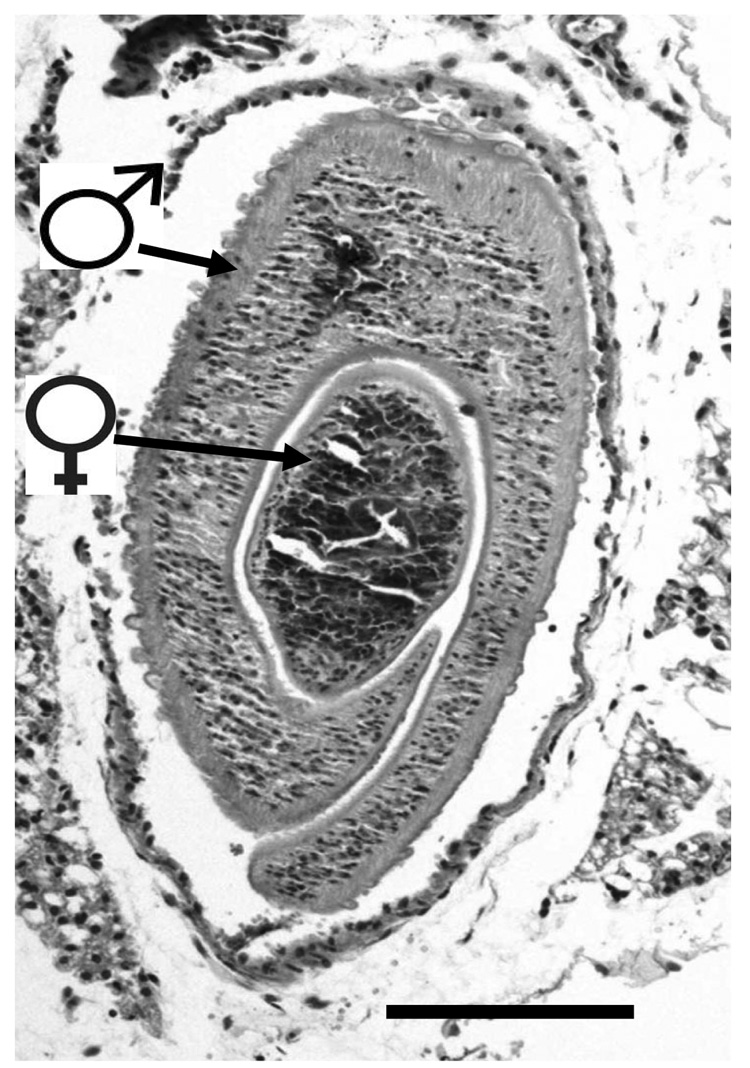
Schistosomes differ from other pathogens in a number of ways. First, they are large. Mature males, for instance, are approximately 10 mm long. The mature female is cylindrical, longer and more slender than the male and is held in a ventral canal of the male (Fig. 3). In cross section, the male/female pair spans about 1 mm. Second, schistosomes are long lived: individual parasites can survive within the vasculature of an immunocompetent host for years and, sometimes, decades. The parasites are therefore exposed to the repertoire of host immunological effectors for prolonged periods and must possess sophisticated evasion strategies to avoid destruction by these effectors.
FIG. 3.
Cross section through a male and female schistosome couple within a blood vessel of a mouse. The female is held in the gynaecophoric canal of the male. The bar is 0.5 mm. The tegumental covering of both parasites is intact and shows no evidence of overt immunological (e.g. complement-mediated) attack.
Regarding complement, all three pathways of complement activation could theoretically, be initiated by intravascular schistosomes and larval schistosomes do appear susceptible to complement mediated damage in rats but not in mice [27]. Schistosomes have been reported to possess an array of mechanisms to avoid complement-mediated damage [28]. These mechanisms are illustrated in Fig. 2 and are listed in Table 1.
One broad method whereby schistosomes have been proposed to avoid attack by complement and other host effectors is by rapidly turning over of the outer surface covering – the membranocalyx. While original observations suggested a rapid shedding of the membranocalyx in support of this notion, these results now appear largely to be a product of experimental conditions and later observations suggest a much slower turnover process. Some experiments suggested that the turnover of at least some tegumental componenets can take days – probably not rapidly enough to slough off the unimpeded assembly of the terminal components of complement [24].
A second broad mechanism for avoiding complement-mediated damage is the reported low intrinsic immunogenicity of the membranocalyx. This membrane contains few exposed parasite proteins and acquires a coating of host molecules [24]. As a result, intravascular parasites are reported to attract few host immunological effectors [26].
To counter the action of circulating complement components that do manage to associate with them, schistosomes are reported to further possess a collection of regulatory proteins, including selected molecules acquired from the host, that impede complement action by binding (and thereby inactivating or inhibiting) selected constituents of the complement cascade. A C2 binding protein, a C3 binding protein and a C8/C9 binding protein have all been reported to be present at the surface of schistosome parasites within the host vasculature [28–31]. It is important to note that while some of these parasite proteins have been isolated and shown to interact with complement components in vitro, the definitive protective role of none of them in vivo has been proven.
The putative C2-binding protein is a 286 amino acid protein designated CRIT (for complement C2 receptor inhibiting trispanning) [29]. The recombinant extracellular domain of CRIT has been reported to inhibit classical pathway-mediated hemolysis of sheep red blood cells in a dose-dependent manner. In addition, peptides derived from the C-terminus of CRIT were demonstrated to inhibit complement activation in vitro [29]. To inhibit complement as proposed, CRIT must be exposed at the host-interactive surface but this molecule has not been detected in recent proteomic analysis of parasite surface membrane extracts [32].
The presence of a schistosome C3-binding protein at the parasite surface is controversial. Some groups reported the presence of a C3-binding molecule on intravascular parasites while others fail to confirm this [24–26, 28]. Nonetheless, labelled surface extracts were reported to contain a surface-associated 130 kDa protein that bound to C3 sepharose [31]. This molecule remains uncharacterized.
A ~ 94 kDa schistosome C8 and C9 binding protein (originally designated schistosome complement inhibitory protein-1 (SCIP-1)), with antigenic and functional similarities to the human complement inhibitor protein CD59 (also called protectin), was reported to bind to purified human C8 and C9 and inhibit lysis of sheep and rabbit red blood cells by human complement [30]. Sequence analysis of purified SCIP-1 revealed it to be the previously described, 97 kDa myofibrillar protein, paramyosin. Native and recombinant paramyosin can bind human C8 and C9 and inhibit C9 polymerization onto red blood cells. The C9 binding domain has been mapped to the carboxyl terminus [30]. A second mechanism whereby paramyosin could impede complement activation was suggested by earlier work, in which the molecule was identified as a surface, Fc-binding protein to which host immunoglobulin bound [33]. Such binding would limit Fc domain access to complement components and therefore the ability of immunoglobulin to activate the classical pathway. However, the ability to detect paramyosin at the schistosome surface where it could engage immunoglobulin and complement is controversial and has not been confirmed in other studies [24]. Furthermore, paramyosin has not been detected in recent proteomic analysis of parasite surface membrane preparations [32]. Adding to the controversy is the inability of other workers to even detect immunoglobulin bound to the parasite surface (either bound via their Fc receptors or otherwise) [24]. These latter studies suggest that schistosomes may not permit antibody to bind to their surface in any manner - an ideal outcome for the parasites to avoid complement activation via the classical pathway.
In addition to molecules that the parasites themselves produce to inhibit complement activation, schistosomes are reported to possess the remarkable property of acquiring molecules from their hosts for this purpose. One study has reported that the host complement-regulating protein DAF (delay accelerating factor) is found at the parasite surface where it may dissociate C3 convertase, and thereby impede the complement cascade [34]. Exactly how host DAF might be acquired by schistosomes is not known and proteomic analysis of the schistosome tegument has not detected DAF [32].
Pertinent host molecules that have been detected by proteomics at the tegumental surface of living worms include the alpha chain C3c/C3dg fragment of C3 [32]. This suggests that C3 can be both activated by C3 convertase and covalently linked to the parasite surface, but subsequently becomes inactivated by RCAs that are presumably recruited by schistosomes from host plasma. Complement receptor-related protein y (Crry) is one such regulatory protein and this has also been detected in adult schistosome tegumental membranes extracts by proteomic analysis [32].
In summary, intravascular schistosomes possess a host-interactive covering of low intrinsic immunogenicity as well as a collection of molecules that are proposed to impede complement action, should components of the complement cascade manage to bind to that covering. Therefore, it is perhaps no surprise that there is no significant difference in parasite development in C3-deficient transgenic mice compared with wild-type mice [35]. A study of S. mansoni development in C-5 deficient mice similarly concluded that C5 plays no role in defence against a primary infection in mice [36].
Ticks and complement
Ticks are obligate blood feeding ectoparasites. They are vectors of viral, bacterial, protozoan and nematode pathogens of medical and veterinary importance. There are two main families of ticks, the Argasidae or soft ticks and the Ixodidae or hard ticks. Argasidae typically feed for a few hours whereas Ixodidae mouthparts remain embedded in host skin for up to two weeks. The long blood meal of ticks implies that they are able to deregulate host physiological processes such as hemostasis, vasoconstriction, inflammation, pain perception and immunity. These processes are targeted by bioactive molecules secreted in tick saliva [37]. Below, we describe the molecules involved in complement inhibition (Fig. 2 and Table 1).
Early studies have highlighted the importance of complement inhibition by tick saliva for completion of the blood meal [38, 39] or the key role of the complement system in cases of acquired resistance to ticks [40–42]. Over the last decades, anticomplement proteins were identified in the saliva of different tick species. A 16.77 kDa protein termed OmCI was characterized in salivary gland extracts of the soft tick Ornithodoros moubata [43]. This protein targets the terminal pathway of the complement system by inhibiting C5 cleavage. OmCI belongs to the lipocalin family and is the first member of this protein family reported to inhibit complement activation.
Most tick salivary anticomplement proteins cloned to date have been identified in ticks belonging to the Ixodes ricinus species complex, a group of closely related hard ticks comprising at least 14 species distributed throughout the world [44, 45]. The ability of tick saliva to inhibit the alternative pathway has been demonstrated for several Ixodes species [39]. More interestingly, the host range of Ixodes ticks was shown to correlate with the ability of their saliva to inhibit activation of the alternative pathway of their most common host species [46].
The first anticomplement protein cloned from ticks belonging to the Ixodes ricinus species complex was reported in 2000. In a very elegant study, Valenzuela et al. [47] described the characterization of a novel salivary anticomplement protein from the American tick I. scapularis [47]. This protein was termed I. scapularis anticomplement protein or Isac. It behaves as a regulator of the alternative pathway by preventing C3b and factor B deposition and by removal of prebound factor B [47]. Isac had no effect on the classical pathway. This protein is relatively small with a predicted and observed molecular mass of 18.5 kDa and 65 kDa, respectively [47]. Interestingly, Isac has no similarity neither to the OmCI protein described above or to any previously described anticomplement molecules. However, the presence of four cysteins in Isac suggests that it is a functional homologue of SCR acquired by convergent evolution. Convergent evolution is the process whereby organisms that are not monophyletic (not closely related phylogenetically) independently acquired sequences encoding proteins with similar function despite having low or no homology.
The property of Isac to inhibit complement could facilitate tick feeding and may predetermine vector competency to transmit pathogens including the Lyme disease spirochete (Borrelia burgdorferi). These hypotheses were addressed by oral administration of dsRNA to silence Isac expression in I. scapularis nymphs [48]. Isac silencing effected subsequent protein expression within the salivary glands and significantly interfered with tick feeding as revealed by a reduction of fed-tick weight. However, Isac silencing did not prevent the transmission of B. burgdorferi by infected nymphal ticks despite a reduction in spirochete load in Isac-silenced infected nymphs compared to controls. This observation does not precluded that Isac homologues play a key role in the transmission of B. burgdorferi by infected ticks. Indeed, the failure to block transmission could be explained either by a partial efficiency of RNAi silencing and/or the activity of paralogues which could elicit the interference.
Since the publication of Isac [47], more than 40 homologous sequences have been described among cDNAs collected from I. scapularis [48–51], I. pacificus [52] and I. ricinus salivary glands [53, 54] isolated from nymphs or adult ticks. In comparison to Isac, these sequences exhibit percentages of nucleotide and amino acid sequence identity ranging from 49% to 95% and 36% to 90%, respectively. Interestingly, the four cysteins reported in Isac are conserved in these sequences. The studies listed above led to the concept of the Isac-like protein family and to the hypothesis that Ixodes ticks co-expressed anticomplement paralogous proteins during blood feeding. Below, we review the studies that addressed the biological activities of Isac-like sequences. We will successively address Isac homologues described in I. scapularis and I. ricinus. Please note that this order of presentation does not always reflect the chronology of the discoveries.
The Salp20 sequence sharing homology with Isac was identified from a nymphal I. scapularis salivary gland cDNA library [49]. Salp20 is a protein with 83% amino acid sequence identity to Isac. Its observed molecular mass is 48 kDa with N- and O-linked glycans that make up almost half the molecular mass of the mature protein [51]. As observed for Isac, recombinant Salp20 inhibited the alternative pathway by preventing C3b and factor B deposition and by removal of prebound factor B [51]. Interestingly, the same study reported the ability of recombinant Salp20 to partially protect in vitro a serum sensitive species of Borrelia from lysis by human serum. This observation led to the hypothesis that Salp20 could contribute in vivo to the survival of tick transmitted pathogens. In a very recent study, Tyson et al. characterized the specific mechanism by which Salp20 inhibits the alternative pathway. They demonstrated that Salp20 directly binds with high affinity to properdin [55]. Properdin is a positive regulator of the alternative pathway that binds and stabilizes the C3 convertase, significantly increasing its half-life [56, 57]. By binding to properdin, Salp20 was shown to displace it from the C3 convertase, thus accelerating the decay of the alternative pathway C3 convertase [55].
Isac homologues were also identified and studied in I. ricinus [54]. Two Isac homologues were identified in I. ricinus tick salivary glands and designated IRAC I and IRAC II (for Ixodes ricinus anticomplement proteins I and II). Both proteins inhibit the alternative pathway of the human complement similarly as reported initially for Isac (inhibition of C3b and factor B deposition and removal of prebound factor B). Using monoclonal antibodies specific of IRAC I and IRAC II, we showed that the two proteins are co-expressed constitutively in I. ricinus adult female salivary glands and are up-regulated during blood feeding. The extent of divergence between the IRAC I and IRAC II sequences is much greater than would be expected for alleles from one locus, strongly suggesting that these proteins result from the expression of two different genes. To test this hypothesis, ticks collected from different locations in Belgium were microdissected: for each tick, left and right salivary glands were isolated and stained with monoclonal antibodies specific to IRAC I and IRAC II, respectively. All ticks were found to express both IRAC I and IRAC II thereby demonstrating that IRAC I and IRAC II are encoded by separate genes that are co-expressed.
Importantly, phylogenetic analyses of Isac homologous sequences encoded by I. scapularis, I. ricinus and I. pacificus demonstrate that ticks belonging to the Ixodes ricinus complex encode a family of relatively small anticomplement molecules that have evolved by positive Darwinian selection as revealed by a constraint in nonsynonymous substitutions [54]. Positive Darwinian selection implies that after gene duplication, paralogous sequences are subjected to selection of coding mutations leading to the expression of paralogous proteins with different biological properties.
Positive Darwinian selection applied to duplicate genes may possibly lead to homologous proteins with complementary biological properties. In our recent study, we tested the hypothesis that IRAC I and IRAC II paralogues may have different inhibitory activities against the complement of different natural host species. IRAC I and II were tested against the complement of eight I. ricinus natural host species (six mammals and two birds). When tested on human complement, IRAC I was slightly more active than IRAC II, while they inhibited canine complement comparably. Interestingly, when tested on sheep, pig and horse sera, IRAC I was a better inhibitor than IRAC II, yet the opposite result was observed with pheasant serum. These results demonstrate that IRAC I and IRAC II have broad and complementary inhibition activities against the complement of different host species [58].
The sequences homologous to Isac described above exhibit amino acid identity > 56%. Very recently, a group of 5 additional sequences homologous to Isac was reported in I. ricinus [53]. When analysed with the other Isac homologues, the 5 new sequences (termed IxAC-B1 to 5) segregated into a relatively distant phylogenic group exhibiting around 40% amino acid identity with the former sequences (see figure 1 of Couvreur et al. 2008). These new sequences were shown to evolve, as reported earlier for other members of the Isac-like family [54], by positive Darwinian selection. Couvreur et al. concluded that diversification of these sequences was associated with differences in antigenicity [53]. As reported earlier for Isac, Salp20 and IRACs, IxACs were shown to inhibit the alternative pathway, but not the classical pathway of complement. Interestingly, IxAC-B1 and IRAC II [53] were shown to inhibit the alternative pathway through binding and inhibition of properdin as described above for Salp20 [55].
All together, the studies reported above suggest that multiple Ixodes species produce families of anticomplement proteins that bind properdin. Tyson et al. [55], proposed that Isac-like proteins specifically bind the thrombospondin type I repeats of properdin through the glycans that make up almost half the molecular weight of the mature proteins. Further studies are required to test this hypothesis and to identify the roles of Isac-like paralogues in vivo.
Calreticulin secreted by hematophagous parasites as complement inhibitor
Calreticulin (CRT) is well known as a multifunctional and abundant protein of the endoplasmic reticulum where it acts as a molecular chaperone and Ca2+-signaling molecule. However, CRT has also been found in other organelles, at the cell surface and in the extracellular compartment, where it has been shown to exert a number of physiological and pathological effects (for a review see [59]). In relation to the complement system, it has been demonstrated that extracellular CRT can bind to C1q [60], and furthermore, can compete with antibodies for binding to C1q and inhibition of C1q mediated hemolysis [61, 62]. It has been suggested that through this mechanism CRT might contribute to the progression of autoimmune disease by preventing immune complex clearance.
There is a remarkable conservation of both genomic organization and amino acid sequence of CRT throughout evolution from plants to mammals. The human CRT consists of a 17 amino acid residue hydrophobic ER signal sequence followed by the 400 amino acids of the mature protein. The latter consists of three domains called N- (for N terminal), P- (for proline rich) and C- (for C terminal) domains. A region, termed the S-domain spanning the N and the P intersection was shown to interact with C1q [63, 64].
It has been demonstrated that the hematophagous nematodes Necator americanus [65] and Haemonchus contortus [66] secrete CRT homologues in excretory/secretory products. These molecules could contribute to blood feeding by inhibition of host physiological processes such as hemostasis and complement activation.
The hookworm Necator americanus inhabits the small intestines of humans. Infection causes severe morbidity in the tropical regions of Africa, Asia and the Americas, due to the development of iron deficiency anaemia in malnourished hosts. Kasper et al. studied the biological activities of N. americanus CRT expressed as a recombinant protein in a prokaryotic system [67]. While they did not detect Ca2+ binding activity, they demonstrated the ability of the protein to bind to human C1q and to inhibit C1q mediated hemolysis of sensitized sheep red blood cells.
Haemonchus contortus is a blood sucking gastroinstestinal parasite of domestic animals mainly sheep and goat. H. contortus CRT was shown to increase plasma coagulation time by binding to Ca2+ and clotting factors. This property was mapped to the C-domain of the protein [66]. The protein was also shown to bind to C1q and to inhibit C1q mediated lysis of sensitized sheep erythrocytes, these functions were mapped to the N-domain [66].
While the secretion and the anticomplement activity have been demonstrated for the CRT expressed by N. americanus and H. contortus, the expression of CRT in the sialome (salivary proteome) of other hematophagous parasites has been suggested by direct or indirect observations. Whether CRT secreted by these parasites inhibits complement activation still needs to be determined. CRT has been identified in the saliva of tick species belonging to different genus of the Ixodidae family [68, 69] and infected hosts were shown to produce anti-CRT antibodies [70–72]. In a very recent study, Gao et al. demonstrated that immunization of sheep against CRT expressed by the Ixodidae tick Haemaphysalis qinghaiensis induced a partially protective immunity against ticks, resulting in 54.3% mortality in adult ticks, compared to the 38.7% death rate in the control group [73]. Finally, transcriptomic studies performed on Aedes aegypti and Anopheles gambiae mosquitoes [74, 75] and in the cat flea Ctenophalides felis [76] suggested that CRT might also be expressed as a secreted protein in the saliva of these hematophagous parasites. All together, the studies described above suggest that secreted CRT could be expressed by multiple families of hematophagous parasites. Further studies are required to determine the extend of this phenomenon and to unravel the roles of salivary CRT in host-parasite interactions.
Conclusions
The complement system is an extremely powerful component of immunity that needs to be tightly regulated by host protective molecules to control its potentially damaging effects at the surface of host cells. This review illustrates the variety of the mechanisms by which hematophagous parasites are reported to evade complement attack either by avoiding recognition and/or by expressing a collection of molecules that impede complement activation. The latter molecules are either host molecules recruited by the parasites or parasite encoded proteins. Analysis of some of the anticomplement molecules encoded by hematophagous parasites suggest that they have been acquired through a process of convergent evolution and positive Darwinian selection. It is very likely that new mechanisms of complement subversion expressed by hematophagous parasites will be discovered in the future. Supporting this prediction, anticomplement activities have been described in insect saliva [77] (Table 1). The mechanism of action and the active proteins still need to be identified. Genome sequencing projects will certainly contribute to the characterization of new anticomplement molecules encoded by pathogens.
While the biological function of the anticomplement proteins described in this review are well defined in vitro, their definitive roles in vivo still need to be investigated in detail. Inhibition of complement by hematophagous parasites could play critical roles at the host-parasite interface. Inhibition of complement could be of course critical for completion of the blood meal by inhibition of inflammation. Alternatively, complement inhibition could also be crucial for protection of the parasite gut from ingested host complement. Finally, complement inhibition could play a key role in the competency of some hematophagous parasites as vectors of pathogens.
In conclusion, the characterization of how hematophagous parasites evade complement attack is a rapidly developing field of current research. The identification of such molecules and the characterization of their roles at the host-pathogen interface is an important field that not only contributes to our knowledge in fundamental biology but also identifies new targets to develop original anti-pathogen strategies such as vaccines targeting these virulent factors and provides new anti-inflammatory molecule candidates.
Acknowledgments
The research on tick complement inhibitory proteins performed by Dr A. Vanderplasschen’s lab was supported by a grant from the Belgian Walloon Region (Convention Région Wallonne 315543). Dr. P. Skelly is supported by grant AI-056273 from the NIH-NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nonaka M, Yoshizaki F. Evolution of the complement system. Mol Immunol. 2004;40(12):897–902. doi: 10.1016/j.molimm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Rother K, Till GO, Hänsch GM. The Complement System. Berlin: Springer; 1998. [Google Scholar]
- 3.Houle J, Hoffmann E. Evidence for restriction of the ability of complement to lyse homologous erythrocytes. J Immunol. 1984;133(3):1444–1452. [PubMed] [Google Scholar]
- 4.Yamamoto H, Blaas P, Nicholson-Weller A, Hansch GM. Homologous species restriction of the complement-mediated killing of nucleated cells. Immunology. 1990;70(4):422–426. [PMC free article] [PubMed] [Google Scholar]
- 5.Meri S, Jarva H. Complement regulation. Vol. 74. Vox Sang; 1998. pp. 291–302. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez de Cordoba S, Lublin D, Rubinstein P, Atkinson J. Human genes for three complement components that regulate the activation of C3 are tightly linked. J. Exp. Med. 1985;161(5):1189–1195. doi: 10.1084/jem.161.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krushkal J, Bat O, Gigli I. Evolutionary Relationships Among Proteins Encoded by the Regulator of Complement Activation Gene Cluster. Mol Biol Evol. 2000;17(11):1718–1730. doi: 10.1093/oxfordjournals.molbev.a026270. [DOI] [PubMed] [Google Scholar]
- 8.Perkins SJ, Gilbert HE, Aslam M, Hannan J, Holers VM, Goodship TH. Solution structures of complement components by X-ray and neutron scattering and analytical ultracentrifugation. Biochem Soc Trans. 2002;30(Pt 6):996–1001. doi: 10.1042/bst0300996. [DOI] [PubMed] [Google Scholar]
- 9.Cooper NR. Complement evasion strategies of microorganisms. Immunol Today. 1991;12(9):327–331. doi: 10.1016/0167-5699(91)90010-Q. [DOI] [PubMed] [Google Scholar]
- 10.Blom AM. Strategies developed by bacteria and virus for protection from the human complement system. Scand J Clin Lab Invest. 2004;64(5):479–496. doi: 10.1080/00365510410002904. [DOI] [PubMed] [Google Scholar]
- 11.Thern A, Stenberg L, Dahlback B, Lindahl G. Ig-binding surface proteins of Streptococcus pyogenes also bind human C4b-binding protein (C4BP), a regulatory component of the complement system. J Immunol. 1995;154(1):375–386. [PubMed] [Google Scholar]
- 12.Vanderplasschen A, Mathew E, Hollinshead M, Sim RB, Smith GL. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc Natl Acad Sci U S A. 1998;95(13):7544–7549. doi: 10.1073/pnas.95.13.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellwage J, Meri T, Heikkila T, Alitalo A, Panelius J, Lahdenne P, Seppala IJ, Meri S. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J Biol Chem. 2001;276(11):8427–8435. doi: 10.1074/jbc.M007994200. [DOI] [PubMed] [Google Scholar]
- 14.Kraiczy P, Skerka C, Kirschfink M, Brade V, Zipfel PF. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. Eur J Immunol. 2001;31(6):1674–1684. doi: 10.1002/1521-4141(200106)31:6<1674::aid-immu1674>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Kotwal GJ, Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988;335(6186):176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- 16.Bernet J, Mullick J, Singh AK, Sahu A. Viral mimicry of the complement system. J Biosci. 2003;28(3):249–264. doi: 10.1007/BF02970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favoreel HW, Van de Walle GR, Nauwynck HJ, Pensaert MB. Virus complement evasion strategies. J Gen Virol. 2003;84(1):1–15. doi: 10.1099/vir.0.18709-0. [DOI] [PubMed] [Google Scholar]
- 18.Inal JM. Parasite interaction with host complement: beyond attack regulation. Trends Parasitol. 2004;20(9):407–412. doi: 10.1016/j.pt.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124(4):767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 20.Kraiczy P, Wurzner R. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Molecular Immunology. 2006;43(1–2):31–44. doi: 10.1016/j.molimm.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Rooijakkers SHM, van Strijp JAG. Bacterial complement evasion. Molecular Immunology. XXI International Complement Workshop; October 22–26, 2006; Beijing, China. 2007. pp. 23–32. [DOI] [PubMed] [Google Scholar]
- 22.Zipfel PF, Wurzner R, Skerka C. Complement evasion of pathogens: common strategies are shared by diverse organisms. Mol Immunol. 2007;44(16):3850–3857. doi: 10.1016/j.molimm.2007.06.149. [DOI] [PubMed] [Google Scholar]
- 23.Samuelson JC, Caulfield JP. Cercarial glycocalyx of Schistosoma mansoni activates human complement. Infect Immun. 1986;51(1):181–186. doi: 10.1128/iai.51.1.181-186.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skelly PJ, Wilson AR, Baker JR, Muller R, Rollinson D. Advances in Parasitology. UK: Academic Press; 2006. Making Sense of the Schistosome Surface; pp. 185–284. [DOI] [PubMed] [Google Scholar]
- 25.Dessein A, Samuelson JC, Butterworth AE, Hogan M, Sherry BA, Vadas MA, David JR. Immune evasion by Schistosoma mansoni: loss of susceptibility to antibody or complement-dependent eosinophil attack by schistosomula cultured in medium free of macromolecules. Parasitology. 1981;82(Pt 3):357–374. doi: 10.1017/s0031182000066890. [DOI] [PubMed] [Google Scholar]
- 26.Keating JH, Wilson RA, Skelly PJ. No overt cellular inflammation around intravascular schistosomes in vivo. J Parasitol. 2006;92(6):1365–1369. doi: 10.1645/GE-864R.1. [DOI] [PubMed] [Google Scholar]
- 27.Vignali DA, Bickle QD, Taylor MG, Tennent G, Pepys MB. Comparison of the role of complement in immunity to Schistosoma mansoni in rats and mice. Immunology. 1988;63(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- 28.Skelly PJ. Intravascular schistosomes and complement. Trends Parasitol. 2004;20(8):370–374. doi: 10.1016/j.pt.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Inal J. Complement C2 receptor inhibitor trispanning: from man to schistosome. Springer Seminars in Immunopathology. 2006;27(4):509–510. doi: 10.1007/s00281-005-0009-9. [DOI] [PubMed] [Google Scholar]
- 30.Deng J, Gold D, LoVerde PT, Fishelson Z. Mapping of the complement C9 binding domain in paramyosin of the blood fluke Schistosoma mansoni. Int J Parasitol. 2007;37(1):67–75. doi: 10.1016/j.ijpara.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Silva EE, Clarke MW, Podesta RB. Characterization of a C3 receptor on the envelope of Schistosoma mansoni. J Immunol. 1993;151(12):7057–7066. [PubMed] [Google Scholar]
- 32.Braschi S, Wilson RA. Proteins Exposed at the Adult Schistosome Surface Revealed by Biotinylation. Mol Cell Proteomics. 2006;5(2):347–356. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Loukas A, Jones MK, King LT, Brindley PJ, McManus DP. Receptor for Fc on the surfaces of schistosomes. Infect Immun. 2001;69(6):3646–3651. doi: 10.1128/IAI.69.6.3646-3651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearce EJ, Hall BF, Sher A. Host-specific evasion of the alternative complement pathway by schistosomes correlates with the presence of a phospholipase C-sensitive surface molecule resembling human decay accelerating factor. J Immunol. 1990;144(7):2751–2756. [PubMed] [Google Scholar]
- 35.La Flamme AC, MacDonald AS, Huxtable CR, Carroll M, Pearce EJ. Lack of C3 affects Th2 response development and the sequelae of chemotherapy in schistosomiasis. J Immunol. 2003;170(1):470–476. doi: 10.4049/jimmunol.170.1.470. [DOI] [PubMed] [Google Scholar]
- 36.Ruppel A, Rother U, Diesfeld HJ. Schistosoma mansoni: development of primary infections in mice genetically deficient or intact in the fifth component of complement. Parasitology. 1982;85(Pt 2):315–323. doi: 10.1017/s0031182000055293. [DOI] [PubMed] [Google Scholar]
- 37.Ribeiro JM, Makoul GT, Levine J, Robinson DR, Spielman A. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J Exp Med. 1985;161(2):332–344. doi: 10.1084/jem.161.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro JM, Spielman A. Ixodes dammini: salivary anaphylatoxin inactivating activity. Exp Parasitol. 1986;62(2):292–297. doi: 10.1016/0014-4894(86)90034-2. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro JM. Ixodes dammini: salivary anti-complement activity. Exp Parasitol. 1987;64(3):347–353. doi: 10.1016/0014-4894(87)90046-4. [DOI] [PubMed] [Google Scholar]
- 40.Wikel SK, Allen JR. Acquired resistance to ticks. iii. Cobra venom factor and the resistance response. Immunology. 1977;32(4):457–465. [PubMed] [Google Scholar]
- 41.Wikel SK. Acquired resistance to ticks: expression of resistance by C4-deficient guinea pigs. Am J Trop Med Hyg. 1979;28(3):586–590. [PubMed] [Google Scholar]
- 42.Papatheodorou V, Brossard M. C3 levels in the sera of rabbits infested and reinfested with Ixodes ricinus L. and in midguts of fed ticks. Exp Appl Acarol. 1987;3(1):53–59. doi: 10.1007/BF01200413. [DOI] [PubMed] [Google Scholar]
- 43.Nunn MA, Sharma A, Paesen GC, Adamson S, Lissina O, Willis AC, Nuttall PA. Complement Inhibitor of C5 Activation from the Soft Tick Ornithodoros moubata. J Immunol. 2005;174(4):2084–2091. doi: 10.4049/jimmunol.174.4.2084. [DOI] [PubMed] [Google Scholar]
- 44.Keirans JE, Needham GR, Oliver JHJ, Needham GR, Michell R, Horn DJ, Welborn WC. Symposium Proceedings IX International Congress of Acarology. Colombus, Ohio: Ohio Biological Survey; 1999. The Ixodes (Ixodes) ricinus complex worldwide: diagnosis of the species in the complex, hosts and distribution; pp. 341–347. [Google Scholar]
- 45.Xu G, Fang QQ, Keirans JE, Durden LA. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89(3):452–457. doi: 10.1645/0022-3395(2003)089[0452:MPAITT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Lawrie CH, Randolph SE, Nuttall PA. Ixodes Ticks: Serum Species Sensitivity of Anticomplement Activity. Experimental Parasitology. 1999;93(4):207–214. doi: 10.1006/expr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 47.Valenzuela JG, Charlab R, Mather TN, Ribeiro JM. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem. 2000;275(25):18717–18723. doi: 10.1074/jbc.M001486200. [DOI] [PubMed] [Google Scholar]
- 48.Soares CA, Lima CM, Dolan MC, Piesman J, Beard CB, Zeidner NS. Capillary feeding of specific dsRNA induces silencing of the isac gene in nymphal Ixodes scapularis ticks. Insect Mol Biol. 2005;14(4):443–452. doi: 10.1111/j.1365-2583.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 49.Das S, Banerjee G, DePonte K, Marcantonio N, Kantor FS, Fikrig E. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J Infect Dis. 2001;184(8):1056–1064. doi: 10.1086/323351. [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36(2):111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Tyson K, Elkins C, Patterson H, Fikrig E, de Silva A. Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol Biol. 2007;16(4):469–479. doi: 10.1111/j.1365-2583.2007.00742.x. [DOI] [PubMed] [Google Scholar]
- 52.Francischetti IMB, My Pham V, Mans BJ, Andersen JF, Mather TN, Lane RS, Ribeiro JMC. The transcriptome of the salivary glands of the female western black-legged tick Ixodes pacificus (Acari: Ixodidae) Insect Biochemistry and Molecular Biology. 2005;35(10):1142–1161. doi: 10.1016/j.ibmb.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Couvreur B, Beaufays Jrm, Charon Cd. Variability and Action Mechanism of a Family of Anticomplement Proteins in Ixodes ricinus. PLoS ONE. 2008;3(1):e1400. doi: 10.1371/journal.pone.0001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daix V, Schroeder H, Praet N, Georgin JP, Chiappino I, Gillet L, de Fays K, Decrem Y, Leboulle G, Godfroid E, Bollen A, Pastoret PP, Gern L, Sharp PM, Vanderplasschen A. Ixodes ticks belonging to the Ixodes ricinus complex encode a family of anticomplement proteins. Insect Mol Biol. 2007;16(2):155–166. doi: 10.1111/j.1365-2583.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 55.Tyson KR, Elkins C, de Silva AM. A novel mechanism of complement inhibition unmasked by a tick salivary protein that binds to properdin. J Immunol. 2008;180(6):3964–3968. doi: 10.4049/jimmunol.180.6.3964. [DOI] [PubMed] [Google Scholar]
- 56.Fearon D, Austen K. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J. Exp. Med. 1975;142(4):856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hourcade DE. The Role of Properdin in the Assembly of the Alternative Pathway C3 Convertases of Complement. J. Biol. Chem. 2006;281(4):2128–2132. doi: 10.1074/jbc.M508928200. [DOI] [PubMed] [Google Scholar]
- 58.Schroeder H, Daix V, Gillet L, Renauld JC, Vanderplasschen A. The paralogous salivary anti-complement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species. Microbes Infect. 2007;9(2):247–250. doi: 10.1016/j.micinf.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Johnson S, Michalak M, Opas M, Eggleton P. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 2001;11(3):122–129. doi: 10.1016/s0962-8924(01)01926-2. [DOI] [PubMed] [Google Scholar]
- 60.Eggleton P, Lieu TS, Zappi EG, Sastry K, Coburn J, Zaner KS, Sontheimer RD, Capra JD, Ghebrehiwet B, Tauber AI. Calreticulin is released from activated neutrophils and binds to C1q and mannan-binding protein. Clin Immunol Immunopathol. 1994;72(3):405–409. doi: 10.1006/clin.1994.1160. [DOI] [PubMed] [Google Scholar]
- 61.Kishore U, Sontheimer RD, Sastry KN, Zaner KS, Zappi EG, Hughes GR, Khamashta MA, Strong P, Reid KB, Eggleton P. Release of calreticulin from neutrophils may alter C1q-mediated immune functions. Biochem J. 1997;322(Pt 2):543–550. doi: 10.1042/bj3220543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kovacs H, Campbell ID, Strong P, Johnson S, Ward FJ, Reid KB, Eggleton P. Evidence that C1q binds specifically to CH2-like immunoglobulin gamma motifs present in the autoantigen calreticulin and interferes with complement activation. Biochemistry. 1998;37(51):17865–17874. doi: 10.1021/bi973197p. [DOI] [PubMed] [Google Scholar]
- 63.Coppolino MG, Dedhar S. Calreticulin. Int J Biochem Cell Biol. 1998;30(5):553–558. doi: 10.1016/s1357-2725(97)00153-2. [DOI] [PubMed] [Google Scholar]
- 64.Stuart GR, Lynch NJ, Lu J, Geick A, Moffatt BE, Sim RB, Schwaeble WJ. Localisation of the C1q binding site within C1q receptor/calreticulin. FEBS Lett. 1996;397(2–3):245–249. doi: 10.1016/s0014-5793(96)01156-8. [DOI] [PubMed] [Google Scholar]
- 65.Pritchard DI, Brown A, Kasper G, McElroy P, Loukas A, Hewitt C, Berry C, Fullkrug R, Beck E. A hookworm allergen which strongly resembles calreticulin. Parasite Immunol. 1999;21(9):439–450. doi: 10.1046/j.1365-3024.1999.00238.x. [DOI] [PubMed] [Google Scholar]
- 66.Suchitra S, Joshi P. Characterization of Haemonchus contortus calreticulin suggests its role in feeding and immune evasion by the parasite. Biochim Biophys Acta. 2005;1722(3):293–303. doi: 10.1016/j.bbagen.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 67.Kasper G, Brown A, Eberl M, Vallar L, Kieffer N, Berry C, Girdwood K, Eggleton P, Quinnell R, Pritchard DI. A calreticulin-like molecule from the human hookworm Necator americanus interacts with C1q and the cytoplasmic signalling domains of some integrins. Parasite Immunol. 2001;23(3):141–152. doi: 10.1046/j.1365-3024.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 68.Jaworski D, Simmen F, Lamoreaux W, Coons L, Muller M, Needham G. A Secreted Calreticulin Protein in Ixodid Tick (Amblyomma americanum) Saliva. Journal of Insect Physiology. 1995;41:369–375. [Google Scholar]
- 69.Ferreira CA, Da Silva Vaz I, da Silva SS, Haag KL, Valenzuela JG, Masuda A. Cloning and partial characterization of a Boophilus microplus (Acari: Ixodidae) calreticulin. Exp Parasitol. 2002;101(1):25–34. doi: 10.1016/s0014-4894(02)00032-2. [DOI] [PubMed] [Google Scholar]
- 70.Sanders ML, Glass GE, Nadelman RB, Wormser GP, Scott AL, Raha S, Ritchie BC, Jaworski DC, Schwartz BS. Antibody Levels to Recombinant Tick Calreticulin Increase in Humans after Exposure to Ixodes scapularis (Say) and Are Correlated with Tick Engorgement Indices. Am. J. Epidemiol. 1999;149(8):777–784. doi: 10.1093/oxfordjournals.aje.a009887. [DOI] [PubMed] [Google Scholar]
- 71.Sanders ML, Jaworski DC, Sanchez JL, DeFraites RF, Glass GE, Scott AL, Raha S, Ritchie BC, Needham GR, Schwartz BS. Antibody to a cDNA-derived calreticulin protein from Amblyomma americanum as a biomarker of tick exposure in humans. Am J Trop Med Hyg. 1998;59(2):279–285. doi: 10.4269/ajtmh.1998.59.279. [DOI] [PubMed] [Google Scholar]
- 72.Alarcon-Chaidez F, Ryan R, Wikel S, Dardick K, Lawler C, Foppa IM, Tomas P, Cushman A, Hsieh A, Spielman A, Bouchard KR, Dias F, Aslanzadeh J, Krause PJ. Confirmation of Tick Bite by Detection of Antibody to Ixodes Calreticulin Salivary Protein. Clin. Vaccine Immunol. 2006;13(11):1217–1222. doi: 10.1128/CVI.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao J, Luo J, Fan R, Fingerle V, Guan G, Liu Z, Li Y, Zhao H, Ma M, Liu J, Liu A, Ren Q, Dang Z, Sugimoto C, Yin H. Cloning and characterization of a cDNA clone encoding calreticulin from Haemaphysalis qinghaiensis (Acari: Ixodidae) Parasitol Res. 2008;102(4):737–746. doi: 10.1007/s00436-007-0826-y. [DOI] [PubMed] [Google Scholar]
- 74.Valenzuela JG, Pham VM, Garfield MK, Francischetti IM, Ribeiro JM. Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect Biochem Mol Biol. 2002;32(9):1101–1122. doi: 10.1016/s0965-1748(02)00047-4. [DOI] [PubMed] [Google Scholar]
- 75.Francischetti IM, Valenzuela JG, Pham VM, Garfield MK, Ribeiro JM. Toward a catalog for the transcripts and proteins (sialome) from the salivary gland of the malaria vector Anopheles gambiae. J Exp Biol. 2002;205(Pt 16):2429–2451. doi: 10.1242/jeb.205.16.2429. [DOI] [PubMed] [Google Scholar]
- 76.Jaworski DC, Higgins JA, Radulovic S, Vaughan JA, Azad AF. Presence of calreticulin in vector fleas (Siphonaptera) J Med Entomol. 1996;33(3):482–489. doi: 10.1093/jmedent/33.3.482. [DOI] [PubMed] [Google Scholar]
- 77.Cavalcante RR, Pereira NH, Gontijo NF. Anti-complement activity in the saliva of phlebotomine sand flies and other haematophagous insects. Parasitology. 2003;127(Pt 1):87–93. doi: 10.1017/s0031182003003329. [DOI] [PubMed] [Google Scholar]