Abstract
When unimpaired participants name pictures quickly, they produce many perseverations that bear a semantic relation to the target, especially when the pictures are blocked by semantic category. These “semantic perseverations” have not shown the steep decay over lags (distance from prior occurrence) that typify the perseverations produced by people with aphasia on standard naming tasks (Cohen & Dehaene, 1998). To reconcile the discrepant findings, we studied semantic perseverations generated by participants with aphasia on a naming task that featured semantic blocking [Schnur, T. T., Schwartz, M. F., Brecher, A., & Hodgson, C. (2006). Semantic interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language, 54, 199–227]. The temporal properties of these perseverations were investigated by analyzing their lag function and the influence of time (response-stimulus interval) on this function. To separate out the influence of chance on the observed lag distributions, chance data sets were created for individual participants by reshuffling whole trials (i.e., stimulus-response pairs) in a manner that preserved unique features of the blocking design. Analyses of chance-corrected lag functions revealed the expected recency bias, i.e., higher perseveration frequencies at short lags. Importantly, there was no difference between the lag functions for perseverations generated with a 5 s, compared to 1 s, response-stimulus interval. This combination of recency and insensitivity to elapsed time indicates that the perseveratory impetus in a named response does not passively decay with time but rather is diminished by interference from related trials. We offer an incremental learning account of these findings.
Keywords: Perseveration, semantic blocking, aphasia, naming, priming, incremental learning
Introduction
Studies of naming errors bring to light the interplay of cooperative and competitive mental representations that underpin lexical access. Errors known as recurrent lexical perseveration (Sandson & Albert, 1984), which repeat a response given earlier in the list, reveal that processes from the past persist and have the potential to intrude on the present. To elucidate the nature of those persisting processes and their temporal dynamics, researchers typically derive a lag function, which reveals how perseveration probability is affected by the number of trials that intervene between the error and its source (Cohen & Dehaene, 1998; Gotts, della Rocchetta & Cipolotti, 2002). A few studies have also experimentally manipulated response-stimulus interval (RSI) for the purpose of exploring how the passage of time affects the perseveration lag function (Campbell & Clark, 1989; Gotts et al., 2002; Vitkovitch, Kirby & Tyrell, 1996).
The investigations of perseveration lag functions do not tell a consistent story, however. They have yielded one set of results when applied to the recurrent lexical perseverations produced by people with aphasia, and quite different results when applied to those produced by healthy individuals on naming tasks designed to promote perseveration. These perseveration-promoting manipulations frequently include the blocking of semantic competitors on successive or nearby trials, such that earlier named competitors, through priming, have a heightened probability of intruding as perseverations, specifically, semantic perseverations, since they are related to the names they replace. The goal of the present study was to confront conflicting findings in these two literatures regarding the temporal characteristics of lexical perseverations. To achieve this goal, we analyzed data collected from 18 individuals with aphasia during performance of task that involved semantic blocking and that elicited a large number of semantic perseverations (Schnur, Schwartz, Hodgson & Brecher, 2006, Experiment 2). Analysis of the semantic-perseveration lag function and its relation to RSI affords direct comparison with Vitkovitch et al. (1996) and clarifies the similarities and differences across participant groups.
Perseverations Elicited by Semantic Blocked Naming
Neurologically healthy individuals do not make frequent errors when naming pictures of familiar objects. However, certain experimental manipulations can induce errors that are not unlike those seen in aphasia. One such manipulation is speeded naming, wherein participants name pictures to a fast deadline. This manipulation increases semantic errors, including semantic perseverations (Moses, Nickels, & Sheard, 2004). The manipulation works because picture naming is a semantically-driven task, and so there is natural competition among words that share semantic features (e.g., Dell, Schwartz, Martin, Saffran & Gagnon, 1997; Humphreys, Riddoch & Quinlan, 1988; Levelt, Roelofs & Meyer, 1991). It takes time for the target to accumulate enough input from semantics to emerge as the winner in the competition for selection, especially when a competing word experiences priming from having been named on an earlier trial (Wheeldon & Monsell, 1994). A fast deadline increases the probability that a semantic competitor, and particularly a primed semantic competitor, will be erroneously selected for output.
The probability of semantic perseveration in normal naming can be increased still further by combining speeded naming with a semantic blocking manipulation (Vitkovitch & Humphreys, 1991; Vitkovitch et al., 1996). Here, multiple semantic competitors, typically exemplars from the same superordinate category, are presented for naming on adjacent or nearby trials. As successive competitors are named, each is primed, and the presence of multiple primed competitors prolongs the time required for the target win the competition (Brown, 1981; Howard, Nickels, Coltheart, & Cole-Virtue, 2006). With the requirement to respond quickly, it becomes more likely that one of the primed competitors will replace the target as the naming response, resulting in a semantic perseveration.
Entailed in the foregoing account is the idea that name priming persists across time and intervening trials. Vitkovitch and colleagues’ seminal studies of blocking-induced semantic perseverations strongly support this. In Vitkovitch and Humphreys (1991), participants named pictures in two consecutive 20-item blocks, where each block contained multiple, non-identical exemplars from a small set of categories. The authors predicted that competitors primed by naming in block 1 would retain this priming advantage into block 2, whereupon they would exert interference in the naming of related block-2 targets. In support of this prediction, they observed an above-chance incidence of semantic perseverations in block 2 that duplicated a response produced back in block 1.
Vitkovitch et al. (1996) performed a follow-up study that focused on the temporal characteristics of semantic perseverations induced by blocking. Two groups of healthy participants named pictures of 30 different 4-legged animals under speeded naming conditions (600 msec deadline). The groups experienced different response-stimulus intervals (RSI 7 s or 4 s). Semantic perseverations, which accounted for a majority of errors, were analyzed for how far back the source occurred; an error whose source was on the preceding trial was coded as having lag 1. They found that semantic perseverations in excess of chance occurred with lags between 4 and 12. There were zero lag 1 perseverations, significantly below chance (see also Cambell & Clark, 1989). The frequency distribution peaked at lag 11, which, in the longer RSI condition corresponds to about 90 s between source and error. In fact, the frequency distribution did not differ as a function of RSI, i.e., in the ANOVA, the Lag by RSI interaction was not significant.
Perseverations in Aphasia
From an aphasia perspective, these are surprising findings. At least since Cohen and Dehaene’s (1998) seminal study of the temporal characteristics of lexical perseverations in aphasia, it has been generally accepted that perseverations exhibit a strong recency bias, occurring with highest frequency at lag 1 and declining exponentially with increasing lag. Cohen and Dehaene (1998) collected perseveration data from three individuals with aphasia using naming tasks in which there was neither semantic blocking nor a fast deadline. They computed lag functions from actual data and from chance data created by randomly shuffling trials (i.e., stimulus-response pairs). Consistently, the plots of the observed vs. chance lag distributions revealed that short lags were over-represented in the actual data. Actual frequencies differed from chance frequencies at the shortest lags and declined to chance levels by lag 6 or so (depending on the individual and the analysis).
The recency bias in the perseveration lag function can be understood as showing that time matters, i.e., that a word is highly primed shortly after having been named and, with the passage of time, loses its priming activation and, consequently, its perseveratory impetus. Contrary to this, the data reported in Vitkovitch et al. (1996) indicate that the priming advantage that is conferred by naming and bolstered by semantic blocking is not biased towards recency and that, within limits, elapsed time (as manipulated by RSI) does not diminish the perseveratory impetus of primed competitors.
How are we to understand the difference across studies? Is it because the perseverations that Vitkovitch and colleagues analyzed were generated by healthy participants, as opposed to individuals with aphasia? Or is it because the perseverations in their study were induced by semantic blocking? To address this question, the present study analyzed semantic perseverations generated by individuals with aphasia during performance of the semantic blocked naming task (Schnur et al., 2006, Experiment 2). The next section describes the methods used in that study and the findings that laid the groundwork for the present investigation.
Schnur, Schwartz, Hodgson and Brecher (2006)
The semantic blocked naming experiment that Schnur and colleagues conducted was inspired by similar experiments run with unimpaired speakers (e.g., Damian, Vigliocco, & Levelt, 2001; Kroll & Stewart, 1994) and individuals with aphasia (McCarthy & Kartsounis, 2000; Wilshire & McCarthy, 2002). Theirs was the first study to demonstrate that participants with aphasia as a group experience reduced naming accuracy as a consequence of semantic blocking. For a complete description of participants and procedures, readers should consult Schnur et al. (2006). What follows is a summary of details relevant to the present follow-up study.
Programmed in Psyscope and run on a Macintosh computer, the experiment consisted of multiple blocks, each comprising 24 consecutive naming trials. On each trial, a single target was presented for naming within a 5 s deadline, without feedback. In each block, 6 unique targets were named once (cycle 1), then again in a different random order (cycle 2), and so on for a total of 4 cycles (24 trials). Blocks were of two types: homogeneous and mixed. In a homogeneous block, targets were 6 exemplars from the same category (e.g., 6 animals or 6 vehicles); in a mixed block, targets were 6 exemplars from different categories (1 animal, 1 vehicle, etc.) There were 12 homogeneous blocks, each containing targets from a different category. (Categories and targets are shown in Appendix A.) There were also 12 mixed blocks, created by rearranging the targets of the homogeneous blocks. Phonological overlap within blocks was kept to a minimum. In each experimental run, all 24 blocks were named, with the homogeneous-mixed presentation order randomly varied. For example, one participant named 3 homogeneous blocks followed by 3 mixed blocks, whereas another participant named a homogeneous block followed by a mixed block then another mixed block, and so on. Between blocks, participants were given as much rest time as they required. Every participant completed two runs of the experiment. In one run, the interval programmed between the response and the following stimulus (response-stimulus interval, RSI) was only 1 s; in the other run-through, it was five times that (5 s). Order of RSI conditions varied across participants. In summary, over the entire experiment, each participant named all 24 sets twice (once with each RSI) for a total of 48 blocks, 1152 trials per subject. The number of sessions required to complete the experiment ranged from 2 to 7.
Participants were 18 individuals with post-stroke, chronic aphasia who had lesions to the left hemisphere and were right-handed native speakers of English (details in Tables 1 and 2). Following up previous evidence linking aphasia of the nonfluent type to enhanced vulnerability to semantic competition (McCarthy & Kartsounis, 2002; Robinson, Shallice & Cipolotti, 2005; Wilshire & McCarthy, 2002), the participants were divided into two groups, differing in aphasia diagnosis but matched on relevant language measures (e.g., picture naming; semantic processing). The 7 participants in the Broca group all carried a diagnosis of nonfluent Broca’s aphasia; the 11 in the “Non Broca’s” group carried one of the fluent diagnoses: Anomic, Conduction, or Wernicke’s aphasia. In the ANOVA model, Group (Broca’s, Non Broca’s) was included as a between-subjects factor; Condition (homogeneous, mixed) and RSI (1 s, 5 s) were within-subjects factors.
Table 1.
Demographic information and clinical profiles, as characterized by the Western Aphasia Battery (Kertesz, 1992) and Quantitative Production Analysis (Saffran, Berndt & Schwartz, 1989). Low values of the Aphasia Quotient indicate greater severity.
| Western Aphasia Battery
|
Quantitative Production Analysis
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participant | Sex | Age | Education | Mos. Post Onset | Classification | Aphasia Quotient | Wds Per Min | Mdn L U | Prop Cl Class | Prop Wds in Ss |
| BAC | M | 57 | 19 | 118 | A | 86.6 | 39.2 | 7.0 | 0.55 | 0.99 |
| TB | F | 35 | 12 | 10 | A | 75.1 | 100.4 | 5.0 | 0.55 | 1.00 |
| MD | M | 54 | 12 | 96 | A | 81.6 | 45.9 | 5.0 | 0.56 | 0.79 |
| KAC | M | 61 | 11 | 16 | C | 72.9 | 55.4 | 5.0 | 0.51 | 0.80 |
| OE | M | 60 | 11 | 29 | A | 93.6 | 34.9 | 5.0 | 0.55 | 0.95 |
| MX | F | 39 | 12 | 49 | B | 67.2 | 16.3 | 2.0 | 0.40 | 0.50 |
| TG | F | 68 | 12 | 20 | A | 91.2 | 85.8 | 5.0 | 0.52 | 0.93 |
| CT | F | 41 | 10 | 22 | B | 69.7 | 14.4 | 4.0 | 0.45 | 0.42 |
| MO | M | 60 | 13 | 135 | B | 70.0 | 42.3 | 4.0 | 0.54 | 0.71 |
| SL | M | 50 | 12 | 36 | A | 92.7 | 55.8 | 5.0 | 0.45 | 0.82 |
| EC | F | 56 | 17 | 175 | B | 68.2 | 32.2 | 3.0 | 0.46 | 0.52 |
| EAC | F | 50 | 17 | 52 | A | 84.4 | 45.4 | 4.5 | 0.52 | 0.75 |
| DD | M | 56 | 16 | 28 | B | 53.1 | 19.9 | 1.0 | 0.29 | 0.23 |
| ED | F | 80 | 16 | 15 | W | 39.3 | 164.8 | 5.5 | 0.59 | 0.96 |
| LF | M | 53 | 20 | 25 | W | 66.1 | 32.5 | 8.5 | 0.52 | 0.88 |
| BT | M | 68 | 14 | 82 | B | 68.8 | 17.9 | 1.0 | 0.21 | 0.10 |
| DAN | F | 75 | 11 | 19 | C | 66.7 | 71.0 | 3.5 | 0.52 | 0.71 |
| NQ | F | 62 | 12 | 62 | B | 63.6 | 32.8 | 3.0 | 0.44 | 0.40 |
B (Broca), A (Anomic), W (Wernicke), C (Conduction), Mdn L U (Median length of utterance), Prop Cl Class (Proportion closed class words, relative to all scored words), Prop Wds in Ss (Proportion of words that fall within sentence boundaries).
Table 2.
Performance on the Philadelphia Naming Test (Roach, Schwartz, Martin, Grewal & Brecher, 1996) and Pyramids and Palms Tests (Howard & Patterson, 1992).
| Philadelphia Naming Test
|
Pyramids and Palms Test
|
|||||
|---|---|---|---|---|---|---|
| Participant | Correct | Semantic Err | Persev - U | Persev - All | All Pictures | Word to Pictures |
| BAC | 0.93 | 0.03 | 0.00 | 0.01 | 0.96 | 0.98 |
| TB | 0.79 | 0.10 | 0.00 | 0.02 | 0.92 | 0.87 |
| MD | 0.89 | 0.02 | 0.00 | 0.03 | 0.90 | 0.94 |
| KAC | 0.38 | 0.02 | 0.00 | 0.01 | 0.90 | 0.85 |
| OE | 0.82 | 0.03 | 0.00 | 0.02 | 0.92 | 0.96 |
| MX | 0.74 | 0.06 | 0.00 | 0.01 | 0.77 | 0.85 |
| TG | 0.71 | 0.07 | 0.00 | 0.05 | 0.94 | 0.92 |
| CT | 0.80 | 0.06 | 0.00 | 0.02 | 0.90 | 0.88 |
| MO | 0.77 | 0.04 | 0.00 | 0.00 | 0.96 | 0.92 |
| SL | 0.87 | 0.07 | 0.00 | 0.01 | 0.92 | 0.88 |
| EC | 0.62 | 0.05 | 0.01 | 0.05 | 0.88 | 0.90 |
| EAC | 0.79 | 0.04 | 0.00 | 0.04 | 0.90 | 0.87 |
| DD | 0.39 | 0.11 | 0.01 | 0.06 | 0.88 | 0.85 |
| ED | 0.07 | 0.09 | 0.05 | 0.12 | 0.88 | 0.83 |
| LF | 0.64 | 0.05 | 0.01 | 0.01 | 0.83 | n/a |
| BT | 0.69 | 0.13 | 0.17 | 0.29 | 0.96 | 0.90 |
| DAN | 0.26 | 0.03 | 0.03 | 0.06 | 0.87 | 0.79 |
| NQ | 0.62 | 0.06 | 0.01 | 0.03 | 0.83 | 0.77 |
Persev-U, Proportion of whole word perseverations unrelated to the target, relative to total responses; Persev-All, Proportion of whole word perseverations unrelated or related (semantically or phonologically) to the target.
Across all 18 participants, significantly more errors were made in the homogeneous condition, compared to the mixed condition. The difference was greater in the Broca’s group than in the group of Non Broca’s, but there was considerable overlap between groups. Collapsed across groups, the homogeneous-mixed difference (indexing the blocking effect) was found to increase across repetition cycles. This increase was later shown to be associated with damage in the left inferior frontal gyrus (Schnur, Lee, Coslett, Schwartz, & Thompson-Schill, 2005; Schnur, Schwartz, Kimberg, Hirshorn, Coslett, & Thompson-Schill, submitted).
Schnur et al. (2006) also carried out separate analyses of the error types of interest. For present purposes, the most interesting errors are those that duplicate other items from the same set. These “within-set substitutions” occurred primarily in homogeneous blocks (e.g., DOG → “horse”, where “horse” was one of the six items in the animal set featured in that block). A much smaller number occurred in mixed blocks (e.g., DOG → “toaster”, where “toaster” was another member of the mixed set featured in that block). The vast majority of the within-set substitutions were perseverations of responses produced earlier in the block. These are the perseverations that we analyzed in the present study.
Schnur et al’s (2006) analysis demonstrated that the semantic blocking manipulation lowered accuracy in part by eliciting semantic within-set intrusions, which, as we said, were primarily of a perseveratory nature. Schnur et al. did not, however, analyze the temporal characteristics of these semantic perseverations. We took up that issue here, using analytic methods inspired by Cohen and Dehaene (1999) and Vitkovitch et al., (1996). First, we compared the semantic-perseveration lag functions to chance, looking for evidence of decay akin to what Cohen and Dehaene (1998) observed. Finding evidence for this, we then performed an ANOVA across subjects to determine whether RSI modulates the lag function and whether any such modulation differs for semantic perseverations versus the (semantically unrelated) perseverations produced in the mixed condition. RSI did not modulate the lag function for either semantic or unrelated perseverations. In the discussion, we consider what these findings reveal about the mechanisms that underpin semantic perseveration in competitor priming tasks and about perseveration production in normality and pathology.
Methods
Participants
Tables 1 and 2 report background information on the 18 individuals with post-stroke aphasia who participated in the blocked naming experiment (Schnur et al., 2006); Table 3 shows their overall error rates in that experiment and the percentage and number of perseverations each contributed to the present analysis (see Perseveration Analysis, below). All three tables list participants in order of total perseveration production, from least to most.
Table 3.
For the individual participants, total percent errors in the semantic blocked naming experiment (Schnur et al., 2006, Experiment 2) and percent and number of perseverations from that experiment that were analyzed in the current study.
| No. Perseverations
|
|||||
|---|---|---|---|---|---|
| Participant | Total Errors | Total Persev | Hmg | Mxd | Total |
| BAC | 4.6% | 0.1% | 1 | 0 | 1 |
| TB | 3.2% | 0.2% | 2 | 0 | 2 |
| MD | 2.6% | 0.2% | 2 | 0 | 2 |
| KAC | 20.3% | 0.4% | 5 | 0 | 5 |
| OE | 12.1% | 0.5% | 6 | 0 | 6 |
| MX | 15.1% | 0.5% | 4 | 2 | 6 |
| TG | 7.4% | 0.8% | 8 | 1 | 9 |
| CT | 18.2% | 0.9% | 7 | 3 | 10 |
| MO | 25.8% | 1.0% | 10 | 1 | 11 |
| SL | 11.9% | 1.2% | 9 | 5 | 14 |
| EC | 33.6% | 1.8% | 21 | 0 | 21 |
| EAC | 28.1% | 2.1% | 20 | 4 | 24 |
| DD | 55.1% | 2.1% | 21 | 3 | 24 |
| ED | 89.7% | 2.5% | 23 | 6 | 29 |
| LF | 36.8% | 3.6% | 34 | 7 | 41 |
| BT | 31.4% | 3.6% | 39 | 2 | 41 |
| DAN | 57.2% | 3.9% | 37 | 8 | 45 |
| NQ | 46.8% | 6.5% | 67 | 8 | 75 |
Hmg. Perseverations produced in the homogeneous condition.
Mxd. Perseverations produced in the mixed condition.
As can be seen in Tables 1 and 2, the participants ranged widely in aphasia severity (Aphasia Quotient), fluency and grammar (Quantitative Production Analysis), and picture naming accuracy (Philadelphia Naming Test, PNT). On the PNT, they produced whole word perseverations at rates in the low to moderate range (see Table 2, columns 4 and 5), with no evidence of verbal stereotypy or iteration of the same response across trials (Sandson & Albert, 1984). Table 3 shows that in the blocked naming task, their rates of perseveration (as defined above) were similarly low as a percentage of total trials (.1% - 6.5%), although the actual number of perseverations was reasonably high, due to the large number of trials (1152 per participant).
Perseveration Analysis
Schnur et al. (2006) scored the first complete response on each trial of the experiment. The error taxonomy coded word errors by their relation to the target (semantic, phonological, or unrelated) and also contained codes for nonwords (neologisms), omissions, descriptions, and miscellaneous others. Secondary codes were used to designate within-set substitutions and other features of interest.
For reasons that will be explained shortly, our analysis necessitated a recoding of their data. Using their trial-by-trial listing of targets and phonetically transcribed responses, we replaced any nonword that strongly approximated (at least 50% phoneme overlap) the name of an item in the current set with the actual name. We then identified the within-set substitutions (substituted words that named another target from the current set) and coded as perseverations those that matched a response produced earlier in the block. Note that perseverations of responses outside of the current set, e.g., matching a response produced in a prior block, were not counted as perseverations in this study. For each coded perseveration, we counted back to the most recent occurrence of the response to find the “lag” for that perseveration. Let us take as an example the following trial sequence from a mixed block:
DOG – dog
TOASTER - toaster,
BUSH – bush
BED – shoaster toaster
The replacement of “shoaster” by “toaster” allowed us to capture the correspondence between that response and the earlier one; an automated matching procedure identified the BED→ toaster error as a within-set substitution, and a perseveration with lag of 2. The replacement rule had the desirable consequence of avoiding overestimation of long-lag perseverations. (Imagine another perseveration of “toaster” two trials later; with the replacement, lag = 3, without it, “shoaster” is passed over and lag = 5.) In any case, replacement affected only 2% of all responses, so the impact of this modification was small.
For each participant, we tabulated the number of perseverations that occurred with lag 1 across blocks, then repeated this for perseverations with lag 2, lag 3, etc. up to lag 23 (recall that there are 24 items per block). Separate tabulations were performed for homogenous and mixed blocks at each RSI. This yielded four summary lag distributions per participant (homogeneous RSI 1, 5; mixed RSI 1, 5)
Chance
Chance data sets are typically generated by repeatedly repairing targets and responses, so as to determine whether observed target-error relationships (e.g., phonological relatedness) are real or due to chance. In Cohen and Dehaene’s (1998) study, the question was rather whether relationships observed across trials were real or due to chance, and so they generated the chance corpus by reshuffling whole trials (i.e., stimulus-response pairs). We used their method, but modified it so that the reshuffling was done within a block and in a manner that preserved the cyclic structure of the block. Table 4 illustrates the procedure: For a reshuffled trial list to be legal, each target in the current set had to be presented once before any was presented again, and so on for all four cycles. In other words, each target appeared exactly once within each quarter of each legal reshuffled trial list, together with its original response. Thirty legal reshuffled trial lists were generated per participant per each of the 48 blocks (12 homogeneous, 12 mixed at each of 2 RSI conditions). In each reshuffled list, we identified perseverations and determined their lags, calculated their frequency at each lag, and then averaged these across the 30 lists per block to derive the mean perseveration frequency at each lag that was due to chance.
Table 4.
Example of a legal reshufled list for a given block of trials. Note that the reshuffling preserves the target-response pairings from the original list (e.g., cat3 -> HORSE) as well as the cyclic order of targets (all five targets presented in each cycle). In the Response columns, within-set substitutions are highlighted, perseverations with rectangles, nonperseverations with circles. For each perseveration, in each list, the corresponding lag is shown.
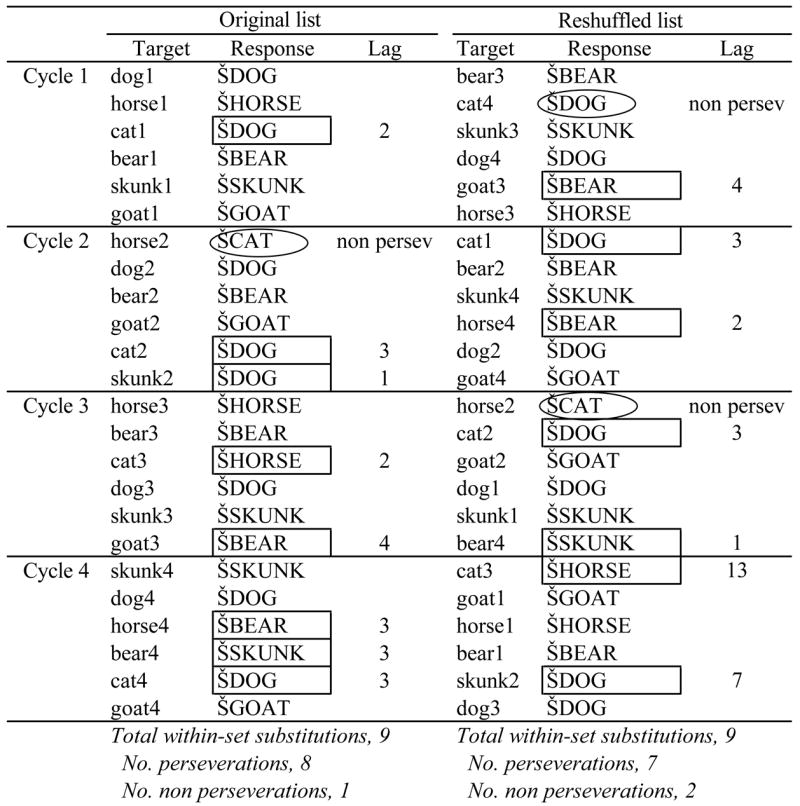
|
Total within-set substitutions, 9
Total within-set substitutions, 9
No. perseverations, 8
No. perseverations, 7
No. non perseverations, 1
No. non perseverations, 2
When the lag calculated for a particular perseveration is x, this means not only that the response in question matched an earlier response at lag x, but that it did not match any responses at shorter lags. Thus, the comparison of perseveration frequencies in the observed and chance data sets at a particular lag must take into account the differing number of within-set substitutions that have yet to be matched to an earlier response. For example, in both the observed and chance data sets, a certain proportion of within-set substitutions will match the previous response (i.e., with lag 1). The number of perseverations with lag 1 is directly comparable between the two data sets because both began with the same number of within-set substitutions. However, the resulting numbers of unmatched within-set substitutions are now different, so that the number of perseverations at the following lag (here, lag 2) in the chance data set must be adjusted as described next, so that the observed data set can be directly compared with it.
Adjusted chance frequency
For each individual’s data, we adjusted the mean within-set perseveration frequencies derived from the reshuffled data sets using Cohen and Dehaene’s (1998) procedure. Table 5 illustrates the procedure in relation to the data in Table 4. (Note that our terminology differs somewhat from what Cohen & Dehaene used in their text and their Table 1.) Consider the boxed example in Table 5: In the reshuffled list, the frequency of lag 3 perseveration was 2, and the adjustment was done by expressing this value as a proportion of the remaining errors in the reshuffled list (7) multiplied by the remaining errors in the observed data set (6). The resulting value (1.17) is the number of perseverations that would be generated by chance, given the actual number of remaining errors at this lag and the probability of generating perseverations by chance at that lag. We call this the adjusted chance frequency. For statistical analysis, we subtracted adjusted chance frequency from observed frequency to create the dependent variable, chance-corrected frequency.
Table 5.
Using the data from Table 4, this table illustrates the procedure used to adjust the reshuffled (chance) data for the differing numbers of errors remaining in the chance vs. original lists. The procedure, explained in the text, is taken from Cohen & Dehaene (1998), and the table is based on their Table 1.
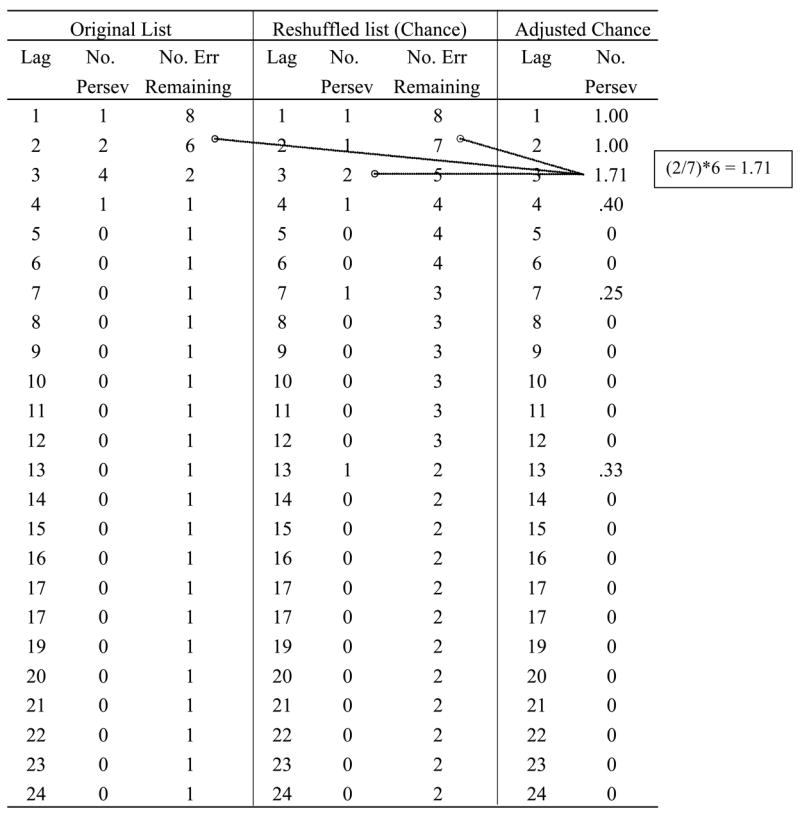
|
The methods used to estimate chance, including the re-shuffling of stimulus-response pairs, ensured that the following properties of the original data set were preserved: 1) the number and nature of errors, 2) the response vocabulary (and therefore any given subject’s bias towards producing one name over another), 3) the cyclic structure of stimulus presentation, and 4) opportunities to perseverate. There are more opportunities to perseverate at short lags than long ones for several reasons. Firstly, within each block of 24 trials, there are 24 – x trials in which it is possible to produce a perseveration with lag x. When x is high (lag is long), this value is small. Secondly, as noted earlier, in order for a response to be considered a perseveration with lag x, it must not only match the response produced x trials earlier, but must also not have matched any of the responses produced in the intervening trials. Since the probability of a response not matching any of the intervening responses is lower at longer lags, this, too, favors short-lag perseverations. This bias is further amplified by the cyclic presentation of stimuli, as the repetition of targets spaced on average six trials apart makes it even less likely that perseverations would occur at lags of more than six trials.Critically, given our method of estimating chance, all these factors should affect both the observed and chance lag frequency distributions in exactly the same manner. Any differences between them must therefore reflect a temporal bias that is present only in the actual data.
Results
A total of 366 perseverations was produced (316 in the homogeneous condition, 50 in mixed). Table 3 shows that the contributions to this total from individual participants varied considerably (1 – 75). The four highest perseveration producers (last four in the table) account for more than half the total, with NQ alone accounting for 20%. Every participant made more perseverations in the homogeneous condition than in the mixed condition. In the analysis of individual and group lag functions described below, we focus on the homogeneous-condition perseverations (i.e., semantic perseverations), collapsed across RSI levels.
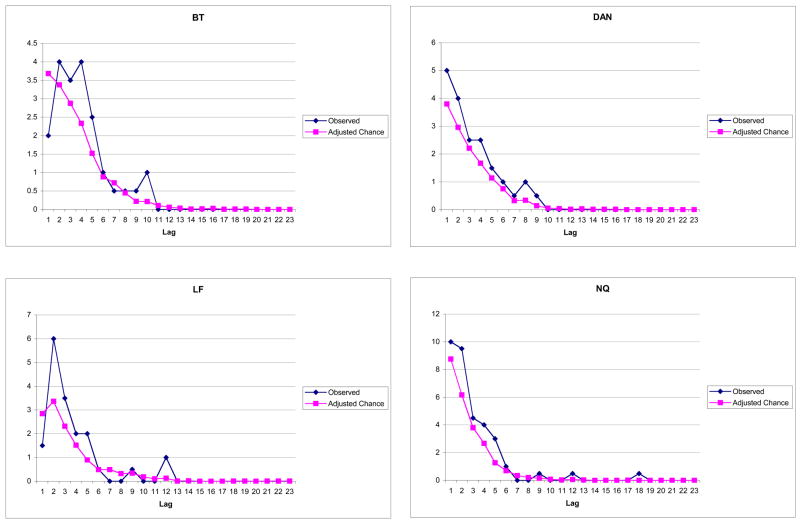
Individual lag functions for the four highest perseveration producers are shown in Figure 1. Looking first at the plots for adjusted chance, it is apparent that the frequencies are highest at short lags and decline to near zero by lag 9 or thereabouts. This confirms that our method of estimating chance did in fact preserve the differential opportunities at short versus long lags. The curves for the observed data are similarly shaped and, importantly, fall above the chance curves primarily at the shorter lags. In the case of immediate (lag 1) perseverations, there is inconsistency across the four participants: For participants DAN and NQ, the observed lag function has its peak at lag 1; for the other two, the peak is at lag 2 and the lag 1 frequency is below chance.
Figure 1.
Individual lag plots for the four highest perseveration producers, representing observed and adjusted-chance frequencies of semantic perseverations (i.e., those from the homogeneous condition), averaged across RSI 1 s and 5 s.
To explore the lag 1 position in a larger number of participants, we performed a median split on total perseverations (homogeneous and mixed condition) and examined the combined lag distributions for individuals in the top half, who together accounted for 86% of all perseverations. In five of the top 9, there were many fewer perseverations at lag 1 than at lag 2. In the remaining four, lag 1 perseverations equaled or exceeded lag 2 perseverations. In view of the widespread inconsistency at lag 1, we omitted this position from the correlational analysis of the recency bias, described next.
We began with the top four error producers, whose semantic perseveration counts were high enough to warrant statistical analysis. For each individual, we correlated lag value against chance-corrected perseveration frequencies, excluding lag 1. Computed over lags 2–23, the correlation was strongly negative for all four participants (Pearson r between -.52 and −.72; p < .05 for all). It remained strong (r between −.49 and −.82) when computed over just lags 2–9 (i.e., excluding the long lags where chance was near zero). This demonstrates that at the level of individual participants, there was a significant trend toward higher chance-corrected frequency at short lags, i.e., a recency bias.
Figure 2 plots the observed and adjusted-chance lag functions averaged across all 18 participants. At lag 1, the observed and adjusted-chance values are about the same, reflecting the averaging of above- and below-chance trends in the individual data. Thereafter, the curves diverge, with observed frequencies exceeding chance at shorter lags. In the correlation analysis, mean chance-corrected frequencies were strongly correlated with lag value for lags in the range 2–23 (r = −.62, p < .01) and 2–9 (r = −.81, p < .05), confirming that for the group, as in the individual data, shorter lags were associated with higher frequencies.
Figure 2.
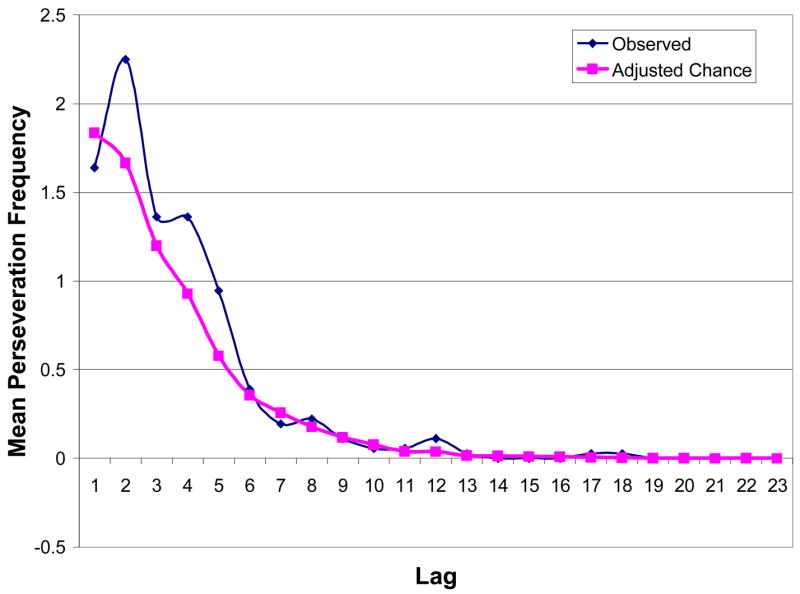
Smoothed plots of the means across all 18 participants, representing observed and adjusted-chance frequencies of semantic perseverations (i.e., those from the homogeneous condition), averaged across RSI 1 s and 5 s.
To investigate how the lag function was affected by RSI, we performed an ANOVA on the chance-corrected frequencies, using the linear mixed-model program of SPSS. Within-subjects factors of primary interest were Lag (1 through 23) and RSI (1 s, 5 s). For completeness, we included a third within-subjects factor, Condition (homogeneous, mixed).
Table 8 (left panel) shows the results of this analysis with all lags included. There was a significant effect of Lag and a significant Lag by Condition interaction. Importantly, there was no main effect of RSI and no Lag by RSI interaction. As a check on whether these results might have been overly influenced by the data from NQ, who contributed 20% of total perseverations, we redid the ANOVA with her data excluded. The results were unchanged.
Table 8.
ANOVA results using chance-corrected frequency as the dependent variable. Asterisk indicate the significant effects at the p < .05 levels.
| All Lags | Excluding Lag 1 | |||||
|---|---|---|---|---|---|---|
| df | F | df | F | |||
| Variable | Num | Den | Num | Den | ||
| Condition | 1 | 1545.838 | 3.022 | 1 | 1477.865 | 7.610* |
| RSI | 1 | 1545.838 | 0.100 | 1 | 1477.865 | 0.260 |
| Lag | 22 | 1545.838 | 4.403* | 21 | 1477.865 | 5.978* |
| Condition x RSI | 1 | 1545.838 | 0.022 | 1 | 1477.865 | 0.031 |
| Condition x Lag | 22 | 1545.838 | 1.700* | 21 | 1477.865 | 1.720* |
| RSI x Lag | 22 | 1545.838 | 0.093 | 21 | 1477.865 | 0.105 |
| Condition x RSI x Lag | 22 | 1545.838 | 0.362 | 21 | 1477.865 | 0.381 |
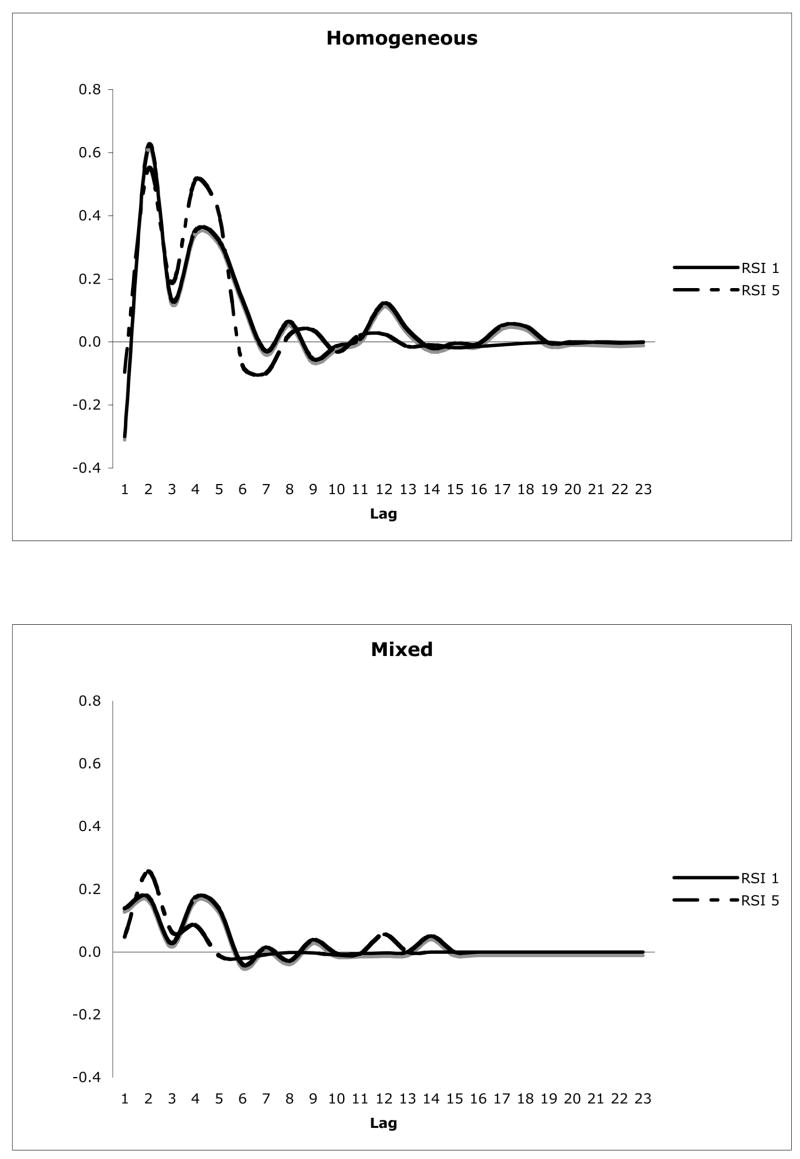
As mentioned, the ANOVA yielded a significant Lag by Condition interaction. Due to the low perseveration counts (low power) in the mixed condition, we did not further analyze this interaction with post hoc tests. Examination of the lag functions in Figure 3 raises the possibility that the interaction might reflect the dip at lag 1, which is obviously lower in the homogeneous condition. However, repeating the ANOVA with lag 1 data removed did not eliminate either the Lag by Condition interaction or the other significant effects (see Table 8, right panel). Given this, and looking again at Figure 3, a plausible interpretation of the Lag by Condition interaction is that chance-corrected frequencies are higher for homogeneous compared to mixed at short lags (2–5) but not longer ones. This speculation aside, the important feature in Figure 3 is the near overlap in the lag plots at RSI 1 and RSI 5, which goes along with the low F-value for the Lag by RSI interaction.
Figure 3.
Smoothed plot of chance-corrected perseveration frequencies, split by condition and RSI.
Discussion
Recency Bias
We analyzed the lag function for semantic perseverations generated in Schnur et al. (2006) to determine if it would exhibit decay such as was previously documented in people with aphasia on more standard naming tasks (Cohen & Dehaene, 1998). We found that immediate perseverations aside, it did, from which we conclude that the recency bias survives the semantic blocking manipulation. However, when Vitkovitch et al., (1996) induced healthy participants to produce semantic perseverations by the combination of semantic blocking and a brief naming deadline, the resulting lag function did not peak until lag 11 and chance was not exceeded until lag 4. Other perseveration-elicitation studies involving unimpaired speakers have also reported under-representation of short-lag perseverations (Campbell and Clark, 1989; Moses et al, 2004; Vitkovitch & Humphreys, 1991; Vitkovitch, Rutter & Read, 2001; see also Wheeldon & Monsell, 1994). Does this represent a fundamental difference in the nature of perseverations elicited from healthy participants versus people with aphasia?
Of possible relevance is the observation that some of the participants in the current study also produced lag 1 (immediate) perseverations with frequency at or below chance. We will consider some possible explanations for this, starting with the special properties of tasks like this one that create a predisposition for semantic perseveration by the mechanism of competitor priming.
Competitor priming paradigms are known to produce opposing facilitative and competitive (interference) effects on different time scales (Damian & Als, 2005; Wheeldon & Monsell, 1994). For example, Wheeldon and Monsell’s (1994) seminal paper on competitor priming showed that naming was slowed on the second of two related items (“whale”, following “shark”) when multiple unrelated items intervened between them but not when they occupied adjacent positions in the list. Their explanation for the interference (slowing) effect was post-naming priming of a lemma-level competitor (“shark” competing with “whale”). The absence of competitor priming with adjacent pairs was attributed to an opposing effect – facilitative priming of WHALE by SHARK at the semantic-conceptual level – which, unlike competitor priming, persists for one trial only. Extending this argument to the present context, one could say that naming “horse” on trial i of a homogeneous block would, through semantic priming, facilitate the production of a different animal name on trial j (target or homogeneous setmate), thereby reducing the probability of repeating “horse” and making a lag 1 perseveration.1 Note, however, that as adjacent items in the mixed condition would not be expected to benefit from semantic facilitation, this account has difficulty with the present evidence, which indicates a similar lag 1 dip in the Mixed condition (see Figure 3).
Another possibility is that study participants whose lag function did not dip at lag 1 were deficient in a process whose effect is normally to reduce the probability of immediate perseveration (e.g., synaptic depression: Gotts & Plaut, 2002; automatic inhibition: Arbuthnott, 1996; MacKay, 1986). One difficulty with this account is that it has nothing to say about why short lag perseverations are under-represented in unimpaired individuals. Another is that insofar as the hypothesized deficit had the effect of maintaining the response in an activated state, it predicts that the same name should be repeated over and over (Gotts et al., 2002). While such “continuous perseveration” is not uncommon in neurological patients (Sandson & Albert, 1984), it occurred rarely in the current study (Schnur et al., 2006, p. 216).
A more plausible account of the opposing trends we observed at lag 1 is that some of the study participants deliberately avoided or suppressed immediate perseverations whereas others did not. Avoidance of immediate repetition would have been an adaptive strategy in the Schnur et al. (2006) experiment, because only 2.2% of trials repeated the preceding target. It would also have been adaptive in the speeded naming study by Vitkovitch et al. (1996), where participants named animals in a non-repeating list. Widespread adoption of the avoidance strategy in that study would explain why none of the many perseverations recorded were of the immediate (lag 1) type and why perseveration frequency at lags 1, 2, and 3 did not exceed chance; the young and neurologically intact participants were presumably capable of remembering what they named across a string of trials and disinclined to repeat the recent, not just immediate, past. Although it is unlikely to explain all situations in which perseveration frequency is reduced at short lags (e.g., Arbuthnott, 1996), the avoidance hypothesis succeeds in explaining the deviations from recency in Vitkovitch et al., (1996) without invoking fundamental differences in the temporal properties of perseverations in healthy and impaired individuals.
Effect of RSI
The lag function analyses that Cohen and Dehaene (1998) conducted led them to the following conclusion: “At any processing level, the probability that an error is a perseveration from a previous trial is a decreasing function of the lag between the two trials considered. This suggests that an exponentially decaying variable, such as an internal level of activation, is responsible for the recurrence of perseverations.” While mention of “an exponentially decaying variable” invites the inference that the variable in question is sensitive to time, Cohen and Dehaene went on to note that “a specific experiment would be needed to distinguish the effects of elapsed time versus elapsed number of trials on the decay of perseveration probability.” (Cohen & Dehaene, 1998, p. 1655).
The manipulation of RSI in the current study constitutes the experiment that Cohen and Dehaene (1998) called for. If long-lag perseverations are less probable than short-lag perseverations on account of passive decay in activation that happens naturally with the passage of time, then spacing trials further apart by lengthening RSI should result in fewer perseverations overall, since that would add time for activation to decay and thereby render past items less competitive. Lengthening the RSI should also cause the lag function to fall to chance levels more quickly, yielding a steeper lag-decay function.
In partial support of these predictions, Santo Pietro and Rigrodsky (1986) obtained fewer perseverations in people with aphasia when RSI was long (RSI 10 s compared with 1 s), indicating that time is important. On the other hand, the RSI manipulation in Vitkovitch et al. (1996) (4 s vs. 7 s) had no effect: perseveration rates did not differ in the two conditions and the RSI by Lag interaction was not significant. Our findings agree with those of Vitkovitch et al. (1996): the 5-fold difference in RSI did not impact perseveration probability. In the ANOVA performed on chance-corrected perseveration frequency, neither the main effect for RSI, nor the RSI by Lag interaction came close to significance (F < 1 for both; Table 2). Graphical depiction of the recency effect showed closely overlapping functions for 5-sec and 1-sec RSI in homogeneous and mixed conditions (Figure 3).
The absence of RSI effects in our study is especially noteworthy because this null result coincides with a Cohen and Dehaene (1998) type lag-decay function. It points to the conclusion that the decay in perseveration probability across lags is not due to elapsed time but instead to the elapsed number of trials. This conclusion is reinforced by an investigation of perseverations that Gotts et al., (2002) carried out with EB, an individual with aphasia. EB performed several naming experiments that involved semantic blocking and a comparison of short (1 s) and long (10 s or 15 s) RSIs. She made numerous perseverations, which unlike the present study, did not tend to resemble the target semantically. When analyzed by lag, these unrelated perseverations showed the expected exponential decay; and the 10+-fold difference in RSI values did not affect the frequency of her perseverations or the shape of the lag function. These results add weight to the conclusion that the perseveratory impetus is stronger for recent responses not because the earlier responses are further removed in time from the current response but because those earlier responses have had more opportunity to be weakened by interference from intervening trials.
Activation Persistence in Competitor Priming
At several points, we have tied the explanation for why semantic blocking encourages semantic perseveration to the mechanism of competitor priming, which rests on the notion that a word is primed by virtue of having been named. The apparent insensitivity of the perseveration lag function to time is relevant to how one conceives of such priming in neural network terms. Specifically, such priming is unlikely to depend on internal activation levels, which are generally thought to decay quickly and spontaneously (e.g., Bock & Griffin, 2000). More likely, it depends on parameters of networks that encode long-term processing biases, for example, connection weights, activation thresholds, or resting levels (all of which would be neurally implemented through long-term synaptic changes). Connection weight changes, in particular, have been invoked to explain the persistence of competitor priming effects across time and trials (e.g., Damian & Als, 2005; Howard et al., 2006; Vitkovitch & Humphreys, 1991; Vitkovitch et al., 1996; Wheeldon & Monsell, 1994).
The study that Howard et al., (2006) conducted is instructive. Unimpaired speakers were given a sequence of 165 pictures to name. Items from the same semantic categories (“category coordinates”) were interspersed throughout the list, with a predetermined spacing that the authors refer to as “lag”; for example, when successive category coordinate targets were separated by two different-category items, the lag was 2. Lags varied from 2–8. Items did not repeat. There were two critical findings: first, with each successive category coordinate named, mean naming times slowed by about 25 ms on average; second, the size of the effect was unrelated to the lag between one category coordinate and the previous one. The authors modeled the cumulative, linear interference effect with a simple connectionist network that updated its lexical-semantic knowledge after each naming trial by strengthening the connection between the named target’s semantic representation and its name. Such updating of a network in response to experience is sometimes called “incremental learning” (see Damian and Als (2005) for related evidence of incremental learning, this time in the blocked naming paradigm.)
An Error-based Incremental Learning Account
The weight-change model that Howard et al. (2006) proposed is consistent with the null effects for RSI that we and others observed, since connection weights do not decay passively with time. However, without some modification, that model can not handle the evidence for the recency bias in semantic perseverations, which, as we argued, indicates that the perseveratory impetus is unlearned or forgotten across intervening related trials. The desired result can be achieved by a model that incrementally adjusts its weights through error-based learning, e.g., using the delta-rule. Examples of such models can be found in Dell, Oppenheim and Kittredge, 2008; Gordon and Dell (2003); and Oppenheim, Dell and Schwartz (2007).
In these models, weights from distributed semantic features to words are tuned whenever a word is produced, such that there are increases in weights from the features to the target word and decreases in weights from the features to words that are erroneously activated. So, any under-activation of the target, or activation of a competitor word, stimulates the system to tweak the weights. The production of a word i therefore primes its representation in a manner that is undiminished by time (weight changes do not passively decay) and by subsequent unrelated trials (an unrelated item is not assumed to share features with the target). This comports with the evidence that competitor priming accumulates and is undiminished by intervening unrelated trials (Damian & Als, 2005; Howard et al., 2006). Critically, though, error-based learning ensures that a subsequent related trial (word j) will lessen the perseverative impetus of word i for replacing future related targets, because i will become activated when j is the target, stimulating weight changes that decrease i’s tendency to be active on future related trials. Thus, incremental error-based learning is consistent with the observed recency effect in semantic perseverations, as well as its insensitivity to time.
A prediction from the incremental, error-based learning account is that the recency bias should be weaker for perseverations produced in the mixed condition of semantic blocked naming, relative to the homogeneous condition. In the mixed condition, targets that follow word i share fewer of its features, so their production should stimulate less unlearning of i, hence less reduction in its perseverative impetus. The ANOVA on chance-corrected perseverations did yield a significant Condition by Lag interaction in the predicted direction; but further analysis was limited by the paucity of perseverations in the mixed condition. A definitive test of the prediction that the recency effect is weaker in the mixed condition will require experiments that generate more mixed-condition perseverations to analyze.
Conclusions and Future Directions
We found that the lag function for semantic perseverations resembles the negative exponential decay curve described by Cohen and Dehaene (1998) and that the 5-fold difference in RSI did not alter the shape of the lag function. These two findings constrain the explanation of how priming operates in semantic blocked naming to make the past competitive with the present. We maintain that responses are strengthened through a process of incremental learning, affecting connection strength or resting activation, and that with the processing of successive trials, there is a degree of unlearning that accounts for the recency gradient.
It remains to be seen whether the evidence that motivates the incremental learning hypothesis of name priming – a perseveration lag function that decays and that is relatively insensitive to time – is also seen in naming tasks that do not include exotic manipulations like semantic blocking and short naming deadlines. Further research also is needed to determine whether the combination of recency bias and time-insensitivity is reliably seen in the data from individual participants with aphasia. Answering these questions will require a massive data gathering effort; with over 1000 trials per participant, the Schnur et al., (2006) study generated too few perseverations to afford adequately powered analysis of the mixed-condition perseverations or patterns of individual differences.
As Howard and colleagues demonstrated, priming by incremental learning is one of three legs on which a complete model of competitor priming rests (Howard et al., 2006). Also required is a mechanism for top-down activation sharing among related competitors (to explain relatedness effects), and a competitive selection mechanism that is slowed by the presence of primed competitors (to explain response time effects in competitor priming paradigms; see also Wheeldon & Monsell, 1994). What must one add to such a model to simulate the heightened frequency of perseverations in people with aphasia? According to one widely held view, what is needed is nothing more than to instantiate a retrieval deficit that lessens the advantage of the current target relative to primed past responses, particularly, those that are also semantic competitors (e.g., Cohen & Dehaene, 1998; Dell, Burger & Svec, 1997; Moses et al., 2004; Martin & Dell, 2007; Martin, Roach, Brecher & Lowery, 1998; Schwartz, Saffran, Bloch, & Dell, 1994). In the incremental, error-based learning model of semantic blocking developed by Oppenheim and colleagues, such a retrieval deficit is simulated by adding noise to the activations of network units (Oppenheim et al., 2007). The result is a high rate of perseveration errors generated without altering the process by which the past is primed (error-based learning) and with no recourse to inhibition or disinhibition of the primed past. It will be interesting to see whether a model constructed along these lines has adequate explanatory power to explain the totality of facts about lexical perseverations, including the yet to be explored individual differences.
Table 6.
Means and standard deviations for the Homogeneous condition.
| Observed | Chance | Adjusted Chance | Chance-Corrected | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LAG | N | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 1 | 36 | 1.64 | 3.07 | 1.84 | 2.23 | 1.84 | 2.23 | −0.20 | 1.47 |
| 2 | 36 | 2.25 | 2.60 | 1.65 | 1.72 | 1.67 | 1.62 | 0.58 | 1.42 |
| 3 | 36 | 1.36 | 1.53 | 1.31 | 1.33 | 1.20 | 1.15 | 0.16 | 0.77 |
| 4 | 36 | 1.36 | 1.57 | 1.03 | 0.99 | 0.93 | 0.85 | 0.43 | 1.02 |
| 5 | 36 | 0.94 | 1.24 | 0.72 | 0.65 | 0.58 | 0.52 | 0.36 | 0.92 |
| 6 | 36 | 0.39 | 0.60 | 0.53 | 0.50 | 0.36 | 0.39 | 0.03 | 0.48 |
| 7 | 36 | 0.19 | 0.47 | 0.38 | 0.33 | 0.26 | 0.27 | −0.06 | 0.38 |
| 8 | 36 | 0.22 | 0.54 | 0.24 | 0.20 | 0.18 | 0.19 | 0.04 | 0.50 |
| 9 | 36 | 0.11 | 0.32 | 0.16 | 0.18 | 0.12 | 0.13 | −0.01 | 0.30 |
| 10 | 36 | 0.06 | 0.23 | 0.11 | 0.10 | 0.08 | 0.10 | −0.02 | 0.22 |
| 11 | 36 | 0.06 | 0.23 | 0.05 | 0.07 | 0.04 | 0.07 | 0.02 | 0.23 |
| 12 | 36 | 0.11 | 0.40 | 0.06 | 0.07 | 0.04 | 0.07 | 0.07 | 0.36 |
| 13 | 36 | 0.03 | 0.17 | 0.03 | 0.05 | 0.02 | 0.03 | 0.01 | 0.17 |
| 14 | 36 | 0.00 | 0.00 | 0.02 | 0.02 | 0.01 | 0.03 | −0.01 | 0.03 |
| 15 | 36 | 0.00 | 0.00 | 0.01 | 0.03 | 0.01 | 0.03 | −0.01 | 0.03 |
| 16 | 36 | 0.00 | 0.00 | 0.01 | 0.02 | 0.01 | 0.02 | −0.01 | 0.02 |
| 17 | 36 | 0.03 | 0.17 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.17 |
| 18 | 36 | 0.03 | 0.17 | 0.01 | 0.02 | 0.00 | 0.01 | 0.02 | 0.17 |
| 19 | 36 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 |
| 20 | 36 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 |
| 21 | 36 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| 22 | 36 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 |
| 23 | 36 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
Note: Chance-Corrected frequency is equal to the difference between Observed and Adjusted Chance.
Table 7.
Means and standard deviations for the Mixed condition.
| Observed | Chance | Adjusted Chance | Chance Corrected | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LAG | N | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 1 | 36 | 0.36 | 0.72 | 0.27 | 0.38 | 0.27 | 0.38 | 0.09 | 0.57 |
| 2 | 36 | 0.42 | 0.73 | 0.22 | 0.33 | 0.20 | 0.35 | 0.22 | 0.55 |
| 3 | 36 | 0.17 | 0.45 | 0.18 | 0.26 | 0.12 | 0.24 | 0.05 | 0.36 |
| 4 | 36 | 0.22 | 0.49 | 0.14 | 0.20 | 0.09 | 0.19 | 0.13 | 0.40 |
| 5 | 36 | 0.11 | 0.40 | 0.12 | 0.15 | 0.05 | 0.11 | 0.06 | 0.32 |
| 6 | 36 | 0.00 | 0.00 | 0.08 | 0.11 | 0.03 | 0.09 | −0.03 | 0.09 |
| 7 | 36 | 0.03 | 0.17 | 0.06 | 0.10 | 0.02 | 0.08 | 0.00 | 0.11 |
| 8 | 36 | 0.00 | 0.00 | 0.04 | 0.06 | 0.02 | 0.04 | −0.02 | 0.04 |
| 9 | 36 | 0.03 | 0.17 | 0.03 | 0.04 | 0.01 | 0.03 | 0.02 | 0.14 |
| 10 | 36 | 0.00 | 0.00 | 0.02 | 0.03 | 0.01 | 0.02 | −0.01 | 0.02 |
| 11 | 36 | 0.00 | 0.00 | 0.01 | 0.04 | 0.00 | 0.02 | 0.00 | 0.02 |
| 12 | 36 | 0.03 | 0.17 | 0.00 | 0.01 | 0.00 | 0.01 | 0.03 | 0.17 |
| 13 | 36 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| 14 | 36 | 0.03 | 0.17 | 0.00 | 0.02 | 0.00 | 0.01 | 0.03 | 0.16 |
| 15 | 36 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| 16 | 36 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| 17 | 36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 18 | 36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 19 | 36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 20 | 36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 21 | 36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 22 | 36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 23 | 36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Note: Chance-Corrected frequency is equal to the difference between Observed and Adjusted Chance.
Acknowledgments
This research was funded by a grant from the National Institutes of Health’s National Institute for Deafness and Other Communication Disorders: R01 DC000191-26 (M.F. Schwartz). Aspects of this study were reported in a poster presentation at the Psychonomics Society meeting, November 2005, in Toronto, Canada (Lee, E.Y., Schnur, T.T, & Schwartz, M.F., “Recency of production influences semantic substitutions in blocked-cyclic naming”) and in a symposium paper at the Academy of Aphasia, October 2007, Washington DC (Lee, E.Y., Schnur, T.T., & Schwartz, M.F., “The temporal analysis of semantic perseverations in blocked-cyclic naming”, delivered by TTS). We gratefully acknowledge Adelyn Brecher’s contribution to the analysis of errors.
We wish to thank Marcus Damian for suggesting this account of the lag 1 dip for semantic perseverations.
Appendix A
A list of the 12 categories and 72 targets used in Schnur et al. (2006)
Animals: bear, cat, dog, goat, horse, skunk
Appliances: fan, iron, radio, scale, toaster, vacuum
Body Parts: arm, chin, ear, nose, thumb, toe
Clothing: coat, dress, glove, hat, skirt, sock
Food: bread, cake, cheese, pie, shrimp, soup
Furniture: bed, chair, crib, sofa, stool, table
Nature: cloud, mountain, pond, sun, volcano, waterfall
Plants: bush, cactus, fern, flower, mushroom, tree
Roles: bride, clown, judge, nun, nurse, soldier
Shapes: arrow, circle, cone, cross, heart, star
Toys: ball, bat, blocks, doll, kite, top
Utensils: cup, fork, glass, knife, pitcher, spoon
References
- Arbuthnott KD. To repeat or not to repeat: Repetition, facilitation and inhibition in sequential retrieval. Journal of Experimental Psychology: General. 1996;125:261–283. [Google Scholar]
- Bock K, Griffin ZM. The persistence of structural priming: Transient activation or implicit learning? Journal of Experimental Psychology: General. 2000;129:177–192. doi: 10.1037//0096-3445.129.2.177. [DOI] [PubMed] [Google Scholar]
- Brown AS. Inhibition in cued retrieval. Journal of Experimental Psychology: Human Learning and Memory. 1981;7:204–215. [Google Scholar]
- Campbell JID, Clark JM. Time course of error priming in number-fact retrieval: Evidence for excitatory and inhibitory mechanisms. Journal of Experimental Psychology: Learning, Memory & Cognition. 1989;15:920–929. [Google Scholar]
- Cohen L, Dehaene S. Competition between past and present - assessment and interpretation of verbal perseverations. Brain. 1998;121:1641–1659. doi: 10.1093/brain/121.9.1641. [DOI] [PubMed] [Google Scholar]
- Damian MF, Als LC. Long-lasting semantic context effects in the spoken production of object names. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:1372–1384. doi: 10.1037/0278-7393.31.6.1372. [DOI] [PubMed] [Google Scholar]
- Damian MF, Vigliocco G, Levelt WJM. Effects of semantic context in the naming of pictures and words. Cognition. 2001;81:B77–B86. doi: 10.1016/s0010-0277(01)00135-4. [DOI] [PubMed] [Google Scholar]
- Dell GS, Burger LK, Svec WR. Language production and serial order: A functional analysis and a model. Psychological Review. 1997a;104:123–147. doi: 10.1037/0033-295x.104.1.123. [DOI] [PubMed] [Google Scholar]
- Dell GS, Oppenheim GM, Kittredge AK. Saying the right word at the right time: Syntagmatic and paradigmatic interference in sentence production. Language and Cognitive Processes. 2008;23:583–608. doi: 10.1080/01690960801920735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell GS, Schwartz MF, Martin N, Saffran EM, Gagnon DA. Lexical access in aphasic and nonaphasic speakers. Psychological Review. 1997b;104:801–838. doi: 10.1037/0033-295x.104.4.801. [DOI] [PubMed] [Google Scholar]
- Gordon JK, Dell GS. Learning to divide the labor: An account of deficits in light and heavy verb production. Cognitive Science. 2003;27:1–40. [PubMed] [Google Scholar]
- Gotts SJ, della Rocchetta AI, Cipolotti L. Mechanisms underlying perseveration in aphasia: Evidence from a single case study. Neuropsychologia. 2002;40:1930–1947. doi: 10.1016/s0028-3932(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Plaut DC. The impact of synaptic depression following brain damage: A connectionist account of “access/refractory” and “degraded store” semantic impairments. Cognitive, Affective, & Behavioral Neuroscience. 2002;2:187–213. doi: 10.3758/cabn.2.3.187. [DOI] [PubMed] [Google Scholar]
- Howard D, Nickels L, Coltheart M, Cole-Virtue J. Cumulative semantic inhibition in picture naming: Experimental and computational studies. Cognition. 2006;100:464–482. doi: 10.1016/j.cognition.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and palm trees: A test of semantic access from pictures and words. Bury St. Edmunds, U.K: Thames Valley Test Company; 1992. [Google Scholar]
- Humphreys GW, Riddoch J, Quinlan PT. Cascade processes in picture identification. Cognitive Neuropsychology. 1988;5:67–103. [Google Scholar]
- Kertesz A. Western Aphasia Battery. New York: Grune & Stratton; 1982. [Google Scholar]
- Kroll JF, Stewart E. Category interference in translation and picture naming: Evidence for asymmetric connections between bilingual memory representations. Journal of Memory and Language. 1994;33:149–174. [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22:1–75. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- MacKay DG. Self-inhibition and the disruptive effects of internal and external feedback in skilled behavior. In: Heuer H, Fromm C, editors. Generation and modulation of action patterns. Berlin: Springer-Verlag; 1986. pp. 174–186. [Google Scholar]
- Martin N, Dell GS. Common mechanisms underlying perseverative and non-perseverative sound and word substitutions. Aphasiology. 2007;21:1002–1017. [Google Scholar]
- Martin N, Roach A, Brecher A, Lowery J. Lexical retrieval mechanisms underlying whole-word perseveration errors in anomic aphasia. Aphasiology. 1998;12:319–333. [Google Scholar]
- McCarthy RA, Kartsounis LD. Wobbly words: Refractory anomia with preserved semantics. Neurocase. 2000;6:487–497. [Google Scholar]
- Moses MS, Nickels LA, Sheard C. I’m sitting here feeling aphasic!” - a study of recurrent perseverative errors elicited in unimpaired speakers. Brain and Language. 2004;89:157–173. doi: 10.1016/S0093-934X(03)00364-X. [DOI] [PubMed] [Google Scholar]
- Oppenheim GM, Dell GS, Schwartz MF. Cumulative semantic interference as learning. Brain and Language. 2007;103:175–176. Long abstract. [Google Scholar]
- Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia Naming Test: Scoring and rationale. Clinical Aphasiology. 1996;24:121–133. [Google Scholar]
- Robinson G, Shallice T, Cipolotti L. A failure of high level verbal response selection in progressive dynamic aphasia. Cognitive Neuropsychology. 2005;22:661–694. doi: 10.1080/02643290442000239. [DOI] [PubMed] [Google Scholar]
- Saffran EM, Berndt RS, Schwartz MF. The quantitative analysis of agrammatic production: Procedure and data. Brain and Language. 1989;37:440–479. doi: 10.1016/0093-934x(89)90030-8. [DOI] [PubMed] [Google Scholar]
- Sandson J, Albert ML. Varieties of perseveration. Neuropsychologia. 1984;22:715–732. doi: 10.1016/0028-3932(84)90098-8. [DOI] [PubMed] [Google Scholar]
- Santo Pietro MJ, Rigrodsky S. Patterns of oral-verbal perseveration in adult aphasics. Brain and Language. 1986;29:1–17. doi: 10.1016/0093-934x(86)90030-1. [DOI] [PubMed] [Google Scholar]
- Schnur TT, Lee E, Coslett HB, Schwartz MF, Thompson-Schill SL. When lexical selection gets tough, the LIFG gets going: a lesion analysis study of interference during word production. Brain and Language. 2005;95:12–13. [Google Scholar]
- Schnur TT, Schwartz MF, Brecher A, Hodgson C. Semantic interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language. 2006;54:199–227. [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. doi: 10.1073/pnas.0805874106. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Saffran EM, Bloch DE, Dell GS. Disordered speech production in aphasic and normal speakers. Brain and Language. 1994;47:52–88. doi: 10.1006/brln.1994.1042. [DOI] [PubMed] [Google Scholar]
- Vitkovitch M, Humphreys GW. Perseverant responding in speeded naming of pictures - its in the links. Journal of Experimental Psychology-Learning Memory and Cognition. 1991;17:664–680. [Google Scholar]
- Vitkovitch M, Kirby A, Tyrrell L. Patterns of excitation and inhibition in picture naming. Visual Cognition. 1996;3:61–80. [Google Scholar]
- Vitkovitch M, Rutter C, Read A. Inhibitory effects during object name retrieval: The effect of interval between prime and target on picture naming responses. British Journal of Psychology. 2001;92:483–506. [PubMed] [Google Scholar]
- Wheeldon LR, Monsell S. Inhibition of spoken word production by priming a semantic competitor. Journal of Memory and Language. 1994;33:332–356. [Google Scholar]
- Wilshire CE, McCarthy RA. Evidence for a context-sensitive word retrieval disorder in a case of nonfluent aphasia. Cognitive Neuropsychology. 2002;19:165–186. doi: 10.1080/02643290143000169. [DOI] [PubMed] [Google Scholar]