Abstract
The neural network controlling breathing exhibits plasticity in response to environmental or physiological challenges. For example, while hypoxia initiates rapid and robust increases in respiratory motor output to defend against hypoxemia, it also triggers persistent changes, or plasticity, in chemosensory neurons and integrative pathways that transmit brainstem respiratory activity to respiratory motor neurons. Frequently studied models of hypoxia-induced respiratory plasticity include: 1) carotid chemosensory plasticity and metaplasticity induced by chronic intermittent hypoxia (CIH), and 2) acute intermittent hypoxia (AIH) induced phrenic long-term facilitation (pLTF) in naïve and CIH preconditioned rats. These forms of plasticity share some mechanistic elements, although they differ in anatomical location and the requirement for CIH preconditioning. Both forms of plasticity require serotonin receptor activation and formation of reactive oxygen species (ROS). While the cellular sources and targets of ROS are not well known, recent evidence suggests that ROS modify the balance of protein phosphatase and kinase activities, shifting the balance towards net phosphorylation and favoring cellular reactions that induce and/or maintain plasticity. Here, we review possible sources of ROS, and the impact of ROS on phosphorylation events relevant to respiratory plasticity.
Keywords: ROS, kinase, phosphatase, serotonin, phrenic, long-term facilitation, carotid body, NADPH oxidase
1. Introduction
An important property of the respiratory control system is its capacity to adapt in face of adverse environmental or physiological conditions. Indeed, some stimuli are capable of inducing respiratory plasticity (Mitchell and Johnson, 2003), a long-lasting change in respiratory behavior after the proximate stimulus is no longer present. Two well-studied forms of respiratory plasticity are induced by intermittent hypoxia, including carotid chemosensory plasticity following chronic intermittent hypoxia (CIH; Peng et al., 2003) and long-term facilitation (LTF) of respiratory motor output following acute intermittent hypoxia (AIH; Mahamed and Mitchell, 2007). In brief, chemosensory plasticity following CIH is expressed as increased basal discharge, hypoxic sensitivity and the capacity to express sensory long-term facilitation (Peng et al. 2003). In contrast, LTF in phrenic nerve activity is a central neural (spinal) mechanism expressed as a progressive and sustained increase in phrenic motor output that is independent of changes in chemoafferent input (Mitchell et al., 2001). These forms of plasticity occur at distinct anatomical sites in different time-domains, and differ in their requirement for pretreatment with chronic intermittent hypoxia (CIH). For example, AIH induces carotid chemosensory facilitation only in rats preconditioned with CIH, whereas LTF of phrenic and hypoglossal (XII) motor output is elicited in naïve as well as CIH pretreated rats (Ling et al., 2001).
On the other hand, both forms of plasticity share similarities, such as the requirement for episodic hypoxia (i.e. phasic hypoxia/re-oxygenation; Mitchell et al., 2001; Prabhakar et al, 2007a) and serotonin receptor activation (Feldman et al., 2003; Lovett-Barr et al., 2006; Peng et al., 2006). Hypoxic pattern-sensitivity may relate, at least in part, to greater formation of reactive oxygen species (ROS) during and/or following intermittent versus sustained hypoxia. Indeed, ROS formation is necessary for both carotid chemosensory plasticity induced by CIH (Peng et al., 2003; Prabhakar et al., 2007b) and AIH-induced phrenic LTF (pLTF) (MacFarlane and Mitchell, 2007a; MacFarlane et al., 2007). A major goal of this review is to compare and contrast potential roles of ROS in these forms of respiratory plasticity, and to consider some of the relevant cellular processes modulated by ROS. New evidence will be presented suggesting that one major role of ROS in AIH-induced pLTF is through its inhibitory actions on serine-threonine protein phosphatases, releasing a constraint to cell signaling cascades that underlie pLTF (Wilkerson et al., 2007, 2008).
2. Two forms of respiratory plasticity induced by intermittent hypoxia
2.1. CIH-induced carotid chemosensory plasticity
The first documented example of ROS-dependence in respiratory plasticity was carotid chemosensory plasticity elicited by CIH (Peng et al., 2003). CIH-induced carotid chemosensory plasticity has been observed experimentally in mice (Peng et al., 2006), cats (Rey et al., 2004) and rats (Peng et al., 2003). After 10 consecutive days of intense poikilocapnic CIH, carotid chemoafferent activity expresses three distinct indicators of plasticity or metaplasticity: 1) basal carotid body discharge is increased in normoxia; 2) the carotid chemosensory response to acute hypoxia is augmented (hypoxic sensory response, HSR; Fig. 1); and 3) chemosensory long-term facilitation is observed following AIH (i.e. an expression of metaplasticity as defined by (Mitchell and Johnson, 2003, Fig. 1). These effects are not seen in naïve rats that were not pretreated with CIH (Peng et al., 2003; Peng and Prabhakar 2004). The carotid chemosensory response to hypercapnia is unaffected by CIH, suggesting that it selectively enhances hypoxic versus hypercapnic carotid chemosensitivity (Peng and Prabhakar 2004). Overall, CIH-induced changes in carotid chemosensory function augment chemoafferent synaptic inputs to the central nervous system, thereby increasing resting breathing and the magnitude of the hypoxic ventilatory response.
Figure 1.
Carotid chemosensory plasticity in rats exposed to poikilocapnic chronic intermittent hypoxia (CIH). A: Carotid chemoafferent neuron activity (in vivo) during an acute intermittent hypoxia (AIH) protocol in rats pretreated with CIH (10 days; closed symbols). AIH responses in untreated rats are shown for comparison (open symbols). The magnitude of the carotid chemosensory response (HSR) to AIH is enhanced in CIH-treated rats compared to untreated rats; further, the sustained increase in baseline activity post-AIH (sensory facilitation) is observed only in CIH pre-treated rats (Note, values are normalized to baseline carotid discharge frequency (i.e. pre-AIH) and, thus, the figure does not illustrate enhanced basal discharge (ie. prior to AIH). B: Daily intraperitoneal injections of the SOD mimetic, MnTMPyP (5mg/kg/day), attenuated the carotid HSR and abolished post-AIH sensory facilitation in CIH pre-treated rats. Modified from Peng et al., (2003). Copyright © 1993-2008 by The National Academy of Sciences of the United States of America, all rights reserved.
The pattern, duration and severity of CIH influence carotid chemosensory plasticity; the most frequently studied protocol consists of daily exposure (up to 10 consecutive days) to hypoxic episodes (5% O2; 15 seconds at the nadir) at 5 minute intervals. Since the hypoxic episodes are repeated 9 times per hour for 8 hours/day, the cumulative duration of hypoxia during each hour is approximately 2 minutes. This CIH protocol has been suggested to mimic important aspects of recurrent apneas experienced during sleep disordered breathing. Carotid chemosensory plasticity induced by CIH is pattern sensitive (particularly metaplasticity in sensory LTF) since sustained hypoxia of equivalent cumulative duration does not induce similar plasticity or metaplasticity (Peng and Prabhakar, 2004). The duration of CIH exposure affects the magnitude of carotid chemosensory plasticity since sensory LTF was not observed after 1 day of CIH, was present after 3 days and was maximal after 10 days (Peng et al., 2003).
Little is known concerning cell signaling mechanisms of CIH-induced carotid chemosensory plasticity. Carotid sensory long-term facilitation can be elicited by episodic serotonin receptor activation by a PKC-dependent mechanism (Peng et al., 2006). Phosphorylation of p47phox, a sub-unit of the ROS generating enzyme, NADPH oxidase, was also increased following episodic serotonin receptor activation, suggesting a serotonergic stimulus for ROS formation which may account for CIH-induced chemosensory plasticity. It is not currently known if episodic serotonin receptor activation is necessary for CIH-induced carotid chemosensory plasticity, although bath applied serotonin prolonged the return of chemosensory activity to baseline levels post-hypoxia in ex vivo carotid bodies (Jacono et al., 2005). Further work is necessary to understand detailed cellular mechanisms of carotid chemosensory plasticity, although the role of ROS will be discussed further below.
2.2. AIH-induced phrenic long-term facilitation
AIH elicits pLTF (Fig. 2) (Mahamed and Mitchell, 2007), and its magnitude is enhanced in rats pretreated with CIH (Ling et al., 2001). Since pLTF was first demonstrated following episodic stimulation of the cut, carotid sinus nerve in cats (Millhorn et al., 1980a,b; Millhorn and Eldridge, 1986) and rats (Hayashi et al., 1993; Ling et al., 1997), at least a portion of pLTF results from central neural mechanisms that do not require persistent increases in carotid chemoafferent activity. Further, AIH-induced pLTF is reduced, but not eliminated, by carotid denervation (Bavis and Mitchell 2003), suggesting a role of additional mechanisms not associated with carotid chemosensory function. Thus, AIH is a physiologically relevant stimulus that elicits CNS plasticity and is not specifically dependent upon changes in chemoafferent neuron activity, nor on CIH.
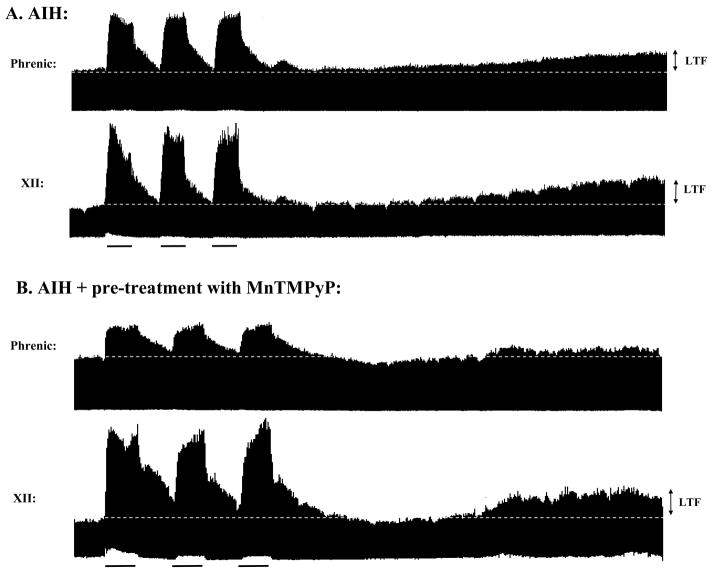
Figure 2.
AIH-induced phrenic and XII long-term facilitation (LTF) in anesthetized rats. A: Isocapnic AIH induced phrenic and XII LTF, expressed as a progressive and sustained increase in integrated phrenic and XII motor activity for at least 60 minutes post-AIH. AIH consisted of 3, 5 minute episodes of 10% O2, each separated by 5 minutes return to baseline O2 (~50% O2); isocapnia was maintained during the hypoxic episodes. B: Phrenic (but not XII) LTF is blocked in rats pre-treated with an intrathecal injection of the SOD mimetic, MnTMPyP (5.5mM, 15μl volume, ~20 minutes prior to AIH), suggesting ROS exert their effects on phrenic LTF within the region of the phrenic motor nucleus (MacFarlane and Mitchell, 2007a).
pLTF is pattern sensitive since it is elicited by AIH, but not a similar cumulative duration of sustained hypoxia (Baker and Mitchell, 2000). pLTF is commonly elicited with three, five minute hypoxic episodes (~10–12% inspired O2), separated by 5 minute intervals (Bach and Mitchell, 1996; Baker and Mitchell, 2000). However, pLTF is also induced by three-minute hypoxic episodes (Baker and Mitchell, 2000), repetitive 15 second hypoxic episodes (Peng and Prabhakar, 2003), or three to six ventilator apneas of 25 seconds duration (Mahamed and Mitchell, 2008). In meta-analyses of large data sets from our laboratory, pLTF is insensitive to the severity of hypoxic episodes, at least between 28 and nearly 70 mmHg (Fuller et al., 2000; Baker-Herman and Mitchell, 2008). Thus, available evidence indicates that repetitive hypoxia/re-oxygenation, and not the severity or duration of hypoxia, is a more critical determinant of pLTF.
Our working model of AIH-induced pLTF has been summarized in recent reviews (Mitchell et al., 2001; Feldman et al., 2003; Mahamed and Mitchell, 2007; Mitchell, 2007). Each hypoxic episode activates medullary raphe neurons and triggers serotonin release near phrenic motor neurons in the cervical spinal cord (Erickson and Millhorn, 1994; Kinkead and Mitchell, 1999). We postulate that subsequent activation of 5-HT2 receptors on phrenic motor neurons initiates intracellular signaling cascades giving rise to pLTF (Fuller et al., 2001; Baker-Herman and Mitchell, 2002). 5-HT2 receptors activate phospholipase C-gamma as well as protein kinase C (PKC). PKC activity appears to be necessary for AIH-induced pLTF (McGuire and Ling, 2004). Downstream signaling mechanisms include new protein synthesis, particularly brain derived neurotrophic factor (BDNF; Baker-Herman et al., 2004), and activation of the high affinity BDNF receptor, TrkB. We speculate that TrkB activation phosphorylates and activates MAP kinases (ERK 1 and 2) and protein kinase B (i.e. Akt) (Mahamed and Mitchell, 2007; Wilkerson et al., 2007). These kinases may phosphorylate glutamate receptor subunits, increasing glutamate receptor currents and/or increasing post-synaptic glutamate receptor densities via trafficking and membrane insertion (Fuller et al., 2000; Mahamed and Mitchell, 2007). Increased glutamatergic synaptic transmission during pLTF is consistent with observations that NMDA receptors are necessary to maintain AIH-induced pLTF in anesthetized rats (McGuire et al., 2005), and that episodic serotonin or alpha-1 adrenergic receptor activation induces XII LTF in rhythmogenic brainstem slices by increasing AMPA receptor currents in XII motor neurons (Bocchiaro and Feldman, 2004; Neverova et al., 2007). Increased post-synaptic glutamate receptor function presumably increases synaptic strength between brainstem respiratory pre-motor neurons and phrenic motor neurons, resulting in pLTF.
A prominent role of serine/threonine phosphatases in constraining pLTF has been demonstrated (Wilkerson et al., 2007; 2008). Specifically, after inhibiting okadaic acid sensitive serine/threonine phosphatases in the cervical spinal cord, pLTF was revealed even after sustained hypoxia (25 minutes duration), a stimulus not able to elicit pLTF (Wilkerson et al., 2008). On the other hand, intrathecal okadaic acid had no effect on AIH-induced pLTF, demonstrating that the phosphatase constraint is somehow relieved during AIH (Wilkerson et al., 2008). Thus, the balance of kinase/phosphatase activation is a critical regulator of pLTF following acute intermittent versus sustained hypoxia (Wilkerson et al., 2007). Protein phosphatase inhibition with okadaic acid increases inspiratory drive currents in Pre-Bötzinger inspiratory neurons and hypoglossal motor neurons (Ge and Feldman, 1998), confirming the relevance of phosphatases in important classes of respiratory neurons.
Although less well investigated in the context of respiratory plasticity, protein kinases exert powerful modulatory influences on respiratory neurons. For example, both PKA and PKC modulate respiratory drive currents in medullary respiratory neurons, including expiratory neurons in the caudal medulla (Lalley et al., 1997; Richter et al., 1997), inspiratory neurons in the Pre-Bötzinger complex (Shao et al., 2003), and hypoglossal motor neurons (Saywell and Feldman 2004; Feldman et al., 2005). The respective roles of protein kinases and phosphatases in pLTF regulation are of considerable interest, largely because of their sensitivity to oxidation by ROS.
3. Types and Sources of ROS
Most cells generate ROS, and the balance of formation and removal via enzymatic activity or antioxidants establishes constitutive ROS levels (Schafer and Buettner, 2001). Different cell types exhibit inherently different capacities to generate ROS. Here we describe ROS types and their most common sources, some of which have been implicated in respiratory plasticity.
3.1. Types of ROS
ROS molecules and the reactions that form them are summarized in Figure 3. Superoxide anions (O2−•) are the most common ROS, but can be readily converted to other types by enzymatic (e.g. superoxide dismutase) and non-enzymatic reactions (Valko et al., 2005, 2007). O2−• is formed by metabolic processes (especially in mitochondria) or by enzymatic reactions (e.g. NADPH oxidase). O2−• is catalyzed to form hydrogen peroxide (H2O2) by multiple isoforms of superoxide dismutase (SOD; Valko et al., 2007). Hydroxyl radicals (•OH) are formed from the breakdown of H2O2 by transition metals such as iron (Fe2+) or copper (Cu+) through Fenton reactions; this is a particularly reactive form of ROS with a short half life. •OH, in turn, can form from O2−• and H2O2 via the Haber-Weiss reaction (Valko et al., 2007).
Figure 3.
Schematic representing chemical reactions leading to ROS formation. When an electron is added to O2, superoxide anion (O2−•) is formed; superoxide dismutase (SOD; types 1, 2 and 3) catalyzes the conversion of O2−• to hydrogen peroxide (H2O2). In the presence of catalase or glutathione peroxidase, H2O2 is subsequently converted to H2O and molecular O2. In the Fenton reaction, iron (Fe2+) converts H2O2 to hydroxyl radicals (•OH); •OH can also be formed from O2−• by the Haber-Weiss reaction. Nitric oxide (NO•) combines with O2−• to form peroxynitrite (ONOO−), a highly reactive molecule. ROS levels depend on multiple factors, including their rates of production, availability of their precursors (e.g. O2−• is a precursor for H2O2), and breakdown (antioxidant availability). Simplified from Valko et al., 2007.
Reactive nitrogen species (RNS), such as nitric oxide (NO•), are of considerable importance when considering the biological impact of ROS. NO• is formed under the influence of nitric oxide synthase (Valko et al., 2007). NO• is more stable (>15s) at low O2 levels and can combine with superoxide to form peroxynitrite (ONOO−), a potent oxidizer responsible for lipid peroxidation and DNA fragmentation (Carr et al., 2000).
Despite the potential of both ROS and RNS to elicit cell damage, low levels of these molecular species are involved in normal cell signaling mechanisms (Valko et al., 2007). Thus, complex regulatory mechanisms exist to maintain both ROS and RNS at homeostatic levels, including active control of both synthesis and removal as appropriate to optimize biological function.
3.2. Cellular sources of ROS
Mitochondria
In addition to their role as primary providers of ATP, mitochondria are a prominent source of ROS (Cadenas and Sies, 1998). Approximately 1–3% of the electrons that pass along the electron transport chain “leak” to molecular O2, primarily at complex I and III, with lesser contributions at complex IV (Cadenas and Sies, 1998). Mitochondrial ROS formation has been implicated in CIH-induced carotid chemosensory plasticity (Peng et al., 2003).
NADPH oxidase
The NADPH oxidase complex is a prominent source of ROS formation in both phagocytic and non-phagocytic cells. In phagocytic cells, NADPH oxidase generates the “respiratory burst” of O2−• upon activation in an inflammatory environment (Keisari et al., 1983) or in response to environmental pathogens (Linsley and Ledbetter, 1993). However, there is also increasing awareness of the essential role played by NADPH oxidase in the regulation of normal cellular functions of non-phagocytic cells (Thannickal and Fanburg, 1995). NADPH oxidase is regulated differently in phagocytic versus non-phagocytic cells; for example, non-phagocytic NADPH oxidase exhibits constitutive activity whereas phagocytic isoforms are normally quiescent, and require activation by a pathogenic or inflammatory stimulus (Valko et al., 2007).
The mechanism of activation and subsequent ROS formation by NADPH oxidase is complex. Multiple membrane-bound (e.g. gp91phox and p22phox) and cytosolic (p20phox, p47phox, p67phox, RAC1 and RAC2) sub-units are involved, but the relative contribution of each sub-unit in phagocytic versus non-phagocytic cells is not fully understood. Generally, when the cytosolic subunits translocate to the membrane and bind with gp91phox, an electron is passed from NADPH to molecular O2, thus forming the superoxide anion (Valko et al., 2007). Different gp91phox isoforms exist (termed NOX), which vary considerably among cell types. NADPH oxidase subunits (gp91phox, rac, and p47phox) have been identified in neurons of the cervical spinal cord (MacFarlane, et al., 2007) and brain (Tejada-Simon et al., 2005). The NADPH oxidase isoforms required for neuronal ROS formation are largely unknown at this time.
Xanthine/xanthine oxidase (X/XO)
The enzymatic formation of ROS from X/XO occurs largely within the cardiovascular system and is a prominent factor in ischemia/reperfusion injury (Valko et al., 2007). During hypoxia/ischemia, rapid ATP consumption in face of limited O2 availability leads to an accumulation in hypoxanthine and xanthine. Upon re-oxygenation, the surge of available O2 allows xanthine and hypoxanthine to be metabolized by xanthine oxidase, thereby generating O2−• and H2O2 (Granger et al., 2001).
4. Regulation of ROS Formation
While a number of biological processes generate ROS, mechanisms responsible for regulating their formation are complex. Intermittent hypoxia and re-oxygenation represent distinct stimuli that initiate ROS formation from different cellular sources. Thus, both hypoxia and re-oxygenation may be important regulators of ROS-dependent respiratory neuroplasticity under different circumstances.
4.1. Hypoxia and re-oxygenation
Both hypoxia (Dirnagl et al., 1995; Fabian et al., 1995) and re-oxygenation (Fabian et al., 2004) are distinct stimuli capable of stimulating ROS formation in brain tissue. Hypoxia stimulates ROS formation from mitochondria and xanthine oxidase in the cortex, whereas re-oxygenation induces NADPH oxidase-derived ROS formation (Abramov et al., 2007). The NADPH oxidase complex responds differentially to hypoxia versus re-oxygenation in different cell types. For example, whereas re-oxygenation triggers increased ROS formation via NADPH oxidase activity in vascular cells, hypoxia per se is a potent NADPH oxidase activator in carotid bodies (see Dinger et al., 2007).
Although single bouts of hypoxia or re-oxygenation increase ROS formation, repetitive hypoxia/re-oxygenation events amplify this effect (Xu et al., 2004; Yuan et al., 2004; Masaoka et al., 2005; Zhan et al., 2005). Greater ROS formation during and/or following repeated hypoxia and re-oxygenation may contribute to the pattern-sensitivity of hypoxia-induced respiratory plasticity (see below).
4.2. Membrane Bound Receptors
Activation of many membrane-bound receptors increases ROS formation. For example, cytokine and growth factor receptors increase ROS formation in non-phagocytic cells, including epidermal growth factor, platelet-derived growth factor and vascular endothelial growth factor receptors (Neufeld et al., 1999). Similarly, activation of the high affinity BDNF receptor (TrkB) increases extracellular ROS formation via the NADPH oxidase complex, an observation of particular importance given the critical role of BDNF in pLTF (Baker-Herman et al., 2004). Although TrkB induced ROS formation has been associated with BDNF-induced cell death (Kim et al., 2002), more moderate TrkB receptor activation may generate lower levels of ROS and promote plasticity. ROS may also influence endogenous release of growth/trophic factors; for example, intermittent hypoxia increases BDNF secretion in differentiated PC12 cells by a ROS-dependent mechanism (Wang et al., 2006).
Neurotransmitter receptor activation may also trigger increased ROS formation. For example, glutamate receptor activation increases O2−• production in hippocampal slices (Bindokas et al., 1996). Furthermore, serotonin receptor (5-HT2A) activation induces ROS formation via NADPH oxidase in renal mesangial cells (Grewal et al., 1999). Serotonin receptor activation also increases NAPDH oxidase-derived ROS formation in ex vivo carotid bodies without changes in oxygenation (Peng et al., 2006). Thus, serotonin receptors regulate NADPH oxidase activity and ROS formation in cells known to play a role in ventilatory control. Overall, multiple receptor types, many of which are critical for respiratory plasticity, also regulate cellular ROS formation.
5. Role of ROS in neuroplasticity
NADPH oxidase-derived ROS are necessary for hippocampal long-term potentiation, a prominent model of activity-dependent synaptic plasticity thought to underlie learning and memory (Otani et al. 1993; Klann and Thiels, 1999; Klann et al. 1998; Knapp and Klann, 2002; Kishida and Klann, 2007). Although intermittent hypoxia protocols inducing carotid chemosensory plasticity and pLTF differ considerably in the number of hypoxic episodes, both stimulus protocols involve repetitive bouts of hypoxia followed by re-oxygenation. Although we do not yet have a full understanding of the respective roles of hypoxia, re-oxygenation or receptor activation in generating the ROS relevant to respiratory plasticity, we are beginning to gain insights.
5.1. CIH-induced carotid chemosensory plasticity
CIH-induced chemosensory plasticity is blocked by daily treatment with the SOD mimetic, MnTMPyP (Fig 1B; Peng et al., 2003), demonstrating a role of ROS (superoxide) in its underlying mechanism. In agreement, CIH decreases aconitase activity, an inverse indicator of O2−• formation (Peng et al., 2003). Since CIH reduces mitochondrial complex I (but not complex III) activity, mitochondria may be an important source of relevant ROS in CIH-induced chemosensory plasticity (Peng et al., 2003). More recently, episodic serotonin receptor (5-HT2A) activation was shown to elicit carotid chemosensory plasticity similar to CIH-induced sensory facilitation (Peng et al., 2006) by a PKC-dependent increase in NADPH oxidase activity. Further work is necessary to determine if NADPH oxidase-derived ROS are necessary for induction of CIH-induced carotid chemosensory plasticity.
5.2. AIH-induced pLTF
Since intravenous MnTMPyP also blocks AIH-induced pLTF, ROS formation is necessary for its induction and/or maintenance (MacFarlane and Mitchell, 2007a). Intravenous MnTMPyP injections also block XII LTF, indicating similar ROS-dependence (Fig. 2). Localized cervical injections of MnTMPyP block pLTF without affecting XII LTF (Fig. 2B), demonstrating that the relevant ROS for pLTF are localized to cervical spinal regions associated with the phrenic motor nucleus. Indeed, presumptive phrenic motor neurons labeled with the superoxide indicator, dihydroethidium, exhibit greater fluorescence following AIH versus normoxia or sustained hypoxia (MacFarlane, Satriotomo and Mitchell, unpublished observations).
When NADPH oxidase inhibitors such as apocynin or DPI are applied to the cervical spinal cord, AIH-induced pLTF is attenuated in a dose-dependent manner without affecting XII LTF (MacFarlane et al., 2007b). Consistent with these functional observations, presumptive phrenic motor neurons (large NeuN-positive neurons in the C4 ventral horn) express gp91phox and p47 phox (MacFarlane et al., 2007). Thus, formation of adequate cervical spinal ROS to enable AIH-induced pLTF requires NADPH oxidase activity.
Spinal serotonin receptor activation is sufficient to induce phrenic and intercostal LTF in in vitro brainstem spinal cord preparations (Lovett-Barr et al., 2006). Similarly, episodic serotonin injections (3 × 5μl injections, 5 min intervals) into the intrathecal space of the cervical spinal cord elicit phrenic (but not XII) motor facilitation in anesthetized adult rats, an effect remarkably similar to AIH-induced pLTF (MacFarlane and Mitchell 2007b). Since serotonin-induced phrenic motor facilitation is blocked by intrathecal application of NADPH oxidase inhibitors, we suggest that serotonin receptor activation during AIH induces NADPH oxidase activity in the phrenic motor nucleus, thereby contributing to pLTF. Unfortunately, the experimental approaches used to date do not distinguish between a permissive role for constitutive NADPH oxidase activity versus NADPH oxidase activation per se.
6. Are protein kinases and phosphatases key ROS targets in respiratory plasticity?
6.1. ROS regulate the kinase/phosphatase balance
ROS modulate critical cellular functions, particularly processes dependent on redox sensitive molecules including protein kinases and phosphatases. Protein kinases and phosphatases are exquisitely sensitive to oxidation by ROS (for reviews see Klann and Thiels, 1999; Kishida and Klann, 2007). Since the balance of kinase and phosphatase activities determines the net phosphorylation state of proteins (Halliwell and Gutteridge, 1984), the capacity of ROS to differentially modulate kinase and phosphatase activities confers a unique ability to regulate critical cellular functions (Barford, 2001; Cohen, 2002).
6.2. ROS inhibit okadaic acid sensitive phosphatases relevant to AIH-induced pLTF
O2−• and H2O2 alter the activity of protein phosphatases, including protein tyrosine phosphatase and multiple okadaic acid sensitive serine/threonine protein phosphatases (Klann and Thiels, 1999). Our working hypothesis is that ROS formation via the NADPH oxidase complex inhibits okadaic acid-sensitive protein phosphatases and releases an important constraint on the cellular mechanism of pLTF.
Evidence that at least some forms of ROS regulate potentially relevant okadaic acid-sensitive protein phosphatases in the cervical spinal cord is presented in Figure 4. We found that, in tissue homogenates from the ventral cervical spinal cord, administration of a stable hydrogen peroxide analogue (tert-butyl H2O2) inhibited protein phosphatase 2A activity (Fig. 4). Further work is necessary to determine if other ROS species have a similar impact on this or other okadaic acid-sensitive phosphatases.
Figure 4.
Tert-Butyl H2O2, a stable form of hydrogen peroxide, applied to tissue homogenates of the ventral cervical spinal cord containing the phrenic motor nucleus reduces the activity of protein phosphatase 2A (PP2A, Wilkerson and Mitchell, unpublished data). These data confirm that at least one form of ROS inhibits PP2A in spinal regions involved in pLTF. PP2A is a serine/threonine protein phosphatase thought to constrain phrenic LTF (Wilkerson et al., 2007). Tissues were collected, processed and analyzed for phosphatase activity as described in Wilkerson et al. 2008. Briefly, cytosolic fraction samples (n=8) were stripped of free phosphates, then treated in vitro with 1 μL of 70% TBHP. PP2A activity was then measured as the picomoles of phosphate generated from a provided phosphopeptide substrate per minute and normalized to total protein within the sample; data are presented as the mean ± SEM of the change in PP2A activity as a percentage of PP2A activity relative to untreated samples from the same animal. *denotes significant difference from basal (i.e. untreated) levels of PP2A activity (one-sample t-test, p<0.05).
Additional evidence that ROS-inhibition of okadaic acid-sensitive protein phosphatases plays a critical role in AIH-induced pLTF is presented in Figure 5. In urethane-anesthetized rats pre-treated with the SOD mimetic MnTMPyP (10mg/kg. i.v.), phrenic and XII LTF were both abolished as reported previously (MacFarlane and Mitchell, 2007a). However, when rats were pre-treated with both intravenous MnTMPyP and intrathecal okadaic acid (C4; 25nM; 10μl volume), phrenic (but not XII) LTF was restored (Fig. 5). Thus, ROS generated during and/or following AIH enable pLTF, at least in part, by inhibiting okadaic acid-sensitive protein phosphatases. By inhibiting relevant serine/threonine phosphatases, their constraint on pLTF was removed, thereby revealing pLTF following AIH, even in the presence of the SOD mimetic. ROS formation may be critical to enable many forms of respiratory plasticity, inhibiting key protein phosphatases and shifting the kinase/phosphatase balance. The implied constitutive constraint on spinal respiratory plasticity may be an important feature in a neural system where constitutive activity-dependent plasticity may not be appropriate (Mitchell and Johnson, 2003). Thus, serine/threonine protein phosphatases may represent a “gate keeper,” maintaining a constraint on plasticity until necessary conditions are satisfied (ROS formation), and the phosphatase inhibition is released (Wilkerson et al., 2007; 2008).
Figure 5.
ROS may be necessary for pLTF because they inhibit okadaic-acid sensitive protein phosphatases. Intravenous MnTMPyP blocks phrenic (left panel) and XII (right panel) LTF in anesthetized rats confirming our previous report that both phrenic and XII LTF require ROS formation (MacFarlane and Mitchell, 2007). Also reported previously, an intrathecal injection of MnTMPyP (5.5mM; 15μl) blocked phrenic, but not XII LTF, demonstrating that ROS exert their effects on phrenic LTF near the phrenic motor nucleus (MacFarlane and Mitchell, 2007). However, when phrenic and XII LTF are both blocked by an intravenous injection of MnTMPyP (10mg/kg), phrenic (but not XII) LTF is restored by a simultaneous cervical spinal injection of the serine/threonine phosphatase inhibitor, okadaic acid (25nM, 10μl, i.t.; original data; for experimental details see MacFarlane and Mitchell, 2007 and Wilkerson et al., 2008). These data strongly suggest that ROS exert their effects on phrenic LTF, at least in part, via regulation of okadaic acid-sensitive protein phosphatases in the cervical spinal cord. *significant difference from baseline; #significant difference from AIH-treated rats (p<0.05; Two-way repeated measures ANOVA).
6.3. ROS activate protein kinases relevant to AIH-induced pLTF
Although we know little concerning the possible role of protein kinases in ROS-dependence of respiratory plasticity, ROS are capable of increasing protein kinase activities through amino acid oxidation, particularly of cysteine and tyrosine residues (Droge, 2002; Spickett et al., 2006). For example, O2−• regulates PKC activity by increasing co-factor dependent and autonomous PKC activity, largely due to thiol oxidation of a cysteine-rich region outside the catalytic domain (Knapp and Klann, 2000). On the other hand, the impact of ROS on PKC activity is highly sensitive to the duration of ROS exposure (see below). While ROS can directly modify protein kinases such as PKC via direct oxidation of amino acid residues, ROS may also modulate kinase activity indirectly through ROS-dependent inhibition of protein phosphatases.
7. ROS, phosphatases and pattern sensitivity of pLTF
The hypoxic pattern-sensitivity of pLTF may be due to differential ROS formation and subsequent phosphatase inhibition during or following hypoxia/re-oxygenation. For example, AIH exposes an animal to multiple re-oxygenation events whereas sustained hypoxia creates a single re-oxygenation event at the end of the exposure. Since re-oxygenation per se activates NADPH oxidase and increases superoxide formation (Abramov et al., 2007), intermittent hypoxia may activate NADPH oxidase more than sustained hypoxia. As a result, sustained hypoxia may not generate sufficient ROS to relieve the inhibitory constraint of the protein phosphatases.
8. ROS Regulation: costs versus benefits
Given that ROS have both deleterious and beneficial effects on biological functions, it is not surprising that ROS formation is subject to homeostatic regulation within limits consistent with maintaining critical cellular functions and (at the same time) minimizing cellular damage. Deviations from homeostatic levels may shift the balance in phosphorylation-dependent cellular processes, leading to pathological consequences. For example, ROS exert dose- and time-dependent effects on hippocampal synaptic plasticity through their effects on kinases and phosphatases. Whereas brief exposures to low concentrations of H2O2 oxidize PKC at the regulatory domain, increasing autonomous PKC activity (Whisler et al. 1995), longer exposures selectively oxidize the catalytic domain and inactivate the kinase (Gopalakrishna and Anderson, 1989). Hydroxyl radicals also inactivate PKC (Gopalakrishna and Anderson, 1989). Thus, the duration and oxidative strength of ROS both determine the site of oxidation on PKC and, ultimately, whether the net result is activation versus inactivation.
The impact of ROS on cellular processes depends on: 1) the type of ROS; 2) availability of antioxidants; 3) levels and duration of exposure, and 4) the proximity of the ROS to their cellular targets. Thus, differences in any of these factors could explain why some forms of ROS-dependent respiratory plasticity exhibit time/dose dependence (Peng et al., 2003), whereas others do not (Mahamed and Mitchell, 2008). While ROS formation is necessary for important forms of respiratory plasticity induced by intermittent hypoxia (Prabhakar, 2001; Wilkerson et al., 2007), excessive ROS formation elicits cardiorespiratory pathology (i.e. oxidative “stress”) such as systemic hypertension (Fletcher et al., 1992; Kumar et al., 2006; for review, see Prabhakar, 2001), increased sympathetic nerve activity (Greenberg et al., 1999) and elevated circulating catecholamines (Bao et al., 1997). Oxidative stress associated with CIH also causes hippocampal cell death and learning deficits, particularly in young animals (Gozal and O’Brien, 2004). On the other hand, less severe protocols of CIH, such as repetitive acute intermittent hypoxia, have the potential to elicit respiratory plasticity without adverse consequences attendant to more severe protocols of CIH (Mahamed and Mitchell, 2007; Mitchell, 2007). The concept of titrating intermittent hypoxia exposures to maximize beneficial effects, while minimizing pathological consequences, may have important therapeutic implications for certain ventilatory control disorders, such as respiratory insufficiency following cervical spinal injury (Mitchell, 2007).
9. Summary and Conclusions
Although ROS are mediators of “oxidative stress,” there is increasing evidence that they play an important role in normal cellular processes that are critical for life. In the context of this review, ROS formation is necessary for important forms of respiratory plasticity, particularly those induced by intermittent hypoxia. Whereas CIH induces ROS-dependent carotid chemosensory plasticity and cardiovascular pathology, AIH elicits ROS-dependent spinal respiratory plasticity (i.e. pLTF) without any evidence for pathology. Although distinct anatomically and functionally, these forms of plasticity share similarities in their underlying mechanisms. Both require: 1) intermittent (versus sustained) hypoxia; 2) serotonin receptor activation; and 3) ROS formation via NADPH oxidase activity. The greater number of hypoxia/re-oxygenation events associated with AIH (versus sustained hypoxia) could be an important component to the pattern-sensitivity in both forms of plasticity, presumably by differential NADPH oxidase activity and ROS formation. Since ROS regulate the balance of kinase/phosphatase activities, they may shift the balance in favor of kinase activation and net phosphorylation of key proteins involved in pLTF. ROS effects on phosphatases may relieve its constraint on the cellular mechanism of pLTF, thereby enabling plasticity and regulating the central neural drive to breathe.
Considerable work is necessary to further our understanding of the mechanisms whereby ROS formation regulates respiratory plasticity. A greater understanding of these mechanisms may advance our understanding that ROS are regulated in a homeostatic manner, similarly to homeostatic regulation of, for example, carbon dioxide. Indeed, appropriate regulation of ROS formation is important to many critical cellular functions, and may play a key regulatory role in mediating adaptive responses to environmental or physiological challenges.
Acknowledgments
Work presented herein has been funded in part by the following grants awarded to G.S. Mitchell: NIH HL80209 AND HL69064.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27(5):1129–38. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104(2–3):251–60. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529(Pt 1):215–9. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22(14):6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol. 2008 doi: 10.1016/j.resp.2008.03.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7(1):48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol. 1997;83(1):95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- Barford D. The mechanism of protein kinase regulation by protein phosphatases. Biochem Soc Trans. 2001;29(Pt 4):385–391. doi: 10.1042/bst0290385. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol. 2003;94(1):399–409. doi: 10.1152/japplphysiol.00374.2002. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Jordán J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16(4):1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA. 2004;101(12):4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E, Sies H. The lag phase. Free Radic Res. 1998;28(6):601–609. doi: 10.3109/10715769809065816. [DOI] [PubMed] [Google Scholar]
- Carr A, McCall MR, Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species-reaction pathways and antioxidant protection. Arterioscl Thromb Vasc Biol. 2000;20:1716–1723. doi: 10.1161/01.atv.20.7.1716. [DOI] [PubMed] [Google Scholar]
- Cohen P. The origins of protein phosphorylation. Nat Cell Biol. 2002;4:E127–130. doi: 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- Dinger B, He L, Chen J, Liu X, Gonzalez C, Obeso A, Sanders K, Hoidal J, Stensaas L, Fidone S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol Neurobiol. 2007;157(1):45–54. doi: 10.1016/j.resp.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Lindauer U, Them A, Schreiber S, Pfister HW, Koedel U, Reszka R, Freyer D, Villringer A. Global cerebral ischemia in the rat: online monitoring of oxygen free radical production using chemiluminescence in vivo. J Cereb Blood Flow Metab. 1995;15(6):929–940. doi: 10.1038/jcbfm.1995.118. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348(2):161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Fabian RH, DeWitt DS, Kent TA. In vivo detection of superoxide anion production by the brain using a cytochrome c electrode. J Cereb Blood Flow Metab. 1995;15(2):242–247. doi: 10.1038/jcbfm.1995.30. [DOI] [PubMed] [Google Scholar]
- Fabian RH, Perez-Polo RJ, Kent TA. Extracellular superoxide concentration increases following cerebral hypoxia but does not affect cerebral blood flow. Int J Devl Neurosci. 2004;22:225–230. doi: 10.1016/j.ijdevneu.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Neverova NV, Saywell SA. Modulation of hypoglossal motoneuron excitability by intracellular signal transduction cascades. Respir Physiol Neurobiol. 2005;147:131–143. doi: 10.1016/j.resp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Qian W, Miller CC, III, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic nerve output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Selected contribution: phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Ge Q, Feldman JL. AMPA receptor activation and phosphatase inhibition affect neonatal rat respiratory rhythm generation. J Physiol. 1998;509(Pt 1):255–266. doi: 10.1111/j.1469-7793.1998.255bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci USA. 1989;86(17):6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, O’Brien LM. Snoring and obstructive sleep apnoea in children: why should we treat? Paediatr Respir Rev. 2004;5(Suppl A):S371–376. doi: 10.1016/s1526-0542(04)90066-8. [DOI] [PubMed] [Google Scholar]
- Granger DN, Stokes KY, Shigematsu T, Cerwinka WH, Tailor A, Krieglstein CF. Splanchnic ischaemia-reperfusion injury: Mechanistic insights provided by mutant mice. Acta Physiol Scand. 2001;173:83–91. doi: 10.1046/j.1365-201X.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol. 1999;86:298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- Grewal JS, Mukhin YV, Garnovskaya MN, Raymond JR, Greene EL. Serotonin 5-HT2A receptor induces TGF-beta1 expression in mesangial cells via ERK: proliferative and fibrotic signals. Am J Physiol. 1999;276(6 Pt 2):F922–930. doi: 10.1152/ajprenal.1999.276.6.F922. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Free radicals, lipid peroxidation, and cell damage. Lancet. 1984;2(8411):1095. doi: 10.1016/s0140-6736(84)91530-7. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol. 1993;265:R811–819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Jacono FJ, Peng YJ, Kumar GK, Prabhakar NR. Modulation of the hypoxic sensory response of the carotid body by 5-hydroxytryptamine: role of the 5-HT2 receptor. Respir Physiol Neurobiol. 2005;145(2–3):135–42. doi: 10.1016/j.resp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Keisari Y, Braun L, Flescher E. The oxidative burst and related phenomena in mouse macrophages elicited by different sterile inflammatory stimuli. Immunobiol. 1983;165:78–89. doi: 10.1016/S0171-2985(83)80048-5. [DOI] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Sohn S, Kwon HJ, Lee JY, Park JH, Gwag BJ. Brain-derived neurotrophic factor can act as a pronecrotic factor through transcriptional and translational activation of NADPH oxidase. J Cell Biol. 2002;159(5):821–831. doi: 10.1083/jcb.200112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am J Physiol. 1999;277(3 Pt 2):R658–666. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antiox Redox Signal. 2007;9(2):233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:359–376. doi: 10.1016/s0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem. 2000;275(31):24136–24145. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J. Neurosci Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- Klann E, Roberson ED, Knapp LT, Sweatt JD. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J Biol Chem. 1998;273(8):4516–4522. doi: 10.1074/jbc.273.8.4516. [DOI] [PubMed] [Google Scholar]
- Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575(Pt 1):229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Pierrefiche O, Bischoff AM, Richter DW. cAMP-dependent protein kinase modulates expiratory neurons in vivo. J Neurophysiol. 1997;77:1119–1131. doi: 10.1152/jn.1997.77.3.1119. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Integrated phrenic responses to carotid afferent stimulation in adult rats following perinatal hyperoxia. J Physiol. 1997;500(Pt 3):787–796. doi: 10.1113/jphysiol.1997.sp022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21(14):5381–8. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Ann Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Mitchell GS, Satriotomo I, Johnson SM. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neurosci. 2006;142(3):885–892. doi: 10.1016/j.neuroscience.2006.06.036. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neurosci. 2007a doi: 10.1016/j.neuroscience.2007.12.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Serotonin-induced phrenic long-term facilitation requires reactive oxygen species signaling via the NADPH oxidase complex. Abstract, Society for Neuroscience 2007b [Google Scholar]
- MacFarlane PM, Satriotomo I, Mitchell GS. Reactive oxygen species generated by NADPH oxidase are necessary for phrenic long-term facilitation following acute intermittent hypoxia. Experimental Biology meeting abstract 2007 [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp. Physiol. 2007;92(1):27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Simulated apneas induce serotonin dependent respiratory long term facilitation in rats. J Physiol. 2008 doi: 10.1113/jphysiol.2007.149047. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka N, Nakajima Y, Hayakawa Y, Ohgame S, Hamano S, Nagaishi M, Yamamoto T. Transplacental effects of allopurinol on suppression of oxygen free radical production in chronically instrumented fetal lamb brains during intermittent umbilical cord occlusion. J Matern Fetal Neonatal Med. 2005;18(1):1–7. doi: 10.1080/14767050500127716. [DOI] [PubMed] [Google Scholar]
- McGuire M, Ling L. Activation of protein kinase C near/in phrenic motoneurons is required for phrenic Long-term facilitation in rats. Am J Respir Crit Care Med (abstr suppl) 2004;169(7):A433. [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Phrenic long-term facilitation requires NMDA receptors in the phrenic motonucleus in rats. J Physiol. 2005;567(Pt 2):599–611. doi: 10.1113/jphysiol.2005.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL. Role of ventrolateral medulla in regulation of respiratory and cardiovascular systems. J Appl Physiol. 1986;61(4):1249–1263. doi: 10.1152/jappl.1986.61.4.1249. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980a;42:171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980b;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94(1):358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Gaultier C, editor. Genetic Basis for Respiratory Control Disorders. New York: Springer Publishing Company; 2007. pp. 291–311. [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22. [PubMed] [Google Scholar]
- Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of alpha1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci. 2007;27(16):4435–4442. doi: 10.1523/JNEUROSCI.2803-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Ben-Ari Y, Roisin-Lallemand M-P. Metabotropic receptor stimulation coupled to weak tetanus leads to long-term potentiation and a rapid elevation of cytosolic protein kinase C activity. Brain Res. 1993;613:1–9. doi: 10.1016/0006-8993(93)90446-t. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol. 2003;94(6):2342–2349. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96(3):1236–1242. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body of intermittent hypoxia: implications for recurrent apneas. PNAS. 2003;100(17):10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol. 2004;97(5):2020–2025. doi: 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol. 2006;576(1):289–295. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol. 2001;90(5):1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol. 2007a;92(1):39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antiox Redox Signal. 2007b;9(9):1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Lalley PM, Pierrefiche O, Haji A, Bischoff AM, Wilken B, Hanefeld F. Intracellular signal pathways controlling respiratory neurons. Respir Physiol. 1997;110:113–123. doi: 10.1016/s0034-5687(97)00077-7. [DOI] [PubMed] [Google Scholar]
- Saywell SA, Feldman JL. Dynamic interactions of excitatory and inhibitory inputs in hypoglossal motoneurones: respiratory phasing and modulation by PKA. J Physiol. 2004;554:879–889. doi: 10.1113/jphysiol.2003.054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Shao XM, Ge Q, Feldman JL. Modulation of AMPA receptors by cAMP-dependent protein kinase in preBotzinger complex inspiratory neurons regulates respiratory rhythm in the rat. J Physiol. 2003;547:543–553. doi: 10.1113/jphysiol.2002.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spickett CM, Pitt AR, Morrice N, Kolch W. Proteomic analysis of phosphorylation, oxidation and nitrosylation in signal transduction. Biochem Biophys Acta. 2006;1764:1823–1841. doi: 10.1016/j.bbapap.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29(1):97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270(51):303343033–8. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wang H, Yuan G, Prabhakar NR, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from PC12 cells in response to oxidative stress requires autocrine dopamine signaling. J Neurochem. 2006;96(3):694–705. doi: 10.1111/j.1471-4159.2005.03572.x. [DOI] [PubMed] [Google Scholar]
- Whisler RL, Goyette MA, Grants IS, Newhouse YG. Sublethal levels of oxidant stress stimulate multiple serine/threonine kinases and suppress protein phosphatases in Jurkat T cells. Arch Biochem Biophys. 1995;319:23–35. doi: 10.1006/abbi.1995.1263. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci. 2007;28(11):2949–2958. doi: 10.1523/JNEUROSCI.5539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JER, Baker-Herman TL, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation following sustained hypoxia. Abstract Experimental Biology. 2008 doi: 10.1523/JNEUROSCI.5539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neurosci. 2004;126(2):313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK, Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol. 2004;557(3):773–783. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, Veasey SC. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 2005;172(7):921–929. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]