Abstract
Background
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) shows widespread hypometabolism even in temporal lobe epilepsy (TLE) patients with mesial temporal foci. 18F-trans-4-fluoro-N-2-[4-(2-methoxyphenyl) piperazin-1-yl]ethyl-N-(2-pyridyl)cyclohexanecarboxamide (18F-FCWAY) PET may show more specific 5-HT1A receptor binding reduction in seizure initiation than propagation regions. 18FCWAY PET might be valuable for detecting epileptic foci, and distinguishing mesial from lateral temporal foci in MRI negative TLE patients.
Methods
We performed 18F-FCWAY-PET and 18F-FDG-PET in 12 MRI negative TLE patients who had had either surgery or subdural electrode recording, and 15 healthy volunteers. After partial volume correction for brain atrophy, free fraction-corrected volume of distribution (V/f1) measurement and asymmetry indices (AIs) were computed. We compared 18F-FCWAY-PET and 18F-FDG-PET results with scalp video electroencephalography (EEG), invasive EEG and surgical outcome.
Results
Mean 18F-FCWAY V/f1, compared with normal controls, was decreased significantly in fusiform gyrus, hippocampus and parahippocampus ipsilateral to epileptic foci, and AIs significantly greater in hippocampus, parahippocampus, fusiform gyrus, amygdala and inferior temporal regions. Eleven patients had clearly lateralized epileptogenic zones. Nine had congruent, and two non-lateralized, 18F-FCWAY PET. One patient with bitemporal seizure onset had non-lateralized 18F-FCWAY-PET. 18FFDG-PET showed congruent hypometabolism in 7/11 EEG-lateralized patients, bilateral hypometabolic regions in one, contralateral hypometabolism in one, as well as lateralized hypometabolism in the patient with bitemporal subdural seizure onset. Patients with mesial temporal foci tended to have lower superior and mid temporal 18F-FCWAY V/f1 binding AI than those with lateral or diffuse foci.
Conclusion
18F-FCWAY-PET can detect reduced binding in patients with normal MRI, and may be more accurate than 18F-FDG-PET.
Keywords: Epilepsy, Positron Emission Tomography, Serotonin Receptors, Temporal Lobe, Glucose Metabolism
Introduction
Thirty to forty percent of medically refractory temporal lobe epilepsy (TLE) patients have no evidence of hippocampal sclerosis or other focal lesions on brain magnetic resonance imaging (MRI) (Cascino et al 1991, Carne et al 2004). For these patients, non-invasive pre-surgical identification of seizure foci is particularly challenging. Reported hypometabolism on 18F-FDG-PET in MRI-negative TLE varies widely (Swartz et al 2002, Willmann et al 2007).
Moreover, previous studies have shown that 18F-FDG-PET may not differentiate mesial from lateral neocortical temporal lobe foci even if non-invasive lateralization is possible (Uijl et al 2007, Willmann et al 2007). In these patients, invasive evaluation with depth and subdural electrodes may be needed, increasing the risk, discomfort, and cost of presurgical evaluation. In order to evaluate a new imaging modality in MRI-negative TLE, we performed PET with 18F-trans-4-fluoro-N-2-[4-(2-methoxyphenyl) piperazin-1-yl]ethyl-N-(2-pyridyl) cyclohexanecarboxamide (18F-FCWAY), a selective 5HT1A receptor antagonist.
Serotoninergic neurons have cell bodies in the midbrain dorsal raphe nuclei, projecting to limbic system and neocortex. 5HT1A receptor concentration is highest in limbic regions, especially the CA1 segment of the hippocampus, moderate in neocortex, and lowest in cerebellum (Barnes and Sharp 1989). 5HT1A receptor activation leads to CA1 membrane hyperpolarization and reduces seizure activity in several partial seizure animal models (Barnes and Sharp 1989, Gasbarri et al 1989).
Several previous studies have reported 5HT1A receptor binding is reduced ipsilateral to temporal lobe seizure foci (Toczek et al 2003, Savic et al 2004, Giovacchini et al 2005). The degree of reduction was greatest in the seizure onset zone, and less marked in regions of seizure spread (Merlet et al 2004a). These data suggest that 18F-FCWAY-PET might be valuable for delineating mesial temporal foci, and distinguishing them from lateral temporal neocortical foci in MRI negative TLE patients.
Methods
We studied 12 patients (4 women; mean age ± SD, 30 ± 9 years) with medically refractory TLE, who were referred to the Clinical Epilepsy Section, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH). Four patients had been included in our previous study (Giovacchini et al 2005). All patients underwent prolonged surface ictal video-EEG recording and either temporal lobe resection (nine) or subdural invasive monitoring (ten). Epilepsy duration was 12 ± 7 years. Medications at the time of scan included carbamazepine, oxcarbazepine, lamotrigine, levetiracetam, phenytoin, and valproic acid. Three patients were taking a single drug; the others were taking two drugs. We also studied 15 normal healthy volunteers (10 men; 5 women; mean age ± SD, 37 ± 8 years), screened with history, general physical examination and routine laboratory tests. Ten of the volunteers were included in our previous studies (Toczek et al 2003, Giovacchini et al 2005). The study was approved by the National Institutes of Health Institutional Review Board and the Radiation Safety Committee, under 45 US Code of Federal Regulations part 46.
18F-FCWAY-PET was performed on a General Electric Advance Tomograph (GE Healthcare), with full width at half maximum [FWHM] resolution 6–7 mm, scanning 35 simultaneous slices with 4.25-mm slice separation. Ten millicuries of 18F-FCWAYwere injected over 60 seconds and dynamic frames (1–5 min) acquired in 3D mode for 120 minutes. Thirty radial arterial blood samples were taken to quantify 18F-FCWAY concentration and selected samples used to measure the 18F-fluorocyclohexanecarboxylic acid metabolite (18F-FC) (Giovacchini et al 2005). Unbound 18F-FCWAY fraction plasma protein was measured with ultracentrifugation (Giovacchini et al 2005). All patients underwent 18F-FDG-PET for CMRglu measurement within 2 days of 18F-FCWAY-PET scans. None had experienced seizures for at least two days before PET studies, and patients were observed carefully during scans to exclude ictal activity. CMRglu parametric images were co-registered to MR images. 18F-FDG-PET was not performed on normal volunteers due to radiation dosimetry restrictions. However, control FDG data were available from previous NIH studies (Theodore et al 2001).
1.5-T GE Signa MRI with fluid attenuated inversion recovery (FLAIR), spoiled grass (SPGR), , , T1 and T2-weighted imagines were performed on all patients and controls. All MRIs were read by neuroradiologists who were unaware of ictal EEG and PET results. Anatomical regions of interest (ROI) were drawn on each patient's -co-registered T1, 3D-SPGR MRI, while 18F-FCWAY radioactivity frames were registered to MRI. Brain tissue activity frames were corrected for brain acid metabolite 18F-FC uptake, vascular radioactivity, and 18F-fluoride metabolite spillover from skull. A previously described MRI-based partial volume correction (PVC) algorithm was applied to correct for brain atrophy (Giovacchini et al 2005).
We used V/f1 (where V is receptor volume of distribution, and f1 is the 18F-FCWAY plasma free fraction.) as the outcome measure, rather than binding potential:
For tracers, such as 18F-FCWAY, with low non-specific binding, VCerebell / f1 is very low, and V/f1 very close to BP. Moreover, it has the advantage of obviating potential cerebellar measurement inaccuracies related to spill-over of [18F]fluoride activity, or cerebellar atrophy due to epilepsy or AEDs with consequent binding heterogeneity (Carson et al 2003, Parsey et al 2005).
Asymmetry Indices (AIs) were computed as 200·(I −C)/(I + C) within amygdala, hippocampus, fusiform gyrus, parahippocampus, insula and superior, middle and inferior temporal lobes, where I and C represent PET values in regions ipsilateral and contralateral to the seizure focus, respectively. A more negative number indicates greater 5HT1A binding (on 18F-FCWAY-PET) or CMRglc (on FDG-PET) reduction ipsilateral to the epileptic focus. In control subjects, the right side was arbitrarily called ipsilateral. Two-sample T-tests were used for 18F-FCWAY binding potential and Asymmetry Index comparison between patient and normal controls. For the single subject analysis, mean ±2 SD AI of 18F-FCWAY and 18F-FDG in each ROI in normal controls was used to define the cut-off point for abnormal binding potentials in patients.
Results
All patients had MRIs that showed no evidence of increased signal or structural atrophy. Seven patients had a left, six a right, and one bitemporal epileptogenic zones, confirmed by scalp ictal video EEG, subdural, or intraoperative recordings.
The 18F-FCWAY plasma free fraction (f1) was higher in TLE patients than in healthy controls (0.13±0.04 vs. 0.09± 0.07, respectively, P < 0.10). Mean 18F-FCWAY V/f1, compared with normal controls, was decreased significantly in fusiform gyrus, hippocampus and parahippocampus ipsilateral to epileptic foci, with trends for ipsilateral insula, contralateral insula, and contralateral hippocampus (Table 1). AIs were significantly greater in hippocampus, parahippocampus, fusiform gyrus, amygdala and inferior temporal regions (table 2).
Table 1.
Free-fraction-corrected FCWAY Volume of distribution in healthy controls and patients with TLE
| Region | Controls (n=15) | Patients (n=11)# | p | ||||
|---|---|---|---|---|---|---|---|
| I. frontal | 69.0 | ± | 23.7 | 62.7 | ± | 14.0 | 0.45 |
| C. frontal | 68.3 | ± | 22.0 | 63.0 | ± | 15.1 | 0.50 |
| I. insular | 85.2 | ± | 39.7 | 64.1 | ± | 16.9 | 0.08 |
| C. insular | 87.1 | ± | 38.3 | 68.6 | ± | 15.7 | 0.11 |
| I. hippocampus | 116.2 | ± | 43.1 | 81.3 | ± | 23.3 | 0.02* |
| C. hippocampus | 116.5 | ± | 43.7 | 90.5 | ± | 25.6 | 0.07 |
| I. amygdala | 70.8 | ± | 26.4 | 58.8 | ± | 24.9 | 0.25 |
| C. amygdala | 73.7 | ± | 26.9 | 69.0 | ± | 23.9 | 0.64 |
| I. parahippocampus | 125.8 | ± | 56.2 | 86.7 | ± | 17.2 | 0.02* |
| C. parahippocampus | 125.6 | ± | 58.0 | 99.6 | ± | 21.5 | 0.13 |
| I. superior temporal | 81.1 | ± | 35.8 | 71.7 | ± | 19.3 | 0.40 |
| C. superior temporal | 81.4 | ± | 30.6 | 80.2 | ± | 14.3 | 0.89 |
| I. middle temporal | 88.5 | ± | 36.8 | 77.3 | ± | 22.4 | 0.38 |
| C. middle temporal | 87.8 | ± | 31.1 | 88.1 | ± | 17.3 | 0.98 |
| I. inferior temporal | 95.5 | ± | 36.7 | 81.0 | ± | 24.7 | 0.27 |
| C. inferior temporal | 98.0 | ± | 32.7 | 94.8 | ± | 25.1 | 0.79 |
| I. fusiform | 115.1 | ± | 47.1 | 82.2 | ± | 22.0 | 0.03* |
| C. fusiform | 112.9 | ± | 43.0 | 101.3 | ± | 22.2 | 0.38 |
patient 12 was excluded
I.=ipsilateral to seizure focus (arbitrarily right for controls).
C.=contralateral to seizure focus.
P<0.05, unpaired t-test, patients vs. controls.
Table 2.
18F-FCWAY volume of distribution Asymmetry Index (AI)
| Region | Controls (n=15) | Patients (n=11) | p | ||||
|---|---|---|---|---|---|---|---|
| Frontal | |||||||
| insular | 0.04 | ± | 0.06 | 0.08 | ± | 0.10 | 0.23 |
| hippocampus | 0.00 | ± | 0.11 | 0.11 | ± | 0.11 | 0.02* |
| amygdala | 0.04 | ± | 0.11 | 0.18 | ± | 0.17 | 0.03* |
| parahippocampus | 0.01 | ± | 0.10 | 0.14 | ± | 0.12 | 0.01* |
| superior temporal | 0.03 | ± | 0.14 | 0.13 | ± | 0.20 | 0.22 |
| middle temporal | 0.02 | ± | 0.11 | 0.12 | ± | 0.22 | 0.21 |
| inferior temporal | 0.04 | ± | 0.15 | 0.16 | ± | 0.14 | 0.05* |
| fusiform | 0.01 | ± | 0.13 | 0.22 | ± | 0.15 | 0.01* |
p <0.05 unpaired T-test
Eleven patients had clearly lateralized epileptogenic zones. Nine had congruent lateralized, and two non-lateralized, 18F-FCWAY-PET, defined by an AI greater than two standard deviations beyond the control mean (table 3) (figure 1). One patient with bitemporal seizure onset on subdural electrode recording had non-lateralized 18F-FCWAY-PET. 18F-FDG-PET showed hypometabolism ipsilateral to the epileptogenic zone in seven of the eleven EEG-lateralized patients (including one with negative 18F-FCWAY-PET), equivocal findings in one (#2) contralateral hypometabolism in one (patient #4), and lateralized hypometabolism in the patient (#12) with bitemporal subdural seizure onset‥
table 3.
| Patient | Age | Sex | Epilepsy duration | Regions with FCWAY Vd Δ | Regions with CMRglc Δ | Predominant Subdural focus | Pathology | Surgery outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | M | 29 | R ST ↓ | R Amyg, supT midT ↓, | Right Subtemporal and lateral T | HS | 1 postoperative seizure |
| 2 | 22 | M | 7 | R Ins, amyg ↓ | R infT ↓; L supT ↓ | Right mesial T | cortical dysplasia | Seizure-free |
| 3 | 31 | M | 12 | R Ins, parahipp, midT ↓ | R midT ↓ | Right T | Cortical gliosis | Only nocturnal seizures |
| 4 | 25 | M | 5 | none | L midT, L infT ↓ | Right Lateral T | Cortical gliosis | 75% seizure reduction |
| 5 | 28 | M | 7 | L Fr, ins, amyg, hipp, parahipp, supT, infT, midT ↓ | L Fr, amyg, supT, infT, fusif ↓ | Left Lateral T | Cortical gliosis; | Rare postoperative seizures |
| 6 | 45 | F | 14 | L supT, infT ↓ | L infT, fusif ↓ | Left Lateral T | Cortical gliosis | Seizure-free |
| 7 | 18 | M | 4 | L Amyg ↓ | L Parahipp, infT ↓ | Left mesial T | HS | Seizure-free |
| 8 | 43 | F | 4 | L Ins, parahipp, fusif ↓ | none | Left mesial T | HS | Seizure-free |
| 9 | 24 | M | 9 | L Hipp, supT, midT, fusif ↓ | L Fr, ins, infT, fusif ↓ | Left mesial and lateral T | No surgery | |
| 10 | 23 | M | 19 | L Fr, ins, amyg, infT, fusif ↓ | none | Left Inferior frontal, mesial temporal | No surgery | |
| 11 | 27 | F | 13 | None | L Ins, fusif ↓ | Left mesial T | Diffuse LT and HF Gliosis | Seizure-free |
| 12 | 36 | F | 26 | None | L Hipp, fusif ↓ | bitemporal | No surgery |
MT: mesial temporal
LT: lateral temporal
T: temporal lobe
HS: hippocampal sclerosis
ST: superior temporal gyrus
Amyg: amygdala
Parahipp: parahippocampal gyrus
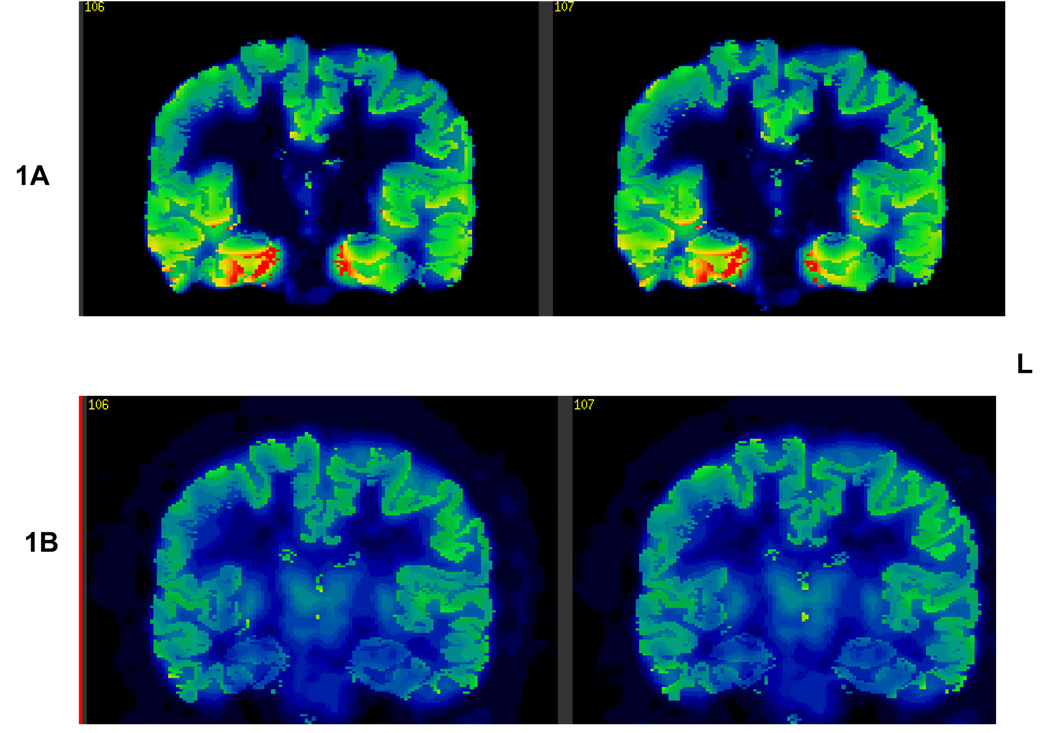
Figure 1.
1A: FCWAY PET showing decreased binding potential in the left mesial temporal region.
1B: FDG PET showing no metabolic asymmetry.
Nine patients had a resection. Five were seizure-free after at least one year, and two additional patients had experienced only rare post-operative seizures. Preoperative 18F-FCWAY-PET detected decreased ipsilateral binding in six, and 18F-FDG-PET hypometabolism in six, of these patients (table 3). Both PET scans found reduced activity ipsilateral to the epileptic focus in one patient who had fluctuating post-operative seizure frequency stabilizing in persistent seizures at night (#3). 18F-FDG-PET found contralateral hypometabolism in one who had only moderate improvement, but was able to become employed after surgery (# 4).
In order to examine the value of the two PET scans for distinguishing mesial from lateral temporal neocortical foci, we compared AI between patients with mesial foci alone (table 3: #2, 7, 8, 11), and lateral or diffuse epileptogenic zones. Patients with mesial temporal foci had lower superior temporal 18F-FCWAY V/f1 AI of 0.02 ± 0.10, and mid temporal AI of −0.03 ± 0.13. Patients with lateral or diffuse foci had superior temporal AI of −0.18 ± 0.21), and mid temporal AI of −0.14 ± 0.27. These trends were non-significant In mesial temporal regions, AI did not differ between the patient groups. 18F-FDG-PET also showed no AI differences in either mesial or lateral temporal regions. . Patients with mesial temporal foci have less reduction of lateral temporal 5HT1A binding ipsilateral to the epileptic focus than those.with lateral (or both lateral and mesial) temporal foci, but it is difficult to derive firm conclusions from a small patient sample.
Discussion
We found decreased ipsilateral temporal 18F-FCWAY-PET 5-HT1A receptor V/f1 and 18F-FDG-PET hypometabolism in comparable proportions of patients with MRI negative temporal lobe epilepsy. Our 18F-FDG-PET results are similar to previous reports that described hypometabolism ipsilateral to EEG foci in 30–90% of patients with MRI-negative TLE (Lamusuo et al 2001, Carne et al 2004, 2007). Thus, both 18F-FCWAY-PET and 18F-FDG-PET may be helpful for locating epileptogenic zones in non-lesional TLE. 18F-FDG-PET did not distinguish mesial from lateral temporal foci, while 18F-FCWAY-PET showed less lateral temporal binding reduction in patients with mesial than lateral foci. 18F-FDG-PET appeared to show potentially misleading data in two cases.
18F-FDG-PET tends to show lateral neocortical hypometabolism even in patients with mesial TLE (Sackellares et al 1990, Kim et al 2003). Patients with mesial foci defined by foramen ovale electrodes had greater mean depression of mesial metabolism than the group with lateral foci, but within the mesial focus group itself lateral hypometabolism was as prominent as mesial (Hajek et al 1993). Patients with mesial temporal sclerosis (MTS) and microdysplasia may have greater lateral temporal hypometabolism than those with MTS alone (Diehl et al 2003). A study using statistical parametric mapping found that medial hypometabolism was less extensive or severe in the lateral than mesial TLE patients, and patients with lateral temporal neocortical foci, as a group, had relatively greater lateral temporal hypometabolism. However, individual variation was too great for 18F-FDG-PET to be used for clinical localization (Kim et al 2003). Pure lateral temporal hypometabolism makes mesial seizure onset less likely.
18F-FDG-PET has been reported to be sensitive to time since last seizure, and to extent of seizure spread, which could have affected the results in two of our patients (#2 and 4) although they had not had seizures detected within two days of the scan (Leiderman et al 1994; Franceschi et al 1995; Savic et al 1997). Occasional hypometabolism contralateral to EEG foci has been reported (Carne et al 2004).
In contrast, 18F-FCWAY-PET results may be related to the specific distribution of receptors, which have their highest concentration in the limbic system and relatively reduced concentration in neocortex (Hall et al 1997). Previous studies showed greater 5HT1A reduction in mesial than lateral temporal lobe in patients with TLE (Toczek et al 2003, Savic et al 2004, Merlet et al 2004a, Giovacchini et al 2005). Thus, patients with both mesial and lateral temporal foci may have mesial 18F-FCWAY binding reduction.
In contrast to previous studies, we did not find significant hippocampal asymmetry ipsilateral to the epileptic focus in the patients with hippocampal sclerosis on pathological examination (Toczek et al 2003, Savic et al 2004, Merlet et al 2004a, Giovacchini et al 2005, Ito et al 2007). This was likely due to the presence of bilateral reduction in hippocampal 18F-FCWAY binding in these patients, also reported in previous studies (Hasler et al 2007). The overall hippocampal AI, was, however, greater than control.
Other factors in addition to the seizure focus itself may lead to reduced 5HT1A binding in patients with TLE. More extended binding reduction may be related to seizure propagation networks (Merlet et al 2004b, Ito et al 2007). Patients with major depressive disorders in addition to epilepsy have reduced binding in ipsilateral and contralateral regions beyond the epileptogenic zone (Hasler et al 2007, Theodore et al 2007). These effects may make the use of asymmetry indices less sensitive for detecting seizure onset regions. In order to use absolute values to detect clinically important abnormalities a larger control population will be needed. However, in a study of nine patients who had depth electrode recordings, 5HT1A receptor binding was significantly lower in seizure onset regions than areas with only interictal or no discharges (Merlet et al 2004a). Even when MRI was normal, 5HT1A receptor binding correlated with the seizure activity. Our results generally support these findings.
PVC could have affected our results in two ways. It appears to have a greater effect on neocortical than mesial temporal structures, possibly because lateral temporal gyral folding leads to greater partial volume effects in uncorrected images (Givoacchini et al 2005). However, 18F-FCWAY BP variability is increased by the procedure. This might have reduced our statistical power, particularly for individual analysis, leading to the slightly greater sensitivity of 18F-FDG-PET. However, the gain in specificity appears to outweigh a small loss of sensitivity.
We found a difference in 18F-FCWAY free fraction between patients and controls, probably due to a protein binding interaction. Although some antiepileptic drugs (AEDs) may have mild influence on serotoninergic neural transmission, they are unlikely to have affected our results. Any drug effect would be likely to be bilateral, and thus not lead to greater binding reduction in the epileptic focus. Moreover, previous studies showed that, after correction for free fraction, there were no significant AED effects on 18F-FCWAY volume of distribution (Theodore et al 2006).
Several patients in our study proved to have hippocampal neuronal loss and focal gliosis on pathological examination, suggesting that reduced 5HT1A receptor binding may occur in early or mild states of MTS. Serotonin receptor loss has been implicated in the hippocampal atrophy that occurs in patients with depression, and might be a contributing mechanism in epilepsy as well (Chugani and Chugani 2003, Husumu et al 2006). 5HT1A receptor activation promotes cell proliferation in the hippocampus by a direct post-synaptic effect, as well as by affecting sensitivity of proliferating cells in the dentate gyrus to corticosterone (Huang and Herbert 2005). Antagonists, including WAY-100635, lead to significant reduction in dentate gyrus proliferating cells, and to cell survival in pilocarpine-induced status epilepticus (Radley and Jacobs 2002, 2003). 5HT1A receptor loss might be an early step in the development of MTS.
Acknowledgments
This study was supported by the NIH NINDS Division of Intramural Research.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Disclosures:
Dr Theodore receives an honorarium from Elsevier as Co-Editor-in-Chief of Epilepsy Research.
None of the other authors has any disclosure.
References
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1989;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Carne RP, O’Brien TJ, Kilpatrick CJ, MacGregor LR, Hicks RJ, Murphy MA, Bowden SC, Kaye AH, Cook MJ. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127:2276–2285. doi: 10.1093/brain/awh257. [DOI] [PubMed] [Google Scholar]
- Carne RP, O'Brien TJ, Kilpatrick CJ, Macgregor LR, Litewka L, Hicks RJ, Cook MJ. 'MRI-negative PET-positive' temporal lobe epilepsy (TLE) and mesial TLE differ with quantitative MRI and PET: a case control study. BMC Neurol. 2007;7:16. doi: 10.1186/1471-2377-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RE, Wu Y, Lang L, Ma Y, Der MG, Herscovitch P, Eckelman WC. Brain uptake of the acid metabolites of [18F]-labeled WAY 100635 analogs. J Cereb Blood Flow Metab. 2003;23:249–260. doi: 10.1097/01.WCB.0000046145.31247.7A. [DOI] [PubMed] [Google Scholar]
- Cascino GD, Jack CR, Jr, Parisi JE. Magnetic resonance imaging-based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol. 1991;30:31–36. doi: 10.1002/ana.410300107. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Chugani HT. Does serotonin have trophic effects in temporal lobe epilepsy? Neurology. 2003;60:736–737. doi: 10.1212/01.wnl.0000057384.83302.34. [DOI] [PubMed] [Google Scholar]
- Diehl B, LaPresto E, Najm I, Raja S, Rona S, Babb T, Ying Z, Bingaman W, Luders HO, Ruggieri P. Neocortical temporal FDG-PET hypometabolism correlates with temporal lobe atrophy in hippocampal sclerosis associated with microscopic cortical dysplasia. Epilepsia. 2003;44:559–564. doi: 10.1046/j.1528-1157.2003.36202.x. [DOI] [PubMed] [Google Scholar]
- Franceschi M, Lucignani G, Del Sole A, Grana C, Bressi S, Minicucci F, Messa C, Canevini MP, Fazio F. Increased interictal cerebral glucose metabolism in a cortical-subcortical network in drug naive patients with cryptogenic temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1995;59:427–431. doi: 10.1136/jnnp.59.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarri A, Campana E, Pacitti F, Pacitti C. Projections of the Tsai tegmental ventral area to the hippocampus: a study of the rat using the Fink-Heimer technic. Boll Soc Ital Biol Sper. 1989;65:639–645. [PubMed] [Google Scholar]
- Giovacchini G, Toczek MT, MD Bonwetsch R, Bagic A, Lang L, Fraser C, Reeves-Tyer P, Herscovitch P, Eckelman WC, Carson RE, Theodore WH. 5-HT1A receptors are reduced in temporal lobe epilepsy after partial volume correction. J Nucl Med. 2005;46:1128–1135. [PMC free article] [PubMed] [Google Scholar]
- Hajek M, Antonini A, Leenders KL, Wieser WG. Mesiobasal versus lateral temporal lobe epilepsy: metabolic differences in the temporal lobe shown by interictal; 18F-FDG positron emission tomography. Neurology. 1993;43:79–86. doi: 10.1212/wnl.43.1_part_1.79. [DOI] [PubMed] [Google Scholar]
- Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Fletcher A, Cliffe IA, Barf T, Wikström H, Sedvall G. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]WAY-100635. Brain Res. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- Hasler G, Bonwetsch R, Giovacchini G, Toczek MT, Bagic A, Luckenbaugh DA, Drevets WC, Theodore WH. 5-HT(1A) Receptor Binding in Temporal Lobe Epilepsy Patients With and Without Major Depression. Biol Psychiatry. 2007;62:1258–1264. doi: 10.1016/j.biopsych.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. The role of 5-HT1A receptors in the proliferation and survival of progenitor cells in the dentate gyrus of the adult hippocampus and their regulation by corticoids. Neuroscience. 2005;135:803–813. doi: 10.1016/j.neuroscience.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Husum H, Aznar S, Høyer-Hansen S, Hald Larsen M, Mikkelsen JD, Møller A, Mathe AA, Wortwein G. Exacerbated loss of cell survival, neuropeptide Y-immunoreactive (IR) cells, and serotonin-IR fiber lengths in the dorsal hippocampus of the aged flinders sensitive line “depressed” rat: implications for the pathophysiology of depression? Journal of Neuroscience Research. 2006;84:1292–1302. doi: 10.1002/jnr.21027. [DOI] [PubMed] [Google Scholar]
- Ito S, Suhara T, Ito H, Yasuno F, Ichimiya T, Takano A, Maehara T, Matsuura M, Okubo Y. Changes in central 5-HT1A receptor binding in mesial temporal epilepsy measured by positron emission tomography with [11C]WAY100635. Epilepsy Research. 2007;73:111–118. doi: 10.1016/j.eplepsyres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lee D, Lee S, Kim S-K, Chung C, Chang K, Choi K, Chung J-K, Lee M. Differential Features of Metabolic Abnormalities between Medial and Lateral Temporal Lobe Epilepsy: Quantitative Analysis of 18F-FDG PET Using SPM. J Nucl Med. 2003;44:1006–1012. [PubMed] [Google Scholar]
- Lamusuo S, Jutila L, Ylinen A, Kalviainen R, Mervaala E, Haaparanta M, Jääskeläinen S, Partanen K, Vapalahti M, Rinne J. [18F]FDG-PET reveals temporal hypometabolism in patients with temporal lobe epilepsy even when quantitative MRI and histopathological analysis show only mild hippocampal damage. Arch Neurol. 2001;58:933–939. doi: 10.1001/archneur.58.6.933. [DOI] [PubMed] [Google Scholar]
- Leiderman DB, Albert P, Balish M, Bromfield E, Theodore WH. The dynamics of metabolic change following seizures as measured by positron emission tomography with fludeoxyglucose F 18. Arch Neurol. 1994;51:932–936. doi: 10.1001/archneur.1994.00540210106019. [DOI] [PubMed] [Google Scholar]
- Merlet I, Ostrowsky K, Costes N, Ryvlin P, Isnard J, Faillenot I, Lavenne F, Dufournel D, Le Bars D, Mauguière F. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F]MPPF-PET study. Brain. 2004a;127:900–913. doi: 10.1093/brain/awh109. [DOI] [PubMed] [Google Scholar]
- Merlet I, Ryvlin P, Costes N, Dufournel D, Isnard J, Faillenot I, Ostrowsky K, Lavenne F, Le Bars D, Mauguiere F. Statistical parametric mapping of 5-HT1A receptor binding in temporal lobe epilepsy with hippocampal ictal onset on intracranial EEG. Neuroimage. 2004b;22:886–896. doi: 10.1016/j.neuroimage.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Arango V, Olvet DM, Maria A, Oquendo MA, Van Heertum RL, Mann JJ. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. Journal of Cerebral Blood Flow & Metabolism. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Research. 2002;955:264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL. Pilocarpine-induced status epilepticus increases cell proliferation in the dentate gyrus of adult rats via a 5-HT1A receptor-dependent mechanism. Brain Research. 2003;966:1–12. doi: 10.1016/s0006-8993(02)03989-6. [DOI] [PubMed] [Google Scholar]
- Sackellares JC, Siegel GJ, Abou-Khalil BW, Hood TW, Gilman S, McKeever PE, Hichwa RD, Hutchins GD. Differences between lateral and mesial temporal hypometabolism interictally in epilepsy of mesial temporal origin. Neurology. 1990;40:1420–1426. doi: 10.1212/wnl.40.9.1420. [DOI] [PubMed] [Google Scholar]
- Savic I, Altshuler L, Baxter L, Engel J., Jr Pattern of interictal hypometabolism in PET scans with fludeoxyglucose F 18 reflects prior seizure types in patients with mesial temporal lobe seizures. Arch Neurol. 1997;54:129–136. doi: 10.1001/archneur.1997.00550140011006. [DOI] [PubMed] [Google Scholar]
- Savic I, Lindström P, Gulyás B, Halldin C, Andrée B, Farde L. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004;62:1343–1351. doi: 10.1212/01.wnl.0000123696.98166.af. [DOI] [PubMed] [Google Scholar]
- Swartz BE, Brown C, Mandelkern MA, Khonsari A, Patell A, Thomas K, Torgersen D, Delgado-Escueta AV, Walsh GO. The use of 2-deoxy-2-[18F]fluoro-D-glucose (FDG-PET) positron emission tomography in the routine diagnosis of epilepsy. Mol Imaging Biol. 2002;4:245–252. doi: 10.1016/s1095-0397(01)00057-7. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Gaillard WD, DeCarli C, Bhatia S, Hatta J. Hippocampal volume and glucose metabolism in temporal lobe epileptic foci. Epilepsia. 2001;42:130–133. doi: 10.1046/j.1528-1157.2001.080874.x. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Giovacchini G, Bonwetsch R, Bagic A, Reeves-Tyer P, Herscovitch P, Carson RE. The Effect of antiepileptic drugs on 5-HT1A Receptor binding Measured by Positron emission tomography. Epilepsia. 2006;47:499–503. doi: 10.1111/j.1528-1167.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Hasler G, Giovacchini G, Kelley K, Reeves-Tyer P, Herscovitch P, Drevets W. Reduced Hippocampal 5HT1A PET Receptor Binding and Depression in Temporal Lobe Epilepsy. Epilepsia. 2007;48:1526–1530. doi: 10.1111/j.1528-1167.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- Toczek MT, Carson RE, Lang L, Ma Y, Spanaki MV, Der MG, Fazilat S, Kopylev L, Herscovitch P, Eckelman WC, Theodore WH. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–756. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- Uijl SG, Leijten FS, Arends JB, Parra J, van Huffelen AC, Moons KG. The added value of [18F]-fluoro-D-deoxyglucose positron emission tomography in screening for temporal lobe epilepsy surgery. Epilepsia. 2007;48:2121–2129. doi: 10.1111/j.1528-1167.2007.01197.x. [DOI] [PubMed] [Google Scholar]
- Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy. A meta-analysis. Seizure. 2007;16:509–520. doi: 10.1016/j.seizure.2007.04.001. [DOI] [PubMed] [Google Scholar]