Abstract
Identifying the major ionotropic neurotransmitter in a respiratory neuron is of critical importance in determining how the neuron fits into the respiratory system, whether in producing or modifying respiratory drive and rhythm. There are now several groups of respiratory neurons whose major neurotransmitters have been identified and in some of these cases, more than one transmitter have been identified in particular neurons. This review will describe the physiologically identified neurons in major respiratory areas that have been phenotyped for major ionotropic transmitters as well as those where more than one transmitter has been identified. Although the purpose of the additional transmitter has not been elucidated for any of the respiratory neurons, some examples from other systems will be discussed.
Keywords: neurotransmitter co-localization, in situ hybridization, peptides, respiration, juxtacellular labeling
1. Introduction
Identification of the major ionotropic, fast synaptic transmitter used by a neuron is a major step forward in understanding the role of the neuron in a functional network. The neurons in the respiratory network were initially characterized as excitatory or inhibitory by using electrophysiological techniques allowing identification of fast inhibitory or excitatory post-synaptic potentials elicited from the identified neuron. In addition to the excitatory or inhibitory nature of the major transmitter, the morphology of some of these physiologically identified neurons was examined, yielding more information about how the neuron might participate in the respiratory network by studying its dendritic fields and axonal projections. A more recent method for identifying the major transmitter is through the use of in situ hybridization (ISH) for definitive markers of glutamatergic, glycinergic or GABAergic transmission combined with cellular labeling of a physiologically identified respiratory neuron. The technique of ISH can be combined with immunocytochemistry and/or double ISH to determine other phenotypic markers for respiratory neurons. ISH has been used to find that other neurotransmitters or neuropeptides with slower kinetics are sometimes co-localized with fast transmitters. In this review, the respiratory areas of the brainstem will be surveyed in terms of definitive characterization of a fast ionotropic transmitter and also those that have been identified with co-localized multiple transmitters.
Most neurons in the brain use glutamate, GABA, and/or glycine as their major ionotropic neurotransmitter. For instance, virtually all spontaneous miniature post synaptic potentials in brainstem slices are blocked by a cocktail of glutamatergic, GABAergic and glycinergic antagonists (Hayar and Guyenet, 1998, 1999; Lin et al., 1998; Doyle and Andresen, 2001). Electron microscopy also reveals that nearly all terminals in brainstem regions contain one of these three transmitters (Llewellyn-Smith et al., 1995, 2001). Although antibodies have been available for GABA, glycine and glutamic acid decarboxylase (GAD), the enzyme present in all GABAergic neurons, these markers are found most prominently in neuronal processes and not in cell bodies. Phosphate activated glutaminase (PAG) has been promoted as useful in identifying glutamatergic neurons but this enzyme has been found in Bötzinger neurons (glycinergic and not glutamatergic)(Pilowsky et al., 1997). Antibodies against glutamate itself have also been used to identify glutamatergic neurons. However, many neurons contain glutamate without it being used as a neurotransmitter, e.g. it is expressed in GABAergic neurons as a precursor of GABA and is used in many metabolic pathways (McKenna, 2007).
These drawbacks for identifying the major ionotropic transmitter can now be overcome by using labeled cRNAs to identify mRNAs coding for GAD 65/67 (Erlander et al., 1991; Esclapez et al., 1993), glycine transporter-2 (GlyT2) (Jursky et al., 1994; Jursky and Nelson, 1995) and three vesicular glutamate transporters (VGluts) (Bellocchio et al., 1998, 2000; Fremeau et al., 2001, 2002, 2004; Herzog et al., 2001, 2004; Varoqui et al., 2002; Oliveira et al., 2003). The major player in terms of markers for glutamatergic transmission in respiratory-related neurons is VGlut2, since in brainstem, VGlut1 is located predominantly in neurons supplying mossy fibers to the cerebellum (Hisano et al., 2002; Stornetta and Guyenet, 2005) and VGlut3 is limited to neurons in raphe magnus, parapyramidal raphe and dorsal and median raphe nuclei (Herzog et al., 2004; Nakamura et al., 2004; Stornetta et al., 2005) and in nitroxidergic neurons in the nucleus of the solitary tract (NTS) (Lin and Talman, 2005). The ISH method for using non-radioactive cRNA probes for GAD 65/67, GlyT2 or VGlut2 is performed using free-floating sections from paraformaldehyde fixed brains. Both digoxigenin and fluoroiso-thiocyanate (FITC) can be coupled to nucleotides and incorporated into cRNA probes. The resulting label from specific hybrids formed in the tissue is amplified by standard immunocytochemical methods. These probes can label neuronal somata with high signal-to-noise ratios and the labeling can be combined with other immunocytochemical and tract-tracing methods (c.f. (Stornetta et al., 2001, 2002a, 2004; Stornetta and Guyenet, 2005)).
Knowing which major ionotropic transmitter the cell uses, glutamate, GABA and/or glycine defines whether the cell is predominantly excitatory or inhibitory. By having information on other signaling molecules, e.g. neuropeptides, in addition to the major transmitter, one begins to gather a better “fingerprint” of a specific cell and perhaps an improvement in assigning the cell to a functional group. However, one must use some caution in trying to determine the function of a neuron based solely on a specific combination of phenotypic markers. One example of the limitations of determining function of brainstem neurons based on co-localization of phenotypic markers is the barosensitive bulbospinal cardiovascular premotor neurons of the ventrolateral medulla. Both VGlut2 and enkephalin have been identified in both C1 (tyrosine hydroxylase-TH-containing) and non-C1 cardiovascular premotor neurons (Stornetta et al., 2001, 2002b). There was no obvious difference between the C1 enkephalin neurons and non-C1 enkephalin neurons in their firing patterns, the response to blood pressure changes or in or in their spinal projections. However, the enkephalin neurons did tend to have a faster conduction velocity regardless of their co-localization with TH. The effects of opiates, glutamate and epinephrine on the sympathetic preganglionic neurons (SPNs) are quite different (although epinephrine as a C1 transmitter is controversial (Sved, 1989)) and these effects may be mediated both pre- and post-synaptically (Backman and Henry, 1983; McCall, 1988; Dun et al., 1992, 1993; Krupp and Feltz, 1995; Miyazaki et al., 1998). Certainly the co-release of enkephalin and glutamate on SPNs could have complex effects; however, the functional significance of this finding in terms of cardiovascular regulation has not been elucidated. Also note that although the effects of neuropeptides such as enkephalin on cardiorespiratory functions have been extensively studied (Hassen et al., 1982, 1984; Feuerstein et al., 1983; Pfeiffer et al., 1983; Iasnetsov et al., 1984; Suzue, 1984; Harfstrand et al., 1985; Hurlë et al., 1985; Sessle and Henry, 1985; Van Bockstaele and Aston-Jones, 1995; Gray et al., 1999; Mellen et al., 2003; Onimaru et al., 2006; Barnes et al., 2007; Bodnar, 2007), their function when co-localized with major transmitters in brainstem neurons has not yet been determined (although see below for discussion of the co-release of these substances in other part of the CNS).
The first indications of the major ionotropic transmitter in respiratory neurons were found using spike-triggered averaging of membrane potentials recorded with intracellular electrodes in neurons identified by their firing pattern in relation to a respiratory output. If the membrane potentials were hyperpolarizing and chloride mediated, these would indicate either glycine or GABA transmission. If the membrane potentials were fast rising, short lasting depolarizations, these were most likely due to ionotropic excitatory (possibly glutamatergic) transmission. A good review of the knowledge obtained by these methods is summarized in Ezure (1990). A newer method for determining the major ionotropic transmitter uses the juxtacellular labeling technique (Pinault, 1996) to allow the phenotypic identification of physiologically characterized respiratory neurons. This method allows one to use extracellular recording which is much easier in whole animals than the intracellular recording technique. Juxtacellular labeling can be done in the whole animal in vivo, thus allowing the study of the intact system. Following the experiment, the animal can be perfused transcardially for optimal histology, surmounting many issues with fixation of slices or other reduced physiological preparations. This technique can also be used in reduced preparations. Using the juxtacellular technique, a neuron can be identified based on both projection (via antidromic activation from the area of projection) as well as its firing pattern in relation to a respiratory outflow (e.g. phrenic nerve activity). The Neurobiotin or biocytin deposited via juxtacellular labeling can be easily combined with other markers visualized with histochemical techniques such as in situ hybridization and immunohistochemistry (for further information on juxtacellular labeling see the review by Guyenet et al. (2004)).
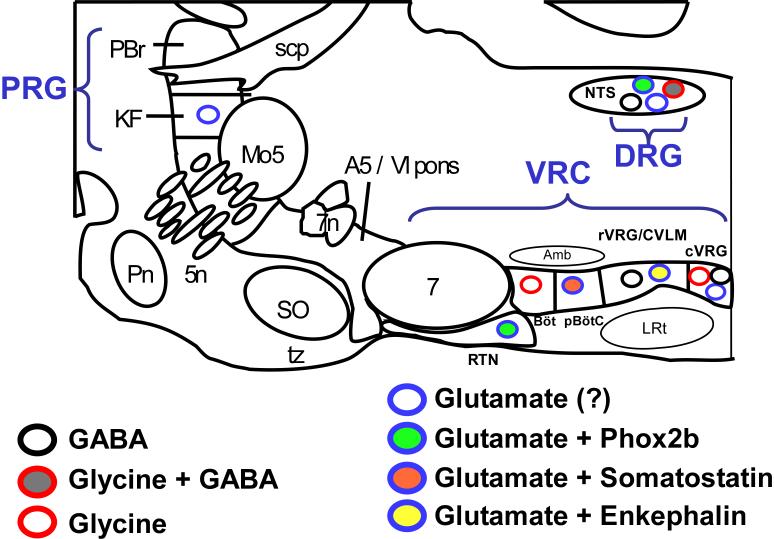
The respiratory network includes the nucleus of the solitary tract (NTS) and a series of contiguous regions located in the ventrolateral medulla and extending into the dorsal pons. The respiratory network in the ventrolateral medulla is referred to as the ventral respiratory column (VRC) in groups designated from caudal to rostral as the caudal ventral respiratory group (cVRG), the rostral ventral respiratory group (rVRG), the preBötzinger complex (preBötC), the Bötzinger complex (BötC), the parafacial respiratory group (pFRG) and the retrotrapezoid nucleus (RTN) (see Fig. 1, for reviews and more thorough anatomical explanations see (Feldman, 1986; Ezure, 1990; Alheid et al., 2002)). Some respiratory neurons (mostly inspiratory) are located in the area of the NTS and are referred to as the dorsal respiratory group (DRG)(de Castro et al., 1994). A prominent group of respiratory-related neurons is located in the pons around the parabrachial nucleus (PBr) and including the Kölliker Fuse (KF) nucleus is called the pontine respiratory group (PRG) (Alheid et al., 2004; Duffin, 2004; Ezure and Tanaka, 2006). The expression (and co-expression) of neurotransmitters will be considered in these general areas.
Figure 1.
Summary of respiratory neurons with identified major transmitters (parasagittal section with landmarks after Feldman). Placement of symbols representing phenotypically identified neurons is not meant to be exactly anatomically correct but to denote the region where this cell type is found. Abbreviations: 5n, motor roots of 5th nerve; 7, facial motor nucleus; 7n, facial nerve; Amb, Ambiguus; Böt, Bötzinger area; CVLM, caudal ventrolateral medulla; cVRG, caudal portion of ventral respiratory group; DRG, dorsal respiratory group; KF, Kölliker Fuse nucleus; LRt, lateral reticular nucleus; Mo5, trigeminal motor nucleus; NTS, nucleus of the solitary tract; PBr, Parabrachial nucleus; Pn, Pontine nuclei; PRG, pontine respiratory group; pBötC, PreBötzinger Complex; rVRG, rostral portion of ventral respiratory group; RTN, retrotrapezoid nucleus; scp, superior cerebellar peduncle; VRC, ventral respiratory column
2. Brainstem respiratory areas with identified major ionotropic transmitters
2.1 cVRG
This area beginning at the level of the obex (rostral to the calamus scriptorius) just caudal to the nucleus ambiguus and extending to the spinal medullary border, contains several different types of neurons with respiratory-related activity. Ezure and coworkers examined neurons with a firing pattern that decreased during expiration (termed expiratory-decrementing or E-DEC neurons) throughout the VRG and found vagal motor neurons in cVRG with E-DEC activity that were cholinergic (identified with immunostaining) and that did not contain either GlyT2 or GAD-67 mRNA (Ezure et al., 2003). The E-DEC cranial motor neurons are also not GABAergic (as determined by immunohistochemical detection of GAD) (Yamazaki et al., 2000; Okazaki et al., 2001). Another group of E-DEC neurons, some of which are located in the cVRG, have propriobulbar (a few additionally with bulbo-spinal) connections and are glycinergic as determined by juxtacellular labeling in combination with ISH for GlyT2 (Ezure et al., 2003). These neurons were not choline acetyltransferase (ChAT) immunoreactive, thus most likely not motor neurons. A few that were tested lacked GAD67 mRNA. Yamazaki and coworkers described a few GAD-immunoreactive neurons with different respiratory-related discharges in the cVRG including firing just following inspiration (post-I), firing increasing during inspiration (inspiratory-augmenting, I-AUG) as well as E-DEC; most of these neurons also contained NMDA receptors (detected by immunoreactivity) and were propriobulbar with no spinal or vagal projection (Yamazaki et al., 2000). Whether or not the E-DEC neurons found by Yamazaki’s group are the same subset of inhibitory neurons described by Ezure and coworkers is uncertain due to the small sample sizes of both studies as Ezure admits he cannot exclude the possibility that some of the glycinergic E-DEC cells in the cVRG might also be GABAergic. Okazaki et al. (2001) found propriobulbar I-AUG neurons in cVRG that were GAD-immunoreactive, consistent with Yamazaki et al. Okazaki and coworkers (2001) also found GAD-immunoreactive, I-AUG neurons extending as far as 600 microns rostral to the obex (in rat) which would correspond to the rVRG.
Most of the neurons found in the cVRG with a firing pattern that increases during expiration (expiratory-augmenting, E-AUG) are excitatory (presumed glutamatergic although the exact phenotype has not been identified) pre-motor neurons projecting to the ventral horn neurons controlling abdominal and other expiratory muscles (Ballantyne and Richter, 1986; Arita et al., 1987; Iscoe, 1998).
2.2 rVRG
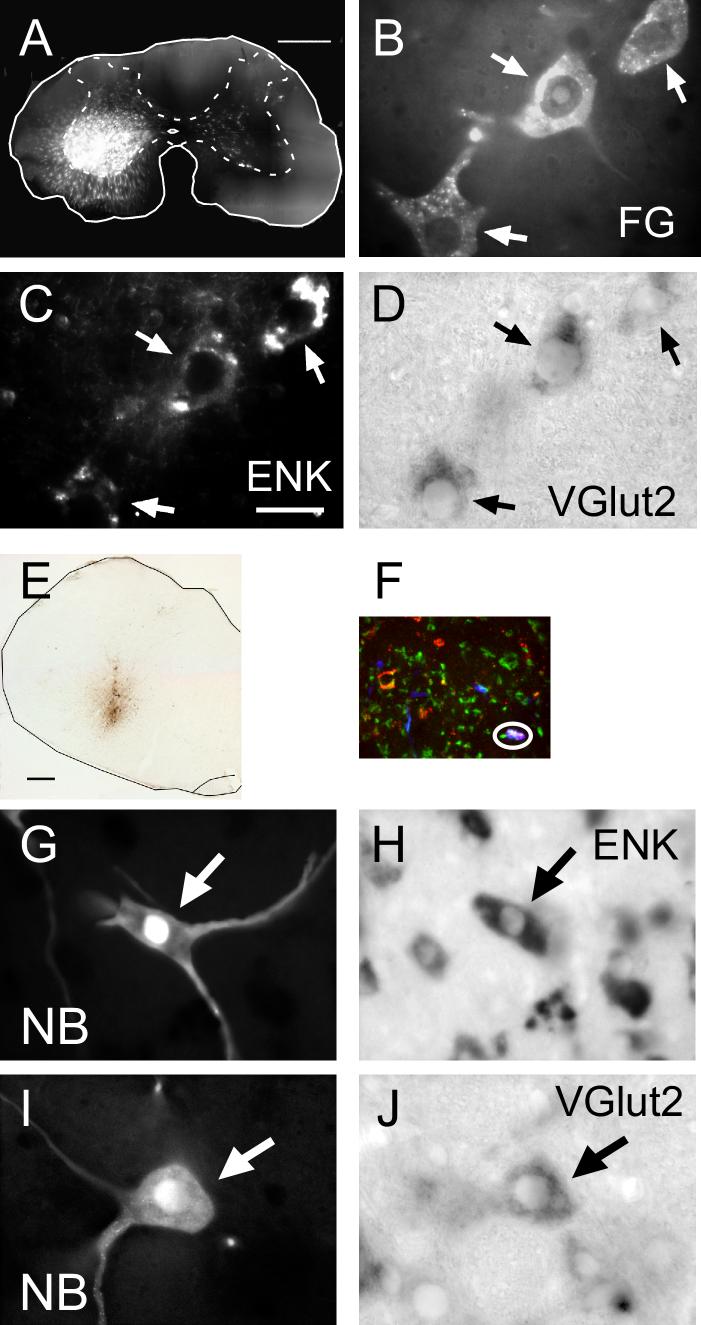
The rVRG, a collection of respiratory neurons located in the ventrolateral medulla, extends from the level of the obex to the rostral portion of the nucleus ambiguus (Ellenberger and Feldman, 1990). The pre-motor, I-AUG neurons in the rVRG have been characterized as excitatory by several different methods. Ono et al. (2006) used spike-triggered averaging to find EPSPs in laryngeal motoneurons elicited from I-AUG neurons in rVRG. Liu et al. (1990) found pharmacological evidence that inspiratory-related bulbospinal input to phrenic motor neurons was from excitatory amino acids. Stornetta et al. (2003b) found further evidence that bulbo-spinal I-AUG neurons are excitatory (glutamatergic). Neurons in the rVRG that project to the phrenic motor neurons in the ventral horn of the cervical spinal cord were identified by the retrograde transport of FluoroGold (FG) injected into the cervical spinal cord at C4-6 (Fig. 2A). These neurons were also labeled with riboprobes for VGlut2 and pre-pro-enkephalin (ENK). Sixty percent of the FG-labeled neurons were found to co-express both VGlut2 and ENK mRNAs (Fig. 2B-D). Biotinylated dextran amine (BDA) iontophoresed into the area of the rVRG (identified by recording of inspiratory-augmenting activity in the area) was transported to terminals found in cervical spinal cord ventral horn to co-express VGlut2 and enkephalin proteins by immunocytochemistry (Fig. 2E, F). Finally, 16/18 physiologically identified I-AUG neurons contained VGlut2 mRNA and 14/14 I-AUG neurons contained ENK mRNA (Fig. 2G-J). These data support the idea that this population of bulbospinal I-AUG neurons of the rVRG are both glutamatergic and enkephalinergic.
Figure 2.
Example of techniques used to determine multiple transmitters in inspiratory-augmenting neurons of the rVRG. A. FluoroGold injection site in C4 cervical spinal cord. B. Neurons in rVRG retrogradely labeled with FG from injection site in A. C. Same neurons as in B labeled using in situ hybridization with an FITC-tagged riboprobe for pre-proenkephalin (ENK). D. Same neurons as in B labeled with a digoxigenin-tagged riboprobe fir VGlut2. E. BDA injection site in rVRG. F. Circled terminal in ventral horn of cervical spinal cord shows labels for BDA, VGlut2 and enkephalin revealed by immunocytochemistry. G. Inspiratory-augmenting neuron in rVRG labeled with Neurobiotin (NB). H. Same cell as G labeled with riboprobe for ENK mRNA. I. Inspiratory-augmenting neuron in rVRG labeled with Neurobiotin. J. Same cell as I labeled with riboprobe for VGlut2 mRNA. (Details in (Stornetta et al., 2003)).
The region of the ventrolateral medulla caudal to the preBötC extending to the forking rostral tips of the lateral reticular nucleus (LRN) is also known as the caudal ventrolateral medulla (CVLM) in the area of cardiovascular regulation. Although the CVLM and the rVRG are not identical, the CVLM overlaps with a portion of the rVRG. The cardiovascular-related neurons in CVLM are usually ventral to the respiratory neurons recorded in the VRG (Verberne et al., 1999; Mandel and Schreihofer, 2006). A group of neurons in the CVLM are GABAergic (GAD-67 mRNA) and provide the critical link in the baroreceptor reflex between the nucleus of the solitary tract (NTS) and the rostral ventrolateral medulla (RVLM) (Schreihofer and Guyenet, 2003). Inhibition of the CVLM increases the sympathetic nerve response to CO2 (Moreira et al., 2006). These baro-activated neurons have recently been described to have various types of inspiratory-modulated activity as evident in averaged extracellular recording of neuronal activity triggered by the onset of the phrenic nerve discharge (PND) and their phenotype determined as GABAergic with juxtacellular labeling followed by GAD-67 ISH (Mandel and Schreihofer, 2006).
2.3 PreBötzinger Complex
The preBötzinger Complex (preBötC) is located in the ventrolateral medulla, just ventral to the compact portion of the nucleus ambiguus and extending caudally to where the lateral reticular nucleus divides. These neurons are essential for the generation of the mammalian respiratory rhythm (Smith et al., 1991; Rekling and Feldman, 1998; Feldman et al., 2003; Ramirez and Viemari, 2005; Feldman and Del Negro, 2006; Koizumi et al., 2008). Many of the neurons in the preBötC express the neurokinin-1 receptor (NK1-R) immunoreactivity (Gray et al., 1999, 2001; Guyenet and Wang, 2001; Liu et al., 2001, 2005; Alheid et al., 2002; Thoby-Brisson et al., 2003; Dehkordi et al., 2004) and have a glutamatergic phenotype, i.e. they co-express VGlut2 (Wang et al., 2001; Guyenet et al., 2002). Some of the NK1-R neurons in this region also express mu-opiate receptors (Gray et al., 1999), nitric-oxide synthase (Liu et al., 2001) and a variety of neurotransmitter receptors besides NK1 (Liu et al., 2001). Most of the NK1-R neurons within the preBötC do not co-express GlyT2, choline acetyltransferase, GAD-67 or tyrosine hydroxylase (Wang et al., 2001). A subpopulation of the glutamatergic NK1-R neurons co-express somatostatin and have propriobulbar but not bulbospinal projections (Stornetta et al., 2003a). While all these described small NK1-R neurons belong to the category of propriobulbar expiratory-inspiratory neurons that are a component of the rhythm generating circuit in the preBötC, there are other populations of NK1-R neurons in this area. Directly adjacent in the rostral most part of the rVRG and sometimes overlapping the preBötC are large I-AUG neurons with direct projections to the phrenic motor nucleus. A fraction of these neurons are also NK1-R immunoreactive (Makeham et al., 2001; Guyenet et al., 2002) and glutamatergic (Guyenet et al., 2002) and some co-express pre-pro-enkephalin (Stornetta et al., 2003b). This data reinforces the report by Gray et al. (1999) that the population of NK1-R neurons in the preBötC /rVRG area is heterogeneous.
There are other cell types within the preBötC, not quite so widely studied as the small NK1-R neurons, including e.g., non-NK1-R neurons with either early or late inspiratory activity (Hayes and Del Negro, 2007) and respiratory neurons whose activity is modified by BDNF through the tyrosine kinase B (TrkB) receptor (Thoby-Brisson et al., 2003). A variety of neuronal phenotypes have also been identified by strictly anatomical methods (no evidence that they are respiratory other than location within the preBötC) that contain a variety of substances and receptors, either with or without NK1-R immunoreactivity (Liu et al., 2001; Liu and Wong-Riley, 2002; Liu et al., 2002, 2004, 2005).
2.4 Bötzinger complex
The Bötzinger complex, the rostral extension of the rVRG from the compact portion of the nucleus ambiguus to just behind the facial motor nucleus, contains neurons with several different respiratory-related firing patterns. The neurons with expiratory-related firing have been well-characterized as inhibitory, first by Merrill et al. (Merrill et al., 1983) who used spike-triggered averaging to show that Bötzinger neurons projected monosynaptically to and inhibited dorsal respiratory group (DRG) inspiratory neurons. Other studies followed using similar techniques to show Bötzinger expiratory neurons elicited monosynaptic inhibitory post-synaptic potentials (IPSPs) in other respiratory area neurons including the inspiratory neurons in the caudal and rostral VRG (Fedorko et al., 1989; Jiang and Lipski, 1990), phrenic motoneurons (Merrill and Fedorko, 1984; Tian et al., 1998), laryngeal motoneurons (Ono et al., 2006; Shiba et al., 2007) and in the expiratory neurons in the caudal VRG (Jiang and Lipski, 1990). The major transmitter of these Bötzinger neurons is glycine as demonstrated by a combination of electrophysiological identification, juxtacellular labeling and ISH for both expiratory-augmenting (Schreihofer et al., 1999) and expiratory-decrementing neurons (Ezure et al., 2003). Although the evidence for glycinergic respiratory Bötzinger neurons is convincing, there is some anatomical evidence that neurons in the Bötzinger region projecting to the area of the DRG in NTS are GABAergic (Livingston and Berger, 1989) and that there are a few bulbospinal GABAergic neurons in the region of the Bötzinger complex (Ellenberger, 1999). However these studies were purely anatomical and did not identify the GAD neurons with the physiological characteristics of Bötzinger respiratory neurons.
2.5 Parafacial respiratory group (pFRG)
The pFRG, as described in the neonate with optical or electrophysiological recordings (Onimaru and Homma, 1987. 2003; Tokumasu et al., 2001; Janczewski et al., 2002), has not been well characterized with respect to exact neurotransmitter phenotype, greatly limiting comparison between studies or conducting specific studies with an identified cell type. Although Onimaru and colleagues have evidence for both inhibitory (Arata et al., 1998) and excitatory-type neurons (Janczewski et al., 2002; Onimaru et al., 2006) in this area, they have provided only correlative physiological data rather than direct recording and histochemical identification of pFRG neurons. A recent study by Guyenet and colleagues (Fortuna et al., 2008) hypothesizes that the pFRG in adult might include some of the glycinergic neurons of the Bötzinger complex that develop a pre-I post-I firing pattern when exposed to severe hypoxia. The idea that neurons with a similar pre-I post-I pattern of firing as observed in the neonate correspond to subgroups of expiratory BötC neurons postulated by Ezure (2004) and Fortuna et al. (2008) remains to be confirmed by identification of unique chemical markers that label these neurons both in the neonate and in the adult.
2.6 Retrotrapezoid nucleus (RTN)
The RTN is the most rostral group of neurons in the VRC with an identified phenotype. The discharge of these neurons is weakly modulated by inhibitory feedback from the central pattern generator (CPG). The RTN neurons are chemosensitive and participate at least in the respiratory component of the chemoreflex (i.e. increased respiration in response to carbon dioxide and to afferents from peripheral chemoreceptors signaling hypoxia) (Mulkey et al., 2004; Stornetta et al., 2006; Takakura et al., 2006). These neurons are glutamatergic (i.e. contain VGlut2 mRNA detected by both ISH and RT-PCR) and express the NK1-R (Mulkey et al.,2007a; Dubreuil et al., 2008; Takakura et al., 2008) but are not serotonergic, cholinergic or catecholaminergic. They express the transcription factor Phox2b (Stornetta et al., 2006; Mulkey et al., 2007b); the gene coding for this transcription factor is mutated in congenital central hypoventilation syndrome (CCHS, (Gozal, 1998; Amiel et al., 2003; Weese-Mayer et al., 2003, 2005; Trang et al., 2004; Matera et al., 2004; Trochet et al., 2005; Gaultier et al., 2005; Bachetti et al., 2005; Todd et al., 2006; Antic et al., 2006; Dubreuil et al., 2008)). Partial loss of this RTN Phox2b neuronal population results in a significant increase in the CO2 threshold for activation of the phrenic nerve under anesthesia (Takakura et al., 2008). Replication in mice of one of the most common mutations observed in humans with CCHS leads a 70% loss of this presumed chemoreceptor population and in turn leads to loss of chemoreflexes and severe hypoventilation in the newborn mice (Dubreuil et al., 2008).
2.7 Dorsal respiratory group (DRG)
Inspiratory neurons in the dorsomedial medulla in and around the NTS have been identified in both cat (Berger et al., 1984) and rat (de Castro et al., 1994), although differences may exist in the projection patterns of these neurons between species. There are several different categories of respiratory-related neurons in the area of the DRG that are distinguished topographically, as well as by their different inputs and firing patterns. Neurons firing synchronously with lung inflation were originally called pump (P) cells (Berger, 1977; Saether et al., 1987) and are unnecessary for the generation of the basic respiratory rhythm. Pump cells receive pulmonary afferents from slowly adapting stretch receptors (SARs) and are responsible for the Hering-Breuer reflex (for reviews see (Coleridge and Coleridge, 1994; Kubin et al., 2006)). The pump cells appear to be mainly inhibitory (Ezure et al., 2002; Tanaka et al., 2003), although there may be an excitatory sub-population not yet definitively characterized (Ezure and Tanaka, 2004). These neurons are located in the ventrolateral NTS in the interstitial subnucleus and slightly rostral to the obex. Their inhibitory nature was confirmed by the observation that they express GAD-67 mRNA and some also co-express GlyT2 mRNA (Ezure and Tanaka, 2004; Takakura et al., 2007). Ezure (2004) suggests that this sub-population of pump cells could co-release GABA and glycine; however, there is no direct evidence of this.
The cells that receive inputs from the rapidly adapting lung stretch receptors (RARs) are quite different from those receiving inputs from SARs. RAR relay neurons are located primarily in the commissural nucleus of the NTS (Lipski et al., 1991; Ezure and Tanaka, 2004) and facilitate inspiratory-related activity. They appear to be neither GAD-67 nor GlyT2-containing and are consequently postulated to be glutamatergic (Ezure and Tanaka, 2004).
Neurons also located caudally within the NTS, mainly within the commissural nucleus, receive inputs from the carotid body chemoreceptors (Finley and Katz, 1992) responding mainly to arterial hypoxia, and project directly (or via higher order relays) to the VRC and to the RTN (Otake et al., 1993; Takakura et al., 2006). The VRC/RTN-projecting neurons are excitatory, i.e. contain VGlut2 mRNA (Takakura et al., 2006), and express the transcription factor Phox 2b (Stornetta et al., 2006).
Cohen and colleagues (Cohen et al., 1974; Cohen and Feldman, 1984) suggested that the DRG inspiratory neurons (i.e. not the pump cells) with direct input from the CPG monosynaptically excited phrenic motor neurons. Lipski and coworkers (Lipski et al., 1983; Duffin and Lipski, 1987) demonstrated that the DRG inspiratory neurons are excitatory using spike-triggered averaging of fast EPSPs in phrenic motoneurons, using action potentials of inspiratory ventrolateral NTS neurons as triggers. There has been no confirmation to date that the excitatory neurotransmitter used by these DRG inspiratory neurons is glutamate.
2.8 Dorsolateral pons- parabrachial nucleus, Kölliker Fuse nucleus
This general area has been termed a “pneumotaxic center” and has been implicated in several aspects of respiratory control including switching between respiratory phases (Lumsden, 1923; Bianchi and St John, 1982; St.John, 1986; Jodkowski et al., 1994) as well as coordination of the breathing pattern (Rybak et al., 2004; Smith et al., 2007). The major respiratory-related projections to this area are from the NTS and from the VRC. Some authors have recorded from identified respiratory neurons in these areas and studied their morphology and projections (Kobayashi et al., 2005; Ezure and Tanaka, 2006; Song et al., 2006). Yokota et al. (2001, 2004, 2007) have data showing glutamatergic neurons in the Kölliker-Fuse nucleus project to rVRG and/or to the phrenic motor nucleus and suggest that these neurons could be respiratory-related, but to date nobody has identified a major transmitter phenotype in physiologically identified cells in the pontine respiratory centers. Since there are several sub-regions of this general area that have different effects on respiration (Chamberlin and Saper, 1994; Chamberlin, 2004) and neurons located throughout the area have different respiratory-related firing patterns, this cries out for more definitive phenotyping for neurotransmitters of identified neurons in this region.
3. Physiological implications of co-localization of neurotransmitters
The finding that some neurotransmitters are co-localized in respiratory-related neurons compels further hypotheses on what the physiological relevance is of this observation. A summary of possible synaptic configurations of multiple transmitters and receptors is elaborated in the review by Davanger (1996). This includes localization of the two transmitters into separate synaptic terminals or co-localization of transmitter in the same synapse but differential post-synaptic localization of receptors, or co-localization of transmitters that have differential effects on the same postsynaptic receptor. Another possibility is differential release of transmitter depending on the properties of the input to the neuron (e.g. high or low firing frequency).
The co-localization of GlyT2 and GAD-67 in brainstem neurons has been noted by several authors (Stornetta et al., 2004; Ezure and Tanaka, 2004; Takakura et al., 2007). The co-localization of these transmitters has also been reported in terminals using electron microscopy (Dumba et al., 1998; Dugue et al., 2005). Jonas and coworkers provided the first evidence that GABA and glycine can be co-released (Jonas et al., 1998). Both these transmitters are active on hypoglossal motor neurons in brainstem (O’Brien and Berger, 1999). GABA and glycine are packaged by the same vesicular transporter (Sagne et al., 1997; McIntire et al., 1997; Chaudhry et al., 1998) and could be co-packaged in the same vesicles (Burger et al., 1991; Wojcik et al., 2006). Although both GABA and glycine receptors are co-localized postsynaptic to terminals that co-express GABA and glycine (Todd et al., 1996), a recent study by Lu et al. (2008) indicates that GABA co-released with glycine in the medial nucleus of the trapezoid body (auditory brainstem) can act directly at glycine receptors to modify their kinetics. Thus both transmitters could work on the same postsynaptic receptor but through different post-synaptic mechanisms. The co-released transmitters also can exhibit specific effects by working at different postsynaptic receptors. Dugue et al. (2005) in their report studying cerebellar synapses suggest that GABA and glycine co-expressed in terminals by the same Golgi cells have differential effects in their contacts on granule cells and unipolar brush cells because these two target cell types differentially express either GABA receptors (in the case of the granule cells) or glycine receptors (associated predominantly with unipolar brush cells). Neither of these reports indicated a differential distribution of GABA and glycine in the presynaptic terminals- both neurotransmitters seemed to be co-localized in the same terminals. Katsurabayashi et al. (2004) found that a single bouton in spinal cord could release GABA only, glycine only or both transmitters together. This study indicates that vesicles in these boutons could contain either GABA or glycine or both together in the same vesicle, thus raising the possibility that GABA, glycine or the combination could be released differentially, although no further experiments were undertaken to verify this idea.
The co-existence of the excitatory neurotransmitter glutamate with the inhibitory neuropeptide enkephalin is puzzling. One possible explanation however, given that peptides are released with higher stimulation frequencies (Lundberg and Hokfelt, 1983; Iverfeldt et al., 1989; Drake et al., 1994; Vilim et al., 2000), is that when the system is undergoing maximal stimulation, the inhibitory peptide could serve as an autoregulatory inhibitory feedback to bring the system back towards normal firing. There is evidence for this in the hippocampus. Glutamate and dynorphin are co-localized in mossy fibers in the hippocampus and there is evidence that they are co-released (Terrian et al., 1988; Gannon and Terrian, 1991; Xie et al., 1991; Conner-Kerr et al., 1993; Terman et al., 2000)). The dynorphin is released from mossy fibers after high-frequency synaptic activation (Drake et al., 1994) and reduces the excitation of granule cells by presynaptic inhibition of excitatory amino acid release (Simmons et al., 1994).
Another possible function of the inhibitory peptide co-expressed with the excitatory neurotransmitter could be an amplification of the excitatory signal through inhibition of pre- or post-synaptic inhibitory neurons. No evidence exists to date of co-release of neurotransmitters with neuropeptides in identified respiratory neurons and the function of the inhibitory neuropeptides that have been found to be co-localized in respiratory neurons is still unknown. It is certainly possible that the inhibitory neuropeptides co-localized in excitatory neurons are a mechanism of inhibitory feedback to return the system to a more normal firing pattern during times of high stress, i.e. if the inspiratory drive is too high, the somatostatin in the rhythm-generating neurons of the preBötC is released by the higher frequency stimulation and this inhibits the VRC neurons downstream as well as potentially inhibiting other preBötC reciprocally connected neurons. Similarly, the inspiratory-augmenting pre-motor neurons that co-express enkephalin and glutamate, may release enkephalin during a similar high stress situation where the inspiratory drive is too high to maintain and results in a more hyperpolarized phrenic motor neurons that would fire less frequently. This topic is wide open for further research in the field of respiratory regulation.
4. Summary and conclusions
A few groups of neurons in the respiratory centers have been identified in terms of major ionotropic transmitter (see Fig. 1 and Table). These include the cholinergic expiratory and inspiratory motor neurons (not illustrated), the glycinergic expiratory-augmenting neurons of the Bötzinger complex, the GABAergic pump cells of the DRG, the glutamatergic peripheral chemoreceptor relay neurons of the DRG, the glutamatergic inspiratory- augmenting premotor neurons of the rVRG, the glycinergic expiratory-decrementing neurons of the cVRG, rVRG and BötC, the glutamatergic inspiratory neurons of the preBötC, and the glutamatergic chemoreceptor neurons of the RTN. A few of these neuronal groups have been further characterized with co-localization of other markers: glutamatergic inspiratory-augmenting neurons of the rVRG express enkephalin mRNA and NK1-r immunoreactivity, glutamatergic, presumed-inspiratory neurons of the preBötC express somatostatin and NK1-R immunoreactivity, glutamatergic peripheral chemoreceptor relay neurons of the DRG and glutamatergic chemoreceptive neurons of the RTN co-express Phox2b.
Table.
Summary of major neurotransmitter phenotypes of identified respiratory neurons
| Location | Firing pattern | Neurotransmitter | Co-localized substance |
|---|---|---|---|
| cVRG | E-DEC | acetylcholine (motor neurons) 1 | - |
| cVRG | E-DEC | glycine 2 | - |
| cVRG | I-AUG, post-I, E-DEC | GABA 3 | - |
| cVRG | E-AUG | glutamate? 4 | - |
| rVRG | I-AUG | glutamate | enkephalin5, NK1-R6 |
| rVRG | E-DEC | glycine 2 | - |
| preBötC | inspiratory | glutamate | somatostatin7, NK1-R6,7 |
| Bötzinger | E-AUG8, E-DEC2 | glycine | - |
| pFRG | pre-I/post-I | glutamate? 9, glycine? 10 | - |
| RTN | tonic, CO2 modulated | glutamate | Phox2b11, NK1-R12 |
| DRG | lung inflation (via SARs) | GABA 13 | glycine14 |
| DRG | lung inflation (via RARs) | glutamate? 14 | - |
| DRG | inspiratory | glutamate? 15 | - |
| Pons | mixed16 | glutamate? 17 | - |
The firing pattern is in relationship to phrenic nerve discharge in terms of inspiration or expiration or as noted with respect to lung inflation. “?” indicates that the neurotransmitter type has not been confirmed by both physiological and histological methods. Abbreviations: cVRG, caudal respiratory group; DRG, dorsal respiratory group; E-AUG, expiratory augmenting; E-DEC, expiratory decrementing; I-AUG, inspiratory augmenting; I, inspiratory; NK1-R, neurokinin1 receptor; preBötC, preBötzinger complex; RARs, rapidly adapting (lung stretch) receptors; SARs, slowly adapting (lung stretch) receptors.
All respiratory motor neurons are cholinergic. Some are expiratory and some are inspiratory but no additional transmitter has been identified in any of them.
Yamazaki et al., 2000. Okazaki et al., 2001. Note that propriobulbar GAD-ir cells with various firing patterns were found throughout the VRC. However, Ezure2 found the E-DEC cells to be mostly glycinergic. These discrepancies could be due to different methods to identify the cells’ phenotypes: Ezure et al. used ISH and the others used GAD immunoreactivity.
Ezure and Tanaka, 2004 (∼ 26% of GABA DRG neurons also contain glycine)
The actual co-release of neurotransmitters in respiratory neurons has not been demonstrated and the physiological ramifications of the co expression of these neurotransmitters require much further study.
Acknowledgment
This work was supported by grants HL28785 & HL74011
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J. Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir. Physiol Neurobiol. 2004;143:105–114. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de PL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat. Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Antic NA, Malow BA, Lange N, McEvoy RD, Olson AL, Turkington P, Windisch W, Samuels M, Stevens CA, Berry-Kravis EM, Weese-Mayer DE. PHOX2B Mutation-confirmed Congenital Central Hypoventilation Syndrome: Presentation in Adulthood. Am. J. Respir. Crit. Care Med. 2006;174:923–927. doi: 10.1164/rccm.200605-607CR. [DOI] [PubMed] [Google Scholar]
- Arata A, Onimaru H, Homma I. Possible synaptic connections of expiratory neurons in the medulla of newborn rat in vitro. NeuroReport. 1998;9:743–746. doi: 10.1097/00001756-199803090-00033. [DOI] [PubMed] [Google Scholar]
- Arita H, Kogo N, Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Res. 1987;401(2):258–266. doi: 10.1016/0006-8993(87)91410-7. [DOI] [PubMed] [Google Scholar]
- Bachetti T, Matera I, Borghini S, Di DM, Ravazzolo R, Ceccherini I. Distinct pathogenetic mechanisms for PHOX2B associated polyalanine expansions and frameshift mutations in congenital central hypoventilation syndrome. Hum. Mol. Genet. 2005;14:1815–1824. doi: 10.1093/hmg/ddi188. [DOI] [PubMed] [Google Scholar]
- Backman SB, Henry JL. Effects of glutamate and aspartate on sympathetic preganglionic neurons in the upper thoracic intermediolateral nucleus of the cat. Brain Res. 1983;277:370–374. doi: 10.1016/0006-8993(83)90948-4. [DOI] [PubMed] [Google Scholar]
- Ballantyne D, Richter DW. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J. Physiol. 1986;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BJ, Tuong CM, Mellen NM. Functional imaging reveals respiratory network activity during hypoxic and opioid challenge in the neonate rat tilted sagittal slab preparation. J. Neurophysiol. 2007;97:2283–2292. doi: 10.1152/jn.01056.2006. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J. Neurosci. 1998;18:8648–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr., Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain Res. 1977;135:231–254. doi: 10.1016/0006-8993(77)91028-9. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Averill DB, Cameron WE. Morphology of inspiratory neurons located in the ventrolateral nucleus of the tractus solitarius of the cat. J. Comp. Neurol. 1984;224:60–70. doi: 10.1002/cne.902240106. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, St John WM. Medullary axonal projections of respiratory neurons of pontile pneumotaxic center. Respir. Physiol. 1982;48:357–373. doi: 10.1016/0034-5687(82)90039-1. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2006. Peptides. 2007;28:2435–2513. doi: 10.1016/j.peptides.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger PM, Hell J, Mehl E, Krasel C, Lottspeich F, Jahn R. GABA and glycine in synaptic vesicles: storage and transport characteristics. Neuron. 1991;7:287–293. doi: 10.1016/0896-6273(91)90267-4. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir. Physiol. Neurobiol. 2004;143:115–125. doi: 10.1016/j.resp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI, Feldman JL. Discharge properties of dorsal medullary inspiratory neurons: relation to pulmonary afferent and phrenic efferent discharge. J. Neurophysiol. 1984;51:753–776. doi: 10.1152/jn.1984.51.4.753. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Piercey MF, Gootman PM, Wolotsky P. Synaptic connections between medullary inspiratory neurons and phrenic motoneurons as revealed by cross-correlation. Brain Res. 1974;81:319–324. doi: 10.1016/0006-8993(74)90946-9. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu. Rev. Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- Conner-Kerr TA, Simmons DR, Peterson GM, Terrian DM. Evidence for the corelease of dynorphin and glutamate from rat hippocampal mossy fiber terminals. J Neurochem. 1993;61:627–636. doi: 10.1111/j.1471-4159.1993.tb02167.x. [DOI] [PubMed] [Google Scholar]
- Davanger S. Colocalization of amino acid signal molecules in neurons and endocrine cells. Anat. Embryol. (Berl) 1996;194:1–12. doi: 10.1007/BF00196310. [DOI] [PubMed] [Google Scholar]
- de Castro D, Lipski J, Kanjhan R. Electrophysiological study of dorsal respiratory neurons in the medulla oblongata of the rat. Brain Res. 1994;639:49–56. doi: 10.1016/0006-8993(94)91763-9. [DOI] [PubMed] [Google Scholar]
- Dehkordi O, Haxhiu MA, Millis RM, Dennis GC, Kc P, Jafri A, Khajavi M, Trouth CO, Zaidi SI. Expression of [alpha]-7 nAChRs on spinal cord-brainstem neurons controlling inspiratory drive to the diaphragm. Respir. Physiol. Neurobiol. 2004;141:21–34. doi: 10.1016/j.resp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J. Neurophysiol. 2001;85:2213–2223. doi: 10.1152/jn.2001.85.5.2213. [DOI] [PubMed] [Google Scholar]
- Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, Chavkin C. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J. Neurosci. 1994;14:3736–3750. doi: 10.1523/JNEUROSCI.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc. Natl. Acad. Sci. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J. Functional organization of respiratory neurones: a brief review of current questions and speculations. Exp. Physiol. 2004;89:517–529. doi: 10.1113/expphysiol.2004.028027. [DOI] [PubMed] [Google Scholar]
- Duffin J, Lipski J. Monosynaptic excitation of thoracic motoneurones by inspiratory neurones of the nucleus tractus solitarius in the cat. J. Physiol. 1987;390:415–431. doi: 10.1113/jphysiol.1987.sp016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugue GP, Dumoulin A, Triller A, Dieudonne S. Target-dependent use of co-released inhibitory transmitters at central synapses. J. Neurosci. 2005;25:6490–6498. doi: 10.1523/JNEUROSCI.1500-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumba JS, Irish PS, Anderson NL, Westrum LE. Electron microscopic analysis of gamma-aminobutyric acid and glycine colocalization in rat trigeminal subnucleus caudalis. Brain Res. 1998;806:16–25. doi: 10.1016/s0006-8993(98)00688-x. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Karczmar AG, Wu SY, Shen E. Putative transmitter systems of mammalian sympathetic preganglionic neurons. Acta Neurobiol. Exp. (Warsz) 1993;53:53–63. [PubMed] [Google Scholar]
- Dun NJ, Wu SY, Shen E, Miyazaki T, Dun SL, Ren C. Synaptic mechanisms in sympathetic preganglionic neurons. Can. J. Physiol. Pharmacol. 1992;70(Suppl):S86–S91. doi: 10.1139/y92-248. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH. Distribution of bulbospinal gamma-aminobutyric acid-synthesizing neurons of the ventral respiratory group of the rat. J. Comp. Neurol. 1999;411:130–144. doi: 10.1002/(sici)1096-9861(19990816)411:1<130::aid-cne10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Tobin AJ, Houser CR. Comparative localization of mRNAs encoding two forms of glutamic acid decarboxylase with nonradioactive in situ hybridization methods. J. Comp. Neurol. 1993;331:339–362. doi: 10.1002/cne.903310305. [DOI] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog. Neurobiol. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neurosci. 2004;127:409–417. doi: 10.1016/j.neuroscience.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neurosci. 2006;141:1011–1023. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Kondo M. Glycine is used as a transmitter by decrementing expiratory neurons of the ventrolateral medulla in the rat. J. Neurosci. 2003;23:8941–8948. doi: 10.1523/JNEUROSCI.23-26-08941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y, Otake K. Axonal projections of pulmonary slowly adapting receptor relay neurons in the rat. J. Comp. Neurol. 2002;446:81–94. doi: 10.1002/cne.10185. [DOI] [PubMed] [Google Scholar]
- Fedorko L, Duffin J, England S. Inhibition of inspiratory neurons of the nucleus retroambigualis by expiratory neurons of the Botzinger complex in the cat. Exp. Neurol. 1989;106:74–77. doi: 10.1016/0014-4886(89)90146-5. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Neurophysiology of breathing in mammals. In: Bloom FE, editor. Handbook of Physiology: The Nervous System. IV. American Physiological Society; Bethesda: 1986. pp. 463–524. [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, Plasticity, Chemosensitivity. Annu. Rev. Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein G, Zerbe RL, Faden AI. Opiate receptors and cardiovascular control in conscious SHR and WKY rats. Hypertension. 1983;5:663–671. doi: 10.1161/01.hyp.5.5.663. [DOI] [PubMed] [Google Scholar]
- Finley JCW, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;571(2):108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Fortuna MG, West GH, Stornetta RL, Guyenet PG. Bötzinger expiratory-augmenting neurons and the parafacial respiratory group. J. Neurosci. 2008;28:2506–2515. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc. Natl. Acad. Sci. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. TINS. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gannon RL, Terrian DM. Presynaptic modulation of glutamate and dynorphin release by excitatory amino acids in the guinea-pig hippocampus. Neurosci. 1991;41:401–410. doi: 10.1016/0306-4522(91)90336-m. [DOI] [PubMed] [Google Scholar]
- Gaultier C, Trang H, Dauger S, Gallego J. Pediatric disorders with autonomic dysfunction: what role for PHOX2B? Pediatr. Res. 2005;58:1–6. doi: 10.1203/01.PDR.0000166755.29277.C4. [DOI] [PubMed] [Google Scholar]
- Gozal D. Congenital central hypoventilation syndrome: an update. Pediatr. Pulmonol. 1998;26:273–282. doi: 10.1002/(sici)1099-0496(199810)26:4<273::aid-ppul7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat. Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the PreBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J. Neurosci. 2002;22:3806–3816. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Weston MC, McQuiston T, Simmons JR. Detection of amino acid and peptide transmitters in physiologically identified brainstem cardiorespiratory neurons. Auton. Neurosci. 2004;114:1–10. doi: 10.1016/j.autneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Wang H. Pre-Botzinger neurons with preinspiratory discharges “in vivo” express NK1 receptors in the rat. J. Neurophysiol. 2001;86:438–446. doi: 10.1152/jn.2001.86.1.438. [DOI] [PubMed] [Google Scholar]
- Harfstrand A, Fuxe K, Kalia M, Agnati LF. Somatostatin induced apnoea: prevention by central and peripheral administration of the opiate receptor blocking agent naloxone. Acta Physiol. Scand. 1985;125:91–95. doi: 10.1111/j.1748-1716.1985.tb07695.x. [DOI] [PubMed] [Google Scholar]
- Hassen AH, Feuerstein G, Faden AI. Selective cardiorespiratory effects mediated by mu opioid receptors in the nucleus ambiguus. Neuropharmacol. 1984;23:407–415. doi: 10.1016/0028-3908(84)90248-x. [DOI] [PubMed] [Google Scholar]
- Hassen AH, Feuerstein G, Pfeiffer A, Faden AI. Delta versus mu receptors: cardiovascular and respiratory effects of opiate agonists microinjected into nucleus tractus solitarius of cats. Regul. Pept. 1982;4:299–309. doi: 10.1016/0167-0115(82)90140-9. [DOI] [PubMed] [Google Scholar]
- Hayar A, Guyenet PG. Pre- and postsynaptic inhibitory actions of methionine-enkephalin on identified bulbospinal neurons of the rat RVL. J. Neurophysiol. 1998;80:2003–2014. doi: 10.1152/jn.1998.80.4.2003. [DOI] [PubMed] [Google Scholar]
- Hayar A, Guyenet PG. α2-adrenoceptor-mediated presynaptic inhibition in bulbospinal neurons of rostral ventrolateral medulla. Am. J. Physiol. Heart Circ. Physiol. 1999;277:H1069–H1080. doi: 10.1152/ajpheart.1999.277.3.H1069. [DOI] [PubMed] [Google Scholar]
- Hayes JA, Del Negro CA. Neurokinin receptor-expressing pre-botzinger complex neurons in neonatal mice studied in vitro. J. Neurophysiol. 2007;97:4215–4224. doi: 10.1152/jn.00228.2007. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J. Neurosci. 2001;21:NIL1–NIL6. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neurosci. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Hisano S, Sawada K, Kawano M, Kanemoto M, Xiong G, Mogi K, Sakata-Haga H, Takeda J, Fukui Y, Nogami H. Expression of inorganic phosphate/vesicular glutamate transporters (BNPI/VGLUT1 and DNPI/VGLUT2) in the cerebellum and precerebellar nuclei of the rat. Brain Res. Mol. Brain Res. 2002;107:23–31. doi: 10.1016/s0169-328x(02)00442-4. [DOI] [PubMed] [Google Scholar]
- Hurlë MA, Mediavilla A, Flörez J. Differential respiratory patterns induced by opioids applied to the ventral medullary and dorsal pontine surfaces of cats. Neuropharmacology. 1985;24:597–606. doi: 10.1016/0028-3908(85)90100-5. [DOI] [PubMed] [Google Scholar]
- Iasnetsov VV, Pravdivtsev VA, Motin VG. Effect of beta-endorphin, enkephalins and their synthetic analogs on the neuronal electrical activity of the respiratory center in the medulla oblongata, Biull. Eksp. Biol. Med. 1984;98:687–690. [PubMed] [Google Scholar]
- Iscoe S. Control of abdominal muscles. Prog. Neurobiol. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Iverfeldt K, Serfozo P, Diaz AL, Bartfai T. Differential release of coexisting neurotransmitters: frequency dependence of the efflux of substance P, thyrotropin releasing hormone and [3H]serotonin from tissue slices of rat ventral spinal cord. Acta Physiol. Scand. 1989;137:63–71. doi: 10.1111/j.1748-1716.1989.tb08721.x. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J. Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Botzinger complex in the cat. Exp. Brain Res. 1990;81(3):639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Coles SK, Dick TE. A ‘pneumotaxic centre’ in rats. Neurosci. Lett. 1994;172:67–72. doi: 10.1016/0304-3940(94)90664-5. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Jursky F, Nelson N. Localization of glycine neurotransmitter transporter (GLYT2) reveals correlation with the distribution of glycine receptor. J. Neurochem. 1995;64:1026–1033. doi: 10.1046/j.1471-4159.1995.64031026.x. [DOI] [PubMed] [Google Scholar]
- Jursky F, Tamura S, Tamura A, Mandiyan S, Nelson H, Nelson N. Structure, function and brain localization of neurotransmitter transporters. J. Exp. Biol. 1994;196:283–295. doi: 10.1242/jeb.196.1.283. [DOI] [PubMed] [Google Scholar]
- Katsurabayashi S, Kubota H, Higashi H, Akaike N, Ito Y. Distinct profiles of refilling of inhibitory neurotransmitters into presynaptic terminals projecting to spinal neurones in immature rats. J. Physiol. 2004;560:469–478. doi: 10.1113/jphysiol.2004.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Onimaru H, Inoue M, Inoue T, Sasa R. Localization and properties of respiratory neurons in the rostral pons of the newborn rat. Neurosci. 2005;134:317–325. doi: 10.1016/j.neuroscience.2005.03.049. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J. Neurosci. 2008;28:2353–2365. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp J, Feltz P. Excitatory postsynaptic currents and glutamate receptors in neonatal rat sympathetic preganglionic neurons in vitro. J. Neurophysiol. 1995;73:1503–1512. doi: 10.1152/jn.1995.73.4.1503. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J. Appl. Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Wu SY, Lai CC, Dun NJ. GABA- and glycine-mediated inhibitory postsynaptic potentials in neonatal rat rostral ventrolateral medulla neurons in vitro. Neurosci. 1998;82:429–442. doi: 10.1016/s0306-4522(97)00294-7. [DOI] [PubMed] [Google Scholar]
- Lin LH, Talman WT. Nitroxidergic neurons in rat nucleus tractus solitarii express vesicular glutamate transporter 3. J. Chem. Neuroanat. 2005;29:179–191. doi: 10.1016/j.jchemneu.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Lipski J, Ezure K, She RBW. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J. Physiol. 1991;443:55–78. doi: 10.1113/jphysiol.1991.sp018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Kubin L, Jodkowski J. Synaptic action of R beta neurons on phrenic motoneurons studied with spike-triggered averaging. Brain Res. 1983;288:105–118. doi: 10.1016/0006-8993(83)90085-9. [DOI] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J. Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Liu JP, Liu YY, Wong-Riley MT, Liu HL, Ju G. A group of neurokinin-1 receptor-immunoreactive neurons expressing phospho-extracellular signal-regulated protein kinases in the pre-Botzinger complex of rats. J. Neurosci. Res. 2005;80:260–267. doi: 10.1002/jnr.20445. [DOI] [PubMed] [Google Scholar]
- Liu QK, Wong-Riley MTT. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Botzinger complex. J. Appl. Physiol. 2002;92:923–934. doi: 10.1152/japplphysiol.00977.2001. [DOI] [PubMed] [Google Scholar]
- Liu YY, Ju G, Wong-Riley MTT. Distribution and colocalization of neurotransmitters and receptors in the pre-Botzinger complex of rats. J. Appl. Physiol. 2001;91:1387–1395. doi: 10.1152/jappl.2001.91.3.1387. [DOI] [PubMed] [Google Scholar]
- Liu YY, Wong-Riley MT, Liu JP, Wei XY, Jia Y, Liu HL, Fujiyama F, Ju G. Substance P and enkephalinergic synapses onto neurokinin-1 receptor-immunoreactive neurons in the pre-Botzinger complex of rats. Eur. J. Neurosci. 2004;19:65–75. doi: 10.1111/j.1460-9568.2004.03099.x. [DOI] [PubMed] [Google Scholar]
- Liu YY, Wong-Riley MTT, Liu JP, Jia Y, Liu HL, Jiao XY, Ju G. GABAergic and glycinergic synapses onto neurokinin-1 receptor-immunoreactive neurons in the pre-Botzinger complex of rats: light and electron microscopic studies. Eur. J. Neurosci. 2002;16:1058–1066. doi: 10.1046/j.1460-9568.2002.02163.x. [DOI] [PubMed] [Google Scholar]
- Livingston CA, Berger AJ. Immunocytochemical localization of GABA in neurons projecting to the ventrolateral nucleus of the solitary tract. Brain Res. 1989;494:43–50. doi: 10.1016/0006-8993(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Minson JB, Pilowsky PM, Arnolda LF, Chalmers JP. The one hundred percent hypothesis: Glutamate or GABA in synapses on sympathetic preganglionic neurons. Clin. Exp. Hypertens. 1995;17:323–333. doi: 10.3109/10641969509087074. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Schreihofer AM, Guyenet PG. Distribution and amino acid content of enkephalin-immunoreactive inputs onto juxtacellularly-labeled bulbospinal barosensitive neurons in rat rostral ventrolateral medulla. Neurosci. 2001;108:307–322. doi: 10.1016/s0306-4522(01)00415-8. [DOI] [PubMed] [Google Scholar]
- Lu T, Rubio ME, Trussell LO. Glycinergic Transmission Shaped by the Corelease of GABA in a Mammalian Auditory Synapse. Neuron. 2008;57:524–535. doi: 10.1016/j.neuron.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Lumsden T. Observations on the respiratory centres in the cat. J. Physiol. 1923;57:153–160. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JM, Hokfelt T. Coexistence of peptides and classical neurotransmitters. TINS. 1983;6:325–333. [Google Scholar]
- Makeham JM, Goodchild AK, Pilowsky PM. NK1 receptor and the ventral medulla of the rat: bulbospinal and catecholaminergic neurons. NeuroReport. 2001;12:3663–3667. doi: 10.1097/00001756-200112040-00012. [DOI] [PubMed] [Google Scholar]
- Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J. Physiol. 2006;572:881–896. doi: 10.1113/jphysiol.2005.103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera I, Bachetti T, Puppo F, Di DM, Morandi F, Casiraghi GM, Cilio MR, Hennekam R, Hofstra R, Schober JG, Ravazzolo R, Ottonello G, Ceccherini I. PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset Central Hypoventilation syndrome. J. Med. Genet. 2004;41:373–380. doi: 10.1136/jmg.2003.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall RB. Effects of putative neurotransmitters on sympathetic preganglionic neurons. Annu. Rev. Physiol. 1988;50:553–564. doi: 10.1146/annurev.ph.50.030188.003005. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: Fates of glutamate in brain. J. Neurosci. Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG, Fedorko L. Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Botzinger neurons. J. Neurosci. 1984;4:2350–2353. doi: 10.1523/JNEUROSCI.04-09-02350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG, Lipski J, Kubin L, Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res. 1983;263:43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kobayashi H, Tosaka T. Presynaptic inhibition by noradrenaline of the EPSC evoked in neonatal rat sympathetic preganglionic neurons. Brain Res. 1998;790:170–177. doi: 10.1016/s0006-8993(97)01549-7. [DOI] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J. Physiol. 2006;577:369–386. doi: 10.1113/jphysiol.2006.115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J. Neurosci. 2007a;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J. Neurosci. 2007b;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J. Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J. Neurophysiol. 1999;82:1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- Okazaki M, Takeda R, Haji A, Yamazaki H. Glutamic acid decarboxylase-immunoreactivity of bulbar respiratory neurons identified by intracellular recording and labeling in rats. Brain Res. 2001;914:34–47. doi: 10.1016/s0006-8993(01)02788-3. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Respiratory rhythm generator neurons in medulla of brainstem- spinal cord preparation from newborn rat. Brain Res. 1987;403:380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J. Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J. Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Ono K, Shiba K, Nakazawa K, Shimoyama I. Synaptic origin of the respiratory-modulated activity of laryngeal motoneurons. Neurosci. 2006;140:1079–1088. doi: 10.1016/j.neuroscience.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y, Ezure K. Projections from the commissural subnucleus of the solitary tract onto catecholamine cell groups of the ventrolateral medulla. Neurosci. Lett. 1993;149:213–216. doi: 10.1016/0304-3940(93)90774-f. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Feuerstein G, Kopin IJ, Faden AI. Cardiovascular and respiratory effects of mu-, delta- and kappa- opiate agonists microinjected into the anterior hypothalamic brain area of awake rats. J. Pharmacol. Exp. Ther. 1983;225:735–741. [PubMed] [Google Scholar]
- Pilowsky P, Sun QJ, Llewellyn-Smith I, Arnolda L, Chalmers J, Minson J. Phosphate-activated glutaminase immunoreactivity in brainstem respiratory neurons. J. Auton. Nerv. Syst. 1997;63:85–90. doi: 10.1016/s0165-1838(96)00136-1. [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J. Neurosci. Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Viemari JC. Determinants of inspiratory activity. Respir. Physiol. Neurobiol. 2005;147:145–157. doi: 10.1016/j.resp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu. Rev. Neurosci. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Paton JF, Dick TE, St-John WM, Morschel M, Dutschmann M. Modeling the ponto-medullary respiratory network. Respir. Physiol. Neurobiol. 2004;143:307–319. doi: 10.1016/j.resp.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Saether K, Hilaire G, Monteau R. Dorsal and ventral respiratory groups of neurons in the medulla of the rat. Brain Res. 1987;419(12):87–96. doi: 10.1016/0006-8993(87)90571-3. [DOI] [PubMed] [Google Scholar]
- Sagne C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Baroactivated neurons with pulse-modulated activity in the rat caudal ventrolateral medulla express GAD67 mRNA. J. Neurophysiol. 2003;89:1265–1277. doi: 10.1152/jn.00737.2002. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Bötzinger neurons express mRNA for glycinergic transporter 2. J. Comp. Neurol. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Henry JL. Effects of enkephalin and 5-hydroxytryptamine on solitary tract neurones involved in respiration and respiratory reflexes. Brain Res. 1985;327:221–230. doi: 10.1016/0006-8993(85)91515-x. [DOI] [PubMed] [Google Scholar]
- Shiba K, Nakazawa K, Ono K, Umezaki T. Multifunctional Laryngeal Premotor Neurons: Their Activities during Breathing, Coughing, Sneezing, and Swallowing. J. Neurosci. 2007;27:5156–5162. doi: 10.1523/JNEUROSCI.0001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons ML, Terman GW, Drake CT, Chavkin C. Inhibition of glutamate release by presynaptic kappa 1-opioid receptors in the guinea pig dentate gyrus. J. Neurophysiol. 1994;72:1697–1705. doi: 10.1152/jn.1994.72.4.1697. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J. Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex--a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J. Neurosci. 2006;26:300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St.John WM. Diffuse pathways convey efferent activity from rostral pontile pneumotaxic center to medullary respiratory regions. Exp. Neurol. 1986;94:155–165. doi: 10.1016/0014-4886(86)90279-7. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Guyenet PG. Non-radioactive in situ hybridization in combination with tract tracing. In: Zaborszky L, Waterloud F, Lanciego JL, editors. Neuroanatomical Tract Tracing 3: Molecules-Neurons-Systems III. Springer; New York: 2005. [Google Scholar]
- Stornetta RL, McQuiston TJ, Guyenet PG. GABAergic and glycinergic presympathetic neurons of rat medulla oblongata identified by retrograde transport of pseudorabies virus and in situ hybridization. J. Comp. Neurol. 2004;479:257–270. doi: 10.1002/cne.20332. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J. Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Simmons JR, McQuiston TJ, Vujovic N, Weston MC, Guyenet PG. Coexpression of vesicular glutamate transporter-3 and gamma-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J. Comp. Neurol. 2005;492:477–494. doi: 10.1002/cne.20742. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Botzinger complex. J. Comp. Neurol. 2003a;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Schreihofer AM, Pelaez NM, Sevigny CP, Guyenet PG. Preproenkephalin mRNA is expressed by C1 and non-C1 barosensitive bulbospinal neurons in the rostral ventrolateral medulla of the rat. J. Comp. Neurol. 2001;435:111–126. doi: 10.1002/cne.1196. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J. Comp. Neurol. 2002a;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Inspiratory augmenting bulbospinal neurons express both Glutamatergic and enkephalinergic phenotypes. J. Comp. Neurol. 2003b;455:113–124. doi: 10.1002/cne.10486. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/GLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J. Comp. Neurol. 2002b;444:207–220. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J. Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved AF. PNMT-containing catecholaminergic neurons are not necessarily adrenergic. Brain Res. 1989;481:113–118. doi: 10.1016/0006-8993(89)90490-3. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J. Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesions of retrotrapezoid Phox2b-expressing neurons raises the apneic threshold in rats. J. Physiol. 2008 doi: 10.1113/jphysiol.2008.153163. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, West GH, Gwilt JM, Colombari E, Stornetta RL, Guyenet PG. GABAergic pump cells of solitary tract nucleus innervate retrotrapezoid nucleus chemoreceptors. J. Neurophysiol. 2007;98:374–381. doi: 10.1152/jn.00322.2007. [DOI] [PubMed] [Google Scholar]
- Tanaka I, Ezure K, Kondo M. Distribution of glycine transporter 2 mRNA-containing neurons in relation to glutamic acid decarboxylase mRNA-containing neurons in rat medulla. Neurosci. Res. 2003;47:139–151. doi: 10.1016/s0168-0102(03)00192-5. [DOI] [PubMed] [Google Scholar]
- Terman GW, Drake CT, Simmons ML, Milner TA, Chavkin C. Opioid modulation of recurrent excitation in the hippocampal dentate gyrus. J. Neurosci. 2000;20:4379–4388. doi: 10.1523/JNEUROSCI.20-12-04379.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrian DM, Johnston D, Claiborne BJ, nsah-Yiadom R, Strittmatter WJ, Rea MA. Glutamate and dynorphin release from a subcellular fraction enriched in hippocampal mossy fiber synaptosomes. Brain Res. Bull. 1988;21:343–351. doi: 10.1016/0361-9230(88)90146-3. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Cauli B, Champagnat J, Fortin G, Katz DM. Expression of Functional Tyrosine Kinase B Receptors by Rhythmically Active Respiratory Neurons in the Pre-Botzinger Complex of Neonatal Mice. J. Neurosci. 2003;23:7685–7689. doi: 10.1523/JNEUROSCI.23-20-07685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Peever JH, Duffin J. Botzinger-complex expiratory neurons monosynaptically inhibit phrenic motoneurons in the decerebrate rat. Exp. Brain Res. 1998;122:149–156. doi: 10.1007/s002210050502. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Watt C, Spike RC, Sieghart W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J. Neurosci. 1996;16:974–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd ES, Weinberg SM, Berry-Kravis EM, Silvestri JM, Kenny AS, Rand CM, Zhou L, Maher BS, Marazita ML, Weese-Mayer DE. Facial phenotype in children and young adults with PHOX2B-determined congenital central hypoventilation syndrome: quantitative pattern of dysmorphology. Pediatr Res. 2006;59:39–45. doi: 10.1203/01.pdr.0000191814.73340.1d. [DOI] [PubMed] [Google Scholar]
- Tokumasu M, Nakazono Y, Ide H, Akagawa K, Onimaru H. Optical recording of spontaneous respiratory neuron activity in the rat brain stem. Jpn. J. Physiol. 2001;51:613–619. doi: 10.2170/jjphysiol.51.613. [DOI] [PubMed] [Google Scholar]
- Trang H, Laudier B, Trochet D, Munnich A, Lyonnet S, Gaultier C, Amiel J. PHOX2B gene mutation in a patient with late-onset central hypoventilation. Pediatr. Pulmonol. 2004;38:349–351. doi: 10.1002/ppul.20074. [DOI] [PubMed] [Google Scholar]
- Trochet D, Hong SJ, Lim JK, Brunet JF, Munnich A, Kim KS, Lyonnet S, Goridis C, Amiel J. Molecular consequences of PHOX2B missense, frameshift and alanine expansion mutations leading to autonomic dysfunction. Hum. Mol. Genet. 2005;14:3697–3708. doi: 10.1093/hmg/ddi401. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Aston-Jones G. Integration in the ventral medulla and coordination of sympathetic, pain and arousal functions. Clin. Exp. Hypertens. 1995;17:153–165. doi: 10.3109/10641969509087062. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schäfer MKH, Zhu HM, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J. Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne AJM, Stornetta RL, Guyenet PG. Properties of C1 and other ventrolateral medullary neurones with hypothalamic projections in the rat. J. Physiol. (Lond) 1999;517:477–494. doi: 10.1111/j.1469-7793.1999.0477t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilim FS, Cropper EC, Price DA, Kupfermann I, Weiss KR. Peptide Cotransmitter Release from Motorneuron B16 in Aplysia californica: Costorage, Corelease, and Functional Implications. J. Neurosci. 2000;20:2036–2042. doi: 10.1523/JNEUROSCI.20-05-02036.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J. Comp. Neurol. 2001;434:128–146. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L. Adult identified with congenital central hypoventilation syndrome--mutation in PHOX2b gene and late-onset CHS. Am J Respir. Crit Care Med. 2005;171:88. doi: 10.1164/ajrccm.171.1.950. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Silvestri JM, Curran ME, Marazita ML. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am. J. Med. Genet. A. 2003;123:267–278. doi: 10.1002/ajmg.a.20527. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A Shared Vesicular Carrier Allows Synaptic Corelease of GABA and Glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Xie CW, McGinty JF, Lee PH, Mitchell CL, Hong JS. A glutamate antagonist blocks perforant path stimulation-induced reduction of dynorphin peptide and prodynorphin mRNA levels in rat hippocampus. Brain Res. 1991;562:243–250. doi: 10.1016/0006-8993(91)90627-8. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Haji A, Okazaki M, Takeda R. Immunoreactivity for glutamic acid decarboxylase and N-methyl-D-aspartate receptors of intracellularly labeled respiratory neurons in the cat. Neurosci. Lett. 2000;293:61–64. doi: 10.1016/s0304-3940(00)01489-0. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y. Glutamatergic neurons in the Kolliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: a combined retrograde tracing and in situ hybridization study in the rat. Neurosci. Res. 2007;59:341–346. doi: 10.1016/j.neures.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Yokota S, Tsumori T, Ono K, Yasui Y. Phrenic motoneurons receive monosynaptic inputs from the Kolliker-Fuse nucleus: a light- and electron-microscopic study in the rat. Brain Res. 2001;888:330–335. doi: 10.1016/s0006-8993(00)03106-1. [DOI] [PubMed] [Google Scholar]
- Yokota S, Tsumori T, Ono K, Yasui Y. Glutamatergic pathways from the Kolliker-Fuse nucleus to the phrenic nucleus in the rat. Brain Res. 2004;995:118–130. doi: 10.1016/j.brainres.2003.09.067. [DOI] [PubMed] [Google Scholar]