SUMMARY
Diverse families of viruses bind immunoglobulin superfamily (IgSF) proteins located in tight junctions (TJs) and adherens junctions of epithelium and endothelium. However, little is known about the roles of these receptors in the pathogenesis of viral disease. Junctional adhesion molecule-A (JAM-A) is an IgSF protein that localizes to TJs and serves as a receptor for mammalian reovirus. We inoculated wild-type (wt) and isogenic JAM-A−/− mice perorally with reovirus and found that JAM-A is dispensable for viral replication in the intestine but required for systemic dissemination. Reovirus replication in the brain and tropism for discrete neural regions are equivalent in wt and JAM-A−/− mice following intracranial inoculation, suggesting a function for JAM-A in reovirus spread to extra-intestinal sites. JAM-A promotes reovirus infection of endothelial cells, providing a conduit for the virus into the bloodstream. These findings indicate that a broadly expressed IgSF viral receptor specifically mediates hematogenous dissemination in the host.
INTRODUCTION
Diverse viruses use immunoglobulin superfamily (IgSF) molecules as receptors (Vogelmann et al., 2004). Many of these proteins localize to tight junctions (TJs) and adherens junctions that link polarized cells. Junctional adhesion molecule-A (JAM-A) is an IgSF member that serves as a receptor for feline calicivirus (Makino et al., 2006) and all three serotypes of mammalian orthoreovirus (reovirus) (Campbell et al., 2005). JAM-A localizes to TJs of tissue endothelium and epithelium and also is found on leukocytes and platelets (Kornecki et al., 1990; Martin-Padura et al., 1998). JAM-A is comprised of two extracellular Ig-like domains, a short transmembrane region, and a cytoplasmic tail possessing a PDZ-domain-binding motif (Martin-Padura et al., 1998). During an inflammatory response, JAM-A redistributes from cellular junctions to the apical surface and engages circulating leukocytes for diapedesis through endothelium (Weber et al., 2007).
Reoviruses form nonenveloped, double-shelled particles containing a genome of 10 segments of double-stranded RNA (Schiff et al., 2007). The viral S1 gene encodes the σ1 protein, which extends from the virion surface (Furlong et al., 1988) and mediates viral attachment to host-cell receptors (Weiner et al., 1980a; Lee et al., 1981). Virtually all mammals, including humans, serve as hosts for reovirus infection (Virgin et al., 1997). However, reovirus causes disease primarily in the very young (Mann et al., 2002; Tyler et al., 2004). Reovirus is a highly tractable experimental model for studies of viral pathogenesis (Virgin et al., 1997) and has been used in clinical trials for the treatment of aggressive human malignancies (Stoeckel and Hay, 2006).
After inoculation of newborn mice, reovirus disseminates to the central nervous system (CNS) and produces serotype-specific patterns of disease. Type 1 (T1) reovirus spreads primarily by hematogenous routes to the CNS where it infects ependymal cells, leading to nonlethal hydrocephalus (Weiner et al., 1980b; Tyler et al., 1986). In contrast, type 3 (T3) reovirus spreads by neural routes to the CNS and infects neurons, causing lethal encephalitis (Weiner et al., 1980b; Tyler et al., 1986; Morrison et al., 1991). The σ1-encoding S1 gene dictates these dichotomous dissemination and disease patterns (Weiner et al., 1980b; Tyler et al., 1986), presumably by selective recognition of cell-surface receptors by σ1.
The σ1 protein forms an elongated fiber with a compact globular head at the C-terminus (Fraser et al., 1990; Chappell et al., 2002). The σ1 head mediates binding to JAM-A (Barton et al., 2001b), whereas residues in the σ1 tail, close to the midpoint of the molecule, mediate binding of T3 σ1 to sialic acid (Chappell et al., 1998; Chappell et al., 2000). A region in the tail just beneath the head mediates T1 σ1 binding to a carbohydrate that has not been determined (Chappell et al., 2000). Residues in the membrane-distal Ig domain of JAM-A that mediate homodimer formation are required for efficient engagement of σ1 (Forrest et al., 2003; Guglielmi et al., 2007). Despite elucidation of these key structural and functional aspects of reovirus-JAM-A interactions, the role of JAM-A in reovirus pathogenesis is unknown.
In this study, we used wild-type (wt) and isogenic JAM-A-null mice to define the function of JAM-A in reovirus disease. We found that JAM-A is not necessary for viral replication in the intestine after peroral inoculation, replication in the brain after intracranial inoculation, or viral access to neural routes of dissemination. However, JAM-A is required for efficient infection of primary endothelial cells, establishment of viremia, and systemic dissemination. These findings highlight a new role for junction-associated virus receptors in mediating hematogenous viral dissemination within the infected host.
RESULTS
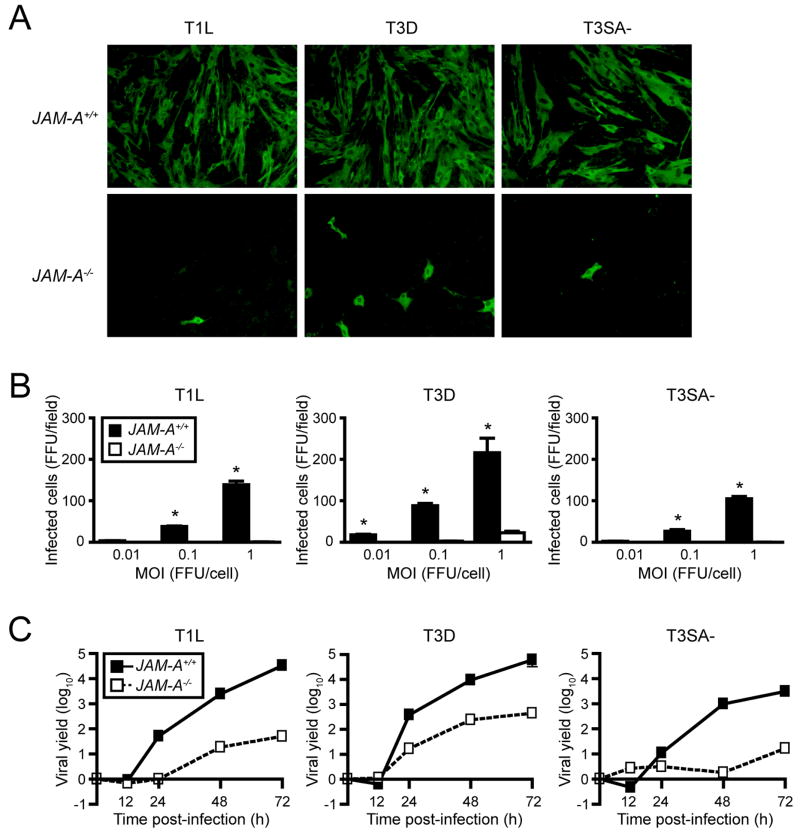
T1 and T3 reoviruses efficiently infect wt but not JAM-A−/− MEFs
To determine whether genetic deletion of JAM-A alters reovirus infection of cells, we infected primary mouse embryonic fibroblasts (MEFs) isolated from wt and isogenic JAM-A−/− mice with T1 and T3 reovirus strains at various multiplicities of infection (MOIs). We used prototype strains T1 Lang (T1L) and T3 Dearing (T3D) and a reassortant virus, T3SA-. The T3SA- genome consists of nine gene segments from T1L and the S1 gene segment from non-sialic-acid-binding strain T3C44 (Barton et al., 2001a). Like T3D, T3SA- binds JAM-A and is neurotropic, but unlike T3D, T3SA- can replicate in the intestine and disseminate systemically from that site (Barton et al., 2003). The absence of JAM-A expression by JAM-A−/− MEFs was confirmed by immunoblotting (Supplemental Fig. 1). All strains of reovirus were capable of infecting wt MEFs but not JAM-A−/− MEFs over the course of a single infectious cycle (Figs. 1A and 1B). We quantified viral yields over several days and found that JAM-A is required for efficient replication of reovirus in MEFs (Fig. 1C). T1 and T3 reovirus strains exhibit differences in infection of cell types that segregate with viral proteins not involved in receptor binding or cell entry (Matoba et al., 1991; Matoba et al., 1993). However, strain T3D, which binds sialic acid (Dermody et al., 1990), replicated to higher titer in JAM-A−/− MEFs than did reovirus strains T1L and T3SA-, which do not bind this carbohydrate (Dermody et al., 1990; Barton et al., 2001a), suggesting that the capacity to bind sialic acid enhances reovirus infection of MEFs.
Figure 1. JAM-A Is Required for Efficient Reovirus Infection of MEFs.
(A) Primary MEFs generated from JAM-A+/+ and JAM-A−/− embryos were adsorbed with reovirus T1L, T3D, or T3SA- at MOIs of 0.01, 0.1, and 1 fluorescent focus unit (FFU)/cell and incubated for 20 hr. Reovirus antigen was detected by indirect immunofluorescence. Representative wells after adsorption with 1 FFU/cell are shown.
(B) Infected cells were quantified in five fields of 200X view for triplicate samples. Results are expressed as the mean FFU/field for triplicate experiments. Error bars indicate SD. *, P < 0.01 as determined by Student’s t test.
(C) Confluent monolayers of JAM-A+/+ and JAM-A−/− MEFs were adsorbed with reovirus T1L, T3D, or T3SA- at an MOI of 2 PFU/cell and incubated for the times shown. Viral titers were determined by plaque assay. Results are expressed as mean viral yields (tx/t0) for triplicate experiments. Error bars indicate SD.
JAM-A is required for lethal T3 reovirus disease
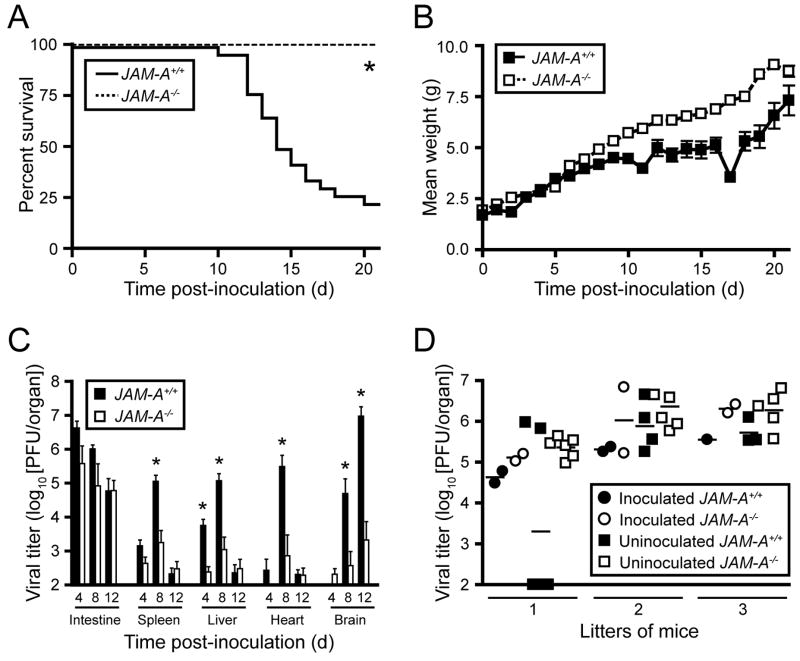
To determine the function of JAM-A in reovirus pathogenesis, we compared susceptibility of wt and JAM-A−/− mice to reovirus infection and disease. We used T3SA-, which is neurotropic but incapable of binding sialic acid, to isolate the role of JAM-A from the role of sialic acid in reovirus pathogenesis. Newborn wt and JAM-A−/− mice were inoculated perorally with escalating doses of T3SA-, from 10 to 109 plaque forming units (PFU), and survival was assessed as a function of dose. The percentage of wt mice that succumbed rose with increasing dose, yielding an LD50 value of 1.9 × 107 PFU (Supplemental Table 1). Infected mice developed clinical signs of encephalitis including lethargy, flaccid paralysis, spastic movements, unsteady gait, and seizures. In sharp contrast, no JAM-A−/− animals succumbed to lethal encephalitis following inoculation with T3SA- at any dose tested (Supplemental Table 1), nor did any JAM-A−/− mouse demonstrate detectable neurological signs. At the highest dose of T3SA- tested, 109 PFU, the majority of wt mice died, whereas all JAM-A−/− mice survived (Fig. 2A). As a quantitative indicator of disease progression, surviving wt mice inoculated with 109 PFU gained weight less rapidly than similarly inoculated JAM-A−/− mice (Fig. 2B). Taken together, these results demonstrate that JAM-A is required for lethal encephalitis following peroral inoculation of T3SA-.
Figure 2. Reovirus T3SA- Is Attenuated following Peroral Inoculation of JAM-A−/− Mice.
(A–B) Newborn JAM-A+/+ and JAM-A−/− mice were inoculated perorally with 109 PFU of reovirus T3SA-. Mice (n = 26 JAM-A+/+ and n = 16 JAM-A−/−) were monitored for survival (A) and weight gain (B). *, P < 0.0001 as determined by log rank test.
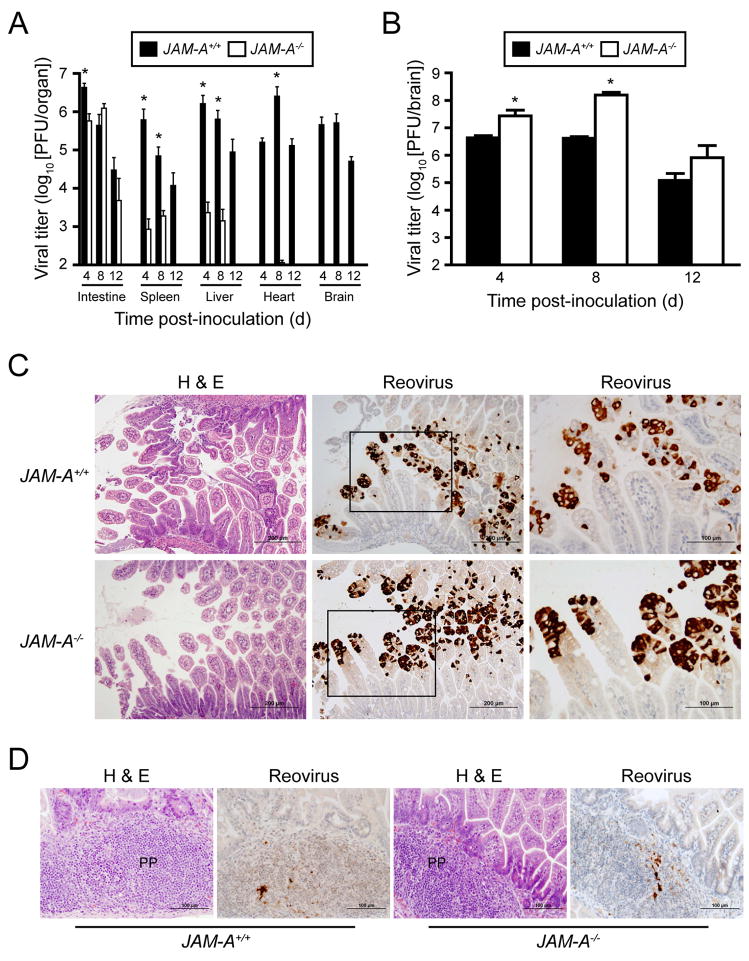
(C) Newborn JAM-A+/+ and JAM-A−/− mice were inoculated perorally with 104 PFU T3SA-. At days 4, 8, and 12 after inoculation, mice were euthanized, organs were resected, and viral titers were determined by plaque assay. Results are expressed as mean viral titers for 6–13 animals for each time point. Error bars indicate SD. *, P < 0.05 by Student’s t test.
(D) One-two newborn JAM-A+/+ and two newborn JAM-A−/− mice from litters of 4 to 8 animals were inoculated perorally with 104 PFU T3SA- and reunited with uninoculated littermates. At day 12 after inoculation, mice were euthanized, intestines were resected, and viral titers were determined by plaque assay. Each data point represents one animal. Horizontal bars indicate the arithmetic mean of log-transformed data.
To determine the extent of viral replication in infected mice, we inoculated newborn wt and JAM-A−/− mice perorally with 104 PFU T3SA-, a sub-LD50 value, and quantified viral titer in organs at various intervals post-inoculation. Titers in the intestine are negligible at days 4 and 8 following peroral inoculation of reovirus strains that do not replicate in the intestine, such as T3D (Bodkin and Fields, 1989). Therefore, viral titer in the intestine at and after 4 days post-inoculation is indicative of reovirus replication at that site. Remarkably, T3SA- produced equivalent titers of virus in the intestine of wt and JAM-A−/− mice. However, viral titers at sites of secondary infection, including the spleen, liver, heart, and brain, were substantially greater in wt animals than in JAM-A−/− mice (Fig. 2C). Thus, following peroral inoculation, JAM-A is dispensable for reovirus replication in the intestine but is required for production of maximal reovirus titer at sites of secondary infection, including the CNS.
To test whether JAM-A promotes transmission of reovirus between infected and uninfected mice, we inoculated one-two newborn wild-type and JAM-A−/− mice from each of three litters perorally with 104 PFU of T3SA- and placed these animals with their littermates immediately after inoculation. Viral titers in intestines of inoculated and uninoculated mice were determined at day 12 post-inoculation. T3SA- replication was observed in the intestines of all inoculated wild-type and JAM-A−/− mice (Fig. 2D). Remarkably, 17 of 17 uninoculated JAM-A−/− littermates and 9 of 13 uninoculated wild-type littermates harbored viral titer in the intestine (Fig. 2D). These results indicate that JAM-A is not required for transmission of reovirus between littermates.
JAM-A is required for efficient dissemination of T3SA- reovirus
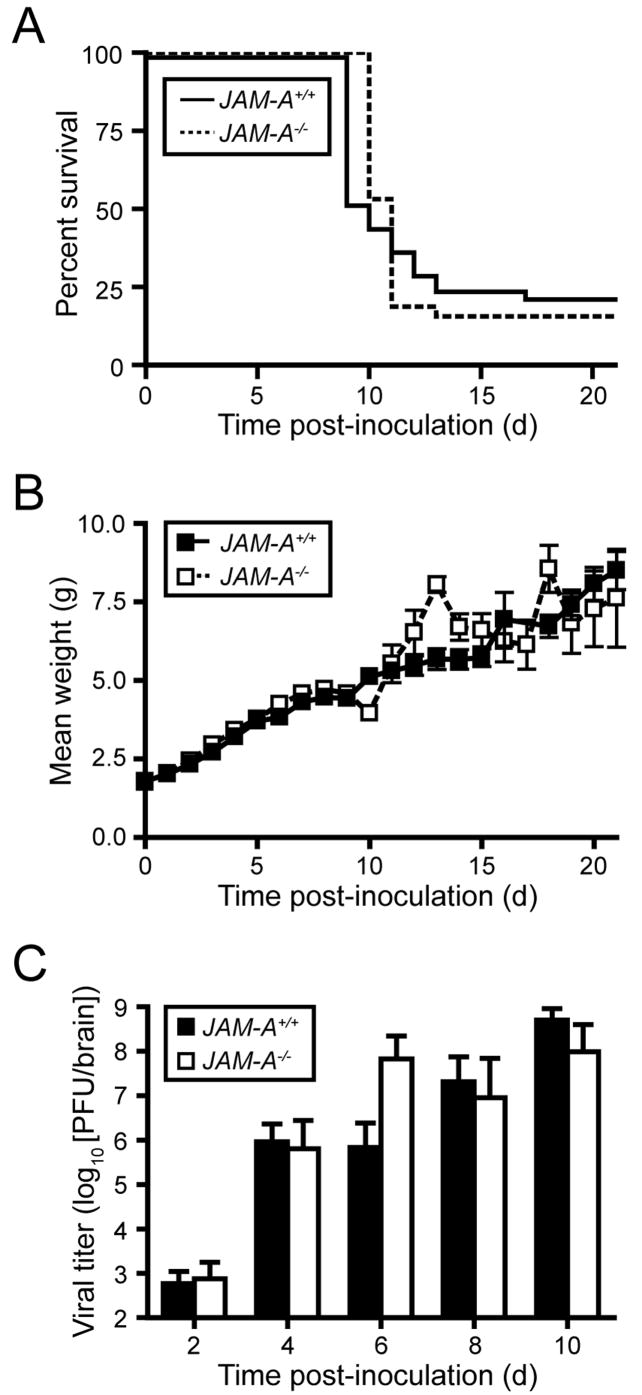
We thought it possible that reovirus might be incapable of either dissemination to sites of secondary infection in JAM-A−/− mice or establishing infection once reaching those sites. To distinguish between these possibilities, we inoculated wt and JAM-A−/− mice intracranially with escalating doses of reovirus T3SA- and assessed survival as a function of dose. Both wt and JAM-A−/− mice succumbed to lethal encephalitis, yielding LD50 values of 52 and 41 PFU, respectively (Supplemental Table 2). At a dose of 100 PFU T3SA- delivered intracranially, wt and JAM-A−/− animal survival and alterations in weight gain were statistically indistinguishable (Figs. 3A and 3B). Concordantly, reovirus reached equivalent titer in the brains of wt and JAM-A−/− mice following intracranial inoculation (Fig. 3C). That reovirus is incapable of replication in the brain of JAM-A−/− mice following peroral inoculation but fully virulent following direct inoculation into the brain of these animals suggests a role for JAM-A in systemic dissemination of reovirus from the intestine to target organs.
Figure 3. Reovirus T3SA- Is Fully Virulent following Intracranial Inoculation of JAM-A−/− Mice.
(A–B) Newborn JAM-A+/+ and JAM-A−/− mice were inoculated intracranially with 100 PFU of reovirus T3SA-. Mice (n = 40 JAM-A+/+ and n = 32 JAM-A−/−) were monitored for survival (A) and weight gain (B).
(C) Newborn JAM-A+/+ and JAM-A−/− mice were inoculated intracranially with 100 PFU T3SA-. At days 2, 4, 6, 8, and 10 after inoculation, mice were euthanized, brains were resected, and viral titers were determined by plaque assay. Results are expressed as mean viral titers for 5–13 animals for each time point. Error bars indicate SD.
T3 reovirus tropism in the brain is not altered in JAM-A−/− mice
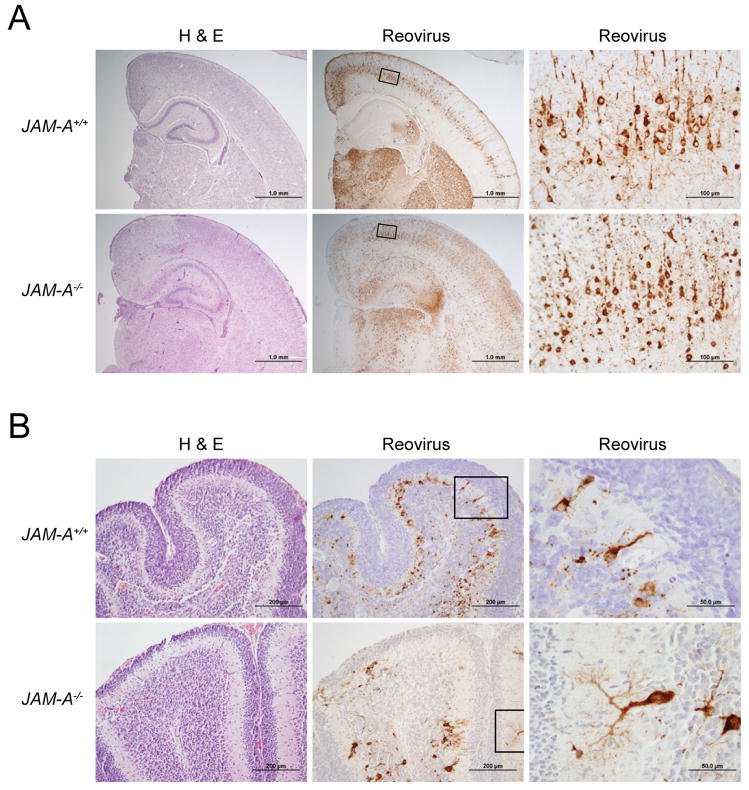
The σ1-encoding S1 gene is the main determinant of reovirus tropism within the CNS (Weiner et al., 1980b). To test whether σ1-JAM-A interactions are required for neural tropism of T3 reovirus, we compared histologic sections of brain from wt and JAM-A−/− mice inoculated intracranially with 104 PFU T3SA-. Brain sections chosen for histologic analysis were matched anatomically based on size and shape of landmarks such as the hippocampus and for viral titer. Hematoxylin and eosin (H&E)-stained sections showed a consistent pattern of injury and inflammation in both wt and JAM-A−/− mice indicative of encephalitis. In both mouse strains, neurons of the hippocampal pyramidal layers were affected, showing abundant eosinophilic, cytoplasmic inclusions and individual cell necrosis and apoptosis. Similar changes were observed in cerebellar Purkinje layer neurons, with relative sparing of granule cells. Other regions consistently affected in both strains of mice included the dorsal thalamic nuclei, hypothalamus, and middle layers of the cerebral cortex (Fig. 4). Immunohistochemistry for reovirus antigen confirmed the presence of virus in hippocampal and cerebellar neurons and cells with neuronal morphology in other affected regions (Fig. 4). Ependymal cells were spared in both genotypes of mice. The reovirus antigen signal appeared more intense in the cortex, especially the outer layers, and in the hippocampus of JAM-A−/− brain when compared to wt brain. There exists some variability in the intensity of antigen staining in individual animals from both strains of mice (Supplemental Fig. 2). However, reovirus targeted the same cell types and brain regions in wt and JAM-A−/− animals. Therefore, JAM-A does not mediate targeting of reovirus to specific regions within the brain.
Figure 4. Histopathology of Reovirus Infection following Intracranial Inoculation.
Newborn JAM-A+/+ and JAM-A−/− mice were inoculated intracranially with 104 PFU T3SA-. Eight days after inoculation, brains of infected mice were resected and bisected sagittally. The left hemisphere was prepared for viral titer determination by plaque assay, and the right hemisphere was processed for histopathology. Consecutive coronal sections were stained with H&E or polyclonal reovirus antiserum. Representative sections of brain hemisphere, matched for hippocampal depth (A), and cerebellum (B) are shown. Boxes indicate areas of enlargement in the panels on the right and show cortical neurons (A) and cerebellar Purkinje neurons (B). JAM-A+/+ brain sections are from brains with left hemisphere viral titers of 4.1 × 109 PFU (A) and 3.0 × 109 PFU (B). JAM-A−/− brain sections are from brains with left hemisphere viral titers of 3.4 × 109 PFU (A) and 1.6 × 109 PFU (B).
JAM-A is required for efficient dissemination of T1L reovirus
To determine whether JAM-A is required for dissemination of reovirus T1L from intestine to brain, we inoculated wt and JAM-A−/− mice perorally with 106 PFU T1L and quantified viral titer in organs at various intervals post-inoculation. Like our results with T3SA-, titers of T1L in the intestines of JAM-A−/− mice were similar to those in wt animals. Titers of T1L at sites of secondary infection, including the spleen, liver, heart, and brain, were substantially greater in wt animals than in JAM-A−/− mice. In fact, there was no detectable T1L titer in the brain of JAM-A−/− mice (Fig. 5A). To confirm that JAM-A is required for T1L reovirus replication in the intestine, we compared histologic sections of intestines from wt and JAM-A−/− mice inoculated perorally with 108 PFU T1L. In both mouse strains, epithelial cells of intestinal villi were affected at early times post-inoculation, showing abundant eosinophilic, cytoplasmic inclusions (Fig. 5C). Immunohistochemistry for reovirus antigen confirmed the presence of virus in villus epithelial cells (Fig. 5C) and mononuclear cells within Peyer’s patches (Fig. 5D).
Figure 5. Reovirus T1L Is Incapable of Dissemination following Peroral Inoculation of JAM-A−/− Mice.
(A) Newborn JAM-A+/+ and JAM-A−/− mice were inoculated perorally with 106 PFU T1L. At days 4, 8, and 12 after inoculation, mice were euthanized, organs were resected, and viral titers were determined by plaque assay. Results are expressed as mean viral titers for 6 animals for each time point. Error bars indicate SD. *, P < 0.005 by Student’s t test. When all values are less than the limit of detection (spleen, liver, heart, and brain in JAM-A−/− mice), a Student’s t test P value cannot be calculated.
(B) Newborn JAM-A+/+ and JAM-A−/− mice were inoculated intracranially with 103 PFU T1L. At days 4, 8, and 12 after inoculation, mice were euthanized, brains were resected, and viral titers were determined by plaque assay. Results are expressed as mean viral titers for 6–11 animals for each time point. Error bars indicate SD. *, P < 0.005 by Student’s t test.
(C–D) Newborn JAM-A+/+ and JAM-A−/− mice were inoculated perorally with 108 PFU T1L. At days 2 and 4 after inoculation, intestines of infected mice were resected and processed for histopathology. Consecutive sections were stained with H&E or polyclonal reovirus antiserum. Representative sections of intestinal villi at day 2 (C) and Peyer’s patches (PP) at day 4 (D) are shown. Boxes indicate areas of enlargement in the panels on the right and show villus epithelial cells.
To determine whether JAM-A is required for T1 reovirus replication in the brain, we inoculated wt and JAM-A−/− mice intracranially with 103 PFU T1L and quantified viral titer in brain homogenates. We found that T1L replicates to equivalent or higher titer in the brain of JAM-A−/− mice in comparison to wt mice (Fig. 5B). That T1L reovirus is incapable of replication in the brains of JAM-A−/− mice following peroral inoculation despite the capacity to replicate in the brain of these animals following intracranial inoculation indicates that JAM-A is required for systemic dissemination of T1 reovirus from the intestine.
Spread of T1 but not T3 reovirus from hindlimb to spinal cord is blunted in JAM-A−/− mice
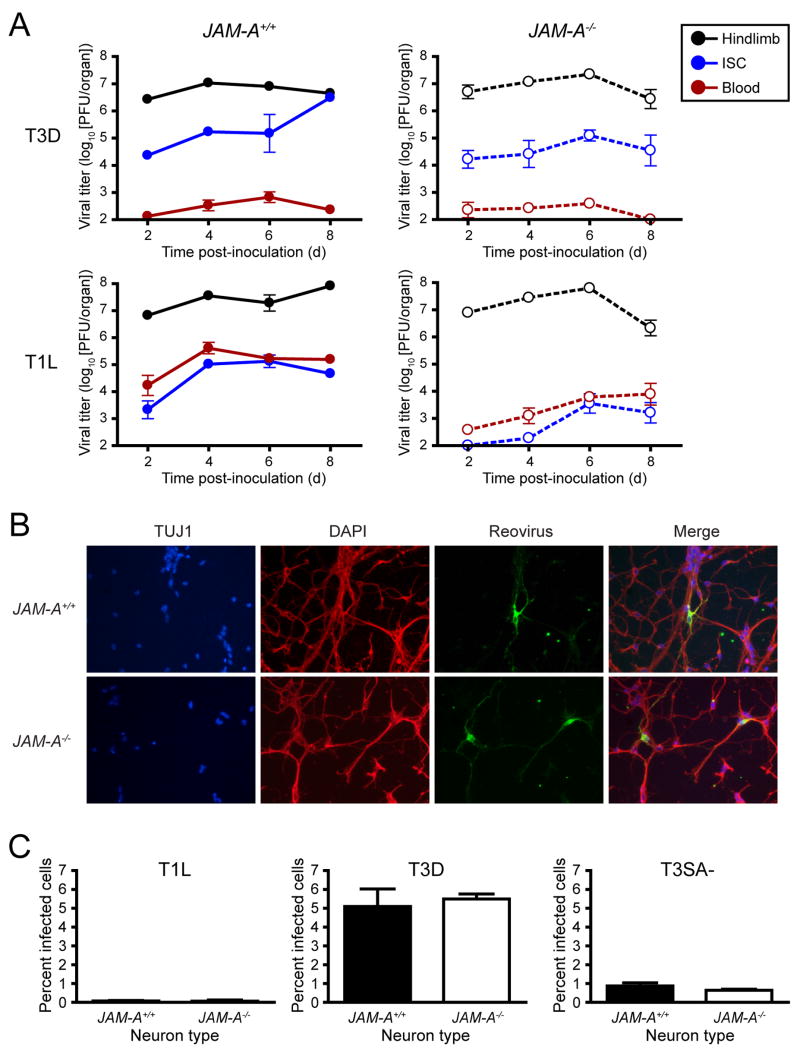
Following inoculation into the hindlimb, section of the sciatic nerve ablates spread of T3D but not T1L to the inferior spinal cord (ISC), indicating that T3D spreads neurally to the spinal cord, whereas T1L spreads primarily hematogenously to that tissue (Tyler et al., 1986). To determine the mechanism by which JAM-A promotes reovirus dissemination, we inoculated wt and JAM-A−/− mice into the hindlimb with 106 PFU of either T1L or T3D and quantified viral titer in the hindlimb, blood, and ISC. After inoculation with T3D, both wt and JAM-A−/− mice harbored high viral titers in the ISC and low titers in the blood, consistent with an intact neural route of spread (Fig. 6A). The ratio of T3D titer in the ISC to titer in the hindlimb is lower on day 8 in JAM-A−/− mice than in wt animals (Supplemental Fig. 3), which may reflect accelerated clearance of T3D in the spinal cord of JAM-A−/− mice in comparison to wt animals. After inoculation of T1L into wt mice, viral titers in blood and ISC increased virtually simultaneously over the experimental timecourse, consistent with a hematogenous route of spread (Fig. 6A). In contrast, the titer of T1L in blood and ISC in JAM-A−/− mice increased with markedly reduced kinetics in comparison to the titer rise in wt animals (Fig. 6A). These findings suggest that JAM-A mediates efficient hematogenous spread of reovirus from the hindlimb but is not required for neural spread from hindlimb to ISC.
Figure 6. JAM-A Is Required for Hematogenous Spread of Reovirus.
(A) Newborn JAM-A+/+ and JAM-A−/− mice were inoculated into the left hindlimb with 106 PFU of either T3D or T1L. At days 2, 4, 6, and 8 after inoculation, mice were euthanized, left hindlimb, blood, and inferior spinal cord (ISC), including the thoracic and lumbosacral cord segments, were resected, and viral titers were determined by plaque assay. Results are expressed as mean viral titers for 6 animals for each time point. Error bars indicate SD.
(B) Primary cortical cultures generated from E15 JAM-A+/+ and JAM-A−/− embryos were cultured in vitro for 5 to 7 days, adsorbed with T3 reovirus at an MOI of 1000 PFU/cell, and incubated for 20 hr. Cells were stained with TUJ1 neural-specific marker to detect neurons (red), 4′,6-diamidino-2-phenylindole (DAPI) to detect nuclei (blue), and polyclonal reovirus antiserum to detect reovirus antigen (green) and visualized using indirect immunofluorescence microscopy. Representative wells from triplicate experiments are shown.
(C) JAM-A+/+ and JAM-A−/− cortical cultures were cultured in vitro for 5 to 7 days and adsorbed with T1L, T3D, or T3SA- at an MOI of 1000 PFU/cell and incubated for 20 hr. The percentage of infected cells was quantified by dividing the number of neurons exhibiting reovirus staining by the total number of cell nuclei exhibiting DAPI staining in three fields of 400X view for triplicate experiments. Fields of view contained between 200 and 600 nuclei. Error bars indicate SD.
Primary cultures of cortical neurons from wt and JAM-A−/− mice support reovirus infection
To directly test whether JAM-A is required for reovirus infection of neurons, we infected primary cortical neuron cultures prepared from embryonic wt and JAM-A−/− mice with T1 and T3 reovirus. The primary cortical neurons displayed classic neuronal morphology and stained for the neuron-specific beta III tubulin marker, TUJ1 (Fig. 6B). The pattern of T3 reovirus antigen staining was identical in wt and JAM-A−/− cortical neurons, which displayed similar morphology and characteristics of reovirus infection (Fig. 6B). In both types of neurons, reovirus-positive inclusions were observed dotting the length of infected axons, a staining pattern consistent with focal sites of reovirus replication (Becker et al., 2003). The total percentage of neurons infected by T3SA- was equivalent in wt and JAM-A−/− cultures (Fig. 6C). As a control, T1L was incapable of infecting mouse cortical neurons regardless of JAM-A expression (Fig. 6C), consistent with previous observations indicating that primary cultures of neurons are refractory to T1 reovirus infection (Dichter and Weiner, 1984). Thus, JAM-A is fully dispensable for neural tropism of T3 reovirus.
Reovirus is detected in draining lymph nodes but not in the bloodstream of JAM-A−/− mice following peroral inoculation
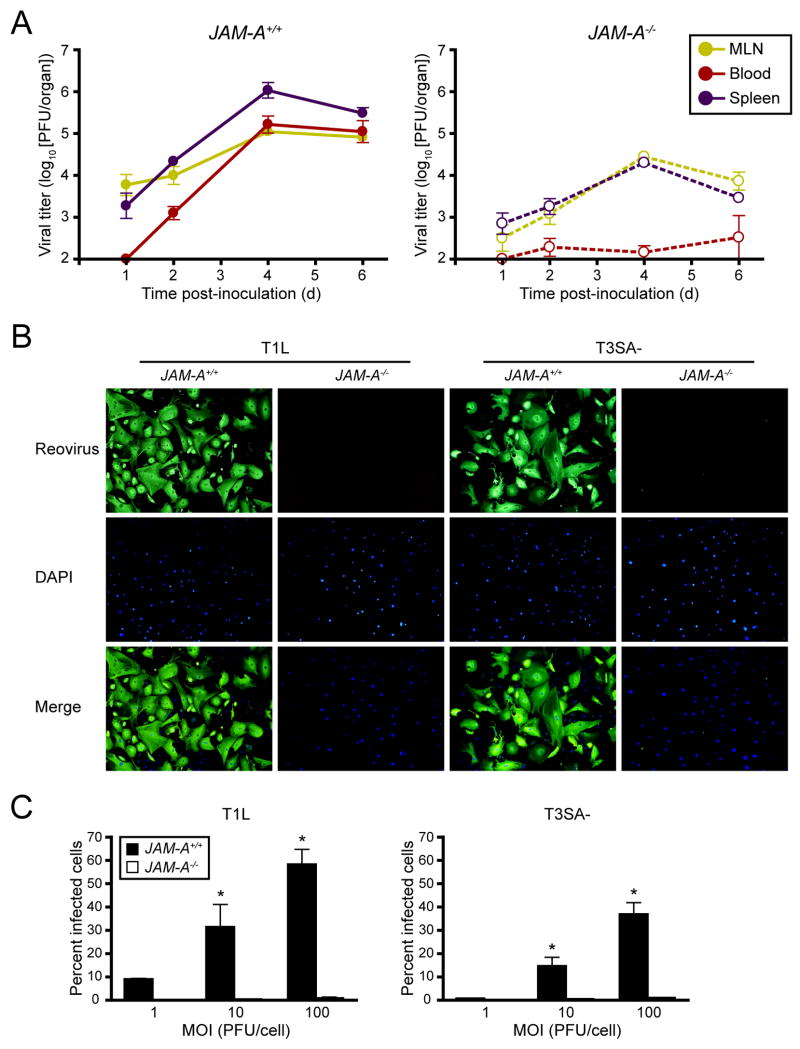
Following peroral inoculation, reovirus T1L disseminates to the mesenteric lymph node (MLN) and spleen within 24 hr (Kauffman et al., 1983). To determine whether JAM-A is required for lymphatic or bloodstream dissemination, we inoculated wt and JAM-A−/− mice perorally with 108 PFU of T1L and quantified viral titer in the MLN, spleen, and blood. In both wt and JAM-A−/− mice, viral titer in the MLN and spleen were detectable at early times post-inoculation and increased over the course of infection, although T1L produced greater titers in wt versus JAM-A−/− mice (Fig. 7A). T1L titer in the blood of wt mice was detectable by day 2 post-inoculation and increased thereafter (Fig. 7A). In sharp contrast, with the exception of one animal per timepoint, there was no detectable titer of T1L in the blood of JAM-A−/− mice (Fig. 7A). Similar results were obtained with T3SA- (Supplemental Fig. 4). Therefore, JAM-A is not required for access to lymphatic routes of dissemination but is required for the establishment of high titer viremia following peroral inoculation of reovirus.
Figure 7. JAM-A Is Required for Viremia and Efficient Reovirus Infection of Endothelial Cells.
(A) Newborn JAM-A+/+ and JAM-A−/− mice were inoculated perorally with 108 PFU of T1L. At days 1, 2, 4, and 6 after inoculation, mice were euthanized, mesenteric lymph node (MLN), blood, and spleen were collected, and viral titers were determined by plaque assay. Results are expressed as mean viral titers for 3–8 animals for each time point. Error bars indicate SD.
(B) Primary endothelial cells generated from JAM-A+/+ and JAM-A−/− mice were adsorbed with either reovirus T1L or T3SA- at an MOI of 100 PFU/cell and incubated for 20 hr. Cells were stained with polyclonal reovirus antiserum to detect reovirus antigen (green) and 4′,6-diamidino-2-phenylindole (DAPI) to detect nuclei (blue) and visualized using indirect immunofluorescence microscopy. Representative wells from triplicate experiments are shown.
(C) JAM-A+/+ and JAM-A−/− primary endothelial cells were adsorbed with T1L or T3SA- at MOIs of 1, 10, and 100 PFU/cell and incubated for 20 hr. The percentage of infected cells was quantified by dividing the number of cells exhibiting reovirus staining by the total number of cell nuclei exhibiting DAPI staining in entire wells of 96-well plates for triplicate experiments. Wells contained between 200 and 1600 nuclei. Error bars indicate SD. *, P < 0.05 as determined by Student’s t test.
JAM-A is required for efficient infection of primary endothelial cells
Endothelial cells (ECs) line blood vessels and may serve as a portal for reovirus entry into the circulation. To test whether JAM-A is required for reovirus infection of ECs, we infected primary mouse lung endothelial cells prepared from adult wt and JAM-A−/− mice with T1L and T3SA-. The ECs displayed classic cobblestone morphology (Supplemental Fig. 5) and differed in JAM-A expression (Supplemental Fig. 5). Both strains of reovirus were capable of infecting wt but not JAM-A−/− ECs over the course of a single infectious cycle (Figs. 7B and 7C). These results indicate that JAM-A expression is required for efficient infection of mouse ECs by reovirus.
DISCUSSION
By virtue of their capacity to infect the intestine and spread systemically to the CNS, reoviruses offer an ideal experimental system to define how neurotropic viruses interact with their hosts at different steps in the disease process. We found that the IgSF TJ protein, JAM-A, is required for reovirus spread from sites of primary replication to target tissues in infected mice. Reovirus strains disseminate systemically via hematogenous, neural, or a combination of both routes (Kauffman et al., 1983; Tyler et al., 1986; Morrison et al., 1991). Spread of T3D from hindlimb to spinal cord, which occurs by neural routes (Tyler et al., 1986), is not dependent on JAM-A. In contrast, spread of T1L from hindlimb to spinal cord, which occurs by hematogenous routes (Tyler et al., 1986), requires JAM-A expression. Thus, JAM-A mediates hematogenous but not neural dissemination of reovirus.
After introduction into the intestinal lumen, reovirus transcytoses through intestinal epithelial M cells that overlie PPs (Wolf et al., 1981; Newberry, 2008). Reovirus infects PP cells (Wolf et al., 1981; Bass et al., 1988) and is thought to spread through lymph or blood vessels to villi surrounding PPs, where significant replication occurs in epithelial cells (Rubin et al., 1985). Reovirus is detectable in MLN and spleen within 24 hr post-inoculation and found subsequently in the blood and virtually every tissue in the infected host (Kauffman et al., 1983). Viruses can establish viremia by transport through lymphatics, infection of endothelial cells, and infection of blood leukocytes (Nathanson and Tyler, 1997). However, mechanisms of reovirus viremia are unknown. Titers of T1L and T3SA- in the blood are diminished or undetectable in all JAM-A−/− mice following peroral inoculation. However, both strains of reovirus are capable of reaching the spleen in JAM-A−/− mice following peroral inoculation, albeit in diminished titers in comparison to those in wt animals. Since the only hematogenous pathway from the intestine to the spleen is via the blood, the presence of virus in the spleen of JAM-A−/− mice following peroral inoculation indicates that some virus likely escapes into the bloodstream in these animals, although titers in blood are reduced below the limit of detection for most JAM-A−/− mice. Lymphatic spread of T1L from intestine to MLN is diminished but not abolished in JAM-A−/− animals in comparison to wt. Most strikingly, JAM-A is required for infection of primary endothelial cells. Thus, two important routes for the establishment of viremia - spread through lymphatics and infection of endothelial cells - are impaired in JAM-A−/− mice.
How might JAM-A promote entry of reovirus into the lymph and bloodstream? We envision three possibilities. First, virus binding to JAM-A might lead to productive infection of lymphatic and blood endothelial cells with subsequent apical release of progeny virions into the circulation. In the case of murine cytomegalovirus, the majority of viral load found in the bloodstream within the first 48 hr post-infection originates in endothelial cells (Sacher et al., 2008). In support of this possibility, reovirus infection of polarized airway epithelial cells leads to apical release of virus with little detectable cytopathic effect (Excoffon et al., 2008). Second, JAM-A might facilitate trafficking of reovirus particles between endothelial cells, analogous to interactions of adenovirus with polarized cells. When the adenovirus attachment protein, fiber, interacts with its TJ-associated receptor, coxsackievirus and adenovirus receptor, junctional integrity is disrupted and virus moves freely between cells (Walters et al., 2002). Although reovirus does not appear to disrupt epithelial TJs (Excoffon et al., 2008), it is possible that reovirus disrupts JAM-A interactions in endothelial TJs to allow bloodstream entry. Third, JAM-A might permit reovirus transport through endothelial cells via a transcellular pathway. Caveolae-mediated transcytosis efficiently transports extracellular molecules across endothelial cells in a receptor-specific manner (Predescu et al., 2007). Adeno-associated virus and HIV-1 can penetrate endothelial cells via transcytosis (Gujuluva et al., 2001; Di Pasquale and Chiorini, 2006). Likewise, reovirus may transcytose through endothelial cells using a JAM-A-dependent mechanism.
Despite the important functions of JAM-A in the host, JAM-A−/− mice are viable, with normal organ development and morphology (Cera et al., 2004). In addition, these mice do not have altered numbers of circulating platelets, lymphocytes, neutrophils, or monocytes (Cera et al., 2004). The intestinal mucosa of JAM-A−/− mice demonstrates normal expression of occludin and claudin-2, upregulation of claudin-10 and claudin-15, and increased permeability to small molecules but not bacteria (Laukoetter et al., 2007). Alterations in the ratios of claudin-10 and claudin-15 can affect the permeability of the epithelium to small molecules (Tsukita et al., 2001; Van Itallie and Anderson, 2006), but the normal expression of TJ structural proteins such as occludin, zonula occludens-1, and E-cadherin (Mandell et al., 2005) suggests that the absence of JAM-A does not lead to overt changes in TJ structure or assembly (Laukoetter et al., 2007). Reovirus virions are ~ 25,000 times larger than the small molecules used in the permeability assays reported previously (Laukoetter et al., 2007) and roughly the size of a TJ. Therefore, we do not think the absence of JAM-A leads to the free flux of reovirus between intestinal epithelium and underlying tissue. This conclusion is supported by intestinal micrographs demonstrating that the absence of JAM-A does not alter the histologic pattern of reovirus intestinal infection. Moreover, any alterations in the permeability of JAM-A−/− intercellular junctions clearly do not enhance the capacity of reovirus to access the lymphatic circulation and the bloodstream following replication in the intestine.
The exquisite neural tropism of reovirus in newborn mice is restricted to T3 strains (Raine and Fields, 1973). The viral gene encoding attachment protein σ1 is the major determinant of reovirus neurovirulence (Weiner et al., 1980b). T3SA- does not bind sialic acid (Barton et al., 2001a), a carbohydrate coreceptor used by some T3 reovirus strains (Dermody et al., 1990). Our finding that JAM-A is not required for reovirus T3SA- replication in the intestine or brain suggests the existence of novel receptors for reovirus at those sites. In parallel with these findings, analysis of histologic brain sections indicates that T3SA- infects the same regions of wt and JAM-A−/− mouse brains: the cortex, CA2-4 regions of the hippocampus, thalamus, and cerebellar Purkinje cells. This regional tropism is identical to that observed using a sialic acid-binding T3 reovirus strain (Richardson-Burns and Tyler, 2004). Moreover, genetic deletion of JAM-A does not alter reovirus infection of primary cultures of neurons. Based on findings reported here, we hypothesize that reovirus replication in neurons is mediated by a serotype-specific receptor other than JAM-A or sialic acid. In addition, since both T1 and T3 reovirus strains can replicate in the intestine of JAM-A−/− mice, we propose that reovirus infection at that site is mediated by a serotype-independent receptor.
Nectin-1, also called poliovirus receptor-related 1 (PVRL1), is a widely expressed IgSF adhesion molecule (Haarr et al., 2001) that serves as a receptor for herpes simplex virus (HSV) (Cocchi et al., 1998; Geraghty et al., 1998). As with reovirus and JAM-A, nectin-1 is required for HSV-2 virulence following intravaginal inoculation (Taylor et al., 2007). However, unlike reovirus and JAM-A, nectin-1 is required for HSV-2 replication at the site of primary replication, the vaginal epithelium (Taylor et al., 2007). Spread of HSV-2 to the dorsal root ganglia and spinal cord from vaginal epithelium is impaired in Pvrl1−/− mice. Although some of these animals succumb to HSV infection, Pvrl1−/− mice are protected from development of external signs of disease, including hair loss, inflammation, and skin lesions, indicating that nectin-1 mediates dissemination from vaginal epithelium to perineal skin and surrounding areas (Taylor et al., 2007). Thus, IgSF member nectin-1 is required for HSV-2 infection of the vaginal epithelium and spread to both perineal skin and the CNS.
The finding that an individual viral receptor can mediate a specific step in pathogenesis has important implications for antiviral strategies. For example, while genetic deletion of JAM-A protects mice from reovirus disease following peroral inoculation, these animals are capable of viral transmission to new hosts. Uninoculated littermates of infected JAM-A−/− mice develop high titers of reovirus in the intestine, suggesting that viral shedding occurs in infected JAM-A−/− mice. In this model, pharmacological blockade of reovirus-JAM-A interactions would diminish disease in treated individuals but have no effect on viral transmission between hosts, an observation that merits consideration in other viral diseases.
Establishment of viremia is a poorly understood process for most viruses. Our work shows that reovirus viremia depends on virus interactions with JAM-A in a limited subset of host tissues. Further dissection of how JAM-A promotes vascular access may provide clues about mechanisms of systemic dissemination of adenovirus, coxsackievirus, HSV, and poliovirus, which also employ junction-associated IgSF proteins as receptors. Moreover, our finding that a broadly-expressed receptor mediates an exquisitely specific aspect of viral pathogenesis suggests that virus-host interactions require multiple receptors that serve unique functions at each step of the disease process.
EXPERIMENTAL PROCEDURES
Cell Lines, Viruses, and Antibodies
L cells were maintained as described (Barton et al., 2001a). Reovirus strains T1L and T3D are laboratory stocks. T3SA- was generated as described (Barton et al., 2001a). Virus was purified after growth in L cells by CsCl-gradient centrifugation (Furlong et al., 1988). Viral titers were determined by either plaque assay (Virgin et al., 1988) or fluorescent focus assay (Barton et al., 2001a). Immunoglobulin G (IgG) fractions of rabbit antisera raised against T1L and T3D (Wetzel et al., 1997) were purified by protein A-Sepharose (Barton et al., 2001a). Fluorescently conjugated secondary Alexa antibodies were obtained from Molecular Probes (Invitrogen).
Mice
C57BL/6J (wt, JAM-A+/+) mice were obtained from Jackson Laboratory. JAM-A−/− mice (Cera et al., 2004) were provided by T. Sato (Cornell University, New York, NY) and backcrossed for ten generations on a C57BL/6J background strain. Disruption of the JAM-A gene was confirmed by PCR and immunoblotting.
MEFs and Primary Cortical Cultures
MEFs were maintained in Dulbecco’s modified Eagle’s medium (Gibco) supplemented to contain 10% FBS (Gibco), 1x MEM non-essential amino acids, 2 mM L-glutamine, 0.1 mM 2-mercaptoethanol, 20 mM HEPES, 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. Cells at passage 3–5 were used for the experiments in this study.
Primary mouse cortical cultures were derived from cortices of E15 wt and JAM-A−/− embryos as described in Supplemental Methods. Viable cells were plated at a density of 2.75 × 105 cells/ml in 24-well plates (Costar) or on glass coverslips placed in 24-well plates (BD). Wells were treated prior to plating with a 10 μg/ml poly-D-lysine solution (BD) and a 1.64 μg/ml laminin solution (BD). Cultures were incubated for the first 24 hr in neurobasal medium (Gibco) supplemented to contain 10% FBS (Gibco), 0.6 mM L-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. Cultures were thereafter maintained in neurobasal medium supplemented to contain 1X B27 (Gibco), 50 units/ml penicillin, and 50 μg/ml streptomycin. A 50% medium change was performed every 3–4 days. Neurons were allowed to mature for 7 days prior to use.
Primary mouse pulmonary microvascular endothelial cells were generated and cultured as described in Supplemental Methods. Cells at passage 3–4 were used for all experiments.
Viral Infectivity
Monolayers of cells were adsorbed with reovirus at various MOIs, fixed after 20 hr, visualized by indirect immunofluorescence, and quantified as described (Barton et al., 2001a).
Virus Replication
Monolayers of cells in 24-well plates (Costar) were adsorbed with reovirus at an MOI of 2 PFU/cell, washed with PBS, and incubated for various intervals. Cells were frozen and thawed twice prior to viral titer determination by plaque assay using L cells. Viral yields were calculated according to the following formula: log10 yieldtx = log10 (PFU/ml)tx − log10 (PFU/ml)t0, where tx is the time post infection.
Infection of Mice
Newborn mice weighing 1.5–2 grams were inoculated perorally (Rubin and Fields, 1980), intracranially (Tyler et al., 1985), or intramuscularly (Tyler et al., 1986) with purified reovirus diluted in PBS. For analysis of virulence, mice were monitored for weight loss and symptoms of disease for 21 days post-inoculation and euthanized when moribund. For analysis of viral replication, mice were euthanized at various intervals following inoculation, and organs were harvested into 1 ml of PBS, frozen and thawed three times, and homogenized by sonication. For analysis of viremia, mice were decapitated at various intervals following inoculation, and whole blood was collected from the neck into a 1 ml syringe containing 100 μl Alsever’s solution (Sigma). Blood in Alsever’s solution was frozen and thawed three times and homogenized by sonication. Viral titers in organ homogenates and blood were determined by plaque assay. Animal husbandry and experimental procedures were performed in accordance with Public Health Service policy and approved by the Vanderbilt University School of Medicine Institutional Animal Care and Use Committee.
Histology
Newborn mice weighing 1.5–2 grams were inoculated intracranially or perorally with reovirus diluted in PBS. At various intervals following inoculation, mice were euthanized and organs were resected. Selected organs were incubated in 10% formalin at RT for 24 hr followed by incubation in 70% ethanol at RT. Fixed organs were embedded in paraffin, and consecutive 6-μm sections were stained with H&E for evaluation of histopathologic changes or processed for immunohistochemical detection of reovirus protein. The left hemispheres of brains were processed for plaque assay.
Supplementary Material
Acknowledgments
We thank Karl Boehme, Jim Chappell, Kristen Guglielmi, Elizabeth Johnson, Kay Washington, and Denise Wetzel for helpful suggestions and review of the manuscript. We thank Tom Sato for providing JAM-A−/− mice. We thank Pam Wirth, Melissa Downing, and Frances Shook from the Vanderbilt Immunohistochemistry Core for sample preparation. This research was supported by Public Health Service awards T32 GM07347 (A.A.R.A.), R37 AI38296, and the Elizabeth B. Lamb Center for Pediatric Research. Additional support was provided by Public Health Service awards P30 CA68485 for the Vanderbilt-Ingram Cancer Center and P60 DK20593 for the Vanderbilt Diabetes Research and Training Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barton ES, Connolly JL, Forrest JC, Chappell JD, Dermody TS. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J Biol Chem. 2001a;276:2200–2211. doi: 10.1074/jbc.M004680200. [DOI] [PubMed] [Google Scholar]
- Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell F, Nusrat A, Parkos CA, Dermody TS. Junction adhesion molecule is a receptor for reovirus. Cell. 2001b;104:441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- Barton ES, Youree BE, Ebert DH, Forrest JC, Connolly JL, Valyi-Nagy T, Washington K, Wetzel JD, Dermody TS. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J Clin Invest. 2003;111:1823–1833. doi: 10.1172/JCI16303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass DM, Trier JS, Dambrauskas R, Wolf JL. Reovirus type 1 infection of small intestinal epithelium in suckling mice and its effect on M cells. Lab Invest. 1988;58:226–235. [PubMed] [Google Scholar]
- Becker MM, Peters TR, Dermody TS. Reovirus σNS and μNS proteins form cytoplasmic inclusion structures in the absence of viral infection. J Virol. 2003;77:5948–5963. doi: 10.1128/JVI.77.10.5948-5963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin DK, Fields BN. Growth and survival of reovirus in intestinal tissue: role of the L2 and S1 genes. J Virol. 1989;63:1188–1193. doi: 10.1128/jvi.63.3.1188-1193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JA, Shelling P, Wetzel JD, Johnson EM, Wilson GAR, Forrest JC, Aurrand-Lions M, Imhof B, Stehle T, Dermody TS. Junctional adhesion molecule-A serves as a receptor for prototype and field-isolate strains of mammalian reovirus. J Virol. 2005;79:7967–7978. doi: 10.1128/JVI.79.13.7967-7978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cera MR, Del Prete A, Vecchi A, Corada M, Martin-Padura I, Motoike T, Tonetti P, Bazzoni G, Vermi W, Gentili F, et al. Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J Clin Invest. 2004;114:729–738. doi: 10.1172/JCI21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell JD, Barton ES, Smith TH, Baer GS, Duong DT, Nibert ML, Dermody TS. Cleavage susceptibility of reovirus attachment protein σ1 during proteolytic disassembly of virions is determined by a sequence polymorphism in the σ1 neck. J Virol. 1998;72:8205–8213. doi: 10.1128/jvi.72.10.8205-8213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell JD, Duong JL, Wright BW, Dermody TS. Identification of carbohydrate-binding domains in the attachment proteins of type 1 and type 3 reoviruses. J Virol. 2000;74:8472–8479. doi: 10.1128/jvi.74.18.8472-8479.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell JD, Prota A, Dermody TS, Stehle T. Crystal structure of reovirus attachment protein σ1 reveals evolutionary relationship to adenovirus fiber. EMBO J. 2002;21:1–11. doi: 10.1093/emboj/21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, Lopez M, Menotti L, Aoubala M, Dubreuil P, Campadelli-Fiume G. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc Natl Acad Sci USA. 1998;95:15700–15705. doi: 10.1073/pnas.95.26.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody TS, Nibert ML, Bassel-Duby R, Fields BN. A sigma 1 region important for hemagglutination by serotype 3 reovirus strains. J Virol. 1990;64:5173–5176. doi: 10.1128/jvi.64.10.5173-5176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale G, Chiorini JA. AAV transcytosis through barrier epithelia and endothelium. Mol Ther. 2006;13:506–516. doi: 10.1016/j.ymthe.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Dichter MA, Weiner HL. Infection of neuronal cell cultures with reovirus mimics in vitro patterns of neurotropism. Ann Neurol. 1984;16:603–610. doi: 10.1002/ana.410160512. [DOI] [PubMed] [Google Scholar]
- Excoffon KJDA, Guglielmi KM, Wetzel JD, Gansemer ND, Campbell JA, Dermody TS, Zabner J. Reovirus preferentially infects the basolateral surface and is released from the apical surface of polarized human respiratory epithelial cells. J Infect Dis. 2008;197:1189–1197. doi: 10.1086/529515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest JC, Campbell JA, Schelling P, Stehle T, Dermody TS. Structure-function analysis of reovirus binding to junctional adhesion molecule 1. Implications for the mechanism of reovirus attachment. J Biol Chem. 2003;278:48434–48444. doi: 10.1074/jbc.M305649200. [DOI] [PubMed] [Google Scholar]
- Fraser RDB, Furlong DB, Trus BL, Nibert ML, Fields BN, Steven AC. Molecular structure of the cell-attachment protein of reovirus: correlation of computer-processed electron micrographs with sequence-based predictions. J Virol. 1990;64:2990–3000. doi: 10.1128/jvi.64.6.2990-3000.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong DB, Nibert ML, Fields BN. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988;62:246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Guglielmi KM, Kirchner E, Holm GH, Stehle T, Dermody TS. Reovirus binding determinants in junctional adhesion molecule-A. J Biol Chem. 2007;282:17930–17940. doi: 10.1074/jbc.M702180200. [DOI] [PubMed] [Google Scholar]
- Gujuluva C, Burns AR, Pushkarsky T, Popik W, Berger O, Bukrinsky M, Graves MC, Fiala M. HIV-1 penetrates coronary artery endothelial cells by transcytosis. Mol Med. 2001;7:169–176. [PMC free article] [PubMed] [Google Scholar]
- Haarr L, Shukla D, Rodahl E, Dal Canto MC, Spear PG. Transcription from the gene encoding the herpesvirus entry receptor nectin-1 (HveC) in nervous tissue of adult mouse. Virology. 2001;287:301–309. doi: 10.1006/viro.2001.1041. [DOI] [PubMed] [Google Scholar]
- Kauffman RS, Wolf JL, Finberg R, Trier JS, Fields BN. The σ1 protein determines the extent of spread of reovirus from the gastrointestinal tract of mice. Virology. 1983;124:403–410. doi: 10.1016/0042-6822(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Kornecki E, Walkowiak B, Naik UP, Ehrlich YH. Activation of human platelets by a stimulatory monoclonal antibody. J Biol Chem. 1990;265:10042–10048. [PubMed] [Google Scholar]
- Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PWK, Hayes EC, Joklik WK. Protein σ1 is the reovirus cell attachment protein. Virology. 1981;108:156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- Makino A, Shimojima M, Miyazawa T, Kato K, Tohya Y, Akashi H. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J Virol. 2006;80:4482–4490. doi: 10.1128/JVI.80.9.4482-4490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell KJ, Babbin BA, Nusrat A, Parkos CA. Junctional adhesion molecule-1 (JAM1) regulates epithelial cell morphology through effects on β1 integrins and Rap1 activity. J Biol Chem. 2005;280:11665–11674. doi: 10.1074/jbc.M412650200. [DOI] [PubMed] [Google Scholar]
- Mann MA, Knipe DM, Fischbach GD, Fields BN. Type 3 reovirus neuroinvasion after intramuscular inoculation: direct invasion of nerve terminals and age-dependent pathogenesis. Virology. 2002;303:222–231. doi: 10.1006/viro.2002.1699. [DOI] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba Y, Colucci WS, Fields BN, Smith TW. The reovirus M1 gene determines the relative capacity of growth of reovirus in cultured bovine aortic endothelial cells. J Clin Invest. 1993;92:2883–2888. doi: 10.1172/JCI116910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba Y, Sherry B, Fields BN, Smith TW. Identification of the viral genes responsible for growth of strains of reovirus in cultured mouse heart cells. J Clin Invest. 1991;87:1628–1633. doi: 10.1172/JCI115177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LA, Sidman RL, Fields BN. Direct spread of reovirus from the intestinal lumen to the central nervous system through vagal autonomic nerve fibers. Proc Natl Acad Sci USA. 1991;88:3852–3856. doi: 10.1073/pnas.88.9.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson N, Tyler KL. Entry, dissemination, shedding, and transmission of viruses. In: Nathanson N, editor. Viral Pathogenesis. Philadelphia: Lippincott-Raven; 1997. pp. 13–33. [Google Scholar]
- Newberry RD. Intestinal lymphoid tissues: is variety an asset or a liability? Curr Opin Gastroenterol. 2008;24:121–128. doi: 10.1097/MOG.0b013e3282f4906d. [DOI] [PubMed] [Google Scholar]
- Predescu SA, Predescu DN, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2007;293:L823–842. doi: 10.1152/ajplung.00436.2006. [DOI] [PubMed] [Google Scholar]
- Raine CS, Fields BN. Reovirus type 3 encephalitis--a virologic and ultrastructural study. J Neuropathol Exp. 1973;32:19–33. doi: 10.1097/00005072-197301000-00002. [DOI] [PubMed] [Google Scholar]
- Richardson-Burns SM, Tyler KL. Regional differences in viral growth and central nervous system injury correlate with apoptosis. J Virol. 2004;78:5466–5475. doi: 10.1128/JVI.78.10.5466-5475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DH, Fields BN. Molecular basis of reovirus virulence: role of the M2 gene. J Exp Med. 1980;152:853–868. doi: 10.1084/jem.152.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DH, Kornstein MJ, Anderson AO. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J Virol. 1985;53:391–398. doi: 10.1128/jvi.53.2.391-398.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher T, Podlech J, Mohr CA, Jordan S, Ruzsics Z, Reddehase MJ, Koszinowski UH. The major virus-producing cell type during murine cytomegalovirus infection, the hepatocyte, is not the source of virus dissemination in the host. Cell Host Microbe. 2008;3:263–272. doi: 10.1016/j.chom.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Schiff LA, Nibert ML, Tyler KL. Orthoreoviruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1853–1915. [Google Scholar]
- Stoeckel J, Hay JG. Drug evaluation: Reolysin--wild-type reovirus as a cancer therapeutic. Curr Opin Mol Ther. 2006;8:249–260. [PubMed] [Google Scholar]
- Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, Ware CF, Pfeffer K, Miyoshi J, Takai Y, Spear PG. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Tyler KL, Barton ES, Ibach ML, Robinson C, Valyi-Nagy T, Campbell JA, Clarke P, O’Donnell SM, Wetzel JD, Dermody TS. Isolation and molecular characterization of a novel type 3 reovirus from a child with meningitis. J Infect Dis. 2004;189:1664–1675. doi: 10.1086/383129. [DOI] [PubMed] [Google Scholar]
- Tyler KL, Bronson RT, Byers KB, Fields BN. Molecular basis of viral neurotropism: experimental reovirus infection. Neurology. 1985;35:88–92. doi: 10.1212/wnl.35.1.88. [DOI] [PubMed] [Google Scholar]
- Tyler KL, McPhee DA, Fields BN. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986;233:770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- Virgin HW, IV, Bassel-Duby R, Fields BN, Tyler KL. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing) J Virol. 1988;62:4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Tyler KL, Dermody TS. Reovirus. In: Nathanson N, editor. Viral Pathogenesis. New York: Lippincott-Raven; 1997. pp. 669–699. [Google Scholar]
- Vogelmann R, Amieva MR, Falkow S, Nelson WJ. Breaking into the epithelial apical-junctional complex--news from pathogen hackers. Curr Opin Cell Biol. 2004;16:86–93. doi: 10.1016/j.ceb.2003.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110:789–799. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Ault KA, Fields BN. Interaction of reovirus with cell surface receptors. I Murine and human lymphocytes have a receptor for the hemagglutinin of reovirus type 3. J Immunol. 1980a;124:2143–2148. [PubMed] [Google Scholar]
- Weiner HL, Powers ML, Fields BN. Absolute linkage of virulence and central nervous system tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980b;141:609–616. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]
- Wetzel JD, Chappell JD, Fogo AB, Dermody TS. Efficiency of viral entry determines the capacity of murine erythroleukemia cells to support persistent infections by mammalian reoviruses. J Virol. 1997;71:299–306. doi: 10.1128/jvi.71.1.299-306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JL, Rubin DH, Finberg R, Kaufman RS, Sharpe AH, Trier JS, Fields BN. Intestinal M cells: a pathway of entry of reovirus into the host. Science. 1981;212:471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.