Abstract
Asthma is a chronic inflammatory disease of the lungs, characterized by airway hyperresponsiveness. Chronic repetitive bouts of acute inflammation lead to airway wall remodeling and possibly the sequelae of fixed airflow obstruction. Nitric oxide (NO) is a reactive molecule synthesized by NO synthases (NOS). NOS are expressed by cells within the airway wall and functionally, two NOS isoforms exist: constitutive and inducible. In asthma, the inducible isoform is over expressed, leading to increased production of NO, which diffuses into the airway lumen, where it can be detected in the exhaled breath. The exhaled NO signal can be partitioned into airway and alveolar components by measuring exhaled NO at multiple flows and applying mathematical models of pulmonary NO dynamics. The airway NO flux and alveolar NO concentration can be elevated in adults and children with asthma and have been correlated with markers of airway inflammation and airflow obstruction in cross-sectional studies. Longitudinal studies which specifically address the clinical potential of partitioning exhaled NO for diagnosis, managing therapy, and predicting exacerbation are needed.
1. ASTHMA BACKGROUND and PATHOPHYSIOLOGY
Asthma is a chronic repetitive inflammatory disease of the airways, characterized by increased responsiveness of the tracheobronchial tree (i.e., airway hyperresponsiveness, AHR) to a wide variety of stimuli. AHR is defined as an increase in the ease and degree of airway narrowing in response to bronchoconstrictor stimuli. Asthma symptoms are usually linked with widespread but heterogeneous bronchoconstriction and airflow obstruction that is at least partially reversible, either spontaneously or with treatment. Asthma exacerbations usually occur at night or in the early morning and these episodes of bronchoconstriction elicit the characteristic clinical symptoms of severe wheezing, dyspnea, chest tightness, and cough. Between the exacerbations, patients may be virtually asymptomatic. The classic exacerbation lasts up to several hours and is followed by prolonged coughing. In its most severe form, called status asthmaticus, the severe acute spasm may last for days or even weeks, and under these circumstances, compromised ventilatory function can lead to sequelae such as respiratory acidosis, cyanosis and even death (Kumar et al., 2005).
Atopic asthma is the most common type (>50%); it usually begins in childhood and is marked by allergic eosinophilic airway inflammation. A positive family history of atopy is common, and asthmatic exacerbations are often preceded by allergic rhinitis (hay fever), urticaria (hives) or eczema. The major etiologic factors of asthma are genetic predisposition to type I hypersensitivity, acute and chronic inflammation and AHR. The remaining types of asthma are lumped together as non-atopic, and can still be associated with inflammation. Symptoms are triggered most commonly by exercise, emotional distress, and viral infection (Kumar et al., 2005).
Atopic asthma exacerbations are triggered by exposure to environmental antigens, to which the patient has been previously sensitized, such as dusts, pollens, animal dander and foods; however any antigen may be implicated. Antigen exposure in the lungs triggers the release of preformed chemical mediators, such as histamine, a potent bronchoconstrictor and inducer of acute inflammation. Additionally, the inflammatory response is marked by immunoglobulin E overproduction and increased production of Th2 cytokines (IL-4, IL-5, IL-9 and IL-13) by CD4+ lymphocytes. These mediators of acute inflammation recruit inflammatory cells, such as eosinophils, that release proteins, such as major basic protein and eosinophil cationic protein, which directly injure the bronchial epithelial cells and may lead to epithelial sloughing and proliferation (Young et al., 1986; Venge, 1998). Chronic asthma is thought to be due to recurrent exposure to these circulating cytokines and mediators of inflammation.
Chronic or recurrent bouts of acute inflammation and bronchoconstriction lead to the characteristic structural changes that manifest as altered composition and organization of the soft tissues (i.e., mucosa and submucosa) of the airway walls. All together these changes result in thickening of the airway wall due to inflammatory cell infiltration, smooth muscle hyperplasia and hypertrophy, subepithelial fibrosis, hyperemia, edema and goblet cell metaplasia (Vignola, 2003). These structural changes are referred to as airway remodeling (Fig. 1), and result in stiffening of the airway wall, narrowing or partial occlusion of the airways, and fixed airflow obstruction that is not responsive to bronchodilators or corticosteroids. Over time, the fixed airflow obstruction may lead to an increase in lung volume, which is reflected as a noticeable increase in the anterior posterior diameter of the thorax.
Figure 1. Airway wall remodeling in asthma.

In asthma, the inflammatory response is marked by increased production of Th2 cytokines such as interleukin-4 (IL-4), IL-5, IL-9 and IL-13, by CD4+ lymphocytes (T-cells) and immunoglobulin E overproduction by CD20+ lymphocytes (B-cells). These mediators of inflammation recruit inflammatory cells, such as eosinophils, that release proteins, such as major basic protein (MBP) and eosinophil cationic protein (ECP), which directly injure the respiratory epithelial cells and may lead to epithelial sloughing. Inflammatory mediators also induce the expression of the inducible nitric oxide synthase (iNOS). Increased NO has been shown to produce cytotoxic effects on respiratory epithelial cells, attributed to the formation peroxynitrite (ONOO−), a highly reactive intermediate generated by a reaction of NO and superoxide anions (O2−). A portion of the NO produced by the epithelium can escape by diffusion to the gas phase and appear in the exhaled breath. Damaged epithelial cells release transforming growth factor β (TGF-β), which is associated with increased collagen production by fibroblasts within the extracellular matrix.
Since the key feature of asthma pathogenesis is inflammation of both the proximal and distal lung airways, iatrogenic treatments have been based on reducing or eliminating the inflammation via the administration of anti-inflammatory agents. Historically, the treatment of choice in the management of asthma associated lung inflammation has been inhaled corticosteroids. However, the decision to initiate corticosteroid therapy is difficult for the clinician due to the potential side effects of this class of drugs. Furthermore, the treatment of asthma in most cases relies on reported symptoms and the results of lung function tests which assess airway patency (i.e., spirometry) (Revised GINA guidelines, 2002), both of which correlate poorly with airway inflammation (Lious et al., 2000; Wilson et al., 2000; van den Toom et al., 2001). The lack of correlation implies that decisions regarding treatment, in particular anti-inflammatory intervention, may often be inappropriate, particularly in populations with limited access to healthcare in which more advanced tests (e.g., sputum eosinophilia) are not routinely available. Thus, there is a need for a new approach to assess the magnitude of inflammation in asthma, guide treatment decisions, minimize side effects, and reduce asthma morbidity (Pijnenburg et al., 2008). It is widely assumed that a surrogate marker of airway inflammation, e.g., exhaled nitric oxide, will lead to better asthma control, particularly in the prevention of the airway wall remodeling that can lead to fixed airflow obstruction (Barnes et al., 1996).
2. SOURCES of EXHALED NITRIC OXIDE
Nitric oxide (NO) is an endogenous, reactive signaling molecule synthesized by the oxidative conversion of L-arginine to L-citrulline by NO synthases (NOS). Functionally, two NOS isoforms exist: constitutive and inducible. Three NOS isoforms have been identified [constitutive endothelial NOS (eNOS or NOSI), inducible NOS (iNOS or NOSII) and constitutive neural NOS (nNOS or NOSIII)] and all three are expressed within the respiratory tract (Suresh et al., 2007). eNOS is found within the bronchial columnar epithelium, type 2 alveolar squamous epithelium, nasal transitional epithelium and endothelial cells of the pulmonary vasculature. nNOS is located in the nerves that supply the lung parenchyma, mesenchyme and associated structures, and is also expressed in the airway epithelium. iNOS is located in bronchial columnar epithelium, type 2 alveolar squamous epithelium, endothelial cells of the pulmonary vasculature, bronchial and vascular smooth muscle cells, macrophages, mast cells, fibroblasts, eosinophils, basophils and neutrophils (Hamid et al., 1993; Asano et al., 1994; Gaston et al., 1994; Kharitonov et al., 2001; Ricciardolo et al., 2004).
In general, nNOS and eNOS are expressed under physiological conditions and their activity is regulated by intracellular calcium and calmodulin. Production of NO by the consititutive isoforms occurs rapidly (seconds), is short lived and occurs in relatively small amounts (picomoles). In contrast the activity of iNOS is calcium independent and its expression is regulated at transcriptional level by the presence of proinflammatory stimuli and cytokines, such as IFN-γ, TNF-α, TNF-β, IL-1 and IL-13 (Morris et al., 1994). Compared to the constitutive forms, the maximum induction of iNOS occurs slowly (hours), is prolonged and generates much higher levels of NO (nanomoles) (Regington, 2006). Thus the amount of NO produced within the lungs, and therefore that which appears in the exhaled breath, will potentially be the greatest in chronic inflammatory conditions such as asthma.
NO is a reactive, free radical gas that modulates many functions in the lungs such as smooth muscle tone (vascular and bronchial), ciliary function, non-cholinergic and non-adrenergic neurotransmission and inflammation (Ricciardolo, 2003). The inducible NOS isoform is purported to be heterogeneously over expressed in asthma and the locally increased production of NO is believed to have deleterious effects within the airways. For example, high concentrations of NO have been shown to produce cytotoxic effects on respiratory epithelial cells (Heiss et al., 1994). The cytotoxic properties of NO have been attributed to the formation of peroxynitrite, a highly reactive intermediate generated by a reaction of NO and superoxide anions (Gaston et al., 1994; Beckman et al., 1996). Peroxynitrite has been shown to cause epithelial desquamation, and has been shown to induce AHR in a guinea-pig model (Sadeghi-Hashjin et al., 1996). Therefore, NO has the potential to mediate cell injury and inflammation, with immunomodulatory effects that predispose AHR.
3. MEASURING EXHALED NO
In biological tissues, NO is highly reactive, making direct determination of NO very difficult. In the gas phase, however, NO is relatively stable. Thus, when NO is formed in the tissues of the airway wall, a portion escapes by diffusion into the lumen where it can be detected in the exhaled breath. The detection of NO in exhaled air was first reported in 1991 (Gustafsson et al., 1991). The most widely used method for measurement of exhaled NO is chemiluminescence. Chemiluminescence is a technique in which NO reacts with ozone to form nitrogen dioxide, a small fraction of which achieves a high energy state which emits a photon when it decays. A photomultiplier tube can easily detect these photons and the signal is proportional to the concentration of NO over several orders of magnitude. This method allows measurement to approximately 1 ppb (Mahut et al., 2004b). Electrochemical detection is another method to measure NO, in particular in the liquid phase, in which the electron flow created by the oxidation of NO on the surface of electrodes is measured.
Following the initial report of NO in exhaled breath in 1991, there were no formal guidelines for the collection and measurement of exhaled NO until 1997. Thus, techniques varied widely from mixed expired concentrations to dynamic oral and nasal exhalation profiles, vital capacity and tidal breathing maneuvers, and a wide range of exhalation flows. However, early research established a strong inverse relationship between the exhaled concentration of NO and the exhalation flow (Fig.2), yet a positive relationship between the elimination rate (product of concentration and flow) and exhalation flow (Silkoff et al., 1997; Tsoukias et al. 1998b).
Figure 2. Flow dependence of exhaled NO.
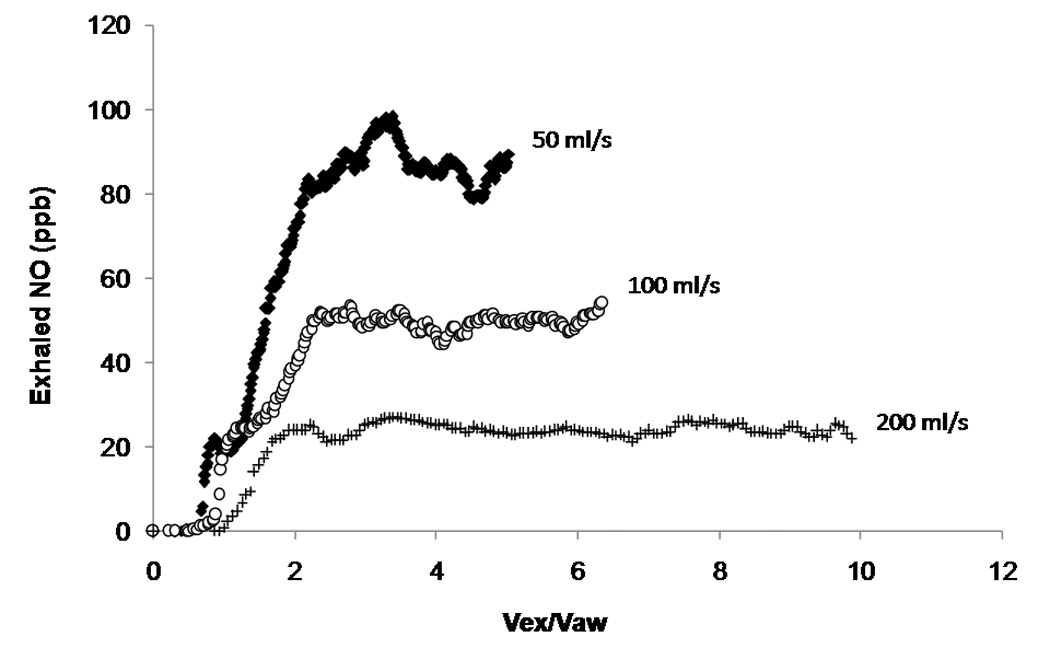
Exhaled NO at three different flows (50 ml/s, 100 ml/s and 200 ml/s) in an asthmatic patient. This plot demonstrates the inverse relationship between the exhaled concentration of NO and the exhalation flow. Vex/Vaw is the airway volume turnover, where Vex is the exhaled volume, and Vaw is the subjects airway volume and is estimated in milliliters as the sum of the subjects ideal body weight in pounds plus age in years (Tsoukias et al., 2001).
In 1997, the European Respiratory Society (ERS) published guidelines for the collection of NO (Karitonov et al., 1997), and the American Thoracic Society (ATS) followed shortly thereafter with a similar set of guidelines (ATS, 1999). The recommendations include several points regarding technique; however, perhaps the most critical factors were isolation of the nasal cavity and maintenance of a constant exhalation flow. The ERS chose an exhalation flow rate of 250 ml/s and the ATS chose 50 ml/s. The reasoning stemmed from the strong dependence of exhaled concentration on exhalation flow (Silkoff et al., 1997); thus, conventional logic dictated that flow must be held constant. The underlying premise was that exhaled concentration was the most effective experimental end-point to characterize exhaled NO. Measurements performed according to these guidelines are referred to as fractional exhaled nitric oxide (FeNO).
Exhaled NO is usually determined during a vital capacity maneuver (exhalation following max inspiration) while holding expiratory flow and pressure constant. The recommended technique involves inspiration of NO-free air via a mouthpiece to total lung capacity, followed immediately by full exhalation at a constant flow rate (50 ml/s) and pressure (>5 cm H2O) through the mouthpiece into the NO measuring device (ATS/ERS, 2005). Inspiration of NO-free air (<5 ppb) is important since levels of NO in ambient air may be high enough to influence the measurement of exhaled NO (Baraldi et al., 1998); this can be achieved by absorption of inspired ambient air through a charcoal-based scrubber to remove ambient NO. Exhalation at a pressure greater than 5 cm H2O is important to ensure closure of the soft palate, thus preventing contamination of the sample with nasal air. This is important as nasal air contains high concentrations of NO, attributed to high concentrations produced within the paranasal sinuses (Little et al., 2000).
Levels of exhaled NO are increased by airway viral infections (Murphy et al., 1998), allergic rhinitis (Henriksen et al., 1999), and a recent intake of a nitrate rich meal (Olin et al. 2001). On the other hand, in the short term, exercise (Terada et al., 2001), smoking (Verleden et al., 1999), spirometry (Deykin et al., 1998; Silkoff et al., 1999; Gabriele et al., 2005; Barreto et al., 2005; Tee et al., 2005), hyperresponsiveness tests (Ho et al., 2000; Piacentini et al., 2002) and sputum induction (Piacentini et al., 2002; Antczak et al., 2005) appear to reduce exhaled NO levels. Therefore, if possible, exhaled NO should be measured prior to lung function tests, histamine or methacholine challenges and/or sputum inductions.
Another important feature of exhaled NO is the significant variability reported within a group of clinically similar individuals (e.g., healthy controls, mild persistent asthma, etc.). As with most biological signals, exhaled NO demonstrates a log normal distribution. At a constant exhalation flow of 50 ml/s, healthy adults usually have FeNO levels between 5 and 35 ppb (Olin et al., 2005), while children have FENO levels that are slightly lower between 5 and 25 ppb (Buchvald et al., 2005). Analysis of this distribution reveals that the upper end of the 95% confidence interval is 35 ppb in adults and 25 ppb in children. This large range suggests many potential endogenous factors which can impact the exhaled concentration.
4. EXHALED NO and ASTHMA
Some of the earliest observations connecting NO with asthma pathophysiology were measurements of NO in the exhaled breath (Alving et al., 1993; Kharitonov et al., 1994) approximately fourteen years ago. These observations demonstrated that NO was a non-invasive biological marker, was significantly elevated (~3–4 fold) in mild untreated asthma, could be reduced to near normal levels following corticosteroid therapy and likely reflected the inflammatory status of the lower airways (Alving et al., 1993; Kharitonov et al., 1994; Artlich et al., 1996; Garnier et al., 1996; Kharitonov et al., 1995; Kharitonov et al., 1996b).
These initial studies provided the foundation for many new investigations, at both the basic science and clinical levels, in an attempt to better understand the source and potential clinical utility of the exhaled NO signal. Several landmark studies demonstrated the complexity and uniqueness of exhaled NO relative to other endogenous gases (e.g., CO2 and N2), such as a significant dependence on exhalation flow that could be attributed to a substantial airway source (Högman et al., 2000; Sacco et al., 2003; Selvestri et. al., 1999; Silvestri et al., 2001; Silvestri et al., 2003; Steerenberg et al., 2003; Thomas et al., 2005), a significant nasal component (Lundberg et al., 1995; Kimberly et al., 1996), as well as an oral component (Olin et al., 2001).
Numerous correlative studies assessing the relationship between levels of exhaled NO and lung function parameters and other biological and physiological markers of airway inflammation followed with mixed results. Some suggested that exhaled NO reflected the inflammatory status of the airways by correlation with eosinophilia in peripheral blood and sputum (Mattes et al., 1999; Silvestri et al., 1999; Silvestri et al., 2001; Sacco et al., 2003; Silvestri et al., 2003; Steerenberg et al., 2003; Barreto et al., 2005; Thomas et al., 2005), while others did not (Lim et al., 2000; Brinke et al., 2001; Lim et al., 2001; Brussee et al., 2005; Lex et al., 2005; Peroni et al, 2005). Furthermore, exhaled NO levels were found to be associated with eosinophilic airway inflammation as determined in bronchoalveolar lavage fluid (Lim et al., 1999), blood (Silvestri et al., 1999), and urine (Lious et al., 2000). However, in other studies no significant relationship has been seen between exhaled NO levels and eosinophils in bronchial biopsy specimens (Lim et al., 2000). There were also inconsistent reports as to whether exhaled NO correlated with spirometry, with some studies finding a correlation (Little et al., 2000; Silvestri et al., 2003) and the majority not (Stirling et al., 1998; Obata et al., 1999; Silvestri et al., 1999; Lim et al., 2001; Dal Negro R et al., 2003; Spergel et al., 2005;). Additionally conflicting evidence regarding the correlation between exhaled NO levels and airway hyperresponsiveness to histamine or methacholine has been reported. A good correlation between exhaled NO and bronchial hyperreactivity has been shown by many investigators (Jatakanon et al., 1998; Salome et al., 1999; Dupont et al, 2003; Fabbri et al., 2003; Langley et al., 2003; Strunk et al., 2003); however, at least two studies show no correlation (van Rensen et al., 1999; Silvestri et al., 2000). Together these findings indicate that increased exhaled NO levels reflect some, but not all aspects of airway inflammation and that our knowledge of the underlying source and determinants of exhaled NO remain crude.
5. USE of EXHALED NO to GUIDE CLINICAL DECISIONS
Several studies suggest that FeNO can be used to diagnose asthma (Dupont et al., 2003; Malmberg et al., 2003; Berkman et al., 2005). In the study by Dupont et al. (2003), among 240 steroid naïve asthmatics, exhaled NO levels were highly predictive of asthma with a sensitivity of 85% and a specificity of 90%. Diagnostic synergy was observed when the combination of an elevated exhaled NO and abnormal spirometry (FEV1 < 80% predicted) was used to diagnose asthma, resulting in an even greater sensitivity (94%) and specificity (93%) (Smith et al., 2004). However, two recent studies suggest exhaled NO alone may not be effective for the diagnosis of asthma, due to confounding influence of other atopic conditions (Prasad et al., 2006; Welsh et al., 2007). Additionally, it is important to note that patients may fulfill the conventional clinical criteria for the diagnosis of asthma, and yet exhaled NO levels will be normal, especially in non-atopic subjects. This highlights the fact that asthma phenotype is heterogeneous, and that exhaled NO measurements provide a perspective on only one aspect of the “asthma syndrome” (Taylor et al., 2006).
Oral and inhaled corticosteroids have been shown to induce a rapid (Kharitonov et al., 1996), reproducible (Silkoff et al., 2001); and dose dependent (Kharitnonv et al, 1996c; Jatakanon et al., 1999; Jones et al., 2002) reduction of exhaled NO. Anti-leukotrienes have also been shown to reduce exhaled NO levels in asthma (Bisgaard et al., 1999; Bratton et al., 1999), albeit to a lesser extent than corticosteroids (Stirling et al., 1998; Bisgaard et al., 1999). Exhaled NO has also been used to monitor spontaneous asthma exacerbations (Massaro et al., 1995) and those induced by corticosteroid treatment reduction (Kharitonov et al., 1996d). In addition, longitudinal studies have begun to investigate the use of FeNO as an effective non-invasive index of asthma control, potentially paving the way for it to be used to titrate the dose of, or predict the response to, corticosteroids (Szefler et al., 2002; Pijnenburg et al., 2005; Smith et al., 2005; Zacharasiewicz et al, 2005). Moreover, at least two longitudinal studies have demonstrated that FeNO was not predictive in reducing the dose of corticosteroid or predicting exacerbation (Leuppi et al., 2001; Shaw et al., 2007). The most recent study by Shaw et. al (2007) was the focus of an editorial (Taylor, 2007) in the Am. J. Resp. Crit. Care Med. that addressed the conflicting reports in the literature and concluded that “…clarifying the scope and interpretation of FeNO measurements remains a highly relevant research endeavor.” This statement summarizes the need for a greater understanding, and perhaps a new approach (e.g., airway and alveolar NO partitioning), for utilizing the exhaled NO signal.
6. CHARACTERIZING the EXHALED NO SIGNAL
Due to the exhalation flow dependence of the NO signal, current guidelines (American Thoracic Society) state that exhaled NO should be collected during a vital capacity maneuver (exhalation following maximum inspiration) while holding expiratory flow (50 ml/s) and pressure (> 5 cm H20) constant. The resultant signal is indicative of NO production, consumption, and release throughout the lungs, and is measured in parts per billion. However, the exhaled NO concentration is inherently non-specific regarding the origin of NO in the lungs, as NO arises from production within the tissues of both the airway and alveolar regions (Robbins et al., 1994; Tsoukias et al., 1998a). Furthermore, the recommended exhalation flow (50 ml/s) is low enough to cause the concentration to be predominately of airway origin; hence, the alveolar contribution is effectively ignored.
For example, two subjects with normal exhaled concentrations of 20 ppb at a flow a 50 ml/s (FENO) might have very different underlying mechanisms. One could have alveolar and airway contributions of 2 and 18 ppb, respectively, whereas the other subject’s pattern of NO production might be 6 and 14 ppb. In the later case, the alveolar concentration would be considered elevated and indicative of inflammation. Moreover, two subjects with a very high FENO of 80 ppb might have alveolar and airway contributions of 2 and 78 ppb, respectively, whereas the other subject might be 12 and 68 ppb. In this case, both individuals have elevated airway inflammation, which is easily detected by FENO, but the later subject also has elevated alveolar NO, and hence a different source of inflammation, which would remain undetected. In other words, the exhaled concentration alone lacks potentially important information about the alveolar concentration, which, even in disease, is normally only a small fraction of exhaled NO, particularly at an exhalation flow of 50 ml/s.
In order to better understand the utility and source of exhaled NO, Tsoukias and George published the two-compartment model (2CM) of NO exchange dynamics in the lungs in 1998a (Fig. 3). NO was assumed to be produced at a constant rate per unit volume and the model consists of two main compartments: 1) a rigid compartment representing the conducting airways and 2) an expansible compartment representing the respiratory bronchioles and alveolar region. The 2CM was able to reproduce the flow dependence of exhaled NO and partition the exhaled NO into an airway and alveolar contribution. The model essentially characterizes NO exchange dynamics with three flow-independent variables: mean steady state alveolar concentration (C̅aNO, the line over the variable denotes mean), mean airway diffusing capacity (D̅awNO ), and either the mean maximum airway flux (J̅' awNO, the prime denotes maximum flux) or the mean airway tissue concentration (C̅awNO, the ratio of J̅' awNO / D̅awNO ).
Figure 3. Schematic of two-compartment model.

During exhalation, a steady state mean alveolar or peripheral concentration (C̅aNO ) enters the airway compartment (net transfer is convection minus diffusion) where upon additional NO is transferred from the airway walls. The alveolar or peripheral concentration represents the acinar region of the lungs (Weibel generations 17–23). The airway compartment represents the oropharynx and Weibel generations 1–16, and considers the increasing surface area per unit volume of the airway tree (i.e., trumpet shape). The contribution from the airways depends on the exhalation flow and is the sum of two terms: J̅' awNO =J̅awNO − D̅awNO *CNO. J̅' awNO is the mean maximum airway flux (picoliters/sec). D̅awNO is the mean airway diffusing capacity (or conductance). CNO is the airway compartment gas phase NO concentration which depends on axial volumetric position (V), and the airway compartment volume is Vaw. (T: trachea; BR: bronchus; BL: bronchiole; TB: terminal bronchiole; RB: respiratory bronchiole; AD alveolar duct; AS: alveolar sac)
The 2CM was attractive because the analytical solution could be easily adapted to create algorithms that analyzed breathing maneuvers with mathematical techniques in which exhaled NO could be partitioned into region specific airway and alveolar contributions. This led to the rapid application of these techniques to characterize the airway and alveolar NO in a range of normal and pathological conditions including exercise (Shin et al., 2003), asthma (Silkoff et al., 1997; Högman et al., 2000; Lehtimäki et al., 2000; Lehtimäki et al., 2001a; Gelb et al., 2004; Shin et al., 2004b), chronic obstructive pulmonary disease (Högman et al., 2002b), cystic fibrosis (Shin et al., 2002) and scleroderma (Girgis et al., 2002).
In an effort to improve upon the 2CM of NO exchange, a series of advanced theoretical and experimental studies were performed to: 1) validate model assumptions and simplifications, 2) to develop breathing techniques that can reliably estimate the parameters, and 3) to interpret the physiological and clinical significance of these parameters (Tsoukias et al., 1998; Tsoukias et al., 2001; Shin et al., 2002; George et al., 2004; Shin et al., 2004b; Shin et al., 2005; Condorelli et al., 2007; Shin et al., 2007). Ultimately, this research led to a refinement of the 2CM, which considers the combination of steady-state flow conditions, the trumpet shape of the airway tree (increasing surface area per unit volume), and axial diffusion of NO in the gas phase. Research has shown (Shin et al., 2002; Shin et al., 2004a; Shin et al., 2005; Shin et al., 2006) that these features of the lungs (trumpet shape and axial diffusion) are important determinants of NO exchange. The trumpet shaped component characterizes the airway geometry by appropriately scaling the lengths and diameters of Weibel’s data (Weibel, 1963) of the human airway tree, based on the volumes of the oral cavity, oropharynx and airway generations 0–16. The axial diffusion component takes into account the diffusion of NO in the gas phase from the airways (high concentration) to the alveolar region (low concentration) in accordance with Fick’s laws of diffusion. The gradient for diffusion is in the opposite direction of the exhaled flow. Thus, by taking into consideration the axial diffusion of NO, one can correct for the “back diffusion” of airway NO and resultant contamination of the alveolar region.
The trumpet model with axial diffusion (TMAD) of NO exchange includes the most relevant anatomical and physical features of the lungs (Condorelli et al., 2007). The model allows partitioning of the exhaled NO signal into its airway and alveolar components, using multiple steady state flow exhalations that are readily performed by children as young as four years of age (Buchvald et al., 2005). Application of the TMAD of NO exchange is quite simple. In this technique, the steady state elimination rate of NO at a constant flow (product of exhalation flow and concentration) is plotted as a function of exhalation flow. For exhalation flows >50–100 ml/s (depending on the airway volume of the subject), the model predicts a linear relationship in which the slope is equivalent to C̅aNO, and the intercept approximates J̅' awNO (Fig. 4). Thus increased airway NO flux should cause a higher intercept value and increased alveolar NO concentration should cause a steeper slope of the regression line.
Figure 4. Illustrative data demonstrating the elimination rate vs. flow technique to estimate mean alveolar concentration and maximum airway flux.

The steady or plateau nitric oxide concentration can be measured at a series of constant exhalation flows, and then the elimination rate, (pl/s, product of concentration × flow) can be plotted as a function of exhalation flow, (ml/s). For flows > ~ 50–100 ml/s in adults, this relationship is approximately linear (solid circles), as predicted by the two-compartment model with axial diffusion. The slope minus a term proportional to the airway flux is an estimate of the mean alveolar concentration (C̅aNO, (pl/s) / (ml/s) = ppb), while the intercept is proportional to the mean maximum airway flux of NO (J̅' awNO, pl/s). Specific values for the coefficients “a” and “b” are described in Condorelli et. al. (2007), and depend on the flow range utilized.
7. OBSERVATIONS made from PARTITIONING FeNO
Numerous potentially clinically significant findings have been reported, highlighting the biological relevance of the exhaled NO signal and the NO model. In the past nine years, there have been no less than 45 reports in the literature (eight so far in 2008) reporting C̅awNO, D̅awNO, C̅aNO and J̅' awNO in different human contexts and diseases such as cigarette smoking (Malinovschi et al., 2006a; Pietropaoli et al., 2007) and smoking cessation (Högman et al., 2002a), patients undergoing cardiopulmonary bypass (Alexiou et al., 2004), anaphylaxis (Rolla et al., 2006), allergic rhinitis (Högman et al., 2002b), severe atopic eczema (Linosalo et al., 2007), cystic fibrosis (Shin et al., 2002; Suri et al., 2007), scleroderma (Girgis et al., 2002), asbestosis (Lehtonen et al., 2007), chronic obstructive pulmonary disease (Högman et al., 2002b; Brindicci et al., 2005; Roy et al., 2007), obstructive sleep apnea syndrome (Foresi et al., 2007), bronchiectasis (Foley et al., 2007), sarcoidosis (Phansalkar et al., 2004), liver cirrhosis (Declaux et al., 2002) and primary billiary cirrhosis (Rolla et al., 2004). The vast majority of the reports involve the study of asthmatic patients and utilize the multiple constant flow exhalation technique described in Fig. 3, to determine the effects of different physiological conditions (e.g., exercise), lung function tests (e.g., spirometry and bronchial hyperactivity tests) and pharmacologic agents (e.g., inhaled corticosteroids) on region specific airway and alveolar nitric oxide concentrations (Pietropaoli et al., 1999; Lehtimäki et al., 2000; Silkoff et al., 2000; Lehtimäki et al., 2001a; Lehtimäki et al., 2001b; Lehtimäki et al., 2001c; Lehtimäki et al., 2002; Gelb et al., 2004; Mahut et al., 2004a; Shin et al., 2004b; Berry et al., 2005; De Blic et al., 2005; Delclaux et al., 2005; Lehtimäki et al., 2005; Gelb et al., 2006; Malinovschi et al., 2006b; Paraskakis et al., 2006; Shin et al., 2006; van Veen et al., 2006; Brindicci et al, 2007a; Brindicci et al., 2007b; Shin et al., 2007; Kerckx et al., 2008; Menzies et al., 2008; Zavorsky et al., 2008).
7.1 Adult asthma
The first report looking at clinical utility of exhaled NO at multiple constant flows in patients with asthma was published in abstract form by Högman et al. (1999). They found significantly higher airway flux (J̅' awNO or intercept of linear regression) and equal alveolar NO concentration (C̅aNO or slope of linear regression) in adult patients with asthma compared to healthy controls. The findings of increased airway flux but normal alveolar NO concentration in adults with asthma were then confirmed in a study of 10 adult patients with newly diagnosed asthma by Lehtimäki et al. (2000). Lehtimäki et al. (2001a) then applied this analysis to a larger group (n=40) of steroid naïve adult asthmatic subjects and reported that there was a correlation between airway NO flux and two separate markers of airway inflammation: bronchial hyperresponsiveness to methacholine and serum eosinophil protein X. However, no correlation was seen with respect to alveolar NO concentrations. Additionally, no correlation was seen between the flow independent exhaled NO parameters and pulmonary volumes determined from spirometry (e.g., FVC or VC).
Shortly following the initial reports of increased airway NO flux, but normal alveolar NO concentrations in steroid naïve asthmatic adults, Lehtimäki et al. (2002) reported that asthmatic patients with nocturnal symptoms exhibit increased alveolar nitric oxide concentration in addition to increased airway NO flux, suggesting that patients with nocturnal symptoms have more peripheral inflammation. Serum levels of myeloperoxidase and IL-6 were found to be significantly greater in asthmatic patients with nocturnal symptoms, suggesting that nocturnal symptoms were related to neutrophilic activation and more active airway inflammation, respectively.
Lehtimäki et al. (2001b) also looked at the effects of inhaled corticosteroids on the flow-independent exhaled NO parameters in 16 steroid naïve adults with newly diagnosed asthma. Compared to normal adults, baseline measurements (i.e., before treatment with inhaled fluticasone) in the asthmatic group revealed that airway NO flux was increased, while the alveolar NO concentration was normal. Following an eight week treatment with inhaled corticosteroids, no change was observed in the alveolar NO concentration but airway NO was decreased and this decrease reached significance following 1 week of treatment. As expected, asthma symptoms decreased following treatment with inhaled corticosteroids and the time course of reduced symptoms was similar to that of reduced airway NO flux. With respect to lung volumes obtained from spirometry, the authors found that treatment with inhaled corticosteroids improved FEV1 % predicted and that there was a trend between the decrease in airway NO flux and increase FEV1 % predicted, but this did not reach significance. Additionally, treatment with inhaled corticosteroids reduced serum levels of eosinophilic cationic protein and eosinophil protein X. A trend was observed between the decrease in airway NO flux and the reduction of eosinophil protein X, however this did not reach significance. This may be because exhaled NO and serum markers of eosinophilic inflammation reflect different aspects of asthmatic airway inflammation, since exhaled NO most likely reflects inflammation induced iNOS expression and serum markers reflect systemic eosinophilic activity.
Silkoff et al. (2000) and Shin et al. (2004b) also studied the effect of inhaled corticosteroids on exhaled NO. In agreement with Lethimaki et al. (2001b) the authors reported that the use of inhaled corticosteroids was associated with a decrease in airway NO flux. Additionally, they also found that treatment with inhaled corticosteroids resulted in a reduced airway NO tissue concentration (C̅awNO ). Interestingly, these two studies reported that the mean airway diffusing capacity of NO (D̅awNO ) was elevated in both groups of asthmatic subjects and was independent of the use of corticosteroids. D̅awNO was also found to be inversely related with both FEV1 and FVC (% predicted), independent of the presence of asthma or steroid use. The studies suggested that D̅awNO may reflect physiologic changes in the lungs that impact lung function independent of the use of corticosteroids and may provide clinical information not available from FENO alone.
In a study assessing 53 nonsmoking, clinically stable individuals with mild-to-severe persistent asthma on inhaled corticosteroids and 13 nonsmoking, clinically stable asthmatic patients who were inhaled corticosteroid naïve, by choice, Gelb et al.(2004) reported an increased airway NO flux and increased alveolar NO concentration in the asthmatic patients without isolated nocturnal symptoms, compared to normal controls. Upon administration of 30 mg prednisone for 5 days, in patients with asthma on inhaled corticosteroids, they noted a significant reduction in the elevated alveolar NO concentration but no significant change in airway NO flux. From these results they concluded that the concurrent increase of bronchial and alveolar NO concentrations in individuals with chronic asthma suggests separate, incompletely or non-suppressed inflammatory sites. Furthermore the increased alveolar NO that was suppressed upon administration of a systemic corticosteroid, suggests that inhaled corticosteroids may suppress iNOS in the large airways but do not penetrate beyond the distal airways into the alveolar region of the lung. A separate end point of the study addressed the reproducibility of the alveolar NO measurements by making exhaled NO measurements within 10 days of each other. No statistical difference in the alveolar NO concentrations were observed, suggesting good reproducibility.
Lehtimäki et al. (2005) also conducted a study assessing the relationship between asthmatic symptoms (cough, shortness of breath, chest tightness or wheezing), airway NO flux and alveolar NO concentration. In the study, 63 nonsmoking patients with asthma symptoms were divided into two populations based on whether or not they fulfilled the diagnostic criteria of asthma: (1) asthma (n=40) and (2) asthmatic symptoms (n=23). Both groups had elevated airway NO flux; however, only the group with asthmatic symptoms but normal lung function had elevated alveolar NO concentration. In the asthmatic symptoms group the elevated alveolar NO concentration was positively correlated with urinary excretion of leukotriene E4 (LTE4) and negatively correlated with FEF 50% and FEF 75% (patients with the highest alveolar NO had the lowest FEF 50% and FEF 75%), suggesting that increased alveolar NO concentration is a measure of inflammation related to small airway disease. These results suggest that patients with asthmatic symptoms have airway inflammation that likely causes their symptoms and that the current tools used to diagnose asthma do not detect all patients with airway inflammation.
In a study of refractory asthmatic patients (asthma not controlled satisfactorily with inhaled corticosteroids), Berry et al. (2005), tested the hypothesis that a crucial element to the pathogenesis of refractory asthma is the presence of inflammation in the distal lung. Analysis of exhaled NO at multiple flows in 27 patients with refractory asthma revealed that despite receiving high dose inhaled corticosteroids, both airway flux and alveolar NO concentration remained elevated. With respect to markers of inflammation, alveolar NO correlated most closely with bronchoalveolar lavage eosinophil counts while airway NO flux was correlated most closely with sputum and bronchial wash eosinophil counts. No correlation was observed between alveolar NO and asthma control as assessed using Juniper asthma control score (Juniper et al., 1999). Treatment of refractory asthma patients with a 2-week course of systemic oral corticosteroids, but not a 1-mounth course of double the dose of inhaled corticosteroids, reduced alveolar NO concentrations. These findings suggest that alveolar NO is a measure of inflammation in the distal lung and is consistent with the view that refractory asthma is associated with distal lung inflammation. Furthermore, the study suggests, in accordance with the findings of Gelb et al. (2004) that inflammation in this site responds to oral but not inhaled corticosteroids.
In a study of 17 adult patients with mild-to-moderate asthma and 14 adult patients with severe asthma, van Veen et al. (2006) investigated the relationship between partitioned exhaled NO and the following lung function tests: spirometry, body plethysmography, bronchial hyperreactivity test, and single breath nitrogen washout. Their results showed that alveolar NO is closely related to parameters of peripheral airway dysfunction, including FRC % predicted and RV/TLC % predicted, in patients with severe asthma, but not in patients with mild-to-moderate asthma. In contrast to other investigators they found no significant difference in alveolar NO levels between patients with mild-to-moderate and severe asthma. They attributed this finding to the large variability of alveolar NO levels amongst the patients with severe asthma in their study. Additionally, another dissimilarity was observed, they reported higher levels of alveolar NO in patients treated with oral corticosteroids as compared to those on inhaled corticosteroids. An explanation was that these patients had severe disease that was not fully controlled by systemic administration of corticosteroids and that asthma is a heterogeneous patient population.
Researchers have also looked at the role of exhaled NO at multiple flows in predicting adult asthma exacerbations. In a prospective study of 53 clinically stable (for at least 6 weeks) asthmatic patients with post hoc data analysis, Gelb et al. (2006) found that in patients with a decreased FENO in combination with a decreased FEV1 % predicted (<76%) there is a 85% chance of an exacerbation within 18 months. They also found that asthma exacerbations are associated with increased airway NO flux and alveolar NO concentration. Moreover, independent of baseline FEV1 % predicted, increased alveolar NO was able to predict increased asthma exacerbations. These results stress the powerful potential role of partitioned exhaled NO in predicting acute asthma exacerbations.
7.2 Pediatric asthma
In a study designed to determine whether airway wall remodeling influenced exhaled NO in pediatric asthma, Mahut et al. (2004a) examined 28 children with refractory atopic asthma (frequent symptoms and/or airflow obstruction despite maximal conventional therapy) treated with an inhaled corticosteroid and a long acting beta-agonist or leukotriene antagonist. The refractory pediatric asthma patients were found to have an elevated airway NO flux and alveolar NO concentration. Alveolar NO concentration and airway NO flux correlated with both airway eosinophilic inflammation (assessed by eosinophilic cationic protein) and remodeling (assessed by reticular basement membrane thickening). However, they found a negative relationship between exhaled NO and IFN-gamma/IL4 ratio, which is surprising considering the experimental findings suggesting that these cytokines stimulate iNOS expression.
Mahut et al. (2004b) also investigated the relationship between exhaled NO and symptoms in 30 asymptomatic and 15 symptomatic (symptomatic was defined as having at least one symptom within 72 hours of testing) asthmatic children. Symptomatic asthmatic children were found to have a significant increase in alveolar NO concentration when compared with normal and asymptomatic asthmatic children, suggesting that a concurrent increase in alveolar NO concentration and symptoms may indicate a loss of asthma control. However, there was no difference in FENO between the symptomatic and asymptomatic asthmatic subjects, limiting the interpretation of exhaled NO at a single flow.
In a study of 37 severe asthmatic children, De Blic et al. (2005), examined the relationship between exhaled NO and airway remodeling assessed by high-resolution computed tomography (HRCT). They found that bronchial wall thickening, estimated using HRCT, was correlated with reticular basement membrane thickening, measured following endobronchial biopsy, and NO production in the airway walls. Partitioning of the exhaled NO signal revealed that airway NO flux correlated with bronchial wall thickening.
A study, by Paraskakis et al. (2006), using exhaled NO at multiple flows to characterize airway NO flux and alveolar NO concentrations in normal children (n=25), atopic children (n=24) and children with asthma (n=83) revealed that the alveolar NO concentration was significantly elevated in children with asthma when compared to the other study groups. The airway NO flux was found to be elevated in both the asthmatic and atopic group when compared to normal subjects, however no statistical difference was seen between the asthmatic and atopic groups. Analysis of the distribution of values provides an estimate for the upper limit of normal for the airway NO flux and alveolar NO concentration as J̅' awNO (<2 nl/s) and CaNO (<3 ppb), in children. Furthermore, the alveolar NO concentration was higher in the subgroup of children whose asthma was poorly controlled (based on bronchodilator use), suggesting that alveolar NO may be indicative of asthma control. Not surprisingly, FENO was found to correlate very well with airway NO (r=0.97, p<0.0001) and thus the authors concluded, airway NO provides little if any novel information. A poor correlation was observed between FENO and alveolar NO concentration, suggesting that the measure of alveolar NO provides unique information. This finding stresses the clinical usefulness of exhaled NO at multiple flows.
7.3 Summary
It has been shown in adults and children that the flow independent parameters of NO exchange, i.e., airway NO flux and alveolar NO concentration, can be calculated by measuring exhaled NO at multiple exhalation flows, and then fitting a regression line to the plot of the elimination rate (product of exhaled NO concentration and flow rate) against the exhalation flow rate. The intercept and the slope of the regression line are estimates of the airway flux and alveolar NO concentration, respectively. These measurements have been shown to be reproducible in normal subjects as well as various lung pathologies, including asthma (Table 1).
Table 1.
Partitioned Exhaled NO Reference Values in Asthma using Slope-Intercept Method (Fig. 4).
| Author, year | Asthmatics | Airway NO (nl/s) | Alveolar NO (ppb) |
|---|---|---|---|
| Lehtimaki et al. 2000 68 | Adults (n=10) Steroid Naive |
2.6 ± 0.6 † | 1.5 ± 0.3 |
| Lehtimaki et al. 2001a 69 | Adults (n=40) Steroid Naïve |
2.5 ± 0.3 † | 1.1 ± 0.2 |
| Gelb et al. 2004 38 | Adults (n=53) ICS Treated |
2.4 ± 3.1 † | 7.0 ± 7.4 † |
| Lehtimaki et al. 2005 73 | Adults (n=65) Steroid Naïve |
2.5 ± 0.3 † | 1.1 ± 0.2 |
| Paraskakis et al. 2006 98 | Children (n=52) ICS Treated |
1.23 [range, 0.2–0.9] † | 2.22 [range, 0.4–6.6] † |
significantly elevated versus controls
ICS: inhaled corticosteroid
Early studies in steroid naïve asthmatic adults demonstrate that the increase in exhaled NO concentration is associated with an increased airway NO flux but not the alveolar NO concentration. However, other studies in children and adults have reported an increased airway NO flux and increased alveolar NO concentration in asthmatic patients, suggesting distinct patterns of airway inflammation in asthma. These observations of increased alveolar NO are particularly relevant as such asthmatic patients have proven to be difficult to manage (e.g., refractory to inhaled corticosteroids) and are hospitalized more frequently. Intriguingly, a concurrent increase in airway NO flux and alveolar NO has been reported in patients with asthmatic symptoms, not fulfilling the diagnostic criteria of asthma. This suggests that a subgroup of patients with asthmatic symptoms, have airway inflammation that likely causes their symptoms, and that the current tools used to diagnose asthma may be inadequate for some individuals.
Treatment of adult asthmatics with inhaled corticosteroids reduces the airway NO flux, and the alveolar NO tissue concentration (C̅awNO ) to near normal, but has no effect on the alveolar NO concentration or the mean airway diffusing capacity of NO (D̅awNO ), which remains elevated D̅awNO and is correlated with worsening lung function. The observations of the airway parameters (C̅awNO and D̅awNO ) are interesting and perhaps clinically relevant, but even more exciting results have involved observations of the alveolar NO concentration. Administration of oral corticosteroids, but not high dose inhaled corticosteroids, to adult asthmatics has been shown to reduce an elevated alveolar NO concentration, implying that asthmatic patients with peripheral or alveolar inflammation may require alternate therapeutic regimens, especially as recent studies have demonstrated that knowledge of regional inflammation (e.g., linked to alveolar NO) can impact the choice of therapy (Gelb et al., 2004).
In adults and children with asthma the airway NO flux and alveolar NO parameters have been correlated with pulmonary and systemic markers of airway inflammation and airway wall remodeling. In patients with asthma there is no correlation between lung volumes obtained from spirometry and FENO, suggesting that exhaled NO is not a marker of airflow obstruction in asthma. However, other studies have shown a correlation between elevated alveolar NO concentration and spirometric markers of small airway obstruction (Lehtimäki et al., 2005; van Veen et al., 2006).
8. MODEL LIMITATIONS
It is important to note that much of the work reviewed in this article has been done with the model of NO exchange in the lungs that neglects gas phase axial molecular diffusion. The impact of axial molecular diffusion on the exhaled concentration, and in particular on the estimate of the alveolar concentration and airway wall flux, has been described in detail by our group (Shin et al, 2002; Shin et al, 2004a), and most recently by Condorelli et al. (2007) and Kerckx et al. (2008), in which experimental evidence and theoretical simulations were used to demonstrate that axial molecular diffusion of NO from the airways can falsely elevate the estimate of the alveolar concentration and decrease the estimate of the airway flux of NO.
The model used to partition NO into central and alveolar components assumes a single path trumpet structure; in other words, heterogeneity in ventilation and inflammation in the proximal or peripheral regions is neglected. Heterogeneity in ventilation and inflammation (NO production) in both the conducting airways (bronchi and bronchioles) and the acinar region (respiratory bronchioles to alveoli) of the lungs significantly alters the shape and magnitude of the exhaled NO profile (Suresh et al., 2008). The variability in ventilation and NO production is exaggerated in asthmatics, and may significantly influence the interpretation of exhaled NO. Therefore, the single path two-compartment model is an idealized representation of the lungs, and may not be adequate for some subjects, particularly those with significant heterogeneity in ventilation. In a recent study of 132 asthmatic children by Paraskakis et al. (2006), it was reported that the data from 15% of the subjects could not be fitted to the two-compartment model of NO exchange in the lungs. In some cases the relationship between NO elimination and exhalation flow was simply non-linear, and in other cases the intercept or the slope was negative (i.e., non-physiological interpretation). The children who did not fit the model had significantly lower absolute and percent predicted FEV1 and higher FENO when compared to those who fit the linear model. The authors suggested that the model may fail in situations of profound heterogeneous ventilation, in which airway obstruction produces a range of airway emptying time constants that are not consistent with a single path model. We have recently published a multicompartment model structure that allows for heterogeneous ventilation and inflammation (Suresh et al., 2008), and may be useful in describing asthmatic subjects, in general, and in particular those for which the single path model is not appropriate.
The high FENo levels in the subjects who did not match the two-compartment model, are likely the result of significant airway inflammation which may contribute to abnormal lung function and ventilation patterns. These subjects would easily be identified by the high FENO as having airway inflammation, and thus the two-compartment may not be necessary. The most difficult subjects to identify are those with peripheral or alveolar inflammation, due to the relatively small level of NO when compared with the airway region. It is not clear yet whether these subjects have abnormal ventilation patterns necessitating a more advanced model.
9. FUTURE DIRECTIONS
The research and clinical potential of partioned exhaled NO is significant, but the success will critically depend on three major areas: 1) model structure, 2) breathing technique, and 3) longitudinal clinical trials. The model structure for NO exchange must maintain simplicity yet capture the necessary features of NO exchange dynamics. We and other groups have clearly demonstrated that gas axial diffusion of NO must be considered in the model, and experimental evidence suggests that heterogeneity in ventilation and inflammation must also be considered. Our most recent multicompartment model of NO exchange which captures ventilation and inflammation heterogeneity (Suresh et al., 2008) has yet to be tested in a large group of asthmatic subjects. Each advance adds complexity to the model making parameter characterization that much more difficult, yet potentially more relevant.
The breathing maneuvers used to collect the exhaled NO and ultimately partition the signal into airway and alveolar components is equally important. While multiple single exhalations at different constant exhalation flows seems to be used by most studies, the technique can require between 6–15 breathing maneuvers depending on the desired accuracy of the parameters. We have described alternate multiple breath maneuvers and single breath maneuvers that involve a breath hold, but these may not be appropriate for subjects with compromised lung function. Perhaps the simplest maneuver is a single breath with a variable flow during exhalation (Tsoukias et al., 2001) which has the potential to provide an accurate assessment of airway and alveolar NO with minimal patient effort.
Finally, longitudinal studies which specifically address the clinical potential of partitioning exhaled NO are needed. These studies may address both the diagnostic and therapeutic potential. A recent study by our group proposes to separate asthmatic subjects into four distinct phenotypes based on their region specific airway and alveolar NO concentrations (Puckett et al., 2008). The studies in asthmatic subjects strongly suggest that a peripheral inflammatory component exists in a subgroup(s) of asthmatics, which we hypothesize, may indicate a separate phenotype, and thus, a different clinical course and therapeutic strategy. Hence, the increased level of specificity provided by the flow-independent variables is important in utilizing exhaled NO as a research and clinical tool.
ACKNOWLEDGEMENTS
This work was supported in part by a grant from the National Institutes of Health (R01 HL070645) and the Medical Scientist Training Program at the University of California, Irvine (T32 GM008620).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alexiou C, Tang AT, Sheppard SV, Haw MP, Gibbs R, Smith DC. A prospective randomized study to evaluate the effect of leukodepletion on the rate of alveolar production of exhaled nitric oxide during cardiopulmonary bypass. Ann. Thorac. Surg. 2004;78:2139–2145. doi: 10.1016/j.athoracsur.2004.05.087. [DOI] [PubMed] [Google Scholar]
- 2.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur. Respir. J. 1993;6:1368–1370. [PubMed] [Google Scholar]
- 3.Antczak A, Kharitonov SA, Montuschi P, Gorski P, Barnes PJ. Inflammatory response to sputum induction measured by exhaled markers. Respiration. 2005;72:594–599. doi: 10.1159/000086721. [DOI] [PubMed] [Google Scholar]
- 4.Artlich A, Hagenah JU, Jonas S, Ahrens P, Gortner L. Exhaled nitric oxide in childhood asthma. Eur. J. Pediatr. 1996;155:698–701. doi: 10.1007/BF01957156. [DOI] [PubMed] [Google Scholar]
- 5.Asano K, Chee CB, Gaston B, Lilly CM, Gerard C, Drazen JM, Stamler JS. Consitutive and inducible nitric oxide synthase gene expression, regulation and activity in human lung epithelial cells. Proc. Natl. Acad. Sci. USA. 1994;91:10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ATS. Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide in Adults and Children. Am. J. Respir. Crit. Care. Med. 1999;160:2104–2117. doi: 10.1164/ajrccm.160.6.ats8-99. [DOI] [PubMed] [Google Scholar]
- 7.ATS/ERS. Recommendations for standardized procedures for the online and offline measurements of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am. J. Respir. Crit. Care. Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 8.Baraldi E, Azzolin NM, Dario C, Carra S, Ongaro R, Biban P, Zacchello F. Effect of atmospheric nitric oxide (NO) on measurements of exhaled NO in asthmatic children. Pediatr. Pulmonol. 1998;26:30–34. doi: 10.1002/(sici)1099-0496(199807)26:1<30::aid-ppul6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ, Kharitonov SA. Exhaled nitric oxide: a new lung function test. Thorax. 1996;51:233–237. doi: 10.1136/thx.51.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barreto M, Villa MP, Monti F, Bohmerova Z, Martella S, Montesano M, Darder MT, Ronchetti R. Additive effect of eosinophilia and atopy on exhaled nitric oxide levels in children with or without a history of respiratory symptoms. Pediatr. Allergy. Immunol. 2005;16:52–58. doi: 10.1111/j.1399-3038.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- 11.Barreto M, Villa MP, Montesano M, Rennerova Z, Monti F, Darder MT, Martella S, Ronchetti R. Reduced exhaled nitric oxide in children after testing of maximal expiratory pressures. Pediatr. Pulmonol. 2006;41:141–145. doi: 10.1002/ppul.20358. [DOI] [PubMed] [Google Scholar]
- 12.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite:the good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 13.Berkman N, Avital A, Breuer R, Bardach E, Springer C, Godfrey S. Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax. 2005;60:383–388. doi: 10.1136/thx.2004.031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry M, Hargadon B, Morgan A, Shelley M, Richter J, Shaw D, Green RH, Brightling C, Wardlaw AJ, Pavord ID. Alveolar nitric oxide in adults with asthma: evidence of distal lung inflammation in refractory asthma. Eur.Respir. J. 2005;25:986–991. doi: 10.1183/09031936.05.00132404. [DOI] [PubMed] [Google Scholar]
- 15.Bisgaard H, Loland L, Oj JA. NO in exhaled air is redced by the leukotriene receptor antagonist montelukast. Am. J. Respir. Crit. Care Med. 1999;160:1227–1231. doi: 10.1164/ajrccm.160.4.9903004. [DOI] [PubMed] [Google Scholar]
- 16.Bratton DL, Lanz MJ, Miyazawa N, Miyazawa N, White CW, Silkoff PE. Exhaled nitric oxide before and after montelukast sodium therapy in school-age children with chronic asthma: a preliminary study. Pediatr. Pulmonol. 1999;28:402–407. doi: 10.1002/(sici)1099-0496(199912)28:6<402::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Brindicci C, Ito K, Barnes PJ, Kharitonov SA. Differential flow analysis of exhaled nitric oxide in patients with asthma of differing severity. Chest. 2007a;131:1353–1362. doi: 10.1378/chest.06-2531. [DOI] [PubMed] [Google Scholar]
- 18.Brindicci C, Ito K, Barnes PJ, Kharitonov SA. Effect of an inducible nitric oxide synthase inhibitor on differential flow-exhaled nitric oxide in asthmatic patients and healthy volunteers. Chest. 2007b;132:581–588. doi: 10.1378/chest.06-3046. [DOI] [PubMed] [Google Scholar]
- 19.Brindicci C, Ito K, Resta O, Pride NB, Barnes PJ, Kharitonov SA. Exhaled nitric oxide from lung periphery is increased in COPD. Eur. Respir. J. 2005;26:52–59. doi: 10.1183/09031936.04.00125304. [DOI] [PubMed] [Google Scholar]
- 20.Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am. J. Respir. Crit.Care Med. 2001;164:744–748. doi: 10.1164/ajrccm.164.5.2011026. [DOI] [PubMed] [Google Scholar]
- 21.Brussee JE, Smit HA, Kerkhof M, Koopman LP, Wijga AH, Postma DS, Gerritsen J, Grobbee DE, Brunekreef B, de Jongste JC. Exhaled nitric oxide in 4-year-old children: relationship with asthma and atopy. Eur. Respir. J. 2005;25:455–461. doi: 10.1183/09031936.05.00079604. [DOI] [PubMed] [Google Scholar]
- 22.Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J. Allergy Clin. Immunol. 2005;115:1130–1136. doi: 10.1016/j.jaci.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Condorelli P, Shin HW, Aledia AS, Silkoff PE, George SC. A simple technique to characterize proximal and peripheral nitric oxide exchange using constant flow exhalations and an axial diffusion model. J. Appl. Physiol. 2007;102:417–425. doi: 10.1152/japplphysiol.00533.2006. [DOI] [PubMed] [Google Scholar]
- 24.Dal Negro R, Micheletto C, Tognella S, Turco P, Rossetti A, Cantini L. Assessment of inhaled BDP-dose dependency of exhaled nitric oxide and local and serum eosinophilic markers in steroids-naive nonatopic asthmatics. Allergy. 2003;58:1018–1022. doi: 10.1034/j.1398-9995.2003.00229.x. [DOI] [PubMed] [Google Scholar]
- 25.De Blic J, Tillie-Leblond I, Emond S, Mahut B, Dang Duy TL, Scheinmann P. High-resolution computed tomography scan and airway remodeling in children with severe asthma. J. Allergy Clin. Immunol. 2005;116:750–754. doi: 10.1016/j.jaci.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Delclaux C, Mahut B, Zerah-Lancner F, Delacourt C, Laoud S, Cherqui D, Duvoux C, Mallat A, Harf A. Increased nitric oxide output from alveolar origin during liver cirrhosis versus bronchial source during asthma. Am. J. Respir.Crit. Care Med. 2002;165:332–337. doi: 10.1164/ajrccm.165.3.2107017. [DOI] [PubMed] [Google Scholar]
- 27.Delclaux C, Zerah-Lancner F, Mahut B, Ribeil S, Dubois A, Larger C, Harf A. Alveolar nitric oxide and effect of deep inspiration during methacholine challenge. Chest. 2005;127:1696–1702. doi: 10.1378/chest.127.5.1696. [DOI] [PubMed] [Google Scholar]
- 28.Deykin A, Halpern O, Massaro AF, Drazen JM, Israel E. Expired nitric oxide after bronchoprovocation and repeated spirometry in patients with asthma. Am. J. Respir. Crit. Care Med. 1998;157:769–775. doi: 10.1164/ajrccm.157.3.9707114. [DOI] [PubMed] [Google Scholar]
- 29.Dupont LJ, Rochette F, Demedts MG, Verleden GM. Exhaled nitric oxide correlates with airway hyperresponsiveness in steroid-naive patients with mild asthma. Am. J. Respir. Crit. Care Med. 1998;157:894–898. doi: 10.1164/ajrccm.157.3.9709064. [DOI] [PubMed] [Google Scholar]
- 30.Dupont LJ, Demedts MG, Verleden GM. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest. 2003;123:751–756. doi: 10.1378/chest.123.3.751. [DOI] [PubMed] [Google Scholar]
- 31.Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, Ligabue G, Ciaccia A, Saetta M, Papi A. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003;167:418–424. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- 32.Foley SC, Hopkins NO, Fitzgerald MX, Donnelly SC, McLoughlin P. Airway nitric oxide output is reduced in bronchiectasis. Respir. Med. 2007;101:1549–1555. doi: 10.1016/j.rmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Foresi A, Leone C, Olivieri D, Cremona G. Alveolar-derived exhaled nitric oxide is reduced in obstructive sleep apnea syndrome. Chest. 2007;132:860–867. doi: 10.1378/chest.06-3124. [DOI] [PubMed] [Google Scholar]
- 34.Gabriele C, Pijnenburg MW, Monti F, Hop W, Bakker ME, de Jongste JC. The effect of spirometry and exercise on exhaled nitric oxide in asthmatic children. Pediatr. Allergy Immunol. 2005;16:243–247. doi: 10.1111/j.1399-3038.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 35.Garnier P, Fajac I, Dessanges JF, Dall'Ava-Santucci J, Lockhart A, Dinh-Xuan AT. Exhaled nitric oxide during acute changes of airways calibre in asthma. Eur. Respir. J. 1996;9:1134–1138. doi: 10.1183/09031936.96.09061134. [DOI] [PubMed] [Google Scholar]
- 36.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am. J. Respir. Crit. Care Med. 1994;149:538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- 37.Gelb AF, Taylor CF, Nussbaum E, Gutierrez C, Schein A, Shinar CM, Schein MJ, Epstein JD, Zamel N. Alveolar and Airway Sites of Nitric Oxide Inflammation in Treated Asthmatics. Am. J. Respir. Crit. Care Med. 2004;170:737–741. doi: 10.1164/rccm.200403-408OC. [DOI] [PubMed] [Google Scholar]
- 38.Gelb AF, Flynn Taylor C, Shinar CM, Gutierrez C, Zamel N. Role of spirometry and exhaled nitric oxide to predict exacerbations in treated asthmatics. Chest. 2006;129:1492–1499. doi: 10.1378/chest.129.6.1492. [DOI] [PubMed] [Google Scholar]
- 39.George SC, Högman M, Permutt S, Silkoff PE. Modeling pulmonary nitric oxide exchange. J. Appl. Physiol. 2004;96:831–839. doi: 10.1152/japplphysiol.00950.2003. [DOI] [PubMed] [Google Scholar]
- 40.Girgis RE, Gugnani MK, Abrams J, Mayes MD. Partitioning of alveolar and conducting airway nitric oxide in scleroderma lung disease. Am. J. Respir.Crit. Care Med. 2002;165:1587–1591. doi: 10.1164/rccm.2104003. [DOI] [PubMed] [Google Scholar]
- 41.Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem. Biophys. Res. Commun. 1991;181:852–857. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- 42.Hamid Q, Springall DR, Riveros-Moreno V, Chanez P, Howarth P, Redington A, Bousquet J, Godard P, Holgate S, Polak JM. Induction of nitric oxide synthase in asthma. Lancet. 1993;342:1510–1513. doi: 10.1016/s0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 43.Heiss LN, Lancaster JR, Corbett JA, Goldman WE. Epithelial autotoxicity of nitric oxide: role in the respiratory cytopathology of pertussis. Proc.Natl. Acad. Sci. 1994;91:267–270. doi: 10.1073/pnas.91.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henriksen AH, Sue-Chu M, Holmen TL, Langhammer A, Bjermer L. Exhaled and nasal NO levels in allergic rhinitis: relation to sensitization, pollen season and bronchial hyperresponsiveness. Eur. Respir. J. 1999;13:301–306. doi: 10.1034/j.1399-3003.1999.13b14.x. [DOI] [PubMed] [Google Scholar]
- 45.Ho LP, Wood FT, Robson A, Innes JA, Greening AP. The current single exhalation method of measuring exhales nitric oxide is affected by airway calibre. Eur. Respir. J. 2000;15:1009–1013. doi: 10.1034/j.1399-3003.2000.01506.x. [DOI] [PubMed] [Google Scholar]
- 46.Högman M, Anderson SD, Hakansson L, Ludviksdottir D, Merilainen P, George SC. Increased airway production of nitric oxide in asthmatics determined by elimination rate flow diagram (abstract) Am. J. Respir. Crit. Care Med. 159:A862. [Google Scholar]
- 47.Högman M, Drca N, Ehrstedt C, Merilainen P. Exhaled nitric oxide partitioned into alveolar, lower airways and nasal contributions. Respir. Med. 2000;94:985–991. doi: 10.1053/rmed.2000.0872. [DOI] [PubMed] [Google Scholar]
- 48.Högman M, Holmkvist T, Walinder R, Merilainen P, Ludviksdottir D, Hakansson L, Hedenstrom H. Increased nitric oxide elimination from the airways after smoking cessation. Clin. Sci. 2002a;103:15–19. doi: 10.1042/cs1030015. [DOI] [PubMed] [Google Scholar]
- 49.Högman M, Holmkvist T, Wegener T, Emtner M, Andersson M, Hedenstrom H, Merilainen P. Extended NO analysis applied to patients with COPD,allergic asthma and allergic rhinitis. Respir. Med. 2002b;96:24–30. doi: 10.1053/rmed.2001.1204. [DOI] [PubMed] [Google Scholar]
- 50.Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53:91–95. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jatakanon A, Kjaritonov SA, Lim S, Barnes PJ. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax. 1999;54:108–114. doi: 10.1136/thx.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones SL, Herbison P, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, Taylor DR. Exhaled NO and assessment of anti-inflammatory effects of inhaled steroid: dose-response relationship. Eur. Respir. J. 2002;20:601–608. doi: 10.1183/09031936.02.00285302. [DOI] [PubMed] [Google Scholar]
- 53.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115(5):1265–1270. doi: 10.1378/chest.115.5.1265. [DOI] [PubMed] [Google Scholar]
- 54.Kerckx Y, Michils A, Van Muylem A. Airway contribution to alveolar nitric oxide in healthy subjects and stable asthma patients. J. Appl. Physiol. 2008;104:918–924. doi: 10.1152/japplphysiol.01032.2007. [DOI] [PubMed] [Google Scholar]
- 55.Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 56.Kharitonov SA, O'Connor BJ, Evans DJ, Barnes PJ. Allergen-induced late asthmatic reactions are associated with elevation of exhaled nitric oxide. Am.J. Respir. Crit. Care Med. 1995;151:1894–1899. doi: 10.1164/ajrccm.151.6.7767537. [DOI] [PubMed] [Google Scholar]
- 57.Kharitonov SA, Barnes PJ, O'Connor BJ. Reduction in exhaled nitric oxide after a single dose of nebulized budesonide in patiehts with asthma. Am.J. Respir. Crit. Care Med. 1996;153:A799. [Google Scholar]
- 58.Kharitonov SA, Chung KF, Evans D, O'Connor BJ, Barnes PJ. Increased exhaled nitric oxide in asthma is mainly derived from the lower respiratory tract. Am. J. Respir. Crit. Care Med. 1996b;153:1773–1780. doi: 10.1164/ajrccm.153.6.8665033. [DOI] [PubMed] [Google Scholar]
- 59.Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am. J. Respir. Crit.Care Med. 1996c;153:454–457. doi: 10.1164/ajrccm.153.1.8542158. [DOI] [PubMed] [Google Scholar]
- 60.Kharitonov SA, Yates DH, Chung KF, Barnes PJ. Changes in the dose of inhaled steroid affect exhaled nitric oxide levels in asthmatic patients. Eur. Respir. J. 1996d;9:196–201. doi: 10.1183/09031936.96.09020196. [DOI] [PubMed] [Google Scholar]
- 61.Kharitonov SA, Alving K, Barnes PJ. Exhaled and nasal nitric oxide measurements: recommendations. The European Respiratory Society Task Force. Eur. Respir. J. 1997;10:1683–1693. doi: 10.1183/09031936.97.10071683. [DOI] [PubMed] [Google Scholar]
- 62.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am.J. Respir. Crit. Care Med. 2001;163:1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 63.Kimberly B, Nejadnik B, Giraud GD, Holden WE. Nasal contribution to exhaled nitric oxide at rest and during breathholding in humans. Am. J. Resp.Crit. Care Med. 1996;153:829–836. doi: 10.1164/ajrccm.153.2.8564139. [DOI] [PubMed] [Google Scholar]
- 64.Kumar V, Abbas AK, Nelson F. Pathologic Basis of Disease. 7th edition. Philadelphia, PA: Elsevier Saunders; 2005. pp. 723–727. [Google Scholar]
- 65.Langley SJ, Goldthorpe S, Craven M, Woodcock A, Custovic A. Relationship among pulmonary function, bronchial reactivity, and exhaled nitric oxide in a large group of asthmatic patients. Ann. Allergy Asthma. Immunol. 91:398–404. doi: 10.1016/S1081-1206(10)61688-2. [DOI] [PubMed] [Google Scholar]
- 66.Lehtimäki L, Turjanmaa V, Kankaanranta H, Sarrelainen S, Hahtola P, Moilanen E. Increased bronchial nitric oxide production in patients with asthma measured with a novel method of different exhalation flow rates. Ann.Med. 2000;32:417–423. doi: 10.3109/07853890008995949. [DOI] [PubMed] [Google Scholar]
- 67.Lehtimäki L, Kankaanranta H, Saarelainen S, Hahtola P, Järvenpää R, Koivula T, Turjanmaa V, Moilanen E. Extended exhaled NO measurement differentiates between alveolar and bronchial inflammation. Am. J.Respir. Crit. Care Med. 2001a;163:1557–1561. doi: 10.1164/ajrccm.163.7.2010171. [DOI] [PubMed] [Google Scholar]
- 68.Lehtimäki L, Kankaanranta H, Saarelainen S, Turjanmaa V, Moilanen E. Inhaled fluticasone decreases bronchial but not alveolar nitric oxide output in asthma. Eur. Respir. J. 2001b;18:635–639. doi: 10.1183/09031936.01.00000201. [DOI] [PubMed] [Google Scholar]
- 69.Lehtimäki L, Kankaanranta H, Sarrelainen S, Hahtola P, Järvenpää R, Koivula T, Turjanmaa V, Moilanen E. Extended exhaled NO measurement differences between alveolar and bronchial inflammation. Am. J. Respir. Crit.Care Med. 2001c;163:1577–1561. doi: 10.1164/ajrccm.163.7.2010171. [DOI] [PubMed] [Google Scholar]
- 70.Lehtimäki L, Kankaanranta H, Saarelainen S, Turjanmaa V, Moilanen E. Increased alveolar nitric oxide concentration in asthmatic patients with nocturnal symptoms. Eur Respir. J. 2002;20(4):841–845. doi: 10.1183/09031936.02.00202002. [DOI] [PubMed] [Google Scholar]
- 71.Lehtimäki L, Kankaanranta H, Saarelainen S, Turjanmaa V, Moilanen E. Peripheral inflammation in patients with asthmatic symptoms but normal lung function. J. Asthma. 2005;42:605–609. doi: 10.1080/02770900500294678. [DOI] [PubMed] [Google Scholar]
- 72.Lehtonen H, Oksa P, Lehtimäki L, Sepponen A, Nieminen R, Kankaanranta H, Saarelainen S, Järvenpää R, Uitti J, Moilanen E. Increased alveolar nitric oxide concentration and high levels of leukotriene B(4) and 8-isoprostane in exhaled breath condensate in patients with asbestosis. Thorax. 2007;62:602–607. doi: 10.1136/thx.2006.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leuppi JD, Salome CM, Jenkins CR, Anderson SD, Xuan W, Marks GB, Koskela H, Brannan JD, Freed R, Andersson M, Chan HK, Woolcock AJ. Predictive markers of asthma exacerbation during stepwise dose reduction of inhaled corticosteroids. Am. J. Respir. Crit. Care Med. 2001;163:406–412. doi: 10.1164/ajrccm.163.2.9912091. [DOI] [PubMed] [Google Scholar]
- 74.Lex C, Payne DN, Zacharasiewicz A, Li AM, Wilson NM, Hansel TT, Bush A. Sputum induction in children with difficult asthma: safety, feasibility, and inflammatory cell pattern. Pediatr. Pulmonol. 2005;39:318–324. doi: 10.1002/ppul.20159. [DOI] [PubMed] [Google Scholar]
- 75.Lim S, Jatakanon A, John M, Gilbey T, O'connor BJ, Chung KF, Barnes PJ. Effect of inhaled budesonide on lung function and airway inflammation. Am. J. Respir. Crit. Care Med. 1999;159:22–30. doi: 10.1164/ajrccm.159.1.9706006. [DOI] [PubMed] [Google Scholar]
- 76.Lim S, Jatakanon A, Meah S, Oates T, Chung KF, Barnes PJ. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in mild to moderately severe asthma. Thorax. 2000;55:184–188. doi: 10.1136/thorax.55.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim S, Tomita K, Caramori G, Jatakanon A, Oliver B, Keller A, Adcock I, Chung KF, Barnes PJ. Low-dose theophylline reduces eosinophilic inflammation but not exhaled nitric oxide in mild asthma. Am. J. Respir. Crit. Care Med. 2001;164:273–276. doi: 10.1164/ajrccm.164.2.2006043. [DOI] [PubMed] [Google Scholar]
- 78.Linkosalo L, Lehtimaki L, Laitinen J, Kaila M, Holm K, Moilanen E. Increased bronchial NO output in severe atopic eczema in children and adolescents. Pediatr Allergy Immunol. 2007 doi: 10.1111/j.1399-3038.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 79.Lious R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanović R. The relationship between airways inflammation and asthma severity. Am.J. Respir. Crit. Care Med. 2000;161:9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- 80.Little SA, Chalmers GW, MacLeod KJ, McSharry C, Thomson NC. Non-invasive markers of airway inflammation as predictors of oral steroid responsiveness in asthma. Thorax. 2000;55:232–234. doi: 10.1136/thorax.55.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lundberg JO, Farkas-Szallasi T, Weitzberg E, Rinder J, Lidholm J, Anggåard A, Hökfelt T, Lundberg JM, Alving K. High nitric oxide production in human paranasal sinuses. Nat. Med. 1995;1:370–373. doi: 10.1038/nm0495-370. [DOI] [PubMed] [Google Scholar]
- 82.Mahut B, Delclaux C, Tillie-Leblond I, Gosset P, Delacourt C, Zerah-Lancner F, Harf A, de Blic J. Both inflammation and remodeling influence nitric oxide output in children with refractory asthma. J. Allergy Clin. Immunol. 2004a;113:252–256. doi: 10.1016/j.jaci.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 83.Mahut B, Delacourt C, Zerah-Lancner F, De Blic J, Harf A, Delclaux C. Increase in alveolar nitric oxide in the presence of symptoms in childhood asthma. Chest. 2004b;125:1012–1018. doi: 10.1378/chest.125.3.1012. [DOI] [PubMed] [Google Scholar]
- 84.Malinovschi A, Janson C, Holmkvist T, Norback D, Merilainen P, Högman M. Effect of smoking on exhaled nitric oxide and flow-independent nitric oxide exchange parameters. Eur. Respir. J. 2006a;28:339–345. doi: 10.1183/09031936.06.00113705. [DOI] [PubMed] [Google Scholar]
- 85.Malinovschi A, Janson C, Holmkvist T, Norback D, Merilainen P, Högman M. IgE sensitisation in relation to flow-independent nitric oxide exchange parameters. Respir. Res. 2006b;7:92. doi: 10.1186/1465-9921-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malmberg LP, Pelkonen AS, Haahtela T, Turpeinen M. Exhaled nitric oxide rather than lung function distinguishes preschool children with probable asthma. Thorax. 2003;58:494–499. doi: 10.1136/thorax.58.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Massaro AF, Gaston B, Kita D, Fanta C, Stamler JS, Drazen JM. Expired nitric oxide levels during treatment of acute asthma. Am. J. Respir. Crit. Care Med. 1995;152:800–803. doi: 10.1164/ajrccm.152.2.7633745. [DOI] [PubMed] [Google Scholar]
- 88.Mattes J, Storm van's Gravesande K, Reining U, Alving K, Ihorst G, Henschen M, Kuehr J. NO in exhaled air is correlated with markers of eosinophilic airway inflammation in corticosteroid-dependent childhood asthma. Eur. Respir. J. 1999;13:1391–1395. [PubMed] [Google Scholar]
- 89.Menzies D, Nair A, Meldrum KT, Hopkinson P, Lipworth BJ. Effect of aspirin on airway inflammation and pulmonary function in patients with persistent asthma. J. Allergy. Clin. Immunol. 2008 doi: 10.1016/j.jaci.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 90.Morris SM, Billar TR. New insights into the regulation of inducible nitric oxide synthesis. Am. J. Physiol. 1994;266:E829–E839. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- 91.Murphy AW, Platts-Mills TA, Lobo M, Hayden F. Respiratory nitric oxide levels in experimental human influenza. Chest. 1998;114:452–456. doi: 10.1378/chest.114.2.452. [DOI] [PubMed] [Google Scholar]
- 92.Obata H, Dittrick M, Chan H, Chan-Yeung M. Sputum eosinophils and exhaled nitric oxide during late asthmatic reaction in patients with western red cedar asthma. Eur. Respir. J. 1999;13:489–495. doi: 10.1183/09031936.99.13348999. [DOI] [PubMed] [Google Scholar]
- 93.Olin AC, Aldenbratt A, Ekman A, Ljungkvist G, Jungersten L, Alving K, Torén K. Increased nitric oxide in exhaled air after intake of a nitrate-rich meal. Respir. Med. 2001;95:153–158. doi: 10.1053/rmed.2000.1010. [DOI] [PubMed] [Google Scholar]
- 94.Olin AC, Andersson M, Wass K. Normal values for fraction of exhaled nitric oxide (FENO) among nonsmoking adults. American Thoracic Society Meeting; San Diego, CA. 2005. [Google Scholar]
- 95.Paraskakis E, Brindicci C, Fleming L, Krol R, Kharitonov SA, Wilson NM, Barnes PJ, Bush A. Measurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. Am. J. Respir. Crit. Care Med. 2006;174:260–267. doi: 10.1164/rccm.200506-962OC. [DOI] [PubMed] [Google Scholar]
- 96.Peroni D, Bodini A, Miraglia Del Giudice M, Loiacono A, Baraldi E, Boner AL, Piacentini G. Effect of budesonide and montelukast in asthmatic children exposed to relevant allergens. Allergy. 2005;60:206–210. doi: 10.1111/j.1398-9995.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 97.Phansalkar AR, Hanson CM, Shakir AR, Johnson RL, Jr, Hsia CC. Nitric oxide diffusing capacity and alveolar microvascular recruitment in sarcoidosis. Am. J. Respir. Crit. Care Med. 2004;169:1034–1040. doi: 10.1164/rccm.200309-1287OC. [DOI] [PubMed] [Google Scholar]
- 98.Piacentini GL, Bodini A, Costella S, Vicentini L, Suzuki Y, Boner AL. Exhaled nitric oxide is reduced after sputum induction in asthmatic children. Pediatr Pulmonol. 2000;29:430–433. doi: 10.1002/(sici)1099-0496(200006)29:6<430::aid-ppul3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 99.Piacentini GL, Bodini A, Peroni DG, Miraglia del Giudice M, Jr, Costella S, Boner AL. Reduction in exhaled nitric oxide immediately after methacholine challenge in asthmatic children. Thorax. 2002;57:771–773. doi: 10.1136/thorax.57.9.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pietropaoli AP, Perillo IB, Torres A, Perkins PT, Frasier LM, Utell MJ, Frampton MW, Hyde RW. Simultaneous measurement of nitric oxide production by conducting and alveolar airways of humans. J. Appl. Physiol. 1999;87:1532–1542. doi: 10.1152/jappl.1999.87.4.1532. [DOI] [PubMed] [Google Scholar]
- 101.Pietropaoli AP, Perillo IB, Perkins PT, Frasier LM, Speers DM, Frampton MW, Utell MJ, Hyde RW. Smokers have reduced nitric oxide production by conducting airways but normal levels in the alveoli. Inhal. Toxicol. 2007;19:533–541. doi: 10.1080/08958370701260673. [DOI] [PubMed] [Google Scholar]
- 102.Pijnenburg MW, Hofhuis W, Hop WC, De Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60:215–218. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pijnenburg MH, De Jongste JC. Exhaled nitric oxide in childhood asthma: a review. Clin. Exp. Allergy. 2008;38(2):246–259. doi: 10.1111/j.1365-2222.2007.02897.x. [DOI] [PubMed] [Google Scholar]
- 104.Prasad A, Langford B, Stradling JR, HoHo LP. Exhaled nitric oxide as a screening tool for asthma in school children. Respir. Med. 2006;100:167–173. doi: 10.1016/j.rmed.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 105.Puckett JL, Aledia AS, Shin HW, Shelly DA, Galant SP, George SC. Airway and Alveolar Nitric Oxide Phenotypes in the Pediatric Asthma Population. Toronto: American Thoracic Society; 2008. [Google Scholar]
- 106.Regington AE. Modulation of nitric oxide pathways: Therapeutic potential in asthma and chronic obstructive pulmonary disease. Eu. J. Pharamacol. 2006;533:263–276. doi: 10.1016/j.ejphar.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 107.National Institutes of Health, National Heart, Lung and Blood Institute; Revised GINA guidelines: Global initiative for asthma. 2002 NIH publication No. 02-3659.
- 108.Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58:175–182. doi: 10.1136/thorax.58.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 110.Robbins RA, Barnes PJ, Springall DR, Warren JB, Kwon OJ, Buttery LD, Wilson AJ, Geller DA, Polak JM. Expression of inducible nitric oxide in human lung epithelial cells. Biochem. Biophys Res. Commun. 1994;203:209–218. doi: 10.1006/bbrc.1994.2169. [DOI] [PubMed] [Google Scholar]
- 111.Rolla G, Brussino L, Scappaticci E, Morello M, Innarella R, Rosina F, Bucca C. Source of exhaled nitric oxide in primary biliary cirrhosis. Chest. 2004;126:1546–1551. doi: 10.1378/chest.126.5.1546. [DOI] [PubMed] [Google Scholar]
- 112.Rolla G, Nebiolo F, Guida G, Heffler E, Bommarito L, Bergia R. Level of exhaled nitric oxide during human anaphylaxis. Ann. Allergy Asthma Immunol. 2006;97:264–265. doi: 10.1016/S1081-1206(10)60025-7. [DOI] [PubMed] [Google Scholar]
- 113.Roy K, Borrill ZL, Starkey C, Hazel AL, Morris J, Vestbo J, Singh D. Use of different exhaled nitric oxide multiple flow rate models in COPD. Eur. Respir. J. 2007;29:651–659. doi: 10.1183/09031936.00149706. [DOI] [PubMed] [Google Scholar]
- 114.Sacco O, Sale R, Silvestri M, Serpero L, Sabatini F, Raynal ME, Biraghi M, Rossi GA. Total and allergen-specific IgE levels in serum reflect blood eosinophilia and fractional exhaled nitric oxide concentrations but not pulmonary functions in allergic asthmatic children sensitized to house dust mites. Pediatr. Allergy Immunol. 2003;14:475–481. doi: 10.1046/j.0905-6157.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 115.Sadeghi-Hashjin G, Folkerts G, Henricks PA, Verheyen AK, van der Linde HJ, van Ark I, Coene A, Nijkamp FP. Peroxynitrite induces airway hyperresponsiveness in guinea pigs in vitro and in vivo. Am. J. Respir. Crit. Care Med. 1996;153:1697–1701. doi: 10.1164/ajrccm.153.5.8630623. [DOI] [PubMed] [Google Scholar]
- 116.Salome CM, Roberts AM, Brown NJ, Dermand J, Marks GB, Woolcock AJ. Exhaled nitric oxide measurements in a population sample of young adults. Am. J. Respir. Crit. Care Med. 1999;159:911–916. doi: 10.1164/ajrccm.159.3.9802108. [DOI] [PubMed] [Google Scholar]
- 117.Shaw DE, Berry MA, Thomas M, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide to guide asthma management:a randomized controlled trial. Am. J. Respir. Crit. Care Med. 2007;176:231–237. doi: 10.1164/rccm.200610-1427OC. [DOI] [PubMed] [Google Scholar]
- 118.Shin HW, George SC. Impact of axial diffusion on nitric oxide exchange in the lungs. J. Appl. Physiol. 2002;93:2070–2080. doi: 10.1152/japplphysiol.00129.2002. [DOI] [PubMed] [Google Scholar]
- 119.Shin HW, Rose-Gottron CM, Sufi RS, Perez F, Cooper DM, Wilson AF, George SC. Flow-independent nitric oxide exchange parameters in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002;165:349–357. doi: 10.1164/ajrccm.165.3.2105098. [DOI] [PubMed] [Google Scholar]
- 120.Shin HW, Rose-Grottron CM, Cooper DM, Hill MA, George SC. Impact of high intensity exercise on flow-independent NO exchange parameters. Med. Sci. Sports Exerc. 2003;35:995–1003. doi: 10.1249/01.MSS.0000072247.46963.CD. [DOI] [PubMed] [Google Scholar]
- 121.Shin HW, Condorelli P, Rose-Gottron CM, Cooper DM, George SC. Probing the impact of axial diffusion on nitric oxide exchange dynamics with heliox. J. Appl. Physiol. 2004a;97:874–882. doi: 10.1152/japplphysiol.01297.2003. [DOI] [PubMed] [Google Scholar]
- 122.Shin HW, Rose-Gottron CM, Cooper DM, Newcomb RL, George SC. Airway diffusing capacity of nitric oxide and steroid therapy in asthma. J. Appl. Physiol. 2004b;96:65–75. doi: 10.1152/japplphysiol.00575.2003. [DOI] [PubMed] [Google Scholar]
- 123.Shin HW, Condorelli P, George SC. A new and more accurate technique to characterize airway nitric oxide using different breath-hold times. J. Appl. Physiol. 2005;98:1869–1877. doi: 10.1152/japplphysiol.01002.2004. [DOI] [PubMed] [Google Scholar]
- 124.Shin HW, Condorelli P, George SC. Examining axial diffusion of nitric oxide in the lungs using heliox and breath hold. J. Appl. Physiol. 2006;100:623–630. doi: 10.1152/japplphysiol.00008.2005. [DOI] [PubMed] [Google Scholar]
- 125.Shin HW, Schwindt CD, Aledia AS, Rose-Gottron CM, Larson JK, Newcomb RL, Cooper DM, George SC. Exercise-induced bronchoconstriction alters airway nitric oxide exchange in a pattern distinct from spirometry. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R1741–R1748. doi: 10.1152/ajpregu.00178.2006. [DOI] [PubMed] [Google Scholar]
- 126.Shin HW, Shelley DA, Henderson EM, Fitzpatrick A, Gaston B, George SC. Airway nitric oxide release is reduced after PBS inhalation in asthma. J. Appl. Physiol. 2007;102:1028–1033. doi: 10.1152/japplphysiol.01012.2006. [DOI] [PubMed] [Google Scholar]
- 127.Silkoff PE, McClean PA, Slutsky AS, Furlott HG, Hoffstein E, Wakita S, Chapman KR, Szalai JP, Zamel N. Marked flow-dependence of exhaled nitric oxide using a new technique to exclude nasal nitric oxide. Am. J. Respir. Crit. Care Med. 1997;155:260–267. doi: 10.1164/ajrccm.155.1.9001322. [DOI] [PubMed] [Google Scholar]
- 128.Silkoff PE, Wakita S, Chatkin J, Ansarin K, Gutierrez C, Caramori M, McClean P, Slutsky AS, Zamel N, Chapman KR. Exhaled nitric oxide after beta2-agonist inhalation and spirometry in asthma. Am. J. Respir. Crit. Care Med. 1999;159:940–944. doi: 10.1164/ajrccm.159.3.9805044. [DOI] [PubMed] [Google Scholar]
- 129.Silkoff PE, Sylvester JT, Zamel N, Permutt S. Airway Nitric Oxide Diffusion in Asthma. Role in pulmonary function and bronchial responsiveness. Am. J. Respir. Crit. Care Med. 2000;161:1218–1228. doi: 10.1164/ajrccm.161.4.9903111. [DOI] [PubMed] [Google Scholar]
- 130.Silkoff PE, McClean P, Spino M, Erlich L, Slutsky AS, Zamel N. Dose-response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled belcomethasone dipropionate therapy in asthma patients. Chest. 2001;119:1322–1328. doi: 10.1378/chest.119.5.1322. [DOI] [PubMed] [Google Scholar]
- 131.Silvestri M, Sabatini F, Sale R, Defilippi AC, Fregonese L, Battistini E, Biraghi MG, Rossi GA. Correlations between exhaled nitric oxide levels, blood eosinophilia, and airway obstruction reversibility in childhood asthma are detectable only in atopic individuals. Pediatr Pulmonol. 2003;35:358–363. doi: 10.1002/ppul.10264. [DOI] [PubMed] [Google Scholar]
- 132.Silvestri M, Spallarossa D, Frangova Y, Battistini E, Fregonese B, Rossi GA. Orally exhaled nitric oxide levels are related to the degree of blood eosinophilia in atopic children with mild-intermittent asthma. Eur. Respir. J. 1999;13:321–326. doi: 10.1034/j.1399-3003.1999.13b17.x. [DOI] [PubMed] [Google Scholar]
- 133.Silvestri M, Spallarossa D, Battistini E, Brusasco V, Rossi GA. Dissociation between exhaled nitric oxide and hyperresponsiveness in children with mild intermittent asthma. Thorax. 2000;55:484–488. doi: 10.1136/thorax.55.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Silvestri M, Sabatini F, Spallarossa D, Fregonese L, Battistini E, Biraghi MG, Rossi GA. Exhaled nitric oxide levels in non-allergic and allergic mono- or polysensitised children with asthma. Thorax. 2001;56:857–862. doi: 10.1136/thorax.56.11.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith AD, Cowan JO, Filsell S, McLachlan C, Monti-Sheehan G, Jackson P, Taylor DR. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am. J. Respir. Crit. Care Med. 2004;169:473–478. doi: 10.1164/rccm.200310-1376OC. [DOI] [PubMed] [Google Scholar]
- 136.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N. Engl. J. Med. 2005;352:2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 137.Spergel JM, Fogg MI, Bokszczanin-Knosala A. Correlation of exhaled nitric oxide, spirometry and asthma symptoms. J. Asthma. 2005;42:879–883. doi: 10.1080/02770900500371344. [DOI] [PubMed] [Google Scholar]
- 138.Steerenberg PA, Janssen NA, de Meer G, Fischer PH, Nierkens S, van Loveren H, Opperhuizen A, Brunekreef B, van Amsterdam JG. Relationship between exhaled NO, respiratory symptoms, lung function, bronchial hyperresponsiveness, and blood eosinophilia in school children. Thorax. 2003;58:242–245. doi: 10.1136/thorax.58.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stirling RG, Kharitonov SA, Campbell D, Robinson DS, Durham SR, Chung KF, Barnes PJ Asthma and Allergy Group. Increase in exhaled nitric oxide levels in patienst with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Thorax. 1998;53:1030–1034. doi: 10.1136/thx.53.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Strunk RC, Szefler SJ, Phillips BR, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J. Allergy Clin. Immunol. 2003;112:883–892. doi: 10.1016/j.jaci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 141.Suresh V, Minh JD, George SC. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 2007;37(1):97–104. doi: 10.1165/rcmb.2006-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Suresh V, Shelley DA, Shin HW, George SC. Effect of Heterogeneous Ventilation and Nitric Oxide Production on Exhaled Nitric Oxide Profiles. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.01355.2007. [DOI] [PubMed] [Google Scholar]
- 143.Suri R, Paraskakis E, Bush A. Alveolar, but not bronchial nitric oxide production is elevated in cystic fibrosis. Pediatr. Pulmonol. 2007;42:1215–1221. doi: 10.1002/ppul.20730. [DOI] [PubMed] [Google Scholar]
- 144.Szefler SJ, Martin RJ, King TS, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J. Allergy Clin. Immunol. 2002;109:410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 145.Taylor DR, Pijnenburg MW, Smith AD, Jongste JCD. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006;61:817–827. doi: 10.1136/thx.2005.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Taylor DR. Exhaled NO: Forward, Backward, or Sideways? Am. J. Respir. Crit. Care Med. 2007;176:221–222. doi: 10.1164/rccm.200702-246ED. [DOI] [PubMed] [Google Scholar]
- 147.Tee AK, Hui KP. Effect of spirometric maneuver, nasal clip, and submaximal inspiratory effort on measurement of exhaled nitric oxide levels in asthma patients. Chest. 2005;127:131–134. doi: 10.1378/chest.127.1.131. [DOI] [PubMed] [Google Scholar]
- 148.Terada A, Fujisawa T, Togashi K, Miyazaki T, Katsumata H, Atsuta J, Iguchi K, Kamiya H, Togari H. Exhaled nitric oxide decreases during exercise-induced bronchoconstriction in children with asthma. Am. J. Respir. Crit. Care Med. 2001;164:1879–1884. doi: 10.1164/ajrccm.164.10.2009105. [DOI] [PubMed] [Google Scholar]
- 149.Thomas PS, Gibson PG, Wang H, Shah S, Henry RL. The relationship of exhaled nitric oxide to airway inflammation and responsiveness in children. J. Asthma. 2005;42:291–295. doi: 10.1081/jas-200057908. [DOI] [PubMed] [Google Scholar]
- 150.Tsoukias NM, George SC. A two-compartment model of pulmonary nitric oxide exchange dynamics. J. Appl. Physiol. 1998a;85:653–666. doi: 10.1152/jappl.1998.85.2.653. [DOI] [PubMed] [Google Scholar]
- 151.Tsoukias NM, Tannous Z, Wilson AF, George SC. Single-exhalation profiles of NO and CO2 in humans: effect of dynamically changing flow rate. J. Appl. Physiol. 1998b;85:642–652. doi: 10.1152/jappl.1998.85.2.642. [DOI] [PubMed] [Google Scholar]
- 152.Tsoukias NM, Shin HW, Wilson AF, George SC. A single breath technique with variable flow rate to characterize nitric oxide exchange dynamics in the lungs. J. Appl. Physiol. 2001;91:477–487. doi: 10.1152/jappl.2001.91.1.477. [DOI] [PubMed] [Google Scholar]
- 153.van den Toom L, Overbeek S, De Jongste JC, Leman K, Hoogsteden HC, Prins JB. Airway inflammation is present during clinical remission of atopic asthma. Am. J. Respir. Crit. Care Med. 2001;164:2107-2013. doi: 10.1164/ajrccm.164.11.2006165. [DOI] [PubMed] [Google Scholar]
- 154.van Rensen EL, Straathof KC, Veselic-Charvat MA, Zwinderman AH, Bel EH, Sterk PJ. Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax. 1999;54:403–408. doi: 10.1136/thx.54.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Veen IH, Sterk PJ, Schot R, Gauw SA, Rabe KF, Bel EH. Alveolar nitric oxide versus measures of peripheral airway dysfunction in severe asthma. Eur. Respir. J. 2006;27:951–956. doi: 10.1183/09031936.06.00087905. [DOI] [PubMed] [Google Scholar]
- 156.Venge P. Epithelial injury by human eosinophils. Am. Rev. Respir. Dis. 1998;138:54–57. doi: 10.1164/ajrccm/138.6_Pt_2.S54. [DOI] [PubMed] [Google Scholar]
- 157.Verleden GM, Dupont LJ, Verpeut AC, Demedts MG. The effect of cigarette smoking on exhaled nitric oxide in mild steroidnaive asthmatics. Chest. 1999;116:59–64. doi: 10.1378/chest.116.1.59. [DOI] [PubMed] [Google Scholar]
- 158.Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, Bonsignore G. Airway remodelling in asthma. Chest. 2003;123:417S–422S. doi: 10.1378/chest.123.3_suppl.417s. [DOI] [PubMed] [Google Scholar]
- 159.Weibel E. Morphometry of the human lung. Berlin: Springler-Verlag; 1963. [Google Scholar]
- 160.Welsh L, Lercher P, Horak E. Exhaled nitric oxide: Interactions between asthma, hayfever, and atopic dermatitis in school children. Pediatr. Pulmonol. 2007;42:693–698. doi: 10.1002/ppul.20632. [DOI] [PubMed] [Google Scholar]
- 161.Wilson NM, Bridge P, Spanevello A, Silverman M. Induced sputum in children: feasibility, repeatability, and relation of findings to asthma severity. Thorax. 2000;55:867–874. doi: 10.1136/thorax.55.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Young JD, Peterson CG, Venge P, Cohn ZA. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986;321:613–616. doi: 10.1038/321613a0. [DOI] [PubMed] [Google Scholar]
- 163.Zacharasiewicz A, Wilson N, Lex C, Erin EM, Li AM, Hansel T, Khan M, Bush A. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am. J. Respir. Crit. Care Med. 2005;171:1077–1082. doi: 10.1164/rccm.200409-1242OC. [DOI] [PubMed] [Google Scholar]
- 164.Zavorsky GS, Cao J, Murias JM. Reference values of pulmonary diffusing capacity for nitric oxide in an adult population. Nitric Oxide. 2008;18:70–79. doi: 10.1016/j.niox.2007.10.002. [DOI] [PubMed] [Google Scholar]


