Abstract
Purpose
Phospholipase A2 (PLA2) is a growing family of lipolytic enzymes that play a key role in various biological processes including general lipid metabolism, membrane homeostasis, and in diseases such as atherosclerosis, arthritis, and acute pancreatitis. Oxidative stress as well as inflammation may be associated with glaucoma pathogenesis. Therefore, our aim was to examine the expression of group IIA secretory PLA2 (sPLA2-IIA), group V secretory PLA2 (sPLA2-V), calcium-independent PLA2 (iPLA2), and cytosolic PLA2 (cPLA2) type in the trabecular meshwork (TM) and the canal of Schlemm in normal eyes and in juxtacanalicular tissue samples from patients with primary open angle glaucoma (POAG) or exfoliation glaucoma (ExG).
Methods
TM tissues were isolated from healthy donor eyes for corneal transplantation. Specimens of inner wall of the Schlemm's canal and the juxtacanalicular tissue were collected during deep sclerectomy from the eyes of patients who had POAG or ExG. Antibodies against PLA2s (sPLA2-IIA, sPLA2-V, iPLA2, and cPLA2) and a standard immunohistochemical procedure were used for the analysis. Quantification of immunoreactions was provided using a Photoshop-based image analysis. Double-staining immunofluorescence of macrophages and sPLA2-IIA was performed by using confocal microscopy.
Results
sPLA2-IIA was not present in normal TM. In contrast, sPLA2-IIA levels were significantly higher in glaucoma patients than in controls. Furthermore, sPLA2-IIA expression was much higher in POAG when compared to ExG. iPLA2 was found to predominate in normal human TM, and it demonstrated strong labeling in the uveal and corneoscleral meshwork. The staining of juxtacanalicular meshwork was only moderate in density. In contrast, expression of the enzyme was significantly decreased in glaucoma patients, especially in ExG, when compared to normal controls or to POAG. In addition, strong regional differences were detected in sPLA2-IIA and iPLA2 levels in POAG, whereas immunostaining of these enzymes was much lower and rather uniform throughout ExG sample. In POAG, sPLA2-IIA staining was restricted to certain parts of the trabecular samples where sPLA2-IIA positive macrophages were also present. Immunostaining of sPLA2-V or cPLA2 was low, and no significant changes were found in levels of these enzymes between normal and glaucomatous samples.
Conclusions
sPLA2-IIA, an oxidative stress marker in atherosclerosis, is overexpressed especially in POAG. This result supports the hypothesis that oxidative stress may play a significant role in the pathogenesis of POAG. In ExG, a dramatic decrease in the expression level of iPLA2, a housekeeping enzyme in phospholipid remodeling, may indicate imbalance in phospholipid turnover and also inhibition of normal physiological functions in the TM. These findings may contribute to understanding the pathogenesis of POAG and ExG and may be important for the development of novel therapeutic strategies to different glaucomas.
Introduction
The term glaucoma is used to describe a heterogeneous group of diseases that have in common a characteristic optic cup neuropathy with loss of visual field defects [1]. Elevated intraocular pressure (IOP) is a strong risk factor for open-angle glaucoma, but some patients with glaucoma have normal IOP and many patients with elevated IOP do not have glaucoma [2,3]. In Finland and other Nordic countries, the most common types of glaucoma are primary open-angle glaucoma (POAG) and exfoliation glaucoma (ExG) [4-6]. Usually ExG is more aggressive; it reacts worse to medical treatment, and optic nerve damage and visual field loss take place earlier than in POAG [7-12]. Elevated IOP in ExG may be attributed to accumulation of the exfoliation material or pigment particles in the angle chamber [13-15].
PLA2 (EC 3.1.1.4) belongs to a superfamily of enzymes that catalyzes the hydrolysis of the sn-2 ester bond in phospholipids. The hydrolysis products are free fatty acids and lysophospholipids [16,17]. Different PLA2 isoenzymes have been found and classified into several groups (from I to XIV) based on their structures, subcellular distributions, cellular functions, and enzymatic characteristics [18,19]. In a simplified classification system, PLA2s can be divided into four major groups: secretory PLA2 (sPLA2), Ca2+-independent PLA2 (iPLA2), cytosolic PLA2 (cPLA2), and a class of PLA2 called platelet-activating factor (PAF) acetylhydrolase (PAF-AH) [20-22]. sPLA2 is optimally active at millimolar Ca2+ concentration and cPLA2 requires micromolar amounts of Ca2+, whereas iPLA2 does not need Ca2+ for activity [23].
PLA2s play a key role in various biological processes. sPLA2 has been implicated in the regulation of a wide array of cellular functions, such as arachidonic acid (AA) metabolism, phospholipids digestion, extracellular matrix (ECM) remodeling, regulation of proliferation and cell contraction, endothelial cell migration, antimicrobial defense, and regulation of acrosome reaction of spermatozoa [23-33]. Elevated levels of sPLA2 have been detected in several diseases including atherosclerosis, inflammatory diseases, arthritis, acute pancreatitis, and neurodegeneration [34-39].
cPLA2 is the only PLA2 that shows significant selectivity toward AA at the sn-2 position of the phospholipid molecule [40]. Therefore, it plays an important role in mediating important cellular processes including eicosanoid biosynthesis [41].
iPLA2 is generally regarded as a housekeeping enzyme as it remodels and maintains membrane phospholipids [42]. Recent studies have suggested the enzyme has other roles. iPLA2 has a proliferative effect and a functional role in cellular signaling cascades, vascular smooth muscle contraction, artery relaxation, and in apoptotic processes [43-48]. Recently, it was reported that iPLA2 is required for activation of store-operated Ca2+ channels to initiate Ca2+ influx [49].
In general, mammalian cells contain more than one PLA2 [17] thus there is considerable interest in determining the role of each PLA2. To our knowledge, although much research has been done to characterize, purify, and clone various forms of PLA2 from diverse sources, virtually nothing has been presented about the existence of PLA2 in the human anterior chamber angle. The anterior segment of the eye is filled with aqueous humor. A major component of the anterior chamber angle is the trabecular meshwork (TM) and the canal of Schlemm. The TM regulates the outflow facility of the aqueous humor and is also responsible for IOP control [50]. Recent notions that oxidative stress may play a role in glaucomatous TM cells have brought new insights to probable pathophysiologic mechanisms behind glaucoma [51]. Interestingly, PLA2s are important mediators of oxidative damage in cells [20,39]. To fill this gap, the goal of the present study was to immunohistochemically analyze the expression of PLA2 in normal human chamber angle and the inner wall of Schlemm's canal and the juxtacanalicular tissue of patients with POAG or ExG. In this work we used antibodies against four distinct PLA2s, including group IIA secretory PLA2 (sPLA2-IIA), group V secretory PLA2 (sPLA2-V), iPLA2, and cPLA2.
Methods
Materials
Monoclonal antibodies against sPLA2-IIA were purchased from Upstate (Lake Placid, NY). Monoclonal antibodies against sPLA2-V and cPLA2 (sc-4-4B-3C, lot number E1704) and rabbit polyclonal antibodies against iPLA2 were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Monoclonal antibodies against CD68 (Lab Vision Corporation) were used for immunohistochemical staining of macrophages. For the double labeling experiments goat antimouse IgG conjugated to Alexa Fluor 488 or Alexa Fluor 594 secondary antibodies (Molecular probes, Eugene OR) were used. For Western blot experiments, horseradish peroxidase-linked antimouse (GE Healthcare, Buckinghamshire, England) or antirabbit (GE Healthcare) secondary antibodies were used.
Samples
Normal human TM tissue samples were obtained from healthy eyes donated for corneal transplantation (n=8). Tissue samples were also obtained from patients with POAG (n=6) and patients with ExG (n=6), who were undergoing elective deep sclerectomy. During the operation, the external wall of Schlemm's canal was opened, and the tissue specimens were taken from the inner wall of Schlemm's canal. The juxtacanalicular meshwork and corneoscleral trabecular layers were taken by direct visual control during the surgery. Tissue specimens were frozen in -70 °C until used, or they were fixed in formalin and embedded in paraffin. Paraffin sections (5 μm) and cryostat (Leica CM3050S, Leica Microsystems, Nussloch, Germany) sections (5 μm) were placed on Super Frost®Plus microscope slides (Menzel GmbH & Co KG, Germany). The procedure for obtaining the tissues was within the tenets of the Declaration of Helsinki.
Clinical findings in primary open angle glaucoma and exfoliation glaucoma patients
Prior to the surgery, clinical data was collected on each patient, including age, gender, use of prostaglandin analogs, number of argon laser trabeculoplasty and other ocular surgical interventions, type and duration of glaucoma, IOP, and visual acuity. Glaucoma classification was based on careful clinical eye examination. All patients underwent slit lamp examination on the day before surgery. All IOPs in the POAG or ExG group exceeded 20 mmHg at the time of surgery. Visual acuity varied from 0.3 to 1.0.
Homogenization and western blot analysis of control samples
Low molecular weight standards were obtained from Amersham Biosciences. Normal human TM tissue samples were homogenized on ice in T-PER Tissue Protein Extraction Reagent with protease inhibitor coctail (Pierce, Rockford, IL). Proteins (10 μg) were separated by SDS-PAGE [52], and after the run, the gels were subjected to western blot. Briefly, the samples were transferred (voltage: 12 V; current: 100 mA) to Hypond ECL (nitrocellulose) membranes (Amersham Biosciences) for 1 h using a semidry blotter (Transblot system, Bio-Rad, Hercules, CA). Transfer buffer was 25 mM Tris containing 192 mM glycine and 20% methanol. The membranes were blocked with 3% milk powder in phosphate-buffered saline (PBS) with 0.3% Tween for 1 h at 25 °C. After blocking the membranes were incubated overnight at 4 °C with antibodies directed against sPLA2-IIA, sPLA2-V, iPLA2, or cPLA2 (each with 1:500 dilution in blocking solution). Membranes were washed in PBS with 0.3% Tween three times for 10 min each. Membranes were probed with the appropriate secondary antibody (antimouse IgG used at 1:50,000 dilution in blocking solution or antirabbit IgG used at 1:20,000 dilution in blocking solution) linked to horseradish peroxidase for 2 h. Membranes were then washed in PBS with 0.3% Tween three times for 10 min each. Proteins were visualized with Immobilon Western Chemiluminescent HPR substrate (Millipore, Billerica, MA) and exposed to Fuji RX film (Fuji, Japan). Purified recombinant human sPLA2-IIA (BioVendor GmbH, Heidelberg, Germany) and sPLA2-V (BioVendor, GmbH) were used as positive controls.
Immunohistochemistry
Cryosections were fixed in ice-cold acetone for 7 min, air-dried, then rinsed twice with tris-buffered saline (TBS). Paraffin sections were dewaxed in xylene and dehydrated in graded ethanols according to standard procedures. Immunostaining was carried out with HistostainTM-Plus Mouse Primary Bulk kit (Zymed Laboratories, South San Francisco, CA) or HistostainTM-Plus Broad Spectrum Bulk kit (Zymed Laboratories) and with DAB substrate kit (Zymed Laboratories) following guidelines described in reference [53]. The antibodies for demonstrating sPLA2-V, iPLA2, and cPLA2 were all used at a dilution of 1:100, and a dilution of 1:400 was used for sPLA2-IIA. The tissue sections were examined and digitally captured using a Nikon Eclipse TE300 inverted microscope (Nikon, Tokyo, Japan) equipped with Nikon E995 digital camera (Nikon), and the images were processed with Adobe Photoshop (version 5.5) software.
Confocal laser scanning microscopy
Double-immunofluorescence was performed for colocalization studies of sPLA2 and macrophages using the method described in Kroeber et al. [54]. Primary antibodies used were anti-sPLA2-IIA (1:400 dilution) and anti-CD68 (1:10 dilution). sPLA2-IIA was detected with antimouse IgG Alexa Fluor 488 (1:200 dilution), and macrophages were detected with Alexa Fluor 594 (1:5 dilution; red fluorescence). In addition, nuclei were stained with a 1 mM solution of far red nucleic acid dye (SYTO 62; Molecular Probes).
For colocalization studies cryosections were fixed in ice-cold acetone for 7 min, air-dried, rinsed twice with TBS and blocked with blocking solution. Colocalization of sPLA2-IIA and macrophages was observed by merged images with UltraVIEW confocal imaging systems (PerkinElmer Life Sciences, Shelton, CT) following guidelines established in reference [55]. Sections were mounted on Vectashield mounting medium (Vector, Burtingame, CA). To verify an absence of cross-reaction between antibodies, we omitted each primary or secondary antibody from the incubation. All control experiments confirmed that there was no cross-reactivity between the antibodies.
Quantification of immunohistochemical staining
The amount of antibody staining was quantified by using Photoshop-based image analysis [53]. All samples were analyzed in triplicate. The final immunostaining intensity (AU) was determined by subtracting the intensity of the negative control.
Statistical analysis
Differences between experimental groups were determined using the Mann-Whitney Rank Sum test (SigmaStat statistical software, SPSS Inc, Chicago, IL). A p less than or equal to 0.05 was considered statistically significant.
Results
Distribution of sPLA2-IIA, sPLA2-V, iPLA2, and cPLA2 in normal human trabecular meshwork
We immunostained tissue sections to assess the immunohistochemical localization of sPLA2-IIA, PLA2-V, iPLA2, and cPLA2 in human TM. sPLA2-IIA (Figure 1A) or sPLA2-V (Figure 1B) immunohistochemical labeling was observed in few macrophages. In contrast, iPLA2 demonstrated strong labeling of the uveal and corneoscleral meshwork, and the staining of juxtacanalicular meshwork was only moderate in density. Uveal TM cells covering the lamellae were more intensely labeled compared to the connective tissue core of the lamellae, and, similarly, the luminal parts of the cells lining Schlemm's canal were noticeably stained with iPLA2. Staining intensity of cPLA2 was considerably weaker when compared to iPLA2. Immunohistochemical staining showed that cPLA2 expression was slightly higher in uveal and corneoscleral meshwork compared to juxtacanalicular meshwork (Figure 1D). The cells lining Schlemm's canal were also faintly stained with cPLA2. Moreover, intense staining of iPLA2 (Figure 1C) or cPLA2 (Figure 1D) was also seen in few macrophages. A similar pattern of PLA2s was found in paraffin-embedded and frozen sections of normal human tissue.
Figure 1.
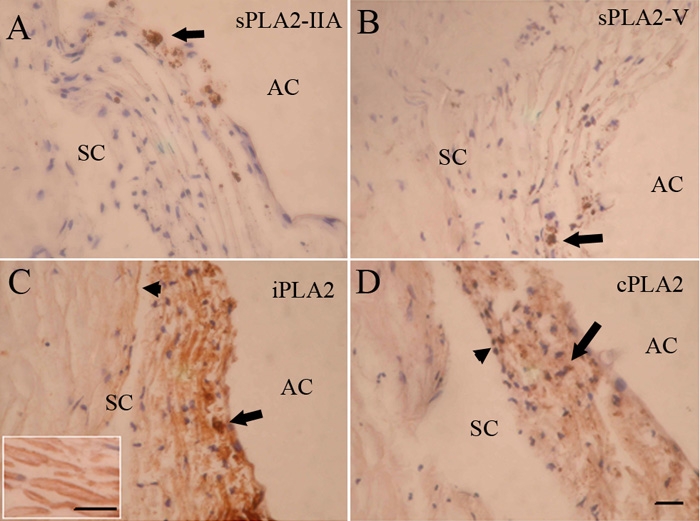
Immunohistochemical localization of PLA2s in normal human trabecular meshwork. Immunostaining for sPLA2-IIA (A) or sPLA2-V (B) was negative in the trabecular meshwork. Intense staining for sPLA2-IIA or sPLA2-V was evident in a few inflammatory-like cells (arrow). iPLA2 immunolabeling was strong (C). Labeling of the uveal and corneoscleral meshwork was stronger compared to the staining of the juxtacanalicular meshwork. Low positive staining was seen in the apical parts of the cells lining Schlemm's canal (arrowhead) as well as in nearby extracellular regions. Positive staining was also seen in a few macrophages (arrow). Inset: A portion of trabecular meshwork lamellae at higher magnification. Uveal trabecular meshwork cells covering the lamellae were more intensely labeled compared to connective tissue core. cPLA2 was weakly positive (D) and staining was slightly higher in uveal and corneoscleral meshwork compared to juxtacanalicular meshwork. The cells lining Schlemm's canal showed weak staining. Positive staining was seen in a few macrophages (arrow). AC, anterior chamber; SC, Schlemm's canal. The scale bar is equal to 50 μm.
Localization of sPLA2-IIA, sPLA2-V, iPLA2, and cPLA2 in trabecular meshwork of primary open angle glaucoma and exfoliation glaucoma patients
We examined the locations of sPLA2-IIA, sPLA2-V, iPLA2, and cPLA2 in TM samples from eyes with glaucoma. POAG and ExG specimens were collected during deep sclerectomy by removing the inner wall of Schlemm's canal and adjacent juctacanalicular tissue and the TM but leaving the inner meshwork intact (Figure 2). In general, we found that immunoreaction of sPLA2-IIA was much heavier in POAG samples than that in ExG samples (Figure 3A,B). In POAG eyes, heavy immunoreactivity was seen in trabecular tissue and around the macrophages, which stained positive for sPLA2-IIA (Figure 3A). In ExG samples, strong positive PLA2-IIA staining was detectable mostly in few macrophage-like cells, and components of extracellular matrix were not so intensively stained compared to POAG samples (Figure 3B).
Figure 2.
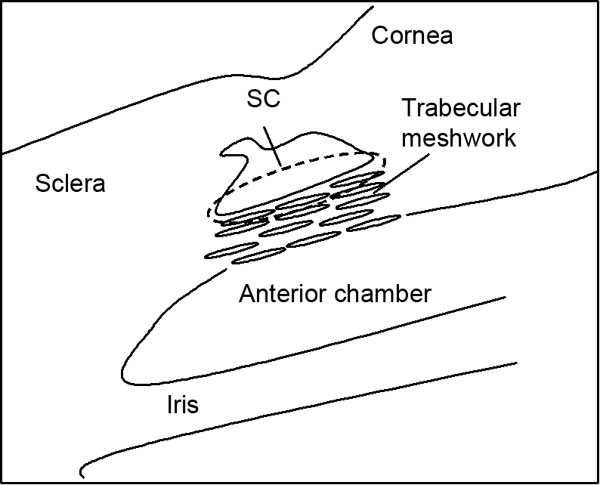
Surgical site. Shown in this schematic line drawing of the chamber angle is the location of the surgical site (dashed line). SC, Schlemm's canal
Figure 3.
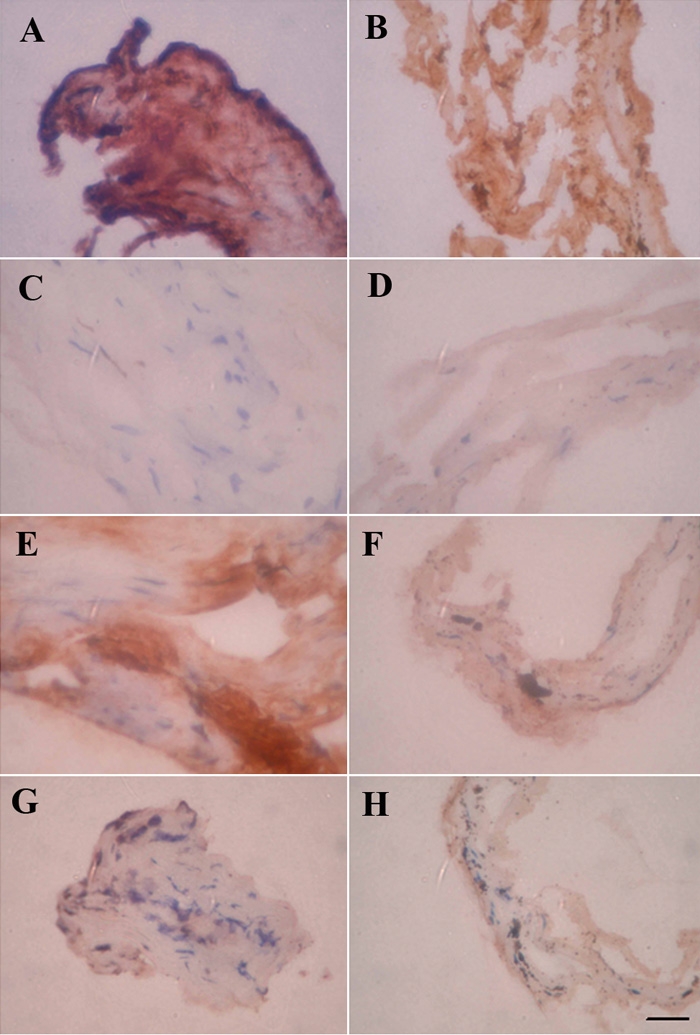
Immunostaining for PLA2s in POAG and ExG. Immunoreaction for sPLA2-IIA is heavier in POAG (A) compared to ExG (B). In trabecular meshwork tissue, immunoreactivity was found near the stained macrophages. PLA2-V staining was weak in POAG (C) and ExG (D). In POAG (E) trabecular meshwork staining for iPLA2 is near positively stained macrophages. In ExG samples (F) iPLA2 staining is in macrophages. Cellular staining of cPLA2 was low in POAG (G) and ExG (H) samples. The scale bar was equal to 50 μm.
No apparent differences were seen in expression levels or localization of sPLA2-V between POAG and ExG samples (Figure 3C,D). Staining for sPLA2-V was weakly positive. The expression pattern of iPLA2 was different in POAG and ExG tissues. In POAG eyes, iPLA2 reactivity was strong in particular areas of tissue where some macrophages also stained positive (Figure 3E). In ExG samples, the staining intensity of trabecular tissue was lower compared to POAG eyes (Figure 3F), and the strongest staining was in few macrophage-like cells. cPLA2 immunoreactivity was barely detectable in trabecular tissue. cPLA2 was stained in few macrophages in POAG (Figure 3G) and ExG samples (Figure 3H).
Photoshop-based image analysis of sPLA2-IIA, sPLA2-V, iPLA2, and cPLA2
Because there were semiquantitative differences in PLA2s levels between POAG and ExG samples, we analyzed the samples via Photoshop-based image analysis [55]. Cryostat sections obtained from healthy donor eyes served as controls. The level of sPLA2-IIA was significantly higher in POAG samples compared to those in ExG (p<0.001) or in control (p<0.001; Figure 4). Expression of sPLA2-IIA in ExG was also statistically higher (p=0.001) compared to control group. The immunostaining of sPLA2-V was slightly higher in POAG samples compared to ExG patients or control, but there were no significant differences between each experimental group (POAG versus ExG, p=0.997; POAG versus control, p=0.301; ExG versus control patients, p=0.317). Expression of iPLA2 was the highest in control group when compared to POAG (p=0.012) or ExG (p<0.001). In POAG, iPLA2 level was also significantly higher (p=0.015) compared to ExG. Analysis of cPLA2 levels showed no significant differences between each experimental group (POAG versus ExG, p=0.367; POAG versus control, p=0.841; ExG versus control patients, p=0.368).
Figure 4.
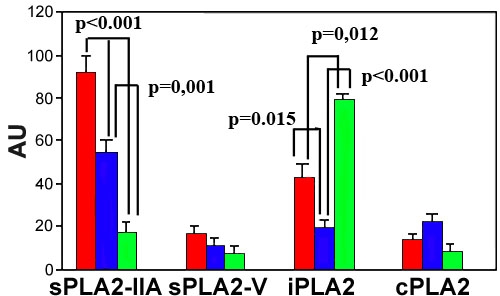
Photoshop-based image analysis of sPLA2-IIA, PLA2-V, iPLA2, and cPLA2 levels in TM. Cryostat sections were obtained from POAG patients (red bars), ExG patients (blue bars), or from healthy donor eyes (green bars). The results are mean (arbitrary units)±SEM of six POAG patients, six ExG patients or three healthy donor eyes. Each set of experiments was performed in triplicate.
Confocal microscopy
Immunohistochemical studies showed increased number of macrophages in POAG samples when compared to ExG or control (Figure 5). Furthermore, our results showed clear differences in expression levels of sPLA2-IIA and iPLA2 in POAG and ExG samples, and immunohistochemical stainings gave indication that both enzymes might also be present in macrophages. Therefore, we next evaluated whether sPLA2-IIA could be in macrophages. We carried out confocal microscopy experiments with double antibody staining for sPLA2-IIA and macrophages to demonstrate their colocalization and to provide further support for the macrophage derived sPLA2-IIA, especially in POAG specimens (Figure 6). Close examination revealed that some of the macrophages showed no colocalization with sPLA2-IIA. Interestingly, immunoreactivity of sPLA2-IIA positive macrophages was unevenly distributed in POAG samples. In contrast, the number of macrophages was lower, and sPLA2-IIA positive macrophages were rarely found in ExG tissue (Figure 6).
Figure 5.
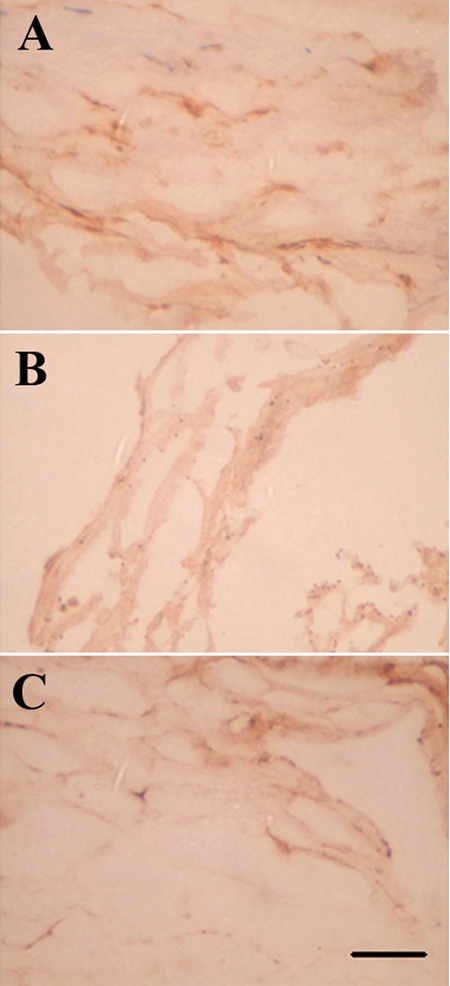
Immunostaining of macrophages. The number of macrophages is increased in POAG (A) when compared to ExG (B) or control samples (C). The scale bar is equal to 50 μm.
Figure 6.
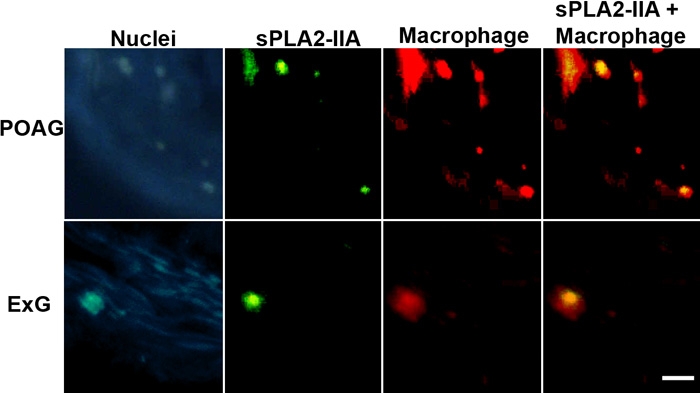
Colocalization of sPLA2-IIA and macrophages in POAG and ExG. The first column shows nuclear staining (far red shown in blue) with SYTO 62. The second column shows sPLA2-IIA staining (green), and the third column shows macrophages (red). Combined image (sPLA2-IIA + macrophage) in the fourth column shows the colocalization of sPLA2-IIA and macrophage (yellow). The scale bar is equal to 50 μm.
Western blot analysis of PLA2s in normal human trabecular meshwork
No expression of sPLA2-IIA or sPLA2-V was detected in normal human TM, while about 85 kDa band corresponding to cPLA2 was detected (Figure 7). Furthermore, we detected a minor (about 80 kDa) band corresponding to full-length iPLA2 and two major (about 55 and 50 kDa) forms. A weak expression of the 40- and 30 kDa forms was also detected. These bands were probably products of alternative splicing or proteolytic degradation.
Figure 7.
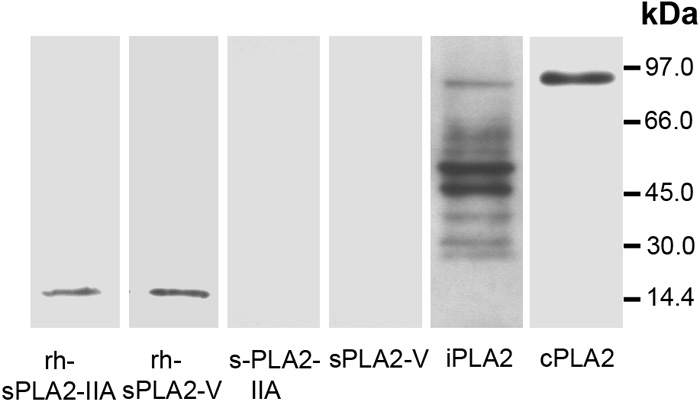
Western blot analysis. Human normal TM samples (10 μg protein) were subjected to SDS-PAGE followed by Western blot analysis using sPLA2-IIA, sPLA2-V, iPLA2, or cPLA2 antibody. Recombinant human sPLA2-IIA (rh-sPLA2-IIA; 200 ng) and sPLA2-V (rh-sPLA2-V; 200 ng) were used as positive controls. Molecular mass markers are indicated on the right. Results are representative of at least three separate experiments.
Discussion
In the present study, we performed immunohistochemistry to determine the expression of PLA2s in chamber angles from normal eyes and in surgical specimens from POAG and ExG patients. To our knowledge, this is the first study to examine this expression. Our results demonstrated significant differences in PLA2 levels. We found (1) sPLA2-IIA or sPLA2-V was not present in normal TM; (2) iPLA2 predominated in normal human TM; (3) labeling was strong in the uveal and corneoscleral meshwork; and (4) staining of juxtacanalicular meshwork was only moderate in density. cPLA2 was also expressed in normal human TM, and the staining intensity of cPLA2 was considerably weaker when compared to iPLA2. Furthermore, expression of macrophage-derived sPLA2-IIA was highly expressed in POAG patients compared to normal controls, and expression of iPLA2 was significantly decreased in ExG (Table 1).
Table 1. Changes in PLA2 levels and macrophage number in POAG and ExG when compared to normal tissue.
| Glaucoma |
sPLA2IIA expression |
sPLA2-V expression |
iPLA2 expression |
cPLA2 expression |
Macrophages |
| POAG |
↑↑ |
Normal |
↓ |
Normal |
↑ |
| ExG | ↑ | Normal | ↓↓ | Normal | Normal |
Our results showed that sPLA2-IIA was significantly increased in glaucomatous tissue compared to normal human TM. sPLA2-IIA has an important role in pathological conditions. Accumulation of sPLA2-IIA has been demonstrated in human inflammatory diseases such as rheumatoid arthritis, ulcerative colitis, and sepsis [56-58]. The reaction products of sPLA2-IIA are lysophospholipids and AA, which are precursors of potent inflammatory mediators such as platelet-activating factor and eicosanoids. Moreover, sPLA2-IIA has a high affinity for several proteoglycans such as glypican, decorin, and versican [59]. The biological actions of sPLA2-IIA might be governed by interactions with these proteoglycans in the ECM of the TM. We found the expression of sPLA2-IIA was significantly higher in POAG samples when compared to ExG or to control. It seems likely that the sPLA2-IIA detected in the TM of POAG patients was primarily macrophage derived because it was not present in healthy TM and expression was seen in macrophages present in the TM. It is well established that macrophages have a great secretory capacity for sPLA2-IIA at certain stages of activation [60]. Furthermore, in atherosclerosis, macrophage-specific sPLA2-IIA has been shown to increase oxidative stress [61]. Thus our results are in concordance with the growing evidence that inflammation and oxidative stress play an important role in the pathogenesis of glaucoma [51,62,63]. In ExG, the lower expression of sPLA2-IIA in ExG does not exclude the role of inflammation in the pathogenesis of ExG; it is different because expression and the number of sPLA2-IIA positive macrophages are lower when compared to POAG (Table 1).
Histological and morphologic studies have demonstrated that POAG differs from ExG histopathologically. Loss of structural stability and flexibility of the TM, disorganization of the normal juxtacanalicular tissue structure, and increased trabecular pigmentation are typical histopathologic clinical findings in ExG [14,15], whereas POAG is characterized by increased juxtacanalicular plaque and decreased cellularity in the TM [64]. However, mechanisms responsible for these differences in the TM are still unknown. Pathophysiological differences in patients with POAG or ExG was supported further by our finding that expression of iPLA2 was significantly lower in ExG samples compared to POAG.
Aqueous humor leaves the eye by passing through intratrabecular spaces in the TM before entering Schlemm's canal [50]. Endothelial cells lining Schlemm's canal and the juxtacanalicular tissue of the TM are expected to be the principal site of outflow resistance [50,65,66]. The physiological functions of trabecular cells are essential for maintaining a normal IOP. It is believed that changes in trabecular ECM, contractility, and cell density may interfere with the normal function of the TM, thereby leading to glaucoma [50,64,67]. It is interesting that iPLA2 may have the potential to participate in monocyte chemotaxis, relaxation, contraction, apoptosis, and calcium entry [45,46,48,49,68]. Therefore, iPLA2 has many functional characteristics that are important for normal TM cells. Our results showed that iPLA2 was expressed in normal human TM, and therefore, we speculate that iPLA2 may have a function to maintain normal physiological functions in the TM. The molecular mass of full-length iPLA2 is about 80 kDa, and it is present predominantly as 50- and 55 kDa forms, which are most likely ankyrin-iPLA2 splice variants [69]. Traditionally, iPLA2 has been regarded as a housekeeping enzyme for remodeling and maintenance of membrane phospholipids [42]. Therefore, in ExG eyes, phospholipids remodeling may be dramatically reduced in TM cells. Based on the biological functions proposed for iPLA2 it is tempting to speculate that dramatic decrease of the enzyme levels in TM cells may enhance the development of these pathological conditions.
During recent years another sPLA2, sPLA2-V has been implicated in inflammatory signaling. sPLA2-V has been shown to be expressed in a species-dependent manner in mouse cells [70]. In humans, sPLA2-V appears to substitute for sPLA2-IIA in airway epithelium cells [71]. cPLA2 is the only PLA2 known to date that is specific for AA at sn-2 position of phospholipids [40]. The activity of cPLA2 is important during inflammation because AA is the substrate for the production of prostaglandins and leukotrienes. We show in the present study that normal expression of sPLA2-V or cPLA2 is low, and there are no significant differences in levels of sPLA2-V or cPLA2 between healthy, POAG, and ExG tissue. Furthermore, we demonstrated that in normal human TM, cPLA2 is a protein with molecular weight about 85 kDa, which is a value typically reported for cPLA2 [18].
In summary, we have studied the expression of sPLA2-IIA, sPLA2-V, iPLA2, and cPLA2 in the TM of POAG and ExG and compared these levels to healthy controls. The present study provides new information about the expression of PLA2s in glaucoma. Distinct levels of sPLA2-IIA and iPLA2 in POAG and ExG further support the hypothesis that POAG and ExG have different pathogenic mechanisms. During oxidative stress iPLA2 recognizes and removes oxidized phospholipids from cell membranes. Due to low expression of iPLA2 in ExG the protection against oxidative stress is much worse compared to that in POAG, enhancing the loss of cell function. IOP tends to be greater in ExG than in POAG, and therefore, decreased expression of iPLA2 may be a link between increased IOP and loss of structural stability and flexibility of TM cells in ExG. sPLA2-IIA has been proposed as an inflammatory marker of cardiovascular disease, and therefore, higher expression of macrophage-derived sPLA2-IIA in POAG compared to normal controls supports the view that vascular diseases and POAG may have common pathophysiological mechanisms. Our findings may provide a biochemical basis for the development of new therapeutic agents for POAG and ExG.
Acknowledgements
This study was generously supported by the National Technology Agency of Finland, the Finnish Eye Research Foundation, Else My Björn Eye Research Fund, and EVO Funds from Kuopio University Hospital. The authors are grateful to Anne-Mari Haapaniemi, Helvi Käsnäen, Anne Kaakkola, Tiina Sistonen, and Aija Parkkinen for technical assistance.
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Coleman AL. Glaucoma. Lancet. 1999;354:1803–10. doi: 10.1016/S0140-6736(99)04240-3. [DOI] [PubMed] [Google Scholar]
- 3.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsius H. Prevalence of pseudoexfoliation of the lens in Finns, Lapps, Icelanders, Eskimos, and Russians. Trans Ophthalmol Soc U K. 1979;99:296–8. [PubMed] [Google Scholar]
- 5.Krause U, Helve J, Forsius H. Pseudoexfoliation of the lens capsule and liberation of iris pigment. Acta Ophthalmol (Copenh) 1973;51:39–46. doi: 10.1111/j.1755-3768.1973.tb08244.x. [DOI] [PubMed] [Google Scholar]
- 6.Krause U, Alanko HI, Karna J, Miettinen R, Larmi T, Jaanio E, Ollila OI, Takala J. Prevalence of exfoliation syndrome in Finland. Acta Ophthalmol Suppl. 1988;184:120–2. doi: 10.1111/j.1755-3768.1988.tb02642.x. [DOI] [PubMed] [Google Scholar]
- 7.Aasved H. The frequency of optic nerve damage and surgical treatment in chronic simple glaucoma and capsular glaucoma. Acta Ophthalmol (Copenh) 1971;49:589–600. doi: 10.1111/j.1755-3768.1971.tb02966.x. [DOI] [PubMed] [Google Scholar]
- 8.Futa R, Shimizu T, Furuyoshi N, Nishiyama M, Hagihara O. Clinical features of capsular glaucoma in comparison with primary open-angle glaucoma in Japan. Acta Ophthalmol (Copenh) 1992;70:214–9. doi: 10.1111/j.1755-3768.1992.tb04126.x. [DOI] [PubMed] [Google Scholar]
- 9.Konstas AG, Stewart WC, Stroman GA, Sine CS. Clinical presentation and initial treatment patterns in patients with exfoliation glaucoma versus primary open-angle glaucoma. Ophthalmic Surg Lasers. 1997;28:111–7. [PubMed] [Google Scholar]
- 10.Lindblom B, Thorburn W. Prevalence of visual field defects due to capsular and simple glaucoma in Halsingland, Sweden. Acta Ophthalmol (Copenh) 1982;60:353–61. doi: 10.1111/j.1755-3768.1982.tb03025.x. [DOI] [PubMed] [Google Scholar]
- 11.Lindblom B, Thorburn W. Functional damage at diagnosis of primary open angle glaucoma. Acta Ophthalmol (Copenh) 1984;62:223–9. doi: 10.1111/j.1755-3768.1984.tb08398.x. [DOI] [PubMed] [Google Scholar]
- 12.Tarkkanen A. Pseudoexfoliation of the lens capsule. A clinical study of 418 patients with special reference to glaucoma, cataract, and changes of the vitreous. Acta Ophthalmol Suppl. 1962;(Suppl 71):1–98. [PubMed] [Google Scholar]
- 13.Repo LP, Suhonen MT, Terasvirta ME, Koivisto KJ. Color Doppler imaging of the ophthalmic artery blood flow spectra of patients who have had a transient ischemic attack. Correlations with generalized iris transluminance and pseudoexfoliation syndrome. Ophthalmology. 1995;102:1199–205. doi: 10.1016/s0161-6420(95)30890-1. [DOI] [PubMed] [Google Scholar]
- 14.Ritch R, Schlotzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 15.Ritch R, Schlotzer-Schrehardt U, Konstas AG. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res. 2003;22:253–75. doi: 10.1016/s1350-9462(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 16.Dennis EA. Phospholipases. In: Boyer P., editor. The Enzymes. Volume 16. New York: Academic Press; 1983. p. 307-51. [Google Scholar]
- 17.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–60. [PubMed] [Google Scholar]
- 18.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 19.Balsinde J, Winstead MV, Dennis EA. Phospholipase A(2) regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/s0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- 20.Cummings BS, McHowat J, Schnellmann RG. Phospholipase A(2)s in cell injury and death. J Pharmacol Exp Ther. 2000;294:793–9. [PubMed] [Google Scholar]
- 21.Taketo MM, Sonoshita M. Phospolipase A2 and apoptosis. Biochim Biophys Acta. 2002;1585:72–6. doi: 10.1016/s1388-1981(02)00326-8. [DOI] [PubMed] [Google Scholar]
- 22.Murakami M. Hot topics in phospholipase A2 field. Biol Pharm Bull. 2004;27:1179–82. doi: 10.1248/bpb.27.1179. [DOI] [PubMed] [Google Scholar]
- 23.Dennis EA. Phospholipase A2 in eicosanoid generation. Am J Respir Crit Care Med. 2000;161:S32–5. doi: 10.1164/ajrccm.161.supplement_1.ltta-7. [DOI] [PubMed] [Google Scholar]
- 24.Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I. The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J Biol Chem. 1998;273:14411–23. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- 25.Murakami M, Kambe T, Shimbara S, Higashino K, Hanasaki K, Arita H, Horiguchi M, Arita M, Arai H, Inoue K, Kudo I. Different functional aspects of the group II subfamily (Types IIA and V) and type X secretory phospholipase A(2)s in regulating arachidonic acid release and prostaglandin generation. Implications of cyclooxygenase-2 induction and phospholipid scramblase-mediated cellular membrane perturbation. J Biol Chem. 1999;274:31435–44. doi: 10.1074/jbc.274.44.31435. [DOI] [PubMed] [Google Scholar]
- 26.Verheij HM, Slotboom AJ, de Haas GH. Structure and function of phospholipase A2. Rev Physiol Biochem Pharmacol. 1981;91:91–203. doi: 10.1007/3-540-10961-7_3. [DOI] [PubMed] [Google Scholar]
- 27.Choi YA, Lim HK, Kim JR, Lee CH, Kim YJ, Kang SS, Baek SH. Group IB secretory phospholipase A2 promotes matrix metalloproteinase-2-mediated cell migration via the phosphatidylinositol 3-kinase and Akt pathway. J Biol Chem. 2004;279:36579–85. doi: 10.1074/jbc.M314235200. [DOI] [PubMed] [Google Scholar]
- 28.Kishino J, Tohkin M, Arita H. Proliferative effect of phospholipase A2 in rat chondrocyte via its specific binding sites. Biochem Biophys Res Commun. 1992;186:1025–31. doi: 10.1016/0006-291x(92)90849-g. [DOI] [PubMed] [Google Scholar]
- 29.Tokumoto H, Croxtall JD, Choudhury Q, Flower RJ. Phospholipase A2-induced stimulation of A549 lung adenocarcinoma cell line proliferation. Biochim Biophys Acta. 1993;1169:236–42. doi: 10.1016/0005-2760(93)90246-6. [DOI] [PubMed] [Google Scholar]
- 30.Kanemasa T, Arimura A, Kishino J, Ohtani M, Arita H. Contraction of guinea pig lung parenchyma by pancreatic type phospholipase A2 via its specific binding site. FEBS Lett. 1992;303:217–20. doi: 10.1016/0014-5793(92)80523-j. [DOI] [PubMed] [Google Scholar]
- 31.Rizzo MT, Nguyen E, Aldo-Benson M, Lambeau G. Secreted phospholipase A(2) induces vascular endothelial cell migration. Blood. 2000;96:3809–15. [PubMed] [Google Scholar]
- 32.Beers SA, Buckland AG, Koduri RS, Cho W, Gelb MH, Wilton DC. The antibacterial properties of secreted phospholipases A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J Biol Chem. 2002;277:1788–93. doi: 10.1074/jbc.M109777200. [DOI] [PubMed] [Google Scholar]
- 33.Masuda S, Murakami M, Matsumoto S, Eguchi N, Urade Y, Lambeau G, Gelb MH, Ishikawa Y, Ishii T, Kudo I. Localization of various secretory phospholipase A2 enzymes in male reproductive organs. Biochim Biophys Acta. 2004;1686:61–76. doi: 10.1016/j.bbalip.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Hurt-Camejo E, Camejo G, Peilot H, Oorni K, Kovanen P. Phospholipase A(2) in vascular disease. Circ Res. 2001;89:298–304. doi: 10.1161/hh1601.095598. [DOI] [PubMed] [Google Scholar]
- 35.Fichtlscherer S, Kaszkin M, Breuer S, Dimmeler S, Zeiher AM. Elevated secretory non-pancreatic type II phospholipase A2 serum activity is associated with impaired endothelial vasodilator function in patients with coronary artery disease. Clin Sci (Lond) 2004;106:511–7. doi: 10.1042/CS20030399. [DOI] [PubMed] [Google Scholar]
- 36.Hamaguchi K, Kuwata H, Yoshihara K, Masuda S, Shimbara S, Oh-ishi S, Murakami M, Kudo I. Induction of distinct sets of secretory phospholipase A(2) in rodents during inflammation. Biochim Biophys Acta. 2003;1635:37–47. doi: 10.1016/j.bbalip.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Kortekangas P, Aro HT, Nevalainen TJ. Group II phospholipase A2 in synovial fluid and serum in acute arthritis. Scand J Rheumatol. 1994;23:68–72. doi: 10.3109/03009749409103030. [DOI] [PubMed] [Google Scholar]
- 38.Schroder T, Somerharju P, Lempinen M. Phospholipase A2 in serum and ascitic exudate in experimental acute haemorrhagic pancreatitis in pigs. Eur Surg Res. 1979;11:172–8. doi: 10.1159/000128064. [DOI] [PubMed] [Google Scholar]
- 39.Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–13. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Kramer RM, Sharp JD. Structure, function and regulation of Ca2+-sensitive cytosolic phospholipase A2 (cPLA2). FEBS Lett. 1997;410:49–53. doi: 10.1016/s0014-5793(97)00322-0. [DOI] [PubMed] [Google Scholar]
- 41.Hirabayashi T, Shimizu T. Localization and regulation of cytosolic phospholipase A(2). Biochim Biophys Acta. 2000;1488:124–38. doi: 10.1016/s1388-1981(00)00115-3. [DOI] [PubMed] [Google Scholar]
- 42.Balsinde J. Roles of various phospholipases A2 in providing lysophospholipid acceptors for fatty acid phospholipid incorporation and remodelling. Biochem J. 2002;364:695–702. doi: 10.1042/BJ20020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roshak AK, Capper EA, Stevenson C, Eichman C, Marshall LA. Human calcium-independent phospholipase A2 mediates lymphocyte proliferation. J Biol Chem. 2000;275:35692–8. doi: 10.1074/jbc.M002273200. [DOI] [PubMed] [Google Scholar]
- 44.Tay HK, Melendez AJ. Fcgamma RI-triggered generation of arachidonic acid and eicosanoids requires iPLA2 but not cPLA2 in human monocytic cells. J Biol Chem. 2004;279:22505–13. doi: 10.1074/jbc.M308788200. [DOI] [PubMed] [Google Scholar]
- 45.Guo Z, Su W, Ma Z, Smith GM, Gong MC. Ca2+-independent phospholipase A2 is required for agonist-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2003;278:1856–63. doi: 10.1074/jbc.M211075200. [DOI] [PubMed] [Google Scholar]
- 46.Seegers HC, Gross RW, Boyle WA. Calcium-independent phospholipase A(2)-derived arachidonic acid is essential for endothelium-dependent relaxation by acetylcholine. J Pharmacol Exp Ther. 2002;302:918–23. doi: 10.1124/jpet.302.3.918. [DOI] [PubMed] [Google Scholar]
- 47.Atsumi G, Murakami M, Kojima K, Hadano A, Tajima M, Kudo I. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IVA cytosolic phospholipase A2alpha inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. J Biol Chem. 2000;275:18248–58. doi: 10.1074/jbc.M000271200. [DOI] [PubMed] [Google Scholar]
- 48.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J Exp Med. 2002;196:655–65. doi: 10.1084/jem.20020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–20. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- 50.Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- 51.Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304–9. doi: 10.1038/85446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 53.Ronkko S, Rekonen P, Kaarniranta K, Puustjarvi T, Terasvirta M, Uusitalo H.Matrix metalloproteinases and their inhibitors in the chamber angle of normal eyes and patients with primary open-angle glaucoma and exfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol 2006[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Kroeber S, Schomerus C, Korf HW. A specific and sensitive double-immunofluorescence method for the demonstration of S-antigen and serotonin in trout and rat pinealocytes by means of primary antibodies from the same donor species. Histochem Cell Biol. 1998;109:309–17. doi: 10.1007/s004180050231. [DOI] [PubMed] [Google Scholar]
- 55.Ruponen M, Ronkko S, Honkakoski P, Pelkonen J, Tammi M, Urtti A. Extracellular glycosaminoglycans modify cellular trafficking of lipoplexes and polyplexes. J Biol Chem. 2001;276:33875–80. doi: 10.1074/jbc.M011553200. [DOI] [PubMed] [Google Scholar]
- 56.Jamal OS, Conaghan PG, Cunningham AM, Brooks PM, Munro VF, Scott KF. Increased expression of human type IIa secretory phospholipase A2 antigen in arthritic synovium. Ann Rheum Dis. 1998;57:550–8. doi: 10.1136/ard.57.9.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi O, Sugimura K, Ishizuka K, Suzuki K, Hasegawa K, Ohtsuka K, Honma T, Asakura H. Correlation between serum phospholipase A(2) IIA levels and histological activity in patients with ulcerative colitis. Int J Colorectal Dis. 2002;17:311–6. doi: 10.1007/s00384-002-0402-y. [DOI] [PubMed] [Google Scholar]
- 58.Dong LW, Yang J, Tong LJ, Hsu HK, Liu MS. Group II phospholipase A2 gene expression is transcriptionally regulated in rat liver during sepsis. Am J Physiol. 1997;273:G706–12. doi: 10.1152/ajpgi.1997.273.3.G706. [DOI] [PubMed] [Google Scholar]
- 59.Fuentes L, Hernandez M, Nieto ML, Sanchez Crespo M. Biological effects of group IIA secreted phosholipase A(2). FEBS Lett. 2002;531:7–11. doi: 10.1016/s0014-5793(02)03401-4. [DOI] [PubMed] [Google Scholar]
- 60.Hidi R, Vargaftig BB, Touqui L. Increased synthesis and secretion of a 14-kDa phospholipase A2 by guinea pig alveolar macrophages. Dissociation from arachidonic acid liberation and modulation by dexamethasone. J Immunol. 1993;151:5613–23. [PubMed] [Google Scholar]
- 61.Tietge UJ, Pratico D, Ding T, Funk CD, Hildebrand RB, Van Berkel T, Van Eck M. Macrophage-specific expression of group IIA sPLA2 results in accelerated atherogenesis by increasing oxidative stress. J Lipid Res. 2005;46:1604–14. doi: 10.1194/jlr.M400469-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612:105–14. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–9. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 64.Rohen JW, Lutjen-Drecoll E, Flugel C, Meyer M, Grierson I. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp Eye Res. 1993;56:683–92. doi: 10.1006/exer.1993.1085. [DOI] [PubMed] [Google Scholar]
- 65.Tripathi RC. Ultrastructure of Schlemm's canal in relation to aqueous outflow. Exp Eye Res. 1968;7:335–41. doi: 10.1016/s0014-4835(68)80047-8. [DOI] [PubMed] [Google Scholar]
- 66.Ethier CR, Kamm RD, Palaszewski BA, Johnson MC, Richardson TM. Calculations of flow resistance in the juxtacanalicular meshwork. Invest Ophthalmol Vis Sci. 1986;27:1741–50. [PubMed] [Google Scholar]
- 67.Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–79. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 68.Carnevale KA, Cathcart MK. Calcium-independent phospholipase A(2) is required for human monocyte chemotaxis to monocyte chemoattractant protein 1. J Immunol. 2001;167:3414–21. doi: 10.4049/jimmunol.167.6.3414. [DOI] [PubMed] [Google Scholar]
- 69.Manguikian AD, Barbour SE. Cell cycle dependence of group VIA calcium-independent phospholipase A2 activity. J Biol Chem. 2004;279:52881–92. doi: 10.1074/jbc.M410659200. [DOI] [PubMed] [Google Scholar]
- 70.Sawada H, Murakami M, Enomoto A, Shimbara S, Kudo I. Regulation of type V phospholipase A2 expression and function by proinflammatory stimuli. Eur J Biochem. 1999;263:826–35. doi: 10.1046/j.1432-1327.1999.00565.x. [DOI] [PubMed] [Google Scholar]
- 71.Masuda S, Murakami M, Mitsuishi M, Komiyama K, Ishikawa Y, Ishii T, Kudo I. Expression of secretory phospholipase A2 enzymes in lungs of humans with pneumonia and their potential prostaglandin-synthetic function in human lung-derived cells. Biochem J. 2005;387:27–38. doi: 10.1042/BJ20041307. [DOI] [PMC free article] [PubMed] [Google Scholar]


