Abstract
We assessed whether or not the sensitivity for identifying luminance-defined and contrast-defined letters improved with training in a group of amblyopic observers who have passed the critical period of development. In Experiment 1, we tracked the contrast threshold for identifying luminance-defined letters with training in a group of 11 amblyopic observers. Following training, six observers showed a reduction in thresholds, averaging 20%, for identifying luminance-defined letters. This improvement transferred extremely well to the untrained task of identifying contrast-defined letters (average improvement = 38%) but did not transfer to an acuity measurement. Seven of the 11 observers were subsequently trained on identifying contrast-defined letters in Experiment 2. Following training, five of these seven observers demonstrated a further improvement, averaging 17%, for identifying contrast-defined letters. This improvement did not transfer to the untrained task of identifying luminance-defined letters. Our findings are consistent with predictions based on the locus of learning for first- and second-order stimuli according to the filter-rectifier-filter model for second-order visual processing.
Keywords: Amblyopia, Perceptual learning, Training, First-order, Second-order, Letter recognition
INTRODUCTION
Amblyopia is a developmental disorder of spatial vision that often results from the presence of strabismus, anisometropia or other form deprivation early in life (Ciuffreda, Levi & Selenow, 1991). Clinically, it presents itself as the presence of visual deficits in one eye that cannot be attributed to an identifiable ocular pathology. The most common forms of visual deficits include a reduction in visual acuity (Gstalder & Green, 1971; Hess, Campbell & Greenhalgh, 1978; Levi & Klein, 1982), contrast sensitivity (Bradley & Freeman, 1981; Hess & Howell, 1977; Levi & Harwerth, 1977), Vernier acuity (Levi & Klein, 1982; 1985; Rentschler & Hilz, 1985) and other position acuities (e.g. Levi & Klein, 1983; 1990), as well as spatial distortion (Bedell & Flom, 1981; 1983; Bedell, Flom & Barbeito, 1985) and abnormal spatial interactions (Bonneh, Sagi & Polat, 2004; Hariharan, Levi & Klein, 2005; Levi, Hariharan & Klein, 2002; Polat, Sagi & Norcia, 1997). Traditionally, treatment of amblyopia is only undertaken in infants and young children who have not passed the critical period for visual development, which is generally believed to be around age 6–8 years in humans (von Noorden, 1981). However, for over a decade, many studies have reported that repetitive practice can improve performance on a variety of visual tasks in adult amblyopes (e.g. Chung, Li & Levi, 2006; Huang, Zhou & Lu, 2008; Levi, 2005; Levi & Polat, 1996; Levi, Polat & Hu, 1997; Li & Levi, 2004; Polat, Ma-Naim, Belkin, & Sagi, 2004; Zhou et al, 2006).
The earliest studies examining perceptual learning in amblyopes used high-contrast targets that were suprathreshold (in the contrast domain) to observers (Levi & Polat, 1996; Levi, Polat & Hu, 1997). Given that suprathreshold contrast processing is largely normal in human amblyopes (Hess & Bradley, 1980; Levi, Klein & Chen, 2007; Loshin & Levi, 1983), potentially properties of perceptual learning such as the time course of learning, the magnitude of improvement and the transfer of the learning effect, could be different between near-threshold and suprathreshold targets. More recent studies have used near-threshold, rather than suprathreshold high-contrast targets for training, including the studies of Polat et al (2004), Zhou et al (2006), Levi (2005) and our previous study (Chung et al, 2006). For example, using a contrast detection task, Polat et al (2004) trained their observers to detect Gabor patches with and without flanking collinear high-contrast Gabor patches, for a range of spatial frequencies. Their premise was to reduce the abnormal lateral inhibition in amblyopic observers through training. Indeed, they found that the inhibition produced by nearby flankers was reduced following training and that the improvements transferred to improved acuities. Zhou et al (2006) also used a contrast detection task to train their amblyopic (all anisometropic) observers. Their stimuli were sinewave gratings and their observers were trained either at one single spatial frequency (one that yielded a contrast threshold of 0.50 during pre-test) or across a range of 9 spatial frequencies (0.5 – 16 c/deg: essentially a contrast sensitivity function measurement). They found that observers who were trained at only one single spatial frequency showed a large amount of improvement (reduction) in contrast threshold at the trained spatial frequency, and that the improvements transferred to other untrained spatial frequencies. There was also a transfer of improvement to an acuity task for these observers. Observers who were trained across a range of spatial frequencies also showed improvements, although the magnitude of improvements was less than that obtained for the group of observers trained at only one spatial frequency.
Levi (2005) trained a group of amblyopic observers to identify luminance-defined letters embedded in various levels of external noise and measured the contrast threshold for performing this task. He found that following 5000 trials of training, observers showed substantial improvement (reduction) of contrast threshold for identifying letters across all external noise levels in the amblyopic eyes, but that the improvement did not transfer to the untrained non-amblyopic eyes. In his study, Levi did not assess whether the improvement transferred to improved visual acuity.
Our recent study examined perceptual learning for a second-order task in a group of amblyopes. Observers were trained to identify contrast-defined letters that were just barely distinguishable from their background (Chung et al, 2006). With this threshold task, eight of the ten amblyopic observers showed a progressive and steady reduction in threshold (minimal difference in contrast between the letters and their background) with training. Comparisons of pre- and post-test thresholds revealed that while the reduction in threshold for identifying contrast-defined letters (the trained task) averaged 33% following training, there was only an average of 5% reduction in threshold for the untrained task of identifying luminance-defined letters (a first-order task) in the trained eyes, suggesting an insignificant amount of transfer of learning to the untrained task. Unfortunately, we did not assess observers’ acuities following training, therefore we do not know if the improvements transferred to an acuity task.
The studies of Polat et al (2004), Zhou et al (2006), Levi (2005) and our previous study clearly indicate that the use of a threshold task is effective in inducing learning in adult amblyopes. Both the studies of Polat et al (2004) and Zhou et al (2006) used grating stimuli and provided evidence that an improvement at the early stage of visual processing could improve acuities in amblyopes. Considering that acuities are often measured using letter stimuli, will the use of letter stimuli be more effective in inducing learning, and of particular interest, will perceptual learning of letter identification transfer to improved visual acuity? In this study, we trained amblyopic observers (strabismic, anisometropic and mixed) to identify near-threshold letters that were larger than the acuity limit to address the primary goal of this study — to determine whether or not improvements in identifying near-threshold letters following learning also improve visual acuity.
A secondary goal of this study was to better understand the processing of second-order visual information. Currently, there exist two conflicting views on the processing pathway for second-order stimuli, and how it interacts with the processing of first-order visual information. One view, widely accepted in psychophysical and modeling studies (e.g. Chung et al, 2006; Ellemberg, Allen & Hess, 2004; McGraw, Levi & Whitaker, 1999; Whitaker, McGraw & Levi, 1997), and corroborated by some physiological (Mareschal & Baker, 1998; Baker & Mareschal, 2001) and brain imaging evidence (Smith et al., 1998), is that second-order visual information is processed via a filter-rectifier-filter pathway (Chubb & Sperling, 1989) where the first-stage filters are linear and with a possible neural substrate in the early cortical areas (Mareschal & Baker, 1998; Schofield, 2000). The output of these first-stage linear filters then undergoes nonlinear processing, possibly rectification, before feeding onto a second-stage filter. The neural site(s) of this second-stage filter in humans and primates have yet to be identified, but brain imaging experiments suggest a possible higher-order extrastriate locus, at least for motion processing (Smith et al, 1998; Dumoulin et al, 2003). Based on this simple model of second-order processing, we hypothesized that improvements on a first-order task are likely to be carried forward to the second-stage of visual processing because the first-order task could be analyzed by the first-stage linear filters of the second-order pathway, in addition to any independent pathways dedicated for analyzing first-order information. In other words, there should be a transfer of improvement from a trained first-order task to an untrained second-order task. Unless there is a feedback mechanism or a subsequent stage where first- and second-order information are pooled, improvements on a second-order task are unlikely to lead to an improvement in an untrained first-order task, as we reported previously (Chung et al, 2006).
An alternative view, supported by some physiological studies, is that the processing of first- and second-order information takes place within the same pathway. For instance, Barraclough, Tinsley, Webb, Vincent and Derrington (2006) found that responses to first-order motion were significantly modulated by the presence of second-order information in neurons as early as in V1, and in V2 and the third visual complex in marmoset visual cortex, arguing against a separate pathway dedicated for second-order motion processing. This model predicts that the transfer of learning between first- and second-order tasks would be bi-directional as learning would improve how the pathway processes information in general, regardless of the type of input information. To test these predictions, we first trained a group of amblyopic observers to identify luminance-defined letters, a first-order task, and determined whether the improvements, if any, transfer to the untrained tasks of identifying contrast-defined letters (a second-order task) and acuity measurement. Then we trained a subset of these observers to identify contrast-defined letters, as in our previous study (Chung et al, 2006) to determine whether the improvements, if any, transfer to the untrained task of identifying luminance-defined letters. This design allowed us to compare whether or not learning first-order stimuli facilitates the learning of second-order stimuli. Also, by comparing the results with our previous study (Chung et al, 2006), the present design allowed us to determine whether or not first-order learning is sufficient to induce the full potential for improvement for second-order stimuli. In addition, in many perceptual learning studies (as in the present study) it is common to find that some observers fail to learn. The “two-stage” learning design maybe helpful in determining whether such individuals are simply incapable of learning, or whether they fail only on a particular stimulus or task. Finally, by assessing whether or not there is a bidirectional cross-over transfer of improvement to an untrained first- and second-order task, we might be able to better understand the structure of the pathway processing second-order information.
METHODS
This study comprised two experiments. In Experiment 1, we trained eleven observers with amblyopia (four with strabismus, four with anisometropia and three with both strabismus and anisometropia), aged between 15 and 58, to identify luminance-defined letters using their amblyopic eyes. All observers are well past the critical period of visual development and did not have any prior experience in psychophysical experiments. Table 1 summarizes the visual characteristics of these observers. Immediately following Experiment 1, seven of the 11 observers were further trained to identify contrast-defined letters in Experiment 2. All observers wore their best optical corrections, appropriate for the viewing distances, during the experiment. Written informed consent was obtained from each observer after the procedures of the experiment were explained and before the commencement of data collection.
Table 1.
Visual characteristics of the 11 amblyopic observers who participated in Experiment 1.
| Observer | Gender | Age (years) | Type | Eye | Visual Acuity (logMAR) | Refractive Errors | Eye Alignment | Stereoacuity (if any) |
|---|---|---|---|---|---|---|---|---|
| CF* | M | 36 | Strab | OD OS |
20/16−2 20/63−1 |
+0.75/−0.50×120 +0.50/−1.00×065 |
4Δ LXT 2Δ LHyperT |
|
| CL* | F | 19 | Strab | OD OS |
20/32−1 20/16 |
−0.75 −0.25/−0.50×055 |
4Δ RET | |
| GW | M | 58 | Strab | OD OS |
20/240+2 20/16−1 |
+0.50 +0.50/−0.75×180 |
4Δ RXT | |
| SCF* | M | 18 | Strab | OD OS |
20/12.5+1 20/125−2 |
−1.50/−1.25×090 pl/−1.00×030 |
6Δ LXT | |
| AM | F | 47 | Aniso | OD OS |
20/12.5−1 20/32−2 |
+0.75/−0.75×095 +2.00 |
200” | |
| HI | M | 19 | Aniso | OD OS |
20/12.5+2 20/100+2 |
pl/−0.50×180 −14.00/−3.75×170 |
||
| MR | M | 30 | Aniso | OD OS |
20/16−1 20/32+1 |
pl +0.50/−0.75×075 |
200” | |
| RAH* | F | 15 | Aniso | OD OS |
20/32+1 20/12.5 |
−2.00/−1.75×155 +0.25/−0.25×060 |
||
| AW* | F | 23 | Strab + Aniso | OD OS |
20/80−1 20/16−1 |
+2.75/−1.00×160 −1.00/−0.50×180 |
4–6Δ RXT 4Δ LHyperT |
|
| GJ* | M | 22 | Strab + Aniso | OD OS |
20/63+1 20/16+1 |
+3.25/−1.00×100 pl/−0.25×100 |
4–5Δ RET | |
| ML* | F | 20 | Strab + Aniso | OD OS |
20/10−1 20/63−2 |
pl +5.50/−3.00×055 |
4–5Δ LET 3–4Δ LHyperT |
Asterisks denoted the seven observers who also participated in Experiment 2.
Stimuli
All stimulus letters, luminance- or contrast-defined, were presented at a background rms noise contrast of 0.07. Details for generating luminance- and contrast-defined letters are described elsewhere (Chung et al, 2006). In brief, luminance-defined letters were generated by assigning a different luminance value to the letter, compared with its mid-gray background (see Figure 1). An array of white noise1 covered both the letter and its background. Hence, contrast threshold for identifying luminance-defined letters was defined as the Weber contrast between the letter and its background, (letter luminance – background luminance)/background luminance. Contrast-defined letters were generated by assigning a different contrast to the white noise that made up the letter, with respect to the contrast of the background (see Figure 1). The mean luminance of the letter and its background were the same. Thus, contrast threshold for identifying contrast-defined letters was defined as the differential contrast (ΔC) that defined the letter from its background.
Figure 1.
A schematic illustration of the basic experimental design of the study. Experiment 1 consisted of learning to identify luminance-defined letters. Experiment 2 immediately succeeded Experiment 1 and consisted of learning to identify contrast-defined letters. In each experiment, a pre-test preceded the training, which was in turn, followed by a post-test. Contrast thresholds for identifying luminance-defined and contrast-defined letters were measured separately for the non-amblyopic (NAE) and amblyopic (AE) eyes during pre- and post-tests.
Apparatus
Stimuli were generated on a Macintosh G4 computer with software written in Matlab (The MathWorks, MA) using the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997), and were displayed on a Sony 17” monitor (model number G400) at a mean luminance of 23 cd/m2. The luminance of the display was measured using a Minolta photometer. By combining the red and blue output of the display using a video attenuator (Pelli & Zhang, 1991) and the use of custom-built software (Tjan, personal communication), we obtained an effective 10 bit luminance resolution after correcting for the gamma of the display. Observers sat at 42 cm from the display during testing (except when letter size thresholds were being determined where the viewing distance ranged between 42 and 400 cm). At this viewing distance, each pixel subtends 2.5 arc min.
Basic experimental design
Before and after each experiment, we determined the size thresholds (acuities) for identifying both luminance- and contrast-defined letters, for the non-amblyopic and amblyopic eyes, respectively. For these measurements, stimulus letters were of a fixed high contrast (0.7 Weber contrast for luminance-defined letters and a differential contrast of 0.7 for contrast-defined letters). The acuity measurements allowed us to examine whether acuity improved following each phase of training.
In addition to acuity measurements, we also determined the contrast thresholds for identifying luminance- and contrast-defined letters, for the non-amblyopic and amblyopic eyes, before and after training. These measurements allowed us to make comparisons of thresholds before and after training to evaluate if there was an improvement due to training. For these measurements and subsequent training, we used the same physical letter size for both luminance- and contrast-defined letters.2 The letter size corresponded to 1.3× the acuity for contrast-defined letters in the amblyopic eye during pre-test. As shown in Chung et al (2006), size thresholds are approximately 6× larger for contrast-defined than for luminance-defined letters, therefore, the letter size used was equivalent to approximately 8× the acuity for luminance-defined letters.
The basic experimental design and training schedule are represented schematically in Figure 1. Essentially, the training schedule was identical for both Experiments 1 (learning to identify luminance-defined letters) and 2 (learning to identify contrast-defined letters) and consisted of a pre-test, training sessions and a post-test.
The pre-test consisted of measurements of contrast thresholds for identifying luminance-and contrast-defined letters in the non-amblyopic and amblyopic eyes. Pre- and post-test threshold reported for each condition (eye × type of letters) represents the average value of at least two blocks of trials (100 trials per block). Training consisted of 80 blocks of trials (100 trials per block, ten blocks per day for eight days) of identifying either luminance-defined (Experiment 1) or contrast-defined (Experiment 2) letters in the amblyopic eyes. Each training session lasted approximately 30–60 min. The post-test, identical to the pre-test, followed the last training session. Due to experimenter’s errors, two observers (SCF and CF) underwent 100 instead of 80 training blocks in Experiment 2. Nevertheless, as can be seen in Figure 6, the thresholds of these two observers at the 80th and the 100th block were fairly similar. Hence, the experimenter’s errors are unlikely to have caused a major difference to our findings.
Figure 6.
Differential contrast threshold (ΔC) for identifying contrast-defined letters is plotted as a function of training block, for the seven observers who took part in Experiment 2. Two observers (SCF and CF) were trained for 100 blocks instead of 80 due to experimenter’s errors (see text for details). Details of the plotting are the same as those in Figure 2.
Testing and psychophysical procedures
Testing and psychophysical procedures were identical for pre-test, training and post-test sessions. Each condition was tested in a separate block of trials. In each block of trials, we used the Method of Constant Stimuli to present the stimulus letter at five stimulus levels (five Weber contrast levels for luminance-defined letters or five differential-contrast (ΔC) for contrast-defined letters), with each stimulus level presented 20 times within the block. On each trial, a single letter of x-height that corresponded to 1.3× the threshold letter size for identifying contrast-defined letters in the amblyopic eye (~8× the acuity for identifying first-order letters), was presented for 150 ms in the center of the display monitor. Letters were randomly chosen with equal probability from the 26 lowercase letters of the Times-Roman alphabet. The task of the observers was to indicate the letter identity using the keyboard. Audio feedback was given to indicate whether or not the response was correct. Testing was monocular, with the non-tested eye covered with a standard black eye-patch. We defined threshold as the contrast (for luminance-defined letters) or ΔC (for contrast-defined letters) that yielded 50%-correct performance (after correction for guessing) on the psychometric function (cumulative Gaussian), constructed based on the data from each block of trials.
RESULTS
In this paper, we follow the color scheme used by McKee, Levi & Movshon (2003) to color-code our amblyopic observers according to the type of amblyopia they exhibited: red for strabismic amblyopia, green for anisometropic amblyopia and blue for strabismic-anisometropic (mixed) amblyopia. The color-coding facilitates visualization of the data to determine if any effects we observed are specific to the type of amblyopia.
Experiment 1: Training on luminance-defined letters
Over the course of an eight-day training period (a total of 8000 trials), six of the 11 observers showed a progressive and steady improvement (reduction) in contrast threshold for identifying luminance-defined letters. In Figure 2, we plot each individual observer’s data as separate panels, and group these panels according to whether or not the observer showed learning (see below for our criterion of “learning”). Data from observers who showed significant learning are represented by circular symbols and are shown in the first two rows in Figure 2. Data from observers who did not show a significant learning effect are plotted as triangles and are shown in the bottom two rows. To determine whether or not there was a learning effect for the entire group of 11 observers and to quantify the improvement, if any, we fit a linear regression function to each set of logarithmic threshold data that included measurements for all training blocks (unfilled symbols), as well as the pre- and post-test thresholds (filled symbols). We then performed a t-test to determine if the slope of these regression functions differed from a slope of zero, an indication that there was no improvement in threshold due to learning. Across the group of 11 observers, the mean slope (± 95% confidence intervals) of the regression lines averaged −0.000089 ± 0.000064, and was statistically significant from a slope of zero (t(df = 10) = 2.74, two-tailed p = 0.021).
Figure 2.
Contrast threshold for identifying luminance-defined letters (Experiment 1) is plotted as a function of training block, for each individual observer. Each unfilled symbol represents threshold obtained for a training block (100 trials). Observers were trained for 10 blocks per day for a total of eight days. Filled symbols in each panel represent thresholds obtained at the pre- and post-test. The solid line in each panel represents the best-fit regression line to each set of data. The slope of this line, if different from zero, represents significant amount of learning (p-value given in each panel). Observers were divided into two groups: those who showed learning (top two rows, data represented by circular symbols) and those who did not show learning (bottom two rows, data represented by triangular symbols). Acuity and letter size used are given in each panel. In this and subsequent figures, observers are color-coded according to the type of amblyopia they exhibited (strabismic, red; anisometropic, green; mixed, blue).
Clearly, not every observer showed learning. We compared the slope of each observer’s regression line with a slope of zero and included the p-value of such comparison in Figure 2. Observers were classified as learners if their p-values were less than 0.05, and non-learners otherwise. Using such a criterion, five of the 11 observers did not reach statistical significance and were thus classified as non-learners. Applying the conservative Bonferroni correction (p-values less than 0.0045 for statistical significance) to correct for the number of observers results in the exclusion of observer ML as a learner.
Our results reveal two clear observations. First, the improvement due to learning does not depend on the type of amblyopia (strabismic, anisometropic or mixed). For each amblyopia type, some observers showed learning (according to our criterion) and some did not. Second, the two oldest observers (47 and 58 years of age respectively) did not show any learning, consistent with the common belief that although the adult visual system still demonstrates plasticity, the plasticity decreases with increased age. We note however that all observers were well past the age at which treatment for amblyopia is normally prescribed.
The fitting of a regression line to describe the change in contrast threshold as training progresses, as in Figure 2, shows the trend of any learning effect. An alternative way to show if learning occurred is to compare the threshold measurements obtained at pre- and post-tests. For our group of 11 observers, a paired t-test reveals that the post-test thresholds are significantly different from the pre-test thresholds for the trained condition (t(df = 10) = 4.44, two-tailed p = 0.001). Note that this analysis included the five “non-learners”. For the six observers who showed learning for luminance-defined letters, a comparison of the contrast threshold ratio obtained at post- and pre-test yielded a group-average (± 95% confidence intervals) value of 0.80 ± 0.07 (see Figure 3). Given that this value is statistically lower than a value of 1 (no improvement due to learning), we conclude that there was a statistically significant amount of improvement, averaging 20% (range: 12–33%), for these six observers.
Figure 3.
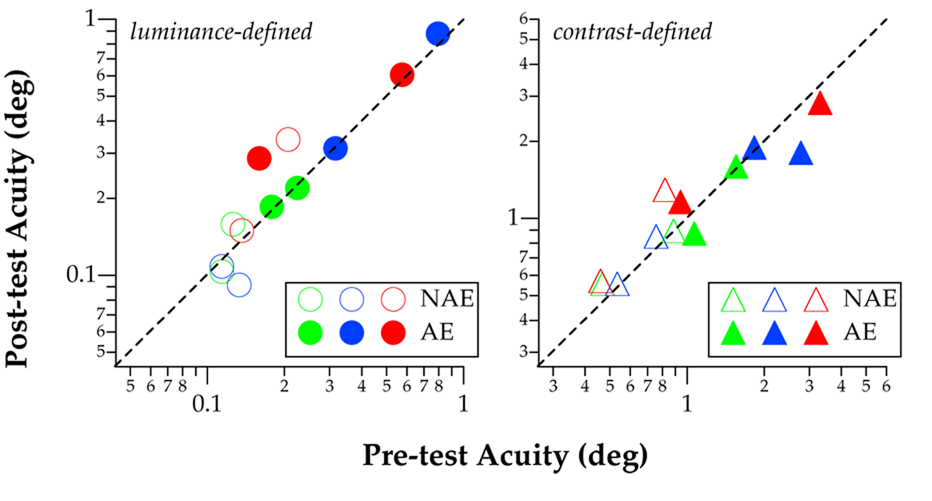
Post-test thresholds are plotted against pre-test thresholds for identifying luminance-defined (trained task: left panel) and contrast-defined letters (untrained task: right panel), for non-amblyopic (NAE: unfilled symbols) and amblyopic eyes (AE: filled symbols), for the six observers who showed significant learning (see text for criterion of “significant” learning). Dashed lines represent the 1:1 lines, indicating no change in thresholds before and after learning.
Two interesting questions follow. Did the improvement in learning to identify first-order letters transfer to the untrained task of identifying second-order letters; and did the improvement transfer to the untrained non-amblyopic eyes? Figure 3 plots the thresholds at post-tests as a function of the thresholds at pre-tests for the tasks of identifying luminance-defined (left panel) and contrast-defined (right panel) letters, and separately for the non-amblyopic (unfilled symbols) and amblyopic (filled symbols) eyes, for the six observers who showed significant learning. Learning can be visualized as data points plotted below the 1:1 line (dashed line) included in each panel. Only data from the six observers who showed learning are included because our goal was to examine whether or not there was a transfer of learning. As such, all the filled symbols (trained amblyopic eyes) fall below the 1:1 line for the task of identifying luminance-defined letters (left panel). More importantly, all the six filled symbols for the task of identifying contrast-defined letters (right panel) also fall below the 1:1 line. The deviations of these symbols from the 1:1 line were of a greater magnitude (averaged post/pre-test threshold ratio = 0.62 ± 0.11) than those for luminance-defined letters (averaged 0.80 ± 0.07)! This finding indicates that the learning effect transfers extremely well to the untrained task of identifying contrast-defined letters in the same trained eyes — the transferred improvement was larger than the direct improvement! However, for the untrained non-amblyopic eyes, there was practically no transfer of the learning effect as the unfilled symbols did not deviate much from the 1:1 line, for both luminance-defined and contrast-defined letters.
Amblyopia is often diagnosed and defined using an acuity criterion. Considering that our amblyopic observers were trained on identifying letters that were approximately 8× larger than the acuity limit, a key question is whether or not the improvement in contrast thresholds for identifying low-contrast large letters transfers to an acuity (a letter size-threshold) task that is usually assessed using high-contrast (supra-threshold) letters. Figure 4 compares pre- and post-test acuities, plotted separately for luminance-defined and contrast-defined letters, for those who showed learning. There was no significant difference (as confirmed using paired t-tests) between pre- and post-test acuities, for any of the conditions. In other words, even though there was an improvement in contrast threshold for identifying luminance-defined letters, the improvement did not help observers identify high-contrast letters near the acuity limit.
Figure 4.
Acuities obtained at post-tests are plotted as a function of those obtained at pre-tests (deg) for the six observers who showed learning in Experiment 1, for luminance-defined and contrast-defined letters. In each panel, data obtained from the non-amblyopic and amblyopic eyes are represented by unfilled and filled symbols, respectively. Dashed lines represent the 1:1 line, indicating no change in acuity before and after learning.
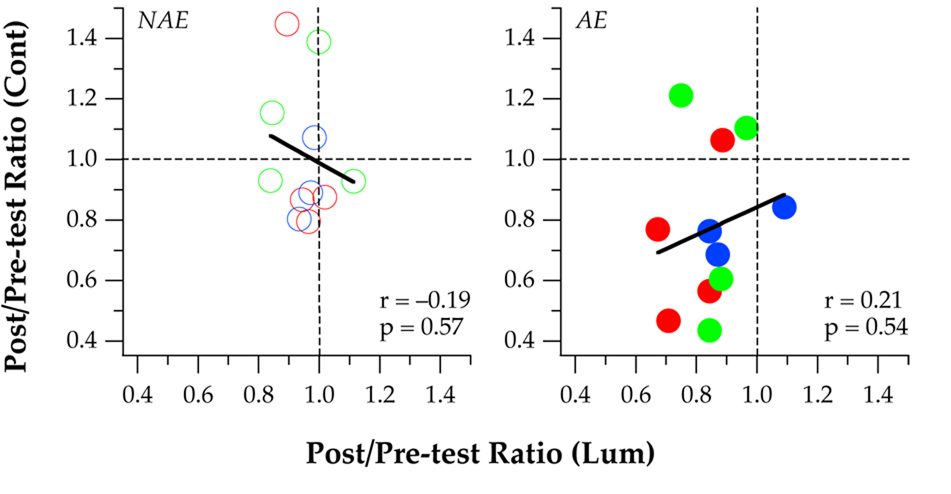
In Figure 3 and Figure 4 we only focused on observers who showed significant learning for identifying luminance-defined letters given our interest in determining whether or not the improvement transferred to an untrained task or the untrained eyes. A more general picture of how the post-test thresholds compared with pre-test thresholds for the entire group of 11 observers is given in Figure 5, where post/pre-test threshold ratios for the untrained task of identifying contrast-defined letters are plotted as a function of the threshold ratios for the trained task of identifying luminance-defined letters. The two dashed lines in each panel divide each panel into four quadrants, with the lower left quadrant representing improvements resulting from the direct learning of identifying luminance-defined letters, as well as the transferred improvement in identifying contrast-defined letters. The right panel clearly shows that the six observers who showed an improvement following learning to identify luminance-defined letters also showed an improvement for the untrained task of identifying contrast-defined letters in the same trained (amblyopic) eyes. The additional data point (red) that also falls within the lower left quadrant belongs to observer CL, who showed no block-to-block improvement in thresholds (see Figure 2) and thus was classified as a non-learner according to our criterion, although she demonstrated a sizeable reduction in post/pretest threshold ratios. As a group, there was practically no correlation between the magnitudes of improvement for identifying luminance- and contrast-defined letters, for non-amblyopic eyes (NAE, left panel) and amblyopic eyes (AE, right panel) alike.
Figure 5.
Post/pre-test threshold ratios for the untrained task of identifying contrast-defined letters (Cont) were plotted as a function of the post/pre-test threshold ratios for the trained task of identifying luminance-defined letters (Lum), for the non-amblyopic eyes (NAE: left panel) and amblyopic eyes (AE: right panel). Each symbol represents the data for one observer. Dashed lines drawn at threshold ratios of 1 divide each panel into four quadrants, with the lower left quadrant representing improvements for both luminance-defined (trained task) and contrast-defined (untrained task) letters following training in Experiment 1. The solid line in each panel represents the best-fit regression line to each set of data, with the correlation coefficient (r) given in the panel.
Experiment 2: Training on contrast-defined letters
Following learning to identify luminance-defined letters, seven of the 11 observers proceeded to train on identifying contrast-defined letters. These included five observers who previously showed learning in Experiment 1 (data plotted as circular symbols) and two who did not (data plotted as triangular symbols). Figure 6 plots their thresholds (ΔC) for identifying contrast-defined letters as a function of training block. As in Figure 2, we arranged the individual panels according to the amount of learning the observers showed, and sorted the panels into those who showed learning (the first two rows) and those who did not (the bottom row). To quantify the amount of learning, we fit a linear regression function to each set of data and performed a t-test to determine if the slope of the regression function differed from a slope of zero (no improvement in threshold due to learning). Using this criterion to define learning, five observers showed improvement (p-value < 0.05). The number drops to four if we apply the Bonferroni correction to correct for the number of observers. Just as in learning to identify luminance-defined letters, here improvements were found for all three types of amblyopia. Among the five observers who showed learning in this experiment, four showed learning previously and one did not show any learning in Experiment 1 (identifying luminance-defined letters). For the two observers who did not show learning in this experiment, one showed learning and one did not show learning in Experiment 1. The observer who showed learning only in Experiment 2 but not in Experiment 1 lends support to refute the argument that the observer could not learn on any tasks in general.
As in Experiment 1, we also assessed whether or not learning occurred by comparing the pre- and post-test thresholds for the trained condition using a paired t-test. Across the seven observers, there is a significant reduction in thresholds following learning (t(df = 5) = 3.05, two-tailed p = 0.023).
For the five observers who demonstrated learning, we compared their thresholds for identifying luminance- and contrast-defined letters in the non-amblyopic and amblyopic eyes before and after learning to identify contrast-defined letters, Thresholds obtained at post-tests are plotted as a function of thresholds obtained at pre-tests for the tasks of identifying luminance-defined (left panel) and contrast-defined (right panel) letters, and separately for the non-amblyopic (unfilled symbols) and amblyopic (filled symbols) eyes, as in Figure 7. Four of the five filled symbols fall below the 1:1 line for the task of identifying contrast-defined letters. The observer whose data fall on, instead of below, the 1:1 line (RAH) displayed marginal improvement, as shown in Figure 6. Averaged across the five observers, the post/pre-test threshold ratio was 0.83 ± 0.13, almost identical to the direct effect of learning luminance-defined letters (Figure 3). This value is statistically lower than 1 and represents an averaged improvement in threshold of 17% (range: 0 to 32%). However, this improvement did not transfer to the untrained task of identifying luminance-defined letters (averaged post/pre-test threshold = 1.02 ± 0.06), or to the untrained non-amblyopic eyes (for either task of identifying luminance-defined or contrast-defined letters). Taking into account the 38% transferred improvement in Experiment 1, our observers demonstrated a very substantial overall improvement in identifying contrast-defined letters of 49% at the end of the study.
Figure 7.
Post-test thresholds are plotted against pre-test thresholds for identifying luminance-defined (untrained task: left panel) and contrast-defined letters (trained task: right panel), for non-amblyopic (NAE: unfilled symbols) and amblyopic eyes (AE: filled symbols), for the five observers who showed significant learning. Dashed lines represent the 1:1 lines, indicating no change in thresholds before and after learning.
DISCUSSION
Letters, being everyday visual stimuli, are over-learned and there is little learning of luminance-defined letters in normal foveal viewing (Dosher & Lu, 2006). However, following learning to identify near-threshold luminance-defined letters in Experiment 1, six of the 11 amblyopic observers showed significant learning, with an average improvement of 20%. This improvement was accompanied by an excellent transfer of improvement (38%) to the untrained task of identifying contrast-defined letters (discussed further below). Interestingly, the two oldest observers (GW and AM) did not show any improvement following training. In spite of the 20% averaged improvement in threshold for identifying low-contrast letters, there was no improvement in acuity (size-threshold measurements) assessed using high-contrast letters among our observers.
Immediately following the completion of Experiment 1, seven observers elected to take part in Experiment 2 in which they were trained to identify contrast-defined letters. Five of these observers showed a further improvement, averaging 17%, following training. This improvement did not transfer to the untrained task of identifying luminance-defined letters and/or to the untrained non-amblyopic eyes.
Plasticity of the second-order visual system
In our previous study (Chung et al, 2006), we reported a mean improvement in threshold of 33% following eight days of learning to identify contrast-defined letters for a group of 10 amblyopic observers. Here in Experiment 2 of the study (learning to identify contrast-defined letters), we observed only a 17% improvement, almost half of what we found in the previous study. Because we used the same methodology and paradigm, the difference in results between the two studies cannot be attributed to differences in how the measurements were obtained. The key difference is the fact that observers in the present study had already shown improvements in identifying contrast-defined letters, an improvement transferred from the learning of identifying luminance-defined letters in Experiment 1. In Experiment 1, the six observers who showed learning demonstrated an average improvement of 20% for the trained condition of identifying luminance-defined letters, but the improvement transferred so well to the untrained task of identifying contrast-defined letters (this was the untrained task of Experiment 1) that it averaged 38%, a magnitude almost twice as large as that for the trained condition. The 38% transferred improvement is very close to what we reported for a direct learning effect of identifying contrast-defined letters in our previous study (Chung et al, 2006)!
Given that the magnitude of the transferred improvement for identifying contrast-defined letters in Experiment 1 was close to what we observed as a result of a direct learning effect of identifying contrast-defined letters in our previous study, it is somewhat surprising that five of the observers were still able to show an additional improvement (averaging 17%) in Experiment 2. This additional improvement suggests that the second-order visual system is more plastic and is more capable of improvement than the first-order system, at least for the amblyopic visual system or for a task that might have been over-learned for the first-order system.
Perceptual learning for first- vs second-order stimuli
Dosher and Lu (2006) examined and contrasted perceptual learning for first- and second-order stimuli in observers with normal vision. They trained their observers at the fovea to discriminate the orientation (normal vs. mirror-reversed) of the letter K rendered using luminance contrast (first-order) or texture contrast (second-order). To identify the mechanism underlying perceptual learning of first- and second-order tasks, they incorporated the external noise paradigm to the training task and analyzed their data using the perceptual template model (Dosher & Lu, 1998, 1999; Lu & Dosher, 2004). Following five days of training to discriminate the orientation of a luminance-defined letter K, their observers (N=4) failed to show any reduction (improvement) in contrast threshold for discriminating the orientation of the letter K. However, for the other four observers who were trained to discriminate the texture-defined letter K, there was a significant improvement in the threshold for performing the task at low external noise, but not at high noise levels. According to the perceptual template model, this result suggests that the improvement of discriminating the orientation of second-order objects was a result of stimulus enhancement. These same four observers who showed improvement on discriminating the orientation of a texture-defined letter K were subsequently trained to discriminate the orientation of a luminance-defined letter K. However, no improvement was found for the luminance-defined task.
The study of Dosher and Lu (2006) differed from our present study in several accounts. The foremost and probably most important difference is that they studied perceptual learning in the normal fovea whereas we studied perceptual learning of the amblyopic visual system. This difference alone may explain why they did not find any improvement following training on a first-order task while our observers showed an average improvement of 20% — namely, that letters are over-learned through years of reading at the normal fovea.
A second difference is that Dosher and Lu (2006) did not examine the cross-over transfer of the learning effect to an untrained task, although after observing an improvement for the texture-defined (second-order) task, they trained the same observers to perform the luminance-defined (first-order) task. An examination of the cross-over transfer effect, as in our study, allows us to test predictions based on the putative filter-rectifier-filter second-order pathway (see below), or the same pathway for first- and second-order hypothesis.
Another difference is that by examining performance in the presence of different amount of external noise, Dosher and Lu (2006) were able to determine the mechanism of perceptual learning for their second-order task. The predominant improvement of threshold at low external noise levels hinted toward an improvement due to stimulus enhancement. In our study, because we only measured thresholds at one external noise level (the background noise level), we were not able to determine whether the observed improvements were attributed to stimulus enhancement (improvement at low external noise levels), external noise exclusion (improvement at high external noise levels) or a reduction of observer’s multiplicative internal noise (coupled improvement at both low and high noise levels). However, identifying the mechanism of perceptual learning for first- vs. second-order tasks was not the goal of the present study.
Model for second-order processing
The uni-directional transfer of the learning effect, from a trained first-order to an untrained second-order task,3 but not the converse, argues against having the same pathway for processing both first- and second-order information. On the contrary, our data provide evidence consistent with a two-stage model, and some new insights into the presumed processing of second-order visual information. With respect to the filter-rectifier-filter pathway postulated to mediate the processing of second-order information, the improvement can occur at the first or the second filtering (perceptual template) stage. As we suggested in the Introduction, if the improvement occurs at the first filtering stage that primarily processes first-order information, then the signals feeding onto the second filtering stage (after the presumed rectification process) would be more reliable, which may in turn facilitate the processing of second-order information of the stimulus. This may explain the extremely strong transfer of improvement to the untrained task of identifying contrast-defined letters in Experiment 1. Alternatively, if the improvement due to learning only occurs at the second filtering stage as a result of direct training for a second-order task, as in Chung et al (2006) or Experiment 2 of the present study, then unless there is a feedback or pooling mechanism in place, the improvement that occurs at the second filtering stage is unlikely to be transferred back to the first filtering stage. This may explain the lack of a transfer of improvement for identifying luminance-defined letters (a first-order task) following learning to identify contrast-defined letters (a second-order task) as reported in Chung et al (2006). Although it can also explain the lack of a transferred improvement that we observed in Experiment 2 of the present study, here we have a confounding factor that observers might have already reached the limit of their improvement in Experiment 1, thereby leaving no room for further (transferred) improvement in Experiment 2. However, we note that for the two observers (CL and GJ) who did not learn in Experiment 1 but still participated in Experiment 2, one (CL) showed some learning in Experiment 2 for identifying contrast-defined letters. Yet, her improvement still did not transfer to the task of identifying luminance-defined letters. Therefore at least for this observer, we can exclude the possibility that the lack of a transfer of improvement to the task of identifying luminance-defined letters in Experiment 2 is due to the fact that observers already reached the limit of their potential for an improvement.
Finally we note that it is widely accepted that there is a stage at which first- and second-order information is pooled (e.g., Baker & Mareschal, 2001; Chung, Li & Levi, 2007; Mareschal & Baker, 1998; Rivest & Cavanagh, 1996; Smith, Clifford & Wenderoth, 2001). A generic model consisting of independent, parallel first- and second-order pathways followed by a pooling stage would predict that the transfer of learning between first- and second-order tasks would be bi-directional when neuronal modifications are located at the pooling stage. In this view, our results (uni-directional transfer) suggest that while first- and second-order signals may be pooled at a later stage, the learning must remain specific and occur in the first-order pathway prior to the pooling stage.
Why does learning of letter identification not transfer to acuity?
One of our main motivations for this study was the hope that learning to identify letters might be more likely to transfer to visual acuity than other tasks such as Vernier acuity or contrast sensitivity, both of which have been shown to transfer to visual acuity in adult amblyopes (Levi et al, 1997; Zhou et al, 2006). To our disappointment, learning letter identification failed to transfer to visual acuity. A recent study (Huang, Zhou & Lu, 2008) suggests that the bandwidth of learning for contrast sensitivity is very broad in observers with amblyopia (≈ 4 octaves) compared to that of normal observers (≈ 1.4 octaves). The broad bandwidth of learning implies more plasticity and broader generalization in the amblyopic visual system, which in turn provides a strong rationale for perceptual learning in amblyopia. Given this broad bandwidth, why did we not see a transfer of improvement to visual acuity in our study? Huang et al (2008) had their observers practice contrast threshold measurements for a sine-wave grating pattern with a spatial frequency close to the observer’s cut-off spatial frequency (their resolution limit). The effect of this training spread to spatial frequencies well below the cutoff (more than 4 octaves below). In the present study, we had observers practice identifying near contrast-threshold luminance-defined letters that were considerably larger (≈ a factor of 8 or 3 octaves) than their resolution limit but were within the 4-octave range. One possible explanation is that letters are different from gratings. However, we would argue that since letters contain multiple spatial frequencies and orientations they should generalize to acuity more readily than gratings. A more likely explanation is that the spread of learning may be uni-directional — spreading from near the acuity limit to lower spatial frequencies (larger objects), but not the other way around. Whether or not this speculation is correct remains to be tested.
Spatial frequency selectivity of perceptual learning
A related issue concerning the generalization of learning based on the spatial frequency content of stimuli is that the luminance- and contrast-defined letters have different spectral composition. The amplitude of the power spectrum of the luminance-defined letters shows a clear peak around 2 c/letter, corresponding to the band of spatial frequencies most useful for letter identification (Chung, Legge & Tjan, 2002; Legge, Pelli, Rubin & Schleske, 1985; Majaj, Pelli, Kurshan & Palomares, 2002; Solomon & Pelli, 1994); whereas the power spectrum of the second-order letters is flat across a range of spatial frequencies, given that the second-order letters are composed of arrays of white noise. Perceptual learning is highly specific with respect to the spatial frequency of the stimulus (Fiorentini & Berardi, 1980, 1981), consequently, our expectations were that improvements following learning to identify luminance-defined letters might not readily transfer to the identification of contrast-defined letters, and vice versa. Here we show that the latter is true, consistent with the finding we reported previously (Chung et al, 2006), but the former is not. Instead, there was an excellent transfer of improvement to the task of identifying contrast-defined letters following learning to identify luminance-defined letters. This transfer cannot be explained by the bandwidth of the spatial frequency selectivity as almost all perceptual learning studies employing first-order stimuli showed rather narrow bandwidths for spatial frequency selectivity. Another factor relevant to spatial frequency is letter size. Because we used a fixed letter size for testing luminance-defined and contrast-defined letters, the stimuli were 1.3× the acuity for contrast-defined letters but approximately 8× the acuity for luminance-defined letters. Despite this 6× difference in the normalized letter size (letter size normalized to acuity for the respective task), improvements following learning readily transferred from a trained first-order to an untrained second-order task, suggesting that it is likely to be the physical letter size that matters, instead of the normalized letter size with respect to the resolution limit.
Concluding remarks
Following training to identify near-threshold luminance-defined letters, our amblyopic observers showed a modest improvement in contrast thresholds for identifying these letters. This improvement transferred very well to the untrained task of identifying contrast-defined letters in the trained eyes, but did not transfer to the untrained task of acuity (size-threshold) measurement. Practically, this result suggests that the use of large low-contrast letters as a training task may have limited clinical application in improving the visual acuity of adults with amblyopia. As noted above, training with near-acuity letters may be more successful.
We also found that despite an excellent transferred improvement in identifying contrast-defined letters following Experiment 1, subsequent direct training on identifying contrast-defined letters in Experiment 2 led to an additional improvement for identifying such letters. The improvement resulting from this direct training did not, however, transfer to the task of identifying luminance-defined letters.
Considering that the magnitude of the transferred improvement on a second-order task (identifying contrast-defined letters) as a result of training on a first-order task (identifying luminance-defined letters) is highly similar to the magnitude of improvement resulting from a direct training on the second-order task (Chung et al, 2006), our results also suggest that it may be more cost-effective to train amblyopic observers simply on a first-order task if time is of a premium, since it will improve performance for both first- and second-order tasks. However, if time is of no object, then it may be desirable to prescribe additional training on a second-order task to further improve performance on second-order tasks. It remains to be seen whether training of first-order letters near the acuity limit will transfer to both larger first- and second-order letters.
Acknowledgments
This study was supported by research grants R01-EY12810 (STLC) and R01-EY01728 (DML) from the National Institutes of Health. We thank Elizabeth Je for her assistance in subject testing and Stanley Klein for statistical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The white noise was generated by first creating a noise array of 256 × 256 pixels, with the luminance of each pixel randomly assigned a value from 0 to 1 according to a rectangular distribution. These luminance values were then scaled to a background maximum luminance contrast of 0.25, which corresponded to a rms contrast of 0.07 (see Chung et al, 2006 for details).
The use of the same physical letter size for both luminance- and contrast-defined letters allowed us to compare data from this study to our previous study (Chung et al, 2006). Given the difference in acuity for luminance- and contrast-defined letters, an alternative way to choose a target letter size is to keep the letter size at a constant multiple above size-threshold for both the luminance- and contrast-defined letters. However, this approach necessitates the use of different physical letter sizes for luminance- and contrast-defined letters, which could lead to other undesirable effects (e.g. different fundamental spatial frequency of the letters) for a perceptual learning study.
In our previous paper, we already reported a lack of a transferred improvement to the untrained task of identifying luminance-defined letters following learning to identify contrast-defined letters (Chung et al, 2006). However, in that paper, one possible explanation of the result is that luminance-defined letters were already over-learned by the observers and thus there was no improvement shown. Consequently, that result alone could not rule out the potential of a bi-directional cross-over transfer effect. In this paper, the fact that observers showed improvements on identifying luminance-defined letters in Experiment 1 suggests that performance on the task could be enhanced through practice, and provides strong evidence that the cross-over transferred effect is indeed uni-directional.
REFERENCES
- Baker CL, Mareschal I. Processing of second-order stimuli in the visual cortex. Progress in Brain Research. 2001;134:171–191. doi: 10.1016/s0079-6123(01)34013-x. [DOI] [PubMed] [Google Scholar]
- Barraclough N, Tinsley C, Webb B, Vincent C, Derrington A. Processing of first-order motion in marmoset visual cortex is influenced by second-order motion. Visual Neuroscience. 2006;23:815–824. doi: 10.1017/S0952523806230141. [DOI] [PubMed] [Google Scholar]
- Bedell HE, Flom MC. Monocular spatial distortion in strabismic amblyoipia. Investigative Ophthalmology & Visual Science. 1981;20:263–268. [PubMed] [Google Scholar]
- Bedell HE, Flom MC. Normal and abnormal space perception. American Journal of Optometry and Physiological Optics. 1983;60:426–435. doi: 10.1097/00006324-198306000-00003. [DOI] [PubMed] [Google Scholar]
- Bedell HE, Flom MC, Barbeito R. Spatial aberrations and acuity in strabismus and amblyoipia. Investigative Ophthalmology & Visual Science. 1985;26:909–916. [PubMed] [Google Scholar]
- Bonneh YS, Sagi D, Polat U. Local and non-local deficits in amblyopia: acuity and spatial interactions. Vision Research. 2004;44:3099–3110. doi: 10.1016/j.visres.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Investigative Ophthalmology & Visual Science. 1981;21:467–476. [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Chubb C, Sperling G. Drift-balanced random stimuli: a general basis for studying non-Fourier motion perception. Journal of the Optical Society of America A. 1988;5:1986–2007. doi: 10.1364/josaa.5.001986. [DOI] [PubMed] [Google Scholar]
- Chung STL, Legge GE, Tjan BS. Spatial-frequency characteristics of letter identification in central and peripheral vision. Vision Research. 2002;42:2137–2152. doi: 10.1016/s0042-6989(02)00092-5. [DOI] [PubMed] [Google Scholar]
- Chung STL, Li RW, Levi DM. Identification of contrast-defined letters benefits from perceptual learning in adults with amblyopia. Vision Research. 2006;46:3853–3861. doi: 10.1016/j.visres.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL, Li RW, Levi DM. Crowding between first- and second-order letter stimuli in normal foveal and peripheral vision. Journal of Vision. 2007;7(2)(10):1–13. doi: 10.1167/7.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda KJ, Levi DM, Selenow A. Amblyopia: basic and clinical aspects. Butterworth-Heinemann: Boston; 1991. [Google Scholar]
- Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proceedings of the National Academy of Science USA. 1998;95:13988–13993. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Mechanisms of perceptual learning. Vision Research. 1999;39:3197–3221. doi: 10.1016/s0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Level and mechanisms of perceptual learning: Learning first-order luminance and second-order texture objects. Vision Research. 2006;46:1996–2007. doi: 10.1016/j.visres.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Baker CL, Hess RF, Evans AC. Cortical specialization for processing first- and second-order motion. Cerebral Cortex. 2003;13:1375–1385. doi: 10.1093/cercor/bhg085. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Allen HA, Hess RF. Investigating local network interactions underlying first- and second-order processing. Vision Research. 2004;44:1787–1797. doi: 10.1016/j.visres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287:43–44. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Learning in grating waveform discrimination: specificity for orientation and spatial frequency. Vision Research. 1981;21:1149–1158. doi: 10.1016/0042-6989(81)90017-1. [DOI] [PubMed] [Google Scholar]
- Gstalder RJ, Green DG. Laser interferometric acuity in amblyopia. Journal of Pediatric Ophthalmology. 1971;8:251–256. [Google Scholar]
- Hariharan S, Levi DM, Klein SA. “Crowding” in normal and amblyopic vision assessed with Gaussian and Gabor C’s. Vision Research. 2005;45:617–633. doi: 10.1016/j.visres.2004.09.035. [DOI] [PubMed] [Google Scholar]
- Hess RF, Bradley A. Contrast perception above threshold is only minimally impaired in human amblyopia. Nature. 1980;287:463–464. doi: 10.1038/287463a0. [DOI] [PubMed] [Google Scholar]
- Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two-type classification. Vision Research. 1977;17:1049–1055. doi: 10.1016/0042-6989(77)90009-8. [DOI] [PubMed] [Google Scholar]
- Hess RF, Campbell FW, Greenhalgh R. On the nature of the neural abnormality in human amblyopia: neural aberrations and neural sensitivity loss. Pflugers Archiv European Journal of Physiology. 1978;377:201–207. doi: 10.1007/BF00584273. [DOI] [PubMed] [Google Scholar]
- Huang C-B, Zhou Y, Lu Z-L. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proceedings of the National Academy of Sciences. 2008;105:4068–4073. doi: 10.1073/pnas.0800824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge GE, Pelli DG, Rubin GS, Schleske MM. Psychophysics of reading – I. Normal vision. Vision Research. 1985;25:239–252. doi: 10.1016/0042-6989(85)90117-8. [DOI] [PubMed] [Google Scholar]
- Levi DM. Perceptual learning in adults with amblyopia: A reevaluation of critical periods in human vision. Developmental Psychobiology. 2005;46:222–232. doi: 10.1002/dev.20050. [DOI] [PubMed] [Google Scholar]
- Levi DM, Hariharan S, Klein SA. Suppressive and facilitatory spatial interactions in amblyopic vision. Vision Research. 2002;42:1379–1394. doi: 10.1016/s0042-6989(02)00061-5. [DOI] [PubMed] [Google Scholar]
- Levi DM, Harwerth RS. Spatio-temporal interactions in anisometropic and strabismic amblyopia. Investigative Ophthalmology & Visual Science. 1977;16:90–95. [PubMed] [Google Scholar]
- Levi DM, Klein SA. Hyperacuity and amblyopia. Nature. 1982;298:268–270. doi: 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Spatial localization in normal and amblyopic vision. Vision Research. 1983;23:1005–1017. doi: 10.1016/0042-6989(83)90011-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Research. 1985;25:979–991. doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Equivalent intrinsic blur in amblyopia. Vision Research. 1990;30:1995–2022. doi: 10.1016/0042-6989(90)90017-f. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Chen I. The response of the amblyopic visual system to noise. Vision Research. 2007;47:2531–2542. doi: 10.1016/j.visres.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proceedings of the National Academy of Sciences. 1996;93:6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Polat U, Hu YS. Improvement in Vernier acuity in adults with amblyopia. Practice makes better. Investigative Ophthalmology & Visual Science. 1997;38:1493–1510. [PubMed] [Google Scholar]
- Li RW, Levi DM. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. Journal of Vision. 2004;4:476–487. doi: 10.1167/4.6.7. [DOI] [PubMed] [Google Scholar]
- Loshin DS, Levi DM. Suprathreshold contrast perception in functional amblyopia. Documenta Ophthalmologica. 1983;55:213–236. doi: 10.1007/BF00140810. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA. Perceptual learning retunes the perceptual template in foveal orientation identification. Journal of Vision. 2004;4:44–56. doi: 10.1167/4.1.5. [DOI] [PubMed] [Google Scholar]
- Majaj NJ, Pelli DG, Kurshan P, Palomares M. The role of spatial frequency channels in letter identification. Vision Research. 2002;42:1165–1184. doi: 10.1016/s0042-6989(02)00045-7. [DOI] [PubMed] [Google Scholar]
- Mareschal I, Baker CL. A cortical locus for the processing of contrast-defined contours. Nature Neuroscience. 1998;1:150–154. doi: 10.1038/401. [DOI] [PubMed] [Google Scholar]
- McGraw PV, Levi DM, Whitaker D. Spatial characteristics of the second-order visual pathway revealed by positional adaptation. Nature Neuroscience. 1999;2:479–484. doi: 10.1038/8150. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. Journal of Vision. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The videotoolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Pelli DG, Zhang L. Accurate control of contrast on microcomputer displays. Vision Research. 1991;31:1337–1350. doi: 10.1016/0042-6989(91)90055-a. [DOI] [PubMed] [Google Scholar]
- Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proceedings of the National Academy of Sciences. 2004;101:6692–6697. doi: 10.1073/pnas.0401200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Sagi D, Norcia AM. Abnormal long-range spatial interactions in amblyopia. Vision Research. 1997;37:737–744. doi: 10.1016/s0042-6989(96)00154-x. [DOI] [PubMed] [Google Scholar]
- Rentschler I, Hilz R. Amblyopic processing of positional information. Part I: Vernier acuity. Experimental Brain Research. 1985;60:270–278. doi: 10.1007/BF00235921. [DOI] [PubMed] [Google Scholar]
- Rivest J, Cavanagh P. Localizing contours defined by more than one attribute. Vision Research. 1996;36:53–66. doi: 10.1016/0042-6989(95)00056-6. [DOI] [PubMed] [Google Scholar]
- Schofield AJ. What does second-order vision see in an image? Perception. 2000;29:1071–1086. doi: 10.1068/p2913. [DOI] [PubMed] [Google Scholar]
- Smith AT, Greenlee MW, Singh KD, Kraemer FM, Hennig J. The processing of first- and second-order motion in human visual cortex assessed by functional magnetic resonance imaging (fMRI) Journal of Neuroscience. 1998;18:3816–3830. doi: 10.1523/JNEUROSCI.18-10-03816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Clifford CWG, Wenderoth P. Interaction between first- and second-order orientation channels revealed by the tilt illusion: psychophysics and computational modeling. Vision Research. 2001;41:1057–1071. doi: 10.1016/s0042-6989(01)00015-3. [DOI] [PubMed] [Google Scholar]
- Solomon JA, Pelli DG. The visual filter mediating letter identification. Nature. 1994;369:395–397. doi: 10.1038/369395a0. [DOI] [PubMed] [Google Scholar]
- Whitaker D, McGraw PV, Levi DM. The influence of adaptation on perceived visual location. Vision Research. 1997;37:2207–2216. doi: 10.1016/s0042-6989(97)00030-8. [DOI] [PubMed] [Google Scholar]
- von Noorden GK. New clinical aspects of stimulus deprivation amblyopia. American Journal of Ophthalmology. 1981;92:416–421. doi: 10.1016/0002-9394(81)90534-1. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Huang C, Xu P, Tao L, Qiu Z, Li X, Lu Z. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Research. 2006;46:739–750. doi: 10.1016/j.visres.2005.07.031. [DOI] [PubMed] [Google Scholar]