Abstract
Elevated nitric oxide (NO) and proton levels in synovial fluid are implicated in joint pathology. However, signaling pathways stimulated by these molecules that mediate inflammation and pain in the temporomandibular joint (TMJ) have not been investigated. The goal of this study was to determine the effect of NO-proton stimulation of trigeminal neurons on the in vivo expression of mitogen-activated protein kinases (MAPKs) and phosphatases (MKPs) in trigeminal ganglion neurons and satellite glial cells. Low levels of the active MAPKs ERK, JNK, and p38 were localized in the cytosol of neurons and satellite glial cells in unstimulated animals. However, increased levels of active ERK and p38, but not JNK, were detected in the cytosol and nucleus of V3 neurons and satellite glial cells 15 min and 2 h following bilateral TMJ injections of a NO donor diluted in pH 5.5 medium. While ERK levels returned to near basal levels 24 h after stimulation, p38 levels remained significantly elevated. In contrast to MKP-2 and MKP-3 levels that were barely detectable in neurons or satellite glial cells, MKP-1 staining was readily observed in satellite glial cells in ganglia from unstimulated animals. However, neuronal and satellite glial cell staining for MKP-1, MKP-2, and MKP-3 were all significantly increased in response to NO-protons. Increased active ERK and p38 levels as well as elevated MKP levels were also detected in neurons and satellite glial cells located in V2 and V1 regions of the ganglion. Our data provide evidence that NO-proton stimulation of V3 neurons results in temporal and spatial changes in expression of active ERK and p38 and MKPs in all regions of the ganglion. We propose that in trigeminal ganglia these cellular events, which are involved in peripheral sensitization as well as control of inflammatory and nociceptive responses, may play a role in TMJ pathology.
Keywords: MAP kinase, MAP kinase phosphatase, trigeminal ganglion neurons, nitric oxide, protons, glia
Sensitization and activation of trigeminal nerves is implicated in the underlying pathology of inflammatory diseases involving the head and face including temporomandibular joint (TMJ) disorders, migraine, and rhinosinusitis. TMJ disorders refer to a cluster of disorders involving the masticatory system, including the TMJ or jaw joint and surrounding tissues (Herb et al., 2006). Pain in the TMJ or jaw joint is frequently reported by individuals with TMJ disorders, which are the most common type of facial pain, affecting an estimated 10–12% of the population (LeResche, 1997). Although the chief symptom of TMJ disorders is chronic pain in the TMJ and face, headaches, limitations in using the jaw comfortably, and joint sounds are frequently reported. Despite the prevalence and significant morbidity associated with TMJ disorders, not much is known about the underlying cellular mechanisms involved in causing inflammation, pain, and destruction in the TMJ. While the TMJ capsule contains a rich supply of nerve fibers and receives inputs from a number of peripheral ganglia, the major sensory innervation is derived from the trigeminal ganglia (Uddman et al., 1998).
The afferent fibers of the trigeminal nerve, except for those associated with pressure and stretch receptors and proprioception, have their cell bodies of origin located in the trigeminal ganglion (Shankland, 2000). The nerve cells of the trigeminal ganglion are classified as pseudounipolar since a single axon divides into a central and peripheral branch (Lazarov, 2002). The central cellular processes exit the concavity of the ganglion before entering the brainstem and terminating in the principal sensory and spinal trigeminal nuclei, while the peripheral fibers leave the convexity of the crescent of the ganglion as the ophthalmic (V1), maxillary (V2), and mandibular (V3) divisions of the trigeminal nerve (Shankland, 2000, Lazarov, 2002). The sensory neurons in the trigeminal ganglion are completely enveloped by a layer of satellite glial cells. The neurons extend numerous processes that increase the surface area considerably and may allow communication in the form of chemical exchange between the neurons and glia (Hanani, 2005). Although previously thought to be mere supportive cells, there is now evidence that satellite glial cells may also communicate with neurons through gap junctions and paracrine signaling (Haydon, 2001, Thalakoti et al., 2007). Moreover, it is now known that satellite glial cells have an essential role in pathological states such as inflammation and pain (Hanani, 2005).
Nitric oxide (NO) is an important messenger involved in the development and maintenance of inflammation and pain (Yun et al., 1996, Liu et al., 2002, Guzik et al., 2003, Naik et al., 2006). It is an unstable free radical gas that mediates many physiological and toxic functions, and is produced by the nitric oxide synthase family of enzymes which includes neuronal nitric oxide synthase (nNOS), endothelial nitric oxide synthase (eNOS), and inducible nitric oxide synthase (iNOS) (Liu et al., 2002). While iNOS is not expressed at high levels in normal human TMJs, iNOS expression in the synovial lining of diseased TMJs is greatly increased (Homma et al., 2001, Nagai et al., 2003, Takahashi et al., 2003). Furthermore, NO levels in synovial fluid obtained from patients with internal derangement and osteoarthritis of their TMJ were significantly increased when compared to control levels and correlated with disease stage and pain in the patients’ joint area (Takahashi et al., 1999, Suenaga et al., 2001). Similarly, elevated proton (acidic pH < 6.0) levels in synovial fluid has been shown to correlate with increased tissue destruction in the joint (Christensen et al., 2005). However, the cellular mechanisms by which NO and protons mediate inflammation and pain in the TMJ and other joints are not well understood.
The cellular signaling pathways collectively known as the mitogen-activated protein kinase (MAPK) signaling cascade are known to play important roles in the initiation and maintenance of inflammation and pain (Seger and Krebs, 1995, Ji, 2004). It is now known that at least four distinctly regulated groups of MAPKs are present in mammalian cells: extracellular signal-regulated kinases (ERK 1/2); Jun amino-terminal kinases (JNK 1/2/3); p38 proteins (p38α/β/δ); and ERK5 (Schaeffer and Weber, 1999, Chang and Karin, 2001). These pathways have been shown to be responsive to many extracellular stimuli, including inflammatory cytokines, ceramides, nitric oxide, and transforming growth factor β (Lewis et al., 1998). As a result, the MAPKs control key cellular functions, including proliferation, differentiation, migration and apoptosis, and participate in a number of disease states including chronic inflammation and cancer (Turjanski et al., 2007). Although these MAPK pathways allow a cell to respond to changes in the extracellular environment in a regulated manner, chronic activation can cause inflammation, pain, and tissue damage.
The magnitude and duration of MAPK stimulation is a crucial determinant of biological outcome. In mammalian cells, negative regulation of MAPKs is primarily achieved by the activity of dual-specificity protein phosphatases through dephosphorylation of key tyrosine and threonine residues of activated MAPKs. The MAPK phosphatases (MKPs) constitute a group of ten active enzymes (Theodosiou and Ashworth, 2002) that function to regulate inflammatory responses mediated by MAPKs. All MKPs share a common structure, and a large number have overlapping substrate specificities (Dickinson and Keyse, 2006). For example, MKP-1 has been shown to preferentially dephosphorylate p38 and JNK while MKP-2 exhibits broad phosphatase activity and is reported to dephosphorylate ERK, JNK, and p38. In contrast, MKP-3 is reported to exhibit selectivity towards ERK (Keyse, 1998, Farooq and Zhou, 2004). It should be noted that these specificities are likely dependent on MKP concentration and the particular cell type (Theodosiou and Ashworth, 2002).
In this study, an in vivo method was utilized to investigate the spatial and temporal changes in several MAPKs and MKPs in a novel model of TMJ pathology. Specifically, the cellular localization and levels of active ERK, JNK, and p38 MAPKs as well as MKP-1, MKP-2, and MKP-3 were determined in both trigeminal ganglion neurons and satellite glial cells in response to NO-proton injection into the TMJ capsule.
EXPERIMENTAL PROCEDURES
Animals
The animal studies were approved by the Institutional Animal Care and Use Committee at Missouri State University in accordance with the guidelines established in the Animal Welfare Act. Adult male Sprague-Dawley rats (250–300 g) were housed in structurally sound, clean plastic cages on a 12 hour light/dark cycle and with unrestricted access to food and water for the duration of the experiment.
TMJ capsule injections
The injections were performed essentially as described in a previously published study (Thalakoti et al., 2007). Briefly, the rats were anesthetized with a 0.25 ml intraperitoneal injection of a ketamine (45 mg/kg) and xylazine (5 mg/kg) solution. A 261/2-gauge needle connected to a microliter syringe was employed to administer bilateral doses of the retrograde tracer True Blue (25 μl, 2 mg/ml dissolved in DMSO, Biotium, Inc., Hayward, CA, USA) in the TMJ capsules, both whisker pads (4 injections; 50 μl total volume), or the brow of each eye (3 injections; 25 μl total volume). For the stimulation studies, animals were either left untreated or were injected with 25 μl of the stimulatory or control compounds directly into the TMJ capsule. The stimulatory solution contained 10 mM sodium nitroprusside (SNP) in HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffered saline (HBS) at pH 5.5. Other animals were either left untreated or were injected with the vehicle, which was HBS at pH 7.4. The concentration of NO donor and pH were chosen since we had previously shown that they can stimulate neuropeptide release from cultured trigeminal ganglia neurons (Bellamy et al., 2006, Vause et al., 2007). The nitrite concentration of the NO-proton solution that was injected into the TMJ capsule was determined using the Griess Reagent System (Promega, Madison, WI, USA) following the manufacturer’s instructions. Absorbance was measured in a Wallac 1420 VICTOR3™ V plate reader (Perkin Elmer, Waltham, MA, USA) at 520–550 nm. The data are reported as average nitrite concentration ± SEM. The experimental procedure was repeated in 4 independent experiments. All statistical tests were performed using Minitab Statistical Software, Release 14.
Immunohistochemistry
For the labeling studies, ganglia were removed from animals 5 days after injections of the dye True Blue into different regions of the head and face. In other studies, both trigeminal ganglia were removed from untreated control rats or from animals 15 min, 2 h, or 24 h after injection of vehicle or NO-proton solution into each capsule. Ganglia were mounted in Neg-50 Frozen Section Medium (Richard Allan Scientific, Kalamazoo, MI, USA), quickly frozen, and stored at −20° C. Serial tissue sections were obtained using a cryostat (Microm HM 525, Richard Allan Scientific). The sections were mounted on microscope slides (Fisherbrand Superfrost, Fisher Scientific, Pittsburgh, PA, USA) with the anterior-posterior axis maintained.
The tissue was incubated in 4% paraformaldehyde for 60 min, and then 0.3% triton X-100 in phosphate buffered saline (PBS) for 60 min to fix and permeabilize the cells. Fixed and permeabilized tissues were then incubated in PBS containing 5% donkey serum for 60 min to reduce non-specific binding of the antibodies. The spatial and temporal changes in levels of active MAPK and MKP were determined by immunohistochemistry.
This procedure was carried out on two sections from each rat. The tissue sections were incubated overnight at 4° C with rabbit primary antibodies directed against the phosphorylated (active) MAPK proteins p38 (diluted 1:100 in PBS, Cell Signaling, Beverly, MA, USA), JNK (Cell Signaling, 1:200), ERK (Cell Signaling, 1:200), MKP-1 (diluted 1:500 in PBS, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), MKP-2 (Santa Cruz, 1:500), or MKP-3 (Santa Cruz, 1:500). Some sections were incubated for 1 h at room temperature with mouse anti-neurofilament 200 antibodies (1:200; Chemicon, Temecula, CA). Sections were then incubated for 1 h at room temperature in Texas Red-conjugated donkey anti-rabbit or donkey anti-mouse Rhodamine Red-X antibodies (diluted 1:100 in PBS, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) to detect immunoreactive proteins via red fluorescence at 555 nm. As a control, some tissue sections were incubated under the same conditions with only the donkey anti-rabbit secondary antibodies. Sections were also viewed at wavelength 350 nm to either identify cells containing True Blue or nuclei costained with 4′, 6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA). Images (40x, 100x, and 400x) were collected using an Olympus DP70 camera mounted on an Olympus BX41 fluorescent microscope (Center Valley, PA, USA). Image analysis was performed using Olympus MicroSuite Five image processing software. Multiple image alignment was utilized to view the entire ganglion in a single image at 40x magnification. Briefly, a total of 12 images were collected and aligned to produce a much larger view of the tissue. Each experimental condition was repeated in at least 3 independent experiments.
Quantification of staining intensity
For intensity measurements, each image was converted to greyscale prior to analysis. The average grey value was measured in 6 regions of interest in the V3 region that contained neurons and satellite glia and 6 regions of interest in the V1/V2 region that contained neurons and satellite glia and normalized to the average grey value in 3 regions of interest that contained only glial Schwann cells, which was used as a background level of staining. The data are reported as a ratio of average greyscale intensity of neurons and satellite glial cells versus Schwann cells ± SEM. Each experimental condition was repeated in at least 3 independent experiments performed in duplicate.
Statistical analysis
Statistical analysis was performed using the parametric Two-Sample T-Test. Differences were considered statistically significant at p < 0.05. All statistical tests were performed using Minitab Statistical Software, Release 14.
RESULTS
Localization of neurons whose fibers innervate the TMJ capsule
Initially, trigeminal ganglia from unstimulated adult rats were isolated and longitudinal sections of the ganglia were stained with the nuclear dye DAPI to show the distribution of the nuclei of all neurons and glial cells in the ganglia. Multiple image alignment was used to obtain images of the entire ganglion so that the unique arrangement of neurons and glial cells in the V1 (anteromedial), V2 (anterolateral), and V3 (posterolateral) regions were clearly visible (Fig. 1A). The nuclei of neuronal cells are typically round and >20 μm while glial cells exhibit more elliptical nuclei that are ~10 μm in diameter. The neuronal cells are arranged in bands within the trigeminal ganglion in all regions. As seen at higher magnification, neuronal cells are completely surrounded by satellite glial cells. To localize neurons whose afferent fibers project to the TMJ capsule, the retrograde tracer True blue was injected into both TMJs 5 days prior to removal of the ganglia. The fluorescent dye was localized primarily in neuronal cell bodies found in a punctate pattern in the V3 region of the ganglion (Fig. 1B). Thus, most of the neurons that provide innervation of the TMJ capsule are located in the V3 region of the ganglion in close association with satellite glial cells.
Fig 1.
Retrograde labeling of neuronal cell bodies within the trigeminal ganglion that provide innervation to the TMJ capsule. Panel A: The fluorescent dye DAPI was used to stain the nuclei of all neuronal and glial cells in the ganglion. A longitudinal section of the entire ganglion of untreated animals is shown in the left panel. The middle figure shows the distribution of neurons and glial cells in the V3 region of the ganglion. Shown in the right figure at higher magnification is the cellular arrangement of neuronal cells (large arrows) and satellite glial cells (small arrows) as identified by the size of their nuclei. Panel B: The distribution of neuronal cell bodies that provide innervation of the TMJ capsule is shown in a ganglion section obtained from an animal injected 5 days earlier with the fluorescent dye True Blue. An enlarged image of the V3 region is seen in the middle figure while an enlarged area of a neuronal cell band is shown in the right figure.
Expression of MAPKs in V3 region of trigeminal ganglia in response to NO-proton stimulation
To investigate the effect of injecting an NO-proton solution into the TMJ on MAPK signaling, ganglia were obtained from animals that were left untreated or injected with 10 mM of the NO donor SNP diluted in HBS pH 5.5 medium. A buffered solution of HBS at pH 5.5 was chosen since we had previously found that this solution could activate cultured trigeminal ganglion neurons and significantly stimulate the release of the neuropeptide calcitonin gene-related peptide (CGRP) (Vause et al., 2007). While SNP spontaneously releases NO when dissolved in an aqueous solution, the amount of NO generated when SNP is dissolved in HBS pH 5.5 medium was not known. The Griess reaction was utilized to determine the concentration of nitrite, a stable metabolite of NO, in acidic HBS. It was determined that under these conditions a nitrite concentration of 6.14 ± 1.95 μM (n = 3) was achieved. This concentration is within the NO range typically reported for synovial samples obtained from patients experiencing TMJ pathology (Suenaga et al., 2001). As seen in Figure 2, active p38 (P-p38) staining was barely detectable in ganglia from unstimulated control animals (A). In contrast, P-p38 staining was readily detectable in the V3 region of the ganglia following NO-proton stimulation for 2 h (B) and even at 24 h (C). At higher magnification, increased P-p38 expression was readily observed in the cytosol and nucleus of both neuronal (thick arrows) and satellite glial cells (thin arrows) in response to NO-proton injection at both time points when compared to control. However, increased P-p38 levels were not detected in the cytosol or nucleus of glial Schwann cells. As a control, no staining was detected in any cell type within the ganglion obtained from NO-proton treated animals when tissues were incubated with only the donkey anti-rabbit secondary antibodies (Figure 2D). Similarly, only minimal staining was observed in ganglia obtained from animals 2 h and 24 h following injection of the vehicle, which was HBS at pH 7.4 (data not shown). In addition, some sections were stained for expression of the neuronal cell marker neurofilament 200 to identify neuronal cells within the ganglion. As seen in Figure 2E, immunoreactivity for neurofilament 200 was detected in neuronal cells characterized by their large round nuclei, but was not detected in glial cells that exhibit smaller elliptical nuclei.
Fig. 2.


Expression of active p38 in trigeminal ganglion neurons and satellite glial cells is increased in response to NO-protons. Sections of ganglia from untreated control (CON) animals (Panel A) or from animals 2 h (Panel B) or 24 h (Panel C) after injection of 10 mM of the NO donor SNP in pH 5.5 medium stained for the active, phosphorylated form of p38 MAPK (P-p38) are shown. The V3 region of the ganglion is shown in the left panels. The next three panels represent the same ganglion section co-stained with p38 antibodies and DAPI and a merged image of the P-p38 and DAPI staining (right panel). Panel D shows images from a ganglion section incubated with only donkey anti-rabbit secondary antibodies while in Panel E images are shown of tissue stained for expression of neurofilament 200 protein (NF 200). Neuronal cells are identified by large arrows, satellite glial cells by smaller long arrows, and Schwann cells by smaller short arrows.
Similar to the results observed for P-p38, the level of active ERK (P-ERK) was greatly increased in the neuronal bands of the V3 region in response to NO-protons 2 h after injection (Fig. 3). The increase at 2 h was observed in the nucleus and cytosol in most neurons and satellite glial cells, but not Schwann cells, within the V3 region. However, by 24 h after injection, the level of P-ERK in both neurons and satellite glial cells had returned to levels observed in ganglia obtained from unstimulated animals.
Fig. 3.
Expression of active ERK in trigeminal ganglion neurons is increased 2 h after injection of NO-protons. Sections of ganglia from untreated control (CON) animals (Panel A) or from animals 2 h (Panel B) or 24 h (Panel C) after injection of NO-protons that were stained for the active, phosphorylated form of ERK MAPK (P-ERK) are shown. The V3 region of the ganglion is shown in the left panels. The next three panels represent the same section co-stained with ERK antibodies and DAPI and a merged image of the P-ERK and DAPI staining (right panel). Neuronal cells are identified by large arrows, satellite glial cells by smaller long arrows, and Schwann cells by smaller short arrows.
In contrast to the stimulatory effect of NO-proton injection on P-p38 and P-ERK, the level of active JNK (P-JNK) was not increased in neurons, satellite glial cells, or Schwann cells found in the V3 region of the ganglion (Fig. 4). Even at higher magnification, no increased P-JNK staining was detected in the cytosol or nucleus of either neuronal or satellite glial cells in the V3 region of the ganglion.
Fig. 4.
NO-proton injection into the TMJ capsule does not cause an increase in the levels of active JNK in trigeminal ganglion neurons or glial cells. Sections of ganglia from untreated control (CON) animals (Panel A) or from animals 2 h (Panel B) or 24 h (Panel C) after NO-proton injection that were stained for the active, phosphorylated form of JNK MAPK (P-JNK) are shown. The V3 region of the ganglion is shown in the left panels. The next three panels represent the same section co-stained with JNK antibodies and DAPI and a merged image of the P-JNK and DAPI staining (right panel). Neuronal cells are identified by large arrows, satellite glial cells by smaller long arrows, and Schwann cells by smaller short arrows.
Expression of MKPs in V3 region of trigeminal ganglia in response to NO-proton stimulation
To determine whether NO-proton injection into the TMJ capsule would lead to increased expression of MKP-1 levels in vivo, other sections from the ganglia obtained from animals injected with NO-protons and used to study MAPK expression were immunostained for the presence of MKPs. In ganglia from untreated animals, low level MKP-1 staining was detected throughout the V3 region (Fig. 5). As seen at higher magnification, MKP-1 was localized primarily in the cytosol and nucleus of satellite glia in control ganglia. However, increased MKP-1 staining especially in neurons was readily observed in the V3 region of the ganglion following NO-proton stimulation for 2 h and 24 h. At 2 h MKP-1 staining was clearly visible in the nuclei and cytosol of most V3 neurons and satellite glia. Even 24 h after injection, MKP-1 levels in the nucleus of many neuronal and satellite glial cells remained elevated compared to control levels. No detectable MKP-1 levels were observed in Schwann cells in ganglia obtained from unstimulated or stimulated animals.
Fig. 5.
Expression of MKP-1 in trigeminal ganglion neurons is increased 2 and 24 h after injection of NO-protons. Sections of ganglia from untreated control (CON) animals (Panel A) or from animals 2 h (Panel B) or 24 h (Panel C) after injection of NO-protons that were stained for MKP-1 are shown. The V3 region of the ganglion is shown in the left panels. The next three panels represent the same section co-stained with MKP-1 antibodies and DAPI and a merged image of the MKP-1 and DAPI staining (right panel). Neuronal cells are identified by large arrows, satellite glial cells by smaller long arrows, and Schwann cells by smaller short arrows.
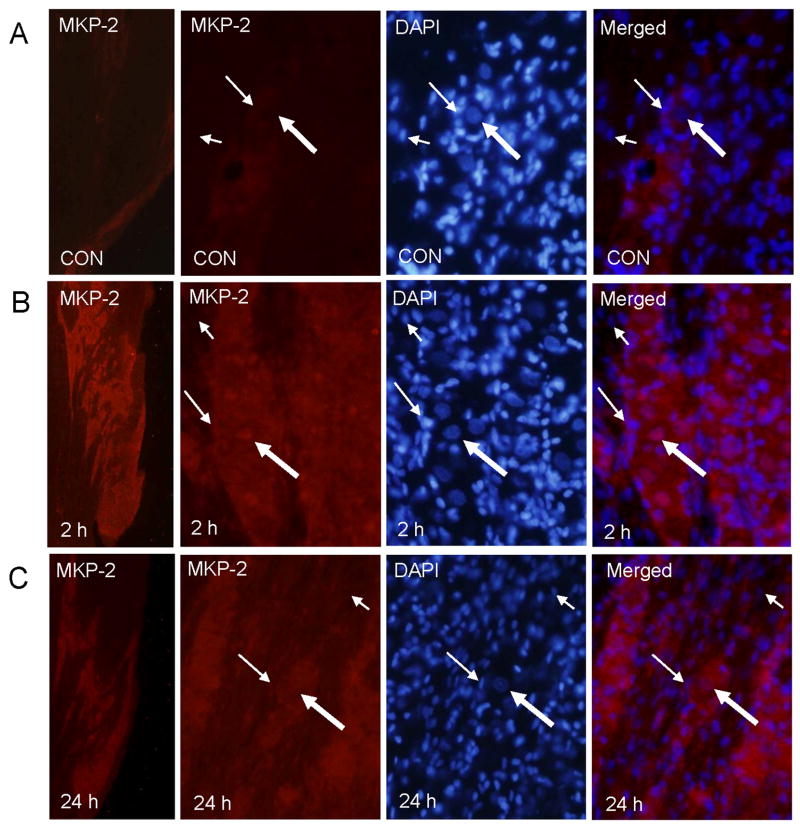
In contrast to the levels of MKP-1 seen in control ganglia, MKP-2 staining was barely detectable in the V3 region in either the neurons or glial cells (Fig. 6). However, increased MKP-2 staining was readily observed in the V3 region of the ganglion following NO-proton stimulation for 2 h and remained elevated at 24 h. At 2 and 24 h MKP-2 staining was detected in the cytosol and nuclei of most V3 neurons and satellite, but not Schwann glial cells. Similar to MKP-2 levels in ganglia from control animals, MKP-3 staining was barely detected in neuronal or glial cells located in the V3 region (Fig. 7). Even at higher magnification, MKP-3 staining was rarely observed in ganglia obtained from control unstimulated animals. However, increased MKP-3 staining was seen in the V3 region of the ganglion following NO-proton stimulation for 2 h and remained elevated at 24 h. At 2 h MKP-3 staining was readily observed in the nuclei and cytosol of most V3 neurons and satellite glia. Even 24 h after injection, MKP-3 was detected in the cytosol and nucleus of neuronal and satellite glia. However, MKP-3 staining of Schwann cells was not seen in any ganglia.
Fig. 6.
MKP-2 expression in trigeminal ganglion neurons and glia is increased 2 and 24 h after injection of NO-protons. Sections of ganglia from untreated control (CON) animals (Panel A) or from animals 2 h (Panel B) or 24 h (Panel C) after injection of NO-protons that were stained for MKP-1 are shown. The V3 region of the ganglion is shown in the left panels. The next three panels represent the same section co-stained with MKP-2 antibodies and DAPI and a merged image of the MKP-2 and DAPI staining (right panel). Neuronal cells are identified by large arrows, satellite glial cells by smaller long arrows, and Schwann cells by smaller short arrows.
Fig. 7.
Expression of MKP-3 in trigeminal ganglion neurons and satellite glia is increased 2 and 24 h after injection of NO-protons. Sections of ganglia from untreated control (CON) animals (Panel A) or from animals 2 h (Panel B) or 24 h (Panel C) after injection of NO-protons that were stained for MKP-3 are shown. The V3 region of the ganglion is shown in the left panels. The next three panels represent the same section co-stained with MKP-3 antibodies and DAPI and a merged image of the MKP-3 and DAPI staining (right panel). Neuronal cells are identified by large arrows, satellite glial cells by smaller long arrows, and Schwann cells by smaller short arrows.
As shown in Table 1, the average intensity of the staining in regions of interest containing neurons and satellite glia in V3 were measured in control ganglia and the NO-proton treated ganglia 15 minutes, 2 h, and 24 h after injection. Analysis of the V3 region demonstrated that active p38 staining was significantly increased in neurons and satellite glia as early as 15 min after TMJ capsule injection when compared to unstimulated control values. The elevated levels of P-p38 remained significantly greater than control levels at 2 and 24 h. While the intensity of P-ERK staining was significantly increased in neurons and satellite glia at 15 min and 2 h, the level at 24 h was similar to control values. The level of P-JNK was not significantly increased in neurons or glia at any time point when compared to control levels. MKP-1 was only significantly increased 2 h after NO-proton injection, while MKP-2 was significantly increased at both the 2 h and 24 h time point. Interestingly, MKP-3 was significantly increased at all three time points following NO-proton stimulation.
Table 1.
Quantification of neuronal and glial MAP kinase and MAP kinase phosphatase staining intensities in the V3 region of the trigeminal ganglion. The values, which are the ratio of the staining intensity of neuron and satellite glial cells to the intensity of Schwann cells in 6 independent fields, are reported as the average staining intensity SEM. Each experimental condition was repeated a minimum of three times.
| V3 Region of Trigeminal Ganglion | ||||||
|---|---|---|---|---|---|---|
| p38 | ERK | JNK | MKP-1 | MKP-2 | MKP-3 | |
| Control | 1.29
0.02 |
2.10
0.13 |
1.42
0.04 |
2.66
0.14 |
1.82
0.10 |
1.96
0.06 |
| NO/proton-15 min | 2.18
0.22* |
3.72
0.13* |
1.32
0.04 |
2.73
0.25 |
2.03
0.10 |
2.68
0.07* |
| NO/proton-2 hrs | 2.25
0.06* |
2.81
0.06* |
1.29
0.02 |
3.31
0.23# |
3.12
0.12* |
2.75
0.22* |
| NO/proton-24hrs | 1.79
0.08* |
1.88
0.22 |
1.17
0.03 |
2.99
0.24 |
2.43
0.04* |
2.86
0.11* |
=p<0.05 or
=p<0.01 when compared to control values.
Expression of MAPKs in trigeminal ganglion cells of the V1 and V2 regions in response to NO-proton stimulation
To localize neurons in the trigeminal ganglion whose afferent fibers project to regions corresponding to the V1 and V2 regions, the retrograde tracer True blue was injected into the whisker pads (V2) and brow of the eyes (V1) five days prior to removal of the ganglia. As seen in Figure 8, the fluorescent dye was localized primarily in neuronal cell bodies found in a punctate pattern in the anteromedial (V1) or anterolateral (V2) regions of the ganglion. An unexpected finding of our studies was the increased expression of P-p38 and P-ERK in all regions of the trigeminal ganglion 2 h after NO-proton injection into the TMJ capsule (data not shown). In control ganglia, P-p38 and P-JNK staining was barely detectable while a low level of P-ERK staining was observed throughout the ganglia. However, trigeminal ganglia obtained from animals injected two h prior with NO-protons into the TMJ capsule exhibited higher levels of P-p38 and P-ERK staining not only in the V3, but V2 and V1 regions. In contrast, no change in P-JNK staining was observed following NO-proton treatment. These results are similar to what was observed in the V3 region.
Fig. 8.
Localization of neuronal cell bodies in the trigeminal ganglion that provide sensory innervation in the whisker pad and eyebrow. A longitudinal section of the entire trigeminal ganglion obtained from an animal injected with True Blue in both the whisker pads and eye brows 5 days earlier is shown in the left panel. An enlarged anterior image is shown in the right panel.
Expression of MKPs in trigeminal ganglion cells of the V1 and V2 regions in response to NO-proton stimulation
Somewhat expectedly, increased expression of MKP-1, MKP-2, and MKP-3 in response to 2 hour treatment with NO-protons was also observed in all regions of the ganglion (data not shown). The level of MKP-1 staining in the V2 and V1 regions was similar to that observed in the V3 region. In control ganglia, the level of MKP-1 was much greater than the level of MKP-2 or MKP-3. However, the intensity of staining was increased for MKP-1, MKP-2, and MKP-3 not only in the V3, but V2 and V1 regions of the ganglia 2 h after injection with NO-protons.
A summary of the changes in MKP levels in the V1 and V2 regions of the trigeminal ganglion are shown in Table 2. The average intensity of the staining in regions of interest containing neurons and glia in V1 and V2 were measured in ganglia from control animals and animals injected with NO-protons. Analysis of the V1 and V2 regions showed p38 intensity to be significantly increased at 15 min, 2 h, and 24 h after NO-proton injection. ERK was significantly increased at 15 min and 2 h, but not at 24 h, while JNK was not significantly increased at 15 min or 24 h after injection, but was slightly elevated at 2 h when compared to the control. Similarly, MKP-1 was significantly increased at only 2 h after NO-proton injection, while both MKP-2 and MKP-3 were significantly increased at all three time points following NO-proton stimulation.
Table 2.
Quantification of neuronal and glial MAP kinase and MAP kinase phosphatase staining intensities in the V1 and V2 regions of the trigeminal ganglion. The values, which are the ratio of the staining intensity of neuron and satellite glial cells to the intensity of Schwann cells in 6 independent fields, are reported as the average staining intensity SEM. Each experimental condition was repeated a minimum of three times.
| V1/V2 Region of Trigeminal Ganglion | ||||||
|---|---|---|---|---|---|---|
| p38 | ERK | JNK | MKP-1 | MKP-2 | MKP-3 | |
| Control | 1.23
0.03 |
1.94
0.09 |
1.27
0.04 |
2.35
0.17 |
1.74
0.09 |
1.62
0.05 |
| NO/proton-15 min | 2.61
0.40* |
3.22
0.19* |
1.34
0.03 |
2.76
0.25 |
2.18
0.8* |
2.67
0.04* |
| NO/proton-2 hrs | 2.47
0.07* |
3.06
0.11* |
1.47
0.06# |
3.25
0.16* |
2.56
0.14* |
2.57
0.12* |
| NO/proton-24 hrs | 1.82
0.09* |
1.62
0.08 |
1.16
0.03 |
2.67
0.24 |
2.14
0.04* |
2.51
0.07* |
=p<0.05 or
=p<0.01 when compared to control values.
DISCUSSION
In this study, a novel in vivo model of acute TMJ inflammation was used to investigate the cell signaling pathways activated in neurons and glia of the trigeminal ganglion in response to NO-protons. The rationale for choosing to study the effects of NO-protons was based on the fact that elevated levels of these molecules are implicated in TMJ inflammation and pain (Takahashi et al., 1999, Arinci et al., 2005). In fact, the most common symptom associated with TMD is pain, which can be spontaneous and is generally aggravated during normal activities such as mastication, biting, yawning, and even speaking (Okeson and de Kanter, 1996). Data from clinical studies has shown that the level of the NO producing enzyme iNOS is greatly increased in synovial fluid obtained from TMD patients (Homma et al., 2001, Takahashi et al., 2003). Importantly, in another study, elevated levels of NO were reported to directly correlate with TMJ pathology (Suenaga et al., 2001). Based on data from these studies, it appears that expression of iNOS and elevated levels of NO represent a pathophysiological feature of the diseased TMJ. In addition, it has been shown that the rat TMJ is richly innervated by trigeminal ganglion nerve fibers that express vanilloid receptor subtypes known to be activated by protons (Ichikawa H et al., 2004). In our study, both TMJs were injected with an NO donor diluted in HBS at pH 5.5. The concentration of NO, as measured as a nitrite metabolite, measured in pH 5.5 medium was ~ 6 μM, which is in the range reported in a study of TMD patients in which most of the patients had a NO level in the TMJ synovial fluid of between 3 and 10 μM (Suenaga et al., 2001). We found that injection of NO-protons into the TMJ capsule caused temporal and spatial changes in the expression of MAPKs and MKPs in both neurons and satellite glial cells in all regions of the trigeminal ganglion. To our knowledge, this is the first study to report on the cellular signaling events mediated by NO-protons in the TMJ capsule that are thought to play an important role in TMJ pathology.
We initially used a fluorescent dye to localize neuronal cell bodies whose afferent fibers project to the TMJ capsule. The majority of labeled cells were located in neuron-satellite glial cell bands in the posterolateral region (V3) of the ventral side of the trigeminal ganglion. This finding is in agreement with previous studies in the rat (Uddman et al., 1998, Liu et al., 2000). We found that the phosphorylated, active forms of p38 and ERK, but not the MAPK JNK, were significantly elevated in the cytosol and nucleus within 15 min of NO-proton injection in trigeminal ganglion neurons. The nuclear levels of P-p38 and P-ERK were still increased at the 2 h time point when compared to control. While cytosolic and nuclear staining for P-p38 remained greater than basal even 24 h after injection, the level of P-ERK staining in the cytosol and nucleus had returned to control levels. In a previous study, we reported increased nuclear localization of P-p38 and P-JNK, but not P-ERK, in cultured trigeminal ganglion neurons in response to 2 h NO stimulation (Bellamy et al., 2006). The difference in MAPK activation are likely due to the loss of the normal cellular arrangements between neuronal and glial cells during the establishment and maintenance of the trigeminal ganglion cultures and the use of NO and protons as a stimulus in this study. While NO can exert direct stimulatory effects on the neurons, the stimulatory effect of protons on trigeminal neurons likely involve activation of the proton-sensitive ion channels TRPV1 or ASIC3, which are known to be expressed by sensory trigeminal ganglion neurons (Ichikawa and Sugimoto, 2002, Bae et al., 2004, Tanimoto et al., 2005, Diogenes et al., 2006, Thalakoti et al., 2007). While only a small percentage of the total number of neurons located in the V3 region provide sensory innervation to the TMJ capsule, injection of NO-protons into the capsule caused spatial and temporal changes in the levels of P-p38 and P-ERK in most neurons located in the V3 region as well as the V1 and V2 regions. This finding is similar to a previous study in which chemical stimulation of afferent fibers in the TMJ capsule caused cellular changes in neurons located in all regions of the ganglion (Thalakoti et al., 2007). In addition, another similarity between the two studies was the fact that stimulation of peripheral afferent fibers located in the TMJ capsule resulted in increased expression of P-p38 and P-ERK in satellite glial cells.
It has been proposed that trigeminal ganglion neurons and the satellite glial cells form a functional unit within the trigeminal ganglion (Hanani, 2005). Satellite glial cells are thought to play an important role in regulating the excitability state of trigeminal neurons (Pannese, 1981, Pannese et al., 2003). For example, it has recently been shown that satellite glial cells mediate enhanced excitability of nociceptive trigeminal ganglion neurons following peripheral inflammation (Takeda et al., 2007). In that study, chemical activation of trigeminal neurons that provide sensory innervation to the whisker pads was found to cause increased expression of interleukin 1-β, which mediates inflammation and hyperalgesia and is known to be MAPK-responsive, in satellite glial cells. Similarly, we have recently shown that stimulation of trigeminal ganglion neurons that provide innervation of the TMJ results in increased neuron-satellite glia communication via gap junctions and possibly paracrine signaling in the trigeminal ganglion (Thalakoti et al., 2007). An interesting finding of this study was that NO-proton stimulation of peripheral trigeminal afferent fibers in the TMJ capsule resulted in an increase in the levels of P-p38 and P-ERK, but not P-JNK, in satellite glial cells associated with neuronal cell bodies located throughout the ganglion. In fact, the temporal and spatial pattern of P-p38 and P-ERK expression were very similar in neurons and satellite glial cells. However, we did not observe any changes in MAP kinase expression in Schwann cells, which are the other major glia cell type found in trigeminal ganglia (Shankland, 2000). Schwann cells are associated with sensory nerve fibers and are responsible for the production of myelin, which increases the speed of nerve conduction. Thus, chemical stimulation of a subpopulation of neurons localized primarily in the V3 region of the ganglion, which provide innervation of the TMJ capsule, caused profound cellular changes in neurons and satellite glial cells throughout the entire ganglion. This type of cross excitation and increased expression of P-p38 and P-ERK is likely to lead to peripheral sensitization, which is characterized by increased sensitivity and excitability of nociceptive neurons and a lower threshold of activation. In this way, activation of only a few neurons leads to a coordinated response throughout the ganglion that involves both neurons and satellite glial cells. In other models of tissue injury and inflammation, sensitizing agents such as NO and protons that are released from inflammatory cells and nerve terminals have been reported to cause activation of multiple protein kinases involved in signal transduction including P-ERK and P-p38 MAPKs (Ji, 2004). While there is little data on peripheral sensitization within the trigeminal ganglion, activation of intracellular signaling pathways involving both P-p38 and P-ERK is thought to participate in synaptic plasticity underlying spinal neuronal sensitization (Garry et al., 2005). Peripheral sensitization involves changes in ion channel function as well as changes in gene expression (Dai et al., 2002). In our study, we observed a significant increase in P-p38 and P-ERK levels within 15 minutes following chemical stimulation. The rapid increase in these MAPKs may be involved in posttranslational events mediated by phosphorylation of ion channels and receptors that are reported to occur within minutes (Ji, 2004). Interestingly, peripheral sensitization in nociceptive terminals is thought to involve a lowering of the threshold of activation of the TRPV1 receptor, which is known to be expressed by trigeminal ganglion neurons (Tanimoto et al., 2005) and is activated in response to protons (Caterina and Julius, 2001). In addition, the increased nuclear expression of P-p38 and PERK in neurons and satellite glial cells are likely to cause increased expression of genes involved in inflammation and pain that is typically not observed until several hours after stimulation. Therefore, it is likely that increased P-p38 and P-ERK activity in neuronal and glial cells in the trigeminal ganglion may contribute to peripheral sensitization of primary sensory neurons. It is interesting to speculate that peripheral sensitization mediated by changes in trigeminal neurons and satellite glia may explain the higher incidence of comorbidity reported for individuals with TMJ disorders and other diseases that involve trigeminal nerve activation such as migraine and sinus pathologies (Cady et al., 2005, Graff-Radford, 2007, Svensson, 2007).
The increased expression of P-p38 and P-ERK in the nucleus of trigeminal neurons is likely to cause increased expression of genes such as CGRP that are known to be MAPK-responsive (Durham and Russo, 2003) and are implicated in the underlying pathology of TMJ disorders (Holmlund et al., 1991, Appelgren et al., 1993). CGRP levels have been reported to be elevated in the synovial fluid of patients suffering from TMJ disorders and correlate with reported pain levels (Appelgren et al., 1993). Similarly, increased expression of P-p38 and P-ERK in satellite glial cells would be expected to lead to induction of pro-inflammatory genes such as cytokines and interleukins that are known to be regulated by these MAPKs and are expressed by satellite glial cells (Kaminska, 2005, Schindler et al., 2007). Importantly, elevated levels of interleukin 1-β, interleukin 6, and TNF-α have been reported in synovial fluid obtained from TMJ patients (Alstergren, 2000, Nishimura et al., 2002). Taken together, these findings support an important role of P-p38 and P-ERK in sustaining the inflammatory response within the TMJ by causing increased expression of genes known to promote inflammation and pain.
Another important finding from our study was that NO-proton stimulation of sensory afferents in the TMJ caused spatial and temporal changes in the levels of MKPs in the neurons and satellite glial cells in all regions of the trigeminal ganglion. To our knowledge, this is the first study that has investigated the expression of MKPs within the trigeminal ganglion under basal and inflammatory conditions. Interestingly, basal levels of MKP-1 were higher in satellite glial cells found throughout the ganglion compared to levels observed in trigeminal neurons. It is possible that this level of MKP-1 is important for regulating the expression and possibly release of cytokines or interleukins from satellite glial cells. Towards this end, MKP-1 is thought to determine the output of cytokines involved in an inflammatory response by limiting the strength and duration of p38 and JNK activation (Wang and Liu, 2007). While only MKP-1 levels were elevated in satellite glial cells under basal conditions, the expression of MKP-1, MKP-2, and MKP-3 were all significantly elevated in both neurons and glial cells in all regions of the ganglion 2 h after peripheral stimulation. However, only the levels of MKP-2 and MKP-3 remained significantly elevated 24 h after injection of inflammatory stimuli. While it is likely that induction of these three MKPs play a role in modulating the levels of p38 and ERK in neuronal and satellite glial cells, it is not possible to know for certain which MKP is responsible for decreasing P-p38 or P-ERK levels in our model. Although we have shown that MKP-1, MKP-2, and MKP-3 are increased in response to NO-proton stimulation, it is possible that other members of the MKP family may also be involved in regulating p38 and ERK MAPKs and hence activation and excitation of trigeminal neuronal and satellite glial cells. Although not tested in this study, induction of MKPs has been reported to be caused to occur in response to MAPK activation but also independently of MAP kinase activation (Owens and Keyse, 2007). Based on our results, it appears that NO-proton stimulation of trigeminal neurons leads to an initial increase in the levels of P-p38 and P-ERK in both neurons and satellite glial cells and induction of several MKPs. The increased levels of these MKPs, and possibly others, would likely be involved in restoring P-p38 and P-ERK to basal levels and be expected to abolish peripheral sensitization in the trigeminal ganglion nerves, inhibit inflammation in the TMJ, and prevent transmission of painful stimuli.
In summary, we have demonstrated that stimulation of neurons in the trigeminal ganglion following injection of NO-protons into the TMJ capsule resulted in temporal changes in the levels of P-p38 and P-ERK in neurons and satellite glia as well as increased MKP-1, MKP-2, and MKP-3 levels in both cell types. These findings demonstrate that injection of inflammatory stimuli, whose levels are known to be elevated in the synovial fluid obtained from TMD patients, can cause activation of cells in the V3 region of the trigeminal ganglion. In addition, changes were also seen in V1 and V2 neurons and glial cells, whose activation are implicated in migraine and allergic rhinitis, a finding that may help explain the significant comorbidity associated with TMJ disorders and these other diseases.
Acknowledgments
We would like to thank Srikanth Thalakoti and Carrie Vause for their technical assistance. Support for these studies was provided by NIH grants R01 DE015385 and RO117805 and a grant from the National Headache Foundation.
Abbreviations
- CGRP
calcitonin gene-related peptide
- DMSO
dimethyl sulfoxide
- ERK
extracellular signal-regulated kinases
- HBS
HEPES-buffered saline
- iNOS
inducible nitric oxide synthase
- JNK
Jun amino-terminal kinases
- MAPK
mitogen-activated protein kinases
- MKP
mitogen-activated protein kinase phosphatases
- PBS
phosphate-buffered saline
- SNP
sodium nitroprusside
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alstergren P. Cytokines in temporomandibular joint arthritis. Oral Dis. 2000;6:331–334. doi: 10.1111/j.1601-0825.2000.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Relation between intra-articular temperature of the arthritic temporomandibular joint and presence of calcitonin gene-related peptide in the joint fluid. A clinical study. Acta Odontol Scand. 1993;51:285–291. doi: 10.3109/00016359309040579. [DOI] [PubMed] [Google Scholar]
- Arinci A, Ademoglu E, Aslan A, Mutlu-Turkoglu U, Karabulut A, Karan A. Molecular correlates of temporomandibular joint disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:666–670. doi: 10.1016/j.tripleo.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Bae Y, Oh J, Hwang S, Shigenage Y, Valtschanoff J. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol. 2004;478:62–71. doi: 10.1002/cne.20272. [DOI] [PubMed] [Google Scholar]
- Bellamy J, Bowen E, Russo A, Durham P. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci. 2006;23:2057–2066. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady R, Dodick D, Levine H, Schreiber P, Eross E, Setzen M, Blumenthal H, Lumry W, Berman G, Durham P. Sinus headache: A neurology, otolaryngology, allergy, and primary care consensus on diagnosis and treatment. Mayo Clin Proc. 2005;80:908–916. doi: 10.4065/80.7.908. [DOI] [PubMed] [Google Scholar]
- Caterina M, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Christensen B, Kochukov M, McNearney T, Taglialatela G, Westlund K. Proton-sensing G protein-coupled receptor mobilizes calcium in human synovial cells. Am J Physiol Cell Physiol. 2005;289:C601–C608. doi: 10.1152/ajpcell.00039.2005. [DOI] [PubMed] [Google Scholar]
- Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci. 2002;22:7737–7745. doi: 10.1523/JNEUROSCI.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson R, Keyse S. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Science. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Patwardhan A, Jeske N, Ruparel N, Goffin V, Akopian A, Hargreaves K. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. The Journal of Neuroscience. 2006;26:8126–8136. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham P, Russo A. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J Neurosci. 2003;23:807–815. doi: 10.1523/JNEUROSCI.23-03-00807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq A, Zhou M. Structure and regulation of MAPK phosphatases. Cell Signaling. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Garry E, Delaney A, Blackburn-Munro G, Dickinson T, Moss A, Nakalenbe I, Robertson D, Rosie R, Robberecht P, Mitchell R, Fleetwood-Walker S. Activation of p38 and p42/44 MAP kinase in neuropathic pain: Involvement of VPAC and NK receptors and mediation by spinal glia. Molecular and Cellular Neuroscience. 2005;30:523–537. doi: 10.1016/j.mcn.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Graff-Radford S. Temporomandibular disorders and headache. Dent Clin North Am. 2007;51:129–144. doi: 10.1016/j.cden.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Guzik T, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Haydon P. Glia: Listening and talking to the synapse. Nature. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Herb K, Cho S, Stiles M. Temporomandibular joint pain and dysfunctioning. Curr Pain and Headache Reports. 2006;10:408–414. doi: 10.1007/s11916-006-0070-7. [DOI] [PubMed] [Google Scholar]
- Holmlund A, Ekblom A, Hansson P, Lind J, Lundenberg T, Theodorsson E. Concentration of neuropeptide substance – P, Neurokinin A, calcitonin gene related peptide, neuropeptide Y and vasoactive intestinal polypeptide in the synovial fluid of the–human temporomandibular joint. Int J Oral Maxillofac Surg. 1991;20:228–231. doi: 10.1016/s0901-5027(05)80181-x. [DOI] [PubMed] [Google Scholar]
- Homma H, Takahashi T, Seki H, Ohtani M, Kondoh T, Fukuda M. Immunohistochemical localization of inducible nitric oxide synthase in synovial tissue of human temporomandibular joints with internal derangement. Arch Oral Biol. 2001;46:93–97. doi: 10.1016/s0003-9969(00)00086-8. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Fukunaga T, Jin H, Fujita M, Takano-Yamamoto T, Sugimoto T. VR1-, VRL-1- and P2X3 receptor-immunoreactive innervation of the rat temporomandibular joint. Brain Res. 2004;1008:131–136. doi: 10.1016/j.brainres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. The co-expression of ASIC3 with calcitonin gene-related peptide and parvalbumin in the rat trigeminal ganglion. Brain Res. 2002;943:287–291. doi: 10.1016/s0006-8993(02)02831-7. [DOI] [PubMed] [Google Scholar]
- Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy. 2004;3:299–303. doi: 10.2174/1568010043343804. [DOI] [PubMed] [Google Scholar]
- Kaminska B. MAPK signaling pathways as molecular targets for anti inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochem Biophsy Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Keyse S. Protein phosphatases and the regulation of MAP kinase activity. Cell & Dev Bio. 1998;9:143–152. doi: 10.1006/scdb.1997.0219. [DOI] [PubMed] [Google Scholar]
- Lazarov N. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002;66:19–59. doi: 10.1016/s0301-0082(01)00021-1. [DOI] [PubMed] [Google Scholar]
- LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- Lewis T, Shapiro P, Ahn N. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Liu B, Gao H, Wang J, Jeohn G, Cooper C, Hong J. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Si F, Zhou S. Distribution of calcitonin gene-related peptide-like immunoreactive fibres in the temporomandibular joint of rat. Zhonghua Kou Qiang Yi Xue Za Zhi. 2000;35:41–43. [PubMed] [Google Scholar]
- Nagai H, Kumamoto H, Fukuda M, Takahashi T. Inducible nitric oxide synthase and apoptosis-related factors in the synovial tissues of temporomandibular joints with internal derangement and osteoarthritis. J Oral Maxillofac Surg. 2003;61:801–807. doi: 10.1016/s0278-2391(03)00155-1. [DOI] [PubMed] [Google Scholar]
- Naik A, Tandan S, Kumar D, Dudhgaonkar S. Nitric oxide and its modulators in chronic constriction injury-induced neuropathic pain in rats. Eur J Pharmacol. 2006;530:59–69. doi: 10.1016/j.ejphar.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Segami N, Keneyama K, Suzuki T, Miyamaru M. Proinflammatory cytokines and arthroscopic findings of patients with internal derangement and osteoarthritis of temporomandibular joint. Br J Oral Maxillofac Surg. 2002;40:68–71. doi: 10.1054/bjom.2001.0742. [DOI] [PubMed] [Google Scholar]
- Okeson J, de Kanter R. Temporomandibular disorders in the medical practice. J Fam Pract. 1996;43:347–356. [PubMed] [Google Scholar]
- Owens D, Keyse S. Differential regulation of MAP kinase signaling by dual specificity protein phophatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- Pannese E. The satellite cells of the sensory ganglia. Adv Anat Embryol Cell Biol. 1981;65:1–111. doi: 10.1007/978-3-642-67750-2. [DOI] [PubMed] [Google Scholar]
- Pannese E, Ledda M, Cherkas P, Huang T, Hanani M. Satellite cell reactions to axon injury of sensory ganglion neurons: increase in number of gap junctions and formation of bridges connecting previously separate perineuronal sheaths. Anat Embryol. 2003;206:337–347. doi: 10.1007/s00429-002-0301-6. [DOI] [PubMed] [Google Scholar]
- Schaeffer H, Weber M. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler J, Monahan J, Smith W. p38 pathway kinases as anti-inflammatory drug targets. J Dent Res. 2007;86:800–811. doi: 10.1177/154405910708600902. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs E. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Shankland W. The trigeminal nerve. Part I: An over-view. Cranio. 2000;18:238–248. doi: 10.1080/08869634.2000.11746137. [DOI] [PubMed] [Google Scholar]
- Suenaga S, Abeyama K, Hamasaki A, Mimura T, Noikura T. Temporomandibular disorders: relationship between joint pain and effusion and nitric oxide concentration in the joint fluid. Dentomaxillofac Radiol. 2001;30:214–218. doi: 10.1038/sj.dmfr.4600610. [DOI] [PubMed] [Google Scholar]
- Svensson P. Muscle pain in the head: overlap between temporomandibular disorders and tension-type headaches. Curr Opin Neuro. 2007;20:320–325. doi: 10.1097/WCO.0b013e328136c1f9. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Homma H, Nagai H, Seki H, Kondoh T, Yamazaki Y, Fukuda M. Specific expression of inducible nitric oxide synthase in the synovium of the diseased temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:174–181. doi: 10.1067/moe.2003.45. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kondoh T, Ohtani M, Homma H, Fukuda M. Association between arthroscopic diagnosis of temporomandibular joint osteoarthritis and synovial fluid nitric oxide levels. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:129–136. doi: 10.1016/s1079-2104(99)70105-8. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, Matsumoto S. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain. 2007;129:155–166. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Tanimoto T, Takeda M, Nasu M, Kadoi J, Matsumoto S. Immunohistochemical co-expression of carbonic anhydrase II with Kv1.4 and TRPV1 in rat small-diameter trigeminal ganglion neurons. Brain Res. 2005;1044:262–265. doi: 10.1016/j.brainres.2005.02.082. [DOI] [PubMed] [Google Scholar]
- Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-Glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biol. 2002;3(7):1–10. doi: 10.1186/gb-2002-3-7-reviews3009. REVIEWS3009: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski A, Vaque J, Gutkind J. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- Uddman R, Grunditz T, Kato J, Sundler F. Distribution and origin of nerve fibers in the rat temporomandibular joint capsule. Anat Embryol. 1998;197:273–282. doi: 10.1007/s004290050137. [DOI] [PubMed] [Google Scholar]
- Vause C, Bowen E, Spierings E, Durham P. Effect of carbon dioxide on calcitonin gene-related peptide secretion from trigeminal neurons. Headache. doi: 10.1111/j.1526-4610.2007.00850.x.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu Y. Regulation of innate immune response by MAP kinase phosphatase-1. Cellular Signalling. 2007;19:1372–1382. doi: 10.1016/j.cellsig.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H-Y, Dawson V, Dawson T. Neurobiology of nitric oxide. Crit Rev Neurobiol. 1996;10:291–316. doi: 10.1615/critrevneurobiol.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]