Abstract
Arginine-grafted bioreducible poly(disulfide amine) (ABP) polymer was synthesized for non-viral gene delivery systems. Its Mw was measured to be 4.45 ×103 Da/mole by FPLC-SEC and its PDI value was 1.49. ABP was able to retard pDNA from a weight ratio of 2 but ABP could not retard pDNA even at a weight ratio of 10 in the presence of DTT, showing that it can be biodegraded in reducing environment such as cytoplasm. ABP was examined to form positively charged nano-sized particles (< 200 nm) with pDNA. ABP showed no significant cytotoxicity and greatly enhanced transfection efficiency in comparison with unmodified poly(cystaminebisacrylamide-diaminohexane) (poly(CBA-DAH)) and PEI25k in mammalian cells. The transfection efficiency of ABP was not much reduced even in the serum condition. Chloroquine treatment was not found to improve the transfection efficiency of ABP. The cellular uptake pattern of ABP polyplexes was almost similar with poly(CBA-DAH), suggesting that greatly enhanced transfection efficiency of ABP is not induced by its high cellular penetrating ability but may be mediated by other factors such as good nuclear localization ability.
Keywords: Gene delivery, bioreducible polymer, arginine-graft, transfection, cytotoxicity
1. Introduction
Development of non-toxic and efficient gene delivery carriers is one of the most important requirements for gene therapy. So far, numbers of non-viral gene delivery carriers based on lipids and polymers have been developed as alternatives of viral gene delivery carriers. They have advantages such as non-immunogenicity, convenience of handling, and unlimited delivery capacity of genetic materials over viral vectors [1-3]. Among them, polymeric gene delivery carriers have multi-functional groups modifiable with bio-functional moieties in their backbones [4].
Despite the advantages, their applications to human gene therapy have been limited because of their cytotoxicity and unsatisfactory transfection efficiency [5]. As a part of the efforts to develop non-toxic and transfection-efficient polymeric gene delivery carriers, various bioreducible polymers possessing internal disulfide bonds have been developed for gene delivery systems [6-11]. Disulfide bonds are known to be cleaved by intracellular reducing agents such as glutathione. Their unique property was reported to reduce the cytotoxicity of polymers due to their biodegradation into small molecules, and to bring about the reducing environment-triggered release of genetic materials from polyplexes, eventually leading to efficient transfection. Recently, bioreducible poly(amido ethylenimine)s, bioreducible poly(amido amine)s, and biodegradable poly(disulfide amine)s have been developed and they showed negligible cytotoxicity and higher transfection efficiency than bPEI25k [12-14]. One of their advantages is convenient to synthesize poly(amido amine)s via simple Michael reaction of commercial monomers in mild condition [15,16]. Additionally, several works to find more efficient and optimized bioreducible polymeric gene delivery carriers have been performed by Engbersen and his co-workers, tuning their monomer structures [17,18].
Meanwhile, cell-penetrating peptide (CPP)s containing cationic arginines and lysines, were known to deliver molecules efficienctly [19,20]. Arginine-rich peptides were also reported to possess great cell-penetrating ability [21]. Cholesteryl oligo-D-arginine (Chol-R9) was presented to reduce tumor growth efficiently by delivering VEGF siRNA in our previous work [22]. These arginine residues were finally applied to polymeric gene delivery carriers. Arginine residues have been conjugated to poly(amido amine) (PAMAM) dendrimer [23], poly(propylene imine) (PPI) dendrimers [24], and poly(l-lysine) (PLL) dendrimer [25] and they showed greatly enhanced transfection efficiency than unmodified dendrimers. For the case of linear polymer, arginine-modified chitosan [26] and arginine-based poly(ester-amide)s [27] also have been reported to display high transfection efficiency, meaning that these conjugated arginines still have an excellent gene delivery potency even though they don’t exist as oligo-peptide forms.
Therefore, combining the unique properties of bioreducible polymers with the advantages of arginine residues, we expect that this arginine-grafted bioreducible poly(disulfide amine) (ABP) would show high transfection efficiency, maintaining its low cytotoxicity.
Here, we report the synthesis of ABP and its characterization for gene delivery systems.
2. Materials and methods
2.1. Materials
Hyperbranched poly(ethylenimine) (bPEI, 25 kDa), tert-Butyl-N-(6-aminohexyl)carbamate (N-Boc-1,6-diaminohexane, N-Boc-DAH), trifluoroacetic acid (TFA), triisopropylsilane, triisobutylsilane, dithiothreitol (DTT), N,N-diisopropylethylamine (DIPEA), piperidine, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), chloroquine diphosphate salt, and trypan blue solution (0.4 %) were purchased from Sigma-Aldrich (St. Louis, MO). N,N’-cystaminebisacrylamide (CBA) was purchased from PolySciences, Inc. (Warrington, PA). 2-(1H-benzotriazole-1-yl)-1, 1, 3, 3-tetramethyluronium hexafluorophosphate (HBTU) was purchased from Novabiochem (San Diego, CA). Fmoc-L-Arg(pbf)-OH was purchased from Anaspec, Inc. (San Jose, CA). The plasmid pCMV-Luc, containing a firefly luciferase reporter gene was amplified in E.coli DH5α and isolated by standard Maxiprep kit (Invitrogen, Carlsbad, CA). Luciferase assay system and reporter lysis buffer were purchased from Promega (Madison, WI). Fetal bovine serum (FBS), Dulbecco’s phosphate buffered saline (DPBS), and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Invitrogen (Carlsbad, Ca). BCA™ protein assay kit was purchased from PIERCE (Rocford, Il). YOYO-1 iodide (1 mM solution in DMSO) was purchased from Molecular Probes (Eugene, OR). All other chemicals were purchased and used without any further purification.
2.2. Synthesis of ABP
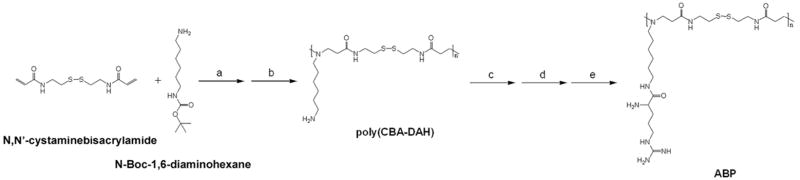
The backbone poly(disulfide amine) polymer, poly(CBA-DAH) was synthesized by the slightly modified method with our previous work [14]. Briefly, poly(CBA-DAH) was synthesized by Michael reaction of equivalent moles of N-Boc-DAH and CBA in MeOH/H2O solution (9:1, v/v). Polymerization reaction was maintained in the dark under nitrogen atmosphere at 60 °C for 5 days. Then, 10 % mole of N-Boc-DAH was added to the reaction mixture to consume unreacted acrylamide functional groups and the reaction was further conducted for 2 days. After precipitation with diethyl ether, the Boc groups of product was removed by the reagent solution (TFA : triisobutylsilane : H2O = 95 : 2.5 : 2.5, v/v) at ice bath for 30 min. After additional precipitation with diethyl ether, the polymer product was dialyzed against ultra-pure water with dialysis membrane (MWCO = 1000, Spectrum Laboratories, Inc., Rancho Dominguez, CA), followed by lyophilization to leave poly(CBA-DAH) as sticky solid.
Then, arginine-graft onto poly(CBA-DAH) was performed by using 4 equiv. of Fmoc-Arg(pbf)-OH and HBTU, and 8 equiv. of DIPEA in DMF at room temperature for 2 days. Then, the reaction product was precipitated with an excess of diethyl ether twice in order to remove unreacted reagents and mixed with an equal volume of piperidine (30% in DMF) at room temperature for 30 min to remove the Fmoc groups of grafted Fmoc-Arg(pbf)-OH. After additional 2 times of precipitation with ether, the reagent solution (TFA : triisopropylsilane : H2O = 95 : 2.5 : 2.5, v/v) was added to the precipitates in order to deprotect the pbf groups of arginine residues at room temperature for 30 min. After another precipitation with ether, the final product, arginine-grafted bioreducible poly(disulfide amine) (ABP) was dialyzed against ultra-pure water overnight and lyophilized before use for analysis and assay. The scheme 1 shows the synthetic scheme of ABP. The synthesis of ABP was confirmed by 1H NMR (400 MHz, D2O).
Scheme 1.
The synthetic scheme of ABP. a) MeOH:H2O = 9:1, v/v, 60 °C, 5 days, b) TFA : triisobutylsilane : H2O = 95 : 2.5 : 2.5, v/v, 0 °C, 30 min, c) Fmoc-Arg(pbf)-OH, HBTU, DIPEA/DMF, r.t., 2 days, d) 15 % piperidine/DMF, r.t., 30 min, e) TFA : triisopropylsilane : H2O = 95 : 2.5 : 2.5, v/v, r.t., 30 min.
2.3. Characterization of ABP
The molecular weight of ABP was estimated by size-exclusion chromatography (SEC) (Superdex 75 column, calibrated with standard poly[N-(2-hydroxypropyl)methacrylamide] (pHPMA)) using AKTA FPLC system. ABP was dissolved at a concentration of 3 mg/mL. 0.1 M acetate buffer (30% Acetonitrile, v/v, pH 5.5) was used as eluent. Flow rate was 0.4 mL/min.
2.4. Agarose gel electrophoresis
pDNA condensing ability of ABP was examined by agarose gel electrophoresis. ABP polyplexes were prepared in Hepes buffered saline (10 mM Hepes, 1 mM NaCl, pH 7.4) at various weight ratios ranging from 0.5 to 20. Agarose gel (0.7 %, w/v) containing ethidium bromide solution (0.5 μg/mL) was prepared in TAE (Tris-Acetate-EDTA) buffer. After 30 min of incubation at room temperature, the samples were electrophoresed at 100 V for 30 min. The identical ABP polyplexes were incubated in the presence of 2.5 mM DTT for 1 h at room temperature and electrophoresed. In this case, poly(CBA-DAH) polyplexes were used as controls. The location of pDNA bands was visualized by UV illuminator (Gel Documentation Systems, Bio-Rad, Hercules, CA).
2.5. Polyplex size measurements
The average sizes of ABP and poly(CBA-DAH) polyplexes were examined by using Zetasizer 3000 (Malvern Instruments, USA) with a He–Ne Laser beam (633 nm, fixed scattering angle of 90°) at 25 °C. 0.5 mL of polyplex solutions (10 μg of pDNA) were prepared in Hepes buffered saline (10 mM Hepes, 1 mM NaCl, pH 7.4) at various weight ratios ranging from 0.5 to 20. After 30 min incubation, polyplex solutions were diluted to final volume of 4 mL before measurement. Measured sizes were presented as the average values of 5 runs.
2.6. Zeta-potential measurements
Zeta-potential measurements of polyplexes were also carried out using a Zetasizer 3000 at 25 °C. ABP and poly(CBA-DAH) polyplexes were prepared by the same method with above size measurement experiments. Potential values were presented as the average values of 3 runs.
2.7. Cytotoxicity
The cytotoxicity of the polymers was measured by MTT assay. C2C12 mouse myoblast cells were seeded in a 96-well tissue culture plate at 1 × 104 cells per well in 90 μL DMEM medium containing 10% FBS. Cells achieving 70~80 % confluence after 24 h were exposed to 10 μL of the polyplex solutions (0.5 μg pDNA) at different weight ratios for 4 h in serum-free DMEM medium. After exchange of medium for fresh DMEM medium containing 10% FBS, the cells were further maintained for 48 h. Then, 25 μL of stock solution of MTT (2 mg/ml in PBS) were added to each well. After 2 h of incubation at 37 °C, each medium was removed carefully and 150 μL of DMSO was added to each well to dissolve the formazan crystal formed by proliferating cells. Absorbance was measured at 570 nm using a microplate reader (Model 680, Bio-Rad Lab, Hercules, CA) and recorded as a percentage relative to untreated control cells. All experiments were performed in quadruplicate.
2.8. Transfection experiments in vitro
C2C12 mouse myoblast cells and NIH3T3 mouse embryonic fibroblasts cells were seeded at a density of 5 × 104 cells/well in a 24-well plate in DMEM medium containing 10% FBS and grown to reach 70~80% confluence prior to transfection. Before transfection, medium of each well was exchanged for fresh serum-free. For transfection in serum condition, the medium was not exchanged at this time. The cells were treated with polyplex solution (0.5 μg pDNA) at different weight ratios for 4 h at 37 °C. After exchange with fresh medium containing 10 % FBS, cells were further incubated for 2 days before assay. Then, the growth medium was removed and the cells were rinsed with DPBS and shaken for 30 min at room temperature in 120 μL of Reporter Lysis Buffer. Luciferase activity was measured by a luminescence assay and a protein quantification assay was performed using a BCA™ Protein Assay Reagent Kit. The luciferase activity of 25 μL cell lysate was measured by using 100 μL of luciferase assay reagent on a luminometer (Dynex Technologies Inc., Chantilly, CA). The final results were reported in terms of RLU/mg cellular protein. All experiments were performed in triplicate.
2.9. Transfection mechanism study
2.9.1. Transfection by using chloroquine
Transfection experiments were performed with or without chloroquine in order to examine the cellular uptake mechanism of ABP polyplex. C2C12 cells were seeded at a density of 5 × 104 cells/well in a 24-well plate in DMEM medium containing 10% FBS and grown to reach 70~80% confluence prior to transfection. Each polyplex was prepared with 0.5 μg of pDNA at a fixed weight ratio (PEI : 1, ABP : 40). Then, 100 μM chloroquine was treated to the cells before transfection and the cells were incubated in the presence or absence of chloroquine during transfection. After 4 h of incubation, all the cells were washed with DPBS and maintained in DMEM containing 10 % FBS at 37 °C for 2 days. Further experiments and assays were performed by the same transfection method mentioned above in the absence of serum. All experiments were performed in triplicate.
2.9.2. Cellular uptake assay
C2C12 cells were seeded at a density of 2 × 105 cells/well in a 6-well plate in DMEM medium containing 10 % FBS and grown to reach 70~80% confluence prior to transfection. Before transfection, medium of each well was exchanged for fresh serum-free medium. pDNA was labeled with YOYO-1 iodide (1 molecule of the dye per 100 base pairs of the nucleotide) [28]. The cells were treated with polyplex solutions (1 μg of pDNA) at different weight ratios for 4 h at 37 °C. Then, medium was aspirated off from the wells. The cells were washed two times with ice-cold DPBS. To quench the fluorescence of polyplexes adsorbed on the cell surface, the cells were incubated with 0.4 % trypan blue solution for 5 min and washed with DPBS again [29]. After trypsinization, the cells were collected by centrifuge at 1500 rpm and suspended in 1 mL DPBS.
The cellular uptake of fluorescence-labeled polyplexes were examined by using the BD FACScan analyzer (Becton Dickinson, San Jose, CA) at a minimum of 1 × 104 cells gated per sample. Analysis was performed by using Becton Dickinson CellQuest software.
3. Results and discussion
3.1. Synthesis and characterization of ABP
Arginine-grafted bioreducible poly(disulfide amine) (ABP) was synthesized by using poly(CBA-DAH) as a backbone polymer. poly(CBA-DAH) was selected as bioreducible backbone polymer because it has modifiable amine groups and showed high transfection efficiency in various cell lines [14]. Briefly, poly(CBA-DAH) was synthesized by using N-Boc-DAH and CBA as repeated monomers. Then, 4 equiv. of Fmoc-Arg(pbf)-OH and HBTU, and 8 equiv. of DIPEA were used for arginine-graft reaction of poly(CBA-DAH) in DMF. Then, protecting groups of arginines were removed. The synthesis of ABP was confirmed by 1H NMR as follows.
ABP; 1H NMR(D2O): δ (NCH2CH2CH2CH2CH2CH2NHCO) = 1.24, δ (NCH2CH2CH2CH2CH2CH2NHCO, arginine(NH2CHCH2CH2CH2NH)) = 1.41~1.53, δ arginine(NH2CHCH2CH2CH2NH) = 1.72, δ (NCH2CH2CONH) = 2.59, δ (CH2SSCH2) = 2.74, δ (NCH2CH2CH2CH2CH2CH2NHCO) = 2.88, δ (NCH2CH2CONH, NCH2CH2CH2CH2CH2CH2NHCO, arginine(NH2CHCH2CH2CH2NH)) = 3.10~3.18, δ (NCH2CH2CONHCH2) = 3.41, δ arginine(NH2CHCH2CH2CH2NH) = 3.71
It was found from the calculation of ratio between the integration of arginine proton peaks and the integration of poly(CBA-DAH) proton peaks that almost 100 % of primary amines of poly(CBA-DAH) were modified with arginine residues.
The molecular weight of ABP was also measured by FPLC-SEC system. The Mw of ABP was estimated to be 4.45 ×103 Da/mole and its PDI value was 1.49. Mw of backbone polymer, poly(CBA-DAH) was reported to be 3.52 kDa/mole in the previous study [14]. With assumption that 100 % primary amines of ABP were modified with arginines, experimental Mw value of ABP was revealed to be lower than theoretical value. In general, positively charged cationic polymers are known to have tendency to appear smaller than they really are in SEC because they may interact with column and consequently they are eluted slowly. Therefore, this result may be due to the strong positive charges of ABP. Higher PDI value of ABP than that of poly(CBA-DAH) (1.13) is also thought to be induced by this interaction.
3.2. Agarose gel electrophoresis
Agarose gel electrophoresis was performed in order to examine pDNA condensing ability of ABP in the absence or presence of DTT. ABP has internal disulfide bonds and it is expected to be degraded in reducing environment such as cytoplasm, which contains 0.1~10 mM glutathione. DTT is a well-known reducing agent. We incubated ABP polyplex in 2.5 mM DTT for 1 h in order to investigate the susceptibility to bioreducing intracellular environment of ABP polyplex. As shown in Figure 1a, ABP can retard pDNA completely from a weight ratio of 2 in the absence of DTT. This result shows that ABP can condense pDNA well via electrostatic interaction between positive charges of ABP and negative charges of pDNA phosphates.
Figure 1.
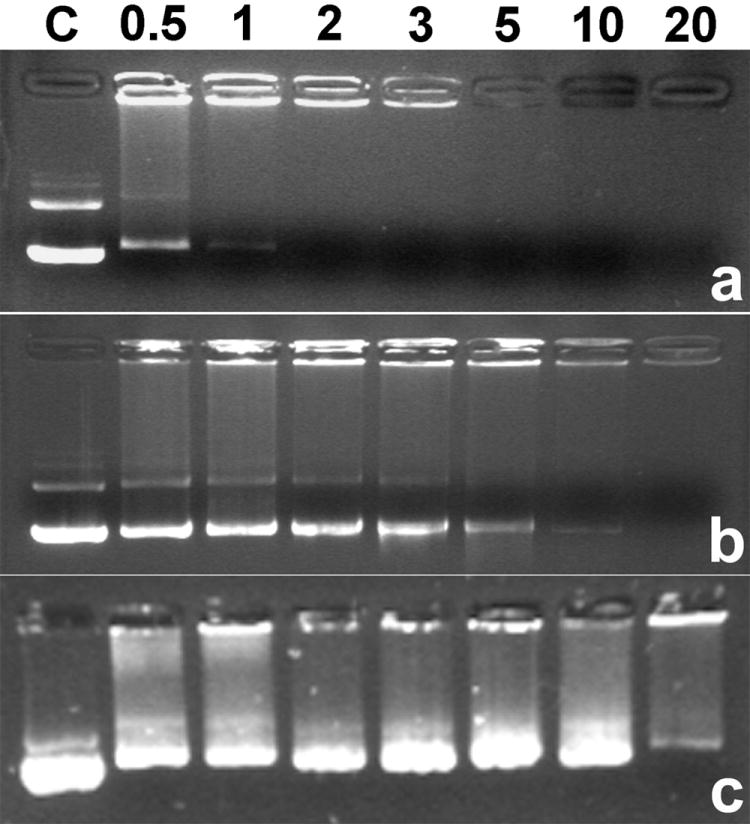
Agarose gel electrophoresis results of ABP polyplex in the absence (a) and in the presence of 2.5 mM DTT (b). (c) shows poly(CBA-DAH) polyplex result in the presence of 2.5 mM DTT. C : pDNA only. Numbers mean weight ratios.
On the other hand, pDNA released from ABP polyplex was observed even at a weight ratio of 10 in the presence of 2.5 mM DTT (Figure 1b). It means that ABP is degraded in reducing environment and that it can’t condense pDNA anymore because of bioreducible cleavage of internal disulfide bonds in the condition. Consequently, it is expected that ABP polyplex can release pDNA by bio-reduction in cytoplasm, which may lead to efficient transfection.
Interestingly, ABP was still able to retard pDNA at a weight ratio of 20 even in the presence of DTT. In contrast with ABP, it was observed that poly(CBA-DAH) could not condense pDNA at the same weight ratio in the presence of DTT (Figure 1c). More molar amount of poly(CBA-DAH) was used for preparing each polyplex than that of ABP at the same weight ratio, because molecular weight of poly(CBA-DAH) is smaller than that of ABP. From this result, it is thought that ABP can form relatively stable polyplexes in comparison with poly(CBA-DAH), which maintain their structures against reduction in that condition. Considering the differences between ABP and poly(CBA-DAH), it may be grafted arginine residues that help ABP form stable polyplexes with pDNA due to their strong positive charges. In general, linear biodegradable polymers are supposed to be degraded into two molecules via just one cleavage of a backbone bond. Their fast degradation rate is believed to bring the possibility of insufficient gene delivery to cell nucleus. Therefore, some cross-linked biodegradable polymers have been developed in order to overcome the structural handicap of linear polymers for efficient gene delivery systems because one cleavage of a backbone bond doesn’t lead to the break of whole polymer structure in cross-linked polymers [30,31]. Considering this fact, this less susceptibility to bio-reduction of ABP may contribute to its higher transfection efficiency than poly(CBA-DAH) although ABP is a linear polymer.
3.3 Average sizes and Zeta-potentials measurements
The average sizes and Zeta-potentials of ABP polyplexes were measured by Zetasizer because polyplexes need to have its proper size for efficient uptake into cells and positive charges of polyplex is thought to be helpful for its absorption to negatively charged cellular membrane, also leading to efficient intracellular trafficking [32]. Backbone polymer, poly(CBA-DAH) was used as a control. ABP polyplexes displayed average sizes less than 200 nm at all weight ratios used (Figure 2). Below a weight ratio of 5, some differences were found in average sizes of both polymers but it seemed that both polyplexes have no significant differences over a weight ratio of 5. This result shows that ABP can condense pDNA into nano-sized particles appropriate for gene delivery.
Figure 2.
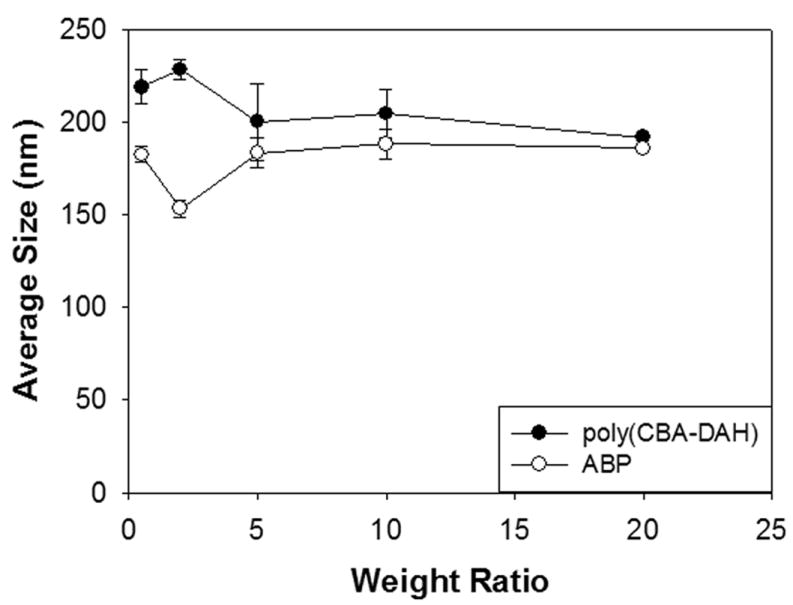
Average sizes measurement of polyplexes.
In the case of Zeta-potential values (Figure 3), ABP polyplex displayed negative value (-15 mV) at a weight ratio of 1, suggesting that stable polyplex is not formed yet at that ratio. It is consistent with the size measurement results. Then, Zeta-potential values of ABP polyplex increased gradually according to the increase of polyplex weight ratios, finally reaching the positive plateau values (30~40 mV) from a weight ratio of 10. Zeta-potential values of ABP and poly(CBA-DAH) polyplex showed no significant differences between them. These results display that ABP forms positively charged polyplexes which can mediated efficient transfection and that arginine-graft onto this poly(CBA-DAH) doesn’t affect on the size or Zeta-potential values of polyplexes.
Figure 3.
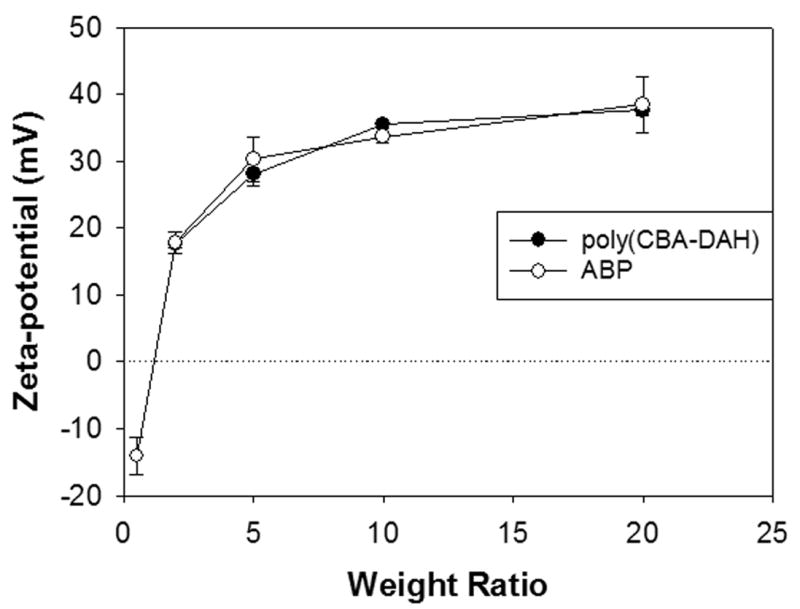
Zeta-potential values measurement of polyplexes.
3.4. Cytotoxicity
MTT assay was performed in order to examine the cytotoxicity of polplexes. In Figure 4, ABP polyplexes were observed to exhibit no considerable cytotoxicity (RCV > 90 %) even at a weight ratio of 80 in C2C12 cells like poly(CBA-DAH). It may be because that ABP can be degraded into nontoxic small molecules in cells. The cytotoxicity of PEI25k was found to be very high as well known. Many works have been reported that biodegradability of polymeric gene carriers can reduce their cytotoxicity [33-35], but hydrolysable polymers showed low water-stability, suggesting that their management will be hard [36]. Unlike them, ABP has high water-stability because it is composed of amide and disulfide bonds which are not susceptible to nuclear attack of water molecules.
Figure 4.
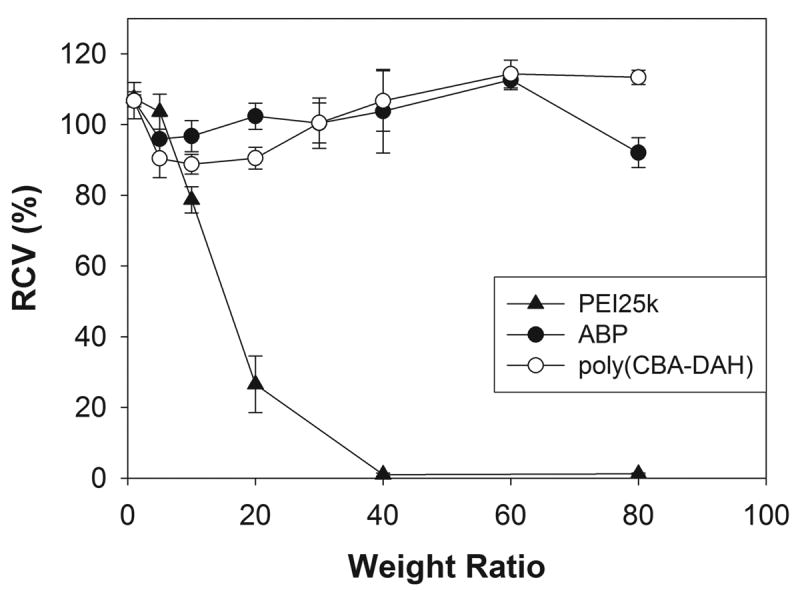
The cytotoxicity assay result in C2C12 cells. RCV means relative cell viability.
This result suggests that ABP does not have cytotoxicity and it can be used for gene delivery system without consideration of cytotoxicity.
3.5. Transfection experiments in vitro
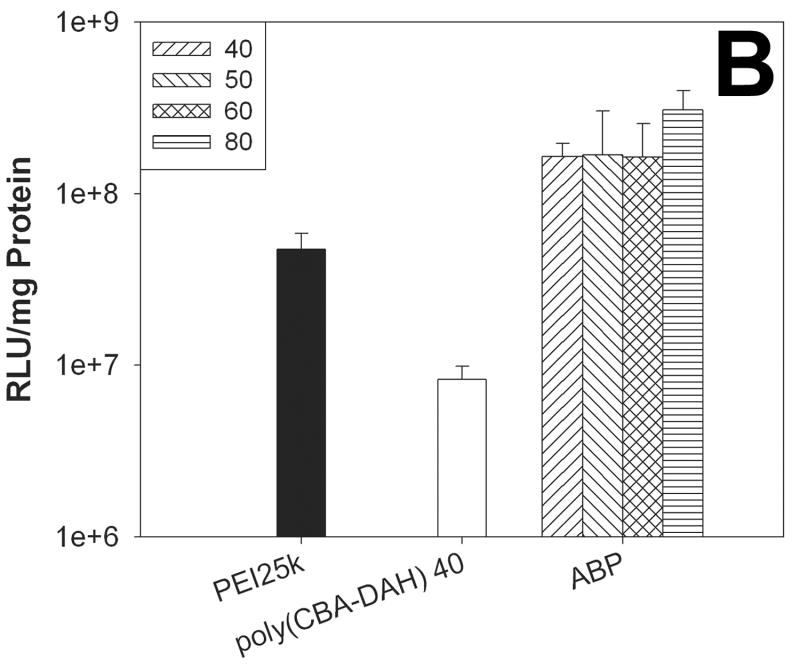
The transfection experiments were performed by using luciferase reporter gene in C2C12 and NIH3T3 cells. PEI25k and poly(CBA-DAH) were used as controls. Polyplexes of PEI25k and poly(CBA-DAH) were prepared at weight ratios of 1 and 40, respectively.
Figure 5A shows the result in C2C12 cells in the absence of serum. The transfection efficiency of ABP at a weight ratio of 50 was found to about 30 times higher than that of PEI25k. ABP displayed about 9 times and 14 times enhanced transfection efficiency than poly(CBA-DAH) at weight ratios of 40 and 50, respectively. In Figure 5B, ABP showed about 3~6 times higher transfection efficiency than PEI25k and 20~37 times enhanced transfection efficiency than poly(CBA-DAH) in NIH3T3 cells in the absence of serum.
Figure 5.
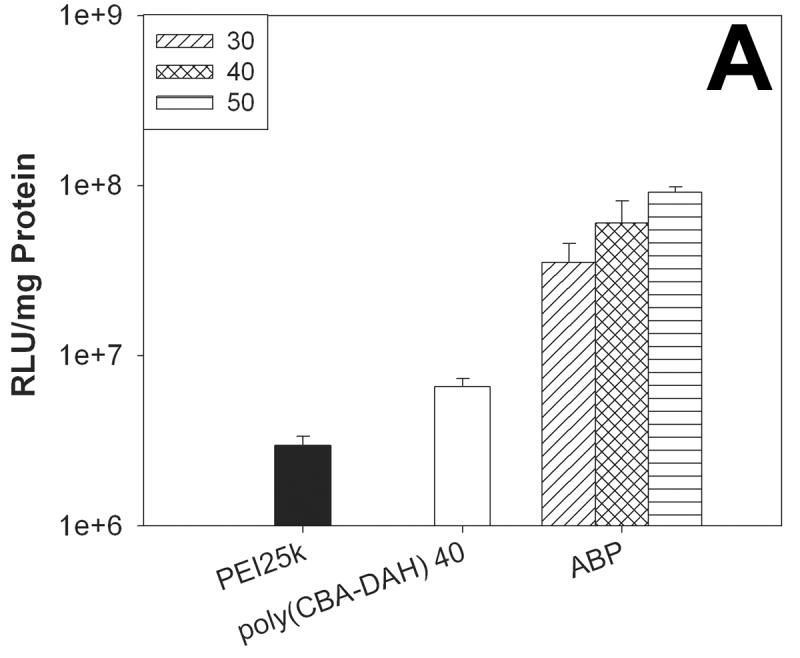
The transfection results in C2C12 cells (A) and NIH3T3 cells (B) in the absence of serum. Numbers in the box mean the weight ratios of ABP polyplexes.
In the presence of serum, the transfection efficiency of ABP was just 15 % reduced in comparison with its efficiency in the absence of serum, although PEI25k showed 90 % reduced efficiency in C2C12 cells (Figure 6). This result means that the transfection process of ABP will not be seriously inhibited from serum interaction and that ABP has a potential for gene delivery systems in vivo as well as in vitro.
Figure 6.
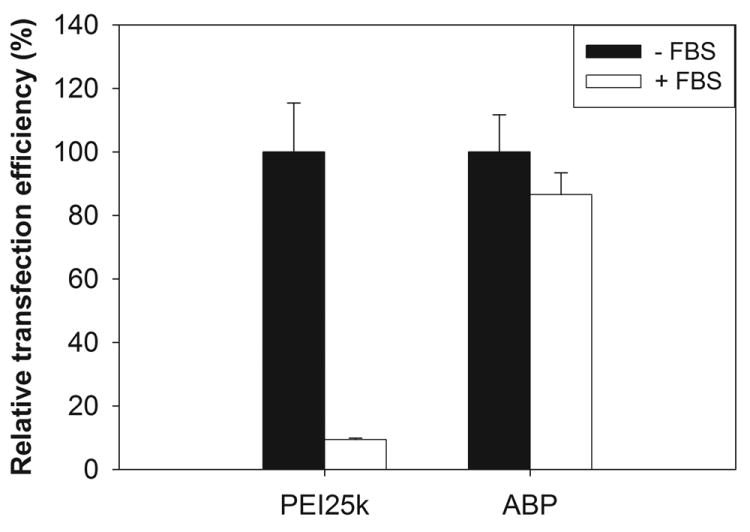
The transfection result in C2C12 cells in the presence of 10 % serum. Relative transfection efficiency means the percentage ratio of the transfection efficiency without serum to with serum.
These results show that arginine-graft to linear poly(CBA-DAH) still can greatly increase the transfection efficiency of the polymer and it can be a promising non-viral gene delivery carrier.
3.6. Transfection mechanism study of ABP
A series of transfection experiments were performed in order to examine the transfection mechanism of ABP polyplex. First, 100 μM chloroquine was added to the cells before treatment of polyplexes and the cells were further incubated for 4 h during transfection. Chloroquine is a well-known endosome disrupting agent which can facilitate the release of polyplexes from endosome and lead to enhanced transfection [37]. PEI25k was used as a control.
As shown in Figure 7, transfection efficiencies of both PEI25k and ABP were found to be reduced to about 60 % in the presence of chloroquine in comparison with the efficiencies in the absence of chloroquine. This result displays that 100 μM chloroquine does not improve the transfection of the polymers used. PEI25k has various internal amines which can act as endosome buffers and a slight decrease of transfection efficiency of PEI25k in treatment of chloroquine has been already reported by Langer and his co-workers [38]. It was also suggested that the backbone polymer of ABP, poly(CBA-DAH) can have endosome buffering moieties in the previous study [14]. Therefore, it is proposed that ABP can function as endosome buffer itself. However, this result doesn’t explain the greatly enhanced transfection of ABP in comparison with poly(CBA-DAH).
Figure 7.
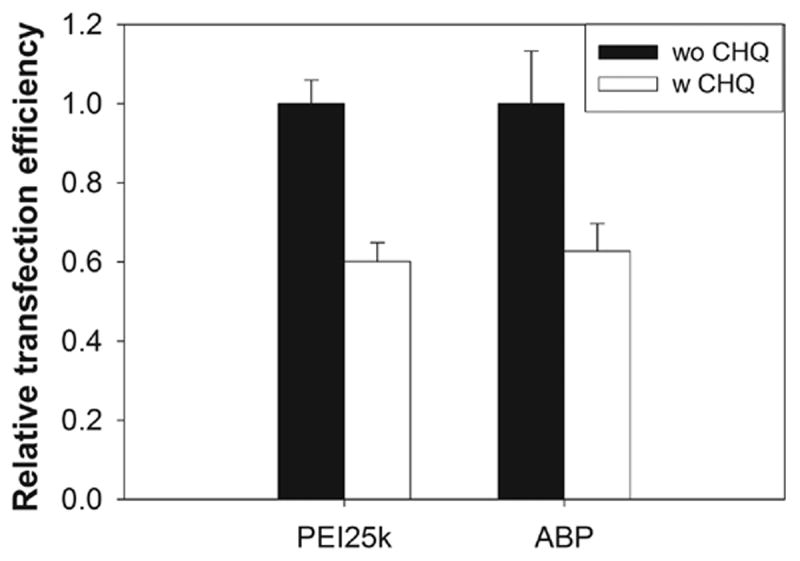
The transfection result with or without 100 μM chloroquine in C2C12 cells.
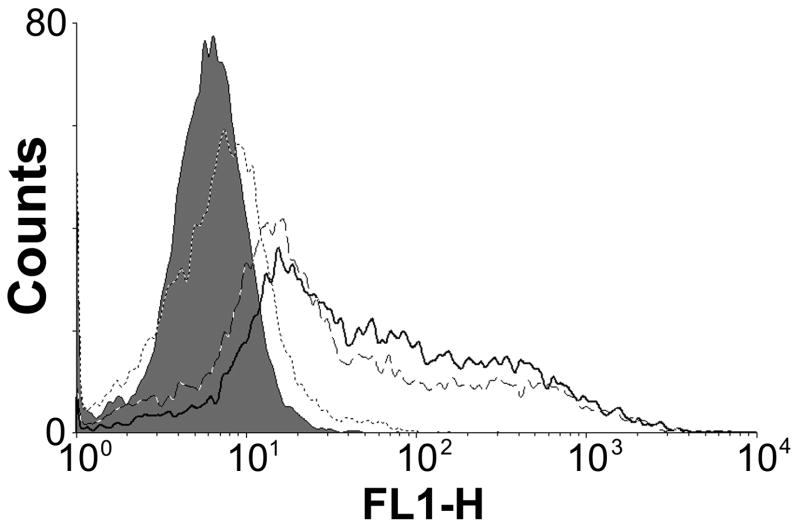
Then, the cellular uptake of polyplexes was investigated by using flow cytometry. YOYO-1 iodide-labeled pDNA was used for fluorescence measurements and poly(CBA-DAH) was used as a control polymer. After treatment of polyplexes, the cells were incubated in 0.4 % trypan blue solution for 5 min in order to quench the fluorescence of extra-cellular polyplexes.
As shown in Figure 8, there seems to be no significant difference of fluorescence peaks between the poly(CBA-DAH) and ABP polyplexes despite their structural differences. This result means that conjugated arginine-residues don’t have any significant functions for cellular uptake of ABP polyplex. Therefore, it is interpreted that greatly enhanced transfection efficiency of ABP is not induced by its high cellular penetrating ability but may be mediated by other factors such as good nuclear localization ability, which is also proposed in the case of arginine-chitosan [26].
Figure 8.
Cellular uptake result by flow cytometry in C2C12 cells. Closed gray peak : cell only, dot line(…) : pDNA only, dashed line(–·–) : poly(CBA-DAH) polyplex, bold line (-) : ABP polyplex.
4. Conclusions
Arginine-grafted bioreducible poly(CBA-DAH) (ABP) polymer was synthesized for non-viral gene delivery systems. ABP was able to condense pDNA into nano-sized (less than 200 nm) and positively charged particles. It was found that ABP was not able to condense pDNA even at a weight ratio of 10 in the presence of DTT, meaning that ABP can be biodegraded in reducing environment such as cytoplasm. The biodegradation of ABP by reductive cleavage can facilitate the efficient release of pDNA from polyplexes and reduce its cytotoxicity. As expected, the cytotoxicity of ABP was examined to be very low. ABP showed greatly enhanced transfection efficiency in comparison with unmodified poly(CBA-DAH) and even PEI25k in mammalian cells. In the serum condition, the transfection efficiency of ABP was not reduced much, not like PEI25k, suggesting that ABP can be also used as an efficient gene delivery carrier for in vivo systems. The transfection efficiency of ABP was not improved by chloroquine treatment and the cellular uptake pattern of ABP polyplex showed no significant difference from that of poly(CBA-DAH). These result displays that ABP can act as an endosome buffer itself and supposes the possibility that greatly enhanced transfection efficiency of ABP is not induced by its high cellular penetrating ability but may be mediated by other factors such as good nuclear localization ability.
Acknowledgments
This work was supported by NIH grant DK 077703. We thank Monika Sima for FPLC-SEC experiments. We also thank Sun Hwa Kim for helpful advice about flow cytometry experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Remy JS, Adballah B, Zanta MA, Boussif O, Behr JP, Demeneix B. Gene transfer with lipospermines and polyethyleneimines. Adv Drug Deliv Rev. 1998;30:85–95. doi: 10.1016/s0169-409x(97)00109-9. [DOI] [PubMed] [Google Scholar]
- 2.Liu F, Huang L. Development of non-viral vectors for systemic gene delivery. J Control Release. 2002;78:259–266. doi: 10.1016/s0168-3659(01)00494-1. [DOI] [PubMed] [Google Scholar]
- 3.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 4.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Wong SY, Pelet JM, Putnam D. Polymer systems for gene delivery-Past, present, and future. Prog Polym Sci. 2007;32:799–837. [Google Scholar]
- 6.Gosselin MA, Guo W, Lee RJ. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjugate Chem. 2001;12:989–994. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- 7.Miyata K, Kakizawa Y, Nishiyama N, Harada A, Yamasaki Y, Koyama H, Kataoka K. Block catiomer polyplexes with regulated densities of charge and disulfide cross-linking directed to enhance gene expression. J Am Chem Soc. 2004;126:2355–2361. doi: 10.1021/ja0379666. [DOI] [PubMed] [Google Scholar]
- 8.Ooya T, Choi HS, Yamashita A, Yui N, Sugaya Y, Kano A, Maruyama A, Akita H, Ito R, Kogure K, Harashima H. Biocleavable polyrotaxane-plasmid DNA polyplex for enhanced gene delivery. J Am Chem Soc. 2006;128:3852–3853. doi: 10.1021/ja055868+. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Mo H, Koo H, Park J-Y, Cho MY, Jin G-w, Park J-S. Visualization of the degradation of a disulfide polymer, linear poly(ethylenimine sulfide), for gene delivery. Bioconjugate Chem. 2007;18:13–18. doi: 10.1021/bc060113t. [DOI] [PubMed] [Google Scholar]
- 10.Zugates GT, Anderson DG, Little SR, Lawhorn IEB, Langer R. Synthesis of poly(β-amino ester)s with thiol-reactive side chains for DNA delivery. J Am Chem Soc. 2006;128:12726–12734. doi: 10.1021/ja061570n. [DOI] [PubMed] [Google Scholar]
- 11.Oupicky D, Parker AL, Seymour LW. Laterally stabilized complexes of DNA with linear reducible polycations: Strategy for triggered intracellular activation of DNA delivery vectors. J Am Chem Soc. 2002;124:8–9. doi: 10.1021/ja016440n. [DOI] [PubMed] [Google Scholar]
- 12.Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong Z, Lin C, Engbersen JF, Feijen J. Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjugate Chem. 2006;17:1233–1240. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- 13.Lin C, Zhong Z, Lok MC, Jiang X, Hennink WE, Feijen J, Engbersen JF. Linear poly(amido amine)s with secondary and tertiary amino groups and variable amounts of disulfide linkages: synthesis and in vitro gene transfer properties. J Control Release. 2006;116:130–137. doi: 10.1016/j.jconrel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Ou M, Wang XL, Xu R, Chang CW, Bull DA, Kim SW. Novel biodegradable poly(disulfide amine)s for gene delivery with high efficiency and low cytotoxicity. Bioconjugate Chem. 2008;19:626–633. doi: 10.1021/bc700397x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danusso F, Ferruti P. Synthesis of tertiary amine polymers. Polymer. 1970;11:88–113. [Google Scholar]
- 16.Ferruti P, Marchisio MA, Barbucci R. Synthesis, physico-chemical properties and biomedical applications of poly(amido-amine)s. Polymer. 1985;26:1336–1348. [Google Scholar]
- 17.Lin C, Zhong Z, Lok MC, Jiang X, Hennink WE, Feijen J, Engbersen JF. Novel bioreducible poly(amido amine)s for highly efficient gene delivery. Bioconjugate Chem. 2007;18:138–145. doi: 10.1021/bc060200l. [DOI] [PubMed] [Google Scholar]
- 18.Lin C, Blaauboer CJ, Timoneda MM, Lok MC, van Steenbergen M, Hennink WE, Zhong Z, Feijen J, Engbersen JF. Bioreducible poly(amido amine)s with oligoamine side chains: synthesis, characterization, and structural effects on gene delivery. J Control Release. 2008;126:166–174. doi: 10.1016/j.jconrel.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Brooks H, Lebleu B, Vive’s E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Delivery Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhao M, Weissleder R. Intracellular cargo delivery using Tat peptide and derivatives. Med Res Rev. 2004;24:1–12. doi: 10.1002/med.10056. [DOI] [PubMed] [Google Scholar]
- 21.Tung C-H, Weissleder R. Arginine containing peptides as delivery vectors. Adv Drug Delivery Rev. 2003;55:281–294. doi: 10.1016/s0169-409x(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 22.Kim WJ, Christensen LV, Jo S, Yockman JW, Jeong JH, Kim Y-H, Kim SW. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA efficiently inhibits tumor growth in colon adenocarcinoma. Mol Ther. 2006;14:343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Choi JS, Nam K, Park JY, Kim JB, Lee JK, Park JS. Enhanced transfection efficiency of PAMAM dendrimer by surface modification with L-arginine. J Control Release. 2004;99:445–456. doi: 10.1016/j.jconrel.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Kim T-i, Baek J-u, Bai CZ, Park J-s. Arginine-conjugated polypropylenimine dendrimer as a non-toxic and efficient gene delivery carrier. Biomaterials. 2007;28:2061–2067. doi: 10.1016/j.biomaterials.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Okuda T, Sugiyama A, Niidome T, Aoyagi H. Characters of dendritic poly(l-lysine) analogues with the terminal lysines replaced with arginines and histidines as gene carriers in vitro. Biomaterials. 2004;25:537–544. doi: 10.1016/s0142-9612(03)00542-8. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Xu Z, Chen S, Gu W, Chen L, Li Y. Arginine-chitosan/DNA self-assemble nanoparticles for gene delivery: In vitro characteristics and transfection efficiency. Int J Pharm. 2008;359:241–246. doi: 10.1016/j.ijpharm.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 27.Yamanouchi D, Wu J, Lazar AN, Craig Kent K, Chu CC, Liu B. Biodegradable arginine-based poly(ester-amide)s as non-viral gene delivery reagents. Biomaterials. 2008;29:3269–3277. doi: 10.1016/j.biomaterials.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Ogris M, Wagner E, Steinlein P. A versatile assay to study cellular uptake of gene transfer complexes by flow cytometry. Biochim Biophys Acta. 2000;1474:237–243. doi: 10.1016/s0304-4165(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 29.Sahlin S, Hed J, Rundquist I. Differentiation between attached and ingested immune complexes by a fluorescence quenching cytofluorometric assay. J Immunol Methods. 1983;60:115–124. doi: 10.1016/0022-1759(83)90340-x. [DOI] [PubMed] [Google Scholar]
- 30.Lim Y-b, Kim S-m, Suh H, Park J-s. Biodegradable endosome disruptive, and cationic network-type polymer as a highly efficient and nontoxic gene delivery carrier. Bioconjugate Chem. 2002;13:952–957. doi: 10.1021/bc025541n. [DOI] [PubMed] [Google Scholar]
- 31.Kim T-i, Seo HJ, Choi JS, Yoon JK, Baek J-u, Kim K, Park J-S. Synthesis of biodegradable cross-linked poly(β-amino ester) for gene delivery and its modification, inducing enhanced transfection efficiency and stepwise degradation. Bioconjugate Chem. 2005;16:1140–1148. doi: 10.1021/bc0497012. [DOI] [PubMed] [Google Scholar]
- 32.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 33.Lim Y-b, Kim S-m, Lee Y, Lee W-k, Yang T-g, Lee M-j, Suh H, Park J-s. Cationic hyperbranched poly(amino ester): a novel class of DNA condensing molecule with cationic surface, biodegradable three-dimensional structure, and tertiary amine groups in the interior. J Am Chem Soc. 2001;123:2460–2461. doi: 10.1021/ja005715g. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Mao H, Leong KW. A novel biodegradable gene carrier based on polyphosphoester. J Am Chem Soc. 2001;123:9480–9481. doi: 10.1021/ja016062m. [DOI] [PubMed] [Google Scholar]
- 35.Lynn DM, Langer R. Degradable poly(β-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122:10761–10768. [Google Scholar]
- 36.Lim Y-b, Kim C-h, Kim K, Kim SW, Park J-s. Development of a safe gene delivery system using biodegradable polymer, poly[α-(4-aminobutyl)-L-glycolic acid] J Am Chem Soc. 2002;122:6524–6525. [Google Scholar]
- 37.Ciftci K, Levy RJ. Enhanced plasmid DNA transfection with lysosomotropic agents in cultured fibroblasts. Int J Pharm. 2001;218:81–92. doi: 10.1016/s0378-5173(01)00623-8. [DOI] [PubMed] [Google Scholar]
- 38.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]