Abstract
The central nervous system (CNS) can be activated by both local and systemic inflammation, resulting in the manifestation of sickness symptoms. The pathways by which the CNS is activated under these two conditions, however, may differ. In this study, we injected casein into the peritoneal cavity (ip) or into a subcutaneous air pouch to induce restricted local inflammation. Both routes of casein injection caused fever and reduced locomotor activity. These responses were not accompanied by the statistically significant induction of the inflammatory cytokine interleukin-1 (IL-1) in the blood and brain. Further, these responses were produced without the induction of brain cyclooxygenase-2 (COX-2), which has been implicated as an obligatory step in systemic inflammation-induced activation of the CNS. Induction of IL-1, IL-6, and COX-2, however, was found consistently at the sites of casein injection. The local inflammation-induced febrile and locomotor activity responses were blunted in animals deficient in functional Toll-Like Receptor 4 (TLR4), IL-1R1, IL-6, or COX-2. Therefore, the observed febrile and locomotor activity effects appear to require local, but not central, IL-1, IL-6, and COX-2. These findings suggest that local inflammation can activate the CNS via pathways distinguishable from those mediating systemic inflammation-induced CNS activation.
Keywords: IL-1, IL-6, COX-2, neuroimmune communication
Introduction
It has been well established that peripheral inflammation stimulates the central nervous system (CNS) via several pathways. These immune-to-brain communication pathways have been demonstrated in studies that used the bacterial endotoxin lipopolysaccharide (LPS) to initiate peripheral inflammation. Systemic administration of LPS results in robust CNS-controlled sickness behaviors accompanied by increases of pro-inflammatory cytokines, IL-1-β, TNF-α and IL-6, in the blood and brain (Givalois et al., 1994; Quan et al., 1994; Lenczowski et al., 1997; Roth et al., 1998; Romanovsky et al., 2000; Johnson et al., 2003; Hopkins, 2007). LPS and the induced inflammatory cytokines then stimulate brain endothelial expression of inflammatory mediators, vagal afferents, and/or blood-brain barrier transport of cytokines to activate the CNS (Quan and Banks, 2007).
Whereas systemic LPS and pro-inflammatory cytokines are known to be present during endotoxic septic shock, a condition that often leads to multiple organ failure and mortality, circulating LPS and pro-inflammatory cytokines are either absent or not correlated with the intensity of localized inflammation (Wiik et al., 2001; Yamamoto et al., 2005; Hedges et al., 2006). Therefore, the neuroimmune pathways that rely on circulating LPS and/or cytokines may not be involved in local inflammation-induced neuroimmune activation.
Limited research has been done to determine the pathways by which restricted local inflammation signals the CNS. LPS has been injected into sterile subcutaneous air pouches in rats (Miller et al., 1997; Cartmell et al., 2000), or into artificial subcutaneous chambers in guinea pigs (Rummel et al., 2005) to produce local inflammation. Other inflammatory stimuli, such as turpentine and complete Freund’s adjuvant, have also been used to induce localized tissue inflammation (Kozak et al., 1995; Leon et al., 1996; Ichitani et al., 1997). Results from these studies hinted that different pathways relay local inflammatory signals to the CNS. For example, deafferentation of C-fiber sensory neurons attenuated fever induced by intramuscular injection of turpentine in rats (Cooper and Rothwell, 1991) and injection of ropivacaine, a local anesthetic, at the site of inflammation suppressed fever induced by low-dose LPS injected into a subcutaneous chamber in guinea pigs (Ross et al., 2000). Therefore, the CNS may be stimulated via primary sensory nerve tracts during localized inflammation.
The use of locally injected LPS to mimic local inflammation, however, may not be ideal. For example, in a model of live E.coli infection, the appearance of significant amounts of LPS was only detected when E.coli was infused intravenously at lethal, but not sublethal, levels (Creasey et al., 1991). Therefore, a localized and contained bacterial infection may not result in the presence of free LPS in the extracelluar fluid at the site of inflammation.
In the present study, we used another inflammatory stimulant, casein, to induce local inflammation. Casein is one of the inflammatory agents found in milk (Wal, 2002), which can induce fever (Moissidis et al., 2005). We hypothesize that local inflammation induced by casein will activate the CNS via pathways that are distinguishable from those mediating systemic inflammation-induced CNS activation. We show here that casein induces pronounced neuroimmune activation without inducing IL-1 in the blood and brain. In addition, casein-induced local inflammation stimulates CNS responses without the induction of COX-2 in the brain, which was thought to be an essential mediator of immune-to-brain signaling.
Material and Methods
Animals
IL-1R1 KO, IL-6 KO, C3H/HeJ, COX-2 KO and FVB normal mice, 6–10 weeks of age and 20–25g of body weight, were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The mice were used in experimental procedures one week after they were acclimated to the animal facility. The ambient temperature was set to 29±1 °C. This ambient temperature is in the normal thermoneutral zone of mice and it is used here to reduce episodic locomotor activity. The reduction of locomotor activity facilitates recording of febrile response because the presence of high core temperatures associated with high locomotor activity in these animals might obscure the appearance of fever. All experiments were conducted in accordance with the NIH Guide on the care and use of animals for research, and an in-house protocol approved by the Ohio State University Animal Care and Use Committee.
Inflammatory agents
Ten grams of casein powder (ICN Biomedicals, Aurora, OH) were dissolved in 80 ml, 50 mM sodium bicarbonate buffer (Sigma, St. Louis, MO). Water was then added to make the final volume of 100 ml. The mixture was stirred in a water bath at 65°C until casein was dissolved. This casein solution was then cleared by filtration through coarse filter paper (Fisher Scientific, Pittsburgh, PA) and stored at −20°C for later injection.
Ten milligrams of lipopolysaccharides (LPS, Sigma, St. Louis, MO) was dissolved in 40 ml of pyrogen-free saline to achieve the final concentration of 0.25 mg/ml and stored at −20°C for later use.
Telemetrical measurements of body temperature and locomotor activity
Mice were anesthetized by intraperitoneal (ip) injection of 2.5mg/25g Nembutal (Abbott Laboratories, Chicago, IL, USA). A PDT-4000 E-mitter (Mini Mitter, Bend, OR) was surgically implanted in the peritoneal cavity. Animals were allowed to recover for 7 days before they were used in any experiments. Signals were calibrated to Celsius (°C) for temperature or counts per minute (CPM) for locomotor activity by the manufacturer using the software VitalView (Data Acquisition System, Mini Mitter, Bend, OR). Data for temperature and activity were sampled at 1 min intervals. For simplicity, the mean values for every 10-min period of a given experiment were plotted.
Inflammation induction
For casein-induced local inflammation, casein at concentration of 10% was administered either intraperitoneally (ip, 0.5 ml/mouse), or into a subcutaneous air pouch (ipo, 0.5 ml/mouse). The same volume of vehicle (pyrogen-free sodium bicarbonate buffer) was injected into the control animals. For LPS-stimulated inflammation, 25 μg of LPS at the concentration of 0.25 mg/ml was injected ip (0.1 ml/mouse). To acclimate the animals to the injection procedure, all mice in the experiment were subjected to mock vehicle injections for 3 consecutive days before the inflammatory agents were injected.
For subcutaneous air pouch preparation, the animals were prepared by shaving the fur on the dorsum between shoulder blades, and a volume of 3 ml sterile air was injected into the subcutaneous space under the shaved skin. After 3 days, 3 ml of sterile air was injected again in order to maintain the patency of the pouch. The air pouch thus made was used for subsequent injections.
Sample collection
Samples in each experiment were collected at both the onset and the plateau of observed febrile responses. These time points were 2, 4, 6 or 8 hrs post-injection depending on the administered inflammatory agents and the routes of the injection.
For blood sample collection, animals were anesthetized with isoflurane (Abbott, North Chicago, IL). Approximately 0.5 ml of whole blood was collected by heart puncture from each animal. The collected blood was divided into two parts, 100 μl for RNA extraction and the rest for plasma fractionation. The blood samples for plasma fractionation were transferred to Vacutainer heparin tubes (Becton Dickinson, Franklin Lakes, NJ). After centrifugation at 2500 rpm for 10 min, plasma samples and blood cell pellets were collected and stored in a −70°C freezer for later analysis of cytokine and prostaglandin E2 (PGE2) levels.
For tissue sample collection, animals were sacrificed. Tissues from brain, liver and spleen were collected. Whole brain was divided from the midline into two equal parts, one for RNA extraction and the other for protein analysis. To further analyze regional gene expression in the brain, tissues of hypothalamus and hippocampus were obtained by micro-dissection. Pituitary glands from separate experimental animals were also collected. Tissue samples were immediately stored in a −70°C freezer for later use. Following tissue collection, 1 ml Hanks’ Balanced Salt Solution (Fisher, Pittsburgh, PA) was used to wash the peritoneal or the air pouch cavity. The lavage fluid was collected and centrifuged at 15000 g for 10 min. Cell pellets from the lavage were used for both RNA extraction and cytokine protein analysis. Supernatants were collected and stored in −70°C freezer for later analysis of cytokine proteins.
Isolation of mRNA and preparation of cDNA
To isolate total RNA from blood, 100 μl of the blood was diluted with 100 μl of RNase and DNase free H2O. The standard protocol from the ZR Whole-Blood Total RNA Kit (ZYMO Research, Orange, CA, USA) was used to extract total RNA. To isolate RNA from solid tissue samples, TRIzol solution was added to each sample. The tissues were homogenized to generate a cell suspension. Conventional TRIzol method (Invitrogen, Carlsbad, CA, USA) was used for the extraction of total RNA from the resulting cell suspension. DNase I (Invitrogen, Carlsbad, CA, USA) was subsequently used to eliminate potential genomic DNA contaminants.
cDNAs were generated by reverse transcription (Promega Corporation, Madison, WI, USA). Briefly, 1 μg of total RNA was added to a 19-μl reaction mixture that contained 4 μl of 25 mM MgCl2, 2 μl of 10X buffer, 2 μl of 10 mM dNTP mixture, 0.5 μl of recombinant ribonuclease inhibitor, 15 units of AMV reverse transcriptase and 0.5 μg of random primers. The reaction was incubated at 42°C for 50 min, followed by denaturation at 75°C for 20 min and cooling at 4°C for 5 min in a PCR-iCycler (Bio-Rad, Hercules, CA).
ELISA
Both extracellular and intracellular cytokine levels were measured by ELISA. Brain tissues and cell pellets from blood or lavage were homogenized in 1 ml 1x PBS and then centrifuged at 30000 g for 30 min at 4°C. Supernatants were directly analyzed by ELISA for levels of extracellular cytokines. The cell pellets from brain, lavage (peritoneal and air pouch) and blood were lysed in 0.5 ml boiling lysis buffer (1% SDS, 10mM Tris pH 7.4) for 5 minutes, then sonicated for 1 minute to make crude cell lysate. Ready to use lysate (for analysis of intracellular cytokines) was prepared by adding 1 volume of the crude lysate to 4 volumes of H2O and 5 volumes of 2X dilution buffer (2% triton X-100, 300 mM NaCL, 20mM Tris PH7.4, 2mM EDTA, 1% NP-40).
ELISA kits (Biosource, Camarillo, CA, USA) were used for analysis of IL-1 and IL-6. Briefly, standards and samples were added to the corresponding wells of the pre-coated plates that contained monoclonal antibodies for either IL-1 or IL-6. The plates were incubated overnight at 4°C. After washing with PBS, a rabbit anti-mouse IL-1 or IL-6 antibody was added to the plates and incubated for 1 h. The labeling antibody was detected with 1:500 goat anti-rabbit antibody conjugated with horseradish peroxidase (HRP). ABTS substrate was then added to the plate. Color was allowed to develop at room temperature for 30 min until positive wells became fully developed. Optical density was read at 450 nm on a Cambridge Technology Model 7520 Microplate Reader (Cambridge Scientific Products, Cambridge, MA, USA). Bradford protein assay was performed to determine total protein concentrations in the samples as previously described (Fleshner et al., 1998).
Immunoassay
PGE2 was measured in plasma by enzyme immunoassay using a commercially available kit (Cayman, Ann Arbor, Michigan). Plasma samples were diluted (1:250) in 100 mM Tris-buffered saline (pH 7.4) before the assay. The levels of plasma PGE2 were assayed following the manufacturer’s protocol. Briefly, the 96-well plates were pre-coated with goat polyclonal anti-mouse IgG and blocked with a proprietary formulation of proteins. The wells were incubated with PGE2- acetylcholinesterase (PGE2 tracer), PGE2 monoclonal antibody, and standard or samples. Then the plates were developed with Ellman’s Reagent, which results in a distinct yellow color and absorbs strongly at 412 nm. The intensity of this color is proportional to the amount of PGE2 tracer bound to the well, which is inversely proportional to the PGE2 levels.
Realtime PCR
The mRNA levels of IL-1β, COX-2, and IL-6 in different tissues were determined by Realtime PCR. To each sample vial, 12.5 μl of 2X TaqMan PCR master mix (Applied Biosystems, Foster City, CA, USA), 125 nM of target probes, 900 nM of primers mix (specific primers for IL-1β, COX-2, and IL-6), 100 nM of internal control probe (specific for the house keeping gene, G3PDH), 900 nM of primers (specific for G3PDH) and 1 μl of cDNA were added. Then, nuclease-free water was added to make the final volume to 25 μl. The PCR parameters were: 40 cycles of chain reaction with denaturation at 95°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 30 s. The primer/probe sets for mouse IL-6 and COX-2 are listed in the following: forward primer: 5′-TATGAAGTTCCTCTCTGCAAGAGA, reverse primer: 5′-TAGGGAAGGCCGTGGTT, probe: 5′-Fam-CCAGCATCAGTCCCAAGAAGGCAA-Tamra-3′ (IL-6); forward primer: 5′-CAGCCAGGCAGCAAATCC, reverse primer: 5′-TCAAATCCTGTGCTCATACATTCC, probe: 5′-Fam-TGCTGTTCCAATCCATGTCAAAACCGT-Tamra-3′ (COX-2). These primers were validated by PCR amplification of IL-6 and COX-2 cDNA clones. For IL-1β and G3PDH, previously established primer/probe sets (Ching et al., 2006) were used. Data were analyzed with the software iCycler 3.0 which was included in the iCycler Detection System (Bio-Rad, Hercules, CA).
Statistical analysis
Data are presented as the means ±SEM. Variations in body temperature and locomotor activity between casein-treated and vehicle-treated mice were evaluated by two-way ANOVA with two grouping factors (time and treatment). Post-hoc analysis (Turkey test) was performed in order to make multiple comparisons between the groups. One way ANOVA was used to analyze changes in PGE2, protein and mRNA levels. The test results were considered significant if p<0.05.
Results
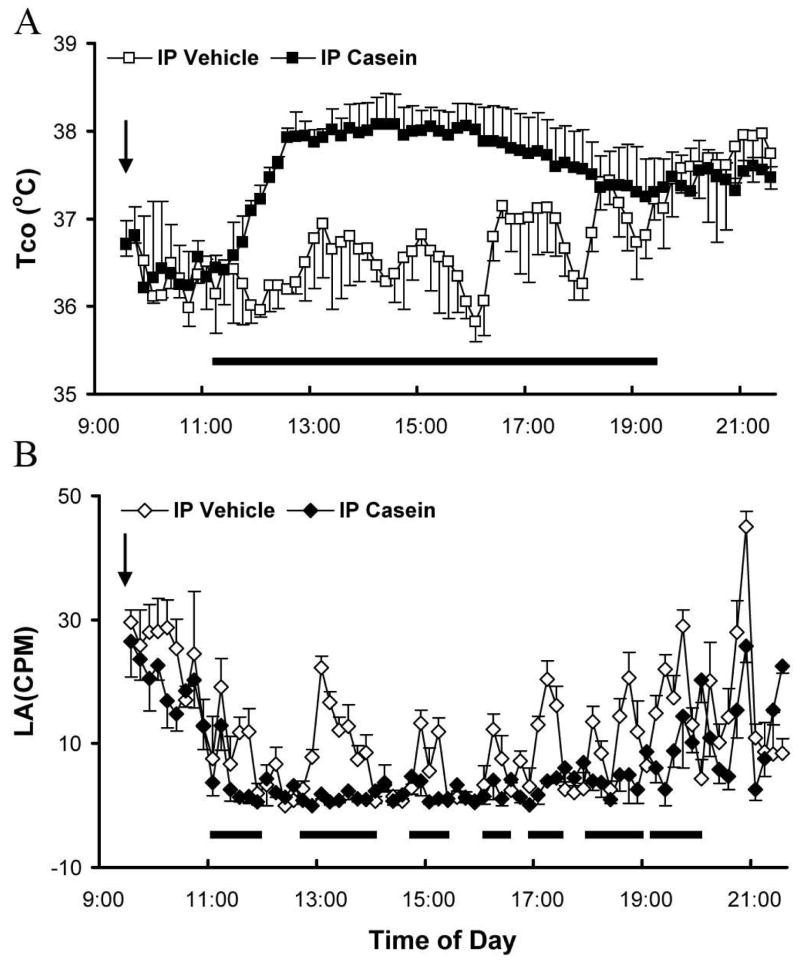
Figure 1A shows the patterns of core body temperature (Tco) following ip administration of casein or vehicle in normal non-transgenic mice. After vehicle injection, Tcos displayed rhythmic changes throughout the day (Fig. 1A, open squares), which were not different from those recorded from non-injected animals (data not shown). The peaks and valleys of the Tcos somewhat correspond to the episodic locomotor activity of these animals (Fig. 1B, open diamonds). Higher Tcos were found after 19:00 during the night (data not shown). In casein-injected animals, the pattern of the rhythmic changes in Tcos disappeared 2 hrs after the injection (Fig.1A, filled squares). Tco reached approximately 2°C above the nadir of Tco vacillation patterns 3 hrs after the injection and stayed at this level for nearly 4 hrs. Tcos from vehicle and casein injected animals gradually became indistinguishable 10 hrs after the injections (data not shown). The time period during which Tco increased significantly in the casein-treated group was indicated by a horizontal bar (Fig 1A, P<0.05).
Figure 1.
Body temperature changes in wild type animals in response to intraperitoneal injection of casein (10%, 500 μl) or vehicle. A. Core body temperature (Tco) after ip casein (filled squares) or vehicle (open squares) injection. B. Locomotor activity (LA) after ip casein (filled diamonds) or vehicle injection (open diamonds). Data are presented as mean ± SEM (n=4). Arrow indicates the time of injection. The horizontal bars indicate time periods during which significant differences (p<0.05) were detected.
Figure 1B shows patterns of locomotor activity (LA) in these animals. Injection of vehicle did not cause any significant change in LA as compared with those from non-injected animals (data not shown). LA in vehicle-injected animals (Fig. 1B, open diamond) exhibited rhythmic changes generally in synchrony with the patterns of Tco changes throughout the day. After casein injection, a reduction of LA was observed, starting approximately 2 hrs post injection (Fig. 1B, filled diamond). This effect lasted approximately 8 hrs. Time periods during which significant differences between casein vs. saline groups were found were indicated by horizontal bars (Fig. 1B, P<0.05).
Levels of inflammatory mediators IL-1, IL-6, and COX-2 in different tissue sites were measured 2 and 6 hrs after the ip casein injection. These time points corresponded with the onset and plateau of the febrile response induced by the ip casein injection. IL-1β mRNA levels in various tissues are shown in Table 1A. Vehicle injection did not induce IL-1β mRNA expression at any time points. Therefore, all data from vehicle-injected animals were combined to show baseline data. The ip casein injection induced a significant increase of IL-1β mRNA in peritoneal cells and in the pituitary (p<0.05), but no significant changes were found in leukocytes, livers, whole brains and spleens. In addition, IL-1β mRNA was not increased in hypothalamus and hippocampus. In contrast, the ip LPS injection caused significant increases in IL-1β mRNA levels in leukocytes and in all tissue sites except hypothalamus.
Table 1.
| Table 1A: Relative IL-1β mRNA expression in various sites
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Leukocytes | Peritoneal Cells | Liver | Spleen | Brain | Hypo-thalamus | Hippo-campus | Pituitary | |
| ip Vehicle | 1.63±0.38 (×10−2) | ND | 7.3±1.6 (×10−4) | 5.5±1.2 (×10−3) | 2.54±0.72 (×10−5) | ND | ND | ND |
| ip casein 2h | 3.27±0.85 (×10−2) | *47.5±16.4 (×10−1) | 3.43±0.85 (×10−4) | 7.4±2.12 (×10−3) | 6.9±2.1 (×10−5) | ND | ND | *23.8±7.2 (×10−4) |
| ip casein 6h | 2.24±0.27 (×10−2) | *43.8±19.5 (×10−1) | 4.12±2.20 (×10−4) | 6.52±3.70 (×10−3) | 4.2±1.6 (×10−5) | ND | ND | *14.4±5.6 (×10−4) |
| ip LPS 2h | *11.4±1.6 (×10−2) | *2.04±0.87 (×10−1) | *87±30 (×10−4) | *230±100 (×10−3) | *70±22 (×10−5) | ND | *12.9±2.1 (×10−4) | *206±8.0 (×10−4) |
| Table 1B: IL-1β protein expression in various sites
| |||||
|---|---|---|---|---|---|
| Plasma (pg/ml) | Peritoneal lavage (pg/ml) | Brain (pg/1mg protein) | Hypothalamus (pg/1mg protein) | Hippocampus (pg/1mg protein) | |
| ip Vehicle | ND | 49±6 | 5.2±0.5 | 5.0±1.6 | 1.6±0.4 |
| ip Casein 2h | ND | *347±101 | 5.9±0.6 | 7.9±2.0 | 1.4±0.5 |
| ip Casein 6h | ND | *403±114 | 5.5±1.2 | 7.8±1.6 | 0.7±0.3 |
| ip LPS 2h | *175±33 | *314 ±30 | *25.0±5.0 | *23.2±2.3 | *20.4±7.5 |
IL-1β mRNA (A, IL-1/G3PDH) and protein (B) levels are shown. Values are means ± SEM (n=4). Asterisk indicates significant difference (p<0.05) between vehicle-injected and inflammatory agents-injected animals. ND. Not Detectable.
Significantly increased IL-1β protein levels were found in peritoneal lavage (Table 1B) after the ip casein injection as compared with those from vehicle-injected animals. Casein failed to induce significant IL-1β protein expression in the plasma and the brain. However, after the ip LPS injection, significantly increased IL-1β protein levels were found in both plasma and brain (Table 1B).
Similarly, no induction of IL-6 mRNA was found after the ip casein injection in leukocytes and in brain. IL-6 mRNA was significantly elevated in the peritoneal lavage cells at both 2 and 6 hrs after the casein injection. Different from IL-1β, ip casein induced IL-6 mRNA expression in the spleen. Ip LPS, on the other hand, induced IL-6 mRNA in peritoneal cells, liver, spleen and pituitary at 2 hrs post-injection, but did not induce IL-6 mRNA expression in the brain. These results are tabulated in Table 2A.
Table 2.
| Table 2A: Relative IL-6 mRNA expression in various sites
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Leukocytes | Peritoneal cells | Liver | Spleen | Brain | Hypo-thalamus | Hippo-campus | Pituitary | |
| ip Vehicle | ND | ND | ND | ND | ND | ND | ND | ND |
| ip Casein 2h | ND | *12.9±3.6 (×10−2) | ND | *8.6±2.4 (×10−3) | ND | ND | ND | ND |
| ip Casein 6h | ND | *9.9±3.6 (×10−2) | ND | *3.6±0.9 (×10−3) | ND | ND | ND | ND |
| ip LPS 2h | ND | *4.83±0.72 (×10−2) | *2.19±0.64 (×10−4) | *26.6±7.1 (×10−3) | ND | ND | ND | *16.8±2.0 (×10−2) |
| Table 2B: IL-6 protein expression in various sites
| |||||
|---|---|---|---|---|---|
| Plasma (pg/ml) | Peritoneal lavage (pg/ml) | Brain (pg/100μg protein) | Hypothalamus (pg/1mg protein) | Hippocampus (pg/1mg protein) | |
| ip Vehicle | 10±3.5 | 43±9 | 0.23±0.05 | 4.9±2.0 | 1.6±0.7 |
| ip Casein 2h | *462±114 | *3800±757.2 | 0.31±0.10 | 8.0±3.5 | 1.5±0.4 |
| ip Casein 6h | *437±193 | *5950±1952 | 0.28±0.08 | 7.8±1.6 | 10.8±3.5 |
| ip LPS 2h | *566±73 | *803±47 | *22.00±2.70 | *53.6±12.2 | *55.2±21.1 |
IL-6 mRNA (A, IL-6/G3PDH) and protein (B) levels are shown. Values are means ± SEM (n=4). Asterisk indicates significant difference (p<0.05) between vehicle-injected and inflammatory agents-injected animals. ND. Not Detectable
After the ip casein injection, increased IL-6 protein levels were found in plasma and peritoneal lavage. In the brain tissues, an increase of IL-6 was observed in hippocampus, although it was not statistically significant (P=0.0513). In contrast, ip LPS resulted in increased levels of IL-6 in plasma, peritoneal lavage and brain tissues. These results are tabulated in Table 2B.
COX-2 mRNA levels are summarized in Table 3. Ip casein induced COX-2 mRNA expression in peritoneal cells, but not in the brain tissues and leukocytes. In contrast, ip LPS induced COX-2 mRNA expression in all the brain tissues examined, spleen, and pituitary.
Table 3.
Relative COX-2 mRNA expression in various sites
| Leukocytes | Peritonea l cells | Liver | Spleen | Brain | Hypo-thalamus | Hippo-campus | Pituitary | |
|---|---|---|---|---|---|---|---|---|
| ip Vehicle | ND | ND | ND | ND | 7.2±0.3 (×10−4) | ND | ND | ND |
| ip Casein 2h | ND | *12.1±5.1 (×10−2) | ND | ND | 8.7±2.4 (×10−4) | ND | ND | ND |
| ip Casein 6h | ND | *24.3±17.8 (×10−2) | ND | ND | 6.6±1.8 (×10−4) | ND | ND | ND |
| ip LPS 2h | ND | *11.2±1.7 (×10−2) | ND | *19.7±5.8 (×10−3) | *44.0±17.0 (×10−4) | *12.5±2.6 (×10−4) | *64.8±22.1 (×10−4) | *29.8±3.5 (×10−3) |
COX-2 mRNA (COX-2/G3PDH) levels are shown. Values are means ± SEM (n=4). Asterisk indicates significant difference (p<0.05) between vehicle-injected and inflammatory agents-injected animals. ND. Not Detectable
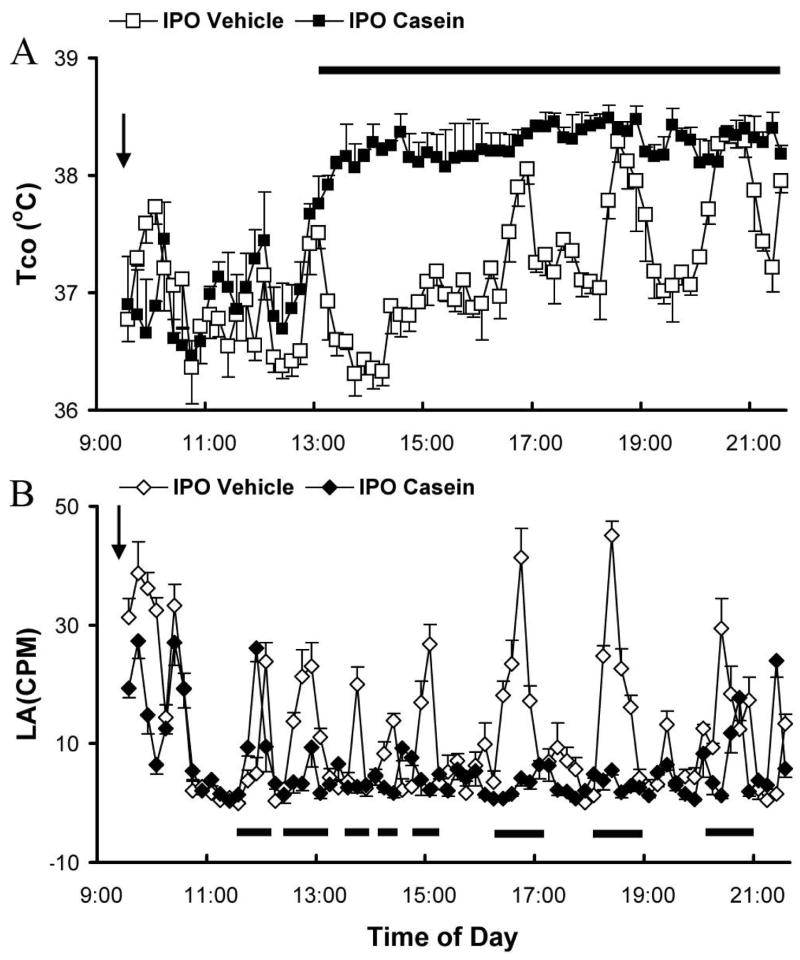
Next, we studied the effects of casein injection into the subcutaneous air pouch (ipo). Figure 2A shows Tco responses after ipo vehicle (open squares) and casein (filled squares) injection. Again, vehicle injection did not change Tco patterns from non-injected animals (data not shown). Ipo casein resulted in the disappearance of the rhythmic pattern of Tco and a sustained rise in Tco. The onset of Tco rise after casein injection occurred 4 hrs post-injection. A 0.5–2°C Tco rise (Fig 3A, filled squares) was observed, lasting until 12 hrs after the casein injection. The Tco in casein-injected animals gradually returned to normal patterns thereafter (data not shown). The time period of significant Tco rise is indicated by a horizontal bar (p<0.5).
Figure 2.
Body temperature changes in wild type animals injected with casein (10%, 500 μl) or vehicle into an air pouch. A. Core body temperature (Tco) after ipo casein (filled squares) or vehicle injection (open squares). B. Locomotor activity (LA) after ipo casein (filled diamonds) or vehicle (open diamonds) injection. Data are presented as mean ± SEM (n=4). Arrow indicates the time of injection. The horizontal bars indicate time periods during which significant differences (p<0.05) were detected.
Figure 3.
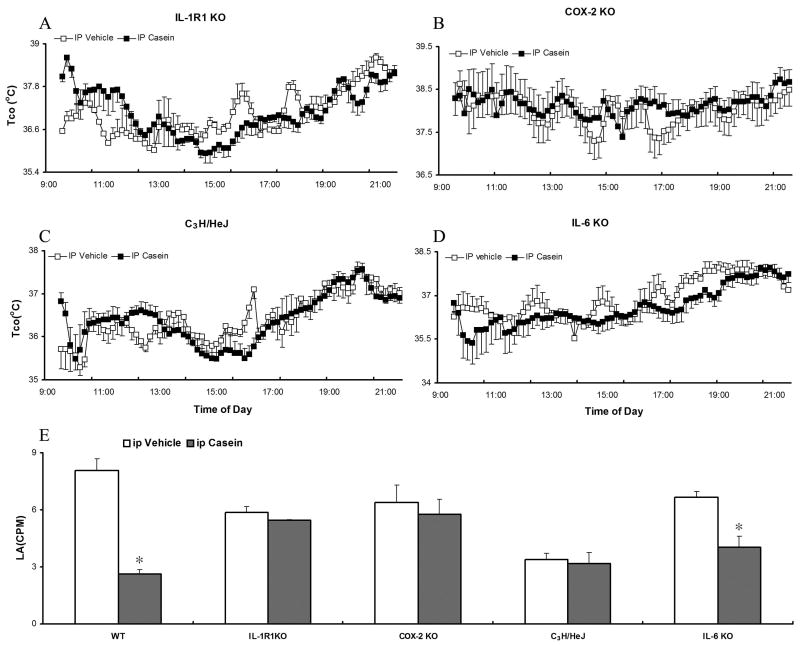
Tco and LA responses in different transgenic mice after ip casein (10%, 500 μl) injection. A-D. Core body temperature (Tco) after ip casein or vehicle injection in IL-1R1 KO, COX-2 KO, C3H/HeJ, and IL-6 KO animals. E. Average LA after ip casein injection in IL-1R1 KO, COX-2 KO, C3H/HeJ, and IL-6 KO animals. Data are presented as mean ± SEM (n=4). *: p<0.05, comparing casein and vehicle group in the same type of animals.
LA after ipo injection of casein (filled diamond) and vehicle (open diamond) is shown in Fig. 2B. In casein treated animals, LA was reduced during the febrile period. Time periods of significant LA reduction were marked by the horizontal bars (Fig. 2B, P<0.05).
IL-1, IL-6, and COX-2 mRNA levels were measured 4 (onset) and 8 (plateau) hrs after the ipo injections. Ipo casein induced IL-1β mRNA expression in the air pouch at both time points and in the pituitary at 4 hrs post-injection, but not in leukocytes and in brain tissues (Table 4A). In contrast, 6 hrs after the ipo LPS injection, IL-1β mRNA was induced in air pouch cells, liver and spleen. Interestingly, ipo LPS did not induce brain and leukocyte IL-1β mRNA expression, although it induced pituitary IL-1β mRNA expression. These results are tabulated in Table 4A. Consistent with these results, increased IL-1β protein levels were found in the air pouch, but not in plasma and brain tissues (Table 4B) after both ipo casein and ipo LPS injections.
Table 4.
| Table 4A: Relative IL-1β mRNA expression in various sites after ipo casein injection
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Leukocytes | Air pouch cells | Liver | Spleen | Brain | Hypo-thalamus | Hippo-campus | ||
| ipo Vehicle | 1.4±0.41 (×10−2) | ND | 3.5±0.8 (×10−4) | 4.4±0.6 (×10−3) | 3.6±0.3 (×10−5) | ND | ND | ND |
| ipo Casein 4h | 3.6±0.6 (×10−2) | *1.2±0.9 (×10−1) | 4.2±1.3 (×10−4) | 6.0±0.2 (×10−3) | 3.2±0.7 (×10−5) | ND | ND | *14.5±2.5 (×10−4) |
| ipo Casein 8h | 5.3±2.2 (×10−2) | *4.2±1.5 (×10−1) | 4.4±0.3 (×10−4) | 4.7±0.4 (×10−3) | 3. 9±0.5 (×10−5) | ND | ND | ND |
| ipo LPS 6h | 2.3±0.6 (×10−2) | *2.1±1.5 (×10−1) | *84±23 (×10−4) | *102±26.7 (×10−3) | 5.2±0.8 (×10−5) | ND | ND | *4.9±1.5 (×10−4) |
| Table 4B: IL-1β protein expression in various sites after ipo casein injection
| |||||
|---|---|---|---|---|---|
| Plasma (pg/ml) | Airpouch lavage (pg/ml) | Brain (pg/1mg protein) | Hypothalamus (pg/1mg protein) | Hippocampus (pg/1mg protein) | |
| ipo Vehicle | ND | 63±12 | 5.1±0.3 | 8.1±1.4 | 2.3±0.6 |
| ipo Casein 4h | ND | *516±36 | 6.2±0.9 | 5.3±1.2 | 0.7±0.2 |
| ipo Casein 8h | ND | *517±42 | 5.9±1.0 | 4.8±0.7 | 1.7±0.3 |
| ipo LPS 6h | ND | *316±51 | 5.1±0.7 | 5.1±2.0 | 2.8±0.6 |
IL-1β mRNA (A, IL-1/G3PDH) and protein (B) levels are shown. Values are means ± SEM (n=4). Asterisk indicates significant difference (p<0.05) between vehicle-injected and inflammatory agents-injected animals. ND. Not Detectable
IL-6 mRNA expression was induced only in the air pouch, but not elsewhere, by either ipo casein or ipo LPS (Table 5A). In contrast, increased IL-6 protein levels were found in the air pouch and plasma after both stimulations (Table 5B). Interestingly, in the plasma, ipo LPS induced much higher levels of IL-6 (246±51 pg/ml) than those induced by ipo casein (87.5±16 pg/ml). Conversely, in the air pouch, ipo casein induced higher levels of IL-6 (6500±736 pg/ml) than those induced by ipo LPS (4253±565 pg/ml).
Table 5.
| Table 5A: Relative IL-6 mRNA expression in various sites after ipo casein injection
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Leukocyt es | Air pouch cells | Liver | Spleen | Brain | Hypothalamus | Hippocampus | Pituitary | |
| ipo Vehicle | ND | ND | ND | ND | ND | ND | ND | ND |
| ipo Casein 4h | ND | *1.9±1.4 (×10−3) | ND | ND | ND | ND | ND | ND |
| ipo Casein 8h | ND | *2.0±0.3 (×10−3) | ND | ND | ND | ND | ND | ND |
| ipo LPS 6h | ND | *1.4±0.2 (×10−3) | ND | ND | ND | ND | ND | ND |
| Table 5B: IL-6 protein expression in various sites after ipo casein injection
| |||||
|---|---|---|---|---|---|
| Plasma (pg/ml) | Airpouch lavage (pg/ml) | Brain (pg/1mg protein) | Hypothalamus (pg/1mg protein) | Hippocampus (pg/1mg protein) | |
| ipo Vehicle | ND | 36±11 | 2.7±0.5 | ND | ND |
| ipo Casein 4h | *64±14 | *6625±554 | 3.5±1.0 | ND | ND |
| ipo Casein 8h | *87.5±16 | *6500±736 | 3.1±0.8 | ND | ND |
| ipo LPS 6h | *246±51 | *4253±565 | 3.6±0.7 | ND | ND |
IL-6 mRNA (A, IL-6/G3PDH) and protein (B) levels are shown. Values are means ± SEM (n=4).
Asterisk indicates significant difference (p<0.05) between vehicle-injected and inflammatory agents-injected animals. ND. Not Detectable
COX-2 mRNA was induced in the cells of the air pouch, but not in the blood and brain after ipo casein. On the other hand, ipo LPS induced COX-2 expression in the air pouch, spleen, pituitary and brain tissues (Table 6).
Table 6.
Relative COX-2 mRNA expression in various tissues after ipo casein injection
| Leukocytes | Air pouch cells | Liver | Spleen | Brain | Hypothalamus | Hippocampus | Pituitary | |
|---|---|---|---|---|---|---|---|---|
| ipo Vehicle | ND | ND | ND | ND | 6.9±0.5 (×10−4) | ND | ND | ND |
| ipo Casein 4h | ND | *2.5±0.4 (×10−1) | ND | ND | 8.4±2.1 (×10−4) | ND | ND | ND |
| ipo Casein 8h | ND | *2.0±0.2 (×10−1) | ND | ND | 6.3±0.5 (×10−4) | ND | ND | ND |
| ipo LPS 6h | ND | *0.8±0.2 (×10−1) | ND | *19.7±5.8 (×10−3) | *32±6.5 (×10−4) | *5.8±0.6 (×10−4) | *55.7±4.4 (×10−4) | *7.3±1.5 (×10−3) |
COX-2 mRNA (COX-2/G3PDH) levels are shown. Values are means ± SEM (n=4). Asterisk indicates significant difference (p<0.05) between vehicle-injected and inflammatory agents-injected animals. ND. Not Detectable
We then studied whether IL-1R1, IL-6, COX-2, and TLR4 are required for casein-induced febrile and locomotor activity changes. Ip casein injections were made in IL-1R1 KO, COX-2 KO, C3H/HeJ (TLR4 mutant), and IL-6 KO animals. In contrast to the responses from wild type mice, ip casein failed to induce significant Tco increases in any of these mice (Fig 3A-D). The means of LA between 2 and 10 hrs after the injections were also presented (Fig 3E). After casein injection, no significant change in locomotor activity was found in IL-1R1 KO, C3H/HeJ and COX-2 KO mice, but dramatic reduction in locomotor activity was found in IL-6 KO mice.
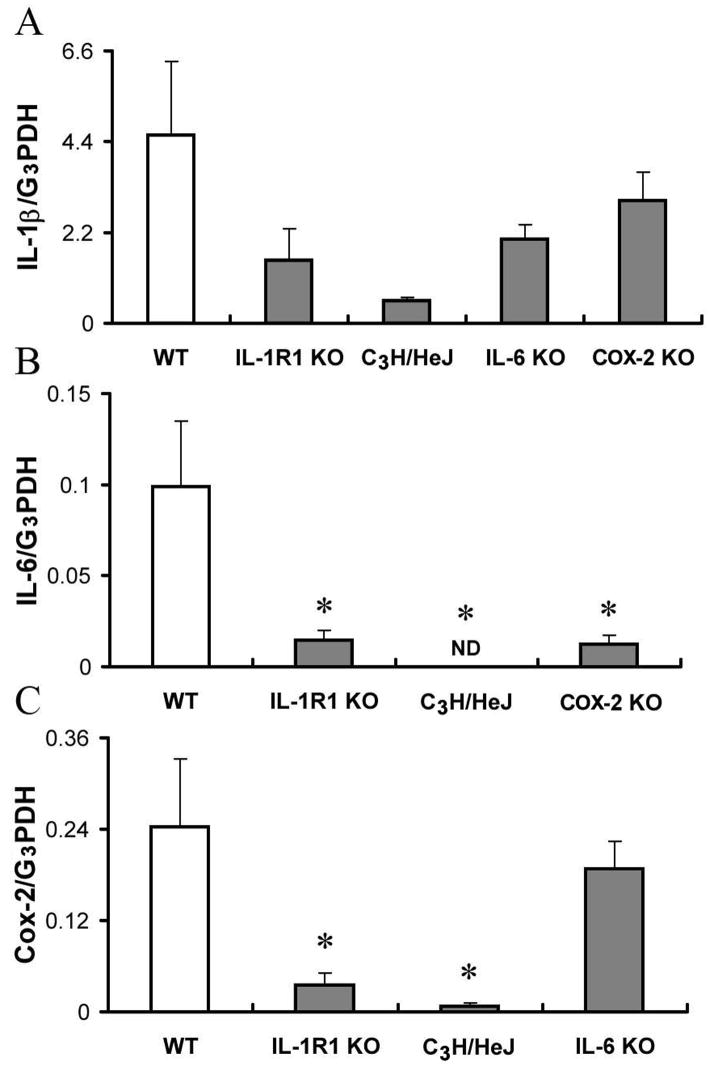
IL-1β, IL-6, and COX-2 mRNA levels in peritoneal cells 6 hrs after ip casein injection in various transgenic animals are shown in Figure 4. In IL-1R1 KO, C3H/HeJ, and IL-6 KO animals, the levels of IL-1β mRNA were lower than in wild type animals, although no significant statistical difference was found (Fig 4A) (p>0.05). In C3H/HeJ mice, IL-6 mRNA was not detectable (ND, Fig. 4B). In IL-1R1 KO and COX-2 KO animals, the levels of IL-6 mRNA were lower than in wild type animals. Again, the differences were not statistically significant (p>0.05). These results are shown in Fig. 4B. In both IL-1R1 KO and C3H/HeJ mice, the levels of COX-2 mRNA were significantly lower (p<0.05) than in wild type animals. In IL-6 KO animals, the level of COX-2 mRNA was not different from wild type animals. These results are shown in Fig. 4C.
Figure 4.
Relative mRNA levels of IL-1β (IL-1β/G3PDH), IL-6 (IL-1β/G3PDH), and COX-2 (COX-2/G3PDH) in peritoneal cells after ip casein injection. Data are presented as mean ± SEM (n=4). *: p<0.05, comparing transgenic animals and the wild type animals (n=4). ND, Not Detectable.
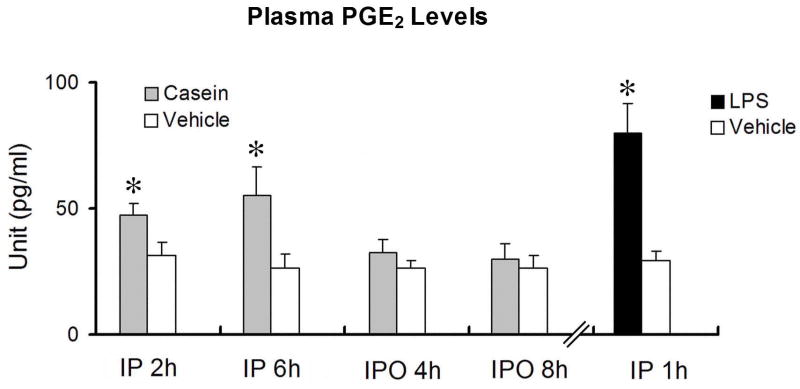
Plasma PGE2 levels after ip or ipo injection of casein were measured in wild type animals. Ipo casein injection did not induce PGE2 increases in the plasma at the onset (4 hrs post-injection) or at the plateau (8 hrs post-injection) of the febrile response. Ip casein, on the other hand, induced significant plasma increases at the onset (2 hrs post-injection) and at the plateau (6 hrs post-injection) of the febrile response. Plasma PGE2 levels were measured 1 h after ip LPS injection as positive controls for the assay. Ip LPS induced a significant increase in plasma PGE2, similar to that reported previously. These results are shown in Fig. 5.
Figure 5.
Levels of PGE2 in the plasma of wild type mice after ip or ipo injection of casein or vehicle. The level of plasma PGE2 1h after ip injection of LPS were also measured as a positive control. Data are presented as mean ± SEM (n=4).
Discussion
Several results in the present study are worth noting: 1) After ip injections of casein or a septic dose of LPS, higher levels of IL-1, IL-6, and COX-2 expression were induced by casein in the peritoneal cavity whereas in the brain and blood, higher IL-1 and COX-2 expression was induced by LPS (Tables 1, 2 and 3). 2) Both ip and ipo casein induced long-lasting fever and reduction in locomotor activity without the significant induction of IL-1, IL-6 and COX-2 in the brain and without the induction of IL-1 in the blood. 3) IL-1R1, COX-2, TLR4, and IL-6 are all required for the expression of febrile responses induced by casein, but casein-induced reduction of locomotor activity does not require IL-6. 4) Ip, but not ipo, casein induced PGE2 increases in the plasma. These results suggest casein injections, especially via ipo route, induce restricted localized inflammation and that inflammatory mediators at the site of inflammation, not in systemic circulation, might activate CNS controlled neuroimmune effects.
Casein has rarely been used as an inflammatory agent in studies concerning neuroimmune activation. An early study by Kitamura et al. showed that ip casein injection in rabbits induced a long-lasting febrile response without eliciting significant amounts of endogenous pyrogens in the blood (Kitamura et al., 1986), although very high levels of endogenous pyrogens were detected in the peritoneal cavity. In the present study, a striking difference between LPS and casein induced effects is illustrated by the results in Table1. Although ip LPS induced less IL-1β mRNA and IL-1 protein in the peritoneal cavity as compared with those induced by ip casein, ip LPS, not ip casein, induced the appearance of IL-1β in the blood. Therefore, high levels of peritoneal IL-1 induced by ip casein are restricted from entering the general circulation. On the contrary, ip LPS appears to facilitate significant spillover of peritoneal IL-1 into circulation. The casein-induced localized IL-1 production profile is consistent with some clinical observations that levels of circulating IL-1 are often far below the levels of IL-1 at the site of localized inflammation (Wiik et al., 2001).
The lack of significant brain cytokine induction in the present study is striking, compared with numerous studies in the literature that showed peripheral inflammation causes the induction of IL-1 and/or IL-6 in the brain (Quan and Banks, 2007). We initially only analyzed the whole brain tissue. But, because cytokine expression in the hypothalamus and hippocampus has been shown to be particularly sensitive to peripheral immune challenges (Takao et al., 1993), we also analyzed cytokine levels in these critical brain regions. The results showed that expression of IL-1 and IL-6 did not increase significantly in the brain after casein injections. In contrast, both ip and ipo LPS induced apparent distal effects—expression of brain COX-2 mRNA was consistently induced by LPS. Thus, casein-induced inflammation could represent a qualitatively different type of inflammation, namely, a restricted localized inflammation.
In the present study, both ip and ipo casein induced the appearance of IL-6 protein in the blood, raising the question in regards to the origin and the role of systemic IL-6 in this model. IL-6 mRNA, on the contrary, was not detected in the blood after either ip or ipo casein injection. Because both IL-6 mRNA and IL-6 protein were found at the sites of casein injection, it is likely that the observed plasma IL-6 protein was derived from cells at these injection sites. Previous studies have suggested that IL-6 leaked into circulation from localized inflammation might mediate fever induction. For example, Rummel et al. showed that febrile response induced by ipo LPS may be mediated by brain COX-2 expression which is induced by circulating IL-6 (Rummel et al., 2006). In the present study the highest circulating levels of IL-6 were 462±114 pg/ml after ip casein and 87.5±16 pg/ml after ipo casein. Cartmell et al. showed previously that a concentration of 5000 pg/ml of IL-6 in the plasma did not induce fever in rats (Cartmell et al., 2000). Therefore, circulating IL-6 levels induced by the casein injections in the present study, especially those induced by ipo casein, are not likely to be sufficient to mediate casein-induced fever. In addition, we found in the present study that both ip and ipo LPS induced plasma IL-6 and brain COX-2. After the casein injections, however, the appearance of plasma IL-6 was not associated with the induction of brain COX-2. Therefore, the pathway involving the induction of brain COX-2 by plasma IL-6 may be operative in CNS activation induced by local injections of LPS, but not casein.
The results of the present study suggest that neuroimmune communication pathway activated by restricted local inflammation is distinct from those activated by the presence of systemic inflammation. Previous studies have shown that systemic inflammation causes the appearance of pro-inflammatory cytokines in the blood and in the brain via multiple pathways (Quan and Banks, 2007). One target of these cytokines is the cerebral endothelium. In response to cytokine or LPS stimulation, brain endothelial cells produce the enzymes COX-2 (Breder et al., 1992; Quan et al., 1998; Dunn et al., 2006) and microsomal prostaglandin E synthase-1 (mPGES-1) (Engblom et al., 2003) to catalyze the production of prostaglandin E2 (PGE2). It has been shown recently that activation of EP3 prostaglandin receptors in the hypothalamus is critical for peripheral LPS-induced fever (Lazarus et al., 2007). Therefore, the generally accepted paradigm is that inflammatory mediators distal to the site of inflammation, such as cytokines in the circulation and PGE2 in the brain, are the primary triggers of neuroimmune effects induced by systemic inflammation. In contrast, other studies showed that localized inflammation may stimulate CNS by local inflammatory factors (Rummel et al., 2005) which transmit signals to the brain via primary sensory (Cooper and Rothwell, 1991; Ross et al., 2000) or vagal sensory afferents (Roth and De Souza, 2001). A limiting factor in these studies for highlighting the non-humoral pathways is the use of LPS as the inflammatory agent. Whereas local inflammation induced by low doses of LPS activate the CNS via vagal (Romanovsky et al., 1997) or primary sensory afferents (Rummel et al., 2005), activation of these neural pathways by high doses of LPS may be masked by the activation of humoral pathways of neuroimmune communication (Romanovsky et al., 1997; Ross et al., 2000).
A major discovery of this study is that robust localized inflammation induces long-lasting febrile responses and reduction in locomotor activity without inducing brain COX-2 expression. Neither ip nor ipo casein caused induction of COX-2 expression in blood cells and in the brain, although COX-2 induction was clearly present in the sites of casein injection. Rummel et al. showed previously that fever induced by local injection of a low dose of LPS is dependent on prostaglandins produced at the site of injection, but fever induced by locally injected high dose of LPS may be a consequence of prostaglandin production from both the site of injection and brain (Rummel et al., 2005). It is important to note that injection of free LPS does not mimic bacterial infection where LPS is bound to the bacterial surface. This is because during infection, bacterial surface LPS does not simply turn into free LPS. In fact, the release of free LPS during live bacterial infection is tightly controlled to be at minimal levels by immune cells and the presence of free LPS is associated with endotoxic shock and distal organ damage (Creasey et al., 1991). Thus, the presence of high levels of free LPS during localized infection may signal that physiological immune responses at the infected location are unable to contain the extent of inflammation and that potentially damaging spread of systemic inflammation to distal organs may ensue. This could explain why casein-induced restricted local inflammation appears to activate CNS differently from that induced by local LPS injections.
Only a few studies have investigated neuroimmune activation after live bacterial infection. Goehler et al. showed that oral inoculation of Campylobacter jejuni activated the CNS via vagal afferents without the induction of pro-inflammatory cytokines in the blood (Goehler et al., 2005). Campisi et al. showed that subcutaneous injection of live E. coli induced neuroimmune effects before the induction of brain and blood pro-inflammatory cytokines (Campisi et al., 2003). Therefore, the results of the present study might mimic aspects of neuroimmune activation induced by these live bacterial infections.
A recently discovered pathway by which peripheral inflammation activates the CNS is via circulating PGE2. The role of circulating PGE2 in CNS activation has long been controversial because intravenous infusion of PGE2 does not cause neuroimmune effects consistently and increase PGE2 levels in the brain parenchyma (Sehic et al., 1996). Recently, Romanovsky et al. showed that PGE2 conjugated to the carrier protein albumin can potently induce fever (Romanovsky et al., 1999). Further, it has been shown that early stages of fever induced by LPS are mediated by circulating PGE2 produced by hepatic and pulmonary macrophages (Ivanov et al., 2002; Steiner et al., 2006b). In addition, Steiner et al. showed that bone marrow derived TLR4 positive cells, not brain endothelial cells, are critical for the initiation of LPS fever (Steiner et al., 2006a). The importance of peripheral PGE2 was further strengthened by Ootsuka et al. who showed that circulating PGE2, not the integrity of the vagus, is critical for the induction of the early phase of fever (Ootsuka et al., 2008). Therefore, circulating PGE2 may act as an endocrine to play a pivotal role in the induction of fever. In the present study, we found that ip, but not ipo, casein induced PGE2 increases in the blood. It is possible that blood PGE2 mediated humoral activation of the CNS is involved in the ip casein-induced effects, but CNS activation by ipo casein appears to be independent of this pathway.
Another plausible pathway for restricted local inflammation to stimulate the CNS is the neural route. A large literature exists supporting the role of vagus afferents in mediating neuroimmune communication (Quan and Banks, 2007). In the present study, both ip and ipo casein induced CNS mediated effects. Therefore, the vagus nerve which innervates the peritoneal cavity and the primary sensory nerve which innervates terminal fields surrounding the air pouch may both participate in mediating the observed immune-to-brain activation.
Our results that local inflammation induced neuroimmune effects can be blocked in IL-1R1 KO and IL-6 KO animals are consistent with previous reports (Kozak et al., 1998). Interestingly, C3H/HeJ animals which show a defective response to the TLR4 ligand were also unresponsive to casein stimulation. Because, casein is not known as a TLR4 ligand, it is not likely that TLR4 is stimulated by casein directly. However, TLR4 is known to participate in responses induced by non-infectious injury by interacting with hyaluronan (Taylor et al., 2007). Therefore, the requirement of TLR4 in local inflammation induced CNS activation suggests that this activity may be gated by tissue injury.
The relationship among the potential mediators of neuroimmune activation was examined in the present study. Interestingly, local production of IL-1β was induced by casein in IL-1R1, IL-6, and COX-2 KO animals as well as C3H/HeJ animals, albeit less IL-1β was induced in COX-2 KO animals. Similarly, COX-2 induction was not completely abolished in IL-6 KO, IL-1R1 KO and C3H/HeJ animals, although IL-1R1 and TLR4 appear to be required for the majority of the COX-2 induction. IL-6 induction also persisted in both IL-1R1 and COX-2 KO animals, but was completely blunted in C3H/HeJ animals. The lack of IL-6 induction by casein in C3H/HeJ animals has been reported previously (Tobita et al., 2006). These data are not consistent with a simple cascade proposed in the literature that stimulation of TLR4 induces IL-1 which then induces IL-6 which induces COX-2. Rather, these data suggest that the induction of one of these factors does not depend exclusively on the presence of the postulated upstream factors. Because casein-induced fever is absent in IL-1R1, IL-6, and COX-2 KO and C3H/HeJ animals, and that casein-induced reduction in locomotor activity is also absent in all these animals except IL-6 KO animals, the combined presence of IL-1R1, IL-6, COX-2, and TLR4 appear to be necessary for localized tissue inflammation to activate febrile responses although IL-6 is not required for the induction of reduced locomotor activity.
Activation of the CNS by systemic neuroimmune pathways and by pathways sensing restricted local inflammation may represent two diametrically opposite scenarios. The presence of LPS and systemic inflammatory cytokines is associated with mortality (Creasey et al., 1991; Sullivan et al., 1992; Pinsky et al., 1993) and distal organ damage (Adembri et al., 2004). Therefore, systemic activation of neuroimmune effects is likely to initiate a general alarm response, aimed at dampening the life threatening sepsis or distal organ damage. Indeed, it has been shown that LPS-induced septic shock is accompanied by high level of circulating IL-6 (Xing et al., 1998)and glucocorticoids (Lazar et al., 1992), which exert critical anti-inflammatory effects that reduce mortality. In contrast, local inflammation as in the present study informs the CNS of the immunological status from the site of immune activity, probably to enlist CNS controlled responses beneficial for the control of local infection. This could be why neither pro-inflammatory cytokines (Strait et al., 1999) nor LPS (Jansen et al., 1996) can be consistently detected in circulation during non-lethal febrile conditions. But fevers lasting from 12 hrs (Pratt and Attia, 2007) to 8 months (Ziai and Noorani, 1972) in duration without systemic complications have been reported. It has been shown elegantly that local injection of bacteria induces febrile responses that is critical for host survival due to enhanced host defense (Kluger et al., 1975). It is possible that pathways relaying local inflammation signals to the CNS, such as those induced in the present study, is not involved in dampening sepsis, but play a major role in increased host defense during localized infection.
The onset of the febrile response is slower after ipo casein than after ip casein. This is probably because that the induction of inflammatory responses may be faster in the peritoneal cavity due to readily present peritoneal cells of innate immune response whereas longer time may be required to move circulating immune cells into the air pouch. Thus, the timing of the elicited CNS effects by local inflammation may be associated with the timing of the development of inflammation at the sites of casein injection.
An unexpected finding is that casein injection induced pituitary IL-1β mRNA expression. Because no increase of IL-1β protein was found in the plasma, IL-1β produced by the pituitary may be insufficient to alter the blood IL-1β levels in the present experiments. The fact the local inflammation causes pituitary IL-1β mRNA expression is highly intriguing. The pathway leading to this induction remains to be identified.
Acknowledgments
This study was supported by an NIH Grant AI059089 to NQ. We thank Michelle Kelly for her careful reading of the manuscript.
Abbreviations
- CNS
Central nervous system
- CPM
Counts per minute
- COX-2
Cyclooxygenase-2
- IL-1
Interleukin-1
- IL-6
Interleukin-6
- ip
Intraperitoneal
- ipo
Intra-airpouch
- KO
Knockout
- LPS
Lipopolysaccharide
- LA
Locomotor activity
- PGE2
Prostaglandin E2
- TLR
Toll like receptor
- IL-1R1
Type I interleukin-1 receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adembri C, Kastamoniti E, Bertolozzi I, Vanni S, Dorigo W, Coppo M, Pratesi C, De Gaudio AR, Gensini GF, Modesti PA. Pulmonary injury follows systemic inflammatory reaction in infrarenal aortic surgery. Crit Care Med. 2004;32:1170–1177. doi: 10.1097/01.ccm.0000124875.98492.11. [DOI] [PubMed] [Google Scholar]
- Breder CD, Smith WL, Raz A, Masferrer J, Seibert K, Needleman P, Saper CB. Distribution and characterization of cyclooxygenase immunoreactivity in the ovine brain. J Comp Neurol. 1992;322:409–438. doi: 10.1002/cne.903220309. [DOI] [PubMed] [Google Scholar]
- Campisi J, Hansen MK, O’Connor KA, Biedenkapp JC, Watkins LR, Maier SF, Fleshner M. Circulating cytokines and endotoxin are not necessary for the activation of the sickness or corticosterone response produced by peripheral E. coli challenge. J Appl Physiol. 2003;95:1873–1882. doi: 10.1152/japplphysiol.00371.2003. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Poole S, Turnbull AV, Rothwell NJ, Luheshi GN. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J Physiol. 2000;526(Pt 3):653–661. doi: 10.1111/j.1469-7793.2000.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Zhang H, Lai W, Quan N. Peripheral injection of lipopolysaccharide prevents brain recruitment of leukocytes induced by central injection of interleukin-1. Neuroscience. 2006;137:717–726. doi: 10.1016/j.neuroscience.2005.08.087. [DOI] [PubMed] [Google Scholar]
- Cooper AL, Rothwell NJ. Mechanisms of early and late hypermetabolism and fever after localized tissue injury in rats. Am J Physiol. 1991;261:E698–705. doi: 10.1152/ajpendo.1991.261.6.E698. [DOI] [PubMed] [Google Scholar]
- Creasey AA, Stevens P, Kenney J, Allison AC, Warren K, Catlett R, Hinshaw L, Taylor FB., Jr Endotoxin and cytokine profile in plasma of baboons challenged with lethal and sublethal Escherichia coli. Circ Shock. 1991;33:84–91. [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, Zhang H, Quan N. Reduced ingestion of sweetened milk induced by interleukin-1 and lipopolysaccharide is associated with induction of cyclooxygenase-2 in brain endothelia. Neuroimmunomodulation. 2006;13:96–104. doi: 10.1159/000096291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom D, Saha S, Engstrom L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci. 2003;6:1137–1138. doi: 10.1038/nn1137. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Nguyen KT, Cotter CS, Watkins LR, Maier SF. Acute stressor exposure both suppresses acquired immunity and potentiates innate immunity. Am J Physiol. 1998;275:R870–878. doi: 10.1152/ajpregu.1998.275.3.R870. [DOI] [PubMed] [Google Scholar]
- Givalois L, Dornand J, Mekaouche M, Solier MD, Bristow AF, Ixart G, Siaud P, Assenmacher I, Barbanel G. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol. 1994;267:R164–170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hedges SR, Barrientes F, Desmond RA, Schwebke JR. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis. 2006;193:556–562. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ. Central nervous system recognition of peripheral inflammation: a neural, hormonal collaboration. Acta Biomed. 2007;78(Suppl 1):231–247. [PubMed] [Google Scholar]
- Ichitani Y, Shi T, Haeggstrom JZ, Samuelsson B, Hokfelt T. Increased levels of cyclooxygenase-2 mRNA in the rat spinal cord after peripheral inflammation: an in situ hybridization study. Neuroreport. 1997;8:2949–2952. doi: 10.1097/00001756-199709080-00028. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Pero RS, Scheck AC, Romanovsky AA. Prostaglandin E(2)-synthesizing enzymes in fever: differential transcriptional regulation. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1104–1117. doi: 10.1152/ajpregu.00347.2002. [DOI] [PubMed] [Google Scholar]
- Jansen PM, Pixley RA, Brouwer M, de Jong IW, Chang AC, Hack CE, Taylor FB, Jr, Colman RW. Inhibition of factor XII in septic baboons attenuates the activation of complement and fibrinolytic systems and reduces the release of interleukin-6 and neutrophil elastase. Blood. 1996;87:2337–2344. [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:R422–432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Goto F, Ohkawara S, Yoshinaga M. Production of pyrogen by polymorphonuclear leukocytes during the course of casein-induced peritonitis in rabbits. Acta Pathol Jpn. 1986;36:791–803. doi: 10.1111/j.1440-1827.1986.tb03114.x. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188:166–168. [PubMed] [Google Scholar]
- Kozak W, Zheng H, Conn CA, Soszynski D, van der Ploeg LH, Kluger MJ. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 beta-deficient mice. Am J Physiol. 1995;269:R969–977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- Kozak W, Kluger MJ, Soszynski D, Conn CA, Rudolph K, Leon LR, Zheng H. IL-6 and IL-1 beta in fever. Studies using cytokine-deficient (knockout) mice. Ann N Y Acad Sci. 1998;856:33–47. doi: 10.1111/j.1749-6632.1998.tb08310.x. [DOI] [PubMed] [Google Scholar]
- Lazar G, Jr, Lazar G, Agarwal MK. Modification of septic shock in mice by the antiglucocorticoid RU 38486. Circ Shock. 1992;36:180–184. [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Lenczowski MJ, Van Dam AM, Poole S, Larrick JW, Tilders FJ. Role of circulating endotoxin and interleukin-6 in the ACTH and corticosterone response to intraperitoneal LPS. Am J Physiol. 1997;273:R1870–1877. doi: 10.1152/ajpregu.1997.273.6.R1870. [DOI] [PubMed] [Google Scholar]
- Leon LR, Conn CA, Glaccum M, Kluger MJ. IL-1 type I receptor mediates acute phase response to turpentine, but not lipopolysaccharide, in mice. Am J Physiol. 1996;271:R1668–1675. doi: 10.1152/ajpregu.1996.271.6.R1668. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Hopkins SJ, Luheshi GN. Sites of action of IL-1 in the development of fever and cytokine responses to tissue inflammation in the rat. Br J Pharmacol. 1997;120:1274–1279. doi: 10.1038/sj.bjp.0701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moissidis I, Chaidaroon D, Vichyanond P, Bahna SL. Milk-induced pulmonary disease in infants (Heiner syndrome) Pediatr Allergy Immunol. 2005;16:545–552. doi: 10.1111/j.1399-3038.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW, Steiner AA, Romanovsky AA. Fever response to intravenous prostaglandin E2 is mediated by the brain but does not require afferent vagal signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1294–1303. doi: 10.1152/ajpregu.00709.2007. [DOI] [PubMed] [Google Scholar]
- Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int. 2007;49:31–35. doi: 10.1111/j.1442-200X.2007.02316.x. [DOI] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Quan N, Sundar SK, Weiss JM. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. J Neuroimmunol. 1994;49:125–134. doi: 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281–293. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Karman EK. Blood-borne, albumin-bound prostaglandin E2 may be involved in fever. Am J Physiol. 1999;276:R1840–1844. doi: 10.1152/ajpregu.1999.276.6.R1840. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol. 1997;273:R407–413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Lenczowski MJ, Kulchitsky VA, Van Dam AM, Poole S, Homer LD, Tilders FJ. Lipopolysaccharide transport from the peritoneal cavity to the blood: is it controlled by the vagus nerve? Auton Neurosci. 2000;85:133–140. doi: 10.1016/S1566-0702(00)00232-0. [DOI] [PubMed] [Google Scholar]
- Ross G, Roth J, Storr B, Voigt K, Zeisberger E. Afferent nerves are involved in the febrile response to injection of LPS into artificial subcutaneous chambers in guinea pigs. Physiol Behav. 2000;71:305–313. doi: 10.1016/s0031-9384(00)00358-9. [DOI] [PubMed] [Google Scholar]
- Roth J, De Souza GE. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz J Med Biol Res. 2001;34:301–314. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- Roth J, Martin D, Storr B, Zeisberger E. Neutralization of pyrogen-induced tumour necrosis factor by its type 1 soluble receptor in guinea-pigs: effects on fever and interleukin-6 release. J Physiol. 1998;509 (Pt 1):267–275. doi: 10.1111/j.1469-7793.1998.267bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel C, Sachot C, Poole S, Luheshi GN. Circulating interleukin-6 induces fever through a STAT3-linked activation of COX-2 in the brain. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1316–1326. doi: 10.1152/ajpregu.00301.2006. [DOI] [PubMed] [Google Scholar]
- Rummel C, Barth SW, Voss T, Korte S, Gerstberger R, Hubschle T, Roth J. Localized vs. systemic inflammation in guinea pigs: a role for prostaglandins at distinct points of the fever induction pathways? Am J Physiol Regul Integr Comp Physiol. 2005;289:R340–R347. doi: 10.1152/ajpregu.00104.2005. [DOI] [PubMed] [Google Scholar]
- Sehic E, Szekely M, Ungar AL, Oladehin A, Blatteis CM. Hypothalamic prostaglandin E2 during lipopolysaccharide-induced fever in guinea pigs. Brain Res Bull. 1996;39:391–399. doi: 10.1016/0361-9230(96)00037-8. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Chakravarty S, Rudaya AY, Herkenham M, Romanovsky AA. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood. 2006a;107:4000–4002. doi: 10.1182/blood-2005-11-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Ivanov AI, Serrats J, Hosokawa H, Phayre AN, Robbins JR, Roberts JL, Kobayashi S, Matsumura K, Sawchenko PE, Romanovsky AA. Cellular and molecular bases of the initiation of fever. PLoS Biol. 2006b;4:e284. doi: 10.1371/journal.pbio.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait RT, Kelly KJ, Kurup VP. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 levels in febrile, young children with and without occult bacteremia. Pediatrics. 1999;104:1321–1326. doi: 10.1542/peds.104.6.1321. [DOI] [PubMed] [Google Scholar]
- Sullivan JS, Kilpatrick L, Costarino AT, Jr, Lee SC, Harris MC. Correlation of plasma cytokine elevations with mortality rate in children with sepsis. J Pediatr. 1992;120:510–515. doi: 10.1016/s0022-3476(05)82476-x. [DOI] [PubMed] [Google Scholar]
- Takao T, Culp SG, De Souza EB. Reciprocal modulation of interleukin-1 beta (IL-1 beta) and IL-1 receptors by lipopolysaccharide (endotoxin) treatment in the mouse brain-endocrine-immune axis. Endocrinology. 1993;132:1497–1504. doi: 10.1210/endo.132.4.8462448. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- Tobita K, Kawahara T, Otani H. Bovine beta-casein (1–28), a casein phosphopeptide, enhances proliferation and IL-6 expression of mouse CD19+ cells via Toll-like receptor 4. J Agric Food Chem. 2006;54:8013–8017. doi: 10.1021/jf0610864. [DOI] [PubMed] [Google Scholar]
- Wal JM. Cow’s milk proteins/allergens. Ann Allergy Asthma Immunol. 2002;89:3–10. doi: 10.1016/s1081-1206(10)62115-1. [DOI] [PubMed] [Google Scholar]
- Wiik H, Karttunen R, Haukipuro K, Syrjala H. Maximal local and minimal systemic cytokine response to colorectal surgery: the influence of perioperative filgrastim. Cytokine. 2001;14:188–192. doi: 10.1006/cyto.2001.0870. [DOI] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Umegae S, Kitagawa T, Matsumoto K. Systemic and local cytokine production in quiescent ulcerative colitis and its relationship to future relapse: a prospective pilot study. Inflamm Bowel Dis. 2005;11:589–596. doi: 10.1097/01.mib.0000161917.97136.e2. [DOI] [PubMed] [Google Scholar]
- Ziai M, Noorani PB. An infant with fever of eight months’ duration. Clin Pediatr (Phila) 1972;11:61–62. doi: 10.1177/000992287201100118. [DOI] [PubMed] [Google Scholar]