Abstract
OBJECTIVES
To test whether the addition of melatonin to bright-light therapy enhances the efficacy in treating rest–activity (circadian) disruption in institutionalized patients with Alzheimer’s disease (AD).
DESIGN
Randomized, controlled trial.
SETTING
Two nursing homes in San Francisco, California.
PARTICIPANTS
Fifty subjects (mean age 86) with AD.
INTERVENTION
Experimental subjects received 1 hour of morning light exposure (≥2,500 lux in gaze direction) Monday to Friday for 10 weeks and 5 mg melatonin (LM, n = 16) or placebo (LP, n = 17) in the evening. Control subjects (n = 17) received usual indoor light (150–200 lux).
MEASUREMENTS
Nighttime sleep variables, day sleep time, day activity, day:night sleep ratio, and rest–activity parameters were determined using actigraphy.
RESULTS
Linear mixed models were employed to test the primary study hypotheses. No significant differences in nighttime sleep variables were found between groups. At the end of the intervention, the LM group showed significant improvement in daytime somnolence as indicated by a reduction in the duration of daytime sleep, an increase in daytime activity, and an improvement in day:night sleep ratio. The LM group also evidenced a significant increase in rest–activity rhythm amplitude and goodness of fit to the cosinor model.
CONCLUSION
Light treatment alone did not improve nighttime sleep, daytime wake, or rest–activity rhythm. Light treatment plus melatonin increased daytime wake time and activity levels and strengthened the rest–activity rhythm. Future studies should resolve the question of whether these improvements can be attributed to melatonin or whether the two zeitgebers interact to amplify efficacy.
Keywords: actigraphy, dementia, sleep, circadian rhythm, nursing home
With Alzheimer’s disease (AD), nighttime sleep is severely fragmented, and daytime activity is disrupted by multiple naps. Leading theories that suggest possible etiologies for the rest–activity disruption include neurological deterioration that underlies the AD process and decreased exposure to external zeitgebers that influence circadian rhythms (e.g., bright light).1 Pharmacological treatments for nighttime sleep disruption have proven only minimally effective and are often associated with unacceptable side effects that are particularly problematic in AD, because sedative medications worsen cognition and contribute to fall risk.1,2 Disturbances in the rest–activity rhythm negatively affect quality of life and are one of the primary reasons caregivers seek institutionalization of patients with AD.3,4 Patients with socially unacceptable rest–activity rhythms (i.e., active during the night and asleep during the day) pose challenges for professional and lay care providers. In an institutional environment, patients experiencing rest–activity disruption can disturb other residents at night. Daytime somnolence also prevents participation in activities and social interaction.5
Exposure of the eyes to light of sufficient intensity and duration at the appropriate time of day can have profound effects on the quality, duration, and timing of sleep. The retinohypothalamic tract mediates the effect of light on the brain, and the daily light–dark cycle is the primary synchronizer responsible for entrainment of circadian rhythms to the 24-hour day. In an institutional environment, where light levels tend to be low, residents may not be exposed to sufficient bright light to entrain to the 24-hour day.6
Therapeutic exposure to bright light has been shown to alter rest–activity rhythms. Results from earlier phases of this study indicated that morning bright-light exposure (9:30–10:30 a.m.) for 10 weeks did not induce an overall improvement in measures of sleep or rest–activity rhythm, although subjects with aberrant timing of their rest–activity rhythm showed significant improvement in rhythm stability and amplitude.7 Subjects who received morning (9:30–10:30 a.m.) or afternoon (3:30–4:30 p.m.) bright-light exposure for 10 weeks were subsequently compared with a control group, and significant stabilization of the rest–activity rhythm acrophase was found in subjects who received bright light.8 Other investigators have reported positive effects of bright light on nighttime sleep time and circadian rhythm variables in subjects with dementia.9–14 In summary, although the appropriate intensity, duration, and timing of exposure to light has not been established, research results indicate that bright light can be an effective treatment strategy for rest–activity disruption in subjects with AD.
Retinal neurons respond to stimuli from the light–dark cycle and project, through the retinohypothalamic tract, to the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, which acts as a pacemaker. The light–dark cycle entrains the pacemaker and its outputs, including melatonin secretion.13,15 The pacemaker thus regulates the pineal gland’s timed secretion of the neurohormone melatonin, which in turn feeds back on melatonin receptors in the SCN. This feedback may be attenuated with older age because of a reduction in serum melatonin concentration and a shift in the melatonin secretion rhythm.15–17 Melatonin secretion is even lower in patients with AD, and this decrease is evident in the early stages of the disease.16,18–20 A further attenuation of the functional feedback signal of melatonin to the SCN in AD may result from the decrease in SCN melatonin receptors.21 Exogenous administration of melatonin in the morning delays circadian rhythms, and administration in the evening advances circadian rhythms.15 Nighttime melatonin administration has also been shown to act as a soporific, increasing sleep propensity, sleep efficiency, and daytime alertness and decreasing sleep onset latency and number of nighttime awakenings.22,23 In patients with dementia, exogenous melatonin administration has been shown to improve sleep in some studies but not in others. In two community-based studies using doses of 2.5 and 10 mg of melatonin and 6 mg of slow-release melatonin, there were no significant effects on nighttime sleep variables.24,25 In nursing home subjects, 6-mg melatonin treatment resulted in better sleep and less sundowning,26 and 1 to 3 mg melatonin resulted in less daytime sleepiness and sundowning but no improvement in nighttime sleep.27
Melatonin is considered to be a safe and nontoxic molecule. In healthy elderly people, low doses (0.2–2 mg) reportedly did not produce improvement in sleep measures, but a higher dose (50 mg) produced a sleep benefit with no adverse effects.24 One study found that 5 mg administered over 1 week trended toward improving sleep in subjects with Parkinson’s disease.28 Few side effects have been observed with low-dose melatonin (≤10 mg). None were noted during the pilot study (n = 8)28 or the subsequent larger study (n = 40).29 Another study24 found in its large sample (n = 157) no difference between tolerability of melatonin and that of placebo. Long-term side effects or interactions of melatonin with other drugs are not known.30 Possible effects of exogenous melatonin in humans include antioxidant properties, drowsiness, reduced glucose tolerance, an increase in peripheral but not cerebral blood flow, and reduction in blood pressure.31–34
Treatment with simultaneous bright light and melatonin in subjects with dementia has also been studied. One study35 reported on motor restless behavior in institutionalized subjects who received bright-light therapy in combination with 2.5 mg of melatonin and bright-light therapy in combination with placebo. Subjects who received melatonin became more aggressive and exhibited more disturbed behavior than subjects who received the placebo, who exhibited less restlessness and better cooperation.
In summary, the results of previous studies on the effects of light and melatonin treatments for sleep disruption in dementia have been equivocal, and treatment durations have been short (a few weeks) and sample sizes small. The purpose of this study was to investigate the effects of bright light and bright light plus melatonin as zeitgebers to strengthen input to the circadian system on actigraphic estimates of night sleep time, daytime activity, day:night sleep ratio, and rest–activity rhythm.
METHOD
Subjects
Staff identified residents of two large long-term care facilities in San Francisco, California, with rest–activity rhythm disruption and a diagnosis of AD. Rest–activity rhythm disruptions included insomnia, frequent nighttime awakenings, wandering at night, unusually early morning awakenings, sundowning, and excessive daytime sleepiness. Chart reviews were conducted to confirm that potential subjects met the following criteria for inclusion: a diagnosis of probable AD according to the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria,36 the ability to perceive light, and a stable medication regimen. Potential subjects were excluded if they had other neurological diagnoses (e.g., Parkinson’s disease) or were regularly taking valerian, melatonin, or sleeping pills. Informed consent was obtained from responsible parties as approved by the institutional review board.
Procedure
This study compared morning bright-light exposure plus evening melatonin (LM) administration with morning bright-light exposure plus evening placebo (LP) administration. A control group received usual indoor light only. Subjects were randomly assigned to one of the three groups.
Light
The study protocol was 11 weeks in duration: a baseline week (Week 1) followed by a 10-week intervention period (Weeks 2–11). Data presented here were collected at baseline (Week 1) and at the end of the intervention period (Week 11). Subjects in the LM and LP experimental conditions received morning (9:30–10:30 a.m.) bright-light exposure (≥2,500 lux in gaze direction) Monday through Friday for 10 weeks. The duration of light treatment, time of day, and number of days per week were chosen for their feasibility of implementation in a nursing home setting. During the intervention, subjects in the experimental groups participated in activities in a brightly lit area outdoors or in an indoor space with windows to let in ample natural light. APOLLO Brite Lite IV (Orem, UT) light boxes were used when necessary to supplement the ambient light. These boxes (23″ × 12″ × 4″) provide 10,000 lux exposure at 26 inches and 2,500 lux exposure at 48 inches. Subjects were positioned at tables facing light boxes located approximately 30 to 34 inches from their eyes. Light levels in gaze direction were monitored for each subject at least once during each bright-light treatment with a Cal LIGHT 400 (Auburn Hills, MI) calibrated precision light meter.
The control group received usual indoor light (150–200 lux) and participated in their regularly scheduled activities in the usual location. During the intervention, the LM and LP groups participated in activities similar to those provided to the control group subjects.
Melatonin
Based on previous findings29 and the unpublished pilot pharmacokinetic data in young and older healthy adults studied at the Oregon Health and Science University, it was decided to use a moderate pharmacological dose (5 mg) of melatonin over a 10-week administration timeframe. This dose should yield peak plasma levels 10 to 1,000 times physiological levels within 1 hour of administration, with supraphysiological levels maintained throughout an 8-hour night.
The University of California, San Francisco Drug Product Services Laboratory provided melatonin (5 mg) and identically appearing lactose placebo capsules. The LM group received 5 mg of melatonin, and the LP group received a lactose placebo, both administered daily at dinnertime (5:00–6:00 p.m.), which was 2 to 3 hours before the scheduled habitual bedtime of 8:00 p.m. The nursing homes’ pharmacies distributed medications, which nursing staff administered. Study staff, nursing home staff, and subjects were all blinded to melatonin treatment group assignment.
Measures
Rest–activity data were collected using the Actiwatch activity monitor (AW-64, Mini Mitter Co., Inc., Bend, OR). Actiwatches are compact, battery-operated activity monitors with physical characteristics similar to a small wristwatch. The devices use an “accelerometer” to monitor occurrence and degree of movement-induced accelerations. Activity counts, representing movement, are stored in memory in the device in 1-minute epochs. Actigraphy has been shown to correlate well with electroencephalogram recordings and direct observation.37 It also provides a feasible technique for studying the rest–activity rhythm in institutionalized patients with dementia and is an appropriate means for assessing treatment effects.38,39
Actiwatches were placed on each subject’s dominant wrist, and a nylon locking cable was affixed through the watchband to deter removal. Subjects wore the Actiwatch continuously during each monitoring period, which consisted of 5 nights and 4 days (Monday 8:00 p.m. through Saturday 8:00 a.m.) for a total of 108 possible hours per subject.
Analyses
Actigraphy data were analyzed using Actiware Sleep Version 3.2 (Mini Mitter Co., Inc.) set at medium sensitivity. Daytime and nighttime were defined as the institutional rise and bed times of 8:00 a.m. and 8:00 p.m. Although calculating the actual sleep episode time for each subject would have been optimal, this was not possible because of staffing constraints. Other investigators have used similar methods to define day and night intervals (e.g.,40), and these methods correlate well with actual nurse recorded time in bed. One study reported actual time in bed to be approximately 12 hours in a study of subjects with dementia residing in a nursing home.12
The nighttime outcome variables included sleep time, wake time, and average number and duration of sleep and wake bouts. Daytime sleep time, wake time, wake and sleep bout duration, total activity, and the day:night sleep time ratio were also calculated.
Additional circadian outcomes were computed using two methods. Traditional parametric cosinor analyses11,40 and nonparametric techniques41 were used to quantify each subject’s 24-hour rest–activity rhythm. For each subject and time condition, the parametric 24-hour fixed period cosinor model was fit to the natural log (ln) transformed actigraphy data (counts per minute). The decision to loge transform the raw count data before the cosinor analysis was based on the strong positive skew in the distribution of the activity count data and was intended to ensure a larger relative weighting of the fit to the conceptually important lower-activity nocturnal period. Natural log transformation makes the effective data-analysis weight of evidence for each time point more uniform and balanced over the entire 24-hour period. The resulting within-subject coefficient estimates were then transformed to compute standard interpretive cosinor parameters (e.g., amplitude, acrophase). These within-subject cosinor summary parameters then became variables in the across-subject analyses for evaluation of treatment effects.
The activity levels that the fitted cosinor model predicts may overestimate the average levels measured during the typically shorter sleep–rest period. This motivates the addition of other summary nonparametric measures that are more sensitive to the characteristics of the empirical 24-hour activity profile. These nonparametric techniques were employed to assess the following circadian parameters, which are significantly related to several indirect parameters of well-being and quality of life in elderly people with dementia.42
Interdaily stability: quantifies the degree of resemblance between activity patterns of individual days (theoretical range 0–1). Higher values indicate a more stable rhythm. Intradaily variability: quantifies the fragmentation of periods of rest and activity (theoretical range near 0 for a sine wave up to 2 for Gaussian noise and even higher values when a definite ultradian component with a period of 2 hours is present). Higher values indicate a more-fragmented rhythm.
L5: sequence of the 5 least-active hours in the 24-hour average activity profile. Average activity during L5 provides an indication of trough or nadir of the rhythm (i.e., regularity and restfulness of sleep periods). Lower values indicate more restful sleep.
M10: sequence of the 10 most-active hours in the 24-hour average activity profile. Average activity during M10 provides an indication of the peak of the rhythm (how active and regular the activity (wake) periods are). Amplitude: the difference between the most-active 10-hour period and the least-active 5-hour period in an average 24-hour pattern.
Relative amplitude: reflects the normalized difference between the most-active 10-hour period and the least-active 5-hour period in an average 24-hour pattern (theoretical range 0–1). Higher values indicate a stronger rhythm.
The main effects of time and group and the time-by-group interactions were analyzed using linear mixed models with full maximum likelihood estimation in SPSS version 14.0 (SPSS Inc., Chicago, IL). The complete model was run with the control group as a reference to compare LP with control and LM with control; the analysis was then repeated with the LM group as a reference to compare LP with LM. Post hoc tests for simple effects were also conducted as linear mixed models with maximum likelihood estimation, following previous recommendations.43
RESULTS
Subjects
Fifty subjects (43 women, 7 men) completed the study. The average age ± standard deviation of the sample as a whole was 86 ± 8 (range 60–100). Despite random assignment of subjects to one of three groups, subjects randomized to the control condition were significantly younger (82 ± 10, post hoc Bonferroni P = .04) than subjects in the LP group (89 ± 7). Age was therefore centered on the grand mean as recommended for quantitative predictors to ease interpretation of the parameter estimates at the intercept (baseline assessment) and included in all subsequent analyses.44,45 The mean Mini-Mental State Examination46 score was 9.3 ± 7.9, and there were no significant differences between groups. Subjects tolerated the Actiwatches well, with 41 of 50 participants never removing the device during the baseline or end-of-the-intervention collection periods. On average, of the total possible 108 hours, there were 105 ± 8 hours of valid data for baseline (range 75–108) and 107 ± 3 hours of valid data at the end of intervention (range 90–108), with no significant differences between the groups.
Exposure to Light Treatment
The median light exposure for the treatment groups was 6,204 ± 2,668 lux. Attendance and approximate percentage of the intervention missed (e.g., eyes closed, toileting time) were recorded for each subject who received bright-light treatment. The percentage (dose) of intervention received was calculated by dividing the hours of intervention received by the total possible number of intervention hours (50 hours over the 10-week intervention period). The mean percentage of intervention received was 82 ± 17% (range 40–97%), and there was no significant difference between the LP and LM groups.
Actigraphy
There was a main effect of time for several dependent variables. To determine whether these main effects could be attributed to seasonal variations, values for sunset, sunrise, day length, and rate of change in day length for both assessment weeks and averaged over the entire treatment periods were tested. There were no significant effects.
Sleep and Wake
Means and standard deviations of the sleep and wake variables are presented in Table 1. Linear mixed-model analyses of the sleep and wake outcomes revealed significant differences between the groups from baseline to end of intervention in the variables presented in Table 2. Daytime sleep time decreased significantly (66 minutes) in the LM group, whereas it increased 25 minutes in the LP group (t = −3.744, P <.001) and 50 minutes in the control group (t = −4.802, P <.001). Post hoc analyses of simple slopes were conducted separately for each group following procedures described previously.43 (That is, change from baseline to end of intervention was tested within each group individually, when the pairwise group-by-time interaction for that group was significant.) Results revealed a significant decrease for daytime sleep time (t = −3.779, P <.001) in the LM group and a significant increase for the control group (t = 3.002, P =.004). There was a significant increase (t = 2.074, P =.04) in the daytime total activity score in the LM group and a significant reduction for the control (t = −2.558, P = .01) and LP (t = −2.790 P = .007) groups. Day:night sleep ratio in the LM group improved significantly (t = −3.871, P < .001).
Table 1.
Sleep/Wake and Rest/Activity Variables
| Control (n = 17)
|
Light and Placebo (n = 18)
|
Light and Melatonin (n = 15)
|
||||
|---|---|---|---|---|---|---|
| Baseline | End of Intervention | Baseline | End of Intervention | Baseline | End of Intervention | |
| Variable | Mean ± Standard Deviation | |||||
| Sleep/wake | ||||||
| Night sleep time, minutes | 480 ± 139 | 512 ± 121 | 493 ± 105 | 521 ± 108 | 459 ± 109 | 489 ± 105 |
| Night sleep bout duration, minutes | 18 ± 13 | 16 ± 8 | 16 ± 7 | 18 ± 8 | 14 ± 9 | 16 ± 10 |
| Night wake bout duration, minutes | 9 ± 7 | 6 ± 4 | 7 ± 4 | 6 ± 4 | 7 ± 4 | 6 ± 2 |
| Number of awakenings at night | 34 ± 15 | 38 ± 12 | 37 ± 12 | 34 ± 9 | 42 ± 16 | 40 ± 15 |
| Day sleep time, minutes | 274 ± 167 | 324 ± 171 | 309 ± 157 | 334 ± 172 | 315 ± 129 | 249 ± 103 |
| Day sleep bout duration, minutes | 7 ± 5 | 8 ± 4 | 8 ± 4 | 8 ± 5 | 7 ± 3 | 6 ± 2 |
| Day wake bout duration, minutes | 9 ± 7 | 12 ± 11 | 7 ± 4 | 10 ± 9 | 8 ± 5 | 14 ± 8 |
| Number of day sleep bouts | 39 ± 18 | 43 ± 14 | 42 ± 16 | 46 ± 15 | 46 ± 15 | 43 ± 16 |
| Day total activity, counts | 99,928 ± 80,025 | 83,513 ± 67,641 | 95,905 ± 77,840 | 78,004 ± 65,672 | 76,880 ± 43,640 | 90,598 ± 39,610 |
| Day sleep time/night sleep time | 0.55 ± 0.28 | 0.63 ± 0.28 | 0.61 ± 0.24 | 0.64 ± 0.29 | 0.70 ± 0.27 | 0.53 ± 0.22 |
| Rest/activity | ||||||
| Parametric | ||||||
| Amplitude | 1.21 ± 0.66 | 1.15 ± 0.64 | 1.13 ± 0.56 | 1.09 ± 0.68 | 0.90 ± 0.64 | 1.26 ± 0.56 |
| Acrophase, decimal time | 13.76 ± 3.30 | 14.11 ± 3.95 | 13.94 ± 1.70 | 13.37 ± 1.71 | 14.57 ± 2.61 | 13.51 ± 1.60 |
| Goodness of fit of the data to the 24-hour cosine curve (R2) | 0.17 ± 0.14 | 0.16 ± 0.13 | 0.13 ± 0.10 | 0.15 ± 0.13 | 0.11 ± 0.13 | 0.17 ± 0.12 |
| Nonparametric | ||||||
| Interdaily stability | 0.53 ± 0.17 | 0.52 ± 0.15 | 0.50 ± 0.20 | 0.49 ± 0.21 | 0.46 ± 0.16 | 0.51 ± 0.14 |
| Intradaily variability | 1.19 ± 0.45 | 1.20 ± 0.34 | 1.36 ± 0.31 | 1.36 ± 0.44 | 1.33 ± 0.38 | 1.30 ± 0.26 |
| Activity during 5 least-active hours | 44.59 ± 60.25 | 27.18 ± 33.79 | 36.33 ± 35.56 | 31.78 ± 47.48 | 40.85 ± 37.07 | 34.82 ± 21.76 |
| Activity during 10 most-active hours | 157.46 ± 118.87 | 133.41 ± 108.16 | 149.71 ± 122.02 | 121.79 ± 102.25 | 124.22 ± 64.18 | 139.22 ± 65.01 |
| Amplitude | 112.87 ± 76.73 | 106.24 ± 86.31 | 113.37 ± 94.26 | 90.01 ± 72.75 | 83.37 ± 51.25 | 104.70 ± 60.02 |
| Relative amplitude | 0.63 ± 0.20 | 0.68 ± 0.15 | 0.60 ± 0.18 | 0.61 ± 0.21 | 0.55 ± 0.25 | 0.61 ± 0.19 |
Table 2.
Significant Results from Mixed-Model Analysis
| Light and Placebo–Control* |
Light and Melatonin–Control* |
Light and Melatonin–Light and Placebo† |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Estimate (SE) | t | P-Value | Estimate (SE) | t | P-Value | Estimate (SE) | t | P-Value |
| Sleep/wake | |||||||||
| Daytime sleep time | −25.59 (23.8) | −1.08 | .29 | −116.09 (24.17) | −4.80 | <.001 | −90.50 (24.17) | −3.74 | <.001 |
| Number of daytime sleep bouts | −0.08 (3.14) | −0.03 | .98 | −6.52 (3.19) | −2.05 | .046 | −6.45 (3.19) | −2.02 | .049 |
| Daytime total activity score | −1,486.67 (9,074.69) | −0.16 | .87 | 30,133.17 (9,215.39) | 3.27 | .002 | 31,619.81 (9,215.39) | 3.43 | <.001 |
| Day/night sleep ratio | −0.05 (0.06) | −0.79 | .43 | −0.25 (0.06) | −4.01 | <.001 | −0.02 (0.06) | −3.23 | .002 |
| Rest activity | |||||||||
| Parametric | |||||||||
| Amplitude | 0.01 (0.13) | 0.11 | .92 | 0.43 (0.13) | 3.33 | .002 | 0.41 (0.13) | 3.23 | .002 |
| Goodness of fit of the data to the 24-hour cosine curve (R2) | 0.03 (0.03) | 1.05 | .30 | 0.07 (0.03) | 2.77 | .008 | 0.05 (0.03) | 1.74 | .09 |
| Nonparametric | |||||||||
| Amplitude | −16.73 (13.80) | −1.21 | .23 | 27.95 (14.01) | 2.00 | .05 | 44.68 (14.01) | 3.19 | .002 |
| Average activity during 10 most-active hours | −3.87 (13.73) | −0.28 | .78 | 39.35 (13.94) | 2.82 | .007 | 43.22 (13.94) | 3.10 | .003 |
Control is the reference group.
Light and placebo is the reference group.
SE = standard error.
Rest–Activity Rhythm
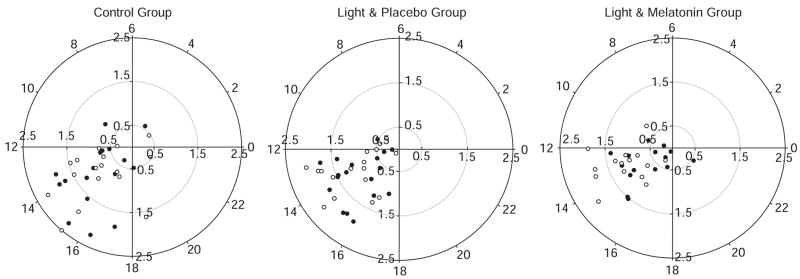
Means and standard deviations for the rest–activity rhythm variables are presented in Table 1. Figure 1 presents polar plots of individual subjects according to group. Figure 2 illustrates group mean activity counts binned in hours. The linear mixed-model analyses of the parametric and non-parametric outcomes revealed significant differences between the groups from baseline to end of intervention for the variables presented in Table 2. Parametric amplitude was significantly better in the LM group than in the LP (t = 3.23, P = .002) and control (t = 3.33, P = .002) groups. Cosinor model goodness of fit (coefficient of determination (R2)) was significantly better in the LM group than in the control group (t = 2.773, P = .008). Nonparametric measure of amplitude also significantly improved for the LM group compared to the LP group (t = 3.19, P = .002). The M10 significantly increased in the LM group compared with the LP (t = 3.099, P = .003) and control (t = 2.822, P = .007) groups. On average, subjects in the LP and LM groups phase advanced (LP 34 minutes, LM 63 minutes), whereas subjects in the control group phase delayed (21 minutes), although these differences were not statistically significant. Post hoc analyses of simple slopes were conducted as described above. Parametric amplitude (t = 3.976, P < .001) and R2 (t = 3.309, P = .002) increased significantly in the LM group. Nonparametric amplitude increased significantly for the LM group (t = 2.120, P = .04) and decreased for the LP group (t = −2.394 P = .02). M10 decreased significantly in the LP (t = −2.875, P = .006) and control (t = −2.477, P = .02) groups.
Figure 1.
Rest–activity rhythm acrophase and amplitude. ● Baseline, ○ End of Intervention. These polar plots provide a visual representation of the individual subject’s acrophase (time of peak activity) and amplitude relative to the 24-hour day at baseline and end of intervention. Position on the circular axis corresponds to 24-hour clock time, with midnight represented as 0 on the far right, 6:00 a.m. on the top, noon on the left, and 6:00 p.m. at the bottom. Position on the radial axis corresponds to rhythm amplitude, smaller amplitudes are closer to the center, and larger amplitudes are more distal.
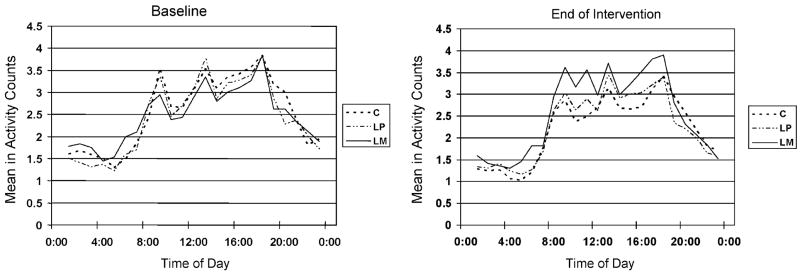
Figure 2.
Hourly activity counts according to group. Mean (5-day, 4-night) hourly natural log (ln) activity counts by time of day and group at baseline and end of intervention. C = control, LP = light and placebo, LM = light and melatonin. The absolute and relative change in the position of the solid line from baseline to end of intervention graphically illustrate the increase in daytime activity in the LM group.
DISCUSSION
In this study, morning bright light plus evening melatonin resulted in more daytime activity and less daytime somnolence, a more-normal diurnal pattern. This was also reflected in an improvement in the goodness of fit (R2) of the data to a traditional cosinor model and greater amplitude of the rest–activity rhythm. Decreased daytime sleep facilitates greater participation in physical and psychosocial activities (e.g., visiting with family, participating in recreational therapy activities). Conversely, engaging in psychosocial activities may prevent napping and ultimately improve nocturnal sleep and the rest–activity rhythm.5 Others have shown these improvements to increase patient well-being and quality of life and the well-being and quality of life of caregivers and family members.27 Evaluation of the nature of behaviors (e.g., increased cooperation, agitation) exhibited by the subjects cannot be determined from actigraphy. Data analyses of neuropsychiatric behaviors are in process and will help to answer this question.
Bright light alone or in combination with melatonin did not significantly improve nighttime sleep variables as assessed using actigraphy. It is therefore unlikely that the daytime improvements found in the group that received bright light plus melatonin resulted from nocturnal improvements in sleep architecture. The old age, severity of dementia, and large intra- and inter-individual differences in our sample may explain, in part, the lack of apparent nighttime treatment effect. Actigraphy-derived measures may also not equate to time asleep. The institutional bed and rise times defined “night,” which most likely does not accurately reflect the nighttime sleep episode. Had it been possible to determine sleep onset and offset and use these to define the nighttime sleep episode, it is possible that the findings might have been more robust. Although using 8:00 p.m. to 8:00 a.m. allowed a standard interval to be examined across subjects, it is not optimal for examining nighttime sleep outcomes. An increase in the number of nights monitored with the actigraph (5 on average in the present study) would be another way to improve the accuracy of sleep estimates.47 In addition, wrist actigraphy does not permit primary sleep disorders (e.g., sleep disordered breathing, periodic limb movements) that the interventions would not be expected to affect to be excluded. Although these findings are consistent with some reports in the literature,24,25,27 other investigators have reported positive nighttime effects with bright-light therapy.9,12 It is possible that, even though the number of weeks of treatment in this study is the longest of any of the work cited, daily treatment (vs only Monday through Friday) might have yielded a stronger result. Indeed, positive effects on the sleep–wake rhythm have been reported in a study that applied light during the entire day, 7 days a week, for 1 month.14
It is also possible that light alone was not powerful enough to produce statistically significant changes, but when combined with melatonin, the effect size increased so that significant results were obtained. It is impossible to determine whether the results in the LM group were due to melatonin alone or the combination of light and melatonin. There remains no accepted standard for the intensity, duration, or timing of bright-light exposure and no accepted standard for melatonin administration dose, formulation, or time of administration. It is possible that subjects respond to light and melatonin differently across the lifespan and range of cognitive impairment. Because the light treatments in this study were administered to all subjects at the same time of day, it is possible that some subjects received light during a sensitive region of their individual phase-response curve and others did not. In future studies, it might be more effective to individualize the timing of light exposure and melatonin administration for subjects based on their endogenous rhythm. When calculating the sensitive phase for morning light treatment, it would be useful to determine each individual’s sleep onset time and sleep duration before deciding the optimal time for light exposure and melatonin administration.
CONCLUSION
These results support the findings of others that 1 hour of morning light treatment alone may not, under all circumstances, be sufficient to improve nighttime sleep, daytime wake, and the rest–activity rhythm. Light treatment combined with melatonin administration increased daytime wake time and activity levels and strengthened the rest–activity rhythm in subjects with AD. It is possible that responses to individual interventions may be small and that combining interventions could produce an additive effect that, in the aggregate, produces more clinically significant effects. Future studies should resolve the question of whether the improvements can be attributed to melatonin per se or whether the two zeitgebers, light and melatonin, interact to amplify their efficacy.
Acknowledgments
The investigators wish to thank the administration and staff at the Jewish Home and Laguna Honda Hospital in San Francisco, California, for their assistance and cooperation.
Funded by the National Institutes of Health, National Institute of Nursing Research (NR002968) (GAD), Netherlands Organization of Scientific Research VIDI Innovation Grant 016.025.0419 (EVS).
Sponsor’s Role: The National Institutes of Health had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of this manuscript.
Footnotes
Conflict of Interest: The editor in chief has reviewed the personal and financial checklist provided by the authors and has determined that none of the authors have any conflicts related to this manuscript.
Author Contributions: Glenna A. Dowling, principal investigator, and Jay S. Luxenberg, co-investigator: study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript. Erin M. Hubbard: acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript. Robert L. Burr, Eus J. W. Van Someren, Judy Mastick, and Bruce A. Cooper: analysis and interpretation of data and preparation of manuscript.
References
- 1.Yesavage JA, Friedman L, Ancoli-Israel S, et al. Development of diagnostic criteria for defining sleep disturbance in Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2003;16:131–139. doi: 10.1177/0891988703255684. [DOI] [PubMed] [Google Scholar]
- 2.McCurry SM, Ancoli-Israel S. Sleep dysfunction in Alzheimer’s disease and other dementias. Curr Treat Options Neurol. 2003;5:261–272. doi: 10.1007/s11940-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 3.Pollak CP, Perlick D. Sleep problems and institutionalization of the elderly. J Geriatr Psychiatry Neurol. 1991;4:204–210. doi: 10.1177/089198879100400405. [DOI] [PubMed] [Google Scholar]
- 4.Hope T, Keene J, Gedling K, et al. Predictors of institutionalization for people with dementia living at home with a carer. Int J Geriatr Psychiatry. 1998;13:682–690. doi: 10.1002/(sici)1099-1166(1998100)13:10<682::aid-gps847>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan SC, Richards KC. Predictors of circadian sleep-wake rhythm maintenance in elders with dementia. Aging Ment Health. 2004;8:143–152. doi: 10.1080/13607860410001649608. [DOI] [PubMed] [Google Scholar]
- 6.Van Someren EJ, Riemersma RF, Swaab DF. Functional plasticity of the circadian timing system in old age: Light exposure. Prog Brain Res. 2002;138:205–231. doi: 10.1016/S0079-6123(02)38080-4. [DOI] [PubMed] [Google Scholar]
- 7.Dowling GA, Hubbard EM, Mastick J, et al. Effect of morning bright light treatment for rest–activity disruption in institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatr. 2005;17:221–236. doi: 10.1017/S1041610205001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowling GA, Mastick J, Hubbard EM, et al. Effect of timed bright light treatment for rest–activity disruption in institutionalized patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2005;20:738–743. doi: 10.1002/gps.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satlin A, Volicer L, Ross V, et al. Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer’s disease. Am J Psychiatry. 1992;149:1028–1032. doi: 10.1176/ajp.149.8.1028. [DOI] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Gehrman P, Martin JL, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 11.Fontana Gasio P, Krauchi K, Cajochen C, et al. Dawn-dusk simulation light therapy of disturbed circadian rest–activity cycles in demented elderly. Exp Gerontol. 2003;38:207–216. doi: 10.1016/s0531-5565(02)00164-x. [DOI] [PubMed] [Google Scholar]
- 12.Fetveit A, Skjerve A, Bjorvatn B, et al. Bright light treatment improves sleep in institutionalized elderly—an open trial. Int J Geriatr Psychiatry. 2003;18:520–526. doi: 10.1002/gps.852. [DOI] [PubMed] [Google Scholar]
- 13.Cagnacci A, Elliott JA, Yen SSC, et al. Melatonin: A major regulator of the circadian rhythm of core temperature in humans. J Endocrinol. 1992;75:447–452. doi: 10.1210/jcem.75.2.1639946. [DOI] [PubMed] [Google Scholar]
- 14.Van Someren EJW, Kessler A, Mirmiran M, et al. Indirect bright light improves circadian rest–activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 15.Lewy AJ, Ahmed S, Jackson JM, et al. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 16.Magri F, Sarra S, Cinchetti W, et al. Qualitative and quantitative changes of melatonin levels in physiological and pathological aging and in centenarians. J Pineal Res. 2004;36:256–261. doi: 10.1111/j.1600-079X.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 17.Skene DJ, Swaab DF. Melatonin rhythmicity: Effect of age and Alzheimer’s disease. Exp Gerontol. 2003;38:199–206. doi: 10.1016/s0531-5565(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J-N, Liu R-Y, Kamphorst W, et al. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res. 2003;35:125–130. doi: 10.1034/j.1600-079x.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 19.Mishima K, Tozawa T, Satch K, et al. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol Psychiatry. 1999;45:417–421. doi: 10.1016/s0006-3223(97)00510-6. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y-H, Feenstra MGP, Zhou J-N, et al. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: Alterations in preclinical and clinical stages. J Clin Endocrinol Metab. 2003;88:1–22. doi: 10.1210/jc.2003-030833. [DOI] [PubMed] [Google Scholar]
- 21.Wu YH, Zhou JN, Van Heerikhuize J, et al. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol Aging. 2007;28:1239–1247. doi: 10.1016/j.neurobiolaging.2006.06.002. Epub July 11, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Tzischinsky O, Lavie P. Melatonin possesses time-dependent hypnotic effects. Sleep. 1994;17:638–645. doi: 10.1093/sleep/17.7.638. [DOI] [PubMed] [Google Scholar]
- 23.Waldhauser F, Saletu B, Trinchard-Logan I, et al. Sleep laboratory investigations on hypnotic properties of melatonin. Psychopharmacology (Berlin) 1990;100:222–226. doi: 10.1007/BF02244410. [DOI] [PubMed] [Google Scholar]
- 24.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serfaty M, Kennell-Webb S, Warner J, et al. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int J Geriatr Psychiatry. 2002;17:1120–1127. doi: 10.1002/gps.760. [DOI] [PubMed] [Google Scholar]
- 26.Cardinali DP, Brusco LI, Liberczuk C, et al. The use of melatoinin in Alzheimer’s disease. Neuro Endocrinol Lett. 2002;23:20–23. [PubMed] [Google Scholar]
- 27.Cohen-Mansfield J, Garfinkel D, Lipson S. Melatonin for treatment of sundowning in elderly persons with dementia—a preliminary study. Arch Gerontol Geriatr. 2000;31:65–76. doi: 10.1016/s0167-4943(00)00068-6. [DOI] [PubMed] [Google Scholar]
- 28.Dowling GA, Mastick J, Aminoff MJ, et al. Melatonin for sleep disturbances in Parkinson’s disease: A pilot study. Sleep Res Online. 2003;5:99–103. doi: 10.1016/j.sleep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Dowling GA, Mastick J, Colling E, et al. Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med. 2005;6:459–466. doi: 10.1016/j.sleep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Arendt J. Melatonin: Characteristics, concerns, and prospects. J Biol Rhythms. 2005;20:291–303. doi: 10.1177/0748730405277492. [DOI] [PubMed] [Google Scholar]
- 31.Cagnacci A, Arangino S, Renzi A, et al. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin Endocrinol (Oxford) 2001;54:339–346. doi: 10.1046/j.1365-2265.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- 32.Pappolla MA, Chyan Y-J, Poeggeler B, et al. An assessment of the antioxidant and the antiamyloidogenic properties of melatonin: Implications for Alzheimers disease. J Neural Transm. 2000;107:201–231. doi: 10.1007/s007020050018. [DOI] [PubMed] [Google Scholar]
- 33.Scheer FA, Van Montfrans GA, Van Someren EJ, et al. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- 34.Van der Helmvan Mil AHM, Van Someren EJW, Van den Boom R, et al. No influence of melatonin on cerebral blood flow in humans. J Clin Endocrinol Metab. 2003;88:5989–5994. doi: 10.1210/jc.2003-031107. [DOI] [PubMed] [Google Scholar]
- 35.Haffmans PMJ, Sival RC, Lucius SAP, et al. Bright light therapy and melatonin in motor restless behaviour in dementia, a placebo-controlled study. Int J Geriatr Psychiatry. 2001;16:106–110. doi: 10.1002/1099-1166(200101)16:1<106::aid-gps288>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 37.Ancoli-Israel S, Clopton P, Klauber MR, et al. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep. 1997;20:24–27. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 39.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: An update for 2002. Sleep. 2003;26:337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 40.Ancoli-Israel S, Martin JL, Kripke DF, et al. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002;50:282–289. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Someren EJW, Swaab DF, Colenda CC, et al. Bright light therapy: Improved sensitivity to its effects on rest–activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16:505–518. doi: 10.3109/07420529908998724. [DOI] [PubMed] [Google Scholar]
- 42.Carvalho-Bos SS, Riemersmavan der Lek RF, Waterhouse J, et al. Strong association of the rest–activity rhythm with well-being in demented elderly women. Am J Geriatr Psychiatry. 2007;15:92–100. doi: 10.1097/01.JGP.0000236584.03432.dc. [DOI] [PubMed] [Google Scholar]
- 43.Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behav Res. 2005;40:373–400. doi: 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- 44.Hox JJ. Multilevel Analysis: Techniques and Applications. Mahwah, NJ: Lawrence Erlbaum; 2002. [Google Scholar]
- 45.Singer JD, Willet JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 46.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 47.Van Someren EJW. Improving actigraphic sleep estimates: How many nights? J Sleep Res. 2007;16:269–275. doi: 10.1111/j.1365-2869.2007.00592.x. [DOI] [PubMed] [Google Scholar]