Abstract
In the pituitary, the transition from proliferating progenitor cell into differentiated hormone producing cell is carefully regulated in a time dependent and spatially restricted manner. We report that two targets of Notch signaling, Hes1 and Prop1, are needed to maintain progenitors within Rathke’s pouch and for the restriction of differentiated cells to the ventral pituitary. We observed ACTH and αGSU producing cells that had prematurely differentiated within Rathke’s pouch along with correlated ectopic expression of Mash1 only when both Prop1 and Hes1 were lost. We also discovered that downregulation of N-cadherin expression in cells as they transition from Rathke’s pouch to the anterior lobe appears to be essential for their movement. In the Prop1 mutant, cells are trapped in Rathke’s pouch and N-cadherin expression remains high. Also, Slug, a marker of epithelial to mesenchymal transition, is absent in the dorsal anterior lobe. When Hes1 is lost in the Prop1 mutant, N-cadherin is downregulated and cells are able to exit Rathke’s pouch but have lost their migrational cues and form ectopic foci surrounding Rathke’s pouch. Our data reveal important overlapping functions of Hes1 and Prop1 in cell differentiation and movement that are critical for pituitary organogenesis.
Keywords: Pituitary, Notch, Hes1, Prop1, cadherin, Slug, Snai2, ACTH
Introduction
Proper development of the pituitary gland is very important because the pituitary is responsible for releasing hormones that affect growth, metabolism, and fertility, as well as the body’s response to stress. In mice, the pituitary gland contains an anterior lobe, an intermediate lobe, and a posterior lobe. The anterior and intermediate lobes are derived from a structure called Rathke’s pouch (RP) which is formed at embryonic day 8.5 (e8.5) from the invagination of the oral ectoderm (Burrows, et al. 1999). During embryonic development, Rathke’s pouch is made up of undifferentiated, proliferating progenitor cells. Many of these cells leave the pouch and move ventrally to the anterior lobe where they differentiate into corticotropes, thyrotropes, somatotropes, lactotropes, and gonadotropes. It is from these cells that adrenocorticotropic hormone (ACTH), thyroid stimulating hormone (TSH), growth hormone (GH), prolactin (PRL), and gonadotropins (FSH and LH) are secreted into the body. Toward the end of prenatal development, the cells that remain in Rathke’s pouch differentiate into melanotropes which make up the intermediate lobe and secrete melanocyte stimulating hormone (MSH).
This transition of cells from the packed, columnar-like cells of Rathke’s pouch to the more loosely distributed round cells of the anterior lobe is not well understood, but resembles epithelial-to-mesenchymal transition (EMT). An important process during embryonic development as well as oncogenesis, EMT is characterized by a loss of cell polarization as well as downregulation of adherens and tight junction markers such as E-cadherin. The purpose of EMT is to allow the cells to change shape and become motile, often accomplished in part by a rearrangement of various cytoskeleton proteins (Kalluri and Neilson, 2003). One gene that has been implicated in this process is Slug (Snai2), a member of the Snail family of zinc finger transcription factors (Nieto, et al. 1994). SLUG has been shown to induce EMT by directly repressing E-cadherin, causing destabilization of cell-cell adhesion which in turn allows for cell migration (Cano, et al. 2000; Bolos, et al. 2003). Although the expression and function of Slug in the rodent pituitary has not been elucidated, E-cadherin and N-cadherin expression patterns have been characterized in the rat pituitary gland. It appears that both E- and N-cadherin are coexpressed at the beginning of pituitary development. Eventually the two become mutually exclusive as there is down-regulation of E-cadherin in the hormone producing cell types and N-cadherin in the marginal cells lining the residual lumen of Rathke’s pouch (Kikuchi, et al. 2006; Kikuchi, et al. 2007). It is possible that SLUG may play a role in the downregulation of E-cadherin in the mouse pituitary which could allow the cells to move to the anterior lobe from Rathke’s pouch.
Previous research into the mechanism by which the Rathke’s pouch progenitor cells remain distinct from differentiating anterior lobe cells has uncovered pivotal roles for the Notch signaling pathway (Zhu, et al. 2006; Raetzman, et al. 2007). Interestingly, SLUG has also been linked to the Notch signaling pathway, through which Notch is able to regulate E-cadherin expression (Leong, et al. 2007). This link to Notch signaling may help illuminate potential roles of SLUG in pituitary cell movement and adhesion. Because Notch signaling has been shown to participate in EMT during development (Timmerman, et al. 2004), it is possible that Notch also plays an active role in the movement of cells from Rathke’s pouch to the anterior lobe. A well characterized target of Notch signaling is HES1, which inhibits transcription of basic helix-loop-helix (bHLH) genes necessary for cell differentiation, such as Mash1. Therefore, it acts to maintain cells in a precursor state (Ishibashi, et al. 1994; Jarriault, et al. 1998). Hes1 is strongly expressed in Rathke’s pouch cells and its expression declines as cells transition to the anterior lobe (Raetzman, et al. 2007).
Another direct target of Notch signaling in the pituitary is PROP1, a pituitary-specific homeobox transcription factor (Zhu, et al. 2006). In humans combined pituitary hormone deficiency (CPHD) can result from a loss of PROP1 (a gene homologous to Prop1 in mice) resulting in hypothyroidism, dwarfism, and infertility (Wu, et al. 1998). Studies on the Ames dwarf mice, which lack functional Prop1, have revealed that Prop1 is critical for pituitary cell differentiation and is responsible for activating the Pit1 lineage: thyrotropes, somatotropes, and lactotropes (Andersen, et al. 1995; Gage, et al. 1996b). Besides its role in Pit1 activation, PROP1 also participates in movement of cells to the anterior lobe. In Ames dwarf mice, there are no noticeable differences in pituitary morphology between wild type and dwarf pituitaries at e12.5. However, by e14.5 Rathke’s pouch is abnormally shaped and hypercellular in dwarfs, likely due to an inability of the cells to leave the pouch (Gage, et al. 1996a; Raetzman, et al. 2002; Ward, et al. 2005). Concurrently, Prop1 deficient anterior lobes are hypocellular at e14.5; reduced to half the size of wildtype anterior lobes at this age (Gage, et al. 1996a). Although downstream targets of PROP1 are emerging (Douglas, et al. 2001; Brinkmeier, et al. 2003; Carninci, et al. 2003), little is definitively known about its role in transitioning cells from Rathke’s pouch to the anterior lobe.
Based on their roles in Notch signaling and Rathke’s pouch progenitor cell expression, we questioned whether Prop1 and Hes1 may interact or have redundant functions in pituitary development. Recent studies have shown that in Prop1 mutants, no significant change is seen in levels of Hes1 mRNA by in situ hybridization (Raetzman, et al. 2006) and Prop1 expression remains in the Hes1 mutant (Zhu, et al. 2006). In order to better determine the roles that Hes1 and Prop1 play in pituitary gland morphogenesis, specifically in cell differentiation and movement, we examined murine pituitaries from wild type, Hes1 mutant, Prop1 mutant, and double mutant embryos. We hypothesized that the two genes function together in the Notch signaling pathway and therefore are both necessary for pituitary organogenesis. Our findings demonstrate that both Hes1 and Prop1 are necessary for proper placement of the anterior lobe. We also show that loss of both of these genes results in severe mislocalization and premature differentiation of αGSU and ACTH producing cells which does not occur with the loss of Hes1 or Prop1 individually. These findings are likely due to a problem with the movement of the newly differentiated Rathke’s pouch cells, some of which move to the wrong place while others do not migrate at all. These changes may be due in part to altered N-cadherin, E-cadherin, and SLUG expression that we observed. Taken together, these data suggest that not only are both Hes1 and Prop1 required for proper pituitary gland development but they also have redundant or overlapping functions within the Notch signaling pathway.
Materials and Methods
Mice
All mice were provided with chow and water ad libitum. A breeding colony of Hes1 null heterozygotes was established from mice obtained from Dr. Ryoichiro Kageyama. A breeding colony of Ames dwarf Prop1 loss of function mutants was established from a colony in Sally Camper’s lab and backcrossed to C57BL6 mice obtained from Jackson Laboratory for at least ten generations. The heterozygous Hes1 progeny were mated with heterozygous Prop1 progeny to obtain mice heterozygous for both genes. These double heterozygote mice were then crossed and their embryos collected and genotyped. Three embryos of each genotype at each age were studied. All procedures involving mice were approved by the University of Illinois IACUC.
To genotype the mice, DNA from tail biopsies was isolated using a DNA salt-out technique. The Hes1 PCR reaction mixture contained 1.56mM of MgCl2, 1 unit of Taq, and 12.5 pmol of each of four primers; two that amplified Hes1: 5’ AGCCAGTGTCAACACGACACC 3’ and 5’ TGTTAAGTGCATCCAAAATCAGTG 3’ ; and two that amplified the neomycin-resistant cassette used to knockout Hes1: 5’ GTCTTGTCGATCAGGATGATCTG 3’ and 5’ CAATATCACGGGTAGCCAACGC 3’. The samples underwent 30 cycles of denaturing at 92°C for 30 seconds, annealing at 55°C for 30 seconds, and elongation at 72°C for 30 seconds followed by a final elongation at 72°C for 10 minutes. The Prop1 PCR reaction mixture contained MgCl2, Taq, and 12.5 pmol of each of two primers that amplified Prop1: 5’ GAGCTGGGGAGACCTAAGCTTTGCC 3’ and 5’ GCCCAGATGTCAGGATACTG 3’. The samples underwent 34 cycles of denaturing at 92°C for 30 seconds, annealing at 56°C for 30 seconds and elongation at 72°C for 30 seconds followed by a final elongation at 72°C for 10 minutes. The samples were then digested with HINF1 overnight before being loaded onto 2% agarose gels and undergoing gel electrophoresis.
Immunohistochemistry and In situ Hybridization
The timing of the pregnancies was monitored and the first day that the copulatory plug was detected was designated e0.5. The embryos were sacrificed at e12.5, e13.5, e14.5, and e16.5 and fixed in 3.7% formaldehyde in phosphate buffered saline (PBS, pH 7.2). They were then dehydrated in a graded series of ethanol before being embedded in paraffin and sectioned sagittally at 6 micrometers. The sections were mounted onto charged slides and prepared for the staining procedures. Hemotoxylin and eosin was used to stain for morphology. For the hormone and PC2 staining procedures, the slides were deparaffinized in xylene and rehydrated in ethanol and PBS. They were then incubated in normal donkey serum (5% w/v) diluted in immunohistochemistry block (IHCB) which consists of PBS, BSA (3%), and Triton-X (0.5%), and followed by incubation overnight at 4°C with a primary antibody against the desired peptide: αGSU (1:1500; National Hormone and Pituitary Program-NHPP), ACTH (1:1000; DAKO), TSH (1:1000; NHPP), GH (1:1000; NHPP), or PC2 (1:100; Chemicon). The antibodies were diluted with IHCB. Next, an anti-rabbit secondary antibody conjugated to biotin (Jackson Immunoresearch) was added to the slides at a dilution of 1:200. Subsequent detection was carried out with a Vectastain kit (Vector Laboratories) diluted in PBS and Sigma Fast 3,3-Diaminobenzidine tablets (Sigma). The slides were counterstained with methyl green and mounted with Permount (Fisher). For colocalization experiments involving hormone antibodies, blocking and icubation of the primary antibody occurred as above, with ACTH being detected by an anti-rabbit secondary antibody conjugated to Cy2 (Jackson Immunoresearch) and αGSU with the TSA Kit #22 (Invitrogen). These slides were mounted with an aqueous mounting medium.
The slides for the LHX3, ISL1, PIT1, N-cadherin, E-cadherin, and SLUG staining procedures required boiling in 10mM citric acid, pH6, for ten minutes before incubation with 5% normal donkey serum diluted in IHCB. They were then incubated overnight at 4°C with a primary antibody against the desired marker: LHX3 (1:500; C651.6DbHn Developmental Studies Hybridoma Bank-DSHB, University of Iowa, Iowa City, IA), ISL1 (1:500; 40.2D6 DSHB), PIT1 (1:800; a gift from Dr. Simon Rhodes), N-cadherin (1:300; ZYMED), E-cadherin (1:100; Cell Signaling Technologies), or SLUG (1:100; Santa Cruz Biotechnology). The antibodies were diluted with IHCB. An anti-mouse secondary antibody conjugated to biotin was used with LHX3, ISL1, and N-cadherin while an anti-rabbit secondary antibody conjugated to biotin was used with PIT1 and E-cadherin. Anti-goat conjugated biotin was used with Slug. Either Strep-CY2 or Strep-CY3 for signal detection was used followed by mounting with an aqueous mounting medium. All secondary antibodies were obtained from Jackson Immunoresearch.
In situ hybridization was performed as previously described (Raetzman, et al. 2004). A Mash1 clone was obtained from a RIKEN embryonic pituitary cDNA library and the probe made from it was labeled with digoxigenin (Carninci, et al. 2003).
After staining as described above, images of the slides were viewed at 200X magnification with a Leica DM2500 microscope, captured with the Retiga 2000R color camera (Q-imaging) attached to the microscope, and the pictures acquired in Q-Capture Pro (Q-imaging). An Axiovert 200M compound microscope, Axiocam HRm camera, and Axiovision software (Carl Zeiss) were used to visualize the slides at 640X magnification.
Results
Anterior lobe placement relies on both Hes1 and Prop1
As the cells in Rathke’s pouch begin to differentiate they also move out along the rostral-caudal axis in a ventrally-restricted manner to form the growing anterior lobe. Generally the anterior lobe forms as a morphologically distinct structure around e12.5. The wildtype pituitary at e12.5 is composed of the Rathke’s pouch (RP), an anterior lobe (AL), and a posterior lobe (PL) (Fig. 1A). The same is true for the Prop1 mutant at this age (Fig. 1C). However, the Hes1 mutant and double mutant both have an anterior lobe that is greatly reduced in size and is not visible as a morphologically distinct structure (Fig. 1B, D). Also, the posterior lobes are stunted in these genotypes.
Fig. 1.
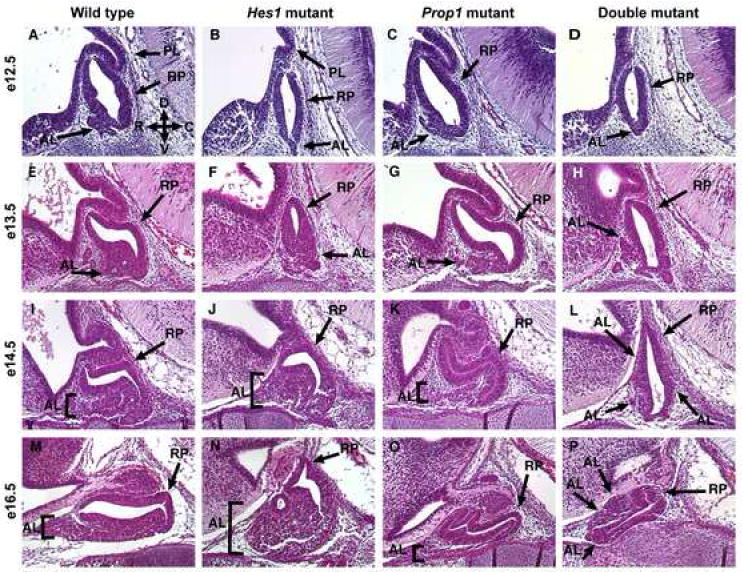
Development of the anterior pituitary requires both Hes1 and Prop1. Embryos were sectioned sagittally at e12.5, e13.5, e14.5, and e16.5 and stained for morphology with hematoxylin and eosin. At e12.5 the wild type consists of an anterior lobe (AL), posterior lobe (PL), and Rathke’s pouch (RP) (A, arrows). In panel A, arrows point to D, V, R, and C which correspond to the directions of dorsal, ventral, rostral, and caudal, respectively. The anterior lobe at e13.5 has grown and Rathke’s pouch has begun to elongate (E). This trend continues with the e14.5 (I) and e16.5 (M) wildtype pituitaries. At e12.5 the Hes1 mutant lacks a distinct anterior lobe and the posterior lobe is noticeably smaller (B). The anterior lobe gets larger at e13.5 (F), e14.5 (J), and e16.5 (N) while Rathke’s pouch gets smaller. At e12.5 the Prop1 mutant (C) is nearly indistinguishable from the wild type, however the anterior lobe doesn’t enlarge much by e13.5 (G). By e14.5 (K) and e16.5 (O) of the Prop1 mutant, Rathke’s pouch is abnormally branched and elongated while the anterior lobe is much smaller than the wildtype anterior lobe. The double mutant at e12.5 (D) and e13.5 (H) has a morphology very similar to the Hes1 mutant at those ages. However, at e14.5 the anterior lobe is forming along the sides of Rathke’s pouch (L, arrows). By e16.5 the Rathke’s pouch is difficult to distinguish from the anterior lobe however, some anterior lobe cells do appear to be located more dorsally than ventrally (P). n=3 Magnification: 200X
By e13.5 the anterior lobe has continued to expand in the wildtype while the size of Rathke’s pouch appears to be maintained (Fig. 1E). The anterior lobe of the Hes1 mutant also has expanded greatly, and this expansion is restricted to the ventral part of the pituitary similar to the wildtype. However, Rathke’s pouch is noticeably much smaller than in the wildtype (Fig. 1F). In the Prop1 mutant, the anterior lobe has failed to expand as much as the wildtype, although the size of Rathke’s pouch is maintained (Fig. 1G). The double mutant no longer closely resembles the Hes1 mutant at this age. The anterior lobe appears to be forming ectopically in the dorsocaudal and dorsorostral Rathke’s pouch (dcRP and drRP, respectively) in addition to the normal ventral expansion along the rostral-caudal axis (Fig. 1H).
At e14.5, the differences in morphology among the four genotypes are even more striking than at e13.5. The wildtype pituitary has maintained the size of Rathke’s pouch while continuing to expand the anterior lobe (Fig. 1I). The anterior lobe of the Hes1 mutant has continued to enlarge while the size of Rathke’s pouch is only slightly larger (Fig. 1J), but is smaller than the wildtype pouch. In the Prop1 mutant the anterior lobe remains nearly the same and, as a result, it is greatly reduced in size compared to the wildtype (Fig. 1K). However, the Rathke’s pouch structure has grown, producing an elongated, often branched structure packed full of the columnar-shaped cells characteristic of Rathke’s pouch. The double mutant is the most interesting at this age, with a morphology different than that of the wildtype or single mutants. The size of Rathke’s pouch doesn’t appear to be greatly altered, but the anterior lobe continues to form ectopically in the dcRP and drRP in addition to the normal ventral expansion (Fig. 1L).
Many of these same growth patterns are seen at e16.5. In the wildtype the anterior lobe and Rathke’s pouch have both expanded (Fig. 1M), although more laterally than ventrally, which cannot be completely appreciated by viewing one sagittal section. The anterior lobe of the Hes1 mutant has continued to enlarge without a noticeable change in Rathke’s pouch structure (Fig. 1N). The Prop1 mutant looks similar to e14.5 with an even more branched, elongated Rathke’s pouch structure as well as an anterior lobe that does not look much larger than that of the e12.5 mutant (Fig. 1O). The morphology of the double mutant has continued to become more distinct with what seems to be a much smaller structure overall. It is difficult to distinguish the anterior lobe from Rathke’s pouch by only viewing the morphology but it appears that the anterior lobe is located along the entire rostral axis in the dorsal and ventral areas.
It is clear from these morphological observations that the loss of either Prop1 or Hes1 has an effect on the size of both Rathke’s pouch and the anterior lobe. It also appears that both of these genes may have redundant functions in restricting the formation of the anterior lobe to the ventral part of the pituitary. This is evidenced by the misplacement of the anterior lobe in the double mutant but not in either single mutant or wildtype.
Aberrant and premature expression of Mash1 correlates with misplacement of ACTH producing cells in the double mutant
To confirm the anterior pituitary identity of the misplaced cells in the double mutant, we examined hormone expression in wildtype, Hes1 mutant, Prop1 mutant, and double mutant pituitaries at e14.5 and e16.5. First, we examined the formation of the corticotrope lineage. Mash1, a bHLH transcription factor, is thought to promote the formation of ACTH producing cells and is known to be directly repressed by Hes1 (Ohsako, et al. 1994; Van Doren, et al. 1994; Liu, et al. 2001). In the wildtype, Hes1, and Prop1 mutants at e13.5 Mash1 is expressed in the anterior lobe and the most dorsal part of Rathke’s pouch (Fig. 2A-C).
Fig. 2.
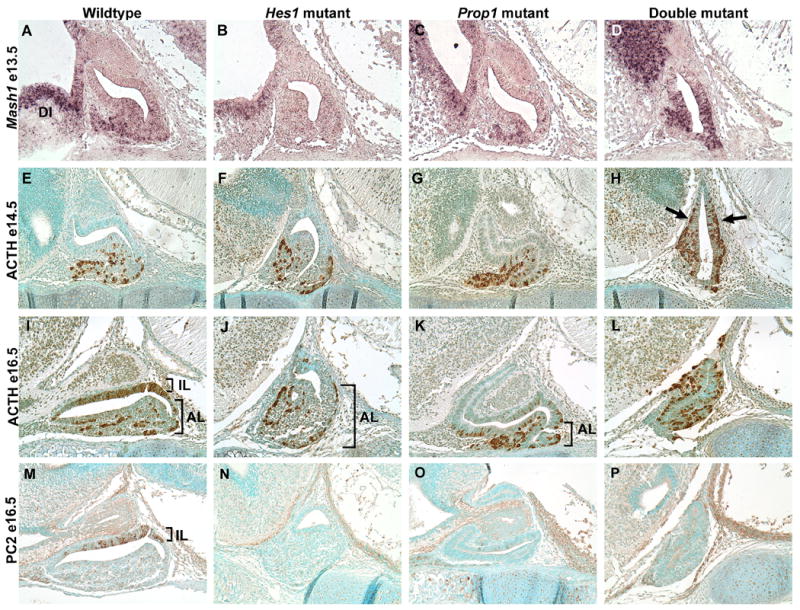
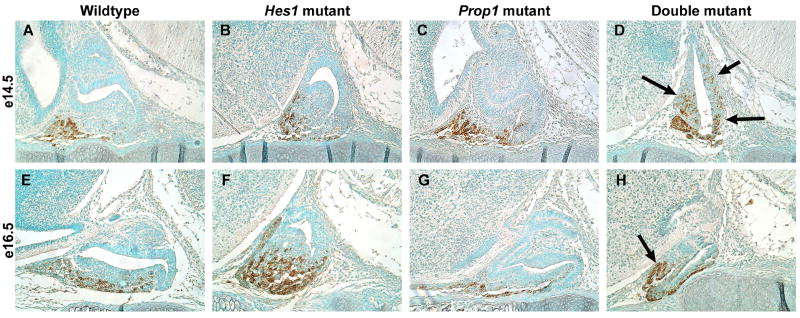
Mash1 and ACTH containing cells are misplaced in double mutants. In situ hybridization on sagittal sections reveals Mash1 mRNA located in the anterior lobe of the wildtype (A), Hes1 mutant (B), Prop1 mutant (C), and double mutant (D) at e13.5 as well as the adjacent diencephalon tissue (DI). However, the double mutant also contains Mash1 within Rathke’s Pouch and the cells that are ectopically located (D). At e14.5 we observe a similar pattern in ACTH with immunohistochemistry on sagittal sections. In the wildtype (E), Hes1 mutant (F), and Prop1 mutant ACTH expression is restricted to the anterior lobe. In the double mutant ACTH is expressed throughout Rathke’s pouch (H, arrows). By e16.5 the melanotropes have differentiated and are detected with the POMC antibody in the intermediate lobe (IL) while the corticotropes are detected in the anterior lobe (AL) in the wildtype (I). The Hes1 mutant lacks melanotropes but contains corticotropes in the anterior lobe (J, bracket). In the Prop1 mutant corticotropes are evident in the anterior lobe (K, bracket). Dark staining likely corresponding to ACTH producing cells is observed throughout an indistinguishable Rathke’s pouch and anterior lobe in the double mutant (L). PC2, marking the melanotropes, is present throughout the intermediate lobe (IL) of the wildtype (M) and is absent in the Hes1 mutant (N). An occasional PC2 positive cell can be observed in the Prop1 mutant (O). Similar to the Hes1 mutant, the double mutant lacks PC2 staining (P). n=3 Magnification: 200X
In the double mutant, Mash1 appears to be expressed throughout much of Rathke’s pouch (Fig. 2D). If Mash1 activates the differentiation of ACTH producing cells, it would be expected that they too would be aberrantly located in the double mutant. In the wildtype pituitary at e14.5, ACTH producing cells are located exclusively in the anterior lobe (Fig. 2E). The same is true for the Hes1 mutant (Fig. 2F) as well as the Prop1 mutant (Fig. 2G). However, in the double mutant the ACTH producing cells are located in the ventral part of the pouch, but they are additionally located ectopically within the dcRP and drRP (Fig. 2H). We also noticed that at e13.5 the double mutant started to have ACTH producing cells that were misplaced (all other phenotypes at this age were the same as at e14.5) (Supp. Fig. 1). At e12.5, we did not notice any difference among the wildtype, Hes1 mutant, Prop1 mutant, or wildtype pituitary concerning the placement of the ACTH producing cells (data not shown). Therefore, it appears that the cells begin to be misplaced in the double mutant at e13.5 and we begin to see a severely abnormal phenotype at e14.5.
By e16.5 there are two populations of POMC containing cells, the corticotropes within the anterior lobe (AL) and the melanotropes within Rathke’s pouch, which is now referred to as the intermediate lobe (IL). The intermediate lobe cells can be distinguished by the presence of prohormone convertase 2 (PC2), which processes POMC into MSH. The wildtype pituitary has POMC immunoreactive cells in the AL that correspond to the corticotropes and cells in the IL that correspond to the melanotropes (Fig. 2I). PC2 expression marks the melanotropes in the IL (Fig. 2M). In the Hes1 mutant, the corticotropes can be visualized in the AL (Fig. 2J). However, as previously reported by Raetzman et al. (2007), when Hes1 is lost, very few, if any, cells in the IL differentiate into melanotropes and express PC2 (Fig. 2N). In the Prop1 mutant at e16.5, ACTH expression appears similar to that of the wildtype pituitary (Fig. 2K), but we are able to see only a few PC2 immunoreactive cells in the IL (Fig. 2O). Ward et al. (2005) demonstrated expression of PC2 in the Prop1 mutant at postnatal day 1 in the mouse, therefore we know that PC2 is expressed in the Prop1 mutant. Low levels at e16.5 or delayed expression may help explain the reduction in PC2 expressing cells that we see. The double mutant contains ACTH positive cells aberrantly expressed throughout the entire pituitary (Fig. 2L) and is devoid of PC2 staining (Fig. 2P).
Clearly, both Hes1 and Prop1 are both required for proper restriction of Mash1 expression to the anterior lobe and ventral part of Rathke’s pouch. They also function redundantly to prohibit differentiation of cells within the pouch and restrict the movement of the ACTH producing cells ventrally from Rathke’s pouch to the anterior lobe.
Hes1 and Prop1 are both required for proper restriction of αGSU positive cells to the anterior lobe
In addition to ACTH, the common alpha subunit of TSH, FSH, and LH (αGSU) is also expressed early in pituitary development. In the wildtype, Hes1, and Prop1 mutant pituitaries at e13.5, αGSU expression is located exclusively in the developing anterior lobe (Supp. Fig. 1). However, we detected misexpression of the hormone subunit within the cells of Rathke’s pouch of the double mutant beginning at this age (Supp. Fig. 1). This misexpression is more evident at e14.5 and e16.5 where the αGSU positive cells are clearly differentiated within the dcRP and drRP in addition to the normal expression in the ventral pituitary (Fig. 3D, H, arrows). In the wildtype, Hes1, and Prop1 mutants at both ages, the αGSU positive cells are restricted to the anterior lobe in the ventral pituitary (Fig. 3A-C, E-G). This data indicates that Hes1 and Prop1 also are required for proper restriction of the αGSU positive cells, in addition to the ACTH producing cells, to the ventral area of the pituitary.
Fig. 3.
αGSU producing cells are misplaced in the double mutant. In the wildtype (A), Hes1 mutant (B), and Prop1 mutant (C) at e14.5 the αGSU immunoreactive cells are located exclusively in the anterior lobe as detected by immunohistochemistry on sagittal sections. However, in the double mutant, αGSU producing cells are detected within Rathke’s pouch (D, arrows). At e16.5 we continue to observe αGSU producing cells strictly within the anterior lobes of wildtype (E), Hes1 mutant (F), and Prop1 mutant (G) pituitaries while in the double mutant, αGSU producing cells appear to be located within Rathke’s pouch (H, arrow) in addition to the anterior lobe. n=3 Magnification: 200X
PIT1 and its lineages are lost in both the Prop1 and double mutants
After examining ACTH and αGSU producing cell types, we wanted to look at other hormones that are expressed in the pituitary to determine if the loss of both Hes1 and Prop1 affects those as well. By e16.5 TSH and GH are detected in the developing pituitary, in addition to ACTH and αGSU. It has been well established that Prop1 is necessary for activation of PIT1 and its cell lineages: thyrotropes, somatotropes, and lactotropes (Gage, et al. 1996b; Sornson, et al. 1996). Although we hypothesized that the function of Prop1 in PIT1 lineage specification is independent of Hes1, we explored this question by looking at hormone specification in double mutants at e16.5.
We first examined PIT1 and found that in the wildtype pituitary at e16.5, PIT1 is expressed throughout the anterior lobe (Fig. 4A). This is also true for the Hes1 mutant, but PIT1 is also expressed in parts of the intermediate lobe (Fig. 4B). However, the Prop1 mutant is completely devoid of PIT1 (Fig. 4C) as is the double mutant (Fig. 4D). We then examined TSH and GH, two of the cell lineages specified by PIT1. There are two distinct populations of cells expressing the TSHβ subunit during pituitary development: one that is PIT1-dependent and secretes functional TSH referred to as the thyrotropes, and one that is PIT1-independent and phenotypically disappears by birth referred to as the rostral tip thyrotropes (Lin, et al. 1994). Both of these cell lineages are visible at e16.5 in the wildtype (Fig. 4E) and Hes1 mutant (Fig. 4F). The thyrotropes are located within the anterior lobe and are characterized by dark, round, distinct staining patterns (denoted with a bracket) while the rostral tip thyrotropes are typically located at the most rostral part of the anterior lobe and are characterized by a hazy staining pattern (denoted with asterisk). The Prop1 mutant has no thyrotropes due to the lack of Pit1 activation by PROP1 (Fig. 4G). However, the rostral tip thyrotropes are present in the rostral tip of the pituitary (asterisk). The double mutant appears to have a similar pattern to that of the Prop1 mutant (Fig. 4H). The brown staining in the rostral part of the double mutant pituitary seems to have a hazy staining pattern similar to that of the rostral tip thyrotropes in the other genotypes. The absence of Prop1, location of this cell population, and staining pattern all indicate that this cell population (denoted with an asterisk) is likely the rostral tip thyrotropes and not the actual hormone-producing thyrotropes of the anterior lobe.
Fig. 4.
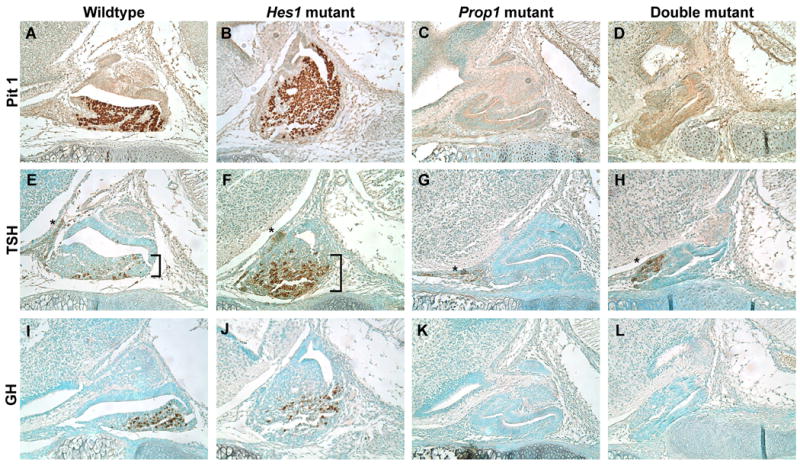
Prop1 and double mutant pituitaries lack PIT1 and its lineages. Sagittal sections at e16.5 stained via immunohistochemistry for PIT1 (A-D), TSHβ (E-H), and GH (I-L). PIT1 is present in the anterior lobes of the wildtype (A) and Hes1 mutant (B) but entirely absent from the Prop1 (C) and double mutant (D) pituitaries. The TSH antibody detects two distinct cell populations, the hormone producing thyrotropes of the anterior lobe (brackets) and the rostral tip thyrotropes (asterisks). In the wildtype (E) and Hes1 mutant (F) the thyrotrope cell population can be seen as darkly stained round cells within the anterior lobe while the rostral tip thyrotropes can be seen as a hazy brown staining in the most rostral part of the pituitary. In the Prop1 mutant (G) and double mutant (H) only the rostral tip thyrotropes appear to be present. These same patterns hold true for GH. In the wildtype (I) and Hes1 mutant (J) GH is located within the anterior lobe while in the Prop1 mutant (K) and double mutant (L) no GH is detected. n=3 Magnification: 200X
Another piece of evidence confirming that the PIT1 lineage is not rescued in the Prop1 mutant when Hes1 is lost is the lack of GH in the double mutant at e16.5 (Fig. 4L). The wildtype and Hes1 mutant both contain GH within their anterior lobes (Fig. 4I, J). The Prop1 mutant and double mutant both completely lack GH (Fig. 4K, L). These results indicate that the pathway of Pit1 activation by PROP1 is independent of Hes1.
Early patterning molecules unaffected by the loss of Hes1 and Prop1
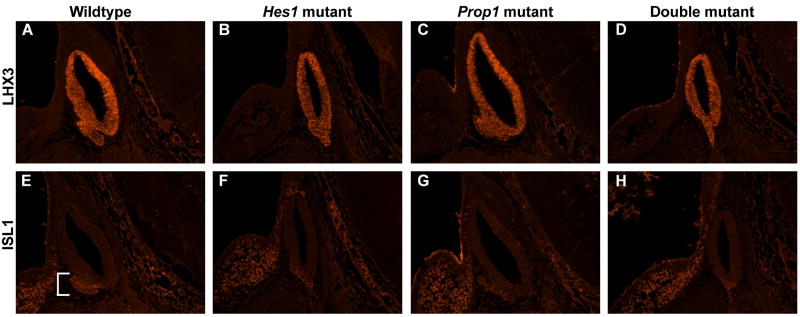
After confirming via hormone staining that the misplaced cells seen in the double mutant at e14.5 and e16.5 were differentiated anterior lobe cells, we examined early dorsal-ventral patterning in the pituitary by the expression of LHX3 and ISL1 at e12.5 (Thor, et al. 1991; Sheng, et al. 1997). Cells expressing LHX3 are found throughout both Rathke’s pouch and the anterior lobe in the e12.5 wildtype (Fig. 5A), Hes1 mutant (Fig. 5B), Prop1 mutant (Fig. 5C), and double mutant pituitaries (Fig. 5D). Unlike LHX3 expression, ISL1 expression is restricted to the ventral anterior lobe in the e12.5 wildtype pituitary (Fig. 5E). The same is true for the Hes1 mutant (Fig. 5F), Prop1 mutant (Fig. 5G), and double mutant (Fig. 5H). Expression of LHX3 and ISL1 do not appear to be affected by the loss of Hes1 or Prop1 individually or by the loss of both at e12.5 or 14.5 (data not shown). Therefore, even though the double mutant has cells differentiating and moving improperly, the pituitary still retains some evidence of dorsal-ventral patterning, such as restricted ISL1 expression.
Fig. 5.
Loss of Hes1 and Prop1 does not affect initial stages of pituitary patterning. Sagittal sections of embryos at e12.5 were stained for expression of LHX3 (A-D) or ISL1 (E-H). LHX3 is detected throughout Rathke’s pouch and the anterior lobe in the wildtype (A), Hes11 mutant (B), Prop1 mutant (C), and double mutant (D). ISL1 expression is restricted to the anterior lobe in the ventral part of the pituitary in the wildtype (E, bracket), Hes1 mutant (F), Prop1 mutant (G), and double mutant (H). n=3 Magnification: 200X
Morphology and movement of Rathke’s Pouch cells correlates with E- and N-cadherin expression
Because the anterior lobe cells appear misplaced in the double mutants we wanted to explore possible causes of the improper cell movement. Cadherins are cell adhesion molecules that play important roles during morphogenesis and have been shown to be present in the rat pituitary (Gumbiner, 2005; Kikuchi, et al. 2006; Kikuchi, et al. 2007). A fine balance in adhesion is necessary for cell movement during development, especially during EMT (Kalluri and Neilson, 2003). It is not yet understood if the pituitary cells of Rathke’s pouch undergo EMT; however, the cells do appear to undergo similar changes: tightly packed columnar cells of Rathke’s pouch transition to groups of round cells as they move to the anterior lobe. After staining for E- and N-cadherin at e13.5, the age when the improper cell movement appears to begin, subtle, yet noticeable differences in expression patterns were observed.
In the wildtype pituitary, we observed E-cadherin (green) expression in an even pattern throughout Rathke’s pouch and the anterior lobe while N-cadherin (red) appears in a strongly concentrated pattern along the lumen lining the dorsal part of Rathke’s pouch. Along the ventral part of the lumen, the concentration of N-cadherin is lost (between the two arrowheads) and instead we observed a more disperse pattern (Fig. 6A-B). This is demonstrated more clearly in Fig. 6C where the cells within Rathke’s pouch and the anterior lobe that are lining both the dorsal and ventral parts of the lumen, respectively, are shown at a higher magnification.
Fig. 6.
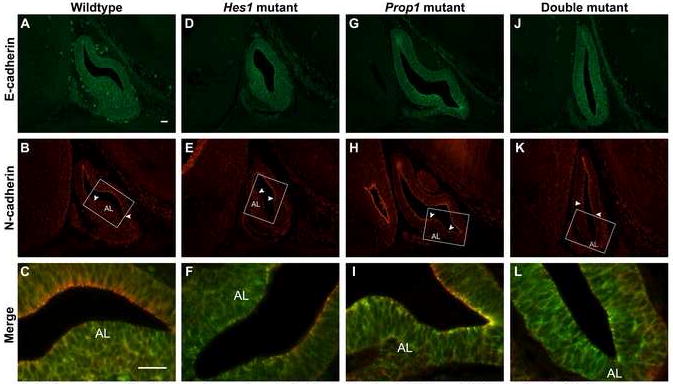
Downregulation of N-cadherin expression correlates with the ability of cells to leave Rathke’s pouch. Sagittal sections of e13.5 embryos were stained via immunohistochemistry for E- and N-cadherin and then the images were merged. In the wildtype pituitary, E-cadherin is dispersed throughout Rathke’s pouch and the anterior lobe (A) while N-cadherin is concentrated along the lumen of Rathke’s pouch except for the ventral area where the anterior lobe (AL) is forming (B, arrowheads mark boundaries of N-cadherin concentration along lumen). The boxes in BE, H, and K correspond to magnified images C, F, I, and L. These images were merged and viewed at higher magnification to show the dorsal aspect of the pouch with N-cadherin concentration and the ventral aspect with only E-cadherin (C). In the Hes1 mutant E-cadherin is dispersed throughout the pouch and anterior lobe (D) while N-cadherin is concentrated along the lumen of the dorsal half of the pouch (E, arrowheads). In the merged image of the Hes1 mutant it is clear that the ventral half of the pouch is void of N-cadherin concentration. The Prop1 mutant contains E-cadherin dispersed throughout the pituitary with areas of concentration along the lumen in the ventral part of the pouch (G). Nearly all of the lumen of Rathke’s Pouch is lined with N-cadherin except for the small area where cells are exiting the pouch for the anterior lobe (H, arrowheads). E-cadherin is dispersed throughout the pouch in the double mutant (J). N-cadherin lines the dorsal half of the lumen of Rathke’s pouch and is absent along the ventral half where the anterior lobe is forming (K, arrowheads). In the merged image of J and K it is clear that there is no N-cadherin concentration along the ventral half of the double mutant Rathke’s pouch (L). n=3 Magnification: 200X (A-H) 640X (I-L), scale bars are 25 μm
In the Hes1 mutant we observed a similar pattern to that of the wildtype (Fig. 6D-F), which includes E-cadherin expression throughout the pituitary and concentrated N-cadherin in the cells lining the dorsal lumen of Rathke’s pouch. However, it appears that N-cadherin may be concentrated in a smaller proportion of the luminal cells than in the wildtype (boundaries marked by arrowheads).
In the Prop1 mutant we notice differences in the expression patterns of the two molecules (Fig. 6G-I). Specifically, we observe strong N-cadherin staining lining almost the entire lumen, except for a small area where anterior lobe cells appear to be leaving the pouch in the rostroventral part of the pituitary (boundaries marked by arrowheads). It also appears that E-cadherin staining is stronger in the ventral part of the pouch, particularly in a concentrated pattern along the lumen of Rathke’s pouch. In the higher magnification image it is clear that there is a strong overlap of concentrated E- and N-cadherin (yellow) along the lumen of Rathke’s pouch that is not seen in the wildtype or Hes1 mutants (Fig. 6I).
We observe what appears to be an opposite staining pattern in the double mutant than that of the Prop1 mutant. There is E-cadherin staining throughout Rathke’s pouch in a pattern that appears to be dispersed throughout the cells (Fig. 6J). Also, a strong N-cadherin signal lines the lumen of the dorsal part of Rathke’s pouch only, as opposed to nearly all of the lumen in the Prop1 mutant (Fig. 6K). The lack of N-cadherin concentration along the ventral half of the lumen is better appreciated in the higher magnified merged image (Fig. 6L). These data point to a possible role of Prop1 in permitting the down-regulation of N-cadherin in cells transitioning from proliferating progenitors to differentiated hormone cells. Hes1 also likely plays an antagonistic role, perhaps by maintaining N-cadherin expression in cells along the lumen and therefore preventing differentiation or movement. We believe that loss of Hes1 in the Prop1 mutant may allow for the downregulation of N-cadherin, which in turn may allow for movement of the cells out of the pouch. However, even though the cells of the Prop1 mutant are able to move with the loss of Hes1, they appear to have lost their directional cues and instead migrate aberrantly.
SLUG is present in the rostral tip but absent in the anterior lobe of the Prop1 and double mutants
After noticing changes in expression pattern of E- and N-cadherin as well as characteristics of EMT we examined the expression of SLUG, a zinc finger transcription factor known to directly repress E-cadherin (Nieto, et al. 1994). SLUG is upregulated during EMT, causing a decrease in E-cadherin expression which allows for cell movement (Bolos, et al. 2003). In the wildtype pituitary at e14.5, we observed dispersed SLUG expression throughout the entire anterior lobe as well as the rostral tip, with Rathke’s pouch cells lacking expression (Fig. 7A). In the Hes1 mutant we observed a similar pattern (Fig. 7B). Surprisingly, we discovered that the only place SLUG is expressed in the Prop1 mutant is in the rostral tip (Fig. 7C). We see the same expression pattern in the double mutant (Fig. 7D). Clearly, Prop1 is necessary for activation of SLUG either directly or indirectly in the anterior lobe except in the cells comprising the rostral tip.
Fig. 7.
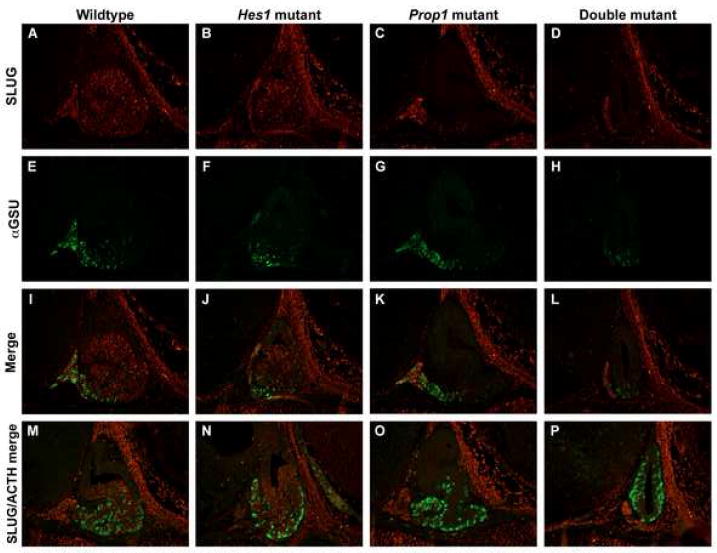
SLUG is absent in Prop1 and double mutant anterior lobe cells. Immunohistochemical staining at e14.5 on sagittal sections revealed that SLUG is present in the anterior lobes of the wildtype (A) and Hes1 (B) mutant pituitaries. In the Prop1 (C) and double mutants (D) SLUG is found only in the most rostral tip of the pituitary. In the wildtype (E), Hes1 mutant (F), and Prop1 mutant (G) pituitaries αGSU is found exclusively in the anterior lobe while in the double mutant (H), αGSU is also expressed in the ventral part of Rathke’s pouch. When these two stainings were merged we saw an overlap of SLUG and αGSU in an occasional cell in the rostral tip in the wildtype (I), Hes1 mutant (J), Prop1 mutant (K), and double mutant (L). We saw no overlap when SLUG and ACTH stainings were merged in the wildtype (M), Hes1 mutant (N), Prop1 mutant (O) and double mutant (P). n=3 Magnification: 200X
We then questioned whether SLUG is typically expressed in cells that have differentiated into hormone producing cells. We hypothesized that the loss of SLUG in αGSU and ACTH producing cells could result in the loss of directional cues seen in these cells in the double mutant. As described in Figs. 2 and 3, the wildtype, Hes1, and Prop1 mutants at e14.5 all have αGSU and ACTH producing cells restricted to the anterior lobe in the ventral pituitary while the double mutant has ectopic ages and ACTH producing cells in the drRP and dcRP in addition to the ventrally located cells (Fig. 7E-H). When we examined the coexpression of SLUG and pituitary hormones, we observed that SLUG appears to colocalize with an occasional αGSU cell in the rostral tip, but not with any cells in the anterior lobe (Fig. 7I-L). Also, we did not observe any colocalization between Slug and ACTH in any of the genotypes (Fig. 7M-P). It is possible that SLUG may be expressed in cells during their transition from proliferating progenitor cell to differentiated hormone producing cell. Also, Prop1 clearly appears to be involved in the activation of SLUG either directly or indirectly.
Discussion
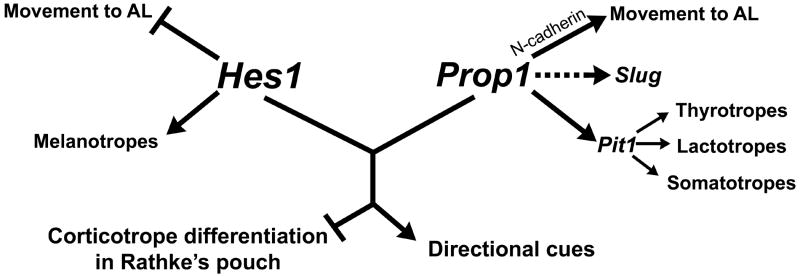
Hes1 and Prop1 are both involved in the Notch signaling pathway as direct targets of the Notch intracellular domain and RBPJ-κ transcriptional activator complex, (Jarriault, et al. 1998; Raetzman, et al. 2004). We demonstrate that Prop1 and Hes1 have overlapping and distinct functions during pituitary organogenesis (Fig. 8). Analysis of pituitary development in mice lacking both of these genes revealed their redundant role in the appropriate timing of pituitary precursor differentiation. We see ectopic ACTH and αGSU expression in the pituitary only when both genes are absent. Although the exact mechanism by which PROP1 and HES1 prevent premature differentiation is unclear, the ectopic corticotropes and αGSU positive cells correlate with the misexpression of Mash1 in the double mutants. It is well established that HES1 represses Mash1 transcription and often Mash1 mRNA levels are upregulated in Hes1 mutants (Ishibashi, et al. 1995; Kageyama and Nakanishi, 1997). For example, in the fetal lung, Mash1 seems to be important for specification of one of the many cell types: the pulmonary neuroendocrine cells (PNEC). In the Hes1 mutant there is increased expression of Mash1 followed by an increase in PNEC in the fetal lung (Ito, et al. 2000). In the pituitary, we observe an increase in Mash1 transcript levels only when both Hes1 and Prop1 are lost, indicating that PROP1 may also play a role in repressing Mash1 expression.
Fig. 8.
The roles of Hes1 and Prop1 during pituitary organogenesis. Alone, Hes1 is likely necessary for preventing movement of cells from Rathke’s pouch to the anterior lobe. Also, it has been shown to be necessary for the differentiation of the melanotrope cell lineage. Prop1 alone is likely necessary for promoting the movement of cells from Rathke’s pouch to the anterior lobe. This may be done through the downregulation of N-cadherin. Prop1 may also be responsible for activating Slug, evidenced by the lack of SLUG in the Prop1 and double mutants. It is also well established that Prop1 is necessary for activation of Pit1, a transcription factor that promotes the thyrotrope, lactotrope, and somatotrope lineages. Together, Hes1 and Prop1 appear to prevent differentiation of the corticotropes within Rathke’s pouch as well as promote factors that provide directional cues to the cells as they transition from Rathke’s pouch to the anterior lobe.
Previous studies have seen a more subtle increase in ACTH immunoreactive cells and Mash1 expression in the Hes1 or Rbpj-κ conditional knockouts similar to our double mutant phenotype but unlike our Hes1 mutant alone (Zhu, et al. 2006; Kita, et al. 2007). One explanation for the discrepancy in our findings is that genetic background plays a significant role in pituitary differentiation. In fact, the phenotype of the Prop1 knockout is altered when moved to different strains of mice, although there is no change in its effect on cell specification (Nasonkin, et al. 2004). Our findings are unique in that neither previous study reports premature differentiation of αGSU containing cells. This strengthens our position that both Hes1 and Prop1 are critical for repressing pituitary precursor differentiation.
A major question in developmental biology is how signaling pathways coordinate complex morphological changes that occur as the organism grows. For the pituitary, the critical organ growth during development is the ventral expansion of the hormone producing cells of the anterior lobe. The placement of the anterior lobe is essential because it must be formed in close apposition with the vasculature to be able to receive signals from the hypothalamus and release its hormone contents into the bloodstream (Treier and Rosenfeld, 1996). Our studies uncovered an interaction between Prop1 and Hes1 in coordinating formation of the anterior lobe in the ventral domain of the developing pituitary. In the Prop1 mutant, few cells are able to leave the pouch to form the anterior lobe, resulting in a hypercellular, dysmorphic Rathke’s pouch during embryonic development. However, after birth there is increased apoptosis in the remnant of Rathke’s pouch which leads to a hypocellular pituitary (Ward, et al. 2005). We observe the opposite problem in the Hes1 mutant where the Rathke’s pouch is much smaller than normal likely due to a premature influx of cells to the anterior lobe around e13.5. When Hes1 is eliminated from the Prop1 mutant, cells are no longer trapped in Rathke’s pouch but they do not migrate ventrally to form the anterior lobe. Instead, the double mutant cells appear to have lost their directional cues and are located both dorsally and ventrally on the rostral and caudal sides of Rathke’s pouch. This phenotype has some similarity with the Lhx3 knockout in which NOTCH2 is not expressed, resulting in abnormal dorsal-ventral patterning and cell movement (Ellsworth, Butts, Camper. 2008). By analyzing the double mutants, we were able to uncover the role of Prop1 and Hes1 in regulating corticotrope movement that was not appreciated in the Rbpj-κ knockout (Zhu, et al. 2006). In this model, Hes1 and Prop1 mRNA were significantly reduced, but not absent like they are in our double mutant system.
During development, expansion of organs relies on differential cell sorting and movement. Changes in the actin cytoskeleton and adhesion molecules on the cell surface play pivotal roles in this process. We suspected that the loss of function of Prop1 might interfere with important cell adhesion or movement cues, thereby preventing the cells from leaving Rathke’s pouch. This would account for not only the branched, elongated structure of the Rathke’s pouch in the Prop1 mutant but also for the hypocellular anterior lobe. We discovered that Prop1 is necessary for downregulating N-cadherin in the cells lining the lumen of Rathke’s pouch. N-cadherin is a classical cadherin molecule known to affect cell-cell adhesion and migration during development. In pituitary tumor formation, reduced expression of N-cadherin can result in an increase in invasiveness of the tumor, likely due to the decrease in cell adhesion (Ezzat, et al. 2004; Gumbiner, 2005; Deramaudt, et al. 2006). However, there appears to be no canonical PROP1 binding sites within 10 kb upstream of N-cadherin, indicating that N-cadherin may not be a direct PROP1 target gene. One mechanism by which PROP1 could influence N-cadherin expression is through its interactions with the Wnt signaling pathway. Knocking out members of the Wnt signaling pathway such as Wnt4 and Wnt5a has produced pituitaries with dysmorphology and/or altered cell specification, similar to the Prop1 mutant (Cha, et al. 2004; Potok, et al. 2008). Additionally, PROP1 can directly interact with β-catenin, a central component of the Wnt signaling pathway (Olson, et al. 2006). N-cadherin is tethered to the cytoskeleton by direct interactions with β-catenin. It is possible that when PROP1 is lost, there is more β-catenin available to interact with N-cadherin, preventing its downregulation at the junction where cells need to exit Rathke’s Pouch (Nagafuchi and Takeichi, 1988; Ozawa, et al. 1990; Douglas, et al. 2001; Gumbiner, 2005; Olson, et al. 2006). Alternatively, loss of Prop1 may influence expression of other pathways that are known to regulate N-cadherin expression such as TGFβ, EGF, metalloproteases, or Rac1 (Grande, et al. 2002; Reiss, et al. 2005; Woods, et al. 2007).
Notch signaling also plays a role in cell movement and segregation. In Drosophila, the imaginal disc relies on Notch to maintain distinct populations of cells along the dorsal-ventral axis as it develops. Loss of Notch results in the absence of F-actin concentration which leads to the mixing of cells between the two compartments (Micchelli and Blair, 1999; Major and Irvine 2005). In Hes1 mutants we observe a similar reduction in the boundary marker N-cadherin which correlates with a greater proportion of cells leaving Rathke’s pouch. Hes1 may have a direct effect on N-cadherin expression, or more likely, an indirect effect. This could include a role in cell sorting or actin cytoskeleton rearrangement, which can lead to changes in N-cadherin expression or function. Interestingly, in the double mutant only about half of the lumen is lined with concentrated N-cadherin staining, despite the lack of Prop1. We speculate that the loss of Hes1 in the Prop1 mutant may permit the cells trapped in Rathke’s pouch to be released by allowing for the downregulation of N-cadherin. It is possible that Prop1 alone is imparts directional cues to Rathke’s pouch cells. However, in the Prop1 mutant, ACTH and αGSU positive cells move to the correct place. This leads us to hypothesize that both Hes1 and Prop1 work together to promote movement of cells ventrally from Rathke’s pouch to the anterior lobe.
The presence of E- and N-cadherin in the pituitary suggests that the transition from the proliferating progenitor cells of Rathke’s pouch to the differentiated cells of the anterior lobe may be an EMT. Classic characteristics of EMT that we are able to observe in the pituitary include presence of epithelial markers such as E-cadherin, loss of cell polarity, and change in cell shape allowing for cell movement or migration. However, we do not observe a downregulation of E-cadherin in the wildtype, which is a typical part of the process (Thiery, 2002; Kalluri and Neilson, 2003). Partial EMT (pEMT) is a similar process that differs from EMT in that often there is only a transient loss of polarity in the epithelial cells and the mesenchymal cells do not display all of their typical characteristics (Leroy and Mostov, 2007). It is possible that one of these processes is occurring in the pituitary.
Both EMT and pEMT involve a protein called SLUG, which is responsible for downregulating E-cadherin in EMT and maintaining cell survival in pEMT (Nieto, et al. 1994; Leroy and Mostov, 2007). We discovered SLUG expression throughout the dorsal area of the anterior lobe where cells appear to be transitioning from Rathke’s pouch to the anterior lobe as well as in the rostral tip in the wildtype and Hes1 mutant pituitaries. This specific expression of SLUG in the pituitary indicates that it could function to support migration like it does in the melanocyte cells (Jiang, et al. 1998). Surprisingly, in the Prop1 and double mutants SLUG was detectable in the rostral tip, but not in the dorsal anterior lobe. This could explain why so many of the cells of Rathke’s pouch fail to transition to the anterior lobe in the Prop1 mutant. There appears to be no canonical PROP1 binding sites within 10 kb upstream of SLUG, therefore PROP1 likely regulates SLUG indirectly via Wnt or TGFβ signaling (Sakai, et al. 2005; Choi, et al. 2007). However, the double mutant also lacks SLUG, yet the cells are able to leave Rathke’s pouch. This may be due to a combinatorial effect of the loss of Hes1 in the Prop1 mutant. Specifically, Mash1 could be playing a role along with SLUG. In addition to regulation of the number and timing of differentiation, MASH1 and other proneural bHLH factors have been implicated in cell migration. A recent study by found that migration of neural precursor cells was enhanced in cell aggregate migration assays by exogenous MASH1 expression (Ge, et al. 2006). In total, our data provides insight into the way in which Notch signaling molecules and cell adhesion molecules can affect cell differentiation and movement (via N-cadherin, Slug, Mash1, etc.) and could be applied when studying Notch in the context of pituitary development and tumors.
Supplementary Material
Misexpression of ACTH and αGSU producing cells begins at e13.5 in the double mutant. Immunohistochemistry, performed on sagittal sections at e13.5, shows that ACTH and αGSU expression is restricted to the anterior lobe in ventral pituitary of the wildtype (A, E), Hes1 mutant (B, F), and Prop1 mutant. However, in the double mutant, ACTH and αGSU positive cells are visible within Rathke’s pouch and along the lateral sides of the pouch (D, H). n=3 Magnification: 200X
Acknowledgments
We would like to thank Barbara Ahrens for help with mouse breeding and Dr. Richard Kollmar for use of his microscope. We would also like to thank Dr. Ryoichiro Kageyama for the Hes1 knockout mice. Antibodies were kindly provided by Dr. A. F. Parlow (National Hormone and Peptide Program, NIDDK), Dr. Simon Rhodes (Indiana University, Purdue University, Indianapolis, IN) and Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). Financial support was received from the National Institute of Health grant R01DK076647A (LTR).
This research was funded by the National Institutes of Health grant R01DK076647A to L.T.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen B, Pearse RV, 2nd, Jenne K, Sornson M, Lin SC, Bartke A, Rosenfeld MG. The Ames Dwarf Gene is Required for Pit-1 Gene Activation. Dev Biol. 1995;172:495–503. doi: 10.1006/dbio.1995.8040. [DOI] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The Transcription Factor Slug Represses E-Cadherin Expression and Induces Epithelial to Mesenchymal Transitions: A Comparison with Snail and E47 Repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Cha KB, Gridley T, Stifani S, Meeldijk J, Clevers H, Camper SA. TCF and Groucho-Related Genes Influence Pituitary Growth and Development. Mol Endocrinol. 2003;17:2152–2161. doi: 10.1210/me.2003-0225. [DOI] [PubMed] [Google Scholar]
- Burrows HL, Douglas KR, Seasholtz AF, Camper SA. Genealogy of the Anterior Pituitary Gland: Tracing a Family Tree. Trends Endocrinol Metab. 1999;10:343–352. doi: 10.1016/s1043-2760(99)00189-7. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The Transcription Factor Snail Controls Epithelial-Mesenchymal Transitions by Repressing E-Cadherin Expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Carninci P, Waki K, Shiraki T, Konno H, Shibata K, Itoh M, Aizawa K, Arakawa T, Ishii Y, Sasaki D, Bono H, Kondo S, Sugahara Y, Saito R, Osato N, Fukuda S, Sato K, Watahiki A, Hirozane-Kishikawa T, Nakamura M, Shibata Y, Yasunishi A, Kikuchi N, Yoshiki A, Kusakabe M, Gustincich S, Beisel K, Pavan W, Aidinis V, Nakagawara A, Held WA, Iwata H, Kono T, Nakauchi H, Lyons P, Wells C, Hume DA, Fagiolini M, Hensch TK, Brinkmeier M, Camper S, Hirota J, Mombaerts P, Muramatsu M, Okazaki Y, Kawai J, Hayashizaki Y. Targeting a Complex Transcriptome: The Construction of the Mouse Full-Length cDNA Encyclopedia. Genome Res. 2003;13:1273–1289. doi: 10.1101/gr.1119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. WNT5A Signaling Affects Pituitary Gland Shape. Mech Dev. 2004;121:183–194. doi: 10.1016/j.mod.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Choi J, Park SY, Joo CK. Transforming Growth Factor-beta1 Represses E-Cadherin Production Via Slug Expression in Lens Epithelial Cells. Invest Ophthalmol Vis Sci. 2007;48:2708–2718. doi: 10.1167/iovs.06-0639. [DOI] [PubMed] [Google Scholar]
- Deramaudt TB, Takaoka M, Upadhyay R, Bowser MJ, Porter J, Lee A, Rhoades B, Johnstone CN, Weissleder R, Hingorani SR, Mahmood U, Rustgi AK. N-Cadherin and Keratinocyte Growth Factor Receptor Mediate the Functional Interplay between Ki-RASG12V and p53V143A in Promoting Pancreatic Cell Migration, Invasion, and Tissue Architecture Disruption. Mol Cell Biol. 2006;26:4185–4200. doi: 10.1128/MCB.01055-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas KR, Brinkmeier ML, Kennell JA, Eswara P, Harrison TA, Patrianakos AI, Sprecher BS, Potok MA, Lyons RH, Jr, MacDougald OA, Camper SA. Identification of Members of the Wnt Signaling Pathway in the Embryonic Pituitary Gland. Mamm Genome. 2001;12:843–851. doi: 10.1007/s00335-001-2076-0. [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Butts DL, Camper SA. Mechanisms Underlying Pituitary Hypoplasia and Failed Cell Specification in Lhx3-Deficient Mice. Dev Biol. 2008;313:118–129. doi: 10.1016/j.ydbio.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzat S, Zheng L, Asa SL. Pituitary Tumor-Derived Fibroblast Growth Factor Receptor 4 Isoform Disrupts Neural Cell-Adhesion molecule/N-Cadherin Signaling to Diminish Cell Adhesiveness: A Mechanism Underlying Pituitary Neoplasia. Mol Endocrinol. 2004;18:2543–2552. doi: 10.1210/me.2004-0182. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA. The Ames Dwarf Gene, Df, is Required Early in Pituitary Ontogeny for the Extinction of Rpx Transcription and Initiation of Lineage-Specific Cell Proliferation. Mol Endocrinol. 1996a;10:1570–1581. doi: 10.1210/mend.10.12.8961267. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Roller ML, Saunders TL, Scarlett LM, Camper SA. Anterior Pituitary Cells Defective in the Cell-Autonomous Factor, Df, Undergo Cell Lineage Specification but Not Expansion. Development. 1996b;122:151–160. doi: 10.1242/dev.122.1.151. [DOI] [PubMed] [Google Scholar]
- Ge W, He F, Kim KJ, Blanchi B, Coskun V, Nguyen L, Wu X, Zhao J, Heng JI, Martinowich K, Tao J, Wu H, Castro D, Sobeih MM, Corfas G, Gleeson JG, Greenberg ME, Guillemot F, Sun YE. Coupling of Cell Migration with Neurogenesis by Proneural bHLH Factors. Proc Natl Acad Sci U S A. 2006;103:1319–1324. doi: 10.1073/pnas.0510419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande M, Franzen A, Karlsson JO, Ericson LE, Heldin NE, Nilsson M. Transforming Growth Factor-Beta and Epidermal Growth Factor Synergistically Stimulate Epithelial to Mesenchymal Transition (EMT) through a MEK-Dependent Mechanism in Primary Cultured Pig Thyrocytes. J Cell Sci. 2002;115:4227–4236. doi: 10.1242/jcs.00091. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of Cadherin-Mediated Adhesion in Morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted Disruption of Mammalian Hairy and Enhancer of Split Homolog-1 (HES-1) Leads to Up-Regulation of Neural Helix-Loop-Helix Factors, Premature Neurogenesis, and Severe Neural Tube Defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Moriyoshi K, Sasai Y, Shiota K, Nakanishi S, Kageyama R. Persistent Expression of Helix-Loop-Helix Factor HES-1 Prevents Mammalian Neural Differentiation in the Central Nervous System. EMBO J. 1994;13:1799–1805. doi: 10.1002/j.1460-2075.1994.tb06448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic Helix-Loop-Helix Transcription Factors Regulate the Neuroendocrine Differentiation of Fetal Mouse Pulmonary Epithelium. Development. 2000;127:3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Le Bail O, Hirsinger E, Pourquie O, Logeat F, Strong CF, Brou C, Seidah NG, Isra lA. Delta-1 Activation of Notch-1 Signaling Results in HES-1 Transactivation. Mol Cell Biol. 1998;18:7423–7431. doi: 10.1128/mcb.18.12.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Lan Y, Norton CR, Sundberg JP, Gridley T. The Slug Gene is Not Essential for Mesoderm Or Neural Crest Development in Mice. Dev Biol. 1998;198:277–285. [PubMed] [Google Scholar]
- Kageyama R, Nakanishi S. Helix-Loop-Helix Factors in Growth and Differentiation of the Vertebrate Nervous System. Curr Opin Genet Dev. 1997;7:659–665. doi: 10.1016/s0959-437x(97)80014-7. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-Mesenchymal Transition and its Implications for Fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Yatabe M, Kouki T, Fujiwara K, Takigami S, Sakamoto A, Yashiro T. Changes in E- and N-Cadherin Expression in Developing Rat Adenohypophysis. Anat Rec (Hoboken) 2007;290:486–490. doi: 10.1002/ar.20516. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Yatabe M, Fujiwara K, Takigami S, Sakamoto A, Soji T, Yashiro T. Distinctive Localization of N- and E-Cadherins in Rat Anterior Pituitary Gland. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1183–1189. doi: 10.1002/ar.a.20384. [DOI] [PubMed] [Google Scholar]
- Kita A, Imayoshi I, Hojo M, Kitagawa M, Kokubu H, Ohsawa R, Ohtsuka T, Kageyama R, Hashimoto N. Hes1 and Hes5 Control the Progenitor Pool, Intermediate Lobe Specification, and Posterior Lobe Formation in the Pituitary Development. Mol Endocrinol. 2007;21:1458–1466. doi: 10.1210/me.2007-0039. [DOI] [PubMed] [Google Scholar]
- Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A. Jagged1-Mediated Notch Activation Induces Epithelial-to-Mesenchymal Transition through Slug-Induced Repression of E-Cadherin. J Exp Med. 2007;204:2935–2948. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy P, Mostov KE. Slug is Required for Cell Survival during Partial Epithelial-Mesenchymal Transition of HGF-Induced Tubulogenesis. Mol Biol Cell. 2007;18:1943–1952. doi: 10.1091/mbc.E06-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Li S, Drolet DW, Rosenfeld MG. Pituitary Ontogeny of the Snell Dwarf Mouse Reveals Pit-1-Independent and Pit-1-Dependent Origins of the Thyrotrope. Development. 1994;120:515–522. doi: 10.1242/dev.120.3.515. [DOI] [PubMed] [Google Scholar]
- Liu J, Lin C, Gleiberman A, Ohgi KA, Herman T, Huang HP, Tsai MJ, Rosenfeld MG. Tbx19, a Tissue-Selective Regulator of POMC Gene Expression. Proc Natl Acad Sci U S A. 2001;98:8674–8679. doi: 10.1073/pnas.141234898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major RJ, Irvine KD. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development. 2005;132:3823–3833. doi: 10.1242/dev.01957. [DOI] [PubMed] [Google Scholar]
- Michelli CA, Blair SS. Dorsoventral lineage restriction in wing imaginal discs requires Notch. Nature. 1999;401:473–476. doi: 10.1038/46779. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Cell Binding Function of E-Cadherin is Regulated by the Cytoplasmic Domain. EMBO J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasonkin IO, Ward RD, Raetzman LT, Seasholtz AF, Saunders TL, Gillespie PJ, Camper SA. Pituitary Hypoplasia and Respiratory Distress Syndrome in Prop1 Knockout Mice. Hum Mol Genet. 2004;13:2727–2735. doi: 10.1093/hmg/ddh311. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of Cell Behavior during Vertebrate Development by Slug, a Zinc Finger Gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Ohsako S, Hyer J, Panganiban G, Oliver I, Caudy M. Hairy Function as a DNA-Binding Helix-Loop-Helix Repressor of Drosophila Sensory Organ Formation. Genes Dev. 1994;8:2743–2755. doi: 10.1101/gad.8.22.2743. [DOI] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG. Homeodomain-Mediated Beta-Catenin-Dependent Switching Events Dictate Cell-Lineage Determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Ringwald M, Kemler R. Uvomorulin-Catenin Complex Formation is Regulated by a Specific Domain in the Cytoplasmic Region of the Cell Adhesion Molecule. Proc Natl Acad Sci U S A. 1990;87:4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potok MA, Cha KB, Hunt A, Brinkmeier ML, Leitges M, Kispert A, Camper SA. WNT Signaling Affects Gene Expression in the Ventral Diencephalon and Pituitary Gland Growth. Dev Dyn. 2008;237:1006–1020. doi: 10.1002/dvdy.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Cai JX, Camper SA. Hes1 is Required for Pituitary Growth and Melanotrope Specification. Dev Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent Expression of Notch2 Delays Gonadotrope Differentiation. Mol Endocrinol. 2006;20:2898–2908. doi: 10.1210/me.2005-0394. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental Regulation of Notch Signaling Genes in the Embryonic Pituitary: Prop1 Deficiency Affects Notch2 Expression. Dev Biol. 2004;265:329–340. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are Required for Cell Survival and Expansion of the Pituitary Primordia. Development. 2002;129:4229–4239. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 Cleavage of N-Cadherin and Regulation of Cell-Cell Adhesion and Beta-Catenin Nuclear Signalling. EMBO J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D, Tanaka Y, Endo Y, Osumi N, Okamoto H, Wakamatsu Y. Regulation of Slug Transcription in Embryonic Ectoderm by Beta-Catenin-Lef/Tcf and BMP-Smad Signaling. Dev Growth Differ. 2005;47:471–482. doi: 10.1111/j.1440-169X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H. Multistep Control of Pituitary Organogenesis. Science. 1997;278:1809–1812. doi: 10.1126/science.278.5344.1809. [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary Lineage Determination by the Prophet of Pit-1 Homeodomain Factor Defective in Ames Dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-Mesenchymal Transitions in Tumour Progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Thor S, Ericson J, Brannstrom T, Edlund T. The Homeodomain LIM Protein Isl-1 is Expressed in Subsets of Neurons and Endocrine Cells in the Adult Rat. Neuron. 1991;7:881–889. doi: 10.1016/0896-6273(91)90334-v. [DOI] [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch Promotes Epithelial-Mesenchymal Transition during Cardiac Development and Oncogenic Transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, Rosenfeld MG. The Hypothalamic-Pituitary Axis: Co-Development of Two Organs. Curr Opin Cell Biol. 1996;8:833–843. doi: 10.1016/s0955-0674(96)80085-8. [DOI] [PubMed] [Google Scholar]
- Van Doren M, Bailey AM, Esnayra J, Ede K, Posakony JW. Negative Regulation of Proneural Gene Activity: Hairy is a Direct Transcriptional Repressor of Achaete. Genes Dev. 1994;8:2729–2742. doi: 10.1101/gad.8.22.2729. [DOI] [PubMed] [Google Scholar]
- Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in Pituitary Gland Growth. Mol Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Dupuis H, Shao Z, Beier F. Rac1 Signaling Stimulates N-Cadherin Expression, Mesenchymal Condensation, and Chondrogenesis. J Biol Chem. 2007;282:23500–23508. doi: 10.1074/jbc.M700680200. [DOI] [PubMed] [Google Scholar]
- Wu W, Cogan JD, Pfaffle RW, Dasen JS, Frisch H, O’Connell SM, Flynn SE, Brown MR, Mullis PE, Parks JS, Phillips JA, 3rd, Rosenfeld MG. Mutations in PROP1 Cause Familial Combined Pituitary Hormone Deficiency. Nat Genet. 1998;18:147–149. doi: 10.1038/ng0298-147. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained Notch Signaling in Progenitors is Required for Sequential Emergence of Distinct Cell Lineages during Organogenesis. Genes Dev. 2006;20:2739–2753. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Misexpression of ACTH and αGSU producing cells begins at e13.5 in the double mutant. Immunohistochemistry, performed on sagittal sections at e13.5, shows that ACTH and αGSU expression is restricted to the anterior lobe in ventral pituitary of the wildtype (A, E), Hes1 mutant (B, F), and Prop1 mutant. However, in the double mutant, ACTH and αGSU positive cells are visible within Rathke’s pouch and along the lateral sides of the pouch (D, H). n=3 Magnification: 200X