Abstract
Aims
Low Carbohydrate Diets (LCD) are a popular intervention for weight loss, but the effect of such diets on myocardial ischemia is not known. Myocardial energy substrates and insulin signaling pathways may be affected by these diets, and both may play a role in protection of ischemic myocardium. We investigated whether LCD increases susceptibility to cardiac injury during ischemia and reperfusion in the isolated rat heart.
Main Methods
Rats were fed LCD (60% kcal from fat / 30% protein / 10% carbohydrate) or a control diet (CONT; 16% / 19% / 65%) for 2 weeks. Hearts from rats fed with LCD or CONT were isolated and subjected to normal perfusion in Langendorff mode, with 30 min global low flow ischemia (LFI; 0.3 ml/min) followed by 60 min reperfusion, or 60 min LFI followed by 120 min reperfusion.
Key Findings
LCD diet led to an increase in 3-hydroxybutyrate and lower circulating insulin. LCD diet also resulted in impaired left ventricular performance during LFI, reduced recovery of function following LFI and reperfusion, and 10- to 20-fold increased injury as measured by lactate dehydrogenase release and histologic infarct area. LCD diet also led to lower myocardial glycogen stores and glycogen utilization during LFI, and lower insulin signaling as assessed by Akt phosphorylation at the end of LFI and reperfusion, but no differences in ERK 1/2 phosphorylation.
Significance
These results demonstrate that LCD affects myocardial energy substrates, affects insulin signaling, and increases myocardial injury following ischemia-reperfusion in the isolated heart.
Keywords: diet, myocardial ischemia, myocardial infarction, obesity, insulin, metabolism
INTRODUCTION
Obesity is a major public health problem and is expected to contribute to an increase in diabetes, hypertension, stroke, and ischemic heart disease. As the obesity epidemic has progressed, the percentage of consumed kilocalories from carbohydrate (CHO) has increased while that from fat has decreased (U.S. Centers for Disease Control and Prevention, 2004). This led to the concept that CHO especially promote weight gain, and resulted in formulation of diets based on low consumption of CHO with more allowance for protein and fats, including the Atkins (Atkins, 2001) and South Beach diets (Agatston, 2003). The safety of such low carbohydrate diets (LCD) has not been adequately assessed, with a meta-analysis of published trials concluding that there is insufficient evidence to recommend either for or against them (Bravata et al., 2003). Questions on LCD safety have focused on effects on circulating lipids; the limited studies have not found a detrimental effect on these, with a possible lowering of triglycerides (Foster et al., 2003; Samaha et al., 2003). Another recent report found that LCD did not lead to increased risk of myocardial infarction or death in women (Halton et al., 2006).
Despite these neutral, or possibly even beneficial, effects of LCD, there are other potential adverse effects of the diet in those with ischemic heart disease. LCD can lead to lower insulin and myocardial glycogen, and higher circulating free fatty acid (FFA) levels, which may reduce the resistance to ischemic injury and worsen the severity of an acute ischemic event. The heart can oxidize a variety of substrates including FFA, glucose, and lactate to generate high energy phosphates. A shift of cardiac energy substrate use from FFA toward CHO results in up to a 12% improvement in “efficiency” of ATP production, (i.e., moles of ATP produced per mole of O2 consumed) (Opie and Lopaschuk, 2004). Thus, lower insulin induced by LCD could be detrimental by increasing the relative importance of FFA oxidation. During severe ischemia, the availability of oxidative substrates and oxygen are tremendously decreased, and endogenous glycogen stores become the predominant energy source (Lloyd et al., 2004; Wang et al., 2005b). Since oxygen is limited in ischemia, glycolysis of glycogen-derived glucose-6-phosphate is the main process for generation of ATP and inhibition of glycolysis may lead to greater ischemic injury (Askenasy, 2001).
Importantly, insulin has direct cardioprotective effects, independent of any action on myocardial metabolism, through the PI3 kinase – Akt and MEK 1/2 – ERK 1/2 signaling cascades (Fujio et al., 2000; Jonassen et al., 2001; Matsui et al., 2001; Hausenloy and Yellon, 2004). If LCD reduces circulating insulin levels or induces insulin resistance at the tissue level (Ouwens et al., 2005), it could further decrease the resistance to ischemic myocardial injury. These potential adverse effects of LCD have not been systematically studied in a model of myocardial ischemia. In this work, we tested the hypothesis that LCD with macronutrient composition similar to that used by humans lowers cardiac glycogen and circulating insulin levels and leads to increased susceptibility to ischemic injury in the isolated perfused rat heart model.
MATERIALS AND METHODS
Animals and diets
Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and followed the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996). Male Sprague-Dawley rats (Taconic Farms) weighing 250 ± 10 g were divided into two groups and fed special diets for two weeks: LCD (TestDiet 5TSY, Richmond, IN, 60% calories from fat, 30% protein, 10% CHO); and a control diet (CONT; TestDiet 5TJM, 16% fat, 19% protein, 65% CHO). The diets have the same concentrations of essential fatty acids and micronutrients and the same relative proportions of amino acids. The macronutrient composition and selected micronutrient content of the diets are listed in Table 1. Rats were kept under twelve hour day / night cycles and had access to diets and water ad libitum. Body weight and food intake were recorded.
Table 1.
Macronutrient compositions of CONT and LCD.
| CONT | LCD | |
|---|---|---|
| Total Fat (% Kcal) | 16 | 60 |
| Saturated fat (% Kcal) | 8 | 30 |
| Protein (% Kcal) | 19 | 30 |
| Carbohydrate (% Kcal) | 65 | 10 |
| Sodium (mg/day) | 210 | 230 |
| Fiber (g/day) | 1.43 | 1.38 |
Sample collection
For initial determination of circulating insulin, glucose, lactate, ketone bodies, triglycerides, and myocardial glycogen, 6 rats from each group were fed LCD or CONT for 14 days. These animals were anesthetized with inhalational isoflurane (4%) and sacrificed by decapitation between 2 and 4 hours after turning on lights. As rats are nocturnal feeders, this was considered the fed state. Blood was collected and centrifuged at 4 °C. Blood glucose and lactate were measured using glucose and lactate meters based on the glucose oxidase and lactate oxidase enzymatic linked methods respectively (FreeStyle Blood Glucose Monitoring System, Roche Diagnostic GmbH; Accutrend Lactate, Sports Resource Group). Hearts were excised, blood rinsed out with chilled buffer, and hearts freeze-clamped with tongs chilled to liquid N2 temperature.
Biochemical analyses
Serum insulin and leptin were measured using radioimmunoassay methods (Linco RI-13K and RI-83K, Linco Research Inc., St. Charles, MO). Serum free fatty acids (FFA) were measured using a colorimetric method (NEFA C, Wako Chemical USA, Inc., Richmond, VA). Coronary effluent (the perfusion buffer collected from the pulmonary artery, after circulating through the heart during the perfusion protocol) lactate dehydrogenase (LDH) was measured by a spectrophotometric method (TOX-7, Sigma) and activities calculated with a standard curve. Spectrophotometric enzymatic-based methods were used to measure effluent lactate and serum 3-hydroxybutyrate (3-HB) (Noll, 1974; Willamson, 1974). Tissue glycogen content was measured from frozen tissue (myocardium or liver) by an enzymatic digestion method (Weinbrenner et al., 1996). Serum triglyceride was measured by use of a commercial kit (Infinity Triglycerides Reagent, Thermo Electron Corp., Melboume, Australia), which is based on coupled lipase-glycerol kinase-glycerophosphate oxidase-peroxidase reactions.
Isolated heart perfusions
Following sacrifice of the initial 6 rats from each diet group to determine the circulating substrate and hormone levels and tissue substrate contents, 5 rats from each diet group were similarly sacrificed and underwent different perfusion protocols. Isolated hearts were perfused in Langendorff mode with a modified Krebs-Henseleit buffer equilibrated with 95% O2-5% CO2 (38°C, pH 7.4) (Lloyd et al., 2004; Wang and Chatham, 2004; Wang et al., 2005b). We prepared perfusion buffers with electrolytes, metabolic substrates, and insulin to be approximately similar to the physiologic, circulating concentrations of each chemical species in vivo. We therefore defined CONT and LCD buffers. Each buffer contained 3% bovine serum albumin (essentially fatty acid free; Intergen, Mansfield, MA) and the following (in mM): NaCl 118, KCl 4.8, MgSO4 1.2, CaCl2 1.4, KH2PO4 1.2, Na2HCO3 25, pyruvate 0.2, palmitate 0.5, glucose 5.0, lactate 2.0, with triglycerides (Intralipid) at 1g/L. Intralipid at higher concentrations was difficult to solubilize in the buffer and resulted in impaired gas exchange across the oxygenation membrane, so this concentration was not exceeded (though somewhat higher concentrations were found circulating in the plasma, see below). Glutamine is the major circulating amino acid and was included at physiologic concentration (0.5 mM) (Kargotich et al., 2005; Le Bacquer et al., 2006) in all groups to model the in vivo setting (Lloyd et al., 2003, 2004; Wang et al., 2005a; Fulop et al., 2007). There were no differences in the circulating concentrations of glucose or lactate in the two groups, but 3-HB and insulin concentrations were different. In vivo, acetoacetate and 3-HB are the main circulating ketone bodies, typically at a ratio of 1:1 (Laffel, 1999). For simplicity, perfusion buffers were formulated with only 3-HB at concentrations of 0.6 mM in CONT buffer and 1.2 mM in LCD buffer. Initial experiments to determine the circulating FFA levels were performed in animals sacrificed at 4 ± 2 hours after lights were turned on, and the FFA concentration in these preliminary analyses was 0.5 mM in both groups; therefore, this concentration of FFA was used in the perfusion experiments (subsequent FFA determination at 2 ± 1 hours after lights on, from blood levels of animals sacrificed for the isolated heart perfusions, revealed lower FFA levels at 0.3 mM, again with no differences between groups). Insulin was 30 µU/ml in CONT buffer and 15 µU/ml in LCD buffer.
Cardiac function was monitored via a fluid filled balloon placed into the left ventricle and connected to a pressure transducer. End-diastolic pressure was set to 0 to 5 mmHg by adjusting balloon volume. Hearts were perfused under constant pressure of 75 mmHg and paced at 330 beats/min (Grass SD9 Stimulator, Quincy, MA) throughout the experiments.
Heart perfusion protocol
Isolated hearts from both groups were equilibrated for 30 min at 75 mmHg perfusion pressure, then subjected to low flow ischemia (LFI, 0.3ml/min) for 30 min followed by 60 min of reperfusion (with flow restored to achieve a perfusion pressure of 75 mmHg; balloon volume was not changed upon reperfusion) as described (Lloyd et al., 2004; Wang et al., 2005b). Perfused hearts were freeze-clamped at the end of either 30 min of baseline perfusion, after 30 min of LFI, or at the end of reperfusion. During the entire perfusion, cardiac function was monitored and recorded (DASYLab, Measurement Computing Corp., Norton, MA). Heart rate (HR), systolic pressure (SP), end diastolic pressure (EDP), and peak positive and negative rates of change of pressure (+dP/dt and −dP/dt) were used to compute indices of ventricular function. From these values, the left ventricular developed pressure (LVDP = SP − EDP), and the rate-pressure product (RPP = LVDP × HR) were used as the primary indices of cardiac systolic function or power. The increase of EDP during LFI was used to determine ischemic cardiac contracture, here defined as > 10 mmHg. The recovery of systolic and diastolic function at the end of reperfusion, expressed as percent of baseline values of RPP and ± dP/dt and by the EDP, were pre-specified endpoints for comparison between groups. In an additional set of isolated hearts (N = 3 each diet), the hearts were quickly sectioned into 2 mm slices following the 30 min LFI / 60 min reperfusion, and stained with 1% triphenyltetrazolium chloride (TTC) to measure infarct size. The sectioned hearts were photographed and the infarct area measured on the digital pictures using ImageJ (Rasband, 1997–2008). This resulted in a small infarct area in both diet groups (< 1% of LV mass). Subsequently, a similar protocol was used but with longer LFI (60 min) followed by 120 min of reperfusion (N = 5 each diet group). This more severe ischemia protocol resulted in larger infarct size.
Coronary effluent samples were collected immediately before ischemia, every five minutes during ischemia, and every ten minutes during reperfusion.
Akt, phospho-Akt, ERK 1/2, phospho-ERK 1/2 assessment
Frozen myocardium (≅50mg) was homogenized in 500 µl lysis buffer containing 1% NP-40, 150mM NaCl, 10 mM Tris (pH7.4), 10% glycerol, 1:100 protease inhibitor cocktail (Sigma), 2 mM sodium orthovanadate, and 10 mM NaF. Following centrifugation at 10,000g for 10 min, supernatants were collected. After protein concentration determination (Bio-Rad Laboratories, Hercules, CA) to normalize protein concentrations, samples were suspended in buffer (Tris HCl, pH 6.8, 40% glycerol, 8% SDS, 0.4% B-2-Me and Bromphenol blue). Lysates (25 µg/lane) were separated by 10% SDS-PAGE (100 V) and transferred to a nitrocellulose membrane (100V, 70 min). Membranes were immunoblotted with anti-Akt and anti-phospho-S473-Akt (Abcam, Cambridge, MA; 1: 2000 dilution), anti-ERK 1/2 and anti-phospho-T202/Y204-ERK 1/2 (Cell Signaling, Danvers, MA; 1:1000 dilution), exposed to film, developed, and digitized. The band intensities were analyzed by ImageJ to quantify Akt and phospho-Akt (p-Akt), ERK 1/2 and phospho-ERK 1/2. Each band intensity was normalized to that of GAPDH (Abcam, Cambridge, MA; 1:20,000 dilution) on the same gel.
Statistics
Data are presented as mean ± SEM. Data were compared for differences by two-way ANOVA (GraphPad Prism, San Diego, CA) or unpaired t test, as appropriate. A p value of less than 0.05 was considered significant. For baseline functional parameters, data from all rat hearts of the appropriate perfusion condition were included (i.e., baseline data from LFI and LFI/reperfusion hearts were also analyzed with the hearts perfused under baseline conditions only), resulting in greater experimental numbers for the baseline groups.
RESULTS
Whole body metabolism following two-week LCD feeding
Rats had body weight of 250 ± 5 g when divided into the two dietary groups for feeding. The whole body metabolic states following 2 weeks of feeding are summarized in Table 2. There was no difference in heart weight between groups. Rats in CONT group ate more food mass than rats in the LCD group (21.1 ± 0.6 g/day vs. 17.7 ± 0.7 g/day, p < 0.01), and total calories consumed were about 9% higher in LCD, though this did not reach statistical significance. Body weight was 6% higher after 2 weeks of LCD (Table 2). Serum FFA, glucose and lactate levels were not different between diet groups, but LCD did lead to a two-fold increase in 3-HB and a 50% decrease in the fed-state insulin level compared with CONT.
Table 2.
Metabolic characteristics after two-week CONT and LCD feeding, with anatomic features and circulating concentrations of insulin and substrates (n= 5 to 6 each group).
| CONT | LCD | |
|---|---|---|
| Body Weight (g) | 363±8 | 383±5* |
| Heart Weight (g) | 1.6±0.1 | 1.7±0.1 |
| Food Intake (Kcal/day) | 82±2 | 89±3 |
| Glucose (mM) | 7.0±0.2 | 6.6±0.2 |
| Lactate (mM) | 4.6±0.3 | 5.1±0.8 |
| FFA (mM) | 0.31±0.02 | 0.33±0.03 |
| 3-HB (mM) | 0.3±0.0 | 0.6±0.1* |
| Triglyceride (g/L) | 1.6±0.2 | 2.0±0.2 |
| Insulin (µU/ml) | 34±3 | 15±2* |
| Leptin (ng/ml) | 4.9±0.4 | 5.2±0.9 |
FFA=Free fatty acids, 3-HB= 3-hydroxybutyrate.
p<0.05 vs. LFD.
Cardiac function under normoxia, LFI and reperfusion conditions
Under normal flow conditions with substrates provided to mimic the in vivo conditions, cardiac power and systolic function (RPP, +dP/dt) and diastolic function (−dP/dt) were not different between the groups (Table 3). The onset of LFI led to a cessation of pulsatile function and an increase in end-diastolic pressure (EDP), which indicates the development of ischemic cardiac contracture (Fig. 1). The time of the beginning of contracture was earlier (7.5 ± 0.5 vs. 12.8 ± 1.8 min; p < 0.01), and the maximum EDP was higher (119 ± 4 vs. 72 ± 7 mmHg; p < 0.001) with shorter time to maximum EDP (14 ± 1 vs. 23 ± 1 min; p < 0.01) in hearts from rats fed LCD and perfused with LCD buffer (designated as diet / buffer, this group being LCD / LCD) as compared to CONT / CONT (Fig. 1A).
Table 3.
Functional parameters of isolated hearts under baseline conditions. (Total N = 16 CONT, N = 14 LCD). P not significant for all parameters.
| CONT | LCD | |
|---|---|---|
| EDP (mmHg) | 4±0.4 | 3±0.4 |
| LVDP (mmHg) | 85±5 | 94±5 |
| RPP (mmHg/min) | 28213±1746 | 30863±1630 |
| +dP/dt (mmHg/s) | 3255±212 | 3578±183 |
| −dP/dt (mmHg/s) | 1752±164 | 1860±94 |
| Coronary Flow (ml/min/g) | 9.2±0.7 | 7.9±0.5 |
Figure 1.
A) Cardiac function in isolated perfused hearts during 30 min LFI. CONT / CONT: hearts from rats fed CONT diet perfused with CONT buffer (N = 10); LCD / LCD: hearts from rats fed LCD perfused with LCD buffer (N = 10). B) Same for hearts with “Reversed” buffers; CONT / LCD: rats fed CONT diet, perfused with LCD buffer (N = 5); LCD / CONT: rats fed LCD, perfused with CONT buffer (N = 5). Note earlier and more severe ischemic contracture (elevation of EDP) in LCD-fed rats regardless of perfusion buffer. *p<0.05 for diet effect; no significant buffer effect or diet-buffer interaction.
Following 30 min of LFI, cardiac pulsatile function began to recover in the first few minutes of reperfusion; the EDP gradually recovered towards the baseline values over the monitored reperfusion time in all groups. At the end of 60 min reperfusion there was lower recovery of RPP and ±dP/dt and higher EDP in LCD / LCD as compared to CONT / CONT (Fig 2). Coronary flow during reperfusion was similar in LCD / LCD and in CONT / CONT (3.38 ± 0.41 and 3.46 ± 0.17 ml /min/gram tissue respectively; p not significant). Total lactate release during LFI and reperfusion was lower in LCD / LCD (28.7 ± 4.4 vs. 37.4 ± 3.6 µmol/g, p < 0.05). Importantly, LCD / LCD led to an overall three-fold greater LDH release during ischemia and reperfusion compared to CONT / CONT (Fig. 3), indicating greater ischemic injury in LCD.
Figure 2.
Percent recovery of baseline A) RPP, B) dP/dt, C) −dP/dt and D) EDP at the end of 60 min reperfusion. N=5 each group. Diet and buffer designation as in Figure 1 and in the text. *p<0.05 for diet effect; no significant buffer effect or diet-buffer interaction.
Figure 3.
Lactate dehydrogenase (LDH) release. Upper panel compares LCD / LCD and CONT / CONT; middle panel compares CONT / LCD and LCD / CONT; lower panel shows total integrated LDH release throughout LFI and reperfusion. N=5 each group. §p<0.05 for diet effect, with no significant buffer effect; a significant diet-buffer interaction was present by two-way ANOVA.
To determine how much of the observed effect on LV function and ischemic injury was due to intrinsic changes in the hearts induced by the diets and how much was due to differences in the circulating factors provided in the perfusion buffer, we performed a series of experiments in which hearts from rats fed CONT diet were perfused with LCD buffer (designated CONT / LCD; N = 5), and vice versa (i.e., rats fed LCD diet, hearts perfused with CONT buffer; designated LCD / CONT; N = 5). The changes of cardiac contracture (Fig. 1B), functional recovery (Fig. 2), and LDH release (Fig. 3) in LCD / CONT were similar to LCD / LCD. Lactate release was also lower in LCD / CONT compared to CONT / LCD (25.9 ± 3.4 vs. 37.9 ± 3.4 µmol/g; p < 0.05). Two-way ANOVA analysis examining the effect of diet and perfusion buffer indicated that there was a significant diet effect, but no perfusion buffer effect for the functional parameters in Fig. 2 and for lactate release. For LDH release (Fig. 3), two-way ANOVA indicated that there was a significant interaction effect between diet and perfusion buffer, along with a significant effect of diet, but no direct effect of the buffer.
Myocardial injury and infarct size
With 30 min LFI protocol, infarct area as measured by TTC staining was small in both groups, less than 1% of total LV mass (data not shown). Therefore a more severe ischemia protocol was next employed, identical to that described above using CONT diet with CONT buffer (CONT / CONT) and LCD diet with LCD buffer (LCD / LCD) but with 60 min of LFI followed by 120 min of reperfusion. At the end of reperfusion, there was lower recovery of all defined measures of systolic and diastolic function in LCD / LCD (Fig. 4). Most significantly, there was a dramatic 20-fold increase in infarction area in LCD / LCD as assessed by TTC staining (Fig. 5A). Similarly, myocardial ischemic injury as measured by LDH washout was markedly (>11-fold) higher in LCD / LCD (Fig 5B).
Figure 4.
Percent recovery of baseline A) RPP, B) dP/dt, C) −dP/dt and D) EDP at the end of 60 min LFI followed by 120 min reperfusion. N=5 each group. *p<0.05 vs. CONT / CONT.
Figure 5.
A) Top: Photographs of sectioned, TTC-stained representative hearts following 60 min LFI and 120 min reperfusion. Note the extensive infarction (pale staining) in LCD / LCD with no visible infarction in CONT / CONT. Bottom: Analysis of infarct data in these hearts shows considerably larger infarction in LCD / LCD (N = 4) than CONT / CONT (N = 6). Black bars, CONT / CONT; open bars, LCD / LCD. *P < 0.01 for comparison. B) Top: Time course of LDH release in same hearts. Bottom: Total time-integrated LDH release during LFI and reperfusion. Black bars, CONT / CONT; open bars, LCD / LCD. * P = 0.03 for comparison.
Myocardial glycogen reserves
Baseline cardiac glycogen content was one-third lower in rats fed LCD than in CONT diet, similar to prior reports (Haddad et al., 1990), but was similar in both diets after 30 min of LFI (Fig. 6) using the CONT / CONT and LCD / LCD schemes. Liver glycogen content was about 30-fold higher than myocardium, and was significantly reduced in LCD as well (45.2 ± 2.0 vs. 72.7 ± 4.8 mg glucose / g wet tissue, p<0.001). During 30 min LFI, cardiac glycogen decreased significantly from baseline levels in both CONT / CONT and LCD / LCD. Total glycogen utilization during 30 minutes of LFI was one-third higher in CONT / CONT as compared to LCD / LCD (1.65 ± 0.07 in CONT / CONT vs. 1.17 ± 0.03 mg/g wet tissue in LCD / LCD, p < 0.001), reflective of the greater glycogen stores in CONT diet. Thus, baseline cardiac glycogen content and glycogen utilization during LFI was significantly lower in LCD / LCD. Glycogen was re-synthesized during reperfusion; after 60 min reperfusion, cardiac glycogen was again lower in LCD / LCD than CONT / CONT (Fig. 6). Based on these measured values, we calculated that glycogen was re-synthesized during reperfusion at a rate of 24 µg/g wet tissue/min in LCD / LCD and 38 µg/g wet tissue/min in CONT / CONT. Interestingly, the glycolysis rate estimated from total lactate release was 60% higher in CONT / CONT than LCD / LCD (2.6 ± 0.2 vs. 1.6 ± 0.1 mg glucose/g wet tissue respectively; p < 0.01).
Figure 6.
Glycogen content in myocardium. Myocardial glycogen was determined at baseline (A), after 30 min LFI (B), and after 60 min reperfusion (C). N = 5 to 6 each group. * p < 0.05 vs. CONT; † p < 0.01 vs. Baseline.
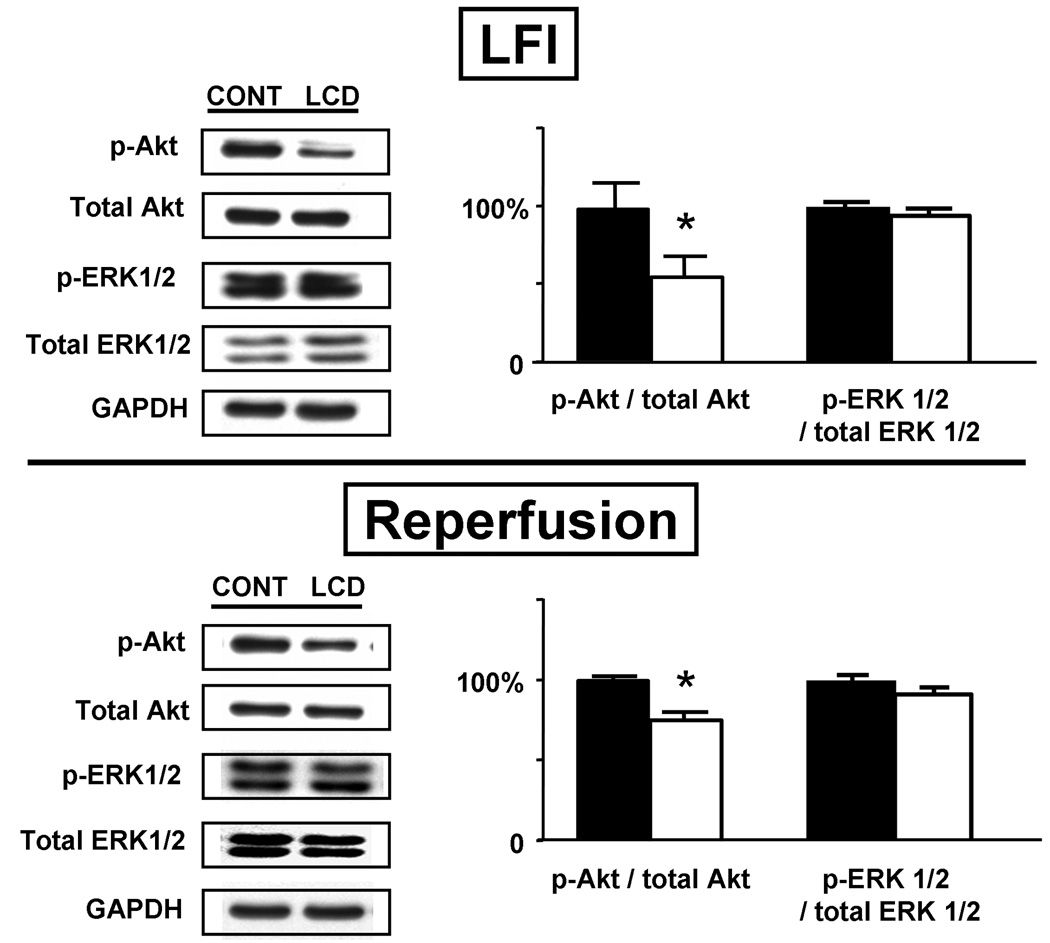
Akt and ERK activation under LFI and reperfusion
There was no difference of total Akt or total ERK 1/2 (normalized to GAPDH) between CONT / CONT and LCD / LCD under LFI and reperfusion conditions. However, p-Akt intensity (normalized to total Akt) was lower both at end of LFI and end of reperfusion in LCD / LCD, with no differences in p-ERK 1/2 between the dietary groups (Fig. 7).
Figure 7.
Left: Representative Akt and ERK 1/2 immunoblots at the end of 30 min LFI and after 60 min Reperfusion. Right: p-Akt and p-ERK intensities shown as the percent of total Akt and ERK, normalized such that CONT / CONT represents 100%. Black bars, CONT / CONT; open bars, LCD / LCD. All data were normalized to intensity of GAPDH run on the same gel. N=5 each group. *p<0.05 vs. CONT / CONT.
DISCUSSION
This study for the first time demonstrated that LCD with a macronutrient composition similar to that in human low carbohydrate diets reduces cardiac glycogen content, lowers fed-state circulating insulin levels and indices of myocardial insulin effect, and leads to greater injury and reduced functional recovery following ischemia-reperfusion as compared to a diet similar to a human low fat diet (Freedman et al., 2001), in the isolated rat heart. The diets have the same concentrations of essential fatty acids and micronutrients, and identical proportions of individual amino acids, so the observed differences between them are due to the different composition of carbohydrate, fat and protein.
We found that 30 min ischemia followed by 60 min reperfusion resulted in lower recovery of cardiac function and greater injury in LCD. These effects were dependent on the diet prior to excision of the heart, and not dependent on the perfusion buffer used. This indicates that these differences are due to intrinsic diet-induced changes in the heart (for example, alterations in myocardial glycogen stores and insulin effect). With prolonged ischemia (60 min LFI followed by 120 min reperfusion), functional recovery was dramatically worse in LCD, with an 11-fold greater release of LDH and 20-fold increased infarct size.
After two weeks of LCD feeding, cardiac glycogen stores were reduced and there was greater glycogen utilization in CONT / CONT compared to LCD / LCD. Beneficial effects of increased cardiac glycogen content have been reported in several studies. Reduced O2 delivery during ischemia lowers oxidative metabolism, leading to dependence on stored glycogen for non-oxidative ATP production through glycolysis (Lloyd et al., 2004; Wang et al., 2005b). The protective effect of glycolysis in ischemic myocardium may occur via a number of mechanisms (Weiss and Hiltbrand, 1985; Uchida and Doi, 1994; Xu et al., 1995; Askenasy, 2001; Vogt et al., 2003). Manipulations that increase cardiac glycogen reduce ischemic cardiac contracture and injury and improve post-ischemic function, though the exact role of glycogen in these effects remains unclear (Lolley et al., 1978; Oldfield et al., 1986; Schneider and Taegtmeyer, 1991; Doenst et al., 1996; Schaefer and Ramasamy, 1997; Eynan et al., 2002).
Other studies have reported that increased glycolysis due to high glycogen may be detrimental during ischemia since the “uncoupling” of glycolysis and TCA-cycle mediated glucose/glycogen oxidation leads to intracellular acidosis (Tani and Neely, 1989; Dyck and Lopaschuk, 1998). Many of the studies demonstrating harmful effects of glycolysis were performed with zero-flow ischemia or complete anoxia. However, clinical data show that even with complete occlusion of a coronary vessel, the infarct zone is supplied by collateral vessels which provide residual flow even in severely ischemic areas (Sabia et al., 1992), and in animal models of acute coronary artery occlusion, perfusion remains at 5 to 20% of the baseline value within the infarct zone (Christian et al., 1997). Thus, a no-flow ischemia model, in which damaging metabolic debris cannot be washed out, may not be representative of ischemia in vivo. We believe that the low flow model used here more accurately reflects in vivo ischemia, with less severe acidosis and some degree of oxidative ATP production (Lloyd et al., 2004).
The protective action of insulin against cardiac ischemia has been well documented (Tune et al., 1998; Doenst et al., 1999; Jonassen et al., 2001; Hausenloy and Yellon, 2004). The effect of insulin will clearly depend on both the circulating insulin level (which was lower in LCD) as well as the specific insulin sensitivity of the tissue. In LCD, we found lower fed-state insulin levels and Akt phosphorylation (at the Ser-473 site, which potentiates Akt activation following phosphorylation of the Thr-308 site), but no change in ERK 1/2 activation. These data indicate that LCD decreases the overall myocardial insulin effect (i.e., the combination of cellular insulin sensitivity and circulating insulin levels) on Akt activation under ischemia and reperfusion; future work will more completely investigate the dietary effect on myocardial insulin sensitivity. Interestingly, Ouwens et al. demonstrated that rats develop impaired cardiac insulin sensitivity (using identical insulin doses in a LCD and CONT diet) after feeding with a diet somewhat similar to the LCD we employed (Ouwens et al., 2005): our study builds on their work by examining the effect of such diets on the myocardial response to ischemia. The fact that LCD did not affect myocardial ERK 1/2 phosphorylation suggests that insulin-related metabolic effects of the diet (e.g., regulation of glycogen and oxidative metabolism, which are modulated via Akt activation, but not through ERK activation) may be more important in determining the ischemic response than the pro-survival effects (modulated by both Akt and ERK) (Hausenloy and Yellon, 2004; Manning and Cantley, 2007). We previously reported that insulin leads to a shift in cardiac metabolism from FFA to CHO utilization, with a 30% decrease in FFA oxidation as insulin increases from 0 to 80 µU/ml (Lloyd et al., 2003). The lower overall insulin effect in LCD could result in increased FFA utilization, even with similar circulating FFA levels in each diet, representing a potentially adverse effect during ischemia.
It has been shown that high-fat diets lead to endothelial dysfunction (Hennig et al., 2001), potentially worsening myocardial perfusion. One study investigating the effect of low carbohydrate diet on endothelial function (by brachial artery flow-mediated dilatation) showed no difference between low carbohydrate and a high carbohydrate, low fat diet in obese humans with risk factors for metabolic syndrome (Keogh et al., 2008), while another work found impaired flow-mediated dilatation in low carbohydrate diet in obese humans without risk factors for cardiovascular disease (Phillips et al., 2008). Focardi et al. found that LCD reversed endothelial dysfunction in coronary arterioles of obese Zucker rats through a mechanism independent of nitric oxide (Focardi et al., 2007). These compelling findings suggest the need for further investigation of these diets on endothelial function and myocardial perfusion during ischemia. It appears that this effect on vascular function is highly dependent on underlying comorbidities and any weight loss, in addition to direct effects of the macronutrients on endothelium. As an index of coronary vascular function, we assessed the coronary flow rate at baseline and during reperfusion; in our model, we did not find differences in these.
We formulated LCD to restrict carbohydrates, as this is predicted to have significant effects on insulin release; however, the diet also has a high fat content. In certain animal models, accumulation of triglycerides within cardiomyocytes leads to cellular apoptosis and cardiac dysfunction under non-ischemic conditions (Zhou et al., 2000; Chiu et al., 2001). We did not find any differences in cardiac function between diets under baseline conditions (Table 3). Furthermore, high-fat feeding in an infarct model of heart failure did not cause cardiac dysfunction under non-ischemic conditions (Morgan et al., 2006). Further work is needed to fully elucidate the relative importance of carbohydrate restriction and elevated dietary fat on the observed effects during acute ischemia.
A strength of our study is the use of an isolated heart model, which allows detailed studies that are not possible in vivo (e.g., our “Reverse” buffer perfusion experiments, with CONT / LCD, and LCD / CONT). Such a model also potentially allows performance of 31P NMR spectroscopy of the beating heart, to determine the evolution of high energy phosphates and tissue pH during ischemia (Murray et al., 2006). These are important determinants of cardiac cellular function and survival and a better understanding of their behavior could provide additional mechanistic information on the response to ischemia, leading us to pursue this model in future work.
This work has several potential limitations, which we do not feel detract from the central findings. Similar to previous work (Focardi et al., 2007), we did not find that LCD-fed animals gained less weight than CONT-fed rats; in fact, our LCD rats gained more weight. However, the point of our study was not to examine the effect of these diets on weight, but rather to investigate the effect of macronutrient composition on the response to ischemia. Though we tried to formulate the perfusion buffers to be similar to in vivo levels of substrates and insulin, technical considerations dictated that we use lower triglyceride; furthermore, the FFA levels used were determined from blood collected up to 6 hours after last feeding opportunity, resulting in higher FFA levels and representing more of a fasting state. Since buffer levels of FFA and triglyceride were identical in both diet groups and only minimally different from physiologic levels, it is unlikely these could have led to the observed differences. Finally, the diets did not have the same concentration of protein, which could lead to alterations in insulin release, endothelial function, cellular metabolism, and kidney function. Justifying our diet composition, typical human low carbohydrate diets are rich in protein. Overall, these factors do not negate the main findings: LCD with macronutrient composition similar to that in human low carbohydrate diets leads to lower circulating insulin and myocardial insulin effect during ischemia and reperfusion, lower cardiac glycogen, and greater myocardial ischemic injury in the isolated heart model.
CONCLUSIONS
We have shown that LCD similar to diets often used by humans reduces cardiac glycogen content, lowers fed-state circulating insulin levels and myocardial Akt activation, and leads to worsened recovery of function and increased myocardial injury during ischemia / reperfusion in the isolated heart. These results suggest that further investigations may be needed to provide optimal dietary recommendations for those with ischemic heart disease.
ACKNOWLEDGMENT
This work was supported in part by NIH grant P30DK056336 and by Scientist Development Grant 0735212N from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agatston A. The South Beach Diet: The Delicious, Doctor-Designed Foolproof Plan for Fast and Healthy Weight Loss. Rodale Press; 2003. [Google Scholar]
- Askenasy N. Glycolysis protects sarcolemmal membrane integrity during total ischemia in the rat heart. Basic Res Cardiol. 2001;96(6):612–622. doi: 10.1007/s003950170013. [DOI] [PubMed] [Google Scholar]
- Atkins RC. Dr. Atkins' New Diet Revolution. New York: Avon; 2001. [Google Scholar]
- Bravata DM, Sanders L, Huang J, Krumholz HM, Olkin I, Gardner CD, Bravata DM. Efficacy and Safety of Low-Carbohydrate Diets: A Systematic Review. JAMA. 2003;289(14):1837–1850. doi: 10.1001/jama.289.14.1837. [DOI] [PubMed] [Google Scholar]
- Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107(7):813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian TF, O'Connor MK, Schwartz RS, Gibbons RJ, Ritman EL. Technetium-99m MIBI to assess coronary collateral flow during acute myocardial infarction in two closed-chest animal models. J Nucl Med. 1997;38:1840–1846. [PubMed] [Google Scholar]
- Doenst T, Guthrie PH, Chemnitius JM, Zech R, Taegtmeyer H. Fasting, lactate, and insulin improve ischemia tolerance in rat heart: a comparison with ischemic preconditioning. Am J Physiol. 1996;270(5 Pt 2):H1607–H1615. doi: 10.1152/ajpheart.1996.270.5.H1607. [DOI] [PubMed] [Google Scholar]
- Doenst T, Richwine RT, Bray MS, Goodwin GW, Frazier OH, Taegtmeyer H. Insulin improves functional and metabolic recovery of reperfused working rat heart. Ann Thorac Surg. 1999;67(6):1682–1688. doi: 10.1016/s0003-4975(99)00326-4. [DOI] [PubMed] [Google Scholar]
- Dyck JR, Lopaschuk GD. Glucose metabolism, H+ production and Na+/H+-exchanger mRNA levels in ischemic hearts from diabetic rats. Mol Cell Biochem. 1998;180(1–2):85–93. [PubMed] [Google Scholar]
- Eynan M, Knubuvetz T, Meiri U, Navon G, Gerstenblith G, Bromberg Z, Hasin Y, Horowitz M. Heat acclimation-induced elevated glycogen, glycolysis, and low thyroxine improve heart ischemic tolerance. J Appl Physiol. 2002;93(6):2095–2104. doi: 10.1152/japplphysiol.00304.2002. [DOI] [PubMed] [Google Scholar]
- Focardi M, Dick GM, Picchi A, Zhang C, Chilian WM. Restoration of coronary endothelial function in obese Zucker rats by a low-carbohydrate diet. Am J Physiol Heart Circ Physiol. 2007;292(5):H2093–H2099. doi: 10.1152/ajpheart.01202.2006. [DOI] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A Randomized Trial of a Low-Carbohydrate Diet for Obesity. N Engl J Med. 2003;348(21):2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- Freedman MR, King J, Kennedy E. Popular diets: a scientific review. Obes Res. 2001;9 Suppl 1:1S–40S. doi: 10.1038/oby.2001.113. [DOI] [PubMed] [Google Scholar]
- Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt Promotes Survival of Cardiomyocytes In Vitro and Protects Against Ischemia-Reperfusion Injury in Mouse Heart. Circulation. 2000;101(6):660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC. Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am J Physiol Cell Physiol. 2007;292(4):C1370–C1378. doi: 10.1152/ajpcell.00422.2006. [DOI] [PubMed] [Google Scholar]
- Haddad F, Baldwin KM, Morris GS. Dietary effects on cardiac metabolic properties in rodents. J Mol Cell Cardiol. 1990;22(3):353–359. doi: 10.1016/0022-2828(90)91468-m. [DOI] [PubMed] [Google Scholar]
- Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low-Carbohydrate-Diet Score and the Risk of Coronary Heart Disease in Women. N Engl J Med. 2006;355(19):1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61(3):448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Hennig B, Toborek M, McClain CJ. High-energy diets, fatty acids and endothelial cell function: implications for atherosclerosis. J Am Coll Nutr. 2001;20(2 Suppl):97–105. doi: 10.1080/07315724.2001.10719021. [DOI] [PubMed] [Google Scholar]
- Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial Protection by Insulin at Reperfusion Requires Early Administration and Is Mediated via Akt and p70s6 Kinase Cell-Survival Signaling. Circ Res. 2001;89(12):1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- Kargotich S, Rowbottom DG, Keast D, Goodman C, Dawson B, Morton AR. Plasma glutamine changes after high-intensity exercise in elite male swimmers. Res Sports Med. 2005;13(1):7–21. doi: 10.1080/15438620590922040. [DOI] [PubMed] [Google Scholar]
- Keogh JB, Brinkworth GD, Noakes M, Belobrajdic DP, Buckley JD, Clifton PM. Effects of weight loss from a very-low-carbohydrate diet on endothelial function and markers of cardiovascular disease risk in subjects with abdominal obesity. Am J Clin Nutr. 2008;87(3):567–576. doi: 10.1093/ajcn/87.3.567. [DOI] [PubMed] [Google Scholar]
- Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412–426. doi: 10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Le Bacquer O, Mauras N, Welch S, Haymond M, Darmaun D. Acute depletion of plasma glutamine increases leucine oxidation in prednisone-treated humans. Clin Nutr. 2006 doi: 10.1016/j.clnu.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S, Brocks C, Chatham JC. Differential modulation of glucose, lactate, and pyruvate oxidation by insulin and dichloroacetate in the rat heart. Am J Physiol Heart Circ Physiol. 2003;285(1):H163–H172. doi: 10.1152/ajpheart.01117.2002. [DOI] [PubMed] [Google Scholar]
- Lloyd SG, Wang P, Zeng H, Chatham JC. Impact of low-flow ischemia on substrate oxidation and glycolysis in the isolated perfused rat heart. Am J Physiol Heart Circ Physiol. 2004;287(1):H351–H362. doi: 10.1152/ajpheart.00983.2003. [DOI] [PubMed] [Google Scholar]
- Lolley DM, Ray JF, Myers WO, Sheldon G, Sautter RD. Reduction of intraoperative myocardial infarction by means of exogenous anaerobic substrate enhancement: prospective randomized study. Ann Thorac Surg. 1978;26:515–524. doi: 10.1016/s0003-4975(10)62937-2. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Tao J, del Monte F, Lee K-H, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt Activation Preserves Cardiac Function and Prevents Injury After Transient Cardiac Ischemia In Vivo. Circulation. 2001;104(3):330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng K-Y, Hoit BD, Stanley WC, Chandler MP. Effects of chronic activation of peroxisome proliferator-activated receptor-{alpha} or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol. 2006;290(5):H1899–H1904. doi: 10.1152/ajpheart.01014.2005. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Lygate CA, Cole MA, Carr CA, Radda GK, Neubauer S, Clarke K. Insulin resistance, abnormal energy metabolism and increased ischemic damage in the chronically infarcted rat heart. Cardiovasc Res. 2006;71(1):149–157. doi: 10.1016/j.cardiores.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Noll F. L-(+)-Lactate. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Weinheim: Deerfield Beach; 1974. pp. 582–588. [Google Scholar]
- Oldfield GS, Commerford PJ, Opie LH. Effects of preoperative glucose-insulin-potassium on myocardial glycogen levels and on complications of mitral valve replacement. J Thorac Cardiovasc Surg. 1986;91(6):874–878. [PubMed] [Google Scholar]
- Opie LH, Lopaschuk GD. Chapter 11: Fuels, Aerobic and Anaerobic Metabolism. In: Opie LH, editor. Heart Physiology: From Cell to Circulation. Philadelphia: Lippincott Williams and Wilkins; 2004. pp. 306–354. [Google Scholar]
- Ouwens DM, Boer C, Fodor M, de Galan P, Heine RJ, Maassen JA, Diamant M. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia. 2005;48(6):1229–1237. doi: 10.1007/s00125-005-1755-x. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Jurva JW, Syed AQ, Syed AQ, Kulinski JP, Pleuss J, Hoffmann RG, Gutterman DD. Benefit of Low-Fat Over Low-Carbohydrate Diet on Endothelial Health in Obesity. Hypertension. 2008;51(2):376–382. doi: 10.1161/HYPERTENSIONAHA.107.101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. Bethesda, MD, USA: ImageJ, U.S. National Institutes of Health. 1997–2008 http://rsb.info.nih.gov/ij/.
- Sabia PJ, Powers ER, Ragosta M, Sarembock IJ, Burwell LR, Kaul S. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992;327:1825–1831. doi: 10.1056/NEJM199212243272601. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L. A Low-Carbohydrate as Compared with a Low-Fat Diet in Severe Obesity. N Engl J Med. 2003;348(21):2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- Schaefer S, Ramasamy R. Glycogen utilization and ischemic injury in the isolated rat heart. Cardiovascular Research. 1997;35(1):90–98. doi: 10.1016/s0008-6363(97)00087-4. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Taegtmeyer H. Fasting in vivo delays myocardial cell damage after brief periods of ischemia in the isolated working rat heart. Circ Res. 1991;68:1045–1050. doi: 10.1161/01.res.68.4.1045. [DOI] [PubMed] [Google Scholar]
- Tani M, Neely JR. Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ Res. 1989;65(4):1045–1056. doi: 10.1161/01.res.65.4.1045. [DOI] [PubMed] [Google Scholar]
- Tune JD, Mallet RT, Downey HF. Insulin improves contractile function during moderate ischemia in canine left ventricle. Am J Physiol Heart Circ Physiol. 1998;274(5):H1574–H1581. doi: 10.1152/ajpheart.1998.274.5.H1574. [DOI] [PubMed] [Google Scholar]
- Uchida K, Doi K. Glycolysis vs. respiration as ATP source for the shape of quiescent cardiomyocytes. Respir Physiol. 1994;97(2):213–223. doi: 10.1016/0034-5687(94)90027-2. [DOI] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control and Prevention. Trends in intake of energy and macronutrients--United States, 1971–2000. MMWR Morb Mortal Wkly Rep. 2004;53:80–82. [PubMed]
- Vogt AM, Elsasser A, Nef H, Bode C, Kubler W, Schaper J. Increased glycolysis as protective adaptation of energy depleted, degenerating human hibernating myocardium. Mol Cell Biochem. 2003;242(1–2):101–107. [PubMed] [Google Scholar]
- Wang P, Chatham JC. Onset of diabetes in Zucker diabetic fatty (ZDF) rats leads to improved recovery of function after ischemia in the isolated perfused heart. Am J Physiol Endocrinol Metab. 2004;286(5):E725–E736. doi: 10.1152/ajpendo.00295.2003. [DOI] [PubMed] [Google Scholar]
- Wang P, Lloyd SG, Chatham JC. Impact of High Glucose/High Insulin and Dichloroacetate Treatment on Carbohydrate Oxidation and Functional Recovery After Low-Flow Ischemia and Reperfusion in the Isolated Perfused Rat Heart. Circulation. 2005a;111(16):2066–2072. doi: 10.1161/01.CIR.0000162466.06150.D4. [DOI] [PubMed] [Google Scholar]
- Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2005b;288(5):H2102–H2110. doi: 10.1152/ajpheart.00935.2004. [DOI] [PubMed] [Google Scholar]
- Weinbrenner C, Wang P, Downey JM. Loss of glycogen during preconditioning is not a prerequisite for protection of the rabbit heart. Basic Res Cardiol. 1996;91(5):374–381. doi: 10.1007/BF00788717. [DOI] [PubMed] [Google Scholar]
- Weiss J, Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest. 1985;75(2):436–447. doi: 10.1172/JCI111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willamson DH, Mellanby J. D-(−)3-Hydroxybutyrate and Acetoacetate. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Weinheim: Deerfield Beach; 1974. pp. 1837–1843. [Google Scholar]
- Xu KY, Zweier JL, Becker LC. Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ Res. 1995;77(1):88–97. doi: 10.1161/01.res.77.1.88. [DOI] [PubMed] [Google Scholar]
- Zhou Y-T, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: Implications for human obesity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(4):1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]