Abstract
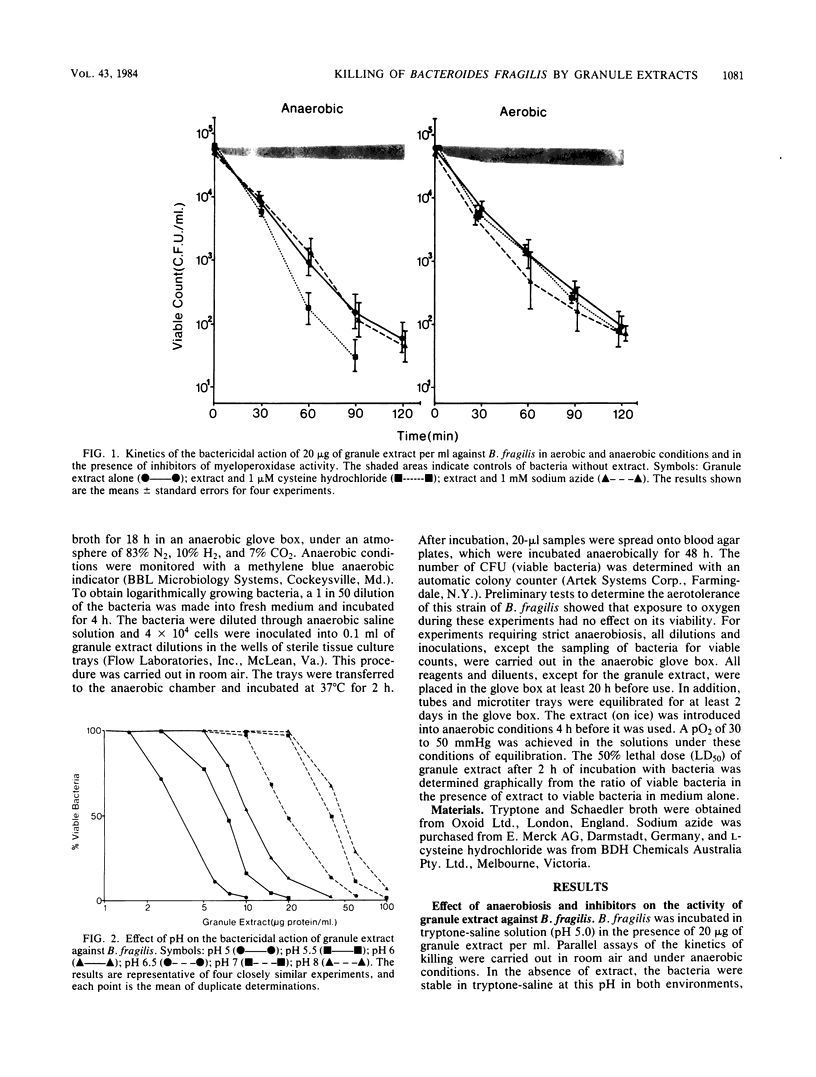
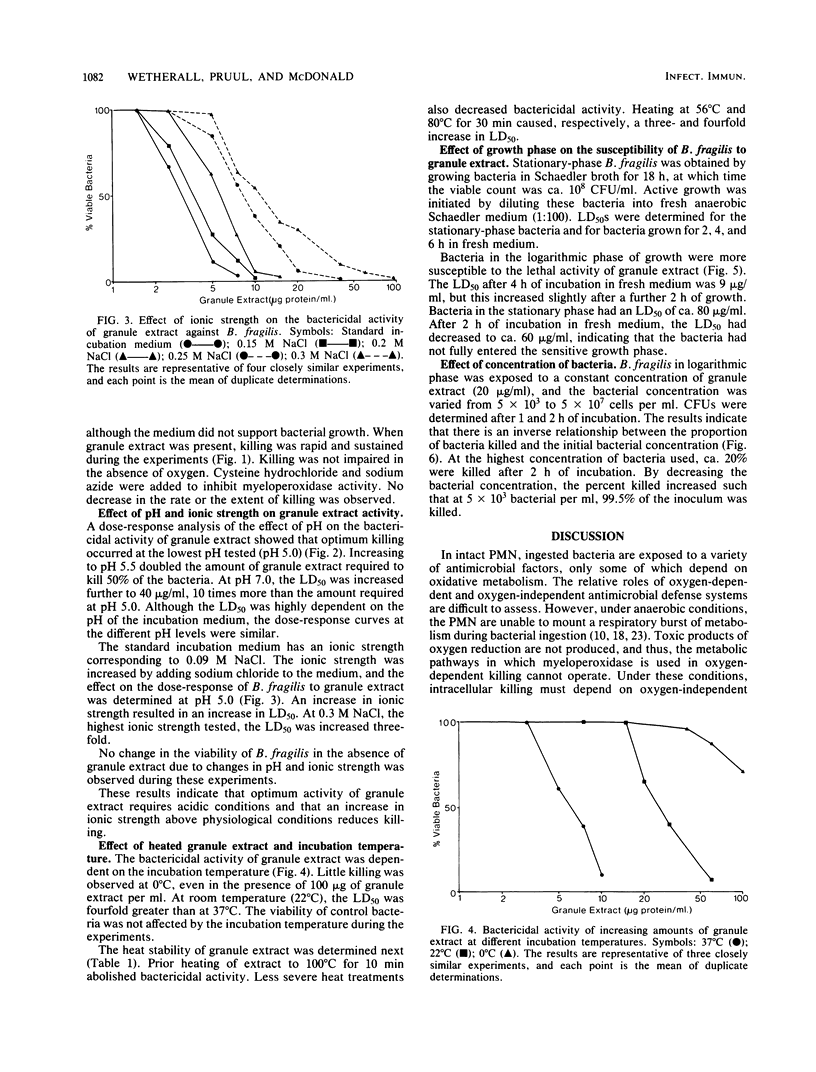
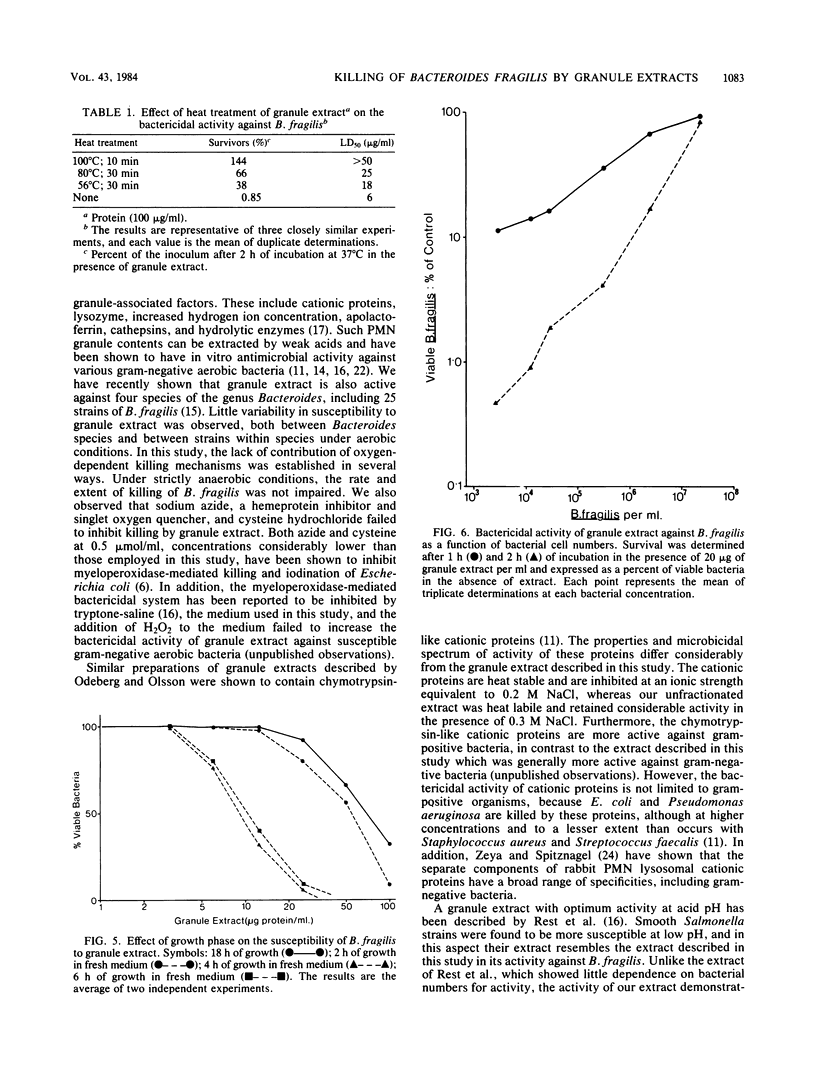
Granule proteins from human neutrophils were prepared by extraction with acetate, and their antibacterial activity against Bacteroides fragilis was determined. Activity was highly dependent on pH; greatest killing occurred at the most acid pH tested (pH 5.0). Optimum activity was observed at physiological ionic strength and low bacterial numbers. Killing was inhibited by incubation temperatures of less than 37 degrees C. Eight times more extract was required to kill 50% of stationary-phase bacteria, compared with those growing in logarithmic phase. The antibacterial effect of granule extract was destroyed by boiling, but some activity was retained after heating to 56 degrees C and 80 degrees C. Granule extract activity was tested under conditions in which oxygen-dependent antibacterial systems were inhibited. The rate and extent of killing was not affected by anaerobiosis, sodium azide, or cysteine hydrochloride. These results suggest that the activity of granule extract is independent of oxidative antibacterial systems, and therefore, under conditions that occur in anaerobic infections, potent leukocyte granule-associated mechanisms exist for the destruction of B. fragilis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjornson A. B., Altemeier W. A., Bjornson H. S. Comparison of the in vitro bactericidal activity of human serum and leukocytes against bacteroides fragilis and Fusobacterium mortiferum in aerobic and anaerobic environments. Infect Immun. 1976 Sep;14(3):843–847. doi: 10.1128/iai.14.3.843-847.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS F. M. THE EFFECT OF THE GROWTH RATE ON THE COMPOSITION OF S. ENTERITIDIS CELL WALLS. Aust J Exp Biol Med Sci. 1964 Apr;42:255–262. doi: 10.1038/icb.1964.27. [DOI] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Middleton R. L., Narang H. K., Codd A. A., Selkon J. B. Phagocytosis and killing of bacteria in aerobic and anaerobic conditions. J Med Microbiol. 1981 Nov;14(4):391–399. doi: 10.1099/00222615-14-4-391. [DOI] [PubMed] [Google Scholar]
- Jacques Y. V., Bainton D. F. Changes in pH within the phagocytic vacuoles of human neutrophils and monocytes. Lab Invest. 1978 Sep;39(3):179–185. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandell G. L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect Immun. 1974 Feb;9(2):337–341. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XI. Relationship between stimulated oxidative metabolism and hydrogen peroxide formation, and intracellular killing. J Bacteriol. 1967 Nov;94(5):1417–1424. doi: 10.1128/jb.94.5.1417-1424.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrzakowski M. C., Cooney M. H., Martin L. E., Spitznagel J. K. Bactericidal activity of fractionated granule contents from human polymorphonuclear leukocytes. Infect Immun. 1979 Mar;23(3):587–591. doi: 10.1128/iai.23.3.587-591.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N., Spitznagel J. K. Outer membrane mutants of Salmonella typhimurium LT2 have lipopolysaccharide-dependent resistance to the bactericidal activity of anaerobic human neutrophils. Infect Immun. 1982 Jun;36(3):1086–1095. doi: 10.1128/iai.36.3.1086-1095.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruul H., Wetherall B. L., McDonald P. J. Bactericidal activity of a granule extract from human polymorphonuclear leukocytes against Bacteroides species. Infect Immun. 1983 Sep;41(3):1373–1375. doi: 10.1128/iai.41.3.1373-1375.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., TOENNIES G. Relations between bacterial cell wall synthesis, growth phase, and autolysis. J Biol Chem. 1958 Feb;230(2):961–977. [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Relationship of glycolytic and oxidative metabolism to particle entry and destruction in phagocytosing cells. Nature. 1966 Sep 17;211(5055):1272–1276. doi: 10.1038/2111272a0. [DOI] [PubMed] [Google Scholar]
- Tofte R. W., Peterson P. K., Schmeling D., Bracke J., Kim Y., Quie P. G. Opsonization of four Bacteroides species: role of the classical complement pathway and immunoglobulin. Infect Immun. 1980 Mar;27(3):784–792. doi: 10.1128/iai.27.3.784-792.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Weiss J., Victor M., Stendhal O., Elsbach P. Killing of gram-negative bacteria by polymorphonuclear leukocytes: role of an O2-independent bactericidal system. J Clin Invest. 1982 Apr;69(4):959–970. doi: 10.1172/JCI110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Arginine-rich proteins of polymorphonuclear leukocyte lysosomes. Antimicrobial specificity and biochemical heterogeneity. J Exp Med. 1968 May 1;127(5):927–941. doi: 10.1084/jem.127.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]


