Abstract
Background/aims
Several studies found hepatitis C (HCV) increases risk of Type II diabetes mellitus (DM). However, others found no or only sub-group specific excess risk. We performed meta-analyses to examine whether HCV infection does increase DM risk in comparison to the general population and in other sub-groups with increased liver disease rates including with hepatitis B (HBV).
Methods
We followed standard guidelines for performance of meta-analyses. Two independent investigators identified eligible studies through structured keyword searches in relevant databases including PubMed.
Results
We identified 34 eligible studies. Pooled estimators indicated significant DM risk in HCV-infected cases in comparison to non-infected controls in both retrospective (ORadjusted=1.68, 95 percent CI 1.15–2.20) and prospective studies (HRadjusted=1.67, 95% CI 1.28–2.06). Excess risk was also observed in comparison to HBV-infected controls (ORadjusted=1.80, 95% CI 1.20–1.40) with suggestive excess observed in HCV+/HIV+ cases in comparison to HIV+ controls (ORunadjusted=1.82, 95 percent CI 1.27–2.38).
Conclusions
Our finding of excess DM risk with HCV infection in comparison to non-infected controls is strengthened by consistency of results from both prospective and retrospective studies. The excess risk observed in comparison to HBV-infected controls suggests a potential direct viral role in promoting DM risk, but this needs to be further examined.
Keywords: hepatitis C; diabetes mellitus, Type 2; meta-analysis; review, systematic; liver diseases; hepatitis B
INTRODUCTION
An estimated 3% of the world’s population (170+ million persons) are infected with the hepatitis C virus (HCV), 55–80% with chronic infection(1). HCV is a significant cause of global morbidity and mortality, responsible for approximately 25% of both chronic liver disease (CLD) and hepatocellular carcinoma (HCC).
HCV infection has also been convincingly linked to several extra-hepatic manifestations including essential mixed cryoglobulinemia, glomeronephritis, and porphyria cutanea tarda(2). Based on early clinical observation, type II diabetes mellitus (DM) was suggested to be another potential extrahepatic manifestation of HCV infection, with excess risk postulated to be due to either direct viral involvement or secondary to HCV-induced liver damage. However, even a small increase in DM risk in HCV-infected patients may be clinically important, as available pharamcotherapies for HCV are less effective with concomitant DM(3) and progression of liver disease has been shown to be worsened(4).
A number of epidemiologic studies have demonstrated significant excess DM risk with HCV infection.(5–15),(16–18) However, others found no significant excess risk(19–26) or excess risk limited to specific segments of the population(27–31). Differences in source of controls, case definition, sample size and underlying target population may explain much of this observed variability among studies. Several general narrative reviews have examined the association between HCV infection and DM. However, they have typically been limited in scope or non-systematic(32, 33). The only published meta-analysis examined the association between HCV and DM in a highly limited sub-population of kidney transplant recipients(34).
Our primary goal was, therefore, to conduct meta-analyses to assess whether HCV infection conveys excess DM risk compared to that observed in the general population. We aimed to quantify and appropriately qualify any observed excess risk, to identify any high-risk sub-groups, and to explore potential sources of between-study heterogeneity. A secondary goal was examining DM risk with HCV infection in comparison to that observed in other sub-groups at risk of CLD, including those mono-infected with HBV or HIV. In addition to providing a greater understanding about the association between HCV and DM risk, the findings of these meta-analyses may also help inform clinical practice guidelines and suggest gaps in current understanding that may be important to address in future research.
METHODS
Eligibility Criteria
We followed published guidelines for the conduct and reporting of meta-analyses(35). All published epidemiologic studies providing, or with data to calculate, an estimate of risk of type II or adult-onset diabetes mellitus (DM) among adults infected with hepatitis C (HCV+) compared to adults without infection (HCV−) or an estimate of risk of HCV among adults with DM compared to adults without DM were considered for possible inclusion in the current meta-analysis. To be eligible, both case and comparison groups (e.g., case-control and cross-sectional studies) or exposed and unexposed groups (e.g., cohort study) had to come from the same geographically- and temporally-defined underlying population. To help assure quality and comparability of data from included studies, we further required: 1) publication as an original, peer-reviewed manuscript, and 2) minimum sample size of 200, with at least 100 cases and 100 controls, or 100 exposed and 100 unexposed.
Studies were excluded if they: included children, post-transplant recipients, dialysis patients, pregnant women, or thalassemia or cancer cases; lacked adequately defined case or comparator groups including those where type II DM could not be distinguished from sub-clinical hyperglycemia or where HCV could not be excluded from other causes of hepatitis; did not provide risk estimates or data necessary to calculate them; or were not published in English. Additionally, as a critical meta-analysis requirement is statistical independence of observations(36), when multiple overlapping reports were available for a single unique study population, we included only the largest or most recent eligible report.
Search Strategy
To identify all potentially eligible studies, two investigators independently conducted structured searches in selected databases including PubMed, ISI Web of Science, and Google Scholar. Searches included combinations of selected key- and text-words including: ‘diabetes’, ‘diabetes mellitus’, ‘diabetes mellitus, type II’, or ‘type II diabetes’ and ‘hepatitis C’, ‘hepatitis C virus’, ‘hepatitis’, or ‘chronic hepatitis’ and ‘*risk’, ‘*rate’, ‘case-control’, ‘cohort’, ‘clinical trial’, ‘cross-sectional’, ‘meta-analysis’, ‘epidemiology’, or ‘review’. Searches were updated as of May 31, 2008.
We also reviewed the bibliographies of eligible studies as well as those of relevant review articles to identify additional studies not captured by our database searches.
Data Abstraction
Two investigators independently reviewed all identified titles, abstracts and manuscripts to determine if an individual study was eligible for inclusion in this meta-analysis. Disagreements about eligibility were resolved by consensus with a third reviewer.
Data on study methods and results were entered into a structured database. When there was insufficient information on methods, relevant information from any earlier or smaller reports was used instead. If specific quantitative results were not reported, when possible we used available data to calculate them.
Analysis
Because we did not place limitations on type of epidemiological design, studies reporting such varied risk estimates as odds ratios (OR), hazards ratios (HR) and incidence rate ratios (IRR) were all eligible for inclusion in our meta-analysis. However, as a valid meta-analysis requires comparability of risk estimators, we divided eligible studies into comparable sub-groups before performing meta-analysis.
For studies where the OR was the applicable risk estimator, we used available data to calculate or confirm the unadjusted estimator. When there was a discrepancy between reported and calculated estimates, the calculated estimates were used in all subsidiary analyses.
Heterogeneity across studies was assessed using the I2 of Higgins and Thompson(37) which quantifies the proportion of total variation attributable to between-study differences or heterogeneity as opposed to random error or chance. An I2>50% was employed to determine if substantive between-study heterogeneity existed. Our decision to perform fixed effects analysis using the Mantel-Haenzel method, or random effects analysis using the Der Simionian and Laird method(38) was based upon our heterogeneity assessment results. Random effects meta-analysis is the preferred method for calculating pooled estimators when there is substantial between-study heterogeneity. All meta-analyses are presented as forest plots with risk estimates for all individual studies as well as the overall pooled estimator. Shaded figures provided for all individual study estimates have dimension proportional to their weight in calculation of the pooled estimator.
Exploratory meta-regression was performed to evaluate potentially important sources of between-study heterogeneity. Variables evaluated included year of publication, source of controls (hospital/clinic-based or not), and region (North America/Europe vs. other). Consonant with widely accepted minimum sample size for regression analysis(39), we performed meta-regression only when there were ≥10 comparable studies per variable assessed. Variables significant at p<0.15 were considered potentially important sources of between-study heterogeneity.
Finally, to help assess validity and reliability of our meta-analyses, we performed an analysis of influence and Egger’s test. An analysis of influence describes how robust the pooled estimator is to removal of individual studies, while Egger’s test, which assesses whether the relationship between effect size and variance differs between large and small studies, was employed to determine if there was potential small study or publication bias(40).
All analyses were conducted using STATA 9.0 (College Station, Texas, USA).
RESULTS
Searches
We identified 223 potentially eligible reports. Review of abstracts and manuscripts resulted in exclusion of 190 reports (85%). The most frequent reasons for exclusion were: publication in an ineligible format including letters/abstracts or the results provided were not from original research including reviews/editorials (n=66); there was no comparator group or else an ineligible case or comparator group (n=45); it contained data on post-transplant patients (n=37); it had a total sample size of less than 200 and/or fewer than 100 cases/exposed and 100 controls/unexposed (n=26); or it was not published in English (n=14).
Study characteristics
Two(5, 10) of the 32(5–18, 20–25, 27–31, 41–47) eligible reports we identified included data from two unique studies. Therefore, 34 unique studies were included in our meta-analysis. Baseline study characteristics are provided in Table 1. Additional information on the definition and identification of DM and HCV in individual studies is reported in Appendix 1.
Table 1.
Characteristics of meta-analysis eligible studies examining association between HCV and DM*
| Ref # | Name | Year | Country | Study Timing (Measure of Association) | Case or Exposed** | Source of identification | Control or Unexposed*** |
|---|---|---|---|---|---|---|---|
| 21 | Akbar DH | 2002 | Saudi Arabia | Retrospective (OR) | HCV+ | Hospital | HBV+ |
| 6 | Antonelli A | 2004 | Italy | Retrospective (OR) | HCV+ with MC | Hospital | HCV− |
| 5 | Antonelli A | 2005 | Italy | Retrospective (OR) | HCV+ with CLD | Clinic | HBV+ with CLD |
| 5 | Antonelli A | 2005 | Italy | Retrospective (OR) | HCV+ with CLD | Clinic | HCV− |
| 41 | Arao M | 2003 | Japan | Retrospective (OR) | HCV+ | Hospital & Clinic | HBV+ |
| 14 | Bigam DL | 2000 | Canada | Retrospective (OR) | HCV+ with LF | Hospital | OLD with LF |
| 42 | Boschi-Pinto C | 2000 | Japan | Prospective (HR) | HCV+ | Miyazaki cohort study | HCV− |
| 43 | Brar I | 2007 | U.S. | Prospective (HR) | HCV+/HIV+ | CPCRA clinical trial | HIV+ |
| 29 | Butt AA | 2004 | U.S. | Retrospective (OR) | HCV+/HIV+ | VA-HIV+ (1992–2001) | HIV+ |
| 44 | Caronia S | 1999 | Italy | Retrospective (OR) | HCV+ with cirrhosis | Hospital | HBV+ with cirrohosis |
| 7 | Chen H-F | 2006 | Taiwan | Retrospective (OR) | DM+ | Hospital | DM− |
| 15 | El-Zayadi ARM | 1998 | Egypt | Retrospective (OR) | HCV+ with CLD | Clinic | OLD with CLD |
| 8 | Howard AA | 2003 | U.S. | Retrospective (OR) | HCV+ | Methadone clinic | HCV− |
| 30 | Huang J-F | 2007 | Taiwan | Retrospective (OR) | DM+ | Kaoshung City cohort study | DM− |
| 31 | Jain MK | 2007 | U.S. | Retrospective (OR) | HCV+/HIV+ | Clinic | HIV+ |
| 27 | Lecube A | 2004 | Spain | Retrospective (OR) | HCV+ | Liver clinic | OLD with CLD |
| 20 | Ledergerber B | 2007 | Switzerland | Prospective (IRR) | HCV+/HIV+ | Swiss HIV cohort study | HIV+ |
| 9 | Li-Ng M | 2007 | U.S. | Retrospective (OR) | DM+ | VA-NY | DM− |
| 47 | Marzouk D | 2007 | Egypt | Retrospective (OR) | HCV+ | Zwyat Razin village cohort study | HCV− |
| 10 | Mason AL | 1999 | U.S. | Retrospective (OR) | DM+ | Clinics-New Orleans & Baton Rouge | DM− |
| 10 | Mason AL | 1999 | U.S. | Retrospective (OR) | HCV+ | VA-St. Louis or Clinic-New Orleans | HBV+ |
| 28 | Mehta SH | 2000 | U.S. | Retrospective (OR) | HCV+ | NHANES III | HCV− |
| 16 | Mehta SH | 2003 | U.S. | Prospective (HR) | DM+ | ARIC cohort study | DM− |
| 12 | Okan V | 2002 | Turkey | Retrospective (OR) | DM+ | Hospital | DM− |
| 22 | Papatheodoridis GV | 2005 | Greece | Retrospective (OR) | HCV+ with CLD | Hospital | HBV+ with CLD |
| 23 | Picerno I | 2002 | Italy | Retrospective (OR) | DM+ | Hospital | DM− |
| 45 | Qureshi F | 2002 | Pakistan | Retrospective (OR) | HCV+ with CLD | Hospital | HBV+ with CLD |
| 11 | Sangiorgio L | 2000 | Italy | Retrospective (OR) | DM+ | Hospital | DM− |
| 13 | Simo R | 1996 | Spain | Retrospective (OR) | DM+ | Hospital | DM− |
| 18 | Butt A | 2007 | U.S. | Retrospective (OR) | HCV+ | VA National Patient Care Database | HCV− |
| 25 | Stapleton JT | 2007 | U.S. | Retrospective (OR) | HCV+/HIV+ | ALLHRT cohort study | HIV+ |
| 46 | Wang C-S | 2007 | Taiwan | Prospective (HR) | HCV+ | A-Lein Township (1997–2003) | HCV− |
| 24 | Corrêa da Costa L | 2008 | Brazil | Retrospective (OR) | DM+ | Hospital | DM− |
| 17 | Imazeki F | 2008 | Japan | Retrospective (OR) | HCV+ | Hospital | HBV+ |
| Source of identification | Matching factors | Case or Exposed # | Outcome present # (%) | Control or Unexposed # |
|---|---|---|---|---|
| Hospital | NA | 153 | 35 (21.2%) | 226 |
| Population >50 yrs | Age and sex | 229 | 33 | 217 |
| Clinic | Age | 564 | 71 (12.6%) | 82 |
| Population | Age | 564 | 71 (12.6%) | 302 |
| Hospital & Clinic | NA | 707 | 148 (20.9%) | 159 |
| Hosptial | NA | 110 | 32 (29%) | 168 |
| Miyazaki cohort study | NA | 222 | 5 (2.3%) | 743 |
| CPCRA clinical trial | NA | 436 | NR | 2,129 |
| VA-HIV+ (1992–2001) | NA | 6153 | NR | 20,835 |
| Hospital | Age and sex | 1,151 | 272 (23.6%) | 181 |
| Hospital | NA | 820 | 56 (5.83%) | 905 |
| Clinic | NA | 591 | 150 (25.4%) | 223 |
| Methadone clinic | NA | 418 | 63 (15%) | 139 |
| Kaoshung City cohort study | NA | 1,237 | 96 (7.8%) | 8,695 |
| Clinic | NA | 388 | 55 (34%) | 1,141 |
| Liver cllinic | NA | 498 | 23 (4.6%) | 144 |
| Swiss HIV cohort study | NA | 123 | 31 (25.2%) | 6,390 |
| VA-NY | NA | 170 | 61 (35.9%) | 331 |
| Zwyat Razin village cohort study | NA | 180 | 9/111 (8.1% viremic), 7/66 (10.6% non-viremic) |
577 |
| Clinics-New Orleans & Baton Rouge | NA | 571 | 25 (4.2%) | 377 |
| VA-St. Louis or Clinic-New Orleans | NA | 604 | 145 (24%) | 486 |
| NHANES III | NA | 230 | NR | 9,551 |
| ARIC cohort study | NA | 15 | 7 (46.7%) | 1,069 |
| Blood donor registry | NA | 692 | 52 (7.5%) | 1,014 |
| Hospital | NA | 260 | 33 (12.7%) | 174 |
| Blood donor registry | NA | 254 | 6 (2.4%) | 223 |
| Blood bank | NA | 302 | 74 (24.5%) | 98 |
| Blood bank | NA | 1,514 | 115 (7.6%) | 1,300 |
| Hospital blood donors | Age, sex, previous transfusion, and IV drug use | 176 | 18 (11.5%) | 6,172 |
| VA National Patient Care Database | Age, race/ethnicity and sex | 126,926 | 36,047 (14.2%) | 126,926 |
| ALLHRT cohort study | NA | 160 | 8 (5%) | 1,274 |
| A-Lein Township (1997–2003) | NA | 812 | 116 (24.5%) | 3,486 |
| Hospital | Age and sex | 206 | 3 (1.4%) | 206 |
| Hospital | NA | 544 | 74 (13.6%) | 286 |
| Outcome present # (%) | Male case or exposed # (%) | Male control or unexposed # (%) | Age case or exposed | Age control or unexposed |
|---|---|---|---|---|
| 33 (14.1%) | 100 (65.3%) | 96 (42.5%) | >40 (67.3%) | >40 (34.1%) |
| 15 | 58 (25.3%) | 217 (23.2%) | 67 +/− 12 | 66+/− 8 |
| 4 (4.9%) | 181 (32,1%) | 30 (36.6%) | 61+/−14 (DM+) | 67+/− 6 (DM+) |
| 22 (7.3%) | 181 (32,1%) | 91 (30.1%) | 61+/−14 (DM+) | 64+/−10 (DM+) |
| 19 (11.9%) | 413 (58%) | 118 (74%) | 40–59 yrs (33%) | 40–59 yrs (46%) |
| 8 (4.8%) | 71 (65%) | 82 (48.9%) | 51 +/− 10 | 48+/− 12 (other CLD), 47+/− 11 (HBV CLD) |
| 6 (0.8%) | 89 (40%) | 300 (40%) | 45–54 yrs (23.0%) | 45–54 yrs (24.5%) |
| NR | 77% -overall cohort | NR | NR | NR |
| NR | NR | NR | <40 35.1% overall cohort | NR |
| 17 (9.4%) | 704 (61.2%) | 135 (74.6%) | median 59+/−8 | median 54+/− 7 |
| 23 (2.56%) | 428 (52.2%) | 503 (55.7%) | 56.7 +/− 11.33 | 53.7 +/− 15.28 |
| 25 ( 11.2%) | 73.3% | 76.0% | 42 +/− 4 | 45 +/− 3 |
| 11 (8%) | 256 (61%) | 62 (45%) | <40 (25%) | <40 (55%) |
| 546 (6.3%) | 640 (51.7%) | 3,652 (42.0%) | 57.1 +/− 6.2 | 54.9 +/− 5.9 |
| 333 (24%) | 310 (80%) | 922 (81%) | 40–50 162 (42%) | 40–50 309 (27%) |
| 18 (12.5%) | 274 (55%) | 71 (49%) | 52.9 +/− 14.1 | 54.7 +/− 15.2 |
| 1,757 (27.5%) | 98 (79.7%) | 4,383 (68.6%) | 45 (IQR 38–53) | 38 (IQR 34–44) |
| 52 (15.7%) | 93.3% male overall | NR | 58.0 Asian overall, 61.0 PI overall | NR |
| 25 (4.7%) | 40.4% male overall | NR | 45–54 61 (19.7%) overall | NR |
| 6 (1.6%) | 12 (48%) HCV+ | 309 (54%) | 59 +/− 15 | 62 +/− 14 |
| 63 (13%) | 386 (64%) | 375 (78%) | 43–49 yrs n=117 (20%) | 43–49 yrs n=74 (19.5%) |
| NR | 49%-overall | NR | 40–49 yrs (19%) -overall | NR |
| 541 (50.6%) | 5 (62.5%) | NR | 44–49 yrs (37.5%) | NR |
| 12 (1.2%) | 260 (37.6%) | 881 (86.9%) | 51.9 yrs | NR |
| 25 (14.4%) | 15 (45.5%) DM+ | 21 (84.0%) DM+ | 53.9 +/− 11.5 (DM+) | 59.4 +/− 10 (DM+) |
| 3 (1.3%) | 148 (58.3%) | 143 (64.1%) | 51.7 +/− 5.8 | 47.2 +/− 6.1 |
| 19 (19.4%) | 65.8% male overall | NR | 42 +/− 13 years overall | NR |
| 30 (2.3%) | 668 (44.1%) | NR | 63.1+/−10.5 | NR |
| 156 (2.5%) | 46.7% (DM+) | 43.2% (DM+) | 46.4 +/−21.2 (DM+) | 48.3 +/− 14.6 (DM−) |
| 33,255 (13.1%) | 245,729 (96.8%) | 245,729 (96.8%) | 51.8 | 50.8 |
| 31 (2%) | 130 (81%) | 1,032 (81%) | 43 median | 37 median |
| 300 (8.6)% | 382 (47.0%) | 1,536 (44.1%) | 58.9 +/− 10.7 | 55.8 +/− 11.5 |
| 2 (1.0%) | 58 (28%) | 58 (28%) | 55 | 54.6 |
| 18 (6.3%) | 164 (57.3%) | 164 (47.2%) | 59.6 +/− 13.1 | 44.5 +/− 13.0 |
See also Appendix 1 for other key study characteristics
Case or exposed dependent on type of design, see also Appendix A for detail on ascertainment
Controls or unexposed dependent on type of design, see also Appendix 1 for details on ascertainment
Abbreviations: HCV (hepatitis C virus infection); DM (diabetes mellitus), OLD (other liver disease including alcohol-induced liver disease and chronic hepatitis); NA (not applicable); NR (not reported); MC (mixed cryoglobulinemia); CLD (chronic liver disease); LF (liver failure); ALT (alanine aminotransferase); HR, hazard ratio; IRR, incidence rate ratio; OR, odds ratio; PI, Pacific Islander
Half of these studies were performed in the U.S. (n=11) or Italy (n=6) and most reported or had data to calculate an applicable prevalence OR (n=29). (Table 1) Among studies with other risk estimates, four reported HRs(16, 29, 30, 42) and the other an incidence rate ratio (IRR)(20).
Sample size was variable among studies with the smallest having a total sample size of 217(14) and the largest with over 252,000(18). (Table 1) A majority of studies (n=24) utilized hospital/clinic-based controls. However, only 6 studies employed a matched design with use of pre-specified criteria to match cases to controls(5, 6, 13, 18, 24, 44).
HCV+ vs. HCV−
Eighteen studies had an estimate of DM risk among individuals with HCV infection in comparison to individuals without infection(5–13, 16, 18, 23, 24, 28, 30, 42, 46, 47). (Table 1) Fifteen had ORs(5–13, 23, 24, 28, 30, 47), while another three had HRs(16, 42, 46). Results of meta-analyses stratified by specific type of risk estimate are provided below.
Meta-analysis results for studies with ORs
ORs from 14 eligible studies were pooled. (5, 7–13, 18, 23, 24, 28, 30, 47) (Fig 1A) We did not include the single remaining study in our meta-analysis because it was restricted to a very limited subgroup of HCV+ cases with symptomatic mixed cryoglobulinemia(6). As the study by Mehta et al(28) reported ORs for two unique and non-overlapping age-groups, both were able to be used to calculate the pooled estimator. The Marzouk et al study(47) also provided stratified estimates, but with the sub-groups defined by the presence or absence of viremia. However, as both the viremic and non-viremic sub-groups were compared to the same underlying control group, only one of these overlapping ORs could be included in our calculation of the pooled estimator. We therefore used the estimate for the viremic sub-group, representing the majority of the HCV cases, to calculate the pooled estimator.
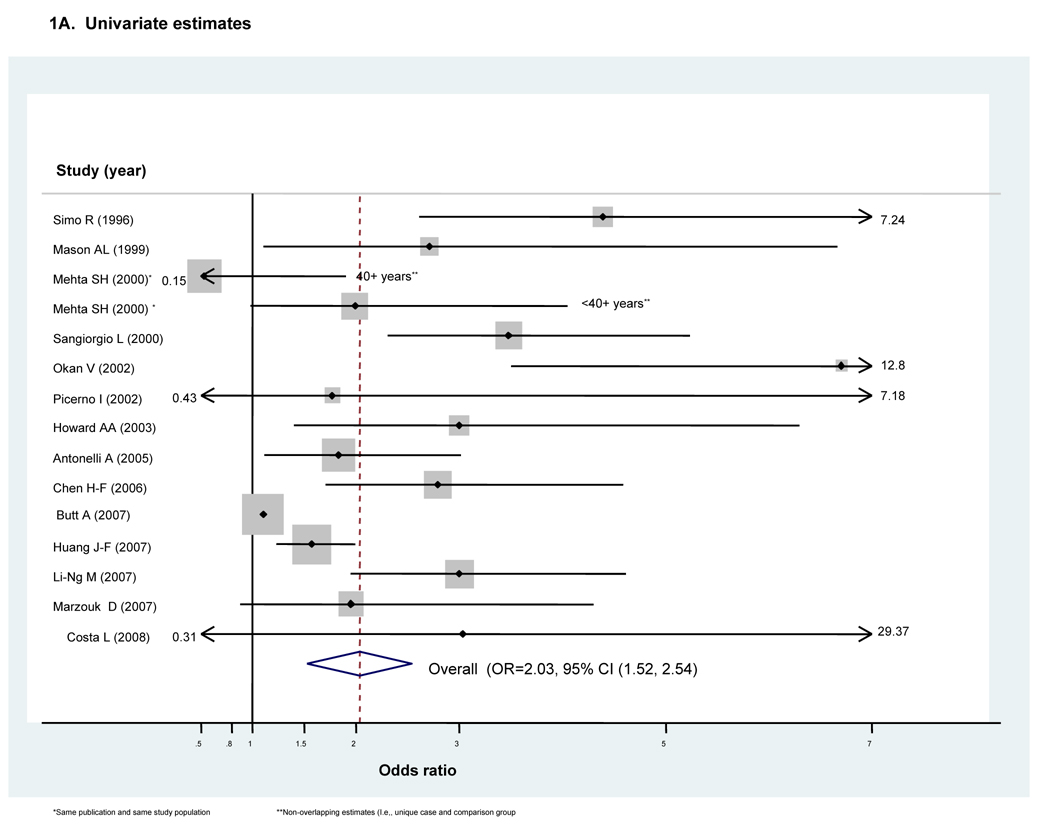
Figure 1. Forest plots for meta-analyses comparing risk of Type II diabetes in HCV infected cases compared to that in non-infected controls in retrospective studies (n=14)^.
^Dimension of shaded odds ratio for individual studies is proportional to their total weight in calculation of the pooled estimator.
The unadjusted OR for DM risk with HCV infection in the 14 included studies ranged between OR=0.67 (95% CI 0.65–0.69)(17) to OR=6.7 (95% CI 3.5–12.8)(12), with statistically significant excess risk reported in 11 studies. (Fig 1A) Because substantial between-study heterogeneity was observed (I2=73%), we employed a random effects meta-analysis. The pooled OR indicated a two-fold excess DM risk with HCV infection (ORunadjusted=2.03, 95 % CI 1.52–2.54). (Fig 1A)
Egger’s test indicated significant small study or publication bias (Egger’s p<0.001). However, none of the variables assessed with exploratory univariate meta-regression (type of controls, region or year of publication) was identified as a potentially important source of between-study heterogeneity. Additionally, our analysis of influence demonstrated that the unadjusted pooled estimator consistently suggested excess DM risk with HCV infection (data not shown).
Given the finding of significant small study or publication bias, we performed sensitivity analyses to ascertain the effects attributable to the single largest study, which had a total sample size of over 252,000(18), and to the single smallest study, which had total sample size of only 477(23). There was still evidence of this bias with removal of the smallest study (pEgger’s<0.001) and between-study heterogeneity was slightly increased (I2=75%) (data not shown). However, there was no longer evidence of small study or publication bias with removal of the largest study (pEgger’s=0.48), with only a small increase in the associated pooled estimator (ORundjusted=1.68, 95 percent CI 1.15–2.20). Further, between-study heterogeneity was demonstrably reduced (I2=57%) (data not shown).
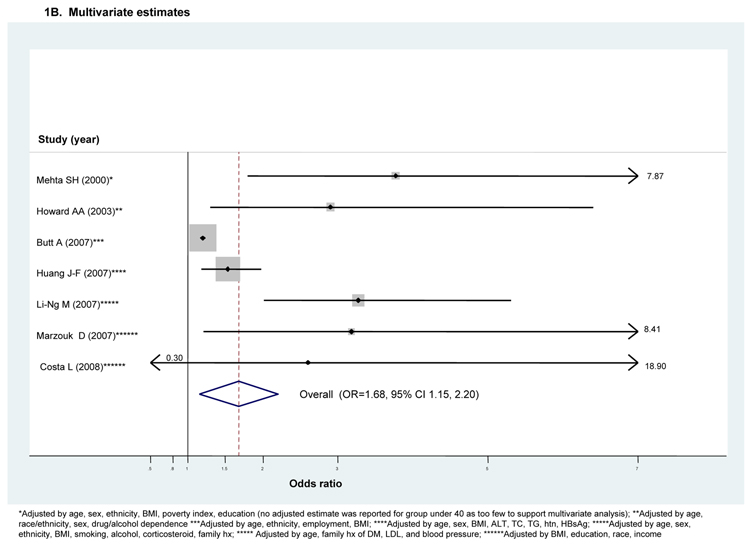
Only 7 studies also provided an adjusted OR, many with a small to modest attenuation in effect. (Fig 1B) Huang et al(30) reported adjusted estimates for sub-groups defined by their viremia status. However, both the viremic and non-viremic sub-groups were compared to the same underlying control group. We therefore used the OR reported for the viremic sub-group as it represented the majority of the HCV cases (74%).
Substantial between-study heterogeneity was also observed among studies reporting adjusted ORs (I2=58.1%) necessitating use of a random effects model. (Fig 1B) The pooled adjusted OR demonstrated a significant though modestly reduced excess risk of DM with HCV infection (ORadjusted=1.68, 95 percent CI 1.15–2.20). However, the pooled OR was reduced slightly and was no longer significant if we used the ORs reported for the smaller non-viremic sub-groups instead of those for the much larger viremic sub-groups for both the Huang et al(30) and the Marzouk et al (47) studies (ORadjusted=1.57, 95 percent CI 0.09–3.05). (data not shown) Although there was again evidence of significant small study or publication bias (p<0.001), this again appears to be explained by the single largest study(18). However, given the limited number of studies, we did not perform meta-regression.
Meta-analysis results for studies reporting HRs
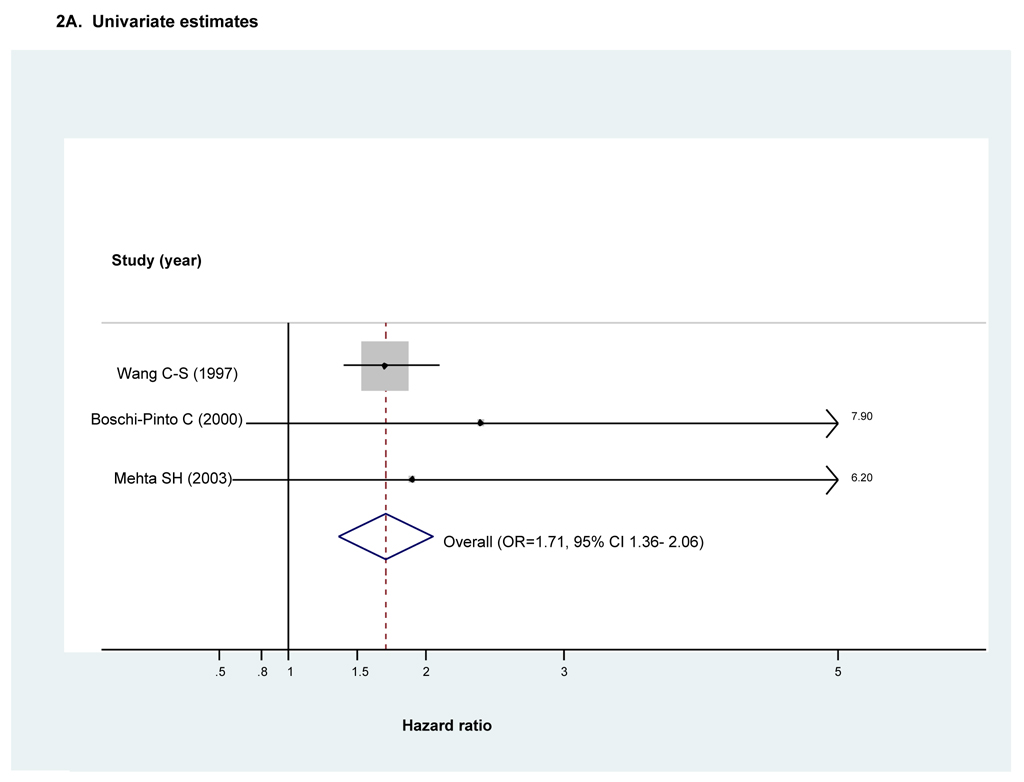
Three studies reported HRs as the measure of association between HCV infection and DM(16, 42, 46). Two were prospective population-based cohort studies performed in Asian populations(42, 46) and the other a case-cohort study based on a sample drawn from the larger Atherosclerosis Risk in Communities (ARIC) cohort study(16) in the U.S. Total follow-up was variable among studies ranging between seven and eleven years. All three excluded prevalent DM cases at baseline and had serologically-confirmed HCV infection.
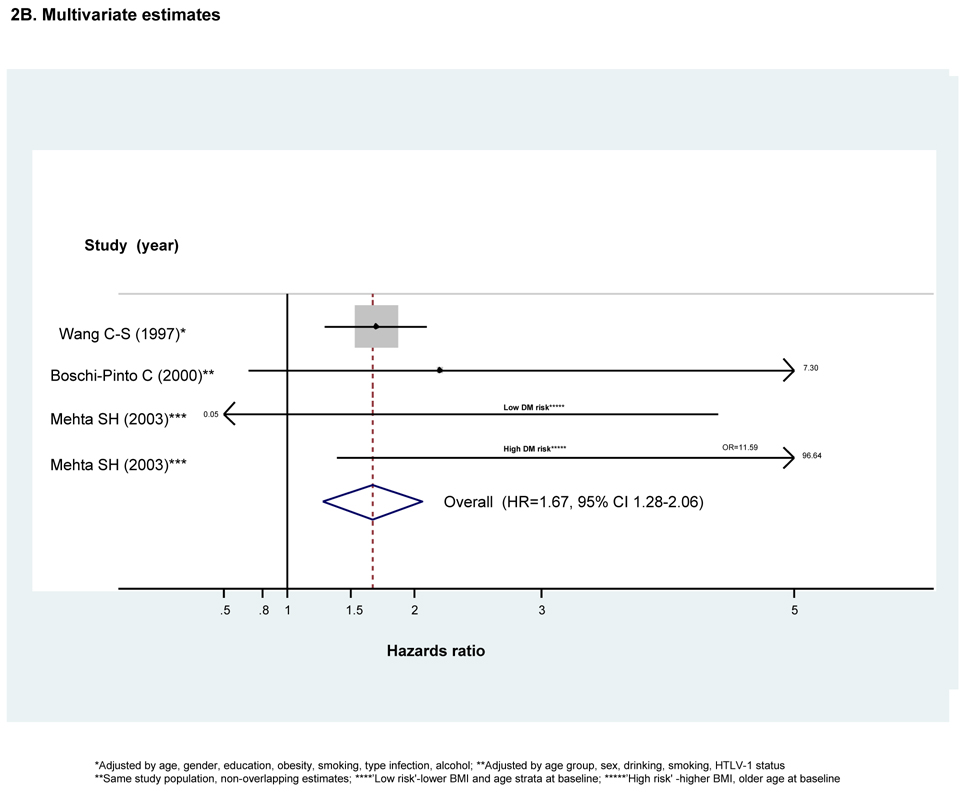
Both unadjusted and adjusted HRs were reported by all three studies (Figs 2A and 2B respectively). However, the adjusted estimates reported by Mehta et al(16) were stratified according to an a priori -specified DM risk category assigned at baseline. As the resulting high- and low- DM risk sub-groups were each compared to their respective and therefore non-overlapping control sub-groups, both estimates were used to calculate the adjusted pooled estimator.
Figure 2. Forest plot of hazard ratios and the overall pooled estimator for longitudinal studies comparing diabetes risk in individuals with HCV infection to that in individuals without HCV infection (n=3)^.
^Dimension of shaded hazards ratio for individual studies is proportional to their total weight in calculation of the pooled estimator.
There was no evidence of substantive between-study heterogeneity when considering either unadjusted or adjusted estimates. We therefore employed fixed effects meta-analysis. Both unadjusted and adjusted pooled estimators demonstrated HCV infection significantly increases risk of developing DM (HRunadjusted=1.71, 95% CI 1.36--2.06 and HRadjusted=1.67, 95% CI 1.28–2.06). (Figs 2A and 2B respectively) While both studies conducted in Asian populations found excess DM risk with HCV infection even after adjusting for BMI (42, 46), the smaller Mehta study(16) found excess risk only in the sub-group at low risk for DM at baseline, including those of younger age or with lower BMI. (Fig 2B) Results for Egger’s test suggested no evidence of publication or small study bias when considering either unadjusted or adjusted HRs (pEgger’s =0.30 and pEgger’s =0.42 respectively). However, given the small number of studies, we did not perform exploratory meta-regression or an analysis of influence.
HCV+ vs. HBV+
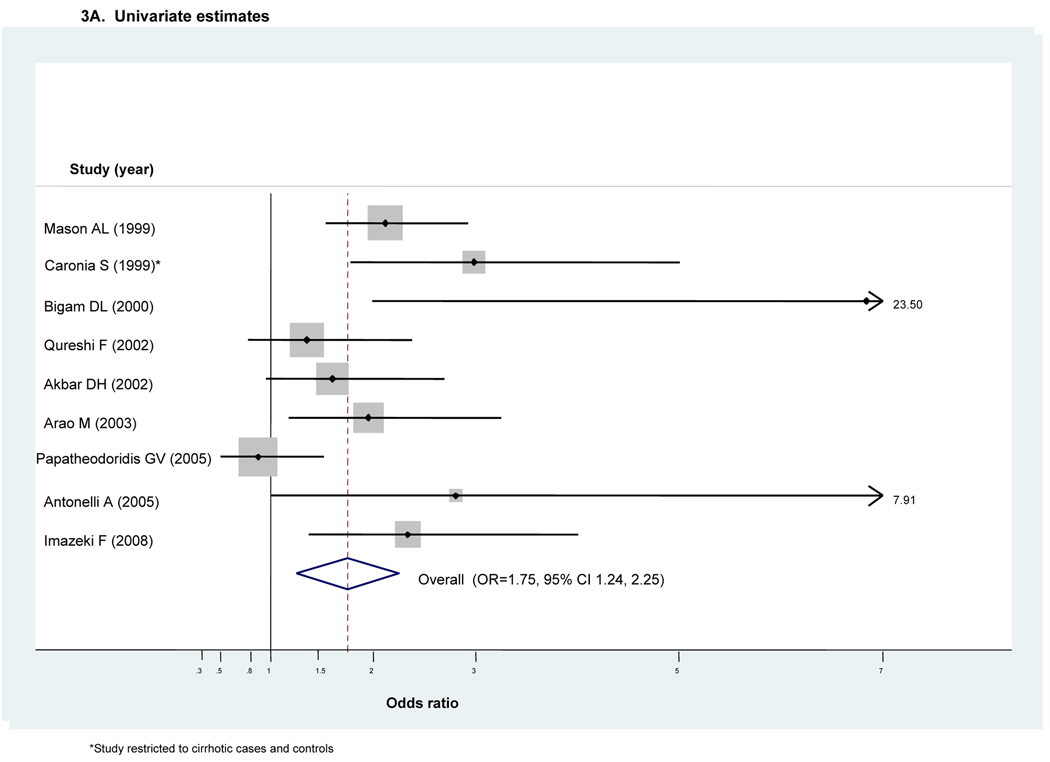
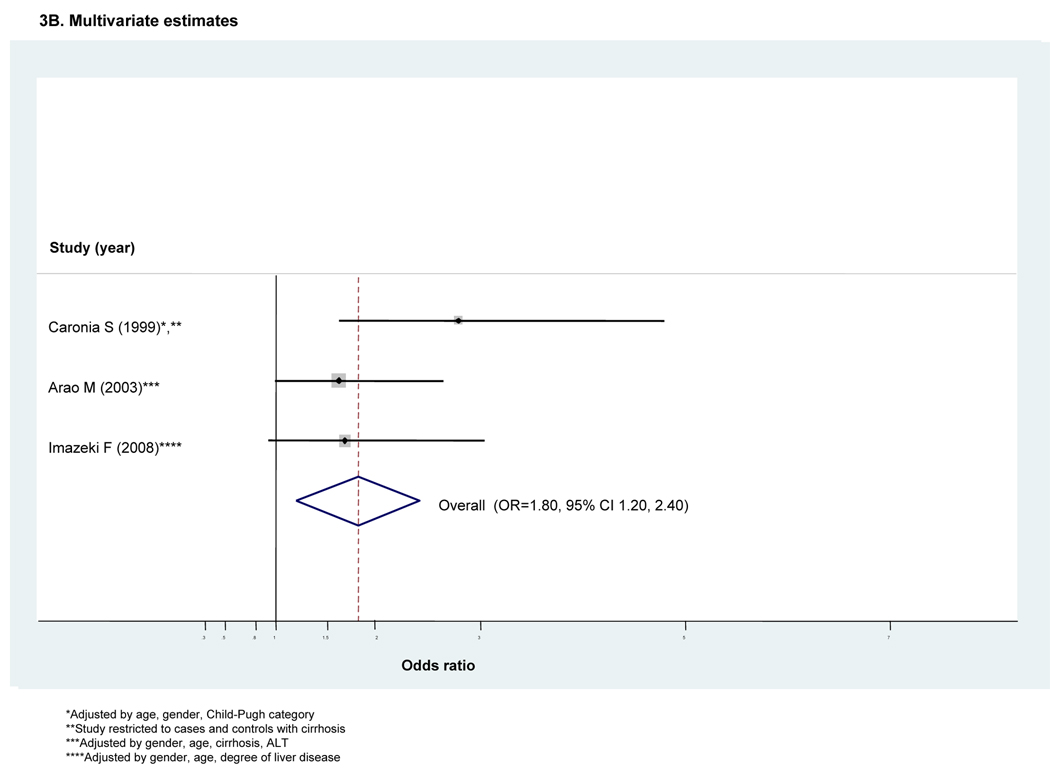
Nine studies assessed DM risk in individuals with HCV infection in comparison to that in individuals with HBV infection(5, 10, 14, 17, 21, 22, 41, 44, 45) (Table 1) Most (n=5) were conducted in European or North American populations.
All nine reported an unadjusted OR (Fig 3A). Given moderate heterogeneity (I2=50.2%), we employed a random effects analysis. It indicated an approximately 1.8-fold excess risk of DM among HCV+ in comparison to those HBV+ (ORunadjusted=1.75, 95 percent CI 1.24–2.25). (Fig 3A) Only three studies provided adjusted estimates(17, 41, 44) with all including adjustment for relative degree of liver pathology. Given only modest heterogeneity (I2<50), we employed a fixed effects analysis. The overall pooled adjusted estimator demonstrated a similarly increased risk of DM (ORadjusted=1.80, 95% CI 1.20–1.40). (Fig 3B)
Figure 3. Forest plot for meta-analyses comparing risk of diabetes in HCV-infected cases compared to that in HBV-infected controls (n=8 retrospective studies)^.
^Dimension of shaded odds ratio for individual studies is proportional to their total weight in calculation of the pooled estimator.
Five studies also provided unadjusted risk estimates stratified according to the degree of liver pathology(10, 22, 41, 44, 45). (Appendix 2A and 2B respectively) The unadjusted pooled estimator in the context of chronic hepatitis and in the context of cirrhosis both suggested modest though non-significant excess DM risk with HCV infection [ORchronic hepatitis =1.28, 95% CI 0.76–1.79 (Appendix 2A) and ORcirrhosis=1.59, 95% CI 0.70–2.49 (Appendix 2B)]. To allow a more direct comparison of DM risk observed in the context of chronic hepatitis versus in the context of cirrhosis, we performed a sensitivity analysis where we removed the single study restricted to cirrhotic cases and controls(44). Its removal resulted in a 74% relative reduction in the pooled estimator for DM risk conveyed by HCV within the context of cirrhosis (ORcirrhosis=1.18, 95% CI 0.47–1.89). (data not shown)
Results for Egger’s test demonstrated no evidence of small study or publication bias in either our unadjusted or adjusted meta-analyses (p=0.38 or p=0.88) and our analysis of influence demonstrated that the pooled estimator was fairly robust to removal of individual studies. (data not shown) However, as there were fewer than 10 studies, we did not perform meta-regression.
HCV+ CLD vs. other cause CLD
Three studies evaluated whether CLD cases attributable to HCV infection had excess DM risk in comparison to CLD cases attributable to other causes of liver disease (OLD), including alcoholic or cholestatic liver.14,15,27 (Table 2) There was evidence of variable increased DM risk with HCV infection in two studies and in a sub-group in the third. However, as the relative proportion of CLD cases attributable to specific other causes was non-comparable across studies, we did not obtain a pooled estimator.
Table 2.
DM risk with HCV infection in comparison to that in two high risk sub-groups: HIV-infected or with other causes of liver disease (OLD)*.
| Ref # | Study (year) | Type of Estimator | Stratified by: | Unadjusted Estimate | 95% CI | Stratified by: | Adjusted Estimate | 95% CI | Adjustment factors: |
|---|---|---|---|---|---|---|---|---|---|
| HCV+/HIV+ vs. HIV+ | |||||||||
| 43 | Brar (2007) | OR | 2.59 | (1.63–4.14) | 1.91 | (1–3.64) | age, BMI, gender, ethnicity, drug use, MSM status, AIDS defining illness | ||
| 29 | Butt (2004) | ||||||||
| Era | Era | ||||||||
| HR | HAART | 1.98 | (1.34–2.92) | HAART | 1.39 | (1.27–1.53) | age, minority race, alcohol or drug dx, end-stage liver disease present | ||
| HR | pre-HAART | 1.23 | (1.13–1.33) | pre-HAART | 1.01 | (0.71–1.36) | age, minority race, alcohol or drug dx, end-stage liver disease present | ||
| 31 | Jain (2007) | OR | 1.6 | (1.1–2.2) | |||||
| DM risk** | |||||||||
| Low | 2.0 | (1.2–3.2) | race | ||||||
| High | 1.2 | (0.7–2.1) | race | ||||||
| 20 | Ledegerber (2007) | IRR | 0.78 | (0.5–1.21) | 1.2 | (0.63–2.29) | sex, age, mode and stage of infection, ethnicity, CD4 (count/nadir), smoking, BMI, central adiposity, time dependent rx | ||
| 25 | Stapleton (2007) | OR | 1.67 | (0.92–3.04) | |||||
| HCV+ vs. OLD* | |||||||||
| 27 | Lecube (2004) | OR | 1.36 | (0.85–2.19) | |||||
| Stage | |||||||||
| Chronic hepatitis | 2.96 | (1.24–7.06) | |||||||
| Cirrhosis | 1.25 | (0.63–2.42) | |||||||
| 14 | Bigam*** (2000) | OR | 8.21 | (3.61–18.6) | |||||
| Sub-type specific | |||||||||
| HBV only | 6.84 | (1.99–23.54) | |||||||
| Cholestatic only | 9.03 | (3.36–24.2) | |||||||
| 15 | El-Zayadi (1998) | OR | 2.7 | (1.7–4.4) | |||||
OR, odds ratio; HR, hazard ratio, IRR, incidence rate ratio; dx, diagnosis; HAART, highly active anti-retroviral therapy; hx, history; MSM, male who has sex with males
OLD, non-HCV related causes of chronic liver disease including alcohol-related, cholestatitic, hepatitis B, etc.
DM risk-a priori specified DM risk category, high risk=yes if (age>60 or BMI>34.9 or family hx) or (age 50–59 & BMI 30–34.9) or (age 55–59 & BMI 25–9.9), low risk=all non-high risk
Pre-transplant time period only.
HCV+/HIV+ vs. HCV−/HIV+
Five studies evaluated whether DM risk was increased in individuals co-infected with HCV and HIV in comparison to individuals mono-infected with HIV(20, 25, 29, 31, 43) (Table 2) Reported risk estimates included ORs (n=3 studies), HRs (n=1 study) and IRRs (n=1 study). Four reported both unadjusted as well as adjusted risk estimates(20, 29, 31, 43). DM risk estimates with HCV/HIV co-infection among these four studies were variable with significant increased risk reported in one study(43), non-significant excess in another(20) and a significant increase only in specific sub-groups in the remaining two studies(29, 31). (Table 2)
A sufficient number of studies to perform meta-analysis existed only for those reporting ORs(25, 31, 43). (Table 2) Our fixed effects meta-analysis demonstrated a 1.8-fold excess risk of DM with dual HCV/HIV infection in comparison to HIV mono-infection (ORunadjusted=1.82, 95 percent CI 1.27–2.38) with no evidence of small study or publication bias (pEgger’s=0.63). (data not shown) As only two studies also provided adjusted estimates, we did not perform meta-analysis to obtain an adjusted pooled estimator.
DISCUSSION
This is the first meta-analysis to specifically examine the association between HCV infection and risk of diabetes (DM) in the general population as well as in sub-groups at particularly increased risk of chronic liver disease (CLD) including those with hepatitis B (HBV) or HIV infection, or with other causes of liver disease (OLD) like alcohol-related liver disease. Among 34 eligible studies identified for this review, eighteen (15 retrospective and 3 prospective) evaluated DM risk in HCV-infected cases in comparison to general controls without HCV infection. Our meta-analysis which combined the adjusted odds ratios from these retrospective studies demonstrated an approximately 1.7-fold significant increase in DM risk with HCV infection. Similarly, the overall unadjusted pooled estimator demonstrated a significant 2-fold excess risk. Although there was evidence of potential small study or publication bias among these retrospective studies, this effect appears to be largely explained by the single largest study(18) removal of which did not change the overall trend. Further, none of the other potential sources of between-study heterogeneity examined including geographic region, year of publication, or type of controls were significant (p>0.15).
Three prospective studies also evaluated whether HCV infection increases risk of developing type II diabetes(16, 42, 46). All had serological confirmation of HCV and exclusion of DM at baseline. Results from our meta-analysis pooling adjusted HRs suggested HCV infection conveys an approximately 1.7-fold excess DM risk. Interestingly, essentially the same significant excess risk was observed by pooling the unadjusted HRs. In contrast to retrospective studies which have well-established limitations, long-term longitudinal studies with prospectively collected data such as these are particularly valuable as they establish a temporal relationship between HCV infection and subsequent occurrence of diabetes and help support an argument of a causal association. The significant excess DM risk observed in our meta-analysis of prospective studies (HRadjusted=1.67) is also highly consistent with the significant excess risk observed in our meta-analysis of retrospective studies (ORadjusted=1.70) and adds further support of those retrospective results. Taken together, the findings of our combined meta-analyses clearly indicate that chronic HCV infection is associated with a modest but significantly increased risk of developing type 2 diabetes in comparison to uninfected controls.
The reasons why chronic HCV infection would induce type 2 diabetes could be manifold. Several experimental studies have suggested a direct role of the virus in promoting DM risk. Within HCV core-transgenic mice, hepatocyte-associated degradation of the HCV core protein leads to negative interaction with insulin signaling by reducing IRS-1 phosphorylation and downstream signaling by Akt(48) and by promoting IRS-1 and IRS-2 degradation(49). In one study, the virus has also been localized in 39% of pancreatic islets in HCV-infected humans and occurs in approximately 54% of all cells within affected islets. Although there is no evidence of increased apoptosis, these HCV+ islet cells exhibit morphologic changes as well as derangement in glucose-stimulated insulin release (β-cell dysregulation)(50). Other experimental studies have suggested a more indirect role of the virus, or rather that it is host response to the virus that promotes DM risk. For example, hepatic levels of pro-inflammatory cytokine TNF-α are doubled in HCV core-transgenic mice with blockade of TNF-α leading to restored hepatic sensitivity to insulin.(51) However, it has also been suggested that HCV infection promotes DM risk as a tertiary consequence of HCV-induced liver damage. Indeed, it is well-established that advanced cirrhosis induces dysregulation of glycemic control which may result in overt diabetes(52). Some support for such a tertiary mechanism comes from a clinical study demonstrating severe fibrosis is the only independent predictor of insulin resistance (IR) as measured by the surrogate marker the HOMA index in HCV-infected patients(53). However, other studies have shown higher IR in HCV-infected patients irrespective of degree of liver injury(4, 27, 54) with increases in IR evident even at early fibrosis stages(55).
If it is characteristics specific to HCV infection itself rather than just the tertiary liver damage it generates that induces insulin resistance and increases diabetes (DM) risk in human populations, then it would be expected that the prevalence of diabetes should be higher with chronic HCV, than, for instance, with other causes of chronic liver disease. Nine retrospective studies evaluated this hypothesis with respect to HBV infection(5, 10, 14, 17, 21, 22, 41, 44, 45). Suggestive evidence in support of this hypothesis came from the 1.7-fold significant excess DM risk conveyed by the unadjusted pooled OR. Only three studies also provided an adjusted estimate, with all three including adjustment for degree of liver pathology(17, 41, 44). Our pooled adjusted OR demonstrated that HCV infection conveys a significant 1.8-fold excess risk of DM beyond that conveyed by relative degree of liver pathology. Four studies also provided unadjusted risk estimates stratified according to the presence of chronic hepatitis or cirrhosis(10, 22, 41, 45). The unadjusted pooled estimators in the context of cirrhosis and in the context of chronic hepatitis both demonstrated only modest non-significant excess risk of DM. However, given low study power as well as lack of adjustment for other possible confounders, these findings are difficult to interpret. The single study comparing DM risk with HCV-infection to that with chronic liver disease (CLD) attributable to a mixture of other causes including alcohol-related disease(27) demonstrated strong and significant excess risk with HCV infection only in the context of chronic hepatitis. Unfortunately, without individual patient data it is not possible to further clarify the impact of liver injury on the relative risk of diabetes associated with HCV infection, both for fibrosis and necro-inflammatory activity.
An estimated 25–30% of HIV cases in the U.S. and Western Europe are co-infected with HCV(56). Highly active anti-retroviral therapy (HAART) used to treat HIV infection is well-known to increase risk of CLD, with development of hepatotoxicity an important reason why HAART is discontinued(57). However, HAART is still recommended for HCV co-infected treatment candidates, with some data suggesting its use may also lessen HCV-related liver disease progression(57). Five studies included in this review evaluated whether HIV cases co-infected with HCV have increased DM risk in comparison to HIV mono-infected cases(20, 25, 29, 31, 43). A small to modest excess risk of DM with HCV co-infection was generally observed though significance of findings was variable. Only three studies provided comparable unadjusted risk estimators (ORs) that were able to be combined in a pooled estimator (25, 31, 43). Two studies included cases and controls prior to initiation of HAART(25, 43) while the third study adjusted for use of HAART(31). This unadjusted meta-analysis demonstrated co-infection with HCV conveys a 1.8-fold significant excess risk of DM in comparison to that observed in HIV mono-infected cases. However, as only two studies provided adjusted estimates, we did not obtain an adjusted pooled estimator and our unadjusted meta-analysis finding of excess DM risk among HIV patients co-infected with HCV must be considered as suggestive only.
In evaluating findings from our meta-analyses, it is important to consider the potential impact of confounders of the relationship between HCV infection and occurrence of DM, particularly from such well-established risk factors as BMI. The three available prospective studies that evaluated diabetes risk in comparison to uninfected controls reached different conclusions as to what categories of HCV-infected individuals are at increased risk. Specifically, the two larger prospective cohort studies both demonstrated HCV infection conveys additional DM risk beyond that conveyed by age or BMI(16, 42, 46). This finding is similar to that observed among all 6 retrospective studies which also included adjustment for these factors(8, 9, 24, 28, 30, 47). In contrast, the much smaller American case-cohort study, which included only 15 HCV-infected cases, showed only individuals who were already at increased diabetes risk (mainly overweight individuals older than 50) have an additional DM risk due to HCV infection(16). Although the preponderance of current evidence suggests hepatitis C infection may convey additional DM risk beyond that conveyed by BMI, additional prospective studies are therefore needed to sort out the important aspect of the interaction between HCV infection and other risk factors for diabetes including current and historical obesity.
The present study has several strengths as well as some limitations. We used exhaustive search methods to identify all eligible studies and attempted to increase comparability and quality of included studies by using pre-specified eligibility criteria including publication in a peer-reviewed journal, a minimum total sample size and presence of appropriate as well as adequately identified case and control groups. To help assess the validity and reliability of our findings, we also performed additional quality control analyses including meta-regression and sensitivity analyses in order to identify possible sources of between-study heterogeneity. Further, we systematically investigated the potential for small study or publication bias and the impact of removal of individual studies on the pooled estimator was also assessed.
Our application of rigorous eligibility criteria to assure the internal validity of our findings has also imposed some potential limitations, particularly with respect to the generalizability of our results. First, our restriction to studies performed in adults means we are unable to extrapolate these findings to HCV-infected children. Second, our restriction to articles published in the English language means it is possible that complex interactions between ethnicity, HCV infection and the occurrence of diabetes could have been missed in particular ethnic groups if these findings were published exclusively in non-English language journals. However, only a small minority (6%) of studies reviewed were excluded specifically because they were not published in English. Further, 62% of all included studies were performed in countries where English is not the primary language. Third, we included only studies with a minimum total sample size of 200, with at least 100 exposed or cases and 100 controls or unexposed. We employed this criterion to help mitigate the potential for small study bias given the greater likelihood that small studies in particular will be published if they report significant or interesting results(58). Although less than 12% of studies were specifically excluded due to sample size, it is therefore possible that our reported effect sizes might actually be increased if we had included these smaller studies. Finally, we selected only studies reporting on prevalence or incidence of overt diabetes. This has the advantage of robust and reproducible clinical definitions of the outcome of interest across studies. However, it can also underestimate the magnitude of the relationship between HCV infection and impaired glucose metabolism.
Other limitations are due to insufficient information provided by the eligible studies themselves. Dose-dependent effects could not be demonstrated because viral load and duration of HCV infection were not typically recorded. Other potentially important viral- (e.g., genotype) or host-related factors (e.g., family history of diabetes and visceral adiposity) could also not be examined. Additional prospective studies are therefore needed to determine what specific combination of viral- and host-related factors explain the observed excess risk of type II DM conveyed by HCV infection.
The findings of this meta-analysis could have important clinical implications. Given the demonstrated increased risk of diabetes conveyed by HCV infection, a strong case can be built for screening for glucose abnormalities in all HCV-infected individuals. Second, these data might provide a better insight into the overall burden of disease in chronic hepatitis C. Indeed, if risk of diabetes increases with the duration of exposure to HCV, then diabetes might become a prominent HCV-induced health problem in some patients like those with a low risk of fibrosis progression (e.g., women contaminated with HCV at a young age). Finally, since some reports have shown that HCV eradication improves insulin sensitivity(3) and reduces the incidence of diabetes(59), a reasonable inference would be that some HCV-infected patients at high risk of diabetes occurrence might benefit from antiviral therapy beyond hepatological reasons. Future work is needed in order to determine if diabetes could be prevented or reversed with successful HCV eradication.
Supplementary Material
Acknowledgment
This research was supported in part by the Houston Center for Quality of Care & Utilization Studies, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs and a Department of Veterans Affairs Merit Review Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1–46. [PubMed] [Google Scholar]
- 2.Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB. Extrahepatic manifestations of Hepatitis C Virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39:2–17. doi: 10.1016/j.dld.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Romero-Gomez M, Del Mar Viloria M, Andrade RJ, Salmeron J, Diago M, Fernandez-Rodriguez CM, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 4.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Antonelli A, Ferri C, Fallahi P, Pampana A, Ferrari SM, Goglia F, et al. Hepatitis C virus infection: evidence for an association with type 2 diabetes. Diabetes Care. 2005;28:2548–2550. doi: 10.2337/diacare.28.10.2548. [DOI] [PubMed] [Google Scholar]
- 6.Antonelli A, Ferri C, Fallahi P, Sebastiani M, Nesti C, Barani L, et al. Type 2 diabetes in hepatitis C-related mixed cryoglobulinaemia patients. Rheumatology (Oxford) 2004;43:238–240. doi: 10.1093/rheumatology/keh011. [DOI] [PubMed] [Google Scholar]
- 7.Chen HF, Li CY, Chen P, See TT, Lee HY. Seroprevalence of hepatitis B and C in type 2 diabetic patients. J Chin Med Assoc. 2006;69:146–152. doi: 10.1016/S1726-4901(09)70195-9. [DOI] [PubMed] [Google Scholar]
- 8.Howard AA, Klein RS, Schoenbaum EE. Association of hepatitis C infection and antiretroviral use with diabetes mellitus in drug users. Clin Infect Dis. 2003;36:1318–1323. doi: 10.1086/374838. [DOI] [PubMed] [Google Scholar]
- 9.Li-Ng M, Tropp S, Danoff A, Bini EJ. Association between chronic hepatitis B virus infection and diabetes among Asian Americans and Pacific Islanders. Dig Liver Dis. 2007;39:549–556. doi: 10.1016/j.dld.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 11.Sangiorgio L, Attardo T, Gangemi R, Rubino C, Barone M, Lunetta M. Increased frequency of HCV and HBV infection in type 2 diabetic patients. Diabetes Res Clin Pract. 2000;48:147–151. doi: 10.1016/s0168-8227(99)00135-7. [DOI] [PubMed] [Google Scholar]
- 12.Okan V, Araz M, Aktaran S, Karsligil T, Meram I, Bayraktaroglu Z, et al. Increased frequency of HCV but not HBV infection in type 2 diabetic patients in Turkey. Int J Clin Pract. 2002;56:175–177. [PubMed] [Google Scholar]
- 13.Simo R, Hernandez C, Genesca J, Jardi R, Mesa J. High prevalence of hepatitis C virus infection in diabetic patients. Diabetes Care. 1996;19:998–1000. doi: 10.2337/diacare.19.9.998. [DOI] [PubMed] [Google Scholar]
- 14.Bigam DL, Pennington JJ, Carpentier A, Wanless IR, Hemming AW, Croxford R, et al. Hepatitis C-related cirrhosis: a predictor of diabetes after liver transplantation. Hepatology. 2000;32:87–90. doi: 10.1053/jhep.2000.8270. [DOI] [PubMed] [Google Scholar]
- 15.el-Zayadi AR, Selim OE, Hamdy H, Dabbous H, Ahdy A, Moniem SA. Association of chronic hepatitis C infection and diabetes mellitus. Trop Gastroenterol. 1998;19:141–144. [PubMed] [Google Scholar]
- 16.Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50–56. doi: 10.1053/jhep.2003.50291. [DOI] [PubMed] [Google Scholar]
- 17.Imazeki F, Yokosuka O, Fukai K, Kanda T, Kojima H, Saisho H. Prevalence of diabetes mellitus and insulin resistance in patients with chronic hepatitis C: comparison with hepatitis B virus-infected and hepatitis C virus-cleared patients. Liver Int. 2008;28:355–362. doi: 10.1111/j.1478-3231.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 18.Butt AA, Khan UA, McGinnis KA, Skanderson M, Kent KC. Co-morbid medical and psychiatric illness and substance abuse in HCV-infected and uninfected veterans. J Viral Hepat. 2007;14:890–896. doi: 10.1111/j.1365-2893.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 19.El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439–1445. doi: 10.1053/jhep.2002.37191. [DOI] [PubMed] [Google Scholar]
- 20.Ledergerber B, Furrer H, Rickenbach M, Lehmann R, Elzi L, Hirschel B et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis. 2007;45:111–119. doi: 10.1086/518619. [DOI] [PubMed] [Google Scholar]
- 21.Akbar DH, Siddique AM, Ahmed MM. Prevalence of Type-2 diabetes in patients with hepatitis C and B virus infection in Jeddah, Saudi Arabia. Med Princ Pract. 2002;11:82–85. doi: 10.1159/000058012. [DOI] [PubMed] [Google Scholar]
- 22.Papatheodoridis GV, Chrysanthos N, Savvas S, Sevastianos V, Kafiri G, Petraki K, et al. Diabetes mellitus in chronic hepatitis B and C: prevalence and potential association with the extent of liver fibrosis. J Viral Hepat. 2006;13:303–310. doi: 10.1111/j.1365-2893.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 23.Picerno I, Di Pietro A, Spataro P, Di Benedetto A, Romano G, Scoglio ME. Is diabetes mellitus a risk factor for HCV infection? Ann Ig. 2002;14:473–477. [PubMed] [Google Scholar]
- 24.Costa LM, Mussi AD, Brianeze MR, Souto FJ. Hepatitis C as a risk factor for diabetes type 2: lack of evidence in a hospital in central-west Brazil. Braz J Infect Dis. 2008;12:24–26. doi: 10.1590/s1413-86702008000100007. [DOI] [PubMed] [Google Scholar]
- 25.Stapleton JT, Bennett K, Bosch RJ, Polgreen PM, Swindells S. Effect of antiretroviral therapy and hepatitis c co-infection on changes in lipid levels in HIV-Infected patients 48 weeks after initiation of therapy. HIV Clin Trials. 2007;8:429–436. doi: 10.1310/hct0806-429. [DOI] [PubMed] [Google Scholar]
- 26.Butt AA, Evans R, Skanderson M, Shakil AO. Comorbid medical and psychiatric conditions and substance abuse in HCV infected persons on dialysis. J Hepatol. 2006;44:864–868. doi: 10.1016/j.jhep.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Lecube A, Hernandez C, Genesca J, Esteban JI, Jardi R, Simo R. High prevalence of glucose abnormalities in patients with hepatitis C virus infection: a multivariate analysis considering the liver injury. Diabetes Care. 2004;27:1171–1175. doi: 10.2337/diacare.27.5.1171. [DOI] [PubMed] [Google Scholar]
- 28.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Hepatology. 2001;33:1554. doi: 10.1053/jhep.2001.0103306le01. [DOI] [PubMed] [Google Scholar]
- 29.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004;40:115–119. doi: 10.1002/hep.20289. [DOI] [PubMed] [Google Scholar]
- 30.Huang JF, Dai CY, Hwang SJ, Ho CK, Hsiao PJ, Hsieh MY, et al. Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication. Am J Gastroenterol. 2007;102:1237–1243. doi: 10.1111/j.1572-0241.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 31.Jain MK, Aragaki C, Fischbach L, Gibson S, Arora R, May L, et al. Hepatitis C is associated with type 2 diabetes mellitus in HIV-infected persons without traditional risk factors. HIV Med. 2007;8:491–497. doi: 10.1111/j.1468-1293.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 32.Noto H, Raskin P. Hepatitis C infection and diabetes. J Diabetes Complications. 2006;20:113–120. doi: 10.1016/j.jdiacomp.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Mehta SH, Strathdee SA, Thomas DL. Association between hepatitis C virus infection and diabetes mellitus. Epidemiol Rev. 2001;23:302–312. doi: 10.1093/oxfordjournals.epirev.a000808. [DOI] [PubMed] [Google Scholar]
- 34.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta-analysis of clinical studies. Am J Transplant. 2005;5:2433–2440. doi: 10.1111/j.1600-6143.2005.01040.x. [DOI] [PubMed] [Google Scholar]
- 35.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 36.Petiti DB. Methods for quantitative synthesis in medicine. Second ed. New York: Oxford University Press; 2000. Meta-analysis, decision analysis and cost-effectiveness analysis. [Google Scholar]
- 37.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 38.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 39.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 40.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arao M, Murase K, Kusakabe A, Yoshioka K, Fukuzawa Y, Ishikawa T, et al. Prevalence of diabetes mellitus in Japanese patients infected chronically with hepatitis C virus. J Gastroenterol. 2003;38:355–360. doi: 10.1007/s005350300063. [DOI] [PubMed] [Google Scholar]
- 42.Boschi-Pinto C, Stuver S, Okayama A, Trichopoulos D, Orav EJ, Tsubouchi H, et al. A follow-up study of morbidity and mortality associated with hepatitis C virus infection and its interaction with human T lymphotropic virus type I in Miyazaki, Japan. J Infect Dis. 2000;181:35–41. doi: 10.1086/315177. [DOI] [PubMed] [Google Scholar]
- 43.Brar I, Shuter J, Thomas A, Daniels E, Absalon J. A comparison of factors associated with prevalent diabetes mellitus among HIV-Infected antiretroviral-naive individuals versus individuals in the National Health and Nutritional Examination Survey cohort. J Acquir Immune Defic Syndr. 2007;45:66–71. doi: 10.1097/QAI.0b013e318031d7e3. [DOI] [PubMed] [Google Scholar]
- 44.Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, et al. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–1063. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- 45.Qureshi H, Ahsan T, Mujeeb SA, Jawad F, Mehdi I, Ahmed W, et al. Diabetes mellitus is equally frequent in chronic HCV and HBV infection. J Pak Med Assoc. 2002;52:280–283. [PubMed] [Google Scholar]
- 46.Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166:196–203. doi: 10.1093/aje/kwm061. [DOI] [PubMed] [Google Scholar]
- 47.Marzouk D, Sass J, Bakr I, El Hosseiny M, Abdel-Hamid M, Rekacewicz C, et al. Metabolic and cardiovascular risk profiles and hepatitis C virus infection in rural Egypt. Gut. 2007;56:1105–1110. doi: 10.1136/gut.2006.091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyamoto H, Moriishi K, Moriya K, Murata S, Tanaka K, Suzuki T, et al. Involvement of the PA28gamma-dependent pathway in insulin resistance induced by hepatitis C virus core protein. J Virol. 2007;81:1727–1735. doi: 10.1128/JVI.01683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawaguchi T, Nagao Y, Tanaka K, Ide T, Harada M, Kumashiro R, et al. Causal relationship between hepatitis C virus core and the development of type 2 diabetes mellitus in a hepatitis C virus hyperendemic area: a pilot study. Int J Mol Med. 2005;16:109–114. [PubMed] [Google Scholar]
- 50.Masini M, Campani D, Boggi U, Menicagli M M, Funel N, Pollera M, et al. Hepatitis C virus infection and human pancreatic beta-cell dysfunction. Diabetes Care. 2005;28:940–941. doi: 10.2337/diacare.28.4.940. [DOI] [PubMed] [Google Scholar]
- 51.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, et al. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 52.Petrides AS, Vogt C, Schulze-Berge D, Matthews D, Strohmeyer G. Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology. 1994;19:616–627. doi: 10.1002/hep.1840190312. [DOI] [PubMed] [Google Scholar]
- 53.Taura N, Ichikawa T, Hamasaki K, Nakao K, Nishimura D, Goto T, et al. Association between liver fibrosis and insulin sensitivity in chronic hepatitis C patients. Am J Gastroenterol. 2006;101:2752–2759. doi: 10.1111/j.1572-0241.2006.00835.x. [DOI] [PubMed] [Google Scholar]
- 54.Harrison SA. Correlation between insulin resistance and hepatitis C viral load. Hepatology. 2006;43:1168–1169. doi: 10.1002/hep.21125. [DOI] [PubMed] [Google Scholar]
- 55.Petit JM, Bour JB, Galland-Jos C, Minello A, Verges B, Guiguet M, et al. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol. 2001;35:279–283. doi: 10.1016/s0168-8278(01)00143-x. [DOI] [PubMed] [Google Scholar]
- 56.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44 1 Suppl:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Soriano V, Puoti M, Garcia-Gasco P, Rockstroh JK, Benhamou Y, Barreiro P, et al. Antiretroviral drugs and liver injury. AIDS. 2008;22:1–13. doi: 10.1097/QAD.0b013e3282f0e2fd. [DOI] [PubMed] [Google Scholar]
- 58.Song F, Easterwood A, Gilbody S, Duley L, Sutton AJ. Publication bias. In: Stevens A, Abrams K, Braziera J, Fitzpatrick R, Lilford R, editors. Handbook of Research Methods for Evidence-based health care - Insights from the NHS HTA Programme. London: Sage Publications; 2001. [Google Scholar]
- 59.Simo R, Lecube A, Genesca J, Esteban JI, Hernandez C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29:2462–2466. doi: 10.2337/dc06-0456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.