Abstract
We have previously shown that keratinocyte-specific deletion of Smad4, a TGFβ/Activin/BMP signaling mediator, results in a progressive alopecia. To further assess the molecular mechanisms of Smad4 loss-mediated alopecia, we examined expression levels of key molecules associated with hair follicle differentiation in Smad4-deleted skin. Among them, Desmoglein 4 (Dsg4) was down-regulated in Smad4-deleted skin prior to the onset of hair follicle abnormalities with gradual depletion coinciding with hair follicle degeneration. Chromatin immunoprecipitation (ChIP) assay showed that Smad4, together with the BMP mediators Smad1 and Smad5, but not the TGFβ/Activin mediators Smad2 or Smad3, bound to the Smad Binding Element (SBE) of the Dsg4 promoter. A Dsg4 reporter assay revealed that Smad4 was required for the maximal transactivation of Dsg4 in cooperation with Smad1 and Smad5. Mutating the SBE of the Dsg4 promoter abrogated Smad4 transactivation of Dsg4. Furthermore, BMP ligands, but not ligands of TGFβ and Activin, induced endogenous Dsg4 expression. Our data demonstrate that in the presence of Smad4, BMP signaling participated in transcriptional regulation of Dsg4. Thus, Smad4 loss-associated Dsg4 depletion contributed, at least in part, to hair follicles degeneration in Smad4 deficient skin.
Keywords: Smad4, Hair Follicle, Desmoglein-4, TGFβ, BMP
Introduction
Epidermal development in mice begins in embryos around E9 with a single layer of the epithelium, and continues with stratification and epidermal barrier formation before birth (Fuchs, 2007). Thereafter, the epidermis undergoes constant self renewal throughout the life of the animal. Epidermal appendages include hair follicles and sebaceous glands. Hair follicle development begins around E14.5 in mice and continues with hair follicle differentiation and production of the hair shaft 1 week after birth (Millar, 2002). Hair follicle cells differentiate into the layers of the outer root sheath (ORS), the inner root sheath (IRS), and the hair shaft. Along with hair follicle morphogenesis, sebaceous glands develop from cells residing in the “bulge” area at the upper portion of the hair follicle. Postnatal hair follicles undergo regenerative cycles of growth (anagen), regression (catagen) and rest (telogen). Following telogen, a new hair shaft is generated adjacent to the previous one through the re-initiation of anagen [for review see (Alonso and Fuchs, 2006)]. Key transcription factors shown to be involved in hair follicle formation and differentiation include Gata-3, Msx-2, FoxN1 among others. Loss of these molecules results in failure of hair shaft formation (Johns et al., 2005; Kaufman et al., 2003; Satokata et al., 2000). Another important molecule in making a normal differentiated hair shaft is Desmoglein-4 (Dsg4). Among the different Dsg isoforms, Dsg4 is the only isoform which is highly expressed in the hair cortex (Green and Simpson, 2007). Loss of function mutations in Dsg4 in humans, rats and mice result in balding due to aberrant hair shaft production (hypotrichosis) (Bazzi et al., 2005).
The process of skin morphogenesis, differentiation and renewal are tightly controlled by multiple signal transduction pathways. Among them, signaling from TGFβ/Activin/BMP, which requires Smad transcription factors as mediators, plays an important role (Li et al., 2003). In the TGFβ family, TGFβ2 has been shown to be required for hair follicle development (Foitzik et al., 1999), whereas TGFβ1 is required for the hair follicle to enter into the catagen phase (Foitzik et al., 2000). Activins have also been shown to play crucial roles in hair follicle development, as knocking out the activin ligand, expressing the activin antagonist, Follistatin, or expressing a dominant negative Activin receptor IB, all give rise to abnormal hair follicle development or cycling (Bamberger et al., 2005; Matzuk et al., 1995a; Matzuk et al., 1995b). In the BMP family, a recent study has revealed that dermal derived BMP2 and BMP4 are cyclically expressed, which regulate stem cell activation during hair regeneration (Plikus et al., 2008). Studies have shown that overexpression of BMP ligands promotes differentiation and that BMP antagonists, such as Noggin, are required to maintain the undifferentiated state of epidermal progenitors [for review see (Botchkarev and Sharov, 2004)]. Consistently, keratinocyte-specific deletion of the type 1A BMP receptor (also known as Activin Like Kinase 3; ALK-3) results in alopecia due to failure of IRS differentiation and hair shaft formation (Andl et al., 2004; Kobielak et al., 2003; Ming Kwan et al., 2004; Yuhki et al., 2004).
It has been shown that TGFβ/activin signals mainly through Smad2 and Smad3, whereas BMP signals mainly through Smad1 and Smad5 (Li et al., 2003). We have previously deleted Smad4, a common Smad that interacts with both TGFβ/activin-specific Smads and BMP-specific Smads, by crossing Smad4 floxed mice with MMTV-Cre mice (Qiao et al., 2006). In addition to directing Cre expression in mammary epithelia, the MMTV promoter targets Cre expression in keratinocytes, around E13.5 (Wagner et al., 2001). These conditional Smad4 knockout mice develop epidermal hyperplasia, progressive hair loss beginning at the first catagen phase on P16, and spontaneous skin tumor formation later in life (Qiao et al., 2006). Similar phenotypes have also been reported in keratinocyte-specific Smad4 knockout mice when using a truncated keratin 5 (K5) targeting vector, which targets Cre expression in keratinocytes around E13.5 (Yang et al., 2005). It remains to be determined whether the loss of hair follicle differentiation markers is the cause or the consequence of hair follicle degeneration in Smad4 null keratinocytes. Further, it remains to be determined whether Smad4 loss affects epidermal differentiation in addition to epidermal hyperproliferation, particularly when it is lost at stages critical for epidermal development. In the present study, we used a K5 promoter to target the CrePR1 transgene that allows inducible Cre expression in keratinocytes including epidermal stem cells as early as E10.5, when a single epithelial layer begins transitioning into a stratified epidermis (Han et al., 2006; Zhou et al., 2002). When we deleted Smad4 in K5.CrePR1/Smad4flox bigenic mice, we found that Smad4 deletion in keratinocytes either in embryos or after birth did not affect epidermal differentiation. However, Smad4 deletion resulted in degeneration of the hair follicles. We then focused on the identification of direct transcriptional targets of Smad4 that are imperative for hair follicle/hair shaft integrity. We found that Dsg4 is a Smad4 target gene and that loss of Dsg4 expression in Smad4 knockout keratinocytes contributed at least in part to hair follicle degeneration.
Materials and methods
Animals
Smad4 homozygous floxed (f/f) mice (Yang et al., 2002) were mated with K5.Cre.PR1 mice (Zhou et al., 2002). Smad4 deletion in keratinocytes was achieved by daily i.p. injection of 100µg/kg RU486 with 0.5mg progesterone in pregnant mice bred from the above two lines at the time points specified in the Results section, or through topical application of 20µg RU486 to neonatal or adult mouse skin (specified in the Results section), once a day for 5 Days. Genotypes of these mice were identified by PCR as previously described to detect the wildtype, floxed allele, the CrePR1 transgene, and cre-mediated Smad4 deletion (Qiao et al., 2006).
Histology and immunostaining
Skin samples were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Immunofluorescence (IF) or immunohistochemistry (IHC) was performed on OCT-embedded frozen sections or paraffin embedded sections as previously described (Wang et al., 1997). Primary antibodies used for immunostaining included: Adipophilin (1:500) and K14 (1:500) from RDI-Fitzgerald; K1 (1:250), K6 (1:250), Loricrin (1:500), and Filaggrin (1:500) from Covance; AE13 (1:100) from Abcam; Gata3 (1:50) from Santa Cruz; Smad4 (1:200) from Upstate; and E-cadherin (1:200) from BD. The AE15 antibody was a gift from Dr. T.T. Sun. Dsg4 staining was performed using mouse monoclonal anti-Dsg4 (18G8, 1:10) as previously described (Bazzi et al., 2006). Alexa Fluor 488 or 594 Secondary antibodies were purchased from Invitrogen.
Real Time PCR
RNA from the dorsal skin was extracted in Trizol and further purified using an RNeasy column with on-column DNase treatment (Qiagen). 50ng of RNA per reaction was analyzed and then amplified with Brilliant II 1-Step QPCR reagent (Stratagene). Analysis was carried out using MxPro Software V4.0 (Stratagene). Samples were analyzed in triplicate and normalized to an internal VIC-labeled Taqman probe for GAPDH. Other probes were FAM-labeled Taqman Assays as follows: Dsg1a: Mm00809994_s1, Dsg2: Mm00514608_m1, Dsg3: Mm00659652_m1, Dsg4: Mm00812608_m1, Msx2: 00442992_m1, Gata-3: 00484683_m1, Krt31: Mm00657991_gH HoxC13: Mm00802798_m1, FoxN1: Mm00433946_m1, Dlx3: Mm00438428_m1, Gli-1: Mm00494645_m1.
ChIP
Fresh skin was removed at postnatal day 6 (anagen), chopped and disrupted using a Dounce homogenizer, and cross-linked with 4% formaldehyde for 20 minutes. The cross-linked chromatin was then sheared using the ChIP-IT Express Enzymatic kit (Active Motif) for 10 minutes. Fifteen µg of sheared chromatin was immunoprecipitated with 1µg of antibody at 4°C for overnight, and precipitated DNA was eluted in 50 µl H2O. Antibodies used for immunoprecipitation included: RNA Pol II, rabbit anti-Smad3, and rabbit anti-Smad4 from Upstate; mouse monoclonal antibodies for Smad1 and Gata-3 were from Santa Cruz; rabbit antibodies for Smad2 and Smad5 from Zymed; and rabbit anti-phospho-Smad1/5 from Cell Signaling. PCR primers encompassing the SBE of the mouse Dsg4 primers are as follows: FWD-5’ ACCCCCTGAAATAAACTGGAGC and REV-5’ GGTAGGTGCTATGGTGACTAAACCC. PCR Primers encompassing a region of -5kb of the promoter, which does not contain SBE, were used as a negative control: FWD-5’ GCTATCGCTGAAACAAAGGTCACAG and REV-5’ TGATGAGGGACTCTGGCTAATGC. DNAs precipitated with individual antibodies were used for PCR. Primers for the BMP responsive element in the Msx2 promoter (Brugger et al., 2004), and for the TGFβ responsive Snail1 promoter, were used as controls for binding of Smad1/5 or Smad2/3, respectively.
Luciferase (Luc) Assay, Site-Directed Mutagenesis, Primary Cell Culture, Growth Factor Treatments
To avoid interference of high levels of endogenous Smad4 with Luc assay, K5.Smad4−/−keratinocytes were used for Luc assay. Primary mouse epidermal keratinocytes were isolated following suggested media protocol (CellNTec/Chemicon). Primary mouse hair follicle keratinocytes were isolated from neonatal K5.Smad4−/− mice (in C57BL/6 background) as previously described (Han et al., 2006) in CnT-07 media (Chemicon). Briefly, following dermal-epidermal separation of neonatal skins, dermal pieces were finely minced and digested with collagenase for 1 hour at 37 degrees. These were then centrifuged in a 4% Ficoll gradient before plating. Prior to transfection, cells were placed in CnT-02. Keratinocytes were transfected with full length individual Smad expression constructs at 50ng/well each (gifts from XH Feng), 500ng of a firefly luciferase reporter construct containing 3kb upstream mouse Dsg4 promoter in PGL4.26 (Promega), and 10ng of the Renilla luc plasmid PGL4.74 (Promega). For site-directed mutagenesis, the SBE sequence CTGT was mutated to TTGA. Twenty-four hours after transfection, cells underwent a calcium switch (1.2 mM calcium in CnT-02 media) for 48 hours to induce Dsg4 promoter activity. Luciferase activity was then normalized to Renilla luciferase activity. Lysates were collected in passive lysis buffer and analyzed on a Glomax™ luminometer using the dual luciferase assay (Promega). Induction of endogenous Dsg4 mRNA was performed by culturing primary keratinocytes isolated from neonatal C57BL/6 (Jax) mice in CnT-02 media in high calcium (1.2mM) for 48 hours with or without treatment of 10ng/mL of TGFβ2, Activin A, and BMP-2 (R&D Systems). Cells were then collected for RNA extraction as previously described (Han et al., 2006). Dsg4 mRNA levels were examined by qRT-PCR as described above.
Statistics
Significant differences between the values obtained in each assay on samples from various genotypes were determined using the Student’s t-test and expressed as mean ± standard deviation of the mean.
Results
Smad4 deletion in keratinocytes resulted in hair follicle degeneration and hypertrophic sebaceous glands
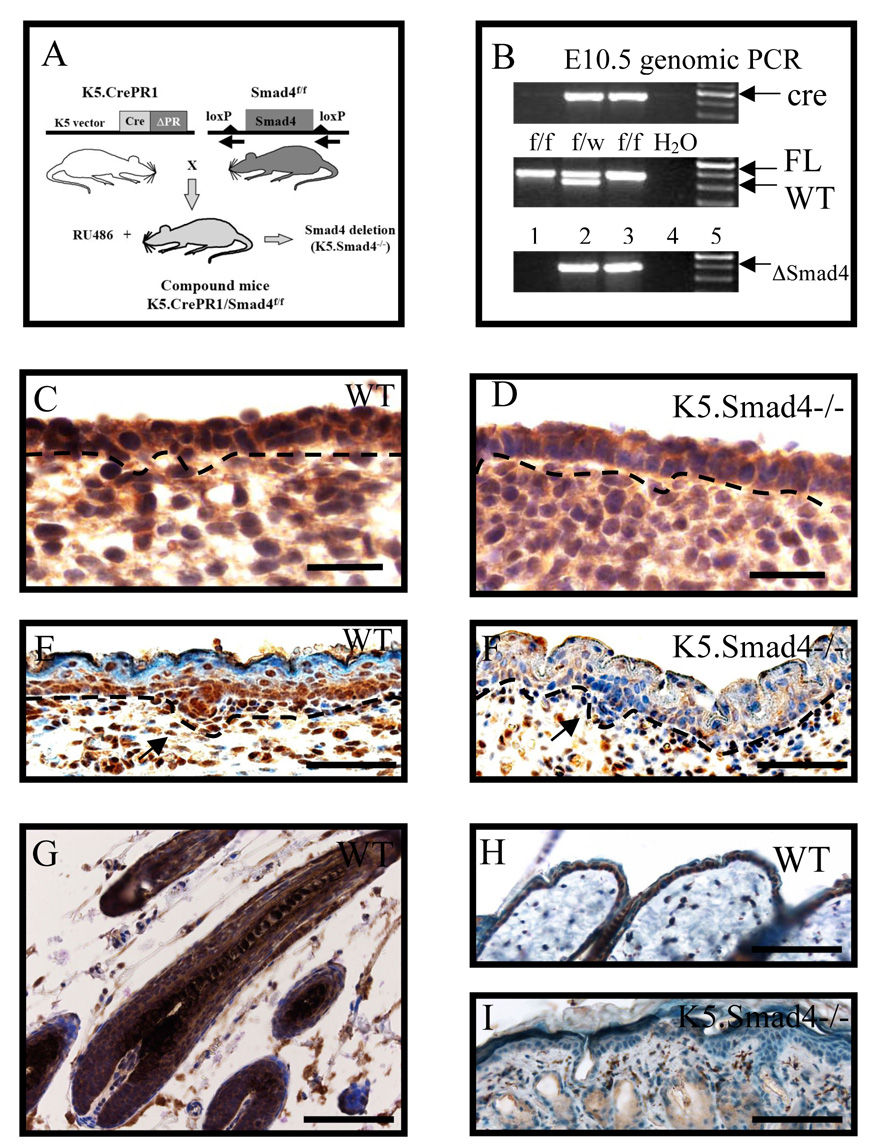
We cross-bred K5-CrePR1 mice (Zhou et al., 2002) with Smad4 floxed mice (Qiao et al., 2006). Smad4 deletion in keratinocytes was induced by either injection of RU486 to pregnant mice from the above mating, or by topical application of RU486 to bigenic mouse skin after birth (designated as K5.Smad4−/−, Fig. 1A). The earliest RU486 application began on E9.5, and the embryos were taken on E10.5, E12.5 and E15.5 to examine Smad4 expression patterns. Smad4 gene deletion in embryonic skin was detected as early as E10.5 (Fig. 1B). Depletion of existing Smad4 protein, as detected by immunostaining, took a few more days. By E12.5, Smad4 protein in wildtype skin was detected uniformly in the nucleus of epidermal cells and also in some of the stromal cells (Fig. 1C). In contrast, Smad4 nuclear positive cells were significantly reduced in K5.Smad4−/− epidermis (Fig. 1D). By E15.5, Smad4 nuclear staining in wildtype skin was prominent in the basal layer of the epidermis and newly formed hair follicle placodes (Fig. 1E). At this stage, uniform depletion of Smad4 protein in K5.Smad4−/− epidermis became obvious, and only stromal cells had persistent Smad4 nuclear staining (Fig. 1F). To verify Smad4 deletion indeed occurs in epidermal stem cells, we applied RU486 to neonatal bigenic skin daily for 5 days, and examined Smad4 expression pattern on P35 skin, when the epidermis had gone through at least two cycles of self renewal and hair follicles had entered into the second postnatal anagen phase. Nuclear Smad4 staining was detected in the epidermis and throughout all layers of the hair follicle (Fig. 1G). At this stage, hair follicles in K5.Smad4−/− skin were degenerated into cysts. The epidermis, hair follicle cysts and sebaceous glands of K5.Smad4−/− skin were negative for Smad4 nuclear staining, whereas Smad4 positive stromal cells were still detectable (Fig. 1H, I). This result indicates that epidermal stem cells harboring Smad4 deletion can repopulate the entire epidermis and hair follicles.
Fig. 1. Keratinocyte-specific Smad4 deletion.
A: Schematic demonstrating generation of K5.Smad4−/− mice by mating K5.CrePR1 mice with Smad4 floxed mice (Smad4f/f), and applying RU486 to bigenic mouse skin. B: Genomic PCR results from E10.5 mouse tail DNA indicate genotype of Smad4 alleles. Lanes 2 and 3 = K5.CrePR1/Smad4f/wt and K5.CrePR1/Smad4f/f, which were heterozygous and homozygous for the floxed allele (FL), respectively and both were positive for the CrePR1 transgene (cre). Smad4 deletion (ΔSmad4) was induced by RU486 application to day 9.5 pregnant bigenic mice. C to I: Immunostaining for Smad4 (brown) of skins of E12.5 (C and D), E15.5 (E and F), and P35 (G to I). Note that Smad4 nuclear staining was uniform in WT E12.5 epidermis, but was patchy and reduced in K5.Smad4−/− E12.5 epidermis. Smad4 nuclear positive cells were predominantly located in the basal layer of the epidermis and the hair follicle placode (arrow), and stromal cells in WT E15.5 skin. Smad4 protein was ablated in the epidermis and placode (arrow) in K5.Smad4 −/− skin, but was still detected stromal cells. In P35 WT skin, Smad4 staining was positive in all layers of the anagen follicle (G), the epidermis (H), and some stromal cells (G and H). In contrast, in P35 K5.Smad4−/− skin, Smad4 staining was negative in the epidermis, degenerated hair follicle cysts and sebaceous glands (I). The stroma remained Smad4 positive cells. Light brown staining in K5.Smad4−/− epidermis, hair follicle cysts and sebaceous glands represents background staining. Scale bars = C–D: 25µm E–F: 50µm G–I: 100µm.)
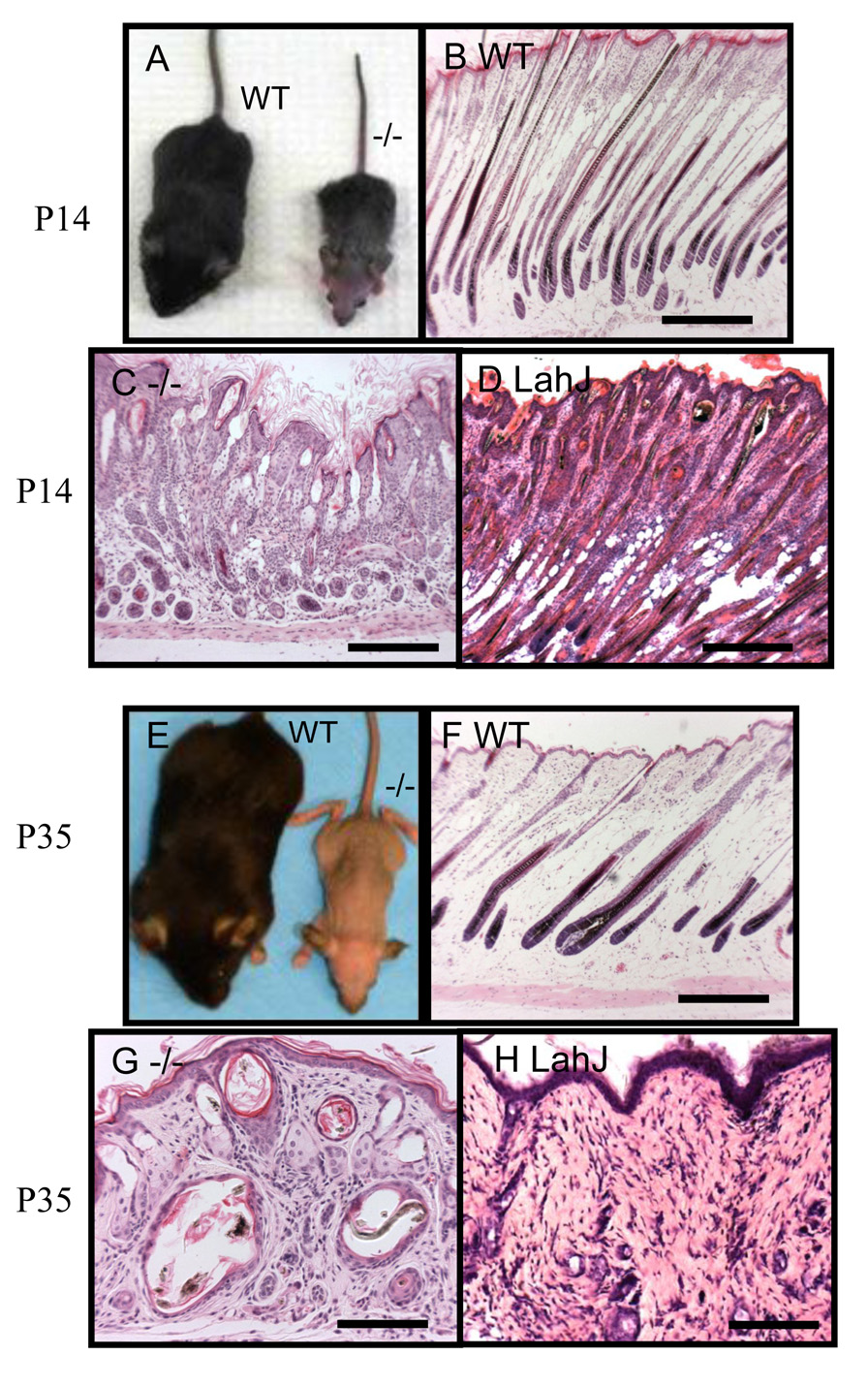
Regardless of Smad4 deletion in embryos, K5.Smad4−/− mice were born normally and showed no visible phenotype in neonates (data not shown). Neonatal K5.Smad4−/− skin displayed normal thickness including dermal and epidermal compartments. Early epidermal differentiation markers, such as keratin 1, (K1), and terminal differentiation markers, such as loricrin and filaggrin, were not altered in K5.Smad4−/− epidermis at all ages even when adult mice developed epidermal hyperplasia (data not shown). However, K5.Smad4−/− mice developed gross hair follicle abnormalities around P6. At this time point, K5.Smad4−/− pups were slightly smaller than their wildtype siblings (Fig. 2A), possibly due to esophageal hyperplasia as previously reported (Yang et al., 2005). Histologically, hair follicles in P6 K5.Smad4−/− skin were shorter and less differentiated (Fig. 2C) than wildtype follicles (Fig. 2B). Some K5.Smad4−/− hair follicles underwent partial degeneration (Fig. 2C, 2D). A similar phenotype was also observed in Dsg4 mutant hair follicles at the same stage (Fig. 2E). By P10, K5.Smad4−/− mice exhibited retarded growth in comparison with their littermates (Fig. 2F). Hair follicles remained shorter in K5.Smad4−/− skin (Fig. 2H) than in wildtype skin (Fig. 2G). Degenerated hair follicles and abnormal hair shafts were broadly observed in P10 K5.Smad4−/− skin (Fig. 2H). Similarly, Dsg4 mutant hair follicles also underwent broad degeneration at this stage (Fig. 2I). By P14, when hair follicles on the head region transitioned from the late anagen to the catagen phase (Fig. 3B), K5.Smad4−/− mice exhibited hair loss in this anterior region (Fig. 3A), which progressed as anterior-to-posterior total alopecia correlated with the anterior-to-posterior order of the catagen phase. At this stage, K5.Smad4−/− hair follicles were degenerated to form canals (Fig. 3C), which were also seen in Dsg4 mutant hair follicles (Fig. 3D). However, hair follicle degeneration in K5.Smad4−/− skin appeared more severe than Dsg4 mutant skin, with shorter hair follicles but prominent sebaceous glands (Fig. 3C). K5.Smad4−/− hairs did not grow back after the first catagen phase. After weaning at P21, K5.Smad4−/− mice were provided with soft food. However, significant retarded growth continued in K5.Smad4−/− mice, which rendered a necessity of euthanizing them around P35. At this stage, K5.Smad4−/− mice were completely hairless (Fig. 3E). Histology shows that while wildtype hair follicles re-entered into the anagen phase (Fig. 3F), K5.Smad4−/− hair follicles further degenerated into cysts, which harbored inward hair shaft growth or degenerated hair shafts (Fig. 3G). Similar phenotypes were also observed in Dsg4 mutant skin with the exception of a lack of enlarged sebaceous glands (Fig. 3H). Topical application of RU486 to P1 bigenic skin gave rise to the same skin phenotypes except without the severe retarded growth phenotype observed when RU486 was administered in utero (data not shown).
Fig. 2. Early onset of hair follicle degeneration in K5.Smad4−/− skin.
A: A P6 K5.Smad4−/− mouse (−/−) was slightly smaller than its wildtype (WT) littermate. The arrow in C points to a degenerated hair follicle, which is enlarged in D. E: an example of a degenerated hair follicle from a P8 Dsg4 mutant (LahJ) skin for comparison. The “[“ symbols in D and E highlight the degenerated region of the hair follicle. F: A P10 K5.Smad4 −/− mouse (−/−) showed a smaller size than its wildtype (WT) littermate. G–I: Histology of P10 skins from WT (G), K5.Smad4−/− (H, −/−) and Dsg4 mutant (I, LahJ) mice. Arrows in H and I point to examples of degenerated hair follicles. Scale bars: B, C, G: 100µm; H, I: 150µm.
Fig. 3. Loss of Smad4 results in progressive alopecia.
A: Anterior to posterior alopecia in P14 K5.Smad4−/− skin (−/−). When wildtype (WT) hair follicles were preparing to enter catagen (B), K5.Smad4 −/− (−/−) hair follicles (C) were shorter than WT follicles in B. K5.Smad4 −/− (−/−) hair follicles formed canals and lacked hair shafts, which was a more severe phenotype than Dsg4 mutant (LahJ) hair follicle degeneration shown in D. K5.Smad4−/− skin also exhibited prominent sebaceous glands, which was not shown in P14 Dsg4 mutant (LahJ) skin (D). E: Complete hairless of P35 K5.Smad4−/− mouse (−/−). F: Wildtype (WT) hair follicles entered into the second anagen growth phase while the Smad4 −/− hair follicles were degenerated (G). H: Adult Dsg4 mutant (LahJ) skin showing that all hair follicles were degenerated into cysts. Scale bars: B–D and F: 300µm. G and H:150 µm.
To determine if the enlarged sebaceous glands found in K5.Smad4−/− skin are a consequence of hair follicle degeneration or represent a direct effect of Smad4 on sebaceous gland development, we performed immunofluorescence staining for Adipophilin, a marker for sebocytes (Heid et al., 1998), at stages before and after sebaceous gland development. The number of sebocytes was comparable between wildtype and K5.Smad4−/− skin up until P6 (data not shown), suggesting that Smad4 loss did not accelerate sebaceous gland formation. However, sebaceous glands in adult K5.Smad4−/− skin became hypertrophic and also contained larger numbers of sebocytes in each gland, when compared with those in wildtype skins (Supplementary Fig. 1B and D compared to A and C). PCNA staining showed increased proliferative sebocytes in K5.Smad4−/− skin (Supplemental Fig. 1F) when compared with wildtype skin (Supplemental Fig. 1E), suggesting that increased sizes and numbers of sebaceous glands were the consequence of increased sebocyte proliferation.
Smad4 loss resulted in failure in maintaining hair shaft integrity
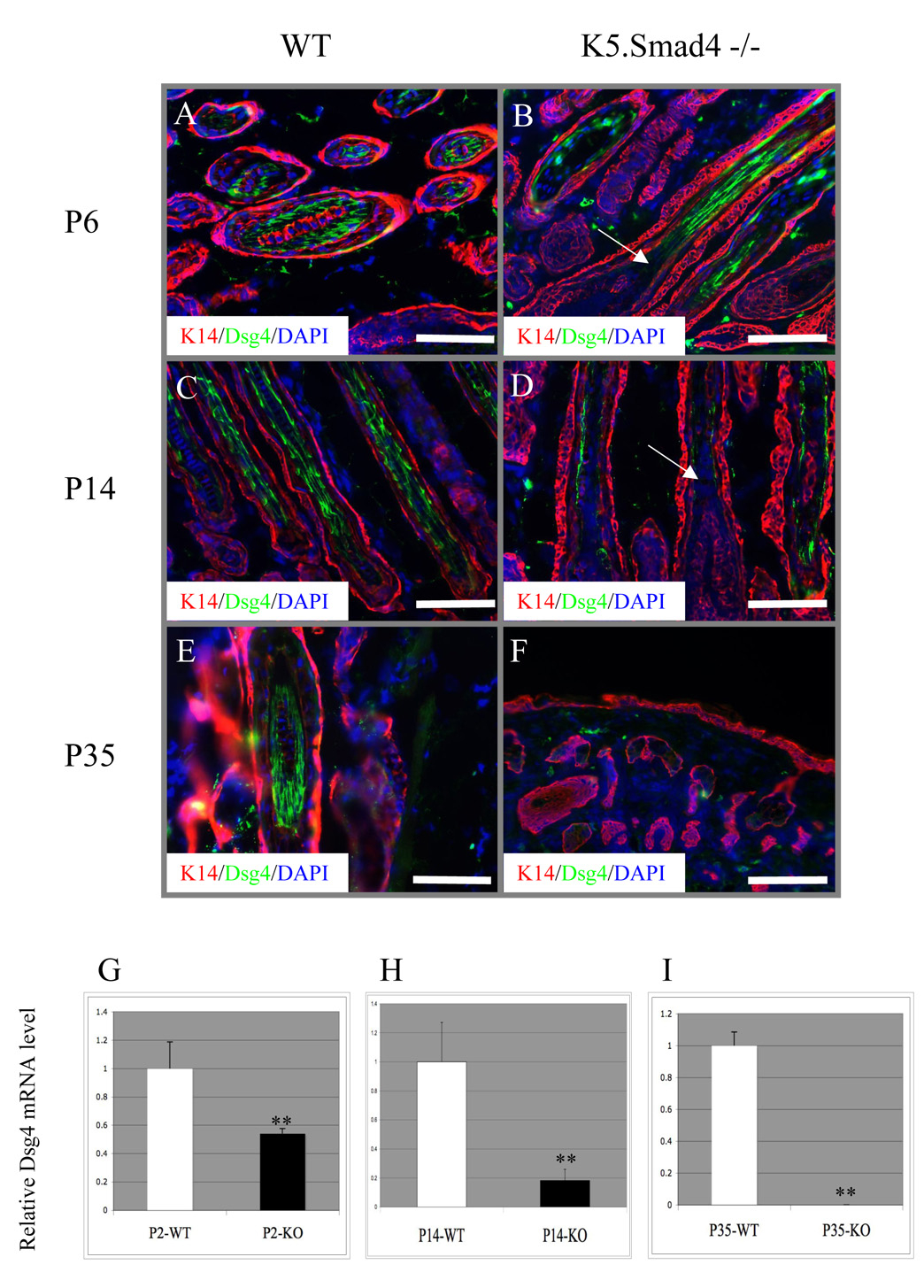
To determine if hair follicle degeneration in K5.Smad4−/− skin is due to defects in hair follicle differentiation, we examined several known hair follicle differentiation markers. Keratin K6, which stained in the companion layer of ORS of wildtype hair follicles (Supplementary Fig. 2), also stained in ORS of K5.Smad4−/− hair follicles at all stages, including the layer surrounding the hair follicle cyst (Supplementary Fig. 2). In wildtype hair follicles, the AE13 antibody stained for cortical acidic keratins, the AE15 (trichohyalin) antibody stained the IRS and medulla cells (Supplementary Fig. 2). These markers were also stained in the same compartments of K5.Smad4−/− hair follicles (e.g., P6) prior to hair follicle collapse (Supplementary Fig. 2), but could not be detected after hair follicles were degenerated into canals and cysts at P35 (Supplementary Fig. 2). Immunofluorescence staining for Dsg4 revealed that Dsg4 was predominantly expressed in the precortex, cortex and cuticle of the hair follicle in wildtype skin (Fig. 4A, C, E), but not in the differentiated layers of the epidermis (data not shown). When the new hair shaft was formed on P6, Dsg4 protein exhibited patchy staining in K5.Smad4−/− hair follicles (Fig. 4B). By P14, it was obvious that hair shafts in K5.Smad4−/− skin have begun to collapse and Dsg4 was significantly reduced. Additionally, K5.Smad4−/− hair follicles, even without obvious degeneration by histology, still exhibited reduction in Dsg4 staining when compared to wild type hair follicles. (Fig. 4D). When the hair follicle eventually collapsed, a complete absence of Dsg4 and inability to produce hair shafts were observed in K5.Smad4−/− skin (Fig. 4F).
Fig. 4. Loss of Dsg4 in K5.Smad4−/−hair follicles.
A–F: Immunofluorescence of Dsg4 (green) counterstained with K5 (red). Arrows in B and D point to the loss of Dsg4 protein in the precortex region of K5.Smad4−/− hair follicles. Scale bars: A, E=50µm B, C & D = 100µm F = 200µm. G–H: qRT-PCR of dsg4 transcripts from K5.Smad4−/− dorsal skins (KO) compared to their wildtype (WT) littermate skins (3 skins/group). **: p<0.01.
Smad4 loss resulted in transcriptional downregulation of Dsg4 prior to hair follicle degeneration
To determine if reduced Dsg4 protein in K5.Smad4−/− skin represents a direct effect of Smad4 on Dsg4 expression, we examined Dsg4 mRNA expression at different stages of K5.Smad4−/− skin. In comparison with wildtype skin at the same time points, the Dsg4 mRNA level in K5.Smad4−/− skin was reduced by 50% on P2, further reduced by 80% on P14, and became undetectable by P35 (Fig. 4G–I). To determine if loss of Smad4 specifically affects Dsg4 or affects Dsg family members in general, we examined other Dsg family members. To exclude molecular changes that are secondary to degenerated hair follicles, we compared P2 wildtype and Smad4−/− skin. At this stage, no delayed hair follicle development or hair follicle degeneration was observed in Smad4−/− skin. We found that mouse Dsg-1α, -2 and -3 were not reduced in K5.Smad4−/− skin (Supplemental Fig. 3). We also examined expression of several other possible Smad target genes that have been shown to regulate hair follicle formation and differentiation and are potential TGFβ/activin/BMP target genes, such as FoxN1, HoxC13, Msx2, GATA3, and Gli1 (Blokzijl et al., 2002; Dennler et al., 2007; Hussein et al., 2003). We did not find reduction of these genes in Smad4−/− skin prior to hair follicle degeneration (Supplemental Fig. 3). In addition, Krt31 (Ha1), a marker for hair shafts, was expressed in Smad4−/− skin at a level similar to wildtype skin at P6 (Supplemental Fig. 4), even when Smad4−/− skin began to show degenerated hair follicles. Further, we examined expression patterns of the E-cadherin protein, another adhesion molecule critical for epidermal and hair follicle integrity (Tinkle, et al.,2004). We found that E-cadherin staining persisted in the cell membrane of K5.Smad4−/− epidermis and hair follicles (Supplemental Fig. 4).
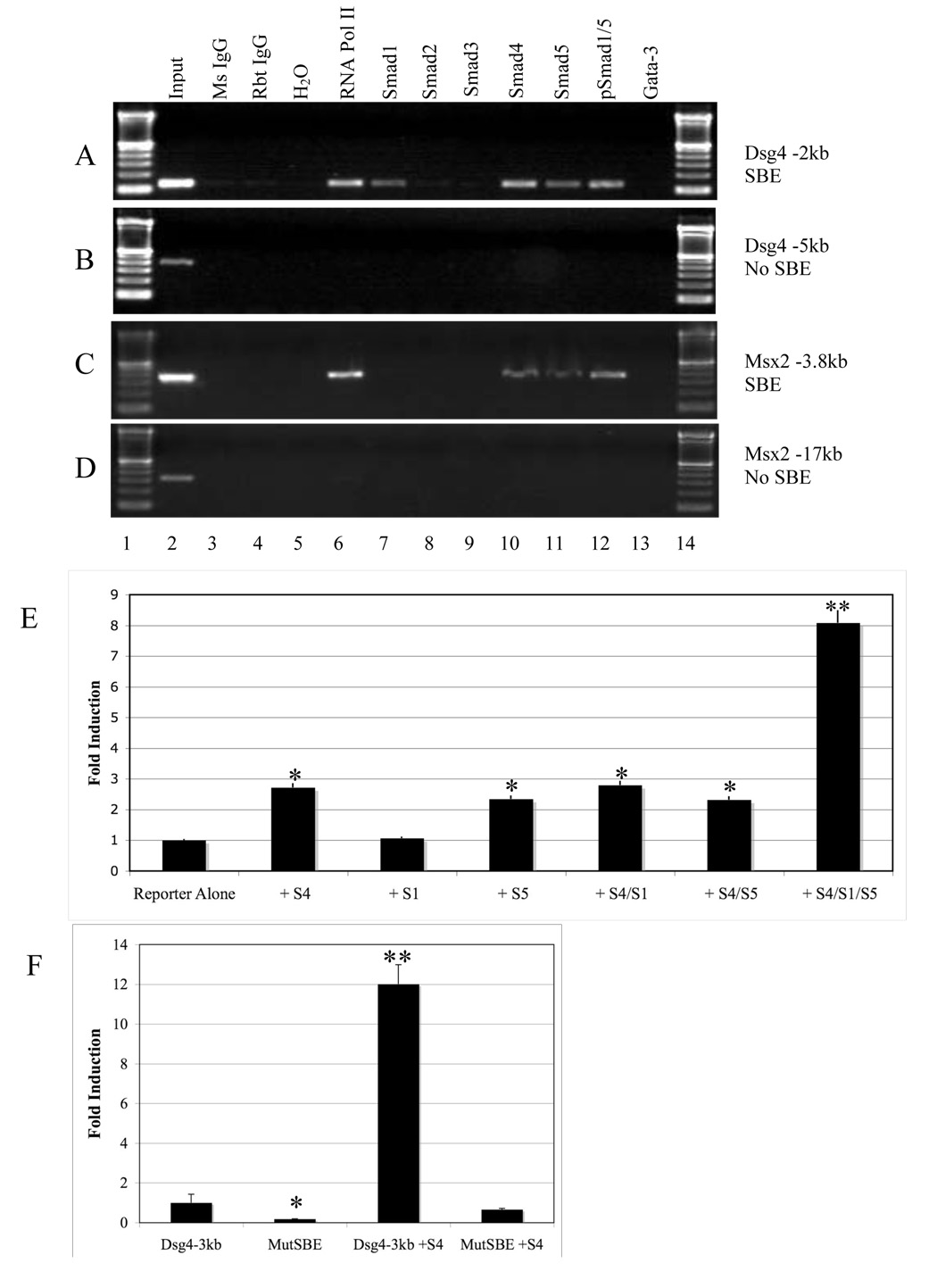
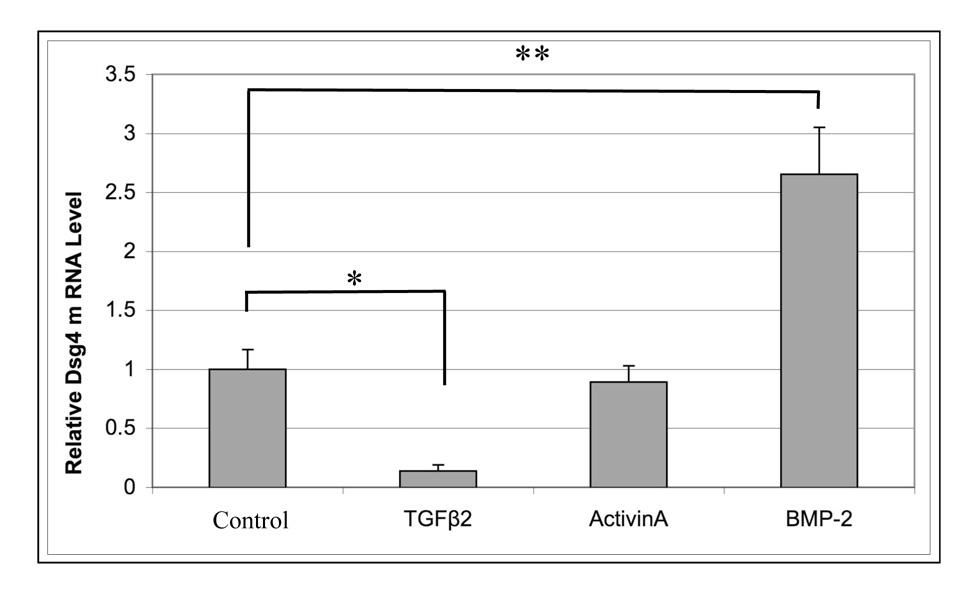
To further determine if Dsg4 was a transcriptional target of Smad4, we analyzed the mouse Dsg4 promoter sequence and found a SBE exists -2173bp upstream of Dsg4 translational start site (TSS). We next sought to identify if endogenous Smads bind to the SBE of the Dsg4 promoter in vivo. We performed in vivo chromatin immunoprecipitation (ChIP) from P6 dorsal skin of wildtype C57BL/6 mice. Smad1, 4 and 5 bound to the SBE of both the Dsg4 promoter and a known SBE in the Msx2 promoter (Fig. 5A and C). In contrast, Smad2 and Smad3 bound to the SBE of the Snail promoter (data not shown) but not the Dsg4 promoter (Fig. 5A). To test if Smad1/4/5 binding results in transactivation of Dsg4 expression, we performed a Dsg4-luc reporter assay, using a 3kb mouse Dsg4 promoter sequence upstream of the Dsg4 TSS. In primary epidermal keratinocytes derived from K5.Smad4−/− newborn skins, we switched the culture medium to high calcium (1.2mM) to induce promoter activity of Dsg4, and transfected Dsg4-luc together with full-length Smad1, 4, or/and 5 expression vectors. Among individual Smads transfected, Smad4 and Smad5 individually resulted in a 3 fold increase in Dsg4-luc activity, whereas Smad1 had no effect on Dsg4-luc activity (Fig. 5). However, combination of Smad1, 4, and 5 resulted in an 8-fold increase in Dsg4 promoter activity (Fig. 5E). This result suggests that Dsg4 transcription is predominantly BMP-dependent and requires Smad4 for full transactivation. Next, we examined if Smad4 induction of Dsg4 occurs in hair follicle cells as well, as these cells predominantly express Dsg4 in mice. Primary mouse hair follicle keratinocytes from neonatal K5.Smad4−/− mice (in C57BL/6 background) were isolated and cultured as previously described (Han et al., 2006). After switching the culture medium to high calcium (1.2 mM) to induce promoter activity of Dsg4, we transfected Smad4 expression vector. Smad4 induced Dsg4 luc activity to a level (Fig. 5F) similar to that shown in cultured epidermal keratinocytes (Fig. 5E). In contrast, mutating the SBE site within the Dsg4 reporter abolished both basal and Smad4-induced luc activity (Fig. 5F), suggesting that Smad4 binding to the SBE is necessary for regulating Dsg4 expression. To further test if this is the case for endogenous Dsg4, we treated primary keratinocytes with different TGFβ family member ligands, and examined endogenous Dsg4 mRNA levels. TGFβ2, which is essential for hair follicle development (Foitzik et al., 1999), resulted in a reduction of Dsg4 mRNA level (Fig. 6). Activin A had no effect on Dsg4 expression (Fig. 6). However, BMP2 increased Dsg4 mRNA expression in comparison with control.
Fig. 5. Smad4 transcriptionally activates Dsg4 expression.
A: ChIP PCR shows that Smad1, 4 and 5, but not Smad2 and 3 bound to the promoter of Dsg4. B: PCR encompassing the Dsg4 promoter region without the SBE shows no Smad binding. Mouse (Ms) IgG and rabbit (Rbt) IgG were used as negative controls for Smad antibody. Antibody to RNA polymerase (pol) II was used as a positive control for ChIP assay. A Gata-3 antibody was used as a control for specificities of individual Smad antibodies. C: Positive control for BMP-specific Smad binding to the Msx2 promoter. D: PCR encompassing the Msx2 promoter region without the SBE shows no Smad binding. E: Luciferase reporter assay in K5.Smad4−/− keratinocytes for Dsg4 transcription with individual Smad (S) expression vectors. *: p<0.05, **: p<.001, in comparison with reporter alone transfection. F: Dsg4 luciferase reporter assay in K5.Smad4−/− hair follicle cells showed that transactivation of the Dsg4 promoter by Smad4 (S4) was dependent on wildtype SBE. MutSBE: mutated SBE in the 3kb Dsg4 promoter. *: p<0.05, **: p<.001, in comparison with transfection with wildtype Dsg4 3kb promoter reporter alone.
Fig. 6. BMP induction of Dsg4.
Πριµαρψ ωιλδτψπε µουσε κερατινοχψτεσ ωερε γροων ιν 1.2µM χαλχιυµ ϕορ 48 η ουρσ ωιτη ορ ωιτηουτ TΓΦβ2, activin A or BMP-2 at a concentration of 10ng/ml. All samples were analyzed in quadruplicate, and normalized to GAPDH internal multiplexed control. *: p<0.05, **: p<0.01 in comparison with no ligand control.
Discussion
In the present study, we deleted Smad4 in keratinocytes at different stages during skin development. Smad4 deletion did not affect epidermal differentiation or initial events of hair follicle morphogenesis. However, hair follicles were degenerated in K5.Smad4−/− skin after birth. In comparison with previous studies (Qiao et al., 2006; Yang et al., 2005), the onset of hair follicle defects appeared earlier and more severe, possibly due to cre-mediated Smad4 excision being more complete by the current K5 promoter (Zhou et al., 2002). The uniform Smad4 deletion in keratinocytes allowed us to further analyze the molecular targets of Smad4 loss-associated hair follicle defects.
Smad4 is required for maximal transactivation of Dsg4 via interaction with BMP-specific Smads
Smad4 is a common Smad that potentially mediates signaling from TGFβ, activin and BMP. We have previously shown that Smad4 loss in keratinocytes results in abrogation of TGFβ-mediated expression of cell cycle inhibitors (Qiao et al., 2006), which explains the hyper-proliferative phenotype in K5.Smad4−/− epidermis, hair follicles and sebaceous glands. However, as ablation of neither TGFβ receptors (Honjo et al., 2007; Wang et al., 1997), Smad2 (our unpublished data), nor Smad3 (Li et al., 2004) resulted in hair follicle abnormalities, Smad4-loss associated hair follicle defects are likely to be a result of blockade of either the BMP pathway alone, or a combination of TGFβ/Activin and BMP. We found that Smad4 loss led to the reduction of Dsg4 mRNA, a protein critical for hair shaft differentiation and adhesion (Kljuic et al., 2003; Qiao et al., 2006), at a stage prior to hair shaft formation in normal mice and to hair follicle degeneration in K5.Smad4−/− mice. Further, Smad1, 4 and 5, but not Smad2 and 3, directly bound to the SBE of the Dsg4 promoter, and the combination of Smad1, 4 and 5 achieved the highest transactivation of the Dsg4 promoter. Lastly, mutating the SBE of the Dsg4 promoter abolished the effect of Smad4 on Dsg4 promoter activity. These data suggest that Dsg4 is a direct transcription target of Smad which is mainly activated by BMP signaling. In supporting of this notion, the ligand of BMP (BMP2), but not TGFβ or Activin, induced endogenous Dsg4 expression. Consistent with our current finding, keratinocyte deletion of the BMP type I receptor, ALK-3, exhibited a similar hair follicle phenotype, i.e., does not affect initial hair follicle development, but results in degeneration of the hair follicle and hair shaft (Andl et al., 2004; Kobielak et al., 2003; Ming Kwan et al., 2004; Yuhki et al., 2004). However, since the combination of Smad-1, -4, and -5 induced the highest transcriptional activity of Dsg4, and knocking out both Smad1 and Smad5 in keratinocytes did not show abnormalities in hair follicle differentiation (our unpublished data), our findings suggest that Smad4 is indispensable for maximal transactivation of Dsg4.
Downregulation of Dsg4 contributes at least in part to the defects in hair shaft differentiation and adhesion in K5.Smad4−/− skin
Dsg4 has been shown to be critical for the proper differentiation and specification of trichocytes of the hair shaft (Kljuic et al., 2003). Mutations in Dsg4 result in balding due to the lack of proper hair shaft formation and adhesion. Therefore, if Dsg4 is a major Smad4 target gene contributing to the role of Smad4 in hair differentiation, it is not surprising that K5.Smad4−/− skin still undergoes normal hair follicle development during embryogenesis, given the fact that Dsg4 is mainly expressed in differentiated pelage hair shaft after birth. Although it remains to be determined what signal plays a major role in switching on Dsg4 expression and how it interacts with Smad4, it appears that the initiating signals for Dsg4 expression in trichocytes are sufficient to support the first wave of hair shaft formation even in the absence of Smad4. Alternatively, Smad4 only regulates Dsg4 expression level modestly or to a limited extent, such that the remaining Smad1 and Smad5 together with other unknown cofactors may transcribe the amount of Dsg4 sufficient for the first wave of hair shaft formation. However, sustained Dsg4 expression during hair follicle regeneration was hindered by the loss of Smad4. It is possible that the defects in hair follicle regeneration in Smad4−/− skin largely prevented the renewal of Dsg4 positive cell types. It is also possible that regeneration of Dsg4 positive cells require maximal transcriptional upregulation of Dsg4.
Unlike Dsg3 knockout mice, which show cyclical balding due to adhesion defects of the club hair anchorage (Koch et al., 1997; Pulkkinen et al., 2002), K5.Smad4−/− hair shafts, which are similar to Dsg4 mutant hair shafts, have the combination of defects in both Dsg-mediated adhesion and associated hair shaft differentiation. At early stages, K5.Smad4−/− hair follicles showed phenotypes similar to Dsg4 mutant mouse and human follicles, i.e., degeneration of hair follicles, inward hair shaft growth, fragile hairs which disappear after the first catagen phase, and hair shaft collapsing in epidermoid cysts highlighting adhesion defects. However, we did not observe changes in E-cadherin expression (Supplemental Fig. 4), suggesting that the adhesion defect is restricted to the Dsg4-mediated adhesion but not to the adhesion complex. This result is consistent with the observation that Smad4 is required for suppression of E-cadherin expression (Deckers et al., 2006). In addition to hair follicle abnormalities, Dsg4 mutant mouse epidermis also showed epidermal hyperplasia, possibly as a secondary effect to hair shaft abnormalities. Similarly, K5.Smad4−/− epidermis also exhibited more obvious epidermal hyperplasia after hair follicle abnormalities became apparent. Therefore, although K5.Smad4−/− epidermis is hyperplastic mainly due to abrogation of TGFβ-mediated growth arrest (Yang et al., 2005), down regulation of Dsg4 in K5.Smad4−/− epidermis could also contribute to epidermal hyperplasia. However, consistent with Dsg4 mutant mice, epidermal differentiation was not altered in K5.Smad4−/− epidermis. Since Dsg4 in differentiated epidermal keratinocytes is expressed at much lower level in mice than in humans (Bazzi et al., 2005), the predominant effect of Dsg4 downregulation was restricted to hair shafts. It is also possible that the epidermis has other Dsg family members that will compensate for Dsg4 loss, whereas Dsg4 is the only predominant Dsg in differentiated hair shafts.
The phenotypes of K5.Smad4−/− skin suggest multiple functions of Smad4 in skin homeostasis
Although K5.Smad4−/− mice had hair follicle defects similar to Dsg4 mutant mice, K5.Samd4−/− mice also showed significant sebaceous hyperplasia. Additionally, there are differences between keratinocyte-specific ALK-3−/− mice and Smad4−/− mice: the former eventually developed hair follicle tumors but did not show expanded sebaceous glands (Andl et al., 2004; Kobielak et al., 2003; Ming Kwan et al., 2004; Yuhki et al., 2004), whereas the latter mainly develop SCC (Qiao et al., 2006; Yang et al., 2005). These observations highlight the multi-functionality of Smad4 and its diversified transcriptional targets that are cell-context specific. For instance, Smad4 loss-associated hyperproliferation and tumorigenesis are largely attributed to its abrogation of Smad2 and Smad3-mediated transcriptional responses to cell cycle arrest (Qiao et al., 2006), as these processes have been shown to require Smad4 (Levy and Hill, 2005). In contrast, Smad4 loss-associated hair follicle defects are the result of abrogation of BMP signaling. In addition, we have previously shown that Smad4 loss leads to down regulation of Lef1 (Qiao et al., 2006), a key transcription factor for the Wnt signaling. In contrast, Smad4 loss did not alter expression of hedgehog signaling components. Perturbing the balance of these two pathways has been shown to switch the epidermal stem cell decision from the hair follicle fate to the sebocyte fate (Niemann et al., 2003). Thus, reduced Wnt signaling and a relative increase in hedgehog signaling in K5.Smad4−/− skin could contribute to sebaceous hyperplasia. However, different from Smad7 transgenic mice in which sebaceous gland development was accelerated (Han et al., 2006), K5.Smad4−/− skin did not show this phenotype. Unlike Smad7, which induces β-catenin degradation via protein-protein interaction thus has a more potent inhibitory effect on Wnt signaling (Han et al., 2006), Smad4 is only known to affect Wnt signaling via transcriptional regulation (Hussein et al., 2003; Lim and Hoffmann, 2006). Therefore, sebaceous gland hyperplasia in K5.Smad4−/− skin could be the result of a combination of mildly reduced Wnt signaling and direct proliferative effect of Smad4 loss due to abrogation of TGFβ-mediated growth arrest. Notably, although K5.Smad4−/− skin has multiple defects, skin development and epidermal differentiation are not altered. Some of the gene products, which are involved in these processes, e.g., such as Msx2, HoxC13, and Gli1, are known or potential Smad target genes (Blokzijl et al., 2002; Dennler et al., 2007; Hussein et al., 2003). However, expression levels of these genes were not changed in K5.Smad4−/− skin prior to hair follicle degeneration while other Smad target genes related to cell cycle control were down regulated (Qiao et al., 2006; Yang et al., 2005), suggesting that transcriptional regulation of these genes could be either Smad4 independent, or dispensable for Smad4 effects due to in vivo compensatory mechanisms. Indeed, Smad4 independent transcriptional regulation of Smad-dependent genes has been observed (Levy and Hill, 2005). Smad4 has also been shown to be in competition with other transcription factors such as TIF1γ for binding to activate Smads 2/3 (Deckers et al., 2006).
In summary, we identified Dsg4 as a direct transcriptional target of Smad4. Such a Smad4 target gene would not necessarily be identified using routine screening for transcriptional targets, given the fact that Dsg4 expression is restricted to certain developmental stages and in specific differentiated cell compartments in vivo. Our data further suggest that loss of Dsg4 expression in K5.Smad4−/− keratinocytes contributes to hair follicle degeneration. However, we should also point out that not all of the phenotypes of K5.Smad4−/− skin can be explained by Dsg4 downregulation and that K5.Smad4−/− and Dsg4 mutant mice have distinguishable phenotypes. These differences highlight the multifunctional nature of Smad4 in the skin and our current study shows the importance of identification of functional Smad transcription targets in a context-specific manner during different developmental stages and physiological/pathological conditions. Future study of expressing a Dsg4 transgene in K5.Smad4−/− skin would ultimately determine to what extent Dsg4 loss mediates hair follicle degeneration associated with Smad4 loss.
Supplementary Material
Acknowledgements
This work is supported by NIH grant CA87849 and AR 47898 to XJW and AR44924 to AMC. P.O. is a recipient of an NIH training grant. E.E. is a Murdock Fellow. We thank Dr. Chuxia Deng for providing Smad4 floxed mice, X.H. Feng for providing Smad expression plasmids, and Dr. T.T. Sun for providing the AE15 antibody.
Abbreviations
- TGFβ
(Transforming Growth Factor β
- BMP
(Bone Morphogenetic Protein)
- Dsg
(Desmoglein)
- SBE
(Smad Binding Element)
- ChIP
(Chromatin Immunoprecipitation)
- ALK
(Activin Like Kinase)
- K5
(Keratin-5)
- TSS
(Transcription Start Site)
- E
(Embryonic Day)
- P
(Postnatal Day)
- ORS
(Outer Root Sheath)
- IRS
(Inner Root Sheath)
- DP
(Dermal Papillae)
- WT
(Wildtype)
- KO
(Knockout)
- luc
(Luciferase)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006;119:391–393. doi: 10.1242/jcs.02793. [DOI] [PubMed] [Google Scholar]
- Andl T, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Bamberger C, et al. Activin controls skin morphogenesis and wound repair predominantly via stromal cells and in a concentration-dependent manner via keratinocytes. Am J Pathol. 2005;167:733–747. doi: 10.1016/S0002-9440(10)62047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi H, et al. Desmoglein 4 is expressed in highly differentiated keratinocytes and trichocytes in human epidermis and hair follicle. Differentiation. 2006;74:129–140. doi: 10.1111/j.1432-0436.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- Bazzi H, et al. Desmoglein 4 mutations underlie localized autosomal recessive hypotrichosis in humans, mice, and rats. J Investig Dermatol Symp Proc. 2005;10:222–224. doi: 10.1111/j.1087-0024.2005.10110.x. [DOI] [PubMed] [Google Scholar]
- Blokzijl A, et al. Physical and functional interaction between GATA-3 and Smad3 allows TGF-beta regulation of GATA target genes. Curr Biol. 2002;12:35–45. doi: 10.1016/s0960-9822(01)00623-6. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation. 2004;72:512–526. doi: 10.1111/j.1432-0436.2004.07209005.x. [DOI] [PubMed] [Google Scholar]
- Brugger SM, et al. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development. 2004;131:5153–5165. doi: 10.1242/dev.01390. [DOI] [PubMed] [Google Scholar]
- Deckers M, et al. The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 2006;66:2202–2209. doi: 10.1158/0008-5472.CAN-05-3560. [DOI] [PubMed] [Google Scholar]
- Dennler S, et al. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- Foitzik K, et al. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. Faseb J. 2000;14:752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- Foitzik K, et al. The TGF-beta2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol. 1999;212:278–289. doi: 10.1006/dbio.1999.9325. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- Han G, et al. Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell. 2006;11:301–312. doi: 10.1016/j.devcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Heid HW, et al. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 1998;294:309–321. doi: 10.1007/s004410051181. [DOI] [PubMed] [Google Scholar]
- Honjo Y, et al. TGF-beta receptor I conditional knockout mice develop spontaneous squamous cell carcinoma. Cell Cycle. 2007;6:1360–1366. doi: 10.4161/cc.6.11.4268. [DOI] [PubMed] [Google Scholar]
- Hussein SM, et al. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J Biol Chem. 2003;278:48805–48814. doi: 10.1074/jbc.M305472200. [DOI] [PubMed] [Google Scholar]
- Johns SA, et al. Foxn1 is required for tissue assembly and desmosomal cadherin expression in the hair shaft. Dev Dyn. 2005;232:1062–1068. doi: 10.1002/dvdy.20278. [DOI] [PubMed] [Google Scholar]
- Kaufman CK, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–2122. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kljuic A, et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell. 2003;113:249–260. doi: 10.1016/s0092-8674(03)00273-3. [DOI] [PubMed] [Google Scholar]
- Kobielak K, et al. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, et al. Targeted disruption of the pemphigus vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to pemphigus vulgaris. J Cell Biol. 1997;137:1091–1102. doi: 10.1083/jcb.137.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L, Hill CS. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25:8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AG, et al. Roles of TGFbeta signaling in epidermal/appendage development. Cytokine Growth Factor Rev. 2003;14:99–111. doi: 10.1016/s1359-6101(03)00005-4. [DOI] [PubMed] [Google Scholar]
- Li AG, et al. Smad3 knockout mice exhibit a resistance to skin chemical carcinogenesis. Cancer Res. 2004;64:7836–7845. doi: 10.1158/0008-5472.CAN-04-1331. [DOI] [PubMed] [Google Scholar]
- Lim SK, Hoffmann FM. Smad4 cooperates with lymphoid enhancer-binding factor 1/T cell-specific factor to increase c-myc expression in the absence of TGF-{beta} signaling. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0604773103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, et al. Functional analysis of activins during mammalian development. Nature. 1995a;374:354–356. doi: 10.1038/374354a0. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, et al. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995b;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Ming Kwan K, et al. Essential roles of BMPR-IA signaling in differentiation and growth of hair follicles and in skin tumorigenesis. Genesis. 2004;39:10–25. doi: 10.1002/gene.20021. [DOI] [PubMed] [Google Scholar]
- Niemann C. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A. 2003;100 Suppl 1:11873–11880. doi: 10.1073/pnas.1834202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen L, et al. Loss of cell adhesion in Dsg3bal-Pas mice with homozygous deletion mutation (2079del14) in the desmoglein 3 gene. J Invest Dermatol. 2002;119:1237–1243. doi: 10.1046/j.1523-1747.2002.19645.x. [DOI] [PubMed] [Google Scholar]
- Qiao W, et al. Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene. 2006;25:207–217. doi: 10.1038/sj.onc.1209029. [DOI] [PubMed] [Google Scholar]
- Satokata I, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Wagner KU, et al. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 2001;10:545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- Wang XJ, et al. Expression of a dominant-negative type II transforming growth factor beta (TGF-beta) receptor in the epidermis of transgenic mice blocks TGF-beta-mediated growth inhibition. Proc Natl Acad Sci U S A. 1997;94:2386–2391. doi: 10.1073/pnas.94.6.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, et al. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res. 2005;65:8671–8678. doi: 10.1158/0008-5472.CAN-05-0800. [DOI] [PubMed] [Google Scholar]
- Yang X, et al. Generation of Smad4/Dpc4 conditional knockout mice. Genesis. 2002;32:80–81. doi: 10.1002/gene.10029. [DOI] [PubMed] [Google Scholar]
- Yuhki M, et al. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development. 2004;131:1825–1833. doi: 10.1242/dev.01079. [DOI] [PubMed] [Google Scholar]
- Zhou Z, et al. In utero activation of K5.CrePR1 induces gene deletion. Genesis. 2002;32:191–192. doi: 10.1002/gene.10064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.