Abstract
5′-O-D- and L-amino acid derivatives and 5′-O-(D- and L-amino acid methyl ester phosphoramidate) derivatives of vidarabine (ara-A) were synthesized as vidarabine prodrugs. Some compounds were equi- or more potent in vitro than vidarabine against two pox viruses and their uptake by cultured cells was improved compared to the parent drug.
Keywords: Vidarabine, ara-A, Amino acid ester prodrug, Phosphoramidate prodrug, Antiviral activity, Selective protection of nucleoside, Levulinate ester, Oral bioavailability, Adenosine deaminase, Arahypoxanthine, ara-H, Caco-2 permeability
There has been continued interest in the synthesis and biological evaluation of nucleoside analogs capable of delivering the corresponding nucleotides inside cells.1 1-Beta-D-arabinofuranosyladenine (vidarabine or ara-A) is an antiviral drug with activity against herpes viruses, poxviruses, and certain rhabdoviruses, hepadnarviruses, and RNA tumor viruses.2–4 Vidarabine also is active against vaccinia virus both in vitro5 and in vivo.6 However, it is more toxic and less metabolically stable than other current antivirals such as acyclovir and ganciclovir; further it is poorly soluble with low oral bioavailability. It is readily deaminated by adenosine deaminase (ADA) to ara-hypoxanthine (ara-H),7 which possesses some antiviral activity but is at least 10-fold less potent than vidarabine. 6–8 Adenosine deaminase (ADA) is a cytosolic enzyme that participates in purine metabolism where it degrades either adenosine or 2′-deoxyadenosine to inosine or 2′-deoxyinosine, respectively. Further metabolism of these deaminated nucleosides leads to hypoxanthine. ADA also degrades vidarabine to ara-H by same mechanism.7
Our current interest in prodrugs of vidarabine was triggered by the report of the activity of vidarabine against cowpox virus9 and by our discovery that vidarabine was 3- to 5-fold more active against vaccinia and cowpox viruses than cidofovir in plaque reduction assays.10 Cidofovir is a broad spectrum antiviral agent,11–13 that is, limited in its usage because of nephrotoxicity and poor oral bioavailability (~2% in humans)14–16 and for which prodrugs have been developed.17 Furthermore, we found that the activity of vidarabine against these viruses was enhanced approximately 10-fold when combined with 2′-deoxycoformycin (pentostatin, a potent inhibitor of ADA), thus providing significant superiority to cidofovir. Based on these results and earlier studies on 5′-substituted vidarabine analogs, we hypothesized that minimizing the conversion of vidarabine to its hypoxanthine analog could yield a significantly more potent anti-pox virus agent. With this goal in mind, we have developed a prodrug strategy that protects the vidarabine from metabolic conversion by making 5′-amino acid esters and 5′-phosphoramidates of the drug. Further, our rationale includes the design of prodrugs that increase aqueous solubility over that of the parent drug and also increase the potential for membrane transport by the dipeptide intestinal transporter.
In order to realize the efficient high throughput synthesis of 5′-amino acid ester and 5′-(phenyl methoxyamino acid)-phosphate derivatives of vidarabine in large quantities (100 mg for each), it is crucial to selectively protect the 2′ and 3′ hydroxyl groups of the arabinoside residue. Such blocking groups have to be easily and quickly removed under non-basic conditions to prevent concomitant cleaving of the acyl groups in the phosphate or amino acid ester moiety. After evaluation of a range of protecting groups, including benzoate and acetate, the final candidate for protection of the 2′, 3′ hydroxyl positions was the levulinate group. The levulinate group can survive the synthesis conditions for these prodrugs and can be easily removed by treating with 1 ml of 2 M hydrazine hydrate in pyridine-acetic acid buffer for 10 min,18,19 conditions under which normal esters are not cleaved.20,21 In addition, the levulinate is less prone to migration between adjacent hydroxyl groups on the sugar residue than other ester protection group such as benzoate and acetate.22,23 Finally, the use of flash chromatography during the purification process was minimized during the parallel synthesis of a representative prodrug library, which significantly improved the overall efficiency of the synthesis.
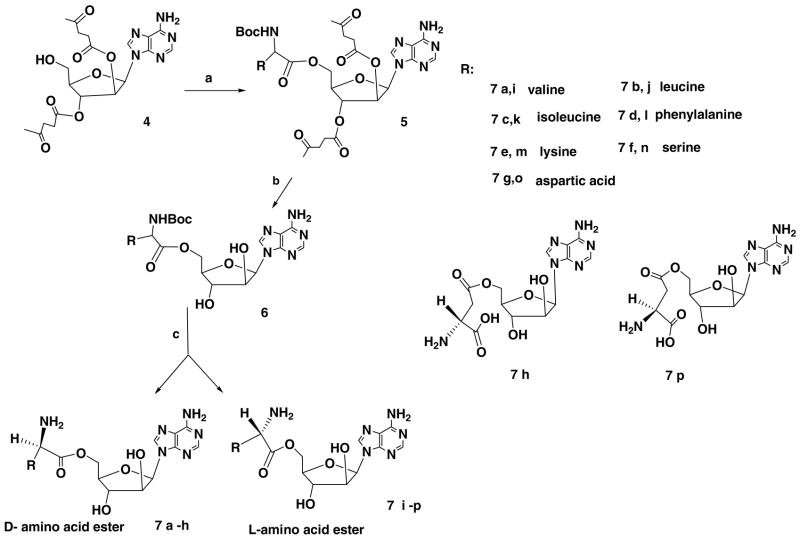
The typical procedure for synthesis of 2′ and 3′ protected vidarabine is depicted in Scheme 1. First, selective protection of the 5′-OH of vidarabine was readily accomplished with tert-butyldimethylsilyl chloride in the presence of imidazole in DMF. The resulting 5′-O-TBDMS-vidarabine 2 was purified with liquid–liquid extraction between water and ethyl acetate, giving a 90% yield. Compound 2 was then acylated with levulinic anhydride, which was generated in situ from levulinic acid with DCC in the presence of DMAP as catalyst to produce the fully blocked 5′-O-TBDMS-2′,3′-dilev-ara A 3. Importantly, the exocyclic amine of the adenine moiety was not levulinated as long as the reaction time was less than two hours. This regioselectivity allowed for the avoidance of protection and deprotection steps of the exoclyclic amine group. Liquid –liquid extraction between saturated ammonium chloride and ethyl acetate was performed, followed by silica gel flash chromatography to purify the product. Selective removal of the 5′-TBDMS group was readily achieved with a mixture of TBAF/acetic acid (1:2 mole ratio) in tetrahydrofuran. In accordance with the observations of other workers,24 we noticed that there were some acyl migration (3′–5′) of the levulinyl group in these arabinoside derivatives when acetic acid was absent during the treatment with TBAF. Addition of acetic acid in the reaction system can prevent the migration from occurring. The 2′,3′-dilevulinyl vidarabine (4) obtained was purified with silica gel flash chromatography eluting with 8% methanol in DCM. The total yield from vidarabine to 2′,3′-dilev vidarabine was 74%.
Scheme 1.
Reagents and condition: (a) TBSCl and Im./DMAP DMF; (b) levulinic acid, DCC, DMAP in ethyl acetate; and (c) TBAF/acetic acid (1:2 mole ratio) in tetrahydrofuran.
After obtaining sufficient quantity of 2′,3′-dilevulinyl vidarabine, a parallel synthesis strategy was applied to synthesize representative libraries of 5′-D- or L-amino acid derivatives (Scheme 2) and 5′-[phenyl (amino acid methyl ester)]-phosphate derivatives (Scheme 3) of vidarabine. The protected D- or L-amino acids were coupled with protected vidarabine using DCC as a coupling reagent and DMAP as a catalyst (Scheme 2). The fully blocked products 5 were isolated with liquid–liquid extraction between saturated ammonium chloride and ethyl acetate and dried with Na2SO4. After removal of solvent, the crude products were treated with 1 ml of 2 M hydrazine hydrate in pyridine–acetic acid buffer for 10 min to produce the uncharged species 6. The excess hydrazine was eliminated through reaction with pentane-2,4-dione for another 10 min. After evaporating the volatile components, the residue was dissolved in EtOAc, washed with saturated ammonium chloride, water and brine. The organic layer was dried over Na2SO4, filtered and evaporated to dryness. Removal of the protection groups on the amino acid promoieties generated the final products 7a–p, which were purified by preparative HPLC.
Scheme 2.
Reagents and condition: (a) alpha N-Boc D- and L-amino acid, DCC, DMAP DMF; (b) 1 ml of 2 M hydrazine hydrate in pyridine–acetic acid buffer; and (c) removal of protection group from amino acid moieties.
Scheme 3.
Reagents and condition: (a) one milliliter of 2 M hydrazine hydrate in pyridine–acetic acid buffer.
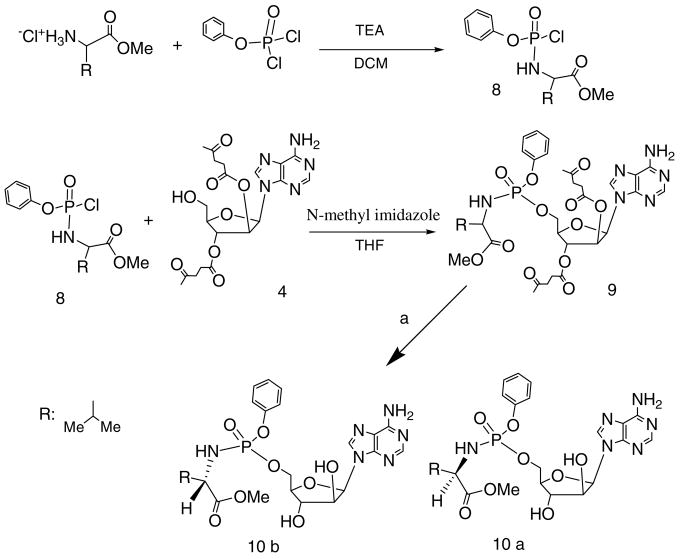
Phosphoramidate prodrugs were synthesized according to the method described in Scheme 3. The phenyl (amino acid methyl ester) phosphochloridates 8, were obtained based on modified literature methodology.25 Compound 8 was then coupled with protected vidarabine 4 in the presence of N-methyl imidazole and THF to yield the blocked phosphoramidate vidarabine prodrugs such as 9. The resultant product 9 was purified by silica gel flash chromatography with 6% methanol in DCM as eluent. After treatment with 1 ml of 2 M hydrazine hydrate in pyridine-acetic acid buffer for 10 min, the same workup method for compound 7 was applied and preparative HPLC was used to obtain the pure products 10a and b. We used both D- and L-amino acids to build the representative libraries for compounds 7 and 10. The detailed synthesis procedures and analytical data can be found in the Supporting Material.
Vidarabine exhibited good activity in cell culture against two small pox related viruses, vaccinia and cowpox (Table 1). As we reported previously for HSV-1,26 addition of the ADA inhibitor deoxycoformycin increased the activity of vidarabine against the poxviruses approximately 5- to 10-fold. Also as shown in Table 1, the amino acid prodrugs were more effective against these viruses than was the parent drug. In some experiments some prodrugs were almost as active as vidarabine plus deoxycoformycin. The apparently greater activity of the prodrugs compared to vidarabine may mean that the prodrugs are not substrates for adenosine deaminase and thereby protect the parent drug from deamination before hydrolysis to vidarabine in cell culture. Alternatively or in addition, this higher potency could be a result of more rapid penetration of the prodrugs into infected cells or the prodrugs could be inhibitors of ADA.
Table 1.
Antiviral activity of amino acid prodrugs of vidarabinea
| Promoiety (Compound) | EC50b (μM) |
CC50b (μM) | |
|---|---|---|---|
| Vaccinia | Cowpox | HFF | |
| Vidarabine (1)c | 6.2 | 12.6 | >100 |
| Vidarabine + dCFc | 1.0 | 1.4 | 90 |
| D-ile (7c) | 2.9 | 3.5 | >100 |
| L-ile (7k) | 4.5 | 2.5 | >100 |
| D-leu (7b) | 3.0 | 2.5 | >100 |
| L-leu (7j) | 3.3 | 3.0 | >100 |
| D-val (7a) | 4.1 | 8.0 | >100 |
| L-val (7i) | 4.8 | 4.0 | >100 |
| D-phe (7d) | 2.3 | 4.0 | >100 |
| L-phe (7l) | 1.8 | 3.5 | >100 |
| D-α-asp (7g) | 2.5 | 4.0 | >100 |
| D-β-asp (7h) | 3.5 | 25 | >100 |
Fifty percent inhibitory concentration by plaque assay against the two viruses grown in human foreskin fibroblasts (HFF’s).
Visual cytotoxicity determined in HFF’s not affected by virus replication expressed as 50% inhibitory concentrations.
Data for vidarabine and vidarabine plus 1 μM deoxycoformycin (dCF) are averages from four to nine experiments. Prodrug data for vaccinia virus are averages of two to four experiments except for the asp prodrugs where data are from a single experiment. Likewise data for cowpox are from one experiment.
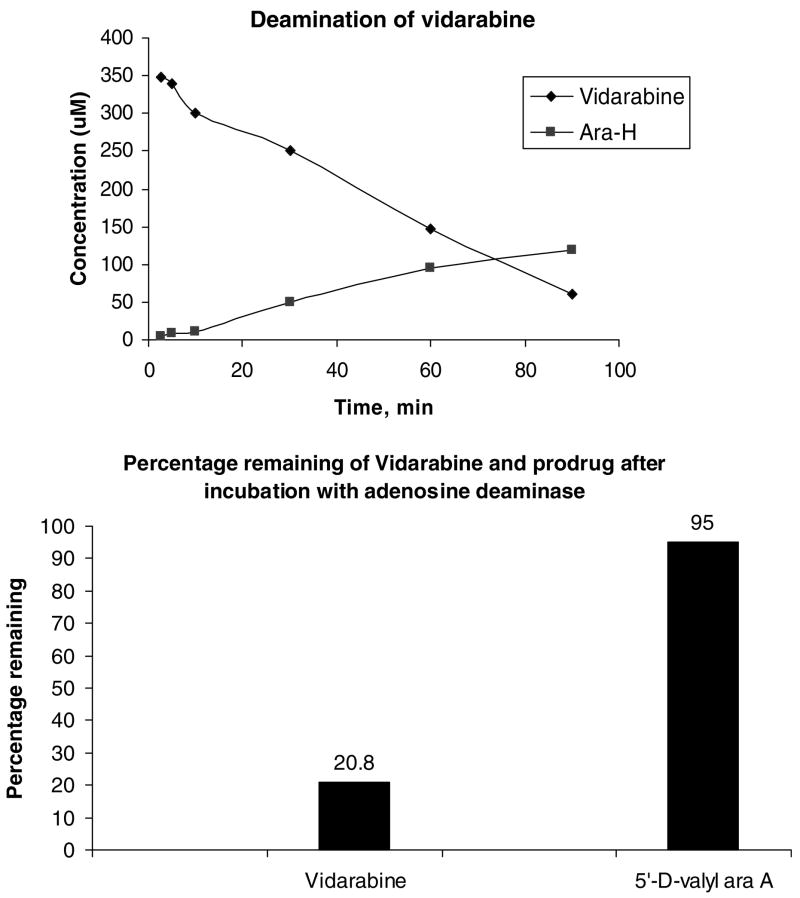
Absorption of vidarabine from the intestine is very poor. In order to achieve sufficiently high plasma levels the drug should be given intravenously or through ocular delivery. The recommended intravenous injection dose is 10–15 mg/kg daily. Due to the poor water solubility, vidarabine must be administered in a large volume of an appropriate intravenous infusion fluid (e.g., glucose 5%). The daily dose is slowly infused in a 12–24 h period for at least 10 days.27 The vidarabine prodrugs are being developed to overcome the biopharmaceutical limitations of vidarabine, including poor solubility, poor intestinal transport and rapid metabolism of the parent vidarabine to ara-H. Vidarabine has a reported solubility of ~0.47 mg/ml in water, with increasing solubility at lower pH.28 The amino acid prodrugs described in this report are all freely soluble in water and have greatly improved solubility at physiological pH, with solubilities >10 mg/ml. Based upon our previous findings with amino acid nucleoside prodrugs,29 it was anticipated that the amino acid prodrugs of vidarabine would be substrates for the dipeptide intestinal transporter, PEPT1. To test this, the uptake of L-valy and L-isoleucyl prodrugs of vidarabine into a HeLa cell line that overexpresses the human PEPT1 transporter was compared to the uptake of valacyclovir, a known substrate of the PEPT1 transporter. 30–35 As seen in Table 2, both the prodrugs showed approximately 20-fold greater uptake over control cells not expressing PEPT1, an increase which was comparable to that seen with valacyclovir. Stability of the promoiety (hydrolysis of prodrug to the parent vidarabine or vidarabine monophosphate) for selected prodrugs was assessed in intestinal and liver homogenates from the rat. As seen in Table 3, the prodrugs showed varying levels of stability to hydrolysis based on the structure of the promoiety. In intestinal homogenates, the phosphoramidate prodrugs (10a and b) were the most stable followed by the D-amino acid prodrugs then the L-amino acid prodrugs. Hydrolysis to parent was more rapid in the liver homogenates for all prodrugs, except the D-valyly phosphoramidate vidarabine, which was stable in both homogenates. However, in general, the phosphoramidate prodrugs were less stable than their amino acid counterparts in plasma. Stability to ADA, which is the major metabolic enzyme for vidarabine and which converts it to ara-H was also examined. While vidarabine was rapidly hydrolyzed with a commensurate rise in the concentration of the deaminated product, ara-H (Fig. 1 top), it was found that substitution at the 5′-OH group made the drug resistant to deamination by ADA (Fig. 1 bottom),36,37 consistent with past reports on 5′-OH prodrugs of vidarabine.8,26
Table 2.
Direct uptake of L-val and L-Ile vidarabine prodrugs in HeLa/hPEPT1 and HeLa cells
| Promoiety (Compound) | HeLa/hPEPT1 (nmol/mg/45 min) | HeLa control (nmol/mg/45 min) | hPEPT1 control |
|---|---|---|---|
| L-val (7i) | 80.3 ± 1.1a | 3.9 ± 0.3 | 20.7 |
| L-ile (7k) | 202 ± 4.8 | 7.5 ± 0.2 | 26.9 |
| Valacyclovirb | 221 ± 8.1 | 11.6 ± 0.5 | 19.0 |
All data are presented as means and standard deviations from three experiments.
Valacyclovir was used as a positive control for hPEPT1 carrier mediated uptake.
Table 3.
Stability of vidarabine and its prodrugs in biological homogenates and plasma from rat
| Promoiety (Compound) | T1/2 (hours) |
||
|---|---|---|---|
| Intestinal homogenate | Liver homogenate | Plasma | |
| Vidarabine (1) | 0.05 | 0.25 | 0.43 |
| D-ile (7c) | 7.90 | 0.31 | 1.61 |
| L-ile (7k) | 0.58 | 0.06 | 3.58 |
| D-leu (7b) | 0.04 | 0.06 | nab |
| L-leu (7j) | 0.03 | 0.05 | nab |
| D-phe (7d) | 0.59 | 0.10 | nab |
| L-phe (7l) | 0.27 | <0.017 | nab |
| L-val (7j) | 0.30 | 0.03 | 0.13 |
| D-val (7a) | Sta | 0.46 | 10.26 |
| P-L valc (10a) | 18.32 | 5.91 | 0.35 |
| P-D valc (10b) | Sta | Sta | 0.30 |
St means stable.
na means not analyzed.
Phosphoramidate prodrug.
Figure 1.
Deamination of vidarabine and its prodrugs by adenosine deaminase1. Top: Concentration (μM) profiles of the disappearance of vidarabine and the appearance of Ara-H in the presence of adenosine deaminase. Bottom: Percent vidarabine or prodrug remaining following incubation with adenosine deaminase1 for 90 min.
The transepithelial transport of some of the prodrugs through Caco-2 monolayers was evaluated as previously described.38 In these experiments, transport studies were performed 21 days post-seeding. The assay was initiated by adding drug transport solution (1.2 mg drug in MES buffer, pH 6.0, containing 5 mM D-glucose, 5 mM MES, 1 mM CaCl2, 1 mM MgCl2, 150 mM NaCl, 3 mM KCl, 1 mM NaH2PO4) to the apical chamber of the Caco-2 insert. Two hundred microliter aliquots were withdrawn from the basolateral chamber at predetermined intervals and replaced with fresh HEPES, pH 7.4, buffer. The epithelial integrity of representative cell monolayers was assessed by monitoring transepithelial resistance. As seen in Table 4, the permeabilities of all tested prodrugs were enhanced by at least 7-fold comparing with the permeability of vidarabine. Considering that the prodrugs would be substrates for the dipeptide intestinal transporter, PEPT1 (Table 2) and those transporters are much more highly expressed in the small intestinal membranes than in Caco-2 membranes, we anticipate significant increases of vidarabine and/or its prodrug in in vivo studies.
Table 4.
Summary of permeability data for vidarabine and selected prodrugs
| Compounds | Caco-2 permeability cm/s × 106 | Fold increase |
|---|---|---|
| Vidarabine (1) | 0.083 | — |
| D-val (7a) | 0.601 | 7.2 |
| L-ile (7k) | 1.026 | 12.4 |
| L-phe (7l) | 0.668 | 8.05 |
These data support our hypothesis that substitution at the 5′-OH group can result in both enhanced uptake of vidarabine or its prodrugs and protection from metabolism by ADA. The next phase of the work will be to examine the in vivo oral bioavailability of the prodrugs and parent compounds and ultimately their effectiveness in animal models of pox viruses.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2008.12.031.
Acknowledgments
This work was supported by a SBIR Grant 1R43AI071400 from the National Institutes of Health.
References and notes
- 1.Kukhanova M, Krayevsky A, Prusoff W, Cheng YC. Curr Pharm Des. 2000;6:585. doi: 10.2174/1381612003400687. [DOI] [PubMed] [Google Scholar]
- 2.Pavan-Langston D, Buchanan RA, Alford CA, editors. Adenine Arabinoside: An Antiviral Agent. Raven Press; New York: 1975. [Google Scholar]
- 3.White OD, Fenner FJ. Medical Virology. 3. Academic Press; San Diego: 1994. p. 267. [Google Scholar]
- 4.Field HJ, De Clercq E. Microbiol Today. 2004;31:58. [Google Scholar]
- 5.Miller FA, Dixon GJ, Ehrlich J, Sloan BJ, McLean IW., Jr Antimicrob Agents Chemother. 1968;8:136. [PubMed] [Google Scholar]
- 6.Sloan BJ. In: Adenine Arabinoside: An Antiviral Agent. Pavan-Langston D, Buchanan RA, Alford CA, editors. Raven Press; New York: 1975. p. 45. [Google Scholar]
- 7.Hubert-Habart M, Cohen SS. Biochim Biophys Acta. 1962;59:468. doi: 10.1016/0006-3002(62)90198-1. [DOI] [PubMed] [Google Scholar]
- 8.Shipman C, Jr, Smith SH, Carlson RH, Drach JC. Antimicrob Agents Chemother. 1976;9:120. doi: 10.1128/aac.9.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smee DF, Sidwell RW. Nucleosides Nucleotides Nucleic Acids. 2004;23:375. doi: 10.1081/ncn-120028334. [DOI] [PubMed] [Google Scholar]
- 10.Hilfinger JM, Wu Z, Kim J, Mitchell S, Breitenbach J, Amidon G, Drach J. Antiviral Res. 2006;70:A14. [Google Scholar]
- 11.Lea AP, Bryson HM. Drugs. 1996;52:225. doi: 10.2165/00003495-199652020-00006. [DOI] [PubMed] [Google Scholar]
- 12.Hitchcock MJM, Jaffe HS, Martin JC, Stagg RJ. Antiviral Chem Chemother. 1996;7:115. [Google Scholar]
- 13.Safrin S, Cherrington J, Jaffe HS. Rev Med Virol. 1997;7:145. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Wachsman M, Petty BG, Cundy KC, Jaffe HS, Fisher PE, Pastelak A, Lietman PS. Antiviral Res. 1996;29:153. doi: 10.1016/0166-3542(95)00829-2. [DOI] [PubMed] [Google Scholar]
- 15.Cundy KC. Clin Pharmacokinet. 1999;36:127. doi: 10.2165/00003088-199936020-00004. [DOI] [PubMed] [Google Scholar]
- 16.Cundy KC, Bidgood AM, Lynch G, Shaw JP, Griffin L, Lee WA. Drug Metab Dispos. 1996;24:745. [PubMed] [Google Scholar]
- 17.Wan WB, Beadle JR, Hartline C, Kern ER, Ciesla SL, Valiaeva N, Hostetler KY. Antimicrob Agents Chemother. 2005;49:656. doi: 10.1128/AAC.49.2.656-662.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassner A, Strand G, Rubinstein M, Patchornik A. J Am Chem Soc. 1975;97:1614. doi: 10.1021/ja00839a077. [DOI] [PubMed] [Google Scholar]
- 19.van Boom JH, Burgers PMJ. Tetrahedron Lett. 1976;52:4875. [Google Scholar]
- 20.Jeker N, Tamm C. Helv Chim Acta. 1988;71:1895. [Google Scholar]
- 21.Jeker N, Tamm C. Helv Chim Acta. 1988;71:1904. [Google Scholar]
- 22.Rej RN, Glushka JN, Chew W, Perlin AS. Carbohydr Res. 1989;189:135. [Google Scholar]
- 23.Glushka JN, Perlin AS. Carbohydr Res. 1990;205:305. doi: 10.1016/0008-6215(90)80149-w. [DOI] [PubMed] [Google Scholar]
- 24.Baker DC, Haskell TH, Putt SR, Sloan BJ. J Med Chem. 1979;22:273. doi: 10.1021/jm00189a011. [DOI] [PubMed] [Google Scholar]
- 25.McGuigan C, Thiery J, Daverio F, Jiang WG, Davies G, Masonb M. Bioorg Med Chem. 2005;13:3219. doi: 10.1016/j.bmc.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PM, Shipman C, Jr, Smith SH, Sandberg JN, Drach JC. Antimicrob Agents Chemother. 1976;10:64. doi: 10.1128/aac.10.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stolk LML, Huisman W, Nordemann HD, Vyth A. Pharmaceutisch Weekblad, Sci Ed. 1983;5:57. doi: 10.1007/BF01960076. [DOI] [PubMed] [Google Scholar]
- 28.Baker DC, Haskell TH, Putt SR. J Med Chem. 1978;21:1218. doi: 10.1021/jm00210a009. [DOI] [PubMed] [Google Scholar]
- 29.Vig BS, Lorenzi PJ, Mittal S, Landowski CP, Shin HC, Mosberg HI, Hilfinger JM, Amidon GL. Pharm Res. 2003;20:1381. doi: 10.1023/a:1025745824632. [DOI] [PubMed] [Google Scholar]
- 30.Song X, Lorenzi PL, Landowski CP, Vig BS, Hilfinger JM, Amidon GL. Mol Pharm. 2005;2:157. doi: 10.1021/mp049888e. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzi PL, Landowski CP, Song X, Borysko KZ, Breitenbach JM, Kim JS, Hilfinger JM, Townsend LB, Drach JC, Amidon GL. J Pharmacol Exp Ther. 2005;314:883. doi: 10.1124/jpet.104.082412. [DOI] [PubMed] [Google Scholar]
- 32.Landowski CP, Song X, Lorenzi PL, Hilfinger JM, Amidon GL. Pharm Res. 2005;22:1510. doi: 10.1007/s11095-005-6156-9. [DOI] [PubMed] [Google Scholar]
- 33.Han HK, De Vrueh RLA, Rhie JK, Covitz KMY, Smith PL, Lee CP, Oh DM, Sadee W, Amidon GL. Pharm Res. 1998;15:1154. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
- 34.Landowski CP, Sun D, Foster DR, Menon SS, Barnett JL, Welage LS, Ramachandran C, Amidon GL. J Pharmacol Exp Ther. 2003;306:778. doi: 10.1124/jpet.103.051011. [DOI] [PubMed] [Google Scholar]
- 35.Song X, Vig BS, Lorenzi PL, Drach JC, Townsend LB, Amidon GL. J Med Chem. 2005;48:1274. doi: 10.1021/jm049450i. [DOI] [PubMed] [Google Scholar]
- 36.Breitenbach JM, Shen W, Hilfinger J, Drach JC. Antiviral Res. 2008;78:A54–A55 . [21st International Conf. Antiviral Res., Montreal, Canada, April 15, 2008]. [Google Scholar]
- 37.Gentry BG, Shen W, Breitenbach JM, Hilfinger J, Drach JC. Antiviral Res. 2008;78:A56. [21st International Conf. Antiviral Res., Montreal, Canada, April 15, 2008. [Google Scholar]
- 38.Han H, de Vrueh RL, Rhie JK, Covitz KM, Smith PL, Lee CP, Oh DM, Sadee W, Amidon GL. Pharm Res. 1998;15:1154. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2008.12.031.