Abstract
OBJECTIVE
To identify research priorities for increasing understanding of the pathogenesis, diagnosis and improved treatment of spasmodic dysphonia.
STUDY DESIGN AND SETTING
A multidisciplinary working group was formed including both scientists and clinicians from multiple disciplines, otolaryngology, neurology, speech pathology, genetics and neuroscience, to review currently available information on spasmodic dysphonia and to identify research priorities.
RESULTS
Operational definitions for spasmodic dysphonia at different levels of certainty were recommended for diagnosis and recommendations made for a multi-center multidisciplinary validation study.
CONCLUSIONS
The highest priority is to characterize the disorder and identify risk factors that may contribute to its onset. Future research should compare and contrast spasmodic dysphonia with other forms of focal dystonia. Development of animal models is recommended to explore hypotheses related to pathogenesis. Improved understanding of the pathophysiology of SD should provide the basis for developing new treatment options and exploratory clinical trials.
SIGNIFICANCE
This document should foster future research to improve the care of patients with this chronic debilitating voice and speech disorder by otolaryngology, neurology, and speech pathology.
Keywords: larynx, voice, speech, focal dystonia, diagnosis, epidemiology, surgery
Background
Experts reviewed the current understanding of spasmodic dysphonia (SD), to identify gaps in knowledge and to develop recommendations for research on the pathogenesis and pathophysiology of the disorder for prevention and improved treatment.
I. Diagnosis of Spasmodic Dysphonia
Spasmodic dysphonia (SD) a rare speech disorder, that develops spontaneously in mid-life. Symptoms are uncontrolled voice breaks1 and a marked effort while speaking. Progression is gradual in the first year, then becoming chronic2. SD is usually idiopathic; symptoms rarely occur secondary to brain injury or neuroleptics. Women are affected more than men; between 60-85% are female3,4.
Involuntary spasms in the laryngeal muscles cause intermittent voice breaks5, only during speech6. In adductor SD, spasmodic hyperadductions of the vocal folds produce voice breaks with a choked, strained quality. Abductor SD is less common, with hyperabduction (uncontrolled opening) of the vocal folds prolonging voiceless consonants before vowels7. Very rarely, adductor and abductor spasms occur in the same patient. Voice tremor is often present with SD.
Diagnosis is difficult because other voice disorders have hyperfunctional voice, termed muscle tension dysphonia (MTD)8. Patients with hyperfunctional voice alone do not have phonatory breaks1. SD responds well to local injection of botulinum toxin9 but does not improve with voice therapy alone10. Hyperfunctional voice often improves with voice therapy, which is not beneficial in SD11. Vocal tremor, with constant modulation of voice amplitude and frequency, is most noticeable during prolonged vowels12. Tremor and SD often co-occur13, while hyperfunctional voice is rarely combined with tremor. The following recommendations for the diagnosis of SD comprise a major outcome of the meeting for research on SD.
Recommendations for Diagnosis
Based on a consensus among the participants, a three tiered approach was recommended: screening questions to suggest possible SD; a speech examination to identify probable SD: and nasolaryngoscopy for a definite diagnosis. The value of the possible, probable and diagnostic levels is to progressively narrow down the number of cases to be examined. An SD Study Group, to include neurologists, otolaryngologists and speech pathologists with experts in design and statistical analysis, will need to design and conduct a validation study of these procedures.
i. Screening Questionnaire (Possible SD)
Four screening questions could be made widely available for completion by prospective patients (Table 1). An affirmative answer to the first two questions and a duration greater than three months is required for possible SD; a positive answer to the fourth question is useful but not required. When SD is a possibility, patients should be examined by an otolaryngologist or neurologist specializing in SD.
Table 1.
Screening Questions for Spasmodic Dysphonia
| Question | Required for Spasmodic Dysphonia | Not Expected for Spasmodic Dysphonia |
|---|---|---|
| 1. Does it take a lot of work for you to talk? |
yes | no |
| 2. Is it sometimes easier and sometimes more difficult to talk? |
yes | Sometimes entirely normal without treatment |
| 3. How long has it been difficult for you to talk? |
3 months or more, a chronic problem |
Less than 3 months |
| 4. Can you do any of the following normally? |
Some of the following should be normal |
Affected |
| -shout | normal | Can’t shout |
| -cry | normal | Not normal |
| -laugh | normal | Not normal |
| -whisper | normal | Affected same as speech |
| -sing | normal | More affected than speech |
| -yawn | normal | Not normal |
ii. Clinical Speech Examination (Probable SD)
A specialist will have the patient perform the several speech tasks (Table 2). If patients have a strained effortful voice with voice breaks while speaking but not while shouting or whispering, SD is possible.
Table 2.
Speech Examination Findings Expected for Probable Spasmodic Dysphonia
| Task | Required for Spasmodic Dysphonia |
Hyperfunctional Voice |
|---|---|---|
| Repeating Adductor sentences (glottal stops and vowels) |
Adductor type: Breaks on vowels, 1 or more breaks per 3 sentences |
Equal voice symptoms on vowels and voiceless consonants |
| Repeated Abductor sentences (/p/, /t/, /k/, /s/, /h/, /f/) |
Abductor type: prolonged voiceless consonants, 1 or more breaks per 3 sentences |
Equal voice symptoms on vowels and voiceless consonants |
| Greater difficulty with one set of sentences |
One set is more difficult than another |
Both sets are equally affected, does not find one type of sentences more difficult |
| Shout | Normal | Affected same as speech |
| Strained choked voice | Less strain at a higher pitch | Consistent for all types of speech sounds and pitches |
| Prolonged vowel | May have tremor on prolonged vowels, prolonged vowels are less affected than speech |
Prolonged vowels are similarly affected to speech, no tremor |
| Counting from 1 to 10 | Breaks on vowels or prolonged voiceless consonants |
No voice breaks |
Sentences can elicit adductor voice symptoms and others can elicit abductor voice symptoms (supplementary materials Appendix A). Patients should repeat sentences in their normal speaking voice and in a whisper. One or more voice breaks per 3 sentences during speaking and fewer during whispering are required. The clinician may use as many sentences as required to confirm that particular symptoms are present. The numbers of breaks in 10 sentences will measure severity. Shouting “No” or “Not now” in a loud voice, should be symptom free.
To differentiate between adductor SD, abductor SD and hyperfunctional voice, the patient repeats sentences from the adductor and abductor lists, indicating which type is more effortful. Adductor SD patients report more effort with adductor sentences, abductor SD patients with abductor sentences, and hyperfunctional voice patients report equal difficulty with both.
Ratings of a choked strained voice should be made on a visual analogue scale with anchors of “normal” on the left and “constantly severely strained” on the right to identify MTD without breaks. Prolonged vowels for 5 seconds, such as /i/ “tea”, or /ɑ/“father” at a normal pitch can identify voice tremor, which usually reduces at a high pitches.
iii. Laryngeal Examination (Definite SD)
Fiberoptic nasolaryngoscopy excludes other bases for the voice symptoms (Table 3) and is diagnostic. No anesthesia should be used; a video record is useful but not essential. No anatomical defects to account for abnormal voice and speech should be present; normal vocal fold movement is present during respiration, cough, throat clear and whistling. Vocal fold tremor and spasms may be observed on prolonged vowels and during sentences.
Table 3.
Nasolaryngoscopy Examination Findings Expected for Definite Spasmodic Dysphonia
| Task or Finding | Required for Spasmodic Dysphonia |
Expected for Hyperfunctional Voice |
|---|---|---|
| Structure | Normal | May have erythema or nodules |
| Vocal fold asymmetry | Normal | Normal |
| Whistling | Normal adductor and abductor movement |
Normal adductor and abductor movement |
| Prolonged Vowels | May have tremor or spasms in vocal folds |
Consistent hyperfunctional posture |
| Adductor sentences | Adductor Type: Intermittent hyperadduction on vowels |
Consistent hyperfunctional posture |
| Abductor sentences | Abductor Type: Intermittent abduction on voiceless consonants (/p/, /t/, /k/, /s/, /h/, /f/) |
Consistent hyperfunctional posture |
The purpose of this procedure is to identify patients who have a diagnosis of SD for research. Therefore, patients with a probable SD result from the clinical examination who are not given a Definite SD diagnosis should not be included for research studies on SD because they may have a different disorder or a combination of both SD along with another voice disorder.
Pilot Study of Diagnostic Validity
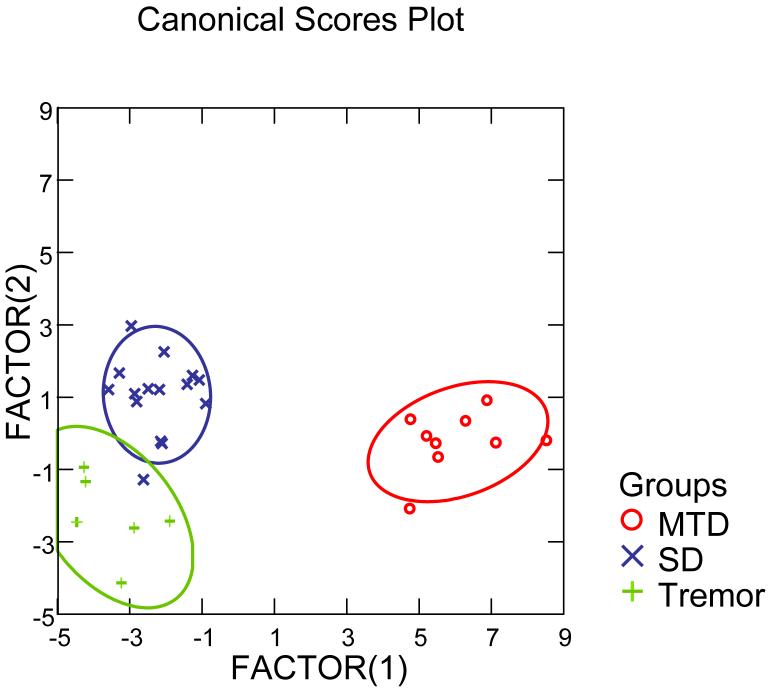
Thirty patients were recruited for the study who had previously been diagnosed as having SD, MTD or tremor based on a multidisciplinary examination and the patients’ responses to treatment with botulinum toxin injection at the National Institutes of Health. All participants provided written informed consent to participate in an Internal Review Board approved research protocol of the National Institute of Neurological Disorders and Stroke. They were examined using the three tiered approach; the patient screening questionnaire, speech recordings of sentences and videotaped nasoendoscopy examinations. A speech pathologist scored the voice characteristics in sentences while blinded to subject identity. In addition, a team of two other speech pathologists and an otolaryngologist reviewed the nasoendoscopy recordings while blind to subject identity and came to a consensus on each of the measures. The ratings were entered into a stepwise linear discriminate function analysis aimed at identifying the factors that could identify the three groups based on the 25 items. The results identified two canonical factors with eigenvalues of 17.834 and 1.842 based on 13 of the 25 measures (Table 4) with canonical correlations of 0.973 and 0.803 and a Wilks’s Lambda of 0.019, F=7.288, p<0.0005. The classification was 97% accurate; one SD patient was misclassified as having tremor (Figure 1).
Table 4.
Results of Discriminant Function Analysis Canonical Discriminant Functions: Standardized by within Variances with Means for Each Measures for the Three Patient Groups
| Measure | Set 1 | Set 2 | Mean Muscular Tension Dysphonia |
Mean Spasmodic Dysphonia |
Mean Tremor |
|---|---|---|---|---|---|
| Alot of Work to Talk | 3.035 | 0.283 | 71.9 | 70.1 | 59.0 |
| Laughing Less Affected than Speech | 4.289 | 0.366 | 25.4 | 20.3 | 38.5 |
| Crying Less Affected than Speech | -2.320 | 0.153 | 32.7 | 28.4 | 34.2 |
| Shouting Less Affected than Speech | -2.384 | 0.740 | 36.9 | 62.5 | 44.8 |
| Whisper Less Affected than Speech | -1.892 | -0.846 | 17.3 | 22.1 | 42.8 |
| Mean number of abductor breaks in 20 sentences |
-3.611 | 1.037 | 1.2 | 2.0 | 1.7 |
| Mean number of adductor breaks in 20 sentences |
3.851 | -1.048 | 1.3 | 2.5 | 1.7 |
| Voice Tremor during Prolonged Vowel |
-2.642 | -0.384 | 21.3 | 29.9 | 52.0 |
| Voice Abnormality during Shouting | 1.227 | -0.251 | 19.1 | 22.4 | 16.8 |
| Functional Vocal Fold Asymmetry | 4.098 | 0.241 | 2.5 | 3.9 | 0.0 |
| Vocal Fold Tremor at Rest | -1.481 | -0.108 | 3.0 | 5.4 | 22.8 |
| Constant Abnormal Laryngeal Posture during Voice |
1.354 | -0.896 | 52.1 | 11.7 | 17.3 |
| Constant Abnormal Laryngeal Posture during Whisper |
-0.842 | 1.379 | 47.2 | 18.2 | 4.2 |
| Group means: | |||||
| Muscular Tension Dysphonia | 6.077 | -0.231 | |||
| Spasmodic Dysphonia | -2.249 | 1.065 | |||
| Tremor | -3.493 | -2.318 |
Figure 1.
Discriminate function analysis results for three patient groups, MTD (muscular tension dysphonia), SD (spasmodic dysphonia) and Tremor.
As can be seen in Figure 1, the first factor (Set 1) displayed on the x axis differentiated the MTD group from the SD and tremor groups. As the group mean values on the individual measures in Table 4 demonstrate, the MTD group had high ratings for Constant Abnormal Laryngeal Posture During Voice and During Whisper in contrast with the SD and tremor groups. The second factor (Set 2) was useful for differentiating between the SD group and the tremor group on the y axis. High ratings for the SD group were found on Shouting Being Less Affected Than Speech, The Mean Number of Adductor Voice Breaks in Sentences, The Mean Number of Abductor Breaks in Sentences And Functional Vocal Fold Asymmetry During Speech. In contrast the tremor group had high ratings on Laughing Was Less Affected than Speech, Whisper Was Less Affected than Speech , Voice Tremor During Prolonged Vowels and Vocal Tremor at Rest. A multi-center validation study is needed to examine these procedures further, although these initial results are promising..
II. Epidemiology and Risk Factors for Spasmodic Dysphonia
Limited data are available on prevalence, incidence, age of onset, gender, race, ethnic and regional variation, and risk factors.
Clinical and Epidemiological Studies
SD is the third most prevalent form of focal dystonia (estimated at 1 per 100,000) following cervical dystonia and blepharospasm14. Underestimates are likely when derived from persons seeking medical attention. SD seems more frequent in persons of European descent15; no reports from Japan include persons with SD16. Reports of the female to male ratios vary from 1:117 to 7:1 in the United States3.
About 10% have a family history of dystonia18 with onset in the 40’s3,14,18 . Ninety percent have adductor SD, 26% also have essential tremor3. At least 30% report having an upper respiratory tract infection or major life stress (21%) prior to onset3, although recall bias is likely.
Because SD may not have a strong genetic component, investigation of potential environmental determinants may be fruitful. Although epidemiologic studies can only identify associations, they provide clues for animal models. Epidemiologic studies on SD will be challenging given the diagnostic difficulties, which also limit the accuracy of patient registries. Clinic-based populations are not generalizable while mandatory health care registries are representative but costly.
Rigorous epidemiologic studies are essential for identifying risk factors19. Patient recall bias is frequent20 and interviewers seek information more intensely from patients than controls. Only prospective examination is verifiable. Incorporation bias depends on population characteristics; patients with SD seen in movement disorders clinics21 may differ from those in voice centers3. Populations with free access to health care and interview studies can minimize this type of bias. Because SD is rare, studies within health care systems are more efficient than door to door surveys.
Genetic Studies of Dystonia
Fifteen “DYT” gene loci with variable penetrance and expressivity have been designated, six characterized by dystonia, although none are specifically linked to SD22. No genetic studies have been reported in large families with just SD; most families have only a few affected members. Of the mapped primary dystonia genes, DYT-1 is associated with early onset dystonia below 13 years, affects the limbs and frequently generalizes although only 10%-20% have voice symptoms similar to sporadic SD. DYT-6 and DYT-13 families have cervical, cranial and arm involvement while late-onset focal dystonia occurs with DYT-7. All three genes are autosomal dominant with reduced penetrance. Laryngeal involvement occurs in large DYT6 families although the voice symptoms have not been characterized23. DYT-4, a whispering dystonia, likely differs from sporadic SD24.
Previously, identifying genes depended on linkage analyses in large families. Using single nucleotide polymorphisms (SNPs) allows for identifying genes from cohorts of unrelated patients25. Whole genome mapping can search for genetic association in a disease cohort compared to a control cohort. This method involves replication, requires a large number of patients with the same phenotype, and can be costly. Finding a gene makes it possible to develop animal and cellular models, study the biochemical cascade of events, hypothesize pathogenesis and intervene in relevant pathways for prevention. Preclinical manifestations can be studied in persons predisposed to the disease to determine the interaction between environmental factors and genetic predisposition.
Recommendations for Population Research
i. Neuropathology Studies
The SD patient and research community should collaborate with existing brain banks that store brains, archive data, and distribute tissue (supplementary materials, Appendix B). Annual exams can determine the health histories of living donors and identify symptoms of other neurological disorders, most notably Parkinson’s disease, tremor, and dementia. Brains can be analyzed for neuropathological markers (e.g. torsin A) or neurotransmitter imbalances. Collecting post mortem larynges from SD patients may be fruitful. The SD Study Group should identify SD brain and tissue banking sites for distribution to the research and patient communities.
ii. Genetic Research
Pursuing a genetic risk factor for SD is a million dollar experiment and considered premature without standardized diagnostic criteria. Although a common etiology may exist between all forms of focal dystonia, this should be determined. For whole genome association studies, 500 cases and 500 controls are needed to identify a candidate gene, and then the same number are needed again to confirm identification through replication. Case collection on this scale for a rare disorder such as SD requires collaboration among centers and a dedicated research team. Standardized clinical criteria with family histories and storage of blood samples in a central public repository are needed.
iii. Epidemiological Study
Ongoing epidemiologic studies on the prevalence of different focal dystonias should include SD using procedures for possible, probable and definite diagnosis. The SD Study Group could enhance continuing collaboration between the disciplines (Neurology, Otolaryngology, Speech Pathology) in planning epidemiological studies.
iv. Patient Registries
A questionnaire on the National Spasmodic Dysphonia Association website, mailed to the membership or placed in voice clinics could establish a voluntary patient registry for research participation. The lack of accurate diagnosis of SD would make the data of little use for population research purposes. The goal is to identify patients willing to participate in clinical trials while protecting patient privacy.
III. Pathophysiology of Focal Dystonia
SD is likely similar to other focal dystonias with a related pathophysiology in the CNS controlling the laryngeal muscles during speech.
The Mammalian Vocalization System
Mammalian vocalization consists of a genetically preprogrammed system for the expression of emotional states, such as alarm, aggression, submission, comfort or need for social contact26. This system is used for nonverbal emotional vocal utterances in humans, such as laughter or cry. In contrast to this innate vocal system, the capacity to produce learned vocal utterances for speech is limited to humans.
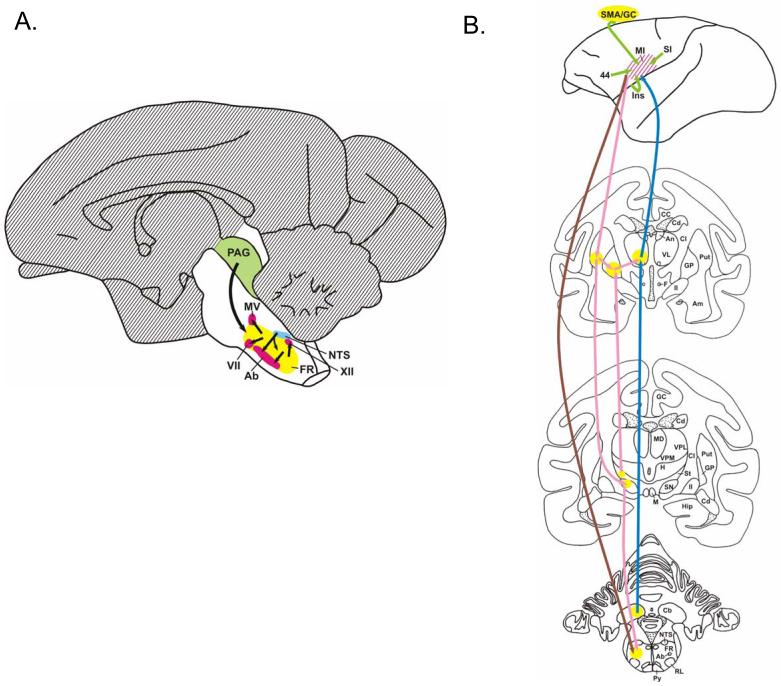
The entire forebrain, cerebellum and rostral midbrain, shown as hatched areas are dispensable for innate vocal patterns in the squirrel monkey27,28 (Figure 2A). Structures indispensable for innate vocalization are: the motor neurons located in the lower brainstem (Ab, the nucleus ambiguus) and ventral horn of the spinal cord; the reticular formation (FR) of the lower brainstem27; the solitary tract nucleus (NTS), a somatosensory relay nucleus of proprioceptive input from the larynx and oral cavity, and stretch receptors in the lungs; and the periaqueductal gray (PAG) of the midbrain29, which triggers vocalization responses to external and internal (motivational) stimuli30.
Figure 2.
A. The mammalian vocalization system includes areas not essential for innate vocalizations (hatched). B. Connections to the primary laryngeal motor control region in non-human primates include: the indirect pathway (pink), the cerebello-thalamo-cortical pathway (blue), and the corticobulbar pathway from M1 to the reticular formation in the brainstem (brown).
Additional structures are involved in speech and not in innate vocal patterns (Figure 2B). With bilateral damage to the “face” area of the primary motor cortex (M1), patients cannot speak or sing. Direct outputs from the face motor cortex involve the pyramidal tract (Py) to the brainstem (shown in brown) while indirect pathways are via the putamen (Put) to the substantia nigra (SN) and reticular formation (FR). Two extensions of the indirect pathway shown in pink are through the putamen and may be important for learned vocalizations: back to motor cortex (via globus pallidus (GP) and ventrolateral thalamus, VL); and between the globus pallidus and subthalamic nuclei (St). The cerebellum (Cb) is also a pathway to the motor cortex (M1) via the ventrolateral thalamus (VL) shown in blue. Abnormalities within this framework should be studied in SD.
Animal models can provide valuable understanding of the complex sensory and motor relationships in SD. Although an animal model for SD is not currently available, several have been developed for other forms of dystonia31. In primates, the toxins MPTP and 3NPA can provoke generalized dystonia, often in combination with parkinsonism32. Models for focal dystonias in primates include hand over-use33, and cervical dystonia due to midbrain lesions34. Rodent models are used for generalized, focal, and even paroxysmal dystonia31. A rat model for blepharospasm, a focal dystonia of the periocular muscles35 may be relevant to SD. Partial striatal dopamine depletion combined with partial injury to the motor nerves to the orbicularis oculi muscles is involved. Similar strategies might generate animal models for SD.
Only speech is affected in SD, whereas innate vocalizations (laughing and crying) are spared. Because vocalizations in primates and rodents are innate rather than learned, it remains unclear if modeling a hypothesized defect in sensorimotor networks will produce a vocal defect resembling SD.
Songbirds have both innate and learned vocalizations and may be considered for modeling SD36. The neuroanatomical basis for song and the molecular and cellular mechanisms underlying neural plasticity during song learning are well studied. The potential of the songbird model for SD depends upon whether the neuroanatomical basis for vocalizations in birds is sufficiently homologous to humans.
Dopaminergic Dysfunction in Focal Dystonia
Evidence for a specific neural pathway involved in dystonia has been emerging37. [18F] spiperone binds preferentially to D2-like dopamine receptors and had a 28% reduction in putamen uptake in persons with cranial or hand dystonia38. An animal model of transient dystonia found a reduction in D2-like dopamine receptors early after neurotoxin-induced nigrostriatal injury39. Dopaminergic abnormalities in the indirect pathway of the cortical-basal ganglia circuits may impair inhibition of unwanted muscle activity during intended movement in dystonia. The possibility of primary defects in this circuit producing behavioral abnormalities37 needs to be examined in SD.
Pathophysiology of Focal Dystonias
The confluence of an inherent genetic factor, and environmental modifiers, may lead to SD. Three physiological mechanisms may be involved in dystonia: decreased inhibition, increased plasticity, and abnormal sensory input. Loss of inhibition is well documented in dystonia and SD40. A variety of spinal and brainstem reflexes are abnormal, and the motor cortex also shows a loss of inhibition41,42. In patients with focal dystonias, measures of intracortical inhibition have revealed deficits43. Current treatments for dystonia generally increase inhibition or reduce excitability44. Center-surround inhibition, expected to play a role in fine motor movements, may be reduced in dystonia43. Motor training of individual finger movements for writer’s cramp, developed out of ideas on center-surround inhibition, substantially improved patients’ writing abilities45.
Increased plasticity, as measured by excitability after paired associative stimulation, was demonstrated in writer’s cramp46. In dystonia, increased plasticity could lead to an abnormal response to repetitive activity or increased use47.
Sensory abnormalities in hand dystonia include decreases in temporal and spatial discrimination48,49. Cerebral responses to vibrotactile hand stimulation were reduced in sensorimotor and supplementary motor areas in hand dystonia50. Sensory evoked potentials from the fingers of both the symptomatic and asymptomatic hands had a deranged somatotopic map49. Because the sensory system is a prime driver of the motor system, disordered sensory input could lead to disordered motor output.
Laryngeal Pathophysiology in SD
SD symptoms are intermittent spasms in the laryngeal muscles5. Current therapies are targeted towards reducing the impact of involuntary muscle spasms on voice. The emerging picture of SD is disordered inhibition in response to sensory feedback51. Muscles in the upper airway used for speech are also required for life-sustaining functions such as swallowing and breathing. Intervention in SD is complicated because of the need to maintain these life sustaining functions.
The larynx is sensitive to many types of stimuli52. Sensory fibers in the internal branch of the superior laryngeal nerve (SLN), terminate in the brainstem in the nucleus tractus solitarius53 and include sensors for pressure on the laryngeal mucosa54; negative supraglottal pressure55; flow receptors56; a lack of chloride ions57; and muscle activity58. Proprioceptive information from the cricoarytenoid joint is conveyed via the SLN, and subglottal afferents are conveyed via the recurrent laryngeal nerve (RLN). Sensory input to the nucleus solitarius is relayed via interneurons to laryngeal motor neurons in the nucleus ambiguus59.
If SD is an abnormality of sensory gating, it may have a similar pathophysiology to other disorders of disinhibition such as chronic cough, tic doloureux, hiccup and cricopharyngeal achalasia. The glottic-stop reflex in cough is similar to the laryngeal adductor reflex60elicited by SLN stimulation. Paroxysmal laryngospasm has been reported following injury to the SLN61.
Reduced inhibition of the laryngeal adductor response to paired electrical SLN stimulation occurred in SD51. Determining the roles of different neurotransmitters in modulating this reflex might identify new avenues for treatment62. Clinical methods for assessment of the laryngeal adductor response to air puff stimuli are available63 and could identify abnormalities in sensori-motor gating in SD. Care must be taken to avoid the confounding effects of higher levels of motor neuron activity likely present in focal dystonia and SD which could augment motor responses in these patients independent from inhibitory gating abnormalities.
Recommendations for Research on the Pathophysiology of SD
i. Animal Models
Although animal models may not precisely mimic the human condition, they can test predictions regarding pathophysiology and treatment. No other mammals have learned vocalizations like humans and cannot reproduce task specificity to speech, a hallmark of SD. However, if the pathophysiological processes hypothesized to underlie SD can be reproduced, the resulting abnormalities could be endpoints for testing hypotheses about SD. Specifically, a model similar to the blepharospasm model with partial lesions of striatal dopamine and damage to laryngeal motor (or sensory) nerves could be considered.
The potential relevance of songbirds should be explored. The effects of specific types of pharmacological and surgical lesions in songbirds should be reviewed from an SD perspective. Any findings suggestive of SD, such as muscle spasms occurring during song, should be examined further. Given the higher occurrence of SD and other focal dystonias in women, research into possible hormonal contributions and their direct/indirect effects on laryngeal sensorimotor control should be pursued.
ii. Integrative Systems Research on the Sensori-Motor Basis for Vocalization
The mammalian vocalization system can be used to study the effects of environmental and neurochemical manipulations on laryngeal sensori-motor function. Fully understanding the anatomy and circuitry for the larynx in the brain is fundamental for further research. The anatomy of laryngeal reflexes, the sensory relay connections to the nucleus ambiguus and descending corticobulbar connections all require further investigation. Although rodents versus primates were debated, higher brain centers that modulate laryngeal motor neurons need to be identified. GABA receptor agonists or glutamate antagonists to block excitatory neurotransmission could determine defects that may be associated with SD. Brain regions identified in animal studies, need to be examined in postmortem brains from SD patients.
iii. Functional Brain Imaging
Two brain imaging studies examined SD comparing brain activation before and after botulinum toxin injection 64,65. Patients with SD had reduced brain activation in sensorimotor regions during symptoms. Studies should be performed to confirm that the pathophysiology in SD is analogous to other dystonias. Writer’s cramp, a task specific focal dystonia, seems to have the closest potential similarity to SD.
As functional neuroimaging methods are applied to this disorder, care must be taken not to confound such studies because of increased speech effort in persons with voice disorders such as SD. Applying sensory stimuli at rest to examine central responses will avoid the confounding effects of the movement disorder. Investigations of selective neurotransmitter receptor systems may be useful, as in other focal dystonias66.
iv. Other Experiments
An autoimmune event may be associated with the development of SD. A study to examine whether a link exists would be worthwhile. Some patients with SD have had over 70 injections of botulinum toxin into their larynx. Animal studies that reproduce multiple injections could assess the cumulative long-term effects of botulinum toxin on laryngeal muscle physiology.
IV. Treatment of Spasmodic Dysphonia
Botulinum Toxin Injections
The most common and effective treatment for SD is injection of botulinum toxin into the laryngeal muscles, for which SD is an off-label indication. By inhibiting acetylcholine release at the neuromuscular junction, the toxins reduce muscle activity. About 90% of patients with adductor SD improve for 3 to 12 months after receiving an injection of botulinum toxin type A18, although voice production is not normal67. The only randomized, controlled and blinded trial in SD was small; demonstrating greater symptom reduction with botulinum toxin type A injection than following saline9. Although the thyroarytenoid muscle is normally injected for adductor SD18, some benefit may occur with injecting other adductor muscles such as the lateral cricoarytenoid (LCA)68. Patients with abductor SD also respond to botulinum toxin injection in the posterior cricoarytenoid (PCA), although treatment is less effective69.
Repeated botulinum toxin injections are needed every 3-6 months18 and dosages range widely across patients. Periods of breathiness and occasional aspiration of liquids follow thyroarytenoid injection for adductor SD while stridor and airway obstruction can follow PCA injections for abductor SD. Long-term results and safety still need to be addressed. Botulinum toxin for patients with voice tremor has less predictable results70. The outcome may relate to the movement disorder characteristics.
Surgical Approaches
The initial surgical approach to symptom control in SD was unilateral RLN section, which was not long lasting71 due to nerve reinnervation72. Current surgical approaches for SD include laryngoplasty73,74, myectomy75, myoplasty76, and denervation/reinnervation77. Laryngoplasty alters the cartilaginous skeleton of the larynx through anterior commissure push back, thyroid cartilage widening, or vocal fold medialization78. Patients with SD usually do not experience lasting benefit following anterior push back74. Myectomies to excise the thyroarytenoid, LCA, or PCA76 may reduce breaks, although vocal harshness and lasting benefit have been unpredictable.
The denervation/reinnervation procedure denervates the RLN branch to the thyroarytenoid muscle bilaterally and then sutures the nerve stump to the ansa cervicalis nerve77. The best candidates are female patients with adductor SD without tremor. Those with mixed adductor and abductor symptoms are not candidates and men have less benefit than women. Later, LCA myotomy was added, to improve outcome. Although 83% of respondents reported improvement for up to 49-52 months79, some continued to receive botulinum toxin injections and others reported mild swallowing difficulties. Controlled trials over 5-years are needed to determine the outcome of this surgery with independent blinded quantitative voice assessments, such as measures of mean number of voice breaks in sentences, the measure specific to SD in the diagnostic procedure (Table 4). Surgical procedures must be considered experimental for SD until controlled studies of the long-term voice outcomes are published.
Recommendations for Treatment Research in SD
Controlled treatment trials are needed to avoid placebo effects, which can be quite large in this patient group, while controlling for differences in previous treatments, concomitant signs and symptoms. At least a three year follow-up is essential, because SD is a chronic condition. Appropriate outcome measures include subjective rating scales80, objective acoustic measures1, and quality of life questionnaires81. Videotapes made before and after treatment can be randomized and rated by an uninvolved investigator. Because few trials of new treatment options have been conducted in this disorder, small, open-label Phase I trials should first explore new approaches before committing to large, randomized, placebo controlled trials.
i. Pharmacological Treatments for SD
Based on clinical observation, only tremor in SD has a modest response to beta-blockers and about 20%-30% of patients may be receiving botulinum toxin injections combined with oral medications. Such combinations may be effective while a drug alone is not. Some patients report symptom improvement with alcohol, suggesting that processes affected by alcohol might respond to pharmacological intervention. Systematic studies of combinations of botulinum toxin injections with oral medications are needed in SD. Immunosuppressive agents also may be worth evaluating particularly in patients with incipient SD. Small exploratory Phase I trials should be encouraged.
ii. Surgical Procedures
Both retrospective information on patients who have already received surgery and prospective studies are needed to determine if procedures are beneficial. Surgeries should be documented and standardized preoperative histories, assessments and postoperative follow-up by blinded independent raters are needed, to gather data on surgical outcomes for physicians and patients.
Trials to evaluate the efficacy of surgical procedures are needed. Surgical trials are difficult compared to medication trials because surgery is irreversible. To yield generalizable results, surgeons at several centers trained to perform similar procedures need to commit to following up patients at regular intervals. Animal studies could determine the short and long term physiological effects of permanent alterations in laryngeal structure.
iii. Deep Brain Stimulation
Deep brain stimulation (DBS) has proven effective in the internal globus pallidus in dystonia82. Exploratory Phase I trials should investigate the use of deep brain stimulation in patients with severe SD who are not adequately treated by botulinum toxin injection. Caution should be used, however, given the reduced benefit of DBS for voice and speech deficits in some cases83 and the need for objective data on the effects of DBS in the internal globus pallidus for voice and speech in dystonia. Mapping speech/voice motor control within the basal ganglia may further both the understanding of SD and the potential effects of DBS.
Supplementary Material
Acknowledgments
Support for the Workshop was from the Office of Rare Diseases of the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, the National Spasmodic Dysphonia Association, the Movement Disorder Society and the National Institute on Deafness and Other Communication Disorders and the Intramural Research Program of the National Institute of Neurological Disorders and Stroke. The authors gratefully acknowledge the expert assistance of Susannah Chang, Ph.D. the medical writer who developed the initial draft of this white paper summarizing the presentations, deliberations and recommendations of each of the presenters and the group discussion during the meeting.
References
- 1.Sapienza CM, Walton S, Murry T. Adductor spasmodic dysphonia and muscular tension dysphonia: acoustic analysis of sustained phonation and reading. J Voice. 2000;14:502–20. doi: 10.1016/s0892-1997(00)80008-9. [DOI] [PubMed] [Google Scholar]
- 2.Izdebski K, Dedo HH, Boles L. Spastic dysphonia: A patient profile of 200 cases. American Journal of Otolaryngology. 1984;5:7–14. doi: 10.1016/s0196-0709(84)80015-0. [DOI] [PubMed] [Google Scholar]
- 3.Schweinfurth JM, Billante M, Courey MS. Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope. 2002;112:220–3. doi: 10.1097/00005537-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Adler CH, Edwards BW, Bansberg SF. Female predominance in spasmodic dysphonia. J Neurol Neurosurg Psychiatry. 1997;63:688. doi: 10.1136/jnnp.63.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shipp T, Izdebski K, Reed C, et al. Intrinsic laryngeal muscle activity in a spastic dysphonic patient. Journal of Speech Hear Dis. 1985;50:54–59. doi: 10.1044/jshd.5001.54. [DOI] [PubMed] [Google Scholar]
- 6.Bloch CS, Hirano M, Gould WJ. Symptom improvement of spastic dysphonia in response to phonatory tasks. Annals Otol,Rhinol Laryngol. 1985;94:51–54. doi: 10.1177/000348948509400111. [DOI] [PubMed] [Google Scholar]
- 7.Edgar JD, Sapienza CM, Bidus K, et al. Acoustic measures of symptoms in abductor spasmodic dysphonia. J Voice. 2001;15:362–72. doi: 10.1016/S0892-1997(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 8.Morrison MD, Rammage LA, Belisle GM, et al. Muscular tension dysphonia. J Otolaryngol. 1983;12:302–06. [PubMed] [Google Scholar]
- 9.Truong DD, Rontal M, Rolnick M, et al. Double-blind controlled study of botulinum toxin in adductor spasmodic dysphonia. Laryngoscope. 1991;101:630–34. doi: 10.1288/00005537-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Murry T, Woodson GE. Combined-modality treatment of adductor spasmodic dysphonia with botulinum toxin and voice therapy. J Voice. 1995;9:460–5. doi: 10.1016/s0892-1997(05)80211-5. [DOI] [PubMed] [Google Scholar]
- 11.Roy N, Bless DM, Heisey D, et al. Manual circumlaryngeal therapy for functional dysphonia: an evaluation of short- and long-term treatment outcomes. J Voice. 1997;11:321–31. doi: 10.1016/s0892-1997(97)80011-2. [DOI] [PubMed] [Google Scholar]
- 12.Ludlow CL, Bassich CJ, Connor NP, et al. Phonatory characteristics of vocal fold tremor. J Phonetics. 1986;14:509–15. [Google Scholar]
- 13.Aronson AE, Hartman DE. Adductor spastic dysphonia as a sign of essential (voice) tremor. J Speech Hear Dis. 1981;46:52–58. doi: 10.1044/jshd.4601.52. [DOI] [PubMed] [Google Scholar]
- 14.Konkiewitz E Castelon, Trender-Gerhard I, Kamm C, et al. Service-based survey of dystonia in Munich. Neuroepidemiology. 2002;21:202–6. doi: 10.1159/000059525. [DOI] [PubMed] [Google Scholar]
- 15.Le KD, Nilsen B, Dietrichs E. Prevalence of primary focal and segmental dystonia in Oslo. Neurology. 2003;61:1294–6. doi: 10.1212/01.wnl.0000090463.05980.59. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto S, Nishimura M, Shibasaki H, et al. Epidemiology of primary dystonias in Japan: comparison with Western countries. Mov Disord. 2003;18:1196–8. doi: 10.1002/mds.10480. [DOI] [PubMed] [Google Scholar]
- 17.Nutt JG, Muenter MD, Aronson A, et al. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov Disord. 1988;3:188–94. doi: 10.1002/mds.870030302. [DOI] [PubMed] [Google Scholar]
- 18.Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope. 1998;108:1435–41. doi: 10.1097/00005537-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher NA, Harding AE, Marsden CD. A case-control study of idiopathic torsion dystonia [see comments] Mov Disord. 1991;6:304–9. doi: 10.1002/mds.870060406. [DOI] [PubMed] [Google Scholar]
- 20.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32:51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]
- 21.Blitzer A, Brin MF, Fahn S, et al. Clinical and laboratory characteristics of focal laryngeal dystonia:Study of 110 cases. Laryngoscope. 1988;98:636–40. doi: 10.1288/00005537-198806000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Bressman SB. Dystonia: phenotypes and genotypes. Rev Neurol (Paris) 2003;159:849–56. [PubMed] [Google Scholar]
- 23.Almasy L, Bressman SB, Raymond D, et al. Idiopathic torsion dystonia linked to chromosome 8 in two Mennonite families. Ann Neurol. 1997;42:670–3. doi: 10.1002/ana.410420421. [DOI] [PubMed] [Google Scholar]
- 24.Parker N. Hereditary whispering dysphonia. J Neurol, Neurosurg Psychiat. 1985;48:218–24. doi: 10.1136/jnnp.48.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craig DW, Stephan DA. Applications of whole-genome high-density SNP genotyping. Expert Rev Mol Diagn. 2005;5:159–70. doi: 10.1586/14737159.5.2.159. [DOI] [PubMed] [Google Scholar]
- 26.Jurgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–58. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 27.Jurgens U, Hage SR. On the role of the reticular formation in vocal pattern generation. Behav Brain Res. 2006 doi: 10.1016/j.bbr.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Hage SR, Jurgens U. Localization of a vocal pattern generator in the pontine brainstem of the squirrel monkey. Eur J Neurosci. 2006;23:840–4. doi: 10.1111/j.1460-9568.2006.04595.x. [DOI] [PubMed] [Google Scholar]
- 29.Siebert S, Jurgens U. Vocalization after periaqueductal grey inactivation with the GABA agonist muscimol in the squirrel monkey. Neurosci Lett. 2003;340:111–4. doi: 10.1016/s0304-3940(03)00071-5. [DOI] [PubMed] [Google Scholar]
- 30.Jurgens U, Pratt R. Role of the periaqueductal grey in vocal expression of emotion. Brain Res. 1979;167:367–78. doi: 10.1016/0006-8993(79)90830-8. [DOI] [PubMed] [Google Scholar]
- 31.Jinnah HA, Hess EJ, Ledoux MS, et al. Rodent models for dystonia research: characteristics, evaluation, and utility. Mov Disord. 2005;20:283–92. doi: 10.1002/mds.20364. [DOI] [PubMed] [Google Scholar]
- 32.Mink JW, Moerlein SM, Perlmutter JS. Pallidal activity in a monkey model of dystonia and parkinsonism. Movt Disord. 2004;19:S77. [Google Scholar]
- 33.Byl NN. Focal hand dystonia may result from aberrant neuroplasticity. Adv Neurol. 2004;94:19–28. [PubMed] [Google Scholar]
- 34.Foltz EL, Knopp LM, Ward AA., Jr Experimental spasmodic torticollis. J Neurosurg. 1959;16:55–67. doi: 10.3171/jns.1959.16.1.0055. [DOI] [PubMed] [Google Scholar]
- 35.Schicatano EJ, Basso MA, Evinger C. Animal model explains the origins of the cranial dystonia benign essential blepharospasm. J Neurophysiol. 1997;77:2842–6. doi: 10.1152/jn.1997.77.5.2842. [DOI] [PubMed] [Google Scholar]
- 36.Doupe AJ, Perkel DJ, Reiner A, et al. Birdbrains could teach basal ganglia research a new song. Trends Neurosci. 2005;28:353–63. doi: 10.1016/j.tins.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Perlmutter JS, Mink JW. Dysfunction of dopaminergic pathways in dystonia. Adv Neurol. 2004;94:163–70. [PubMed] [Google Scholar]
- 38.Perlmutter JS, Stambuk MK, Markham J, et al. Decreased [18F]spiperone binding in putamen in dystonia. Adv Neurol. 1998;78:161–8. [PubMed] [Google Scholar]
- 39.Todd RD, Carl J, Harmon S, et al. Dynamic changes in striatal dopamine D2 and D3 receptor protein and mRNA in response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) denervation in baboons. J Neurosci. 1996;16:7776–82. doi: 10.1523/JNEUROSCI.16-23-07776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen LG, Ludlow CL, Warden M, et al. Blink reflex curves in patients with spasmodic dysphonia. Neurology. 1989;39:572–77. doi: 10.1212/wnl.39.4.572. [DOI] [PubMed] [Google Scholar]
- 41.Ibanez V, Sadato N, Karp B, et al. Deficient activation of the motor cortical network in patients with writer’s cramp. Neurology. 1999;53:96–105. doi: 10.1212/wnl.53.1.96. [DOI] [PubMed] [Google Scholar]
- 42.Butefisch CM, Boroojerdi B, Chen R, et al. Task-dependent intracortical inhibition is impaired in focal hand dystonia. Mov Disord. 2005;20:545–51. doi: 10.1002/mds.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohn YH, Hallett M. Surround inhibition in human motor system. Exp Brain Res. 2004;158:397–404. doi: 10.1007/s00221-004-1909-y. [DOI] [PubMed] [Google Scholar]
- 44.Boroojerdi B, Cohen LG, Hallett M. Effects of botulinum toxin on motor system excitability in patients with writer’s cramp. Neurology. 2003;61:1546–50. doi: 10.1212/01.wnl.0000095965.36574.0f. [DOI] [PubMed] [Google Scholar]
- 45.Zeuner KE, Shill HA, Sohn YH, et al. Motor training as treatment in focal hand dystonia. Mov Disord. 2005;20:335–41. doi: 10.1002/mds.20314. [DOI] [PubMed] [Google Scholar]
- 46.Quartarone A, Bagnato S, Rizzo V, et al. Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain. 2003;126:2586–96. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- 47.Quartarone A, Siebner HR, Rothwell JC. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci. 2006;29:192–9. doi: 10.1016/j.tins.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Bara-Jimenez W, Shelton P, Sanger TD, et al. Sensory discrimination capabilities in patients with focal hand dystonia. Ann Neurol. 2000;47:377–80. [PubMed] [Google Scholar]
- 49.Bara-Jimenez W, Catalan MJ, Hallett M, et al. Abnormal somatosensory homunculus in dystonia of the hand. Ann Neurol. 1998;44:828–31. doi: 10.1002/ana.410440520. [DOI] [PubMed] [Google Scholar]
- 50.Tempel LW, Perlmutter JS. Abnormal vibration-induced cerebral blood flow responses in idiopathic dystonia. Brain. 1990;113:691–707. doi: 10.1093/brain/113.3.691. [DOI] [PubMed] [Google Scholar]
- 51.Ludlow CL, Schulz GM, Yamashita T, et al. Abnormalities in long latency responses to superior laryngeal nerve stimulation in adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1995;104:928–35. doi: 10.1177/000348949510401203. [DOI] [PubMed] [Google Scholar]
- 52.Sant’Ambrogio G. Afferent pathways for the cough reflex. Bull EurPhysiopatholRespir. 1987;23(Suppl 10):19s–23s. [PubMed] [Google Scholar]
- 53.Sessle BJ. Excitatory and inhibitory inputs to single neurones in the solitary tract nucleus and adjacent reticular formation. Brain Res. 1973;53:319–31. doi: 10.1016/0006-8993(73)90217-5. [DOI] [PubMed] [Google Scholar]
- 54.Davis PJ, Nail BS. Quantitative analysis of laryngeal mechanosensitivity in the cat and rabbit. J Physiol (Lond) 1987;388:467–85. doi: 10.1113/jphysiol.1987.sp016625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathew OP, Sant’Ambrogio FB, Sant’Ambrogio G. Laryngeal paralysis on receptor and reflex responses to negative pressure in the upper airway. Respir Physiol. 1988;74:25–34. doi: 10.1016/0034-5687(88)90137-5. [DOI] [PubMed] [Google Scholar]
- 56.Sant’Ambrogio G, Brambilla-Sant’Ambrogio F, Mathew OP. Effect of cold air on laryngeal mechanoreceptors in the dog. Respir Physiol. 1986;64:45–56. doi: 10.1016/0034-5687(86)90059-9. [DOI] [PubMed] [Google Scholar]
- 57.Sant’Ambrogio G, Anderson JW, Sant’Ambrogio FB, et al. Response of laryngeal receptors to water solutions of different osmolality and ionic composition. Respir Med. 1991;85(Suppl A):57–60. doi: 10.1016/s0954-6111(06)80256-8. [DOI] [PubMed] [Google Scholar]
- 58.Sant’Ambrogio G, Mathew OP, Sant’Ambrogio FB. Role of intrinsic muscles and tracheal motion in modulating laryngeal receptors. Respir Physiol. 1985;61:289–300. doi: 10.1016/0034-5687(85)90072-6. [DOI] [PubMed] [Google Scholar]
- 59.Ambalavanar R, Tanaka Y, Selbie WS, et al. Neuronal activation in the medulla oblongata during selective elicitation of the laryngeal adductor response. J Neurophysiol. 2004;92:2920–32. doi: 10.1152/jn.00064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasaki CT, Suzuki M. Laryngeal reflexes in cat, dog and man. Arch Otolaryngol. 1976;102:400–02. doi: 10.1001/archotol.1976.00780120048004. [DOI] [PubMed] [Google Scholar]
- 61.Wani MK, Woodson GE. Paroxysmal laryngospasm after laryngeal nerve injury. Laryngoscope. 1999;109:694–7. doi: 10.1097/00005537-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Ambalavanar R, Purcell L, Miranda M, et al. Selective suppression of late laryngeal adductor responses by NMethyl-D-Asparate receptor blockade in the cat. J Neurophysiol. 2002;87:1252–62. doi: 10.1152/jn.00595.2001. [DOI] [PubMed] [Google Scholar]
- 63.Kearney PR, Poletto CJ, Mann EA, et al. Suppression of thyroarytenoid muscle responses during repeated air pressure stimulation of the laryngeal mucosa in awake humans. Ann Otol Rhinol Laryngol. 2005;114:264–70. doi: 10.1177/000348940511400403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haslinger B, Erhard P, Dresel C, et al. “Silent event-related” fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology. 2005;65:1562–9. doi: 10.1212/01.wnl.0000184478.59063.db. [DOI] [PubMed] [Google Scholar]
- 65.Ali SO, Thomassen M, Schulz GM, et al. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: an H215O PET study. J Speech Lang Hear Res. 2006;49:1127–46. doi: 10.1044/1092-4388(2006/081). [DOI] [PubMed] [Google Scholar]
- 66.Perlmutter JS, Stambuk MK, Markham J, et al. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. J Neurosci. 1997;17:843–50. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langeveld TP, van Rossum M, Houtman EH, et al. Evaluation of voice quality in adductor spasmodic dysphonia before and after botulinum toxin treatment. Ann Otol Rhinol Laryngol. 2001;110:627–34. doi: 10.1177/000348940111000707. [DOI] [PubMed] [Google Scholar]
- 68.Castellanos PF, Gates GA, Esselman G, et al. Anatomic considerations in botulinum toxin type A therapy for spasmodic dysphonia. Laryngoscope. 1994;104:656–62. doi: 10.1288/00005537-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Bielamowicz S, Squire S, Bidus K, et al. Assessment of posterior cricoarytenoid botulinum toxin injections in patients with abductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2001;110:406–12. doi: 10.1177/000348940111000503. [DOI] [PubMed] [Google Scholar]
- 70.Hertegard S, Granqvist S, Lindestad PA. Botulinum toxin injections for essential voice tremor. Ann Otol Rhinol Laryngol. 2000;109:204–9. doi: 10.1177/000348940010900216. [DOI] [PubMed] [Google Scholar]
- 71.Aronson AE, Desanto LW. Adductor spasmodic dysphonia: three years after recurrent nerve section. Laryngoscope. 1983;93:1–8. doi: 10.1288/00005537-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Fritzell B, Hammarberg B, Schiratzki H, et al. Long-term results of recurrent laryngeal nerve resection for adductor spasmodic dysphonia. J Voice. 1993;7:172–78. doi: 10.1016/s0892-1997(05)80348-0. [DOI] [PubMed] [Google Scholar]
- 73.Isshiki N, Tsuji DH, Yamamoto Y, et al. Midline lateralization thyroplasty for adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2000;109:187–93. doi: 10.1177/000348940010900214. [DOI] [PubMed] [Google Scholar]
- 74.Chan SW, Baxter M, Oates J, et al. Long-term results of type II thyroplasty for adductor spasmodic dysphonia. Laryngoscope. 2004;114:1604–8. doi: 10.1097/00005537-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 75.Koufman JA, Rees CJ, Halum SL, et al. Treatment of adductor-type spasmodic dysphonia by surgical myectomy: a preliminary report. Ann Otol Rhinol Laryngol. 2006;115:97–102. doi: 10.1177/000348940611500203. [DOI] [PubMed] [Google Scholar]
- 76.Shaw GY, Sechtem PR, Rideout B. Posterior cricoarytenoid myoplasty with medialization thyroplasty in the management of refractory abductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2003;112:303–6. doi: 10.1177/000348940311200403. [DOI] [PubMed] [Google Scholar]
- 77.Berke GS, Blackwell KE, Gerratt RR, et al. Selective laryngeal adductor denervation-reinnervation: a new surgical treatment for adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1999;108:227–31. doi: 10.1177/000348949910800302. [DOI] [PubMed] [Google Scholar]
- 78.Isshiki N. Recent advances in phonosurgery. Folia Phoniat. 1980;32:119–54. doi: 10.1159/000264334. [DOI] [PubMed] [Google Scholar]
- 79.Chhetri DK, Mendelsohn AH, Blumin JH, et al. Long-term follow-up results of selective laryngeal adductor denervation-reinnervation surgery for adductor spasmodic dysphonia. Laryngoscope. 2006;116:635–42. doi: 10.1097/01.MLG.0000201990.97955.E4. [DOI] [PubMed] [Google Scholar]
- 80.Barkmeier JM, Case JL, Ludlow CL. Identification of symptoms for spasmodic dysphonia and vocal tremor: a comparison of expert and nonexpert judges. J Commun Disord. 2001;34:21–37. doi: 10.1016/s0021-9924(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 81.Jacobson B, Johnson A, Grywalski C, et al. The Voice Handicap Index (VHI): Development and validation. Am J Speech Lang Pathol. 1997;6:66–70. [Google Scholar]
- 82.Coubes P, Cif L, El Fertit H, et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg. 2004;101:189–94. doi: 10.3171/jns.2004.101.2.0189. [DOI] [PubMed] [Google Scholar]
- 83.Deuschl G, Herzog J, Kleiner-Fisman G, et al. Deep brain stimulation: postoperative issues. Mov Disord. 2006;21(Suppl 14):S219–37. doi: 10.1002/mds.20957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.