Summary
Proteins of the DExH/D family are ATPases that can unwind duplex RNA in vitro. Individual members of this family coordinate many steps in ribonucleoprotein enzyme assembly and catalysis in vivo, but it is largely unknown how the action of these co-factors is specified and precisely timed. As a first step to address this question biochemically, we describe the development of a new protein-dependent group I intron splicing system that requires such an ATPase for coordinating successive steps in splicing. While genetic analysis in yeast have shown that at least five nuclear-encoded proteins are required for splicing of the mitochondrial aI5β group I intron, we show that efficient in vitro splicing of aI5β occurs with only two of these co-factors, and furthermore, they fulfill distinct functions in vitro. The Mrs1p protein stabilizes RNA structure and promotes the first step in splicing. In contrast, a DExH/D protein, Mss116p, acts after the first step and, utilizing ATP hydrolysis, specifically enhances the efficiency of exon ligation. An analysis of Mss116p variants with mutations that impair its RNA-stimulated ATP hydrolysis activity or reduce its ability to unwind duplexes show that the efficiency of ATP hydrolysis is a major determinant in promoting exon ligation. These observations suggest that Mss116p acts in aI5β splicing by catalyzing changes in the structure of the RNA/protein splicing intermediate that promote the second step. More broadly, these observations are consistent with a model in which the “functional-timing” of DExH/D-box protein action can be specified by a specific conformation of its substrate due to the “upstream” activity of other co-factors.
Keywords: DExH/D proteins, helicase, catalytic RNAs, exon ligation, RNP assembly and function
Introduction
Functional RNAs are at the heart of numerous essential enzymes that catalyze fundamental cellular processes, such as tRNA processing, translation and pre-mRNA splicing.1 In many examples, controlled and ordered conformational changes in the RNA components are essential for biological activity. In almost all cases, RNA binding proteins are required to promote RNA folding transitions that result in a functional structure.2,3 Protein facilitation of RNA folding has been shown to occur in two broad ways. First, proteins can stabilize one RNA conformation among competing structures through selective and tight binding. On the other hand, RNA chaperone proteins can bind transiently to RNA and accelerate folding steps that ultimately lead to a native structure. Recent experiments show that some proteins also display characteristics of both mechanisms.4,5 In many cases, multiple proteins act synergistically to fold RNA into an active conformation, although the final structure can be arrived at via multiple folding pathways.2,3,6
Once correctly assembled, ribonucleoprotein (RNP) enzymes also require ancillary proteins to coordinate successive steps in an RNP catalytic cycle (e.g. substrate recognition, catalysis and product release). In this regard, proteins that interact with RNA and catalyze hydrolysis of nucleotide triphosphates (NTPs) have been identified as essential co-factors in many cases.7,8 For example, one family of NTPases, designated DExH/D proteins, is involved in almost all aspects of pre-mRNA splicing, including the first and second chemical reactions.7,9,10,
DExH/D proteins have been shown to unwind model RNA duplexes and/or displace RNA binding proteins in vitro and therefore are often referred to as RNA helicases or unwindases.9,11 Structural and biochemical evidence have been used to propose a “two-state” model in which one family of RNA helicases, the DEAD-box proteins, couples ATP hydrolysis to RNA unwinding.12,13 The conserved catalytic core of these proteins consists of two RecA-like domains that form a “closed complex” when ATP and RNA are bound, and, upon ATP hydrolysis and inorganic phosphate release, an open complex that releases a partially melted duplex RNA. High affinity binding of RNA and ATP in the core is largely interdependent, and thus, ATP hydrolysis occurs primarily only in the presence of RNA.13–15 In contrast to these activities, very little is known about how individual DEAD-box proteins engage their native RNP substrates or how their activity is precisely timed or regulated in vivo. Furthermore, the nature of the physical rearrangements that individual proteins catalyze in vivo is also not well understood.
Group I introns are self-splicing catalytic RNAs that generally require protein co-factors for activity in vivo.16 Most co-factors for mitochondrial (mt) introns have been identified by genetic analysis in fungi, and in a few cases, a direct role in splicing has been verified by biochemical analysis with purified proteins, reviewed in 16,17. In general, the proteins bind tightly to specific introns, forming an RNP particle, and facilitate splicing by stabilizing the active structure of the RNA. Once the RNP particle has formed, the intron catalyzes splicing via two transesterification reactions. In the first step, an exogenous guanosine co-factor is bound by the intron and it attacks the 5’ splice site (SS) resulting in covalent attachment of the guanosine to the first base of the intron. The free 3’ hydroxyl of the 5’ exon then attacks the 3’ SS resulting in exon ligation.
In addition to intron-specific protein co-factors, mt group I introns in Neurospora crassa (Nc) and yeast also require DEAD-box proteins for splicing.18–20 Since many group I introns assemble into RNPs with their intron-specific co-factors, they represent excellent model systems to investigate how DExH/D-box proteins can influence both the assembly and function of cognate RNPs. For example, in collaboration with the mt tyrosyl tRNA synthetase (CYT-18), the DEAD-box CYT-19 protein from Neurospora facilitates splicing of the Nc large ribosomal subunit (LSU) intron both in vivo and in vitro, as well as a non-cognate group I intron in vitro.19
The related protein, Mss116p, from yeast is also required for the splicing of mt group I and II introns in vivo and is also thought to play a role in mt translation.18,20 It has been recently demonstrated that Mss116p, in an ATP-dependent manner, increases the efficiency of the in vitro splicing of various group II introns from yeast.21–23 Furthermore, Mss116p can stimulate splicing of the non-cognate Nc mt LSU group I intron complexed with the CYT-18 protein, although ATP hydrolysis is not strictly required.22 In these cases, it has been speculated that Mss116p functions transiently to resolve mis-folded structures that curtail overall catalytic activity or to stabilize on-pathway folded intermediates, properties that have been exemplified by RNA chaperones and intron specific co-factors, respectively.1,3 Interestingly, Mss116p also inhibits the splicing of the Tetrahymena intron in vitro, most likely by catalyzing partial unfolding of the native structure.22,23 Since only a limited number of introns have been tested in vitro(two non-cognate group I and two cognate group II introns), it is difficult to judge whether Mss116p functions in specialized ways for distinct introns. Furthermore, almost all catalytic introns from yeast require additional co-factors for splicing, but it is unknown how Mss116p cooperates with other proteins to promote group I or II intron splicing.
In order to investigate how DEAD-box proteins influence RNP function, we have developed a new in vitro splicing system based on the aI5β group I intron from yeast mt. The aI5β intron resides in the cytochrome oxidase 1 (COX1) gene and genetic analysis has shown that it requires at least five co-factors for efficient splicing in vivo, including Mss116p and a protein, Mrs1p, that is related to DNA four-way junction resolvase enzymes.18,20,24–26 Here we show that the Mrs1p protein efficiently promotes the first step of aI5β splicing, but the efficiency of the second step is poor. Mss116p, in the presence of Mrs1p and in an ATP hydrolysis dependent fashion, facilitates the second step of aI5β splicing, but has little influence on the first step. ATP pulse/depletion experiments provide evidence that Mss116p functions after the first step has occurred. Proteins with mutations in the predicted ATP binding site of Mss116p do not stimulate exon ligation, and both RNA stimulated ATP hydrolysis and duplex unwinding are also abolished. Proteins with mutations at the predicted interface of the two RecA domains have uniformly decreased duplex unwinding activity, but varying negative effects on ATP hydrolysis, the magnitude of which scales with the mutant proteins ability to stimulate exon ligation. Collectively, these results delineate an unanticipated function of Mss116p and suggest that its role in aI5β exon ligation is related to its efficiency to hydrolyze ATP.
Results
The aI5β intron does not efficiently undergo exon ligation
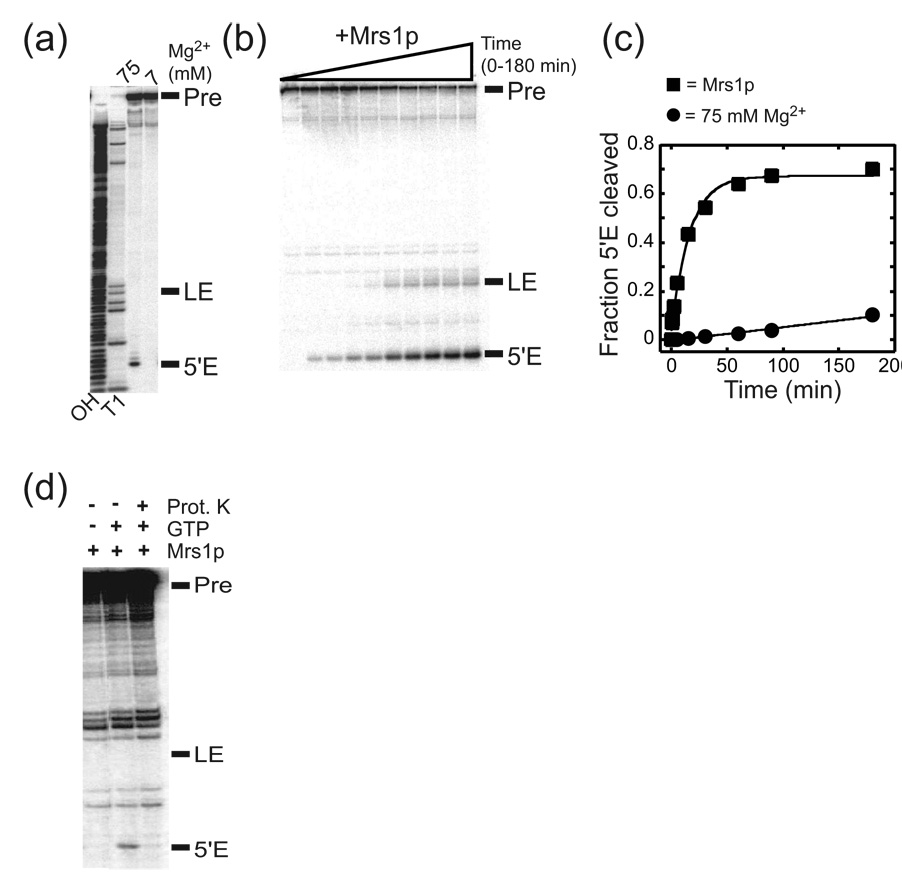
To characterize the in vitro catalytic activity of the aI5β intron, a portion of the mt COXI gene containing the intron was cloned into a vector to generate in vitro transcripts, and the self-splicing reaction was assayed under a variety of conditions. Despite its large size (1574 nts), the aI5β intron catalyzes the first step in splicing, albeit inefficiently at 37 °C in the presence of elevated (75 mM) Mg2+ concentrations but no splicing is observed under more physiological (7 mM) Mg2+ conditions (Figure 1a; Table 1). However, unlike other group I introns, aI5β does not appreciably undergo the second step of splicing under self-splicing conditions (Figure 1a).
Figure 1. Splicing activity of the aI5β intron RNA.
(a) Representative gel image for aI5β self-splicing reactions. 5’ 32P-end-labeled RNA was incubated in reaction buffer at 37 °C containing 75 or 7 mM MgCl2 in the presence of 1 mM GTP. Pre, aI5β pre-RNA; LE, ligated exons; 5’E, free 5’ exon; OH, hydrolysis ladder; T1, ribonuclease T1 sequencing ladder. Note that a band corresponding to ligated exons cannot be seen in the self-splicing reactions. (b) Representative gel image for Mrs1p-dependent splicing of the aI5β intron. (c) Representative plots for self-splicing and Mrs1p-dependent splicing. The total fraction of RNA that had undergone the first step (5'E cleaved) was calculated by summing the amount of radioactivity of the free 5’ exon and ligated exons and dividing this value by the total radioactivity in the lane. The values for total fraction of 5’ exon cleaved were plotted against time and the data were fit to a first order equation: Fraction RNA spliced = A(1 − e−kt) where A is the amplitude of reacted RNA and k represents the pseudo-first order rate constant, kobs. See Table 1 for values and error calculations. (d) Proteinase K treatment abolishes Mrs1p stimulation of splicing. Mrs1p/aI5β pre-RNA were preincubated in the absence of GTP and the complex then digested with Proteinase K. GTP was added and the reactions proceeded for 5 min.
Table 1.
Efficiency of aI5β exon ligation
| Amplitude of | kobs for 5’Ea | Amplitude of | kobs for LEb | |
|---|---|---|---|---|
| Splicing conditions | 5’Ea cleavage | cleavage (min−1) | LEb | (min−1) |
| Autocatalysis (75 mM Mg2+) | N/Ac | 0.0005 ± 0.0001e | N/Ac | N/Ac |
| Mrs1p+ATP | 0.64 ± 0.050d | 0.041 ± 0.011d | 0.14 ± 0.028d | 0.052 ± 0.014d |
| Mrs1p+Mss116p+ATP | 0.75 ± 0.059d | 0.050 ± 0.008d | 0.29 ± 0.026d | 0.071 ± 0.011d |
| Mrs1p+Mss116p+AMP-PNP | 0.82 ± 0.076d | 0.043 ± 0.010d | 0.18 ± 0.022d | 0.049 ± 0.008d |
| Mrs1p+K158A+ATP | 0.72 ± 0.013e | 0.036 ± 0.005e | 0.16 ± 0.008e | 0.057 ± 0.004e |
| Mrs1p+T307A+ATP | 0.74 ± 0.045d | 0.037 ± 0.004d | 0.21 ± 0.014d | 0.045 ± 0.007d |
| Mrs1p+Q412A+ATP | 0.77 ± 0.033d | 0.042 ± 0.002d | 0.26 ± 0.014d | 0.052 ± 0.002d |
| Mrs1p+D441A+ATP | 0.60 ± 0.005e | 0.037 ± 0.012e | 0.14 ± 0.008e | 0.048 ± 0.019e |
5’E – 5’ Exon
LE – Ligated Exons
N/A, not applicable
Errors are the standard deviations calculated from 3–10 independent experiments
Average of two independent experiments with the error estimated by dividing the spread of values by two.
In order to study the roles of individual aI5β protein co-factors, we cloned each gene into bacterial expression vectors and purified the recombinant proteins via a N-terminal His-tag. Remarkably, although the aI5β intron requires multiple proteins to splice in vivo, binding of Mrs1p protein facilitated splicing in vitro in 7 mM Mg2+ at 37 °C (Figure 1b). The Mrs1p protein was more efficient at promoting splicing than elevated Mg2+ concentrations, as the rate of splicing was ~80-fold greater than that of the self-splicing reaction (Figure 1c, Table 1). Importantly, Mrs1p had to remain bound to aI5β to facilitate splicing, since splicing was abolished if Mrs1p was removed from the RNP complex by digestion with proteinase K prior to addition of the guanosine splicing co-factor (Figure 1d). These data imply that Mrs1p does not act transiently as an RNA chaperone to facilitate splicing and that Mrs1p binding most likely stabilizes the catalytically active structure of the aI5β intron.
Despite its ability to promote the first step of aI5β intron splicing, binding of the Mrs1p protein does not efficiently promote the exon ligation reaction of aI5β (Figure 1b; Table 1). While the fraction of RNA that undergoes the first step in splicing is 0.64 (±0.05), the fraction of RNA that proceeds to exon ligation is only 0.14 (±0.03). This surprising lack of ligated exon product cannot be explained by premature hydrolysis of the 3’ exon or exon re-opening reactions, since we did not observe the expected free 3’ exon product when using a 3’ end-labeled pre-mRNA (not shown; see also 27). These data suggest that other aI5β co-factors may be required to promote the second step of splicing.
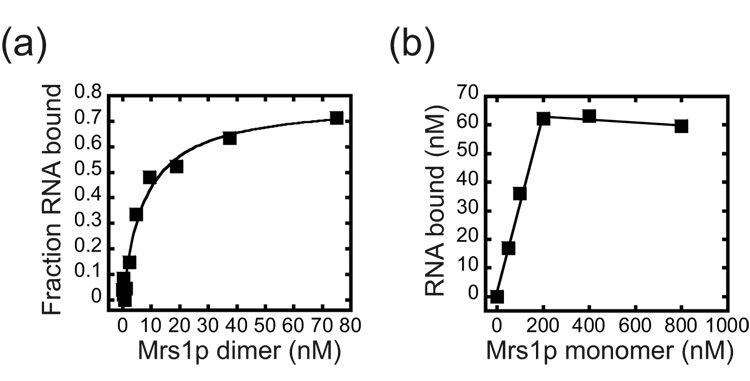
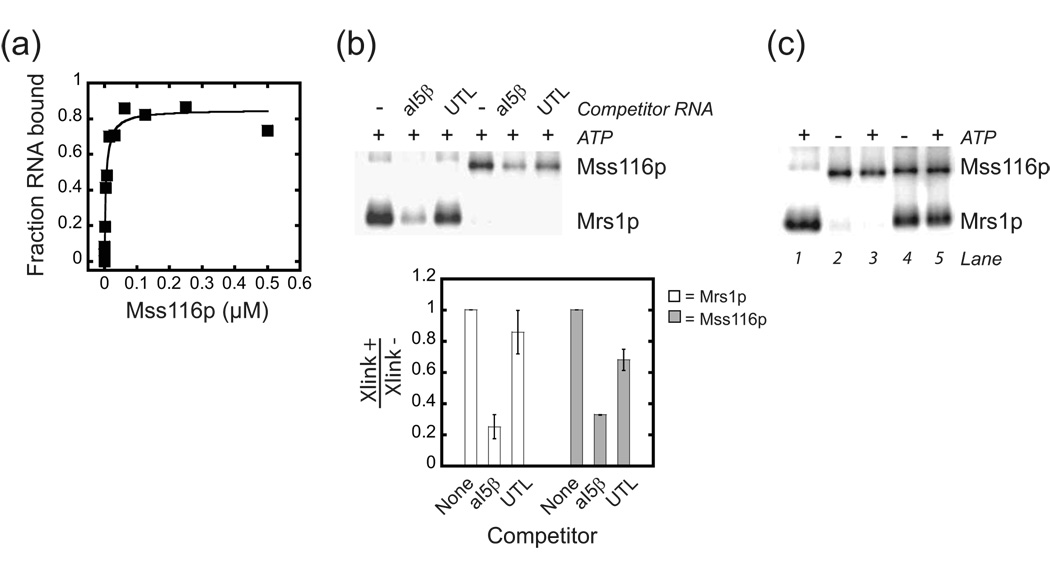
To gain more insight into how Mrs1p facilitates splicing, we measured the apparent equilibrium binding constant (Kdapp) for the complex using a nitrocellulose filter-binding assay (Figure 2a). Mrs1p is a homodimer in solution and titratration experiments showed that, under splicing conditions, Mrs1p bound the aI5β pre-RNA with a Kdapp of 5.6 (±1.7) nM.28 To measure the stoichiometry of the complex, high (80 nM) concentrations of aI5β pre-RNA were incubated with limiting amounts of Mrs1p and the concentration of RNA bound was plotted as a function of the concentration of Mrs1p monomer (Figure 2b). The data were linear up ~ 200 nM and then no further increase in binding was observed. From multiple experiments, the linear portion of the curve yielded an inverse slope of 4.3 (±0.9) providing evidence that four monomers (and thus two dimers) of Mrs1p bound the aI5β RNA. Analysis of the equilibrium binding data using the Hill equation did not show evidence of strong cooperativity (Hill co-efficient of 1.25 (±0.5)) suggesting that two Mrs1p dimers bind independently to the aI5β pre-RNA.
Figure 2. Binding of Mrs1p to aI5β pre-RNA.
(a) Equilibrium binding. Representative plot for Mrs1p binding to aI5β RNA. The Kdapp from three independent experiments was 5.6 (± 1.7) nM. (b) Stoichiometry of the Mrs1p/aI5β pre-RNA complex. Representative plot for binding of 80 nM aI5β RNA with increasing concentrations of Mrs1p (50 – 800 nM, monomer). The reactions were conducted with RNA concentrations greater than 10-fold above the Kdapp of 5.6 nM. The plot was linear up to 200 nM Mrs1p monomers with the inverse of the slope equal to the number of protein monomers bound to the RNA. From three independent experiments, the stoichiometry of the complex was 4.3 (± 0.9) monomers per RNA.
Mss116p facilitates aI5β catalyzed exon ligation
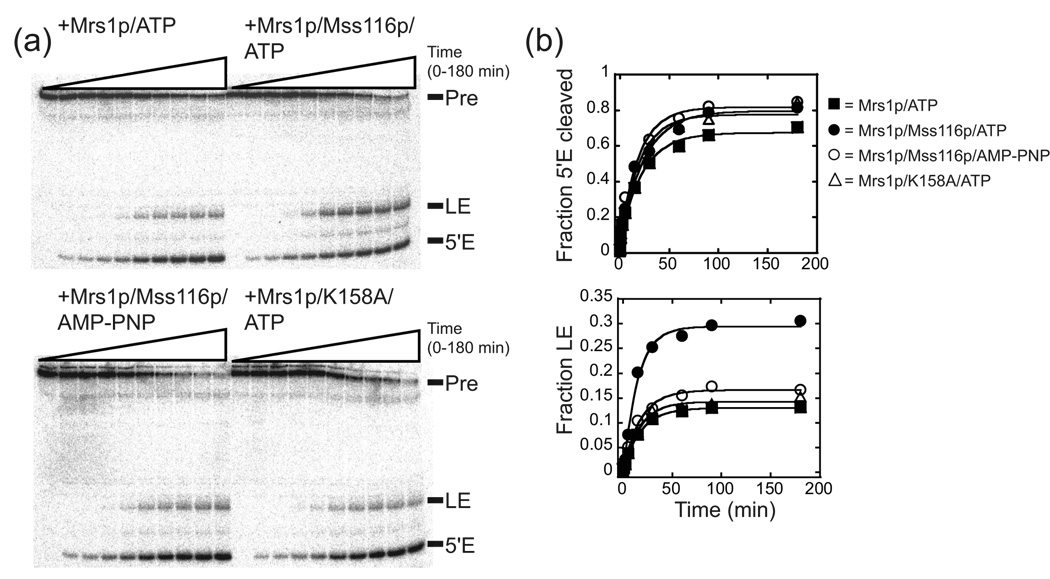
To assess whether the addition of other recombinant proteins could facilitate exon ligation, we individually added recombinant aI5β protein co-factors to the Mrs1p/aI5β pre-RNA splicing reactions. Under the conditions used, inclusion of the Mss116p protein at 1.5 µM increased the amount of RNA that underwent the second step in splicing (Figure 3a, b). The addition of the Mss116p increased the fraction of ligated exon product by 2.1-fold in the presence of ATP, but it had no effect in the presence of the non-hydrolyzable ATP analog AMP-PNP (Figure 3a, b; Table 1). In contrast, there was a modest (less than 1.3-fold) increase in the fraction of RNA that underwent the first step in the presence of either ATP or AMP-PNP indicating that this stimulation was independent of ATP hydrolysis (Figure 3a, b; Table 1). Finally, the apparent rate for exon ligation with Mrs1p in the absence or presence of Mss116p is the same within error than that of the first step, suggesting that ligation is rapid relative to the first step, and thus, possible Mss116p effects on ligation rate could not be observed. The ~2-fold effect is consistent with genetic analysis that showed deletion of the MSS116 gene inhibited the splicing of all group I introns by 20 – 60% in yeast mitochondria.20
Figure 3. Mss116p stimulated exon ligation.
(a) Representative gel image for aI5β splicing in the presence of Mss116p and/or Mrs1p. For the wild-type Mss116p and the ATPase mutant, K158A (see Figure 6), the reactions were carried out in the presence of ATP. The non-hydrolyzable ATP analog, AMP-PNP, was also included in a set of reactions with the wild-type protein. (b) Representative plots for splicing. The fraction of RNA that had undergone the first step (5’ E cleaved) is plotted against time and the data fit to a first order equation to obtain kobs and the total fraction (amplitude) that had undergone the first step (see Figure 1). Likewise, the fraction of ligated exons was plotted against time and the data fit to a first order equation to obtain kobs and the total fraction (amplitude) that had undergone the second step.40 The individual amplitudes of ligated exon shown in this Figure vary less than 12% from the averaged value from multiple experiments for each condition. The average amplitudes were associated with errors of less than 20% for each condition. For values and calculated errors, see Table 1.
A number of control experiments were carried out to further characterize Mss116p stimulation of aI5β exon ligation in the presence of Mrs1p. The dependence of ATP hydrolysis for Mss116p to stimulate exon ligation was also confirmed by testing a variant Mss116p, in which the highly conserved K158 residue in motif I that contacts ATP was mutagenized to alanine.9 This variant failed to show ATP hydrolysis and did not stimulate exon ligation (Figure 3a,b and Figure 6). This variant also showed the same modest increase in the fraction of RNA that underwent the first step, as the wild-type protein, providing more evidence that the minor effect on 5’ exon cleavage was independent of ATP hydrolysis (Table 1). Equilibrium binding experiments showed that Mss116p bound the aI5β pre-RNA with a Kdapp of 18 (±7) nM, and doubling the concentration of Mss116p in the splicing reaction did not change the stimulatory effect on ligation (Figure 4a and data not shown). Taken together, these observations provide evidence that the protein was saturating under splicing conditions. Finally, Mss116p by itself did not facilitate splicing of aI5β containing pre-mRNA (Supplementary Figure 1).
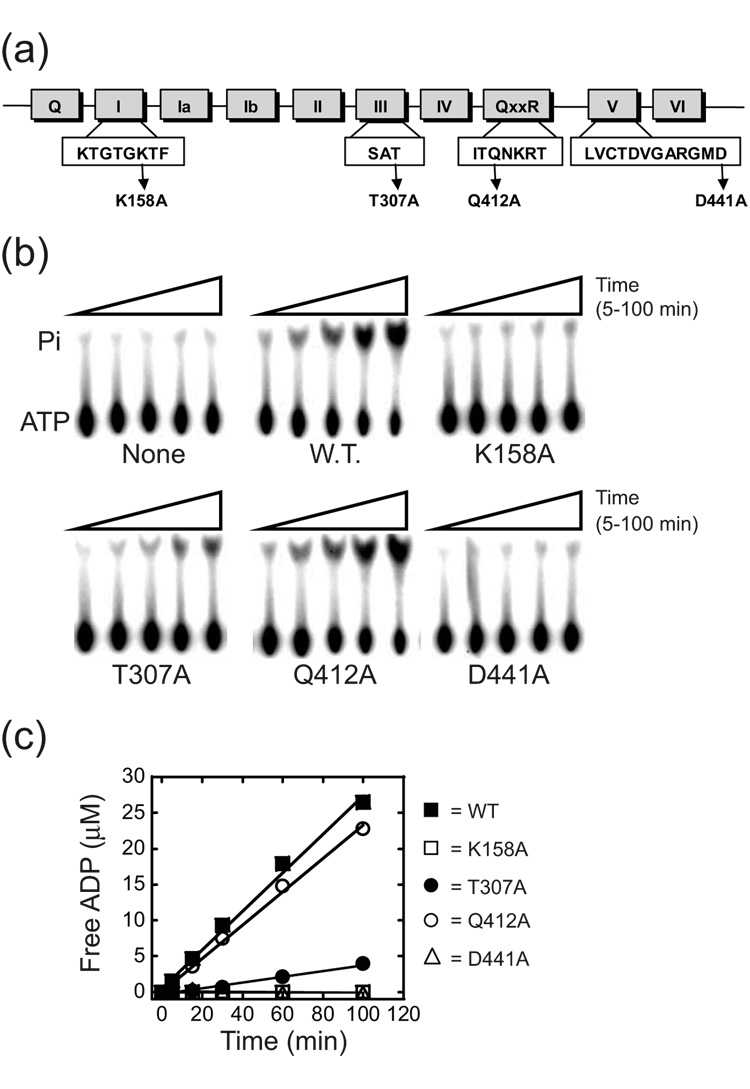
Figure 6. ATPase activity of wild-type and mutant derivatives of Mss116p.
(a) Schematic of Mss116p conserved motifs. Mutated residues are indicated. (b) Representative TLC plate images for each mutant in the presence of 50 µM ATP. Pi, released phosphate; None, no protein added; W.T., wild-type Mss116p. (c) Representative plots of free ADP versus time for reactions with 50 µM ATP. The curves are linear fits to the data, with the slope equal to the initial velocity (vo). For kinetic parameters, see Table 3.
Figure 4. Interaction of Mss116p and Mrs1p with the aI5β pre-RNA.
(a) Representative plot of equilibrium binding of Mss116p to aI5β pre-RNA. The Kdapp from three independent experiments was 18 (± 7.2) nM. (b) Specificity of uv cross-linking of 4-thiouridine substituted aI5β pre-RNA to Mrs1p and Mss116p. Internally 32P-radiolabeled substituted aI5β was incubated with either saturating amounts of Mrs1p (0.75 µM, dimer) or Mss116p (0.75 µM) in the absence or presence of 0.6 µM unlabeled competitor aI5β pre-RNA (aI5β) or COX3 5’ UTL (UTL). Below shows a histogram with the ratio of phosphorimager counts from cross-linked material in the presence (Xlink+) versus absence (Xlink−) of competitor RNAs. The data are from two independent experiments and the error bars represent the range of values divided by two. (c) Evidence that Mrs1p and Mss116p bind simultaneously to aI5β pre-RNA. UV cross-linking reactions were performed with saturating Mrs1p (0.75 µM, dimer, lane 1), Mss116p (0.75 µM, lanes 2,3) or both (lanes 4,5). There are no significant changes in the intensity of cross-linking when both proteins were included in the reactions suggesting that both proteins bind to a single aI5β pre-RNA. In addition, the presence of ATP does not affect the cross-linking efficiency of Mss116p or Mrs1p in the presence of Mss116p. Note that the amount of Mrs1p cross-linked is reduced ~30% in the presence of Mss116p similar to the amount reduced in the presence of the non-specific competitor COX3 UTL in (b). This may reflect that a small percentage of the Mrs1p-cross-link represents a non-specific interaction and Mss116p competes for these sites on the RNA.
Previously, it was shown that, in vitro, Mss116p stimulated splicing of group I and II introns from fungi at more physiological temperatures (e.g. 25 or 30 °C).21,22 Low temperatures have been shown to slow folding of some group I intron RNAs by stabilizing mis-folded intermediates in the folding pathway.29,30 Furthermore, the homologous DEAD-box protein CYT-19p can facilitate folding at lower temperatures by destabilizing such intermediates.19 Therefore, we carried out reactions at a typical yeast growth temperature (30 °C) to assess whether decreasing the temperature affected the splicing behavior of aI5β in the presence of Mrs1p and Mss116p. At 30 °C, there was a slight decrease in the rate of overall splicing, but still a significant fraction of aI5β RNAs that underwent the first step in splicing (Supplementary Figure 2). Again, Mss116p in the presence of ATP increased the fraction of RNA that underwent exon ligation by 2.5 fold. Similar to the results at 37 °C, a small increase (1.5-fold) in the first step was also observed (Supplementary Figure 2). We continued our analysis at 37 °C since overall splicing was more efficient and went to completion under 3 hours thus allowing for a more accurate measure of the fraction of RNA that underwent exon ligation.
The equilibrium binding data presented above suggests that under splicing conditions, both Mrs1p and Mss116p can be bound simultaneously to the aI5β RNA, presumably at different locations in the RNA. UV cross-linking experiments were used to confirm this possibility. At saturating concentrations both Mrs1p and Mss116p independently cross-linked to 4-thiouridine substituted aI5β intron (Figure 4b). The cross-link to Mrs1p was sensitive to excess unlabeled aI5β but not a non-specific RNA (5’ untranslated leader sequence (5’UTL) from the yeast mt COX 3 mRNA) competitor providing evidence that the cross-link reports a specific interaction (Figure 4b).31 For Mss116p, cross-linking efficiency was sensitive to either un-labeled aI5β pre-RNA or COX3 5’UTL, as expected since Mss116p has broad RNA binding specificity in vitro.22 The observation that aI5β was a better competitor than COX3 5’UTL may reflect a binding preference of Mss116 for aI5β. However, we have been unable to identify a sub-domain with the intron that could account for this possibility (data not shown).
When both proteins were included in the reaction, the aI5β pre-RNA cross-inked without significant loss of efficiency to both proteins, and this did not change when ATP was included in the reaction (Figure 4c). Given that both proteins were saturating, it is likely that both proteins bound the RNA at the same time and also that hydrolysis of ATP by Mss116p did not influence the binding of Mrs1p at saturating concentrations used in the splicing reactions.
Mss116p acts after the first step of aI5β splicing
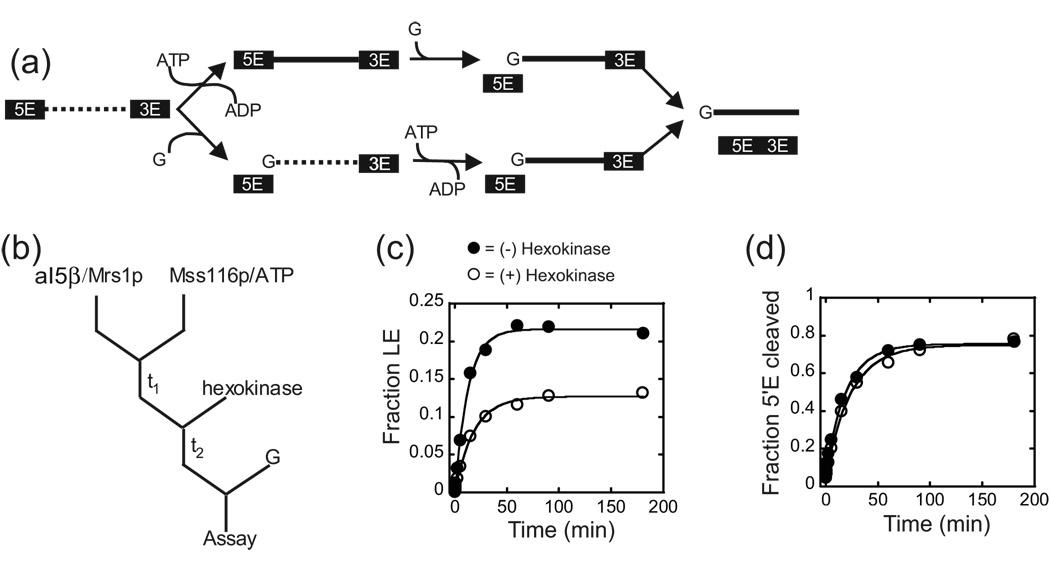
In principle, Mss116p could enhance the second step in two different ways (Figure 5a). First, Mss116p may act to change a fraction of RNP into a conformation that is competent to catalyze both steps of splicing at any time during the splicing cascade. Alternatively, Mss116p may only act on RNPs that have completed the first step (Figure 5a). In order to distinguish between these possibilities, we carried out ATP pulse/depletion experiments. In these experiments, the Mrs1p/aI5β pre-RNA complex was pre-incubated with Mss116p and ATP in the presence of glucose, while splicing was prevented by withholding the GTP co-factor. The ATP was then converted to ADP by the addition of hexokinase that catalyzes the formation of glucose 6-phosphate. Splicing was then initiated by the addition of GTP (Figure 5b). If Mss116p acted after the first step, hexokinase treatment should abolish the second step stimulation. In contrast, if Mss116p, prior to the first step, facilitates a change in the structure of the pre-RNA that makes it competent to undergo exon ligation, the Mss116p dependent stimulation of exon ligation should be unaffected by ATP depletion. As shown in Figure 5c, after a 15 minute pre-incubation with Mss116p and ATP, the addition of hexokinase reduced the amount of ligated exons to levels comparable to reactions with Mrs1p alone (Table 1,Table 2). Similar results were observed when the pre-incubation time was increased to 90 minutes (data not shown). Mock-hexokinase treated control reactions showed that Mss116p in the presence of ATP still promoted exon ligation under these reaction conditions (Figure 5c; Table 2). Finally, the hexokinase treatment had minimal effects on the efficiency of the first step in splicing (Figure 5d; Table 2). These results suggest that Mss116p exerts it influence only after the first step to promote exon ligation. Collectively, these observations are consistent with Mss116p acting in an ATP hydrolysis manner as a second step co-factor for aI5β splicing.
Figure 5. Mss116p acts after the first step in splicing.
(a) Schematic of possible sites of Mss116p action in the splicing cascade. Mss116p could possibly act upon precursor (top) or the intron-3’exon intermediate (bottom) RNAs to facilitate the second step. The dashed and solid lines represent structures of the intron that are incapable or capable of performing exon ligation, respectively. Removing ATP by hexokinase treatment prior to the addition of GTP (G) should abolish the increase in ligated exon, if Mss116p acts after the first step. (b) Outline of the ATP depletion protocol. Mrs1p/aI5β RNA complexes were pre-incubated with Mss116p and ATP (t1). Hexokinase was added, which catalyzes the phosphorylation of glucose via ATP hydrolysis, and, after a brief incubation (t2), splicing initiated by the addition of GTP (G). (c) Representative plot of exon accumulation. The fraction of ligated exons was plotted against time and the data fit to a single exponential to obtain kobs and the total fraction (amplitude) that had undergone the second step. The individual amplitudes of ligated exon shown in this Figure vary less than 8% from the averaged value from multiple experiments for each condition. The average amplitudes were associated with errors of less than 4% for each condition. For values and calculated errors, see Table 2. (d) Representative plots for the first step in splicing. See Table 2 for values and calculated errors.
Table 2.
Efficiency of Mss116p stimulated aI5β exon ligation after ATP depletion
| Amplitude of | kobs for 5’Ea | Amplitude of | kobs for LEb | |
|---|---|---|---|---|
| Splicing conditions | 5’Ea cleavaged | cleavage (min−1)d | LEb,d | (min−1)d |
| (−) HKc (+) ATP | 0.65 ± 0.018 | 0.049 ± 0.002 | 0.21 ± 0.005 | 0.076 ± 0.004 |
| (+) HKc (+) ATP | 0.70 ± 0.008 | 0.036 ± 0.005 | 0.13 ± 0.005 | 0.046 ± 0.009 |
5’E – 5’ Exons
LE – Ligated Exons
HK – Hexokinase
Average of two independent experiments with the error estimated by dividing the spread of values by two.
The efficiency of Mss116p ATP hydrolysis correlates with its ability to promote aI5β RNA exon ligation
To identify which properties of Mss116p are required to stimulate aI5β exon ligation step, we made alanine substitutions in three conserved amino acids that have been implicated in coupling ATP hydrolysis and protein conformational rearrangements required for efficient helix unwinding in DEAD-box proteins.9 Using the crystal structure of the Drosophila DEAD-box VASA protein as a reference, two of these residues, within the conserved SAT and QxxR motifs in the first and second RecA domains (T307 and Q412), respectively, are expected to make distinct interdomain contacts that are important for catalyzing unwinding (Figure 6a).12 A third residue in the second RecA domain, D441 (within motif V) is expected to both contact the first domain as well as the bound ATP (Figure 6a).12 As a control, the highly conserved K158 residue in motif I that contacts ATP was also mutagenized (see above; Figure 6a).12
The RNA-dependent ATPase activity of each mutant was measured under aI5β splicing conditions with saturating concentrations of the intron (Figure 6). We note that some preparations of purified proteins showed hydrolysis in the absence of added RNA (data not shown). This background activity was due to contaminating nucleic acid, since treatment of proteins with micrococcal nuclease prior to the ATPase reactions abolished hydrolysis in the absence of exogenously added RNA (not shown). At 37 °C, wild-type Mss116p hydrolyzed ATP with a kcat of 4.5 min−1 and was characterized by a Km of 100 µM, whereas mutants that are expected to perturb ATP binding (K158A and D441A) showed no detectable activity (Figure 6b,c; Table 3). In contrast, T307A and Q412A retained significant ATPase activity. There were small effects on Km and a 6-fold decrease in kcat relative to wild-type for T307A, while there was no significant decrease in kcat for Q412A (Figure 6b,c; Table 3).
Table 3.
Kinetic parameters for ATP hydrolysis
| Protein | Km (µM)a | kcat (min−1)a |
|---|---|---|
| Wild type | 100 ± 7.5b | 4.5 ± 1.1b |
| K158A | N.Ac | N.Ac |
| T307A | 165 ± 59d | 0.77 ± 0.26b |
| Q412A | 143 ± 3d | 3.4 ± 0.20b |
| D441A | N.Ac | N.Ac |
Parameters were measured at 37°C as described in Materials and Methods.
Errors are the standard deviations calculated from 3–5 independent experiments
N.A., not active.
Average of two independent experiments with the error estimated by dividing the spread of values by two.
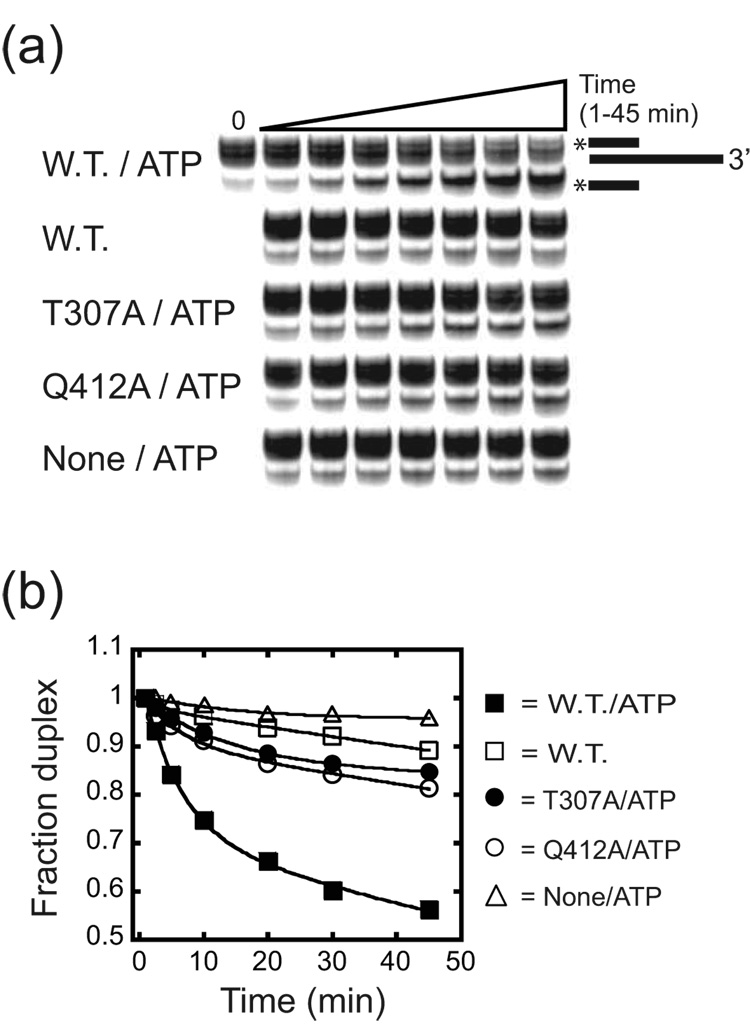
To determine the efficiency of unwinding activity, we carried out a series of duplex unwinding assays using a 32P-labeled, 17 nt DNA oligonucleotide hybridized to a larger, unlabeled RNA such that the duplex had a 30 nt 3’ overhang.32 At 37 °C, the un-catalyzed oligonucleotide dissociation was too great (~30 % after 45 min) to accurately measure the unwinding activity of the mutants. Therefore, we carried out the analysis at 30 °C in which the rates of unwinding were measured under aI5β splicing conditions for each protein. The wild-type Mss116p efficiently catalyzed unwinding of this substrate in the presence of ATP, but showed little activity above the basal unwinding rate in the absence of ATP (Figure 7a, b; Table 4). After 45 min. ~ 50 % of the duplex was unwound and this was defined by two kinetic phases (Table 4). In this regard, the data best fit to a double exponential with ~20 % unwinding at a fast rate (0.16 (µ0.009) min−1) and the remainder at a slower rate (0.006 ± 0.0001 min−1). The fast and slow rates were 160- and 6-fold faster than the un-catalyzed rate (0.001 (±0.0003) min−1; Table 4). Increasing the concentration of the Mss116p did not significantly increase the rates of unwinding or change the relative fraction of molecules in each phase, confirming that the protein was saturating under these conditions (see Materials and Methods).
Figure 7. Unwinding activity of wild-type and mutant derivatives of Mss116p.
(a) Representative gel image for reactions in the presence of ATP. The mobility of the released, 32P-labeled DNA was confirmed by boiling the complex and running that material alongside the starting material (not shown). (b) Representative time course plots. Reactions were initiated by the addition of Mss116p, mutant protein or protein dilution buffer (None) and aliquots removed from 1 to 45 min. The data for Mss116p (W.T.), T307A and Q412A with ATP was fit to a first order equation with a double exponential: Fraction Duplex = A(e−kt) + B(e−kt) where A and B are the amplitude of duplex in each phase and k represents the first order rate constants for each phase. For Mss116p in the absence of ATP and in reactions without protein, the data was fit to a single exponential: Fraction Duplex = A(e−kt) where A is the amplitude and k represents the first order rate constant. For values derived from multiple experiments and calculated errors, see Table 4. Multiple bands in the duplex are due to the addition of non-templated nucleotides to the RNA transcript.
Table 4.
Percentage and rates of duplex unwinding
| 1st Phase |
2nd Phase |
|||
|---|---|---|---|---|
| Protein | % Unwounda | Rate (min−1)a | % Unwounda | Rate (min−1)a |
| None / ATPb | 05.8 ± 1.0 | 0.001 ± 0.0003 | N/Ad | N/Ad |
| W.T. b,e | 13.2 ± 2.3 | 0.003 ± 0.00005 | N/Ad | N/Ad |
| W.T. / ATPc, e | 12.2 ± 0.5 | 0.16 ± 0.009 | 33.5 ± 0.2 | 0.006 ± 0.0001 |
| T307A / ATPc | 01.9 ± 0.4 | 0.10 ± 0.020 | 14.7 ± 0.2 | 0.001 ± 0.0005 |
| Q412A / ATPc | 001.8 ± 0.01 | 0.13 ± 0.033 | 18.2 ± 0.7 | 0.002 ± 0.0001 |
Average of two independent experiments with the error estimated by dividing the spread of values by two.
Percentage of unwound duplex and rates were obtained from fitting the data to a first order single exponential equation; see Legend to Figure 7.
Percentage of unwound duplex and rates were obtained from fitting the data to a first order double exponential equation; see Legend to Figure 7.
N/A, not applicable
W.T., wild-type
The mutant proteins showed a spectrum of activities. The ATP hydrolysis deficient variants (K158A, D441A) did not show unwinding activity above the basal, un-catalyzed rate (not shown). In contrast, the T307A and Q412A were active but extremely inefficient. In both cases, not more than 20% of the duplexes were unwound and the time course data again showed two distinct phases (Table 4). Only ~2% of the duplexes were unwound at a rate that was within two-fold of the fast wild-type rate. The overall majority (~15–18%) was characterized by a slow rate reduced by 6- or 3-fold relative to the wild-type slow rate for T307A and Q412A, respectively (Figure 7a, b; Table 4). In fact, for both mutants, the extent and rate of unwinding in the second phase were not significantly different than that of the wild-type in the absence of ATP (Figure 7a, b; Table 4). Increasing the concentrations of T307A or Q412A did not significantly change the results providing evidence that the proteins were saturating for unwinding (not shown). Thus, the T307A and Q412A mutants, despite the relative differences in ATPase activity, were equally impaired in the extents and rates of unwinding relative to the wild-type.
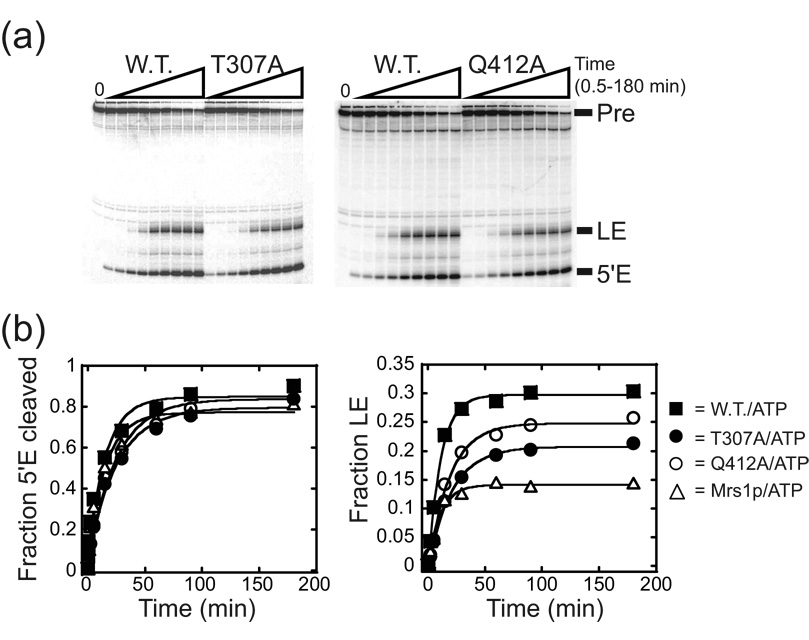
The ability of each mutant to catalyze ATP hydrolysis closely paralleled the ability to promote exon ligation in the presence of the Mrs1p protein (Figure 8). In this regard, neither K158A nor D441A facilitated exon ligation (Figure 3; Table 1). In contrast, Q412A increased the amplitude of ligated exon production by 1.9-fold relative to Mrs1p alone, but not significantly less than the wild-type (Figure 8, Table 1). The T307A mutant increased the amplitude of ligated exon production by 1.5-fold relative to Mrs1p alone, but was only half as efficient as the wild-type protein (Figure 8, Table 1). Control experiments showed that increasing the concentrations of T307A or Q412A did not significantly change the results providing evidence that the proteins were saturating for splicing (not shown). Thus, only those proteins that retain measurable unwinding and significant ATP hydrolysis activities facilitated aI5β exon ligation. Moreover, the relative ability of Mss116p variants to promote exon ligation paralleled their relative ATP hydrolysis efficiencies: the Q412A variant was more efficient at both catalyzing ATP hydrolysis and promoting exon ligation than the T307A mutant protein. These data provide evidence that Mss116p-facilitated exon ligation is exquisitely dependent on the ability of the protein to efficiently hydrolyze ATP.
Figure 8. Splicing activity of wild-type and mutant derivatives of Mss116p.
(a) Representative gel image for time course experiments. The reactions were initiated by addition of Mrs1p and Mss116p proteins and aliquots removed from 0.5 to 180 min. (b) Representative plots of splicing product accumulation. The fraction of RNA species spliced was plotted against time and the data fit to a single exponential to obtain kobs and the total fraction (amplitude) that had undergone either step (see Figure 3). Note that the mutants stimulate the ligated exon production above that in the presence of Mrs1p alone. The individual amplitudes of ligated exon shown in this Figure for each Mss116p variant vary less than 11% from the averaged value from multiple experiments and the average amplitudes were associated with errors of less than 6%. For values and calculated errors, see Table 1.
Discussion
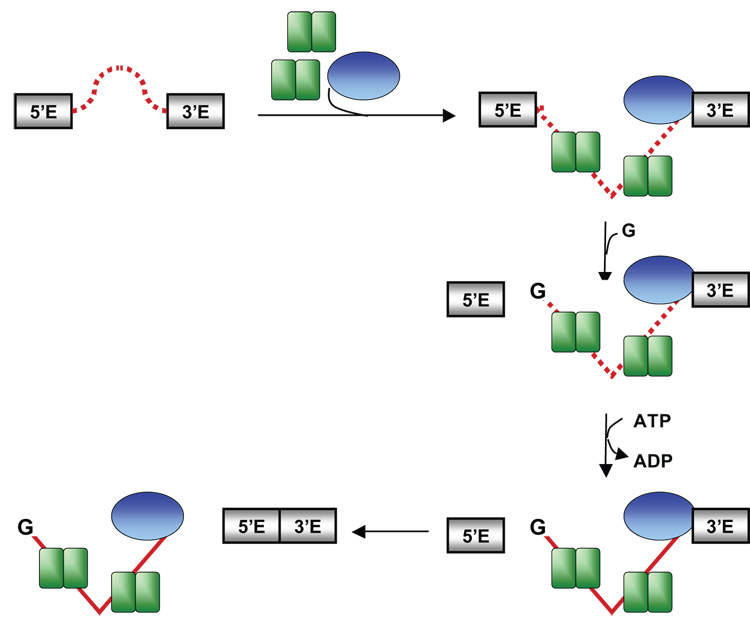
All self-splicing intron RNA co-factors characterized in vitro promote RNA folding that ultimately leads to a catalytically active structure. The studies of such systems have revealed many fundamentally important insights into the mechanisms of protein-dependent RNA folding, RNP assembly and catalysis. Most complex catalytic RNPs, however, require specific co-factors that are required at different steps in the assembly and reaction pathways (e.g. pre-mRNA splicing catalyzed by the spliceosome). The mechanisms by which such co-factors are temporally coordinated are not well understood and no simple in vitro system exists to explore this question. Here, we describe the analysis of a novel in vitro splicing system based on the yeast aI5β group I intron that faithfully recapitulates a division of labor between protein co-factors. Our observations are consistent with a model for how aI5β intron undergoes splicing (Figure 9). The initial intron structure is unable to carry out either step in splicing (dashed line in Figure 9). Binding of two Mrs1p dimers most likely stabilizes the catalytically active structure for the first step. Mss116p, by virtue of its ability to bind non-specifically to RNA (Figure 4; 22) can bind independently to the initial RNA structure, but this interaction has no effect on the first splicing step. After binding of the exogenous guanosine in the intron core and first step catalysis, Mss116p, in an ATP hydrolysis dependent step, facilitates a second conformational change to promote exon ligation (see legend to Figure 9). The timing of Mss116p function may reflect that its target in the intron becomes available only after the first step in splicing. On the other hand, the structural change promoted by Mss116p may be unstable and ligation only proceeds once the 5’ exon has been cleaved and can rapidly attack the 3’SS. Below we discuss aspects of this model in detail.
Figure 9. Protein-dependent splicing of the aI5β intron.
Mrs1p dimers are double green rectangles and Mss116p is a blue oval. The intron initially is structured such that it is unable to carry out either step in splicing (looped, dashed line). Binding of Mrs1p stabilizes the first step, catalytically active structure (bent, dashed line). Mss116p also binds to the starting structure of aI5β, but initially, has no effect on intron activity. After binding of guanosine (G) and first step catalysis, Mss116p, in an ATP hydrolysis dependent manner, facilitates a second conformational change (dashed to solid line) in the intron to promote exon ligation. Mss116p may also facilitate release of the ligated exons, thus preventing reversal of the second step and increase the efficiency of ligation. It may also disrupt the Mrs1p interaction with the intron (not shown, but see text).
The catalytic activity of the aI5β intron is promoted by a co-factor with homology to a 4-way DNA junction binding protein
The aI5β intron inefficiently catalyzes the first step of splicing at high Mg2+ concentrations and no reaction is observed under physiological Mg2+ concentrations. As shown for many other protein-dependent introns, this likely indicates that the intron is un- or mis-folded under these conditions. The addition of recombinant Mrs1p increased the rate of splicing more than 80-fold above that observed in the self-splicing reaction. The observation that Mrs1p must remain bound to the intron to stimulate activity is consistent with the hypothesis that the protein directly stabilizes an aI5β structure(s) that are essential for splicing.
A clue as to how Mrs1p stabilizes aI5β structure comes from the identity of another group I intron that requires the protein for splicing. Genetic analysis has implicated the Mrs1p protein in the splicing of the bI3 intron found within the COB gene.25 Mrs1p binds the bI3 group I intron in vitro, but this complex requires an additional, intron-encoded protein (the COB maturase) for splicing to occur in vitro. 24,28,33 Both the aI5β and bI3 introns are bound by two Mrs1p dimers with Kdapp’s in the low nanomolar range, although the two dimers bind cooperatively in the case of bI3 (Figure 2; 28). Interestingly, there is little sequence similarity between the aI5β and bI3 introns, and they belong to different structural subclasses, IA1 and IB4, respectively.34 Thus, Mrs1p may facilitate splicing of both introns by binding to structural features within the conserved catalytic core that are shared between the RNAs. Interestingly, MRS1 is homologous to the CCE1 gene that encodes a magnesium-dependent endonuclease responsible for the resolution of yeast mt Holliday junctions, although the recombinant Mrs1p does not possess DNA cleavage activity in vitro.28,35 Like a four-way DNA helical junction, the conserved group I intron catalytic core forms two elongated, stacked helices that cross one another resulting in a distorted “X-like” structure that could represent part of the Mrs1p binding site.36–38 Important avenues to pursue will be to determine whether the ancestral four-way junction-binding site of Mrs1p is involved in its splicing function and to dissect the mechanism by which the protein promotes aI5β intron folding.
Coordination of the first and second steps of splicing by a DEAD-box protein
The finding that Mss116p did not appreciably facilitate the first step of Mrs1p-facilitated aI5β splicing was a surprise, as this protein has been observed to promote intron catalytic activity in vitro for the Nc LSU group I and two yeast group II introns in an ATP-dependent manner.21,22 Based on these studies, it had been suggested that Mss116p acts as an unwindase to destabilize non-native structures that “trap” intron RNAs in catalytically inactive conformations.22,23 However, it has also been suggested that Mss116p may also function to stabilize an “on-pathway” intermediate in the folding pathway through ATP-modulated RNA binding.21 Overall, the previous observations suggest that Mss116p plays a role in inducing structural transitions that promote catalytic core assembly, although the precise mechanism of action may be different for individual introns.
The data presented here suggest another function for Mss116p; that it can also act to modulate the structure of the assembled, catalytically active aI5β/Mrs1p RNP to facilitate the second step of splicing. Previous studies with self-splicing group I introns have suggested that efficient exon ligation is achieved by the preferential binding of second step ligands relative to first step- and ligated exon-products in the catalytic core.27,39–42 The mechanisms responsible for inducing the intron conformational changes that modulate the relative affinities of splicing substrate and product ligands are not clear, but it is reasonable to assume that the aI5β intron must be unable to independently carry out one or more of these events since exon ligation was inefficient under both Mrs1p-dependent and self-splicing conditions. Thus, Mss116p likely promotes one of these steps via a mechanism that requires ATP hydrolysis.
Since the ATP depletion experiments suggested that Mss116p functions only after the first step has occurred, it may act by destabilizing or stabilizing specific splicing products or substrate ligands to drive the exon ligation step. In this model, the timing of Mss116p function may reflect that its “target” in the intron becomes available only after the first step in splicing. For example, the simplest iteration of this model is that Mss116p may promote dissociation (either directly or indirectly) of the ligated exons prior to rapid reversal of the second step. On the other hand, Mss116p could also act to promote the association of the 3’ exon with its binding sites within the conserved P1 and P7 helices of the intron that are engaged in mutually exclusive interactions with products of the first step.42 However, we cannot discount that any structural change promoted by Mss116p may be unstable and the RNA readily reverts to its initial, inactive conformation. In this model, ligation only proceeds once the 5’ exon has been cleaved, and can rapidly attack the 3’SS once Mss116p facilitates formation of the functional, yet relatively unstable second step conformation. Finally, Mss116p may function by displacing Mrs1p or altering its interaction with aI5β to facilitate RNA conformational changes required for exon ligation. In this model, high affinity binding of Mrs1p may “hyper-stabilize” structures required for the first step, that then cannot be disrupted to allow transition into the second step conformation. In this regard, DEAD-box proteins have been shown to disrupt RNP complexes.43,44 Delineation of the changes induced by Mss116p will help to distinguish between these models. Nonetheless, our current observations suggest that the “functional-timing” of Mss116p in aI5β splicing is dependent on the structural conformation of the intron due to the “upstream” activity of Mrs1p.
The second step of aI5β splicing requires an “ATPase-competent” DEAD-box protein
As discussed above, the nature of the structural change(s) that Mss116p facilitates is not known for aI5β (or any of the protein’s substrates). To begin to address this issue, it was necessary to define which properties of Mss116p are important for its function in exon ligation. It was clear that ATP hydrolysis is essential for Mss116p’s aI5β splicing function since mutants that abolish the protein’s ATPase activity did not promote exon ligation, and the wild-type was equally ineffective when the non-hydrolyzable analog AMP-PNP was included in the reaction.
The analysis of mutants within the conserved SAT and QxxR motifs that play key roles in the unwinding activity of Mss116p provided evidence that the efficacy of ATP hydrolysis was a critical determinant for its aI5β splicing function. Both mutants were equally ineffective in unwinding a 17 bp RNA/DNA duplex. However, the T307A mutant was impaired in ATP hydrolysis by ~6-fold in kcat, and showed a 1.5-fold defect in ligated exon production. In contrast, the kcat for ATP hydrolysis for the Q412A protein was similar to the wild-type and it was essentially as efficient as the wild-type protein in promoting aI5β exon ligation. Thus there is strong correlation between the efficiency of ATP hydrolysis and promoting aI5β splicing suggesting that the two activities are closely linked. Kinetic and equilibrium characterization of the DEAD-box protein DbpA has revealed that strong RNA binding and helix destabilization are linked to protein conformational changes that occur during the transition to an ATP-hydrolysis competent and/or ADP-Pi hydrolysis product states.13 This analysis was consistent with a model in which conformational changes in the protein precede and limit ATP hydrolysis while release of the inorganic phosphate product favors a change to a weaker binding RNA-binding conformation. Assuming this is true for Mss116p, the strong dependence of exon ligation on ATP cleavage efficiency suggests that changes in the catalytic core of the protein that result in tight binding of the aI5β "target" are primarily responsible for promoting exon ligation. This tight binding may result in RNA helix disruption. However, given that Q412A does not efficiently unwind stable helices but catalyzes ATP hydrolysis and promotes ligation with wild-type efficiency suggests that this target site is composed of few base pairs or that the protein stabilizes a short lived single stranded intermediate long enough for ligation to occur. On the other hand, if Mrs1p binding prevents the transition to the second step conformation, Mss116p may transiently destabilize Mrs1p interaction by binding competitively to an overlapping site or altering structures near Mrs1p's binding site, thereby promoting exon ligation. Further investigations into the structural requirements for aI5β exon ligation will shed light on this interesting function of Mss116p.
Perspective
The need to coordinate both steps of splicing is essential in all types of intron removal. In pre-mRNA splicing in yeast, a DExH/D box protein, Prp16p is exclusively required for exon ligation and it has been postulated that it helps to promote transition of the spliceosome to a conformation that favors the second step, perhaps by rearranging mutually exclusive helices in the U2 snRNP.45–49 The finding that DExH/D-box proteins are required for coordinating both steps in both group I and pre-mRNA splicing suggest that their biochemical properties may be uniquely suited for facilitating the transition from first to second steps of catalysis. Like group I introns, there is a requirement to remove products from the first step of pre-mRNA splicing and replace them with substrates for the second step in the spliceosome.50 Interestingly, both Mss116p and Prp16p unwind RNA duplexes in vitro, which may catalyze these rearrangements either directly or by altering the affinity of other protein co-factors (Figure 7).21,22,51 Continued investigations of Mss116p function in aI5β splicing should provide important new and broadly applicable insights into the role of DExH/D-box proteins in RNA splicing and RNP assembly and function, in general.
Methods
Cloning and Protein Purification
The MRS1 and MSS116 genes were amplified from genomic DNA isolated from the BY4741 yeast strain via PCR and the products cloned into the pET28b vector (EMD Biosciences, Inc. San Diego, CA) downstream of a His6 tag that was used for protein purification. The first 108 nts of the MSS116 gene code for the mt targeting sequence and were deleted in the expression construct (MSS116Δ36).20 The MSS116 mutants were constructed by site-directed PCR mutagenesis,52 using the appropriate mutagenic primers and the MSS116Δ36 plasmid described above, and the products were cloned into the pET28b vector.
Both recombinant Mrs1p and Mss116p were expressed in BL21(DE3) cells (Novagen Inc., Madison, Wisconsin). A final concentration of 1 mM IPTG was used to induce the cultures and the Mrs1p culture was grown at 30°C for 18–20 hr, while the Mss116p culture was grown at 16°C.
The cells containing Mrs1p were harvested at 4°C, and the pellets washed with 20 mL of 150 mM NaCl. The cells were re-suspended in 8 mL of 20 mM Tris-HCl, pH 7.9, 500 mM NaCl (TN) supplemented with 5 mM imidazole and 0.01 % Triton X-100, to which 10 µL of 10 mg/mL lysozyme were added. The cells were further lysed by three freeze/thaw cycles at −70 and 25°C, in which 125 µL of 115 nM phenylmethylsulfonyl fluoride (PMSF) was added after the first freeze thaw, followed by sonication (3– 20 sec pulses) on ice. The insoluble material was removed by centrifugation of the lysate at 16,000 x g for 15 min at 4°C. The Mrs1p protein in the cleared lysate was mixed with 1.0 mL Ni-NTA agarose resin (Qiagen, Valencia, California) at 4°C for 1 hr.
The mixture was loaded onto a 1×5 cm column at 4°C, and the resin was first washed with 200 mL of TN buffer supplemented with 5 mM imidazole and 0.01 % Triton X-100. The resin was then washed with 50 mL of TN supplemented with 15 mM imidazole and 0.01 % Triton X-100, followed by a third wash with 30 mL of TN supplemented with 30 mM imidazole and 0.01 % Triton X-100. Finally, the Mrs1p protein was eluted by three successive 3 mL washes of TN supplemented with only 60, 100, and 1,000 mM imidazole. Fractions containing the pure protein were combined and dialyzed against 250 mL of TN buffer containing 50 % glycerol for 12–18 hr at 4°C. The protein was stored at −20°C.
The purification of Mss116p was performed essentially as described for the purification of Mrs1p, except that Mss116p was eluted in TN supplemented with either 200 or 1,000 mM imidazole.
Protein concentrations were determined by the absorption at 280 nm in 6 M guanidine-HCl, 20 mM Na-phosphate buffer, pH 6.6, using the calculated extinction coefficients of 33,920 and 29,800 M−1cm−1 for Mrs1p and Mss116p, respectively. The Mrs1p is a homodimer in solution, and therefore, unless otherwise indicated, the reported concentrations are dimers.28
RNA preparation
A precursor RNA containing the aI5β intron RNA was generated by in vitro transcription of the paI5FL vector,31 which yields a 1604 nt aI5β pre-RNA that includes a 12 nt 5’ exon, the catalytic core, an open reading frame encoding a degenerate homing endonuclease, and the 3’ SS followed by an 18 nt 3’ exon. The pCOX3UTL plasmid was used to transcribe the COX3 5’UTL RNA.31 Unlabeled RNAs were transcribed as described.31 End-labeled RNAs were transcribed and 32P-labeled at the 5’ end and purified as described.53 High specific activity transcripts used in the cross-linking reactions were transcribed in the presence of 4 µCi/µL [α-32P]ATP (3,000 Ci/mmole; ICN Biomedicals, Irvine, CA), 0.8 mM 4-thioUTP (Ambion, Austin, TX), 0.04 mM ATP, and 0.4 mM of each remaining NTP. High specific activity transcripts used in filter binding experiments were synthesized by adding 4 µCi/µL [α-32P]ATP (3,000 Ci/mmole) to a transcription mix containing 0.04 mM ATP and 0.4 mM of each remaining NTP.
RNA splicing assays
For protein-dependent reactions, 5’-32P-end-labeled aI5β pre-RNA was incubated in TNMSD buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 7 mM MgCl2, 2 mM spermidine, 10 mM DTT) plus 0.7 U/µL of RNase OUT (Invitrogen), 1 mM GTP, and 0.7 µg/µL tRNA, and 1 mM nucleotide cofactors when necessary, for 5 min at 37°C. All nucleotide cofactors included an equal molar concentration of MgCl2. Mss116p (final concentration 1.5 µM) and/or Mrs1p (final concentration 0.5 µM) were added simultaneously to initiate the splicing reaction. Aliquots (5 µL) were quenched with 1 µL of 0.5 M EDTA (pH 8.0) and organic extraction. The reaction products were then added to an equal volume of 2x loading buffer (10 M urea, 1x TBE) and separated in a denaturing 10 % polyacrylamide / 7 M urea / 5 % glycerol gel. The dried gels were visualized with a phosphorimager. Titration experiments confirmed that the concentrations of Mrs1p and Mss116p were saturating (see text). The ~2-fold stimulation of exon ligation was judged optimal as experiments with different concentrations of Mg2+ and/or protein gave similar (or weaker) results. Purification of Mrs1p from frozen cell pellets yielded slightly lower splicing rates and levels of exon ligation than those reported here.31
For the Proteinase K experiments, Mrs1p/aI15β pre-RNA were pre-incubated in the absence of GTP and the complex digested with 15 µg Proteinase K for 20 min at 37°C. GTP was added to a final concentration of 1 mM GTP and the reactions proceeded for 5 min. For self-splicing assays, the TNMSD buffer contained 75 mM MgCl2, and GTP (1 mM final) was used to initiate the reactions.
For the hexokinase reactions, the reaction buffer excluded GTP and included 200 mM glucose and both the Mrs1p and Mss116p proteins. After incubation for 15 min at 37°C, hexokinase (Amresco, Solon, OH) was added to a final concentration of 0.04 U/µL, and the reaction incubated for 1 min at 37°C. GTP was added to a final concentration of 1 mM to initiate the splicing reaction.
ATPase assays
For the ATPase assays, 500 nM aI5β pre-RNA was pre-incubated in a reaction mixture containing TNMSD buffer, 12.5 µM BSA, 0.005 % Nonidet P-40, 0.7 U/µL RNase OUT, 40 or 50 nM [γ-32P]-ATP, and either 50 (subsaturating) or 500 µM (saturating) ATP at 37°C for 5 min. All ATP included an equal molar concentration of MgCl2. Original ATP stocks included 167 µCi/µL of [γ-32P]-ATP (7,000 Ci/mmole) or 10 µCi/µL of [γ-32P]-ATP (25 Ci/mmole) (MP Biomedicals, Irvine, CA). Mss116p protein (50 or 200 nM final) was added to initiate the reactions. Aliquots were withdrawn (at 5 to 100 min) and spotted onto cellulose-polyethyleneimine (PEI) TLC plates (Selecto* Scientific, GA) and dried. The plates were developed with 0.5 M LiCl / 2 M acetic acid for ~ 10 min, dried, visualized, and quantified with a phosphorimager. The amount of released ADP (µM) at each time point was calculated by multiplying the fraction of inorganic phosphate by the ATP concentration. The initial rates (vo) were determined using points in the linear portion of curve.
The values for kcat, and Km were calculated as described.54 Briefly, kcat, was calculated using the relationship: vo = E × kcat, where E is the enzyme concentration. The Km parameter was calculated using the relationship: vo = E × So × kcat/Km, where So is the initial ATP concentration. The vo at 500 and 1000 µM were identical for each Mss116p derivative, thus confirming that 500 µM ATP was saturating. Furthermore, two comprehensive titration experiments with the wild-type protein yielded values very close to those reported in Table 3 (Km = 58 (± 3) µM, kcat = 5.9 (± 0.6) min−1; data not shown). The Km value was similar to that published previously using different reaction conditions. It was previously shown that kcat parameter fluctuates depending on the nature of the RNA included in the reaction: accordingly, the value here is ~ 2 –20-fold less than values measured with different RNAs.22
Unwinding assays
The design for the RNA of the duplex was previously used to characterize the DEAD-box protein Ded1.32 The RNA was made by transcription with hybridized DNA oligonucleotides, resulting in a 49 nt RNA of the sequence: 5’ –GGGCGAAUUCAAAACAAAACAAAACUAGCACCGUAAAGCAAGCCCGGGG. After transcription, the DNA template was digested with rDNase I (2 U/µL; RNase free; USB, Solon, OH) for 45 min at 37°C, the enzymes were removed by organic extraction, and the RNA was centrifuged through a Sephadex G-25 (Sigma Chemical Co., St. Louis, MO) spun column. The DNA oligonucleotide (Hel-a) had the sequence 5’ -TTGTTTTGAATTCGCCC and hybridized to the 5’ end of the RNA. The DNA (114 pmoles) was end-labeled with 8.35 µCi/µL of [γ-32P]-ATP (7,000 Ci/mmole; MP Biomedicals) using T4 polynucleotide kinase (New England Biolabs). After organic extraction, the DNA was centrifuged through a Sephadex G-25 spun column. The RNA/DNA complex was made by heating equimolar amounts of RNA and DNA in 10 mM NaCl at 95°C for 1 min followed by slow cooling to 10°C over 25 min. This resulted in ~90 % of the DNA hybridized to the RNA. The complex was stored on ice or frozen. The hybridization free energy (ΔG°30°C) of the 17 nt duplex is predicted to be −22.69 kcal/mol, using the web-based tool, HYTHER™ version 1, Nicolas Peyret and John SantaLucia, Jr., Wayne State University.55,56
For the unwinding assay, the RNA/DNA complex (~10 nM final concentration) was added to a mixture containing TNMSD buffer, 11.5 µM BSA, 0.01% Nonidet P-40, 20 nM unlabeled Hel-a DNA oligonucleotide (to prevent the labeled Hel-a from re-annealing), 1 mM ATP with an equal molar concentration of MgCl2, and 0.7 U/µL RNase OUT on ice. Mss116p or a mutant derivative was added to a final concentration of 1.5 µM to initiate the reaction. Reactions were incubated at 30°C. At each time point (1 to 20 min), aliquots were added to an equal volume of stop buffer containing 40 % glycerol, 10 mM EDTA, 10 mM aurin tricarboxylic acid, 400 nM Hel-a DNA, and 0.1 % SDS and stored on ice. The reaction products were loaded onto a running 22.5 % polyacrylamide (29:1) / 1 % glycerol gel in 1X TBE at 4°C. The gels were dried onto whatman paper and the products visualized and quantified with a phosphorimager. Unwinding experiments were also performed using 3.0 µM Mss116p or mutant derivatives, which yielded values similar to those reported in Table 4.
UV Cross-linking
For the cross-linking experiments,32P-internally-labeled, 4-thiouridine containing aI5β pre-RNA (0.5–1 nM) was incubated in TNMSD buffer with or without 1 mM ATP with an equal molar concentration of MgCl2. For competition experiments, unlabeled competitor RNAs were included at a final concentration of 0.6 µM. Mrs1p (final concentration 0.75 µM) and/or Mss116p (final concentration 0.75 µM) were added simultaneously and the reactions were incubated for 10 min at 37°C. The reaction mixture (15 µL) was placed in a 96-well plate on ice and irradiated at 312nm in a UV Stratalinker 1800 (Stratagene, La Jolla, CA, USA) for 20 min on ice. The RNA was digested with 2000 U of RNase TI (Ambion, 1000 U/µL) and 20 µg of RNase A for 60 min at 37°C and then an additional 15 min at 50°C. Each sample was incubated in an SDS-loading buffer (final: 50 mM Tris HCl, pH 6.8, 2 % SDS, 10 % glycerol, 0.01 % bromophenol blue) for 2 min at 90°C, and the reaction products separated on a 12 % SDS–PAGE (29:1) gel. The gels were dried onto whatman paper and the products visualized using a phosphorimager.
RNA binding
For equilibrium binding measurements, 2 pM 32P-labeled RNA was incubated with Mrs1p or Mss116p in 50 µL TNMSD buffer supplemented with 0.1 mg/ml bovine serum albumin and 0.01% Nonidet P-40 at 37°C. The complexes were filtered after 30 min through nitrocellulose (BA85; Schleicher and Schuell, Keene, NH, USA), washed with 3 mL of buffer, dried, and counted using a Beckman-Coulter LS6500 scintillation counter (Beckman-Coulter Inc., Fullerton, CA, USA). The data were fit to the following equation: Fraction RNA bound=A × E/(E+Kdapp) where A is the total amplitude bound and E is the concentration of Mrs1p dimer or Mss116p and Kdapp is the apparent equilibrium binding constant. Each titration was repeated three times and errors are expressed as standard deviations. To examine cooperative binding of Mrs1p, the data were also fit to the Hill equation.28
The stoichiometry of the Mrs1p/aI5β pre-RNA complex was measured by incubating 80 nM 32P-RNA with different concentrations of Mrs1p in 25 µL of buffer at 37°C for 15 min. The complexes were filtered and processed as above.
Supplementary Material
Acknowledgements
We thank Maureen Downing for constructing the Mrs1p expression vector. We also thank Dr. Timothy Nilsen (CWRU), members of the Caprara lab, and two anonymous reviewers for insightful comments on the manuscript. A.L.B. was supported in part by a Cell and Molecular Biology Training Grant awarded through NIGMS (T32-GM08056). This work was supported by National Institutes of Health grant GM-62853 to M.G.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caprara MG, Nilsen TW. RNA: versatility in form and function. Nat Struct Biol. 2000;7:831–833. doi: 10.1038/82816. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder R, Grossberger R, Pichler A, Waldsich C. RNA folding in vivo. Curr Opin Struct Biol. 2002;12:296–300. doi: 10.1016/s0959-440x(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder R, Barta A, Semrad K. Strategies for RNA folding and assembly. Nat Rev Mol Cell Biol. 2004;5:908–919. doi: 10.1038/nrm1497. [DOI] [PubMed] [Google Scholar]
- 4.Bokinsky G, Nivon LG, Liu S, Chai G, Hong M, Weeks KM, Zhuang X. Two distinct binding modes of a protein cofactor with its target RNA. J Mol Biol. 2006;361:771–784. doi: 10.1016/j.jmb.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caprara MG, Chatterjee P, Solem A, Brady-Passerini KL, Kaspar BJ. An allosteric-feedback mechanism for protein-assisted group I intron splicing. RNA. 2007;13:211–222. doi: 10.1261/rna.307907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson JR. Assembly of the 30S ribosomal subunit. Q Rev Biophys. 2005;38:397–403. doi: 10.1017/S0033583506004264. [DOI] [PubMed] [Google Scholar]
- 7.Staley JP, Guthrie C. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell. 1998;92:315–326. doi: 10.1016/s0092-8674(00)80925-3. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson J, Nissen P. Elongation factors on the ribosome. Curr Opin Struct Biol. 2005;15:349–354. doi: 10.1016/j.sbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Cordin O, Banroques J, Tanner NK, Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Linder P. Dead-box proteins: a family affair--active and passive players in RNP-remodeling. Nucleic Acids Res. 2006;34:4168–4180. doi: 10.1093/nar/gkl468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanner NK, Linder P. DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol Cell. 2001;8:251–262. doi: 10.1016/s1097-2765(01)00329-x. [DOI] [PubMed] [Google Scholar]
- 12.Sengoku T, Nureki O, Nakamura A, Kobayashi S, Yokoyama S. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell. 2006;125:287–300. doi: 10.1016/j.cell.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Henn A, Cao W, Hackney DD, De La Cruz EM. The ATPase cycle mechanism of the DEAD-box rRNA helicase, DbpA. J Mol Biol. 2008;377:193–205. doi: 10.1016/j.jmb.2007.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorsch JR, Herschlag D. The DEAD box protein eIF4A. 1. A minimal kinetic and thermodynamic framework reveals coupled binding of RNA and nucleotide. Biochemistry. 1998;37:2180–2193. doi: 10.1021/bi972430g. [DOI] [PubMed] [Google Scholar]
- 15.Polach KJ, Uhlenbeck OC. Cooperative binding of ATP and RNA substrates to the DEAD/H protein DbpA. Biochemistry. 2002;41:3693–3702. doi: 10.1021/bi012062n. [DOI] [PubMed] [Google Scholar]
- 16.Lambowitz AM, Caprara MG, Zimmerly S, Perlman PS. Group I and group II ribozymes as RNPs: clues to the past and guides to the future. In: Gesteland RF, Atkins JF, Cech TR, editors. The RNA World II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. pp. 451–485. [Google Scholar]
- 17.Caprara MG, Waring RB. Group I Introns and their maturases: Uninvited, but welcome guests. In: Belfort M, Stoddard BL, Wood DW, Derbyshire V, editors. Homing Endonucleases and Inteins. Berlin, Germany: Springer-Verlag; 2005. pp. 103–119. [Google Scholar]
- 18.Seraphin B, Simon M, Boulet A, Faye G. Mitochondrial splicing requires a protein from a novel helicase family. Nature. 1989;337:84–87. doi: 10.1038/337084a0. [DOI] [PubMed] [Google Scholar]
- 19.Mohr S, Stryker JM, Lambowitz AM. A DEAD-box protein functions as an ATP-dependent RNA chaperone in group I intron splicing. Cell. 2002;109:769–779. doi: 10.1016/s0092-8674(02)00771-7. [DOI] [PubMed] [Google Scholar]
- 20.Huang HR, Rowe CE, Mohr S, Jiang Y, Lambowitz AM, Perlman PS. The splicing of yeast mitochondrial group I and group II introns requires a DEAD-box protein with RNA chaperone function. Proc Natl Acad Sci U S A. 2005;102:163–168. doi: 10.1073/pnas.0407896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solem A, Zingler N, Pyle AM. A DEAD protein that activates intron self-splicing without unwinding RNA. Mol Cell. 2006;24:611–617. doi: 10.1016/j.molcel.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Halls C, Mohr S, Del Campo M, Yang Q, Jankowsky E, Lambowitz AM. Involvement of DEAD-box proteins in group I and group II intron splicing. Biochemical characterization of Mss116p, ATP hydrolysis-dependent and -independent mechanisms, and general RNA chaperone activity. J Mol Biol. 2007;365:835–855. doi: 10.1016/j.jmb.2006.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Campo M, Tijerina P, Bhaskaran H, Mohr S, Yang Q, Jankowsky E, Russell R, Lambowitz AM. Do DEAD-box proteins promote group II intron splicing without unwinding RNA? Mol Cell. 2007;28:159–166. doi: 10.1016/j.molcel.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreike J, Schulze M, Ahne F, Lang BF. A yeast nuclear gene, MRS1, involved in mitochondrial RNA splicing: nucleotide sequence and mutational analysis of two overlapping open reading frames on opposite strands. EMBO J. 1987;6:2123–2129. doi: 10.1002/j.1460-2075.1987.tb02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bousquet I, Dujardin G, Poyton RO, Slonimski PP. Two group I mitochondrial introns in the cob-box and coxI genes require the same MRS1/PET157 nuclear gene product for splicing. Curr Genet. 1990;18:117–124. doi: 10.1007/BF00312599. [DOI] [PubMed] [Google Scholar]
- 26.Wardleworth BN, Kvaratskhelia M, White MF. Site-directed mutagenesis of the yeast resolving enzyme Cce1 reveals catalytic residues and relationship with the intron-splicing factor Mrs1. J Biol Chem. 2000;275:23725–23728. doi: 10.1074/jbc.M002612200. [DOI] [PubMed] [Google Scholar]
- 27.Golden BL, Cech TR. Conformational switches involved in orchestrating the successive steps of group I RNA splicing. Biochemistry. 1996;35:3754–3763. doi: 10.1021/bi952599z. [DOI] [PubMed] [Google Scholar]
- 28.Bassi GS, de Oliveira DM, White MF, Weeks KM. Recruitment of intron-encoded and co-opted proteins in splicing of the bI3 group I intron RNA. Proc Natl Acad Sci U S A. 2002;99:128–133. doi: 10.1073/pnas.012579299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treiber DK, Williamson JR. Exposing the kinetic traps in RNA folding. Curr Opin Struct Biol. 1999;9:339–345. doi: 10.1016/S0959-440X(99)80045-1. [DOI] [PubMed] [Google Scholar]
- 30.Webb AE, Rose MA, Westhof E, Weeks KM. Protein-dependent transition states for ribonucleoprotein assembly. J Mol Biol. 2001;309:1087–1100. doi: 10.1006/jmbi.2001.4714. [DOI] [PubMed] [Google Scholar]
- 31.Kaspar BJ, Bifano AL, Caprara MG. A shared RNA-binding site in the Pet54 protein is required for translational activation and group I intron splicing in yeast mitochondria. Nucleic Acids Res. 2008;36:2958–2968. doi: 10.1093/nar/gkn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordin O, Tanner NK, Doere M, Linder P, Banroques J. The newly discovered Q motif of DEAD-box RNA-helicases regulates RNA-binding and helicase activity. EMBO J. 2004;23:2478–2487. doi: 10.1038/sj.emboj.7600272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazowska J, Claisse M, Gargouri A, Kotylak Z, Spyridakis A, Slonimski PP. Protein encoded by the third intron of cytochrome b gene in Saccharomyces cerevisiae is an mRNA maturase. Analysis of mitochondrial mutants, RNA transcripts proteins and evolutionary relationships. J Mol Biol. 1989;205:275–289. doi: 10.1016/0022-2836(89)90341-0. [DOI] [PubMed] [Google Scholar]
- 34.Michel F, Westhof E. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol. 1990;216:585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- 35.White MF, Lilley DM. The structure-selectivity and sequence-preference of the junction-resolving enzyme CCE1 of Saccharomyces cerevisiae. J Mol Biol. 1996;257:330–341. doi: 10.1006/jmbi.1996.0166. [DOI] [PubMed] [Google Scholar]
- 36.Adams PL, Stahley MR, Gill ML, Kosek AB, Wang J, Strobel SA. Crystal structure of a group I intron splicing intermediate. RNA. 2004;10:1867–1887. doi: 10.1261/rna.7140504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo F, Gooding AR, Cech TR. Structure of the Tetrahymena ribozyme: base triple sandwich and metal ion at the active site. Mol Cell. 2004;16:351–362. doi: 10.1016/j.molcel.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Golden BL, Kim H, Chase E. Crystal structure of a phage Twort group I ribozyme-product complex. Nat Struct Mol Biol. 2005;12:82–89. doi: 10.1038/nsmb868. [DOI] [PubMed] [Google Scholar]
- 39.Emerick VL, Pan J, Woodson SA. Analysis of rate-determining conformational changes during self-splicing of the Tetrahymena intron. Biochemistry. 1996;35:13469–13477. doi: 10.1021/bi960865i. [DOI] [PubMed] [Google Scholar]
- 40.Zarrinkar PP, Sullenger BA. Probing the interplay between the two steps of group I intron splicing: competition of exogenous guanosine with omega G. Biochemistry. 1998;37:18056–18063. doi: 10.1021/bi982193x. [DOI] [PubMed] [Google Scholar]
- 41.Karbstein K, Herschlag D. Extraordinarily slow binding of guanosine to the Tetrahymena group I ribozyme: implications for RNA preorganization and function. Proc Natl Acad Sci U S A. 2003;100:2300–2305. doi: 10.1073/pnas.252749799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hougland JL, Piccirilli JA, Forconi M, Lee J, Herschlag D. How a group I intron works: A case study of RNA structure and function. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA World III. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2006. pp. 133–205. [Google Scholar]
- 43.Fairman ME, Maroney PA, Wang W, Bowers HA, Gollnickm P, Nilsen TW, Jankowsky E. Protein displacement by DExH/D "RNA helicases" without duplex unwinding. Science. 2004;304:730–734. doi: 10.1126/science.1095596. [DOI] [PubMed] [Google Scholar]
- 44.Bowers HA, Maroney PA, Fairman ME, Kastner B, Lührmann R, Nilsen TW, Jankowsky E. Discriminatory RNP remodeling by the DEAD-box protein DED1. RNA. 2006;12:903–912. doi: 10.1261/rna.2323406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwer B, Guthrie C. A conformational rearrangement in the spliceosome is dependent on PRP16 and ATP hydrolysis. EMBO J. 1992;11:5033–5039. doi: 10.1002/j.1460-2075.1992.tb05610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Query CC, Konarska MM. Suppression of multiple substrate mutations by spliceosomal prp8 alleles suggests functional correlations with ribosomal ambiguity mutants. Mol Cell. 2004;14:343–354. doi: 10.1016/s1097-2765(04)00217-5. [DOI] [PubMed] [Google Scholar]
- 47.Konarska MM, Query CC. Insights into the mechanisms of splicing: more lessons from the ribosome. Genes Dev. 2005;19:2255–2260. doi: 10.1101/gad.1363105. [DOI] [PubMed] [Google Scholar]
- 48.Hilliker AK, Mefford MA, Staley JP. U2 toggles iteratively between the stem IIa and stem IIc conformations to promote pre-mRNA splicing. Genes Dev. 2007;21:821–834. doi: 10.1101/gad.1536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perriman RJ, Ares M., Jr Rearrangement of competing U2 RNA helices within the spliceosome promotes multiple steps in splicing. Genes Dev. 2007;21:811–820. doi: 10.1101/gad.1524307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konarska MM, Vilardell J, Query CC. Repositioning of the reaction intermediate within the catalytic center of the spliceosome. Mol Cell. 2006;21:543–553. doi: 10.1016/j.molcel.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Wagner JD, Guthrie C. The DEAH-box splicing factor Prp16 unwinds RNA duplexes in vitro. Curr Biol. 1998;8:441–451. doi: 10.1016/s0960-9822(98)70178-2. [DOI] [PubMed] [Google Scholar]
- 52.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 53.Downing ME, Brady KL, Caprara MG. A C-terminal fragment of an intron-encoded maturase is sufficient for promoting group I intron splicing. RNA. 2005;11:437–446. doi: 10.1261/rna.7225205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner JD, Jankowsky E, Company M, Pyle AM, Abelson JN. The DEAH-box protein PRP22 is an ATPase that mediates ATP-dependent mRNA release from the spliceosome and unwinds RNA duplexes. EMBO J. 1998;17:2926–2937. doi: 10.1093/emboj/17.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.SantaLucia J., Jr A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc Natl Acad Sci U S A. 1998;95:1460–1465. doi: 10.1073/pnas.95.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peyret N, Seneviratne PA, Allawi HT, SantaLucia J., Jr Nearest-neighbor thermodynamics and NMR of DNA sequences with internal A.A, C.C, G.G, and T.T mismatches. Biochemistry. 1999;38:3468–3477. doi: 10.1021/bi9825091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.