Abstract
Rationale
Nucleus accumbens dopamine (DA) participates in the modulation of instrumental behavior, including aspects of behavioral activation and effort-related choice behavior. Rats with impaired accumbens DA transmission reallocate their behavior away from food-reinforced activities that have high response requirements and instead select less-effortful types of food-seeking behavior. Although accumbens DA is considered a critical component of the brain circuitry regulating effort-related processes, emerging evidence also implicates adenosine A2A receptors.
Objective
The present work was undertaken to test the hypothesis that accumbens A2A receptor stimulation would produce effects similar to those produced by DA depletion or antagonism.
Materials and methods
Three experiments assessed the effects of the adenosine A2A agonist CGS 21680 on performance of a concurrent choice task (lever pressing for preferred food vs. intake of less preferred chow) that is known to be sensitive to DA antagonists and accumbens DA depletions.
Results
Systemic injections of CGS 21680 reduced lever pressing but did not increase feeding. In contrast, bilateral infusions of the adenosine A2A receptor agonist CGS 21680 (6.0–24.0 ng) into the nucleus accumbens decreased lever pressing for the preferred food but substantially increased consumption of the less preferred chow. Injections of CGS 21680 into a control site dorsal to the accumbens were ineffective.
Conclusions
Taken together, these results are consistent with the hypothesis that local stimulation of adenosine A2A receptors in nucleus accumbens produces behavioral effects similar to those induced by accumbens DA depletions. Accumbens adenosine A2A receptors appear to be a component of the brain circuitry regulating effort-related choice behavior.
Keywords: Decision making, Motivation, Activation, Anergia, Depression, Nucleus accumbens, Dopamine
Introduction
Motivated behavior can be characterized by vigor, persistence, and high levels of work output. These activational aspects of motivated behavior have enormous adaptive significance because they enable organisms to overcome obstacles or work-related response costs that separate them from significant stimuli (Salamone 1991, 1992; Salamone et al. 1997, 2003, 2007; Salamone and Correa 2002; Van den Bos et al. 2006; Walton et al. 2006). Moreover, pathologies related to behavioral activation, such as psychomotor slowing, anergia, and fatigue, are recognized as critical aspects of depression and other psychiatric disorders (Tylee et al. 1999; Stahl 2002; Salamone et al. 2006, 2007). Over the past two decades, considerable research has demonstrated that nucleus accumbens DA is a critical component of the brain circuitry controlling effort-related behavioral processes (Salamone et al. 1991, 1994, 1997, 2002, 2007; Phillips et al. 2007; Niv et al. 2007). Depletions of DA in nucleus accumbens make animals highly sensitive to ratio requirements in operant schedules (Sokolowski and Salamone 1998; Aberman and Salamone 1999; Correa et al. 2002; Mingote et al. 2005). Furthermore, studies involving choice behavior have shown that rats administered DA receptor antagonists or rats with accumbens DA depletions reallocate their behavior away from food-reinforced tasks that have high response requirements and instead select less-effortful types of food-seeking behavior (Salamone et al. 1991, 1997, 2003, 2005, 2006, 2007). Some of these studies utilized maze tasks to assess effort-related choice (Salamone et al. 1994; Cousins et al. 1996; Denk et al. 2005; Floresco et al. 2007). In addition, several studies have employed a concurrent fixed ratio 5 (FR5)/chow-feeding procedure (Salamone et al. 1991). With this procedure, rats are allowed to choose between completing a FR5 lever-pressing requirement for a highly palatable food (i.e., high carbohydrate operant pellets) or to approach and consume freely available food (i.e., less palatable standard rodent chow). Rats trained using this procedure spend most of their time pressing the lever for the preferred food and eat very little of the concurrently available chow. Low-to-moderate doses of DA antagonists with different selectivity profiles, including haloperidol, cis-flupenthixol, SCH 23390, SCH 39166, raclopride, and eticlopride, all suppress lever pressing for food but actually increase chow intake (Salamone et al. 1991, 2002; Cousins et al. 1994; Koch et al. 2000; Sink et al. 2008). In addition, rats with accumbens DA depletions or those treated with local intra-accumbens injections of DA antagonists also reallocate their responses in this task, showing decreases in lever pressing and increases in chow intake as a result of local interference with DA transmission (Salamone et al. 1991; Cousins et al. 1993; Sokolowski and Salamone 1998; Koch et al. 2000; Nowend et al. 2001). These effects of interference with DA transmission differ substantially from the effects produced by pre-feeding to reduce food motivation (Salamone et al. 1991), as well as appetite suppressants such as amphetamine (Cousins et al. 1994), fenfluramine (Salamone et al. 2002), and cannabinoid CB1 antagonists and inverse agonists (Sink et al. 2008), all of which fail to shift behavior from lever pressing to chow intake.
Several lines of evidence indicate that, in addition to nucleus accumbens DA, other brain areas and transmitters are involved in effort-related processes, including prefrontal cortex, amygdala, and ventral pallidum (Walton et al. 2002, 2003; Schweimer et al. 2005; Schweimer and Hauber 2006; Floresco and Ghods-Sharifi 2007; Farrar et al. 2008). Recent studies also have highlighted the involvement of the purine nucleoside adenosine in this type of function (Farrar et al. 2007). Although there are at least four types of adenosine receptors, adenosine A2A receptors are primarily localized in striatal areas, including both neostriatum and nucleus accumbens (Jarvis and Williams 1989; Schiffmann et al. 1991; DeMet and Chicz-DeMet 2002; Ferré et al. 2004). In these striatal areas, there is considerable evidence of a functional interaction between dopamine D2 and adenosine A2A receptors (Fink et al. 1992; Ferré 1997; Hillion et al. 2002; Fuxe et al. 2003). This interaction has typically been investigated in connection with neostriatal motor functions that are potentially related to parkinsonism (Ferré et al. 1997; Svenningsson et al. 1999; Ferré et al. 2001; Hauber et al. 2001; Morelli and Pinna 2002; Jenner 2003, 2005; Pinna et al. 2005). In these studies, adenosine A2A receptor antagonists have been shown to exert effects consistent with antiparkinsonian actions in animal models (Ferré et al. 1997, 2001; Hauber et al. 2001; Wardas et al. 2001; Jenner 2003; Correa et al. 2004; Pinna et al. 2005; Ishiwari et al. 2007), and adenosine A2A receptor antagonists are being evaluated as antiparkinsonian agents in human clinical trials (Jenner 2005). More recently, researchers have begun to identify potential motivational functions of adenosine A2A receptors (O’Neill and Brown 2006; Harper et al. 2006; Cabeza de Vaca et al. 2007; Farrar et al. 2007). Farrar et al. (2007) described the involvement of adenosine A2A receptors in modulating the effects of DA blockade on behavioral performance using the concurrent FR5/feeding procedure. In this experiment, a low systemic dose of the DA antagonist haloperidol induced the typical shift from lever pressing to approaching and feeding upon the freely available rodent chow. Injections of the adenosine A2A antagonist MSX-3 increased lever pressing and decreased chow intake in haloperidol-treated rats, reversing the haloperidol-induced shift in behavior.
Consistent with these previous findings of an interaction between DA and adenosine receptors, adenosine A2A agonists have been shown to induce effects that resemble those produced by DA antagonists or DA depletions (Heffner et al. 1989; Barraco et al. 1993; Ferré 1997; Rimondini et al. 1997). Administration of the adenosine A2A receptor agonist CGS 21680 into the ventricles inhibited acquisition and expression of wheel running behavior (Cabeza de Vaca et al. 2007). High systemic doses of CGS 21680 have been shown to induce catalepsy (Wardas et al. 2003). In addition, CGS 21680 depressed locomotor activity when infused directly into the nucleus accumbens (Barraco et al. 1993; Hauber and Munkel 1997). Although it seems clear that stimulation of adenosine A2A receptors in nucleus accumbens can suppress locomotor activity, relatively little is known about the role of these receptors in the regulation of effort-related processes. The present studies were undertaken to determine if stimulation of adenosine A2A receptors in nucleus accumbens would produce behavioral effects similar to those produced by DA antagonism and accumbens DA depletions; these experiments all employed the concurrent FR5/chow-feeding choice task described above. The first experiment examined the effects of systemic injections of the adenosine A2A agonist CGS 21680 on the concurrent FR5/chow-feeding task. The second experiment evaluated the effects of intra-accumbens injections of CGS 21680 using the same procedure. It was hypothesized that intra-accumbens injections of GGS 21680 would shift choice behavior from lever pressing to chow intake in a manner similar to that previously shown for dopaminergic manipulations. Finally, in order to demonstrate the anatomic specificity of these effects, the highest dose of CGS 21680 was bilaterally injected into a control site dorsal to the nucleus accumbens.
Materials and methods
Subjects
Male Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN, USA) weighing between 300 and 360 g at the beginning of the study (total n=49) were housed in a colony maintained at 23°C with a 12-h light/dark cycle (lights on at 08:00 h). Rats were food-restricted to 90% body weight prior to training, and after initial training, they were allowed modest growth (e.g., up to 95% of original weight). Water was available ad libitum in the home cages at all times. In experiments 2 and 3, animals were housed in pairs before surgery and in single cages afterwards. All animal protocols were approved by the Institutional Animal Care and Use Committee, and the methods were in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources 1996).
Drugs
The selective adenosine A2A agonist CGS 21680 4-[2-[[6-Amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid hydrochloride] was purchased from Tocris (Ellisville, MO, USA). For the systemic injections (experiment 1), CGS 21680 was dissolved in 2% dimethyl sulfoxide solution (Fisher Scientific, Hampton, NH, USA). This solution also served as the vehicle control. For the intracranial injections (experiments 2 and 3), CGS 21680 was dissolved in a saline solution (0.9%) at room temperature and afterwards was sonicated for 8–10 min. For these experiments, saline was also used as the vehicle control solution.
Behavioral procedure—acquisition phase
All the experiments were conducted in operant chambers (28×23×23 cm; Med Associates). After magazine training, all rats were trained for 4 days to lever press (30 min sessions; 45 mg pellets, Bioserve Inc., Frenchtown, NJ, USA) on a fixed ratio 1 (FR1) schedule of reinforcement. In this schedule for each lever press, the animals receive one operant pellet (45 mg pellets, Bioserve Inc., Frenchtown, NJ, USA). After this initial training, the animals were trained on a FR5 schedule (30-min sessions, 5 days/week) for four additional weeks. Rats were then trained on the concurrent FR5/chow-feeding procedure for at least 1 week before surgery. For this procedure, weighed amounts of lab chow (typically 15–20 g, three large pieces) were concurrently available on the floor of the chamber during the FR5 sessions. At the end of the session, rats were immediately removed from the chamber. Food intake was determined by calculating the difference between pre- and post-session food weight, including spillage, which was collected on paper sheets below the floor of the operant chambers.
Surgery
For guide cannulae implantations in the intracranial injection experiments, animals were anesthetized with a solution (1.0 ml/kg, IP) that was prepared by mixing 10 ml of 100 mg/ml ketamine and 0.75 ml of 20 mg/ml xylazine and placed in a stereotaxic device (David Kopf Instruments, Tujunga, CA, USA). For experiment 1, animals received bilateral implantation of stainless steel guide cannulae (25 gauge; Small Parts, Inc., Miami Lakes, FL, USA) 1.0 mm above the nucleus accumbens (flat skull; AP +1.6 mm from the bregma and ±1.4 mm lateral to bregma and −6.8 mm ventral to skull surface). Coordinates for placements into dorsal control site were AP +1.6 mm from bregma, ML ±1.4 mm lateral from midline, and DV −4.8 mm ventral from the surface of the skull. These coordinates were obtained from the Paxinos and Watson (1998) stereotaxic atlas. All guide cannulae were secured to the skull by stainless steel screws and by Durelon carboxylate cement (3M ESPE Dental products, St. Paul, MN, USA). A stainless steel stylet was inserted through each guide cannula to insure its integrity. Following surgery, animals were allowed to recover for a minimum of 7 days before testing.
Experimental procedures
Experiment 1: effects of systemic administration of the selective adenosine A2A agonist CGS 21680 on the concurrent FR5/chow-feeding procedure
After the initial training with the concurrent FR5/chow-feeding procedure described above, all animals (n=8) received IP injections of the following doses of CGS 21680: vehicle, 0.0125, 0.025, 0.05, 0.1, and 0.2 mg/kg. This experiment used a within-groups design, with all rats receiving all drug treatments in a randomly varied order (one treatment per week). Baseline training (i.e., non-drug) sessions were conducted four additional days per week. All injections were given 15 min before the animals were put in the operant chambers for a 30-min session. Behavioral measures for the concurrent FR5/chow-feeding procedure included number of food pellets obtained, number of lever presses, and grams of lab chow intake as described above.
Experiment 2: effects of intracranial injections of CGS 21680 into the nucleus accumbens on the concurrent FR5/chow-feeding procedure
After recovering from cannulae implantation surgery, rats resumed training on the concurrent FR5/chow-feeding procedure for two additional weeks. Rats were then randomly assigned to one of the following drug treatment groups: vehicle (n=7), 6.0 (n=7), 12.0 (n=7), and 24.0 ng/0.5 μl per side (n=7). On the test day, bilateral injections of vehicle or CGS 21680 into the nucleus accumbens were made through a guide cannula using 30-gauge injectors set to extend 1.0 mm beyond the tips of the cannulae. Each injector was attached via PE10 tubing to a 10.0-μl syringe driven by Harvard syringe pump. All injections were at a flow rate of 0.125 μl/min, and the total volume injected was 0.5 μl per side. After injection, the injector was left in place for one more minute to allow for diffusion from the injection site. Directly following the injection procedure, the animals were placed in the operant boxers for a 30-min session on the concurrent FR5/chow-feeding procedure. At the end of the session, rats were immediately removed from the chamber. Food intake was determined by calculating the difference between pre- and post-session food weight, including spillage, which was collected on paper sheets below the floor of the operant chambers. Other behavioral measures included number of food pellets obtained and number of lever presses.
Experiment 3: effects of intracranial injections of CGS 21680 into the dorsal control sites on the concurrent FR5/chow-feeding procedure
The procedures followed in this experiment were the same as those in experiment 2, except that a different injection site was used. Once animals were recovered from surgery and presented a stable baseline of concurrent FR5/chow feeding, bilateral injections of either saline vehicle (n=7) or 24.0 ng of CGS 21680 (n=6) were given into the control site 2 mm dorsal to the nucleus accumbens, as described above.
Histology
At the completion of behavioral testing in the intracranial injection experiments, each animal was anesthetized with CO2. In experiments 2 and 3, rats were perfused intracardially with physiological saline followed by a 3.7% formaldehyde solution. The brains were removed and stored in formaldehyde and then were sliced with a cryostat in 50-μm sections, which were mounted on glass microscope slides. Following mounting, slides were stained with Cresyl Violet for microscopic observation by an observer who was unaware of the experimental treatment. Any animal with improper cannulae placement or significant damage around the injection site was excluded from the statistical analyses of behavioral data (27% of all implantations were rejected). Final numbers of animals included in the statistical data analyses for each experiment are as follows: experiment 2 (n=28), experiment 3 (n=13).
Data analysis
For the within-subjects study (experiment 1), the total number of lever presses and the quantity of chow intake were analyzed with repeated measures analysis of variance (ANOVA). Non-orthogonal planned comparisons using the overall error term were used, with the number of comparisons being restricted to the number of treatments minus one (Keppel 1991). The behavioral data for experiments 2 and 3 that included number of lever presses and chow consumption quantities were both analyzed using between-subjects analyses. For each measure analyzed in these experiments, a one-way ANOVAs (CGS 21680 doses) was conducted. Non-orthogonal planned comparisons using the overall error term were used, with the number of comparisons being restricted to the number of treatments minus one (Keppel 1991). Finally, correlational analyses were used to measure the relation between lever pressing and chow consumption in rats treated with intra-accumbens CGS 21680. This correlational analysis has been conducted previously to provide an additional statistical marker of the shift from lever pressing to chow intake that was produced by interference with DA transmission (Cousins et al. 1993; Sokolowski and Salamone 1998; Salamone et al. 2002).
Results
Experiment 1: effects of systemic administration of the selective adenosine A2A agonist CGS 21680 on the concurrent FR5/chow-feeding procedure
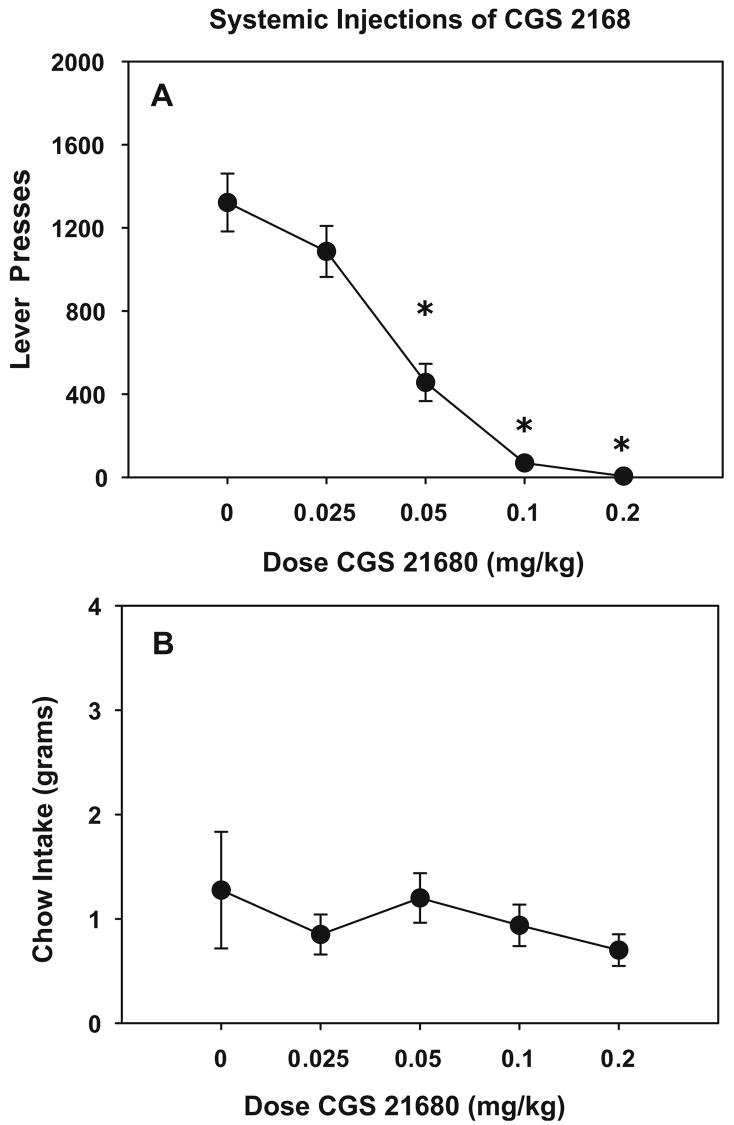
On a concurrent FR5/chow-feeding procedure in which rats can choose between pressing the lever for preferred food and consuming readily available lab chow, systemic injections of CGS 21680 produced a reduction in the number of lever presses [F(4, 28)=46.92, p<0.001) and no statistical change in the amount of chow intake [F(4, 28)= 0.640, p=0.633]. Figure 1a and b show the effects of CGS 21680 on lever presses and chow intake, respectively. Planned comparisons revealed that 0.05, 0.1, and 0.2 mg/kg doses of CGS 21 680 significantly reduced the number of lever presses relative to vehicle control (p<0.05).
Fig. 1.
Systemic injections of CGS 21680. a Effects of systemic injections of CGS 21680 on the lever pressing on the FR5/chow-feeding procedure. Mean (±SEM) number of lever presses after injections of vehicle and different doses of CGS 21680. b Effects of systemic injections of CGS 21680 on chow consumption on the FR5/chow-feeding procedure. Mean (±SEM) weight of chow intake in grams after injections of vehicle and different doses of CGS 21680. Asterisks different from vehicle, p<0.05
Experiment 2: effects of intracranial injections of CGS 21680 into the nucleus accumbens on the concurrent FR5/chow-feeding procedure
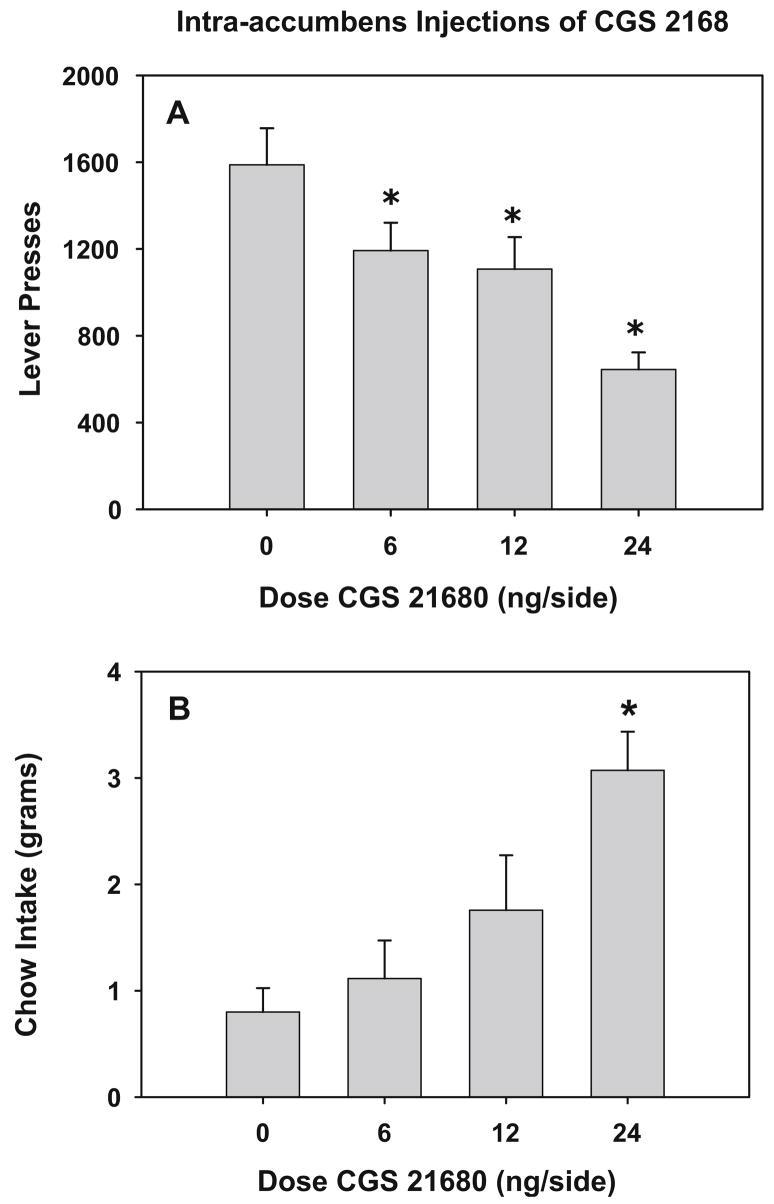
The results of experiment 2 in which rats received injections of CGS 21680 into the nucleus accumbens are presented in Fig. 2a and b. In this experiment, CGS 21680 dose dependently decreased lever pressing [F(3, 24)=8.218, p= 0.001] and also increased chow consumption [F(3, 24)= 7.09, p<0.01]. Planned comparisons revealed that 6.0, 12.0, and 24.0 ng/0.5 μl per side doses of CGS 21680 significantly reduced the number of lever presses relative to vehicle control (p<0.05). In addition, planned comparisons on the chow consumption data showed that the highest dose (24.0 ng CGS 21680) increased chow consumption to quantities that were statistically different from vehicle (p<0.05). Correlational analysis revealed that there was a significant inverse correlation between lever pressing and chow consumption in rats treated with CGS 21680 (Pearson correlation=−0.757, df=19, p<0.001). Additional analyses were performed on the total amount of food consumed (i.e., 45 mg operant pellets plus chow consumed). The mean (+SEM) values for total food consumption were as follows: vehicle, 15.1 (+1.4) g; 6.0 ng CGS 21680, 11.8 (+1.0) g; 12.0 ng CGS 21680, 11.7 (+1.1) g; 24.0 ng CGS 21680, 8.8 (+0.5) g. There was an overall significant effect of CGS 21680 on total food intake (F(3, 24)=5.9, p<0.005). Across all animals, there was a significant positive correlation between lever pressing and total food intake (r=0.97, p<0.01, df=26) and a significant negative correlation between chow intake and total food consumed (r=−0.57, p<0.01, df=26).
Fig. 2.
Intra-accumbens injections of CGS 21680. a Effects of intracranial injections of CGS 21680 into the nucleus accumbens on lever pressing on the FR5/chow-feeding procedure. Mean (±SEM) number of lever presses for rats that received injections of vehicle or different doses of CGS 21680. b Effects of intra-accumbens injections of CGS 21680 on chow consumption on the FR5/chow-feeding procedure. Mean (±SEM) weight of chow intake in grams after injections of vehicle or different doses of CGS 21680. Asterisks different from vehicle, p<0.05
Experiment 3: effects of intracranial injections of CGS 21680 into the dorsal control site on the concurrent FR5/chow-feeding procedure
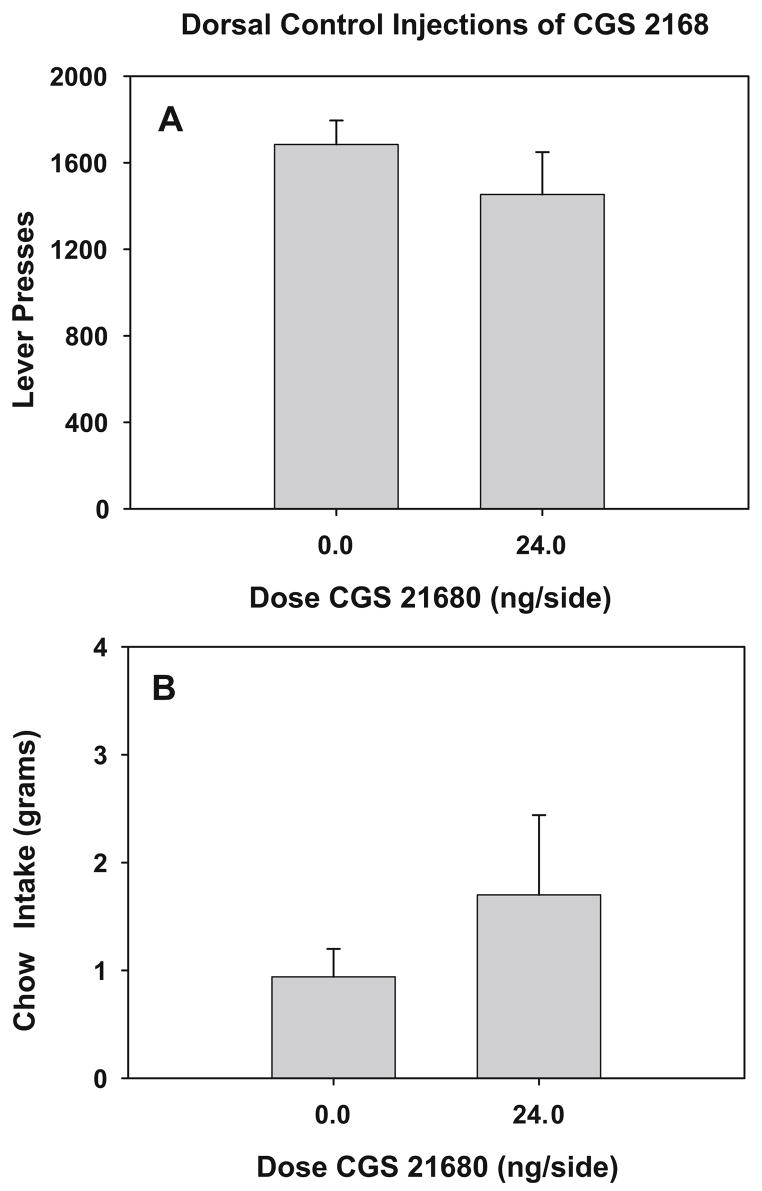
The results of experiment 3 in which rats received injections of CGS 21680 into a control site dorsal to the nucleus accumbens are depicted in Fig. 3a and b. Data analyses demonstrated that CGS 2168 (24.0 ng/0.5 μl per side) microinjected into the dorsal control area had no significant effect on the concurrent lever pressing/chow-feeding choice task (lever pressing: t=1.07, df=11, ns; chow intake: t=−1.1, df=11, ns).
Fig. 3.
Dorsal control injections of CGS 21680. a Effects of intracranial injections of CGS 21680 into a control site dorsal to the accumbens on lever pressing on the FR5/chow-feeding procedure. Mean (±SEM) number of lever presses during a 30-min session in rats treated with vehicle or CGS 21680. b Effects of intracranial injections of CGS 21680 into the dorsal control site on the chow consumption. Mean (±SEM) weight of chow intake in grams during a 30-min session after injections of vehicle or CGS 21680
Analysis of histology and general observations of behavior
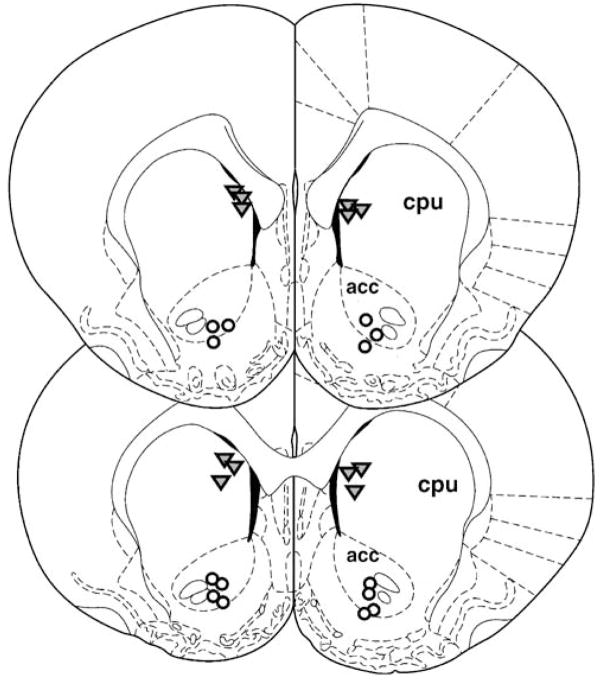
Figure 4 shows the cannula placements for rats that received injection of CGS 21680 into the nucleus accumbens (experiment 2) and the control site dorsal to the accumbens (experiment 3). The rats that received 24 ng CGS 21680 are shown because this is the only dose that showed a significant effect on both lever pressing and chow intake in experiment 2, and it was the only dose used in experiment 3. Placements in the accumbens were generally in core or adjacent areas of dorsomedial shell. Among animals in the dorsal control experiment, cannula placements were generally in the anterior/medial part of the neostriatum dorsal to nucleus accumbens.
Fig. 4.
This figure, which was adapted from Paxinos and Watson (1996), shows the cannulae placements for rats that received bilateral injections of the highest dose of CGS 21680 (24.0 ng) in the nucleus accumbens in experiment 2 (open circles) and the dorsal control site in experiment 3 (gray triangles). cpu Caudate/putamen, acc nucleus accumbens
Overt signs of sedation were noted by experimenters as they placed animals in and out of the test chambers. In experiment 1, some of the rats that received 0.05 mg/kg CGS 21680 and all the animals that received 0.1 and 0.2 mg/kg CGS 21680 showed overt signs of sedation (e.g., eyes partially closed, head lowered). None of the animals in the intracranial experiments (i.e., experiments 2 or 3) showed overt signs of sedation after injections of CGS 21680. In addition, drug-treated rats in each experiment consumed all of the operant pellets that were presented.
Discussion
The present studies demonstrated that systemic injections of CGS 21680 produced a dose-related suppression of FR5 lever pressing but that drug-treated rats with reduced levels of responding failed to show corresponding increases in chow intake. Thus, the effects of systemic administration of an adenosine A2A agonist did not produce effects that closely mimic the actions of low doses of DA antagonists or accumbens DA depletions. Nevertheless, local injections of CGS 21680 into the nucleus accumbens did induce a shift from lever pressing to chow intake. These studies support the hypothesis that stimulation of adenosine A2A receptors in nucleus accumbens can produce effects that resemble those resulting from accumbens DA depletions or DA receptor antagonism and also highlight differences between the effects of systemic and intra-accumbens injections of CGS 21680.
The behavioral procedure used in the present studies provides information about the allocation of behavior in the context of an operant conditioning environment that allows for choices between alternative paths that lead to food. Rats can choose between lever pressing on a FR5 schedule to obtain a preferred food or approaching and feeding upon a less preferred lab chow that is concurrently available. Untreated animals trained on this procedure generally receive most of their food by pressing the lever for food pellets and tend to eat little of the concurrently available but less preferred chow (Salamone et al. 1991; Cousins et al. 1993, 1996; Cousins and Salamone 1994; Salamone et al. 1995, 2002; Nowend et al. 2001; Farrar et al. 2007). However, rats injected with low doses of DA antagonists or rats with accumbens DA depletions reallocate their behavior towards the less-effortful food-seeking behavior, i.e., animals with decreased lever pressing show a substantial increase in feeding upon the rodent chow (Salamone et al. 1991, 2002; Cousins et al. 1993, 1994; Cousins and Salamone 1994; Sokolowski and Salamone 1998; Koch et al. 2000; Nowend et al. 2001). The results of these studies have been interpreted to mean that interference with nucleus accumbens DA transmission changes the pattern of choice behavior, altering the relative allocation of food-related responses away from lever pressing and towards approach and consumption of chow (Salamone et al. 1997, 2003, 2007). Farrar et al. (2007) recently showed that systemic administration of the adenosine A2A antagonist MSX-3 could attenuate the effects of the DA antagonist haloperidol in rats performing on the concurrent FR5/chow intake procedure. This finding suggests that adenosine A2A receptors participate in the effort-related functions of nucleus accumbens, and the present studies were undertaken to determine if stimulation of adenosine A2A receptors in nucleus accumbens could produce effects that resemble those produced by accumbens DA depletions or DA receptor antagonism.
Experiments 1 and 2 examined the effects of systemic and intra-accumbens injections of the adenosine A2A agonist CGS 21680 using the concurrent lever pressing/chow intake procedure. The results of the systemic administration study (experiment 1) showed the predicted dose-related suppression of FR5 lever pressing, which is consistent with a previous paper demonstrating that CGS 21680 could reduce lever-pressing rates in rats responding on cocaine and methamphetamine drug discrimination tasks (Justinova et al. 2003). However, in experiment 1, there were no corresponding increases in chow intake in drug-treated rats. Thus, systemic administration of an adenosine A2A agonist did not produce effects that closely mimic the effects of low doses of a DA antagonist or accumbens DA depletions. Nevertheless, as hypothesized, local injections of CGS 21680 into the nucleus accumbens decreased lever pressing and increased chow intake. Thus, although lever pressing was decreased by intra-accumbens CGS 21680, animals showed a compensatory reallocation of their behavior to the alternative choice (i.e., approaching and eating the freely available chow). In agreement with these observations, correlational analyses revealed a strong inverse relation between lever pressing and chow intake across drug treatments. This robust inverse correlation between lever pressing and chow intake in drug-treated animals has been a reliable statistical marker of the shift from lever pressing to chow intake that is induced by dopaminergic manipulations (Cousins et al. 1993; Nowend et al. 2001; Salamone et al. 2002; Sink et al. 2008). In view of these results, it appears as though the hypothesis that local stimulation of adenosine A2A receptors could produce effects similar to interference with DA transmission was confirmed. Furthermore, in the present study, local infusion of CGS 21680 into a site 1 mm above the nucleus accumbens into anterior/medial dorsal striatum failed to produce any significant effect on lever pressing or chow consumption. For these reasons, it is unlikely that diffusion to other brain areas accounts for the effects observed after intra-accumbens injections. The present studies used a nucleus accumbens placement that was targeted towards central portions of the core, and most of the placements were in the medial core, with a few in adjacent areas of the dorsomedial shell (see Fig. 4). Recent data from our laboratory indicate that the locomotor effects of adenosine A2A antagonists can differ depending upon whether they are injected into core or shell, with core placements being more effective (Ishiwari et al. 2007). Thus, future research in this area should specifically target distinct core and shell placements, as was done previously with studies involving DA antagonists (e.g., Nowend et al. 2001).
In experiment 2, stimulation of adenosine A2A receptors in nucleus accumbens also decreased total food consumed. This observation is consistent with previous studies in which the concurrent lever-pressing chow-feeding procedure was used to study the effects of accumbens DA depletions or antagonism (Salamone et al. 1991; Koch et al. 2000; Nowend et al. 2001). However, as noted previously (Nowend et al. 2001), this effect is merely an artifact of the shift from operant pellet intake to chow consumption and the different baseline amounts of food consumed from this source. With free-feeding, FR1 or FR5 procedures, rats typically consume about 13–15 g of the 45-mg operant pellets in a 30-min session, while the large chow pellets are consumed much more slowly (i.e., 5–7 g per 30 min, see Salamone et al. 1991, 1993). Thus, any manipulation that decreases operant pellet intake and increases chow consumption in animals responding on this task will invariably reduce total food intake because the total amount of food consumed is simply regressing towards the mean chow intake that would occur if only chow were available. This observation is consistent with the correlational analyses showing that, across all animals in experiment 2, lever pressing was positively correlated with total food intake, while chow consumption was negatively correlated with total food intake.
As described above, systemic injections of CGS 21680 had a large suppressive effect on operant responding that was not accompanied by an increase in chow consumption, which was quite distinct from the effects produced by intra-accumbens injections of CGS 21680. There could be several reasons why IP administration of CGS 21680 produced different effects than intra-accumbens injections. One of the likely explanations is that systemic administration of an adenosine A2A agonist such as CGS 21680 produces sedation (i.e., drowsiness). The sedative effects of adenosine have been well characterized (Satoh et al. 1998; Porkka-Heiskanen et al. 2000; Scammell et al. 2001; Hong et al. 2005; Stenberg 2007). Systemic or intraventricular injections of adenosine, as well as local infusions into the basal forebrain, have been shown to induce sleep (Stenberg 2007). Sleep deprivation is associated with increases in the extracellular levels of adenosine, and non-selective adenosine antagonists such as caffeine are used to enhance wakefulness (Stenberg 2007). Evidence indicates that both adenosine A1 and A2A receptors are involved in the regulation of sleep, though probably through different mechanisms and distinct brain areas (Stenberg 2007). Several studies have reported that sleep can be induced by systemic administration of CGS 21680 or local injections of this drug into the basal forebrain (Satoh et al. 1998; Porkka-Heiskanen et al. 2000; Scammell et al. 2001; Hong et al. 2005; Stenberg 2007). Knockout of adenosine A2A receptors in mice resulted in a loss of sensitivity to the sedative effects of adenosine A2A agonists, and in these studies, it also was shown that an adenosine A2A agonist could induce sleep in wild-type mice (Satoh et al. 1998). Thus, it is possible that systemic administration of CGS 21680 decreased lever pressing but failed to increase feeding because of a drug-induced sedation effect (i.e., induction of drowsiness, which produced a non-selective suppression of several behaviors), which is consistent with the behavioral observations of rats treated with systemic CGS 21680 (see above). Interestingly, the atypical antipsychotic clozapine, which is known to produce sedation, decreased the number of lever presses and, like CGS 21680, failed to increase chow consumption in the FR5/chow-feeding procedure at doses that also produced observable sedative effects (Salamone et al. 1996).
Consistent with these observations, recent studies from our laboratory indicate that systemic administration of CGS 21680 produced a broad of suppression of behavior that was accompanied by marked sedation and drowsiness (Mingote et al. 2008). This study employed a sedation rating scale adapted from Salamone et al. (1996) and Chuck et al. (2006), and showed that, in the same systemic dose range that also suppressed FR5 lever pressing and feeding, CGS 21680 (0.05 and 0.1 mg/kg IP) significantly induced overt signs of sedation/drowsiness that included foot dragging, stumbling, ataxia, flattened posture, lowered head, and partially closed eyes. In the Mingote et al. (2008) study, 0.1 mg/kg CGS 21680 produced a level of sedation that was comparable to that induced by 2.0 g/kg ethanol (Chuck et al. 2006), which is a dose of ethanol that also suppressed lever pressing. Thus, the absence of a shift from lever pressing to chow intake following systemic administration of CGS 21680 is likely to be due to a sedation/drowsiness effect. It is possible that intra-accumbens injections of CGS 21680 bypassed these sedative effects, and in fact, the experimenters did not note any overt signs of sedation in rats that received intra-accumbens injections of CGS 21680; this lack of sedation could have allowed the predicted shift in behavior from lever pressing to chow intake to occur.
In addition to sedation, there are other possible mechanisms through which systemic injections of CGS 21680 could be acting to keep levels of feeding low in animals with suppressed operant responding. For example, IP administration of CGS 21680 is likely to be stimulating neostriatal adenosine A2A receptors, as well as those in nucleus accumbens. Several lines of evidence indicate that ventrolateral neostriatum (VLS) is a critical area in regulating orofacial and forepaw motor control (Heimer et al. 1995; McGeorge and Faull 1989; Cousins et al. 1996, 1999). Previous work has demonstrated that DA depletions in the VLS reduce both lever presses and chow consumption in rats responding on the concurrent FR5/chow intake task (Cousins et al. 1993). Depletions of DA in the VLS induced profound deficits in feeding that are related to impairments in feeding rate and a loss of the ability to handle the food (Cousins et al. 1993; Salamone et al. 1993). Consistent with this idea, recent studies have demonstrated that systemic administration of CGS 21680 in doses of 0.05 mg/kg or higher can induce feeding impairments, which are marked by a significant reduction in the rate of feeding (Mingote et al. 2008). Thus, it is possible that motor impairments related to neostriatal functions also contributed to the generally low levels of both lever pressing and feeding after IP injections of CGS 21680. Finally, it is possible that systemic CGS 21680 acted to suppress appetite or produce food aversions. Previous work showed that appetite suppressants such as amphetamine and fenfluramine decreased lever pressing but failed to increase chow intake in animals responding on the choice procedure (Cousins et al. 1994; Salamone et al. 2002). More recent data indicate that CB1 antagonists and inverse agonists, which act either to suppress appetite or induce food aversions, also decrease lever pressing but fail to increase chow intake in rats tested on the concurrent choice procedure (Sink et al. 2008). Any one of these actions (sedation, motor impairment, appetite suppression, or food aversion) could have contributed to the effects of systemic CGS 21680, and it is possible that some combination of these actions led to the results of experiment 1.
The present experiments demonstrated clearly that adenosine transmission can regulate operant responding. Yet, they also illustrated that systemic injections of an adenosine A2A receptor agonist can have different behavioral effects than those produced by injections into the nucleus accumbens. Systemic administration of CGS 21680 produced effects that were quite broad, probably resulting from stimulation of A2A receptors in several distinct brain regions that regulate a variety of different functions. In contrast, the behavioral effects of stimulation of adenosine A2A receptors in the nucleus accumbens tended to be more selective, and they more closely resembled those effects produced by accumbens DA depletions or local DA antagonism. Thus, our data establish a parallel between the behavioral consequences of stimulating adenosine A2A receptors in the nucleus accumbens and earlier findings in which accumbens DA depletions or local DA antagonism altered the relative allocation of food-related responses away from lever pressing and towards approach and consumption of chow. Both dopaminergic and adenosinergic manipulations appear to make animals more sensitive to the work requirements of a task. These findings suggest a possible functional interaction between DA and adenosine in the nucleus accumbens that modulates effort output in motivated behavior. In addition to being involved in the regulation of normal motivated behavior, it also is possible that adenosine transmission in the nucleus accumbens is involved in pathological aspects of behavioral activation, such as anergia or psychomotor slowing in depression (Salamone et al. 2006, 2007). Additional research into the function of adenosine A2A receptors, as well as associated forebrain systems, may lead to a greater understanding of both normal and pathological features of motivation and may also promote the development of novel treatments for effort-related disorders in humans.
Acknowledgments
This research was supported by a grant to JDS from the United States NIH/NIMH (MH078023-01A1). L. Font was supported by a fellowship from Generalitat Valenciana (Conselleria d’ Empresa, Universitat i Ciencia), Spain.
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/S0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Barraco RA, Martens KA, Parizon M, Normile HJ. Adenosine A2A receptors in the nucleus accumbens mediate locomotor depression. Brain Res Bull. 1993;31:397–404. doi: 10.1016/0361-9230(93)90233-2. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Kannan P, Pan Y, Jiang N, Sun Y, Carr KD. The adenosine A2A receptor agonist, CGS-21680, blocks excessive rearing, acquisition of wheel running, and increases nucleus accumbens CREB phosphorylation in chronically food-restricted rats. Brain Res. 2007;1142:100–109. doi: 10.1016/j.brainres.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79:154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Correa M, Carlson BB, Wisniecki A, Salamone JD. Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res. 2002;137:179–187. doi: 10.1016/S0166-4328(02) 00292-9. [DOI] [PubMed] [Google Scholar]
- Correa M, Wisniecki A, Betz A, Dobson DR, O’Neill MF, O’Neill MJ, Salamone JD. The adenosine A2A antagonist KF17837 reverses the locomotor suppression and tremulous jaw movements induced by haloperidol in rats: possible relevance to parkinsonism. Behav Brain Res. 2004;148:47–54. doi: 10.1016/S0166-4328(03)00178-5. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-X. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Sokolowski JD, Salamone JD. Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacol Biochem Behav. 1993;46:953–951. doi: 10.1016/0091-3057(93)90226-J. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Wei W, Salamone JD. Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology. 1994;116:529–537. doi: 10.1007/BF02247489. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res. 1996;74:189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Trevitt J, Atherton A, Salamone JD. Different behavioral functions of dopamine in the nucleus accumbens and ventrolateral striatum: a microdialysis and behavioral investigation. Neuroscience. 1999;91:925–934. doi: 10.1016/S0306-4522(98)00617-4. [DOI] [PubMed] [Google Scholar]
- DeMet EM, Chicz-DeMet A. Localization of adenosine A2A-receptors in rat brain with [3H]ZM-241385. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:478–481. doi: 10.1007/s00210-002-0613-3. [DOI] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Pereira M, Velasco F, Hockemeyer J, Muller CE, Salamone JD. Adenosine A(2A) receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology. 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Font L, Pereira M, Mingote S, Bunce JG, Chrobak JJ, Salamone JD. Forebrain circuitry involved in effort-related choice: injections of the GABAA agonist muscimol into ventral pallidum alters response allocation in food-seeking behavior. Neuroscience. 2008;152:321–330. doi: 10.1016/j.neuroscience.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S. Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology. 1997;133:107–120. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/S0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Giménez-Llort L, Rimondini R, Müller CE, Strömberg I, Ögren SO, Fuxe K. Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2001;7(3):235–241. doi: 10.1016/S1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casado V, Hillion J, Torvinen M, Fanelli F, Benedetti PdP, Goldberg SR, Bouvier M, Fuxe K, Agnati LF, Lluis C, Franco R, Woods A. Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuropsychiatric disorders. Parkinsonism Relat Disord. 2004;10:265–271. doi: 10.1016/j.parkreldis.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2A adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328X(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex. 2007;17:251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301565. in press. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, Tinner-Staines B, Staines W, Rosin D, Terasmaa A, Popoli P, Leo G, Vergoni V, Lluis C, Ciruela F, Franco R, Ferré S. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61:S19–S23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- Harper LK, Beckett SR, Marsden CA, McCreary AC, Alexander SP. Effects of the A 2A adenosine receptor antagonist KW6002 in the nucleus accumbens in vitro and in vivo. Pharmacol Biochem Behav. 2006;83:114–121. doi: 10.1016/j.pbb.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Hauber W, Munkel M. Motor depressant effects mediated by dopamine D2 and adenosine A2A receptors in the nucleus accumbens and the caudate–putamen. Eur J Pharmacol. 1997;323:127–131. doi: 10.1016/S0014-2999(97)00040-X. [DOI] [PubMed] [Google Scholar]
- Hauber W, Neuscheler P, Nagel J, Muller CE. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A2A receptors in the caudate putamen of rats. Eur J Neurosci. 2001;14:1287–1293. doi: 10.1046/j.0953-816x.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Wiley JN, Williams AE, Bruns RF, Coughenour LL, Downs DA. Comparison of the behavioral effects of adenosine agonists and dopamine antagonists in mice. Psychopharmacology. 1989;98:31–37. doi: 10.1007/BF00442002. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Alheid GF. Basal ganglia. In: Paxinos G, editor. The rat nervous system. Academic; New York: 1995. pp. 579–628. [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferré S, Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Hong ZY, Huang ZL, Qu WM, Eguchi N, Urade Y, Hayaishi O. An adenosine A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. Neurochem. 2005;92:1542–1549. doi: 10.1111/j.1471-4159.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- Ishiwari K, Madson LJ, Farrar AM, Mingote SM, Valenta JP, DiGianvittorio MD, Frank LE, Correa M, Hockemeyer J, Muller C, Salamone JD. Injections of the selective adenosine A2A antagonist MSX-3 into the nucleus accumbens core attenuate the locomotor suppression induced by haloperidol in rats. Behav Brain Res. 2007;178:190–199. doi: 10.1016/j.bbr.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Williams M. Direct autoradiographic localization of adenosine A2A receptors in the rat brain using the A2A-selective agonist, [3H]CGS 21680. Eur J Pharmacol. 1989;168:243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- Jenner P. Dopamine agonists, receptor selectivity and dyskinesia induction in Parkinson’s disease. Curr Opin Neurol. 2003;16(Suppl 1):S3–S7. doi: 10.1097/00019052-200312001-00002. [DOI] [PubMed] [Google Scholar]
- Jenner P. Istradefylline, a novel adenosine A2A receptor antagonist, for the treatment of Parkinson’s disease. Expert Opin Investig Drugs. 2005;14:729–738. doi: 10.1517/13543784.14.6.729. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferré S, Segal PN, Antoniou K, Solinas M, Pappas LA, Highkin JL, Hockemeyer J, Munzar P, Goldberg SR. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–986. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researcher’s handbook. Prentice-Hall; Englewood Cliffs, New Jersey: 1991. [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Role of muscles accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RLM. Organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Mingote S, Weber SM, Ishiwari K, Correa M, Salamone JD. Ratio and time requirements on operant schedules: effort-related effects of nucleus accumbens dopamine depletions. Eur J Neurosci. 2005;21:1749–1757. doi: 10.1111/j.1460-9568.2005.03972.x. [DOI] [PubMed] [Google Scholar]
- Mingote S, Pereira M, Farrar AM, McLaughlin PJ, Salamone JD. Systemic administration of the adenosine A2A agonist CGS 21680 induces sedation at doses that suppress lever pressing and food intake. Pharmacol Biochem Behav. 2008;89:345–351. doi: 10.1016/j.pbb.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M, Pinna A. Interaction between dopamine and adenosine A2A receptors as a basis for the treatment of Parkinson’s disease. Neurol Sci. 2002;22:71–72. doi: 10.1007/s100720170052. [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology. 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/S0091-3057(01)00524-X. [DOI] [PubMed] [Google Scholar]
- O’Neill M, Brown VJ. The effect of the adenosine A2A antagonist KW-6002 on motor and motivational processes in the rat. Psychopharmacology. 2006;184:46–55. doi: 10.1007/s00213-005-0240-z. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Compact. 3. Academic Press; San Diego: 1996. The rat brain in stereotaxic coordinates. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic; Spiral Bound, New York: 1998. [Google Scholar]
- Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology. 2007;191:483–495. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- Pinna A, Wardas J, Simola N, Morelli M. New therapies for the treatment of Parkinson’s disease: adenosine A2A receptor antagonists. Life Sci. 2005;77:3259–3267. doi: 10.1016/j.lfs.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/S0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Ferré S, Ogren SO, Fuxe K. Adenosine A2A agonists: a potential new type of atypical antipsychotic. Neuropsychopharmacology. 1997;17:82–91. doi: 10.1016/S0893-133X(97)00033-X. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Behavioral pharmacology of dopamine systems: a new synthesis. In: Willner P, Scheel-Kruger J, editors. The mesolimbic dopamine system: from motivation to action. Cambridge University Press; Cambridge, England: 1991. pp. 599–613. [Google Scholar]
- Salamone JD. Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology. 1992;107:160–174. doi: 10.1007/BF02245133. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology. 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993;44:605–610. doi: 10.1016/0091-3057(93)90174-R. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Kurth P, McCullough LD, Sokolowski JD. The effects of nucleus accumbens dopamine depletions on continuously reinforced operant responding: contrasts with the effects of extinction. Pharmacol Biochem Behav. 1995;50:437–443. doi: 10.1016/0091-3057(94)00294-S. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Maio C, Champion M, Turski T, Kovach J. Different behavioral effects of haloperidol, clozapine and thioridazine in a concurrent lever pressing and feeding procedure. Psychopharmacology. 1996;125:105–112. doi: 10.1007/BF02249408. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/S0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi M, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonsts alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride and fenfluramine on a concurrent choice task. Psychopharmacology. 2002;160:371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber S. Accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural otivation and psychiatry. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j. coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. Nucleus accumbens dopamine and the forebrain circuitry involved in behavioral activation and effort-related decision making: implications for understanding anergia and psychomotor slowing in depression. Curr Psychiatr Rev. 2006;2:267–280. doi: 10.2174/157340006776875914. [DOI] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Hayaishi O. Involvement of adenosine A2A receptor in sleep promotion. Eur J Pharmacol. 1998;351:155–162. doi: 10.1016/S0014-2999(98)00302-1. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayaishi O. An adenosine A2A agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/S0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2A receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1071. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Hauber W. Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn Mem. 2006;12:334–342. doi: 10.1101/lm.90605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer J, Saft S, Hauber W. Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behav Neurosci. 2005;119:1687–1692. doi: 10.1037/0735-7044.119.6.1687. [DOI] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology. 2008;196:565–574. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. The role of nucleus accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav. 1998;59:557–566. doi: 10.1016/S0091-3057(97) 00544-3. [DOI] [PubMed] [Google Scholar]
- Stahl SM. The psychopharmacology of energy and fatigue. J Clin Psychiat. 2002;63:7–8. doi: 10.4088/jcp.v63n0102. [DOI] [PubMed] [Google Scholar]
- Stenberg D. Neuroanatomy and neurochemistry of sleep. Cell Mol Life Sci. 2007;64:1187–11204. doi: 10.1007/s00018-007-6530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/S0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Tylee A, Gastpar M, Lepine JP, Mendlewicz J. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. Int Clin Psychopharmacol. 1999;14:139–151. doi: 10.1097/00004850-199905002-00001. [DOI] [PubMed] [Google Scholar]
- Van den Bos R, van der Harst J, Jonkman S, Schilders M, Spruijt B. Rats assess costs and benefits according to an internal standard. Behav Brain Res. 2006;171:350–354. doi: 10.1016/j.bbr.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–11003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–6479. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PE, Rushworth MF. Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Netw. 2006;19:1302–1314. doi: 10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardas J, Konieczny J, Lorenc-Koci E. SCH 58261, an A2A adenosine receptor antagonist, counteracts parkinsonian-like muscle rigidity in rats. Synapse. 2001;41:160–171. doi: 10.1002/syn.1070. [DOI] [PubMed] [Google Scholar]
- Wardas J, Konieczny J, Pietraszek M. Influence of CGS 21680, a selective adenosine A2A agonist, on the phencyclidine-induced sensorimotor gating deficit and motor behaviour in rats. Psychopharmacology. 2003;168:299–306. doi: 10.1007/s00213-003-1439-5. [DOI] [PubMed] [Google Scholar]