Abstract
Viral infections remain a major cause of morbidity and mortality after hematopoietic stem cell transplantation (HSCT), and conventional small-molecule therapeutics often have modest benefit, high cost and adverse effects. Adoptive transfer of donor-derived virus-specific T cells has been shown to be feasible and safe after HSCT, and to reconstitute immunity against cytomegalovirus, Epstein-Barr virus and adenovirus. Current protocols to generate these cytotoxic T cell (CTL) lines are lengthy, taking up to 12 weeks. Since viral infections often occur <30 days after HSCT, speedy production of virus-specific cytotoxic T cells lacking alloreactivity is highly desirable. We now describe a modified rapid selection method for production and characterization of CD4+ and CD8+ T cells specific for cytomegalovirus, Epstein-Barr virus and adenovirus in a single infusate. We use Ad5f35-pp65/LMP2 vectors in a single procedure over a 48hr time period and manufacture a product suited for clinical use. By simultaneously expanding a portion of the selected product we can characterize phenotype and function of the infused product and link them with subsequent in vivo outcome.
Keywords: Immunotherapy, Viral infection, Stem cell transplantation, IFN-γ selection
Introduction
Successful allogeneic hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic and non-hematologic disorders requires immunosuppression, whose duration and severity correlates with the degree of histoincompatibility between donor and recipient. As a result, recipients of stem cells from unrelated or HLA-mismatched donors are susceptible to a wide array of serious and often lethal infections, many of which are not amenable to conventional small-molecule therapeutics.(1;2)
One approach to prevent and treat these opportunistic infections is donor leukocyte infusion (DLI), which involves infusion of unmanipulated T cells from the stem cell donor. Although DLIs contain virus-specific T-cell precursors, which should be able to respond to and protect against infection, their benefits are limited by the low frequency of specific T cells and by the presence of alloreactive T cells that often cause graft versus host disease (GvHD).(3;4) Consequently, we and others have developed approaches for the specific transfer of virus-specific T cells to safely reconstitute virus-specific immunity post-transplant.(5–14)
In vitro reactivation and expansion of cytotoxic T lymphocytes (CTLs), specific for viral antigens presented on antigen-presenting cells (APC) results in the loss of alloreactive T cells during culture, and such cultured T cells have proved safe and effective in clinical trials. For example, we have expanded and infused tri-virus specific T cell lines, generated by stimulating donor T cells with monocytes and EBV-transformed B lymphoblastoid cell lines (LCLs) transduced with a clinical grade chimeric Ad5f35 vector expressing the cytomegalovirus (CMV) antigen pp65.(14) These CTLs have specificity for Epstein-Barr virus (EBV), CMV and adenovirus (AdV) infected target cells, and following transfer, they expand by up to 2–4 logs within 2 weeks of infusion, thereby providing long-term reconstitution of immunity to the three viruses. Wider clinical applicability, however, will require the current 10 to 12 week manufacturing process to be modified so that we can rapidly and selectively produce virus-specific CTL, devoid of alloreactivity, in numbers sufficient to reproducibly provide therapeutic benefit. Further, these CTL must be isolated from relatively small amounts of donor blood, which could be cryopreserved from any donor at the time of transplant.
Feuchtinger et al used an IFN-γ capture assay to specifically select AdV-specific T cells directly from peripheral blood after brief in vitro stimulation with viral antigen.(15) Small numbers (1.2–50 × 103/kg) of selected cells were subsequently infused to patients with AdV infection/reactivation, and in five of six evaluable patients, there was in vivo expansion of AdV-specific T cells and a decrease in viral load. Although this method of T cell selection was rapid, cells with specificity to only a single virus were produced. Moreover, because of the small numbers of cells selected, it was not possible to characterize the infused product, making it difficult to correlate the phenotype and functional activity of the infused cells with subsequent clinical outcome.
In our current study we extend this rapid selection approach to enable us to rapidly manufacture multivirus-specific T cells, which we are subsequently able to characterize, to enable us to link phenotype, specificity and function with antiviral and immunological outcomes. Our data show that we can efficiently and reproducibly select multivirus-specific T cells over a 48hr time period; that these cells are suited for infusion into immunocompromised patients; and that we can simultaneously expand a portion of the selected product for extensive in vitro characterization.
Materials and Methods
Donors and cell lines
Peripheral blood mononuclear cells (PBMCs) from Epstein-Barr virus (EBV), cytomegalovirus (CMV), and adenovirus (AdV)-seropositive healthy volunteers were obtained with informed consent on a protocol approved by the Baylor College of Medicine IRB. PBMCs were used to generate T-cell lines and EBV transformed lymphoblastoid cell lines (LCLs) and as feeder cells. LCLs were generated using concentrated supernatants of the EBV B95-8 cell line in the presence of cyclosporin A.(16) LCLs were maintained in RPMI 1640 (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (FBS) (Hyclone) and 2mM L-glutamine (GlutaMAX-I, Invitrogen, Carlsbad, CA).
Viruses and vectors
The titer of Ad5f35pp65 vector(17;18) and Ad5f35LMP2 vector(19) was 5 × 1012 virus particles/ml or 5 × 1010 plaque-forming units/ml, and that of the Ad5f35GFP vector was 5 × 109 virus particles/ml or 5 × 107 plaque-forming units/ml.(18)
Generation and selection of multivirus-specific T cells
Bivirus-specific T cells (AdV and CMV)
Monocytes were primed for transduction by overnight (16 to 18 h) culture of bulk PBMC in X-VIVO 15(BioWhittaker, Walkersville, MD) at 2 × 106 cells per well in a plastic, tissue culture treated 24-well plate. PBMCs were then harvested(20), pelleted in a 15 ml Falcon tube, transduced with Ad5f35pp65 at a multiplicity of infection (MOI) of 10 PFU per cell. After 2 hours of incubation, the cells were resuspended in CTL medium (RPMI 1640 supplemented with 45 % Click’s medium, Irvine Scientific, Santa Ana, CA, 2mM GlutaMAX-I, and 5% human serum), and were plated at 1×106 cells per well of a 24-well plate for 16 to 18hours during which time T cells were stimulated by monocytes presenting pp65 from the transgene and virion proteins from the adenovirus vector. Thus PBMCs were served as both stimulators and responders for the generation of bivirus-specific T cells. After overnight stimulation, IFN-γ secreting cells were selected using the IFN-γ secretion assay, cell enrichment and detection kit (Miltenyi Biotec, Bisley, UK) as described previously for CMV or AdV specific cells.(15;21–23) In brief, we harvested the cells transduced with Ad5f35pp65 vector, and labeled them with anti-IFN-γ monoclonal antibody conjugated to CD45 antibody (Miltenyi Biotec). We diluted them in CTL medium, and incubated them at 37 C° for 45min to enable IFN-γ capture. Thereafter cells were labeled with anti-IFN-γ magnetic microbeads (Miltenyi Biotec), incubated for 15 minutes at 4 C°, and IFN-γ positive secreting cells were selected using Miltenyi Mini-MACS column.
Trivirus-specific T cells (AdV, CMV and EBV)
For the generation of trivirus-specific T cells, adhered PBMCs were split into two aliquots, and each transduced with either Ad5f35pp65 (AdV and CMV) or Ad5f35LMP2 (AdV and EBV) vector at a multiplicity of infection (MOI) of 10 PFU per cell. After transduction, the samples were combined, then cultured and selected as described above (see Figure 1).
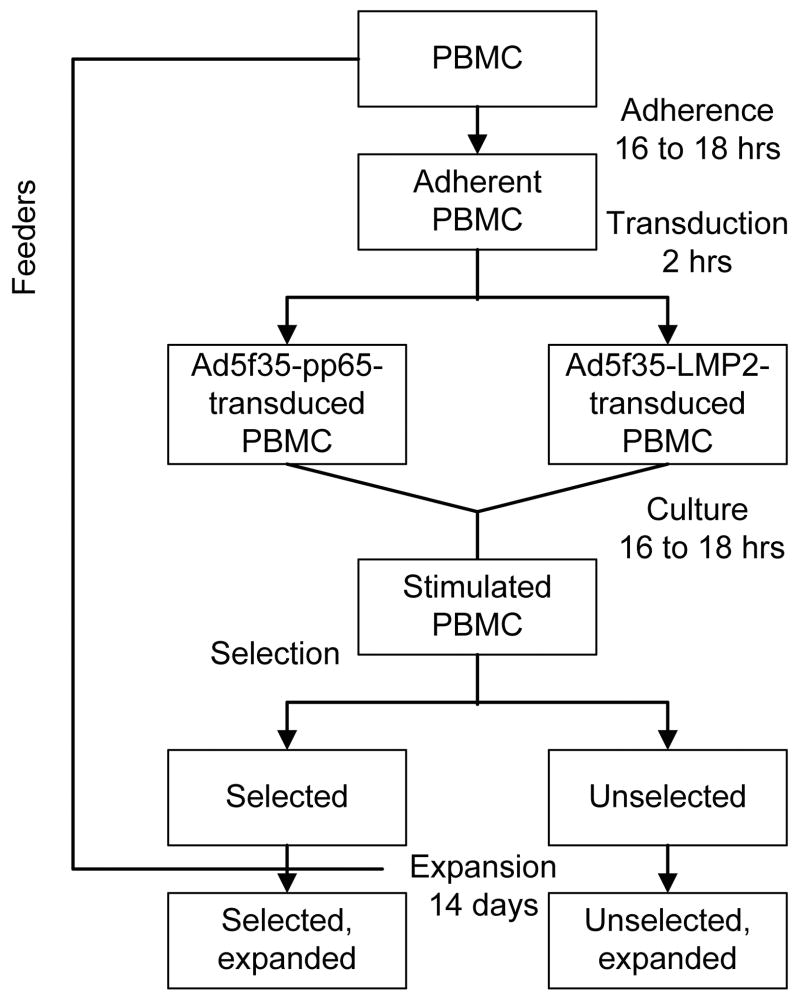
Figure 1. Flow chart outlining the isolation, ex vivo expansion, and characterization of bivirus- and trivirus-specific T cells.
PBMC are adhered overnight to activate the monocyte fraction, which as a result becomes primed for transduction. Adherent PBMC are split in 2 parts and transduced separately with different Adv vectors. After transduction the samples are recombined for overnight culture during which time virus-specific T cells become activated. After 16 to 18 hours the stimulated PBMC are selected by IFN-γ capture, retaining a small fraction of unselected population. Both selected and unselected fractions are then expanded on autologous PBMC feeder cells with IL-15.
Mixed lymphocyte reactions (MLR)
As previously described, (24) we used PBMC or unselected or selected cells as responder cells and cultured them with autologous 30Gy-irradiated PBMCs or third-party 30Gy-irradiated PBMCs at a stimulator: responder ratio of 1:1 for 3 to 4 days in 200μl CTL medium in triplicate in a U- bottom 96-well plate (Nunc, Rochester, NY) at 37°C and 5%CO2. Cell proliferation was analyzed by 3H-thymidine incorporation. Cultures were pulsed with 0.037MBq (1μCi) 3H-thymidine per well (Amersham Biosciences, Piscataway, NJ) and 16 hrs later harvested them onto glass fiber strips using a Brandel PHD cell harvester. 3H-thymidine uptake was measured using a Matrix B liquid scintillation counter (Canberra Packard, Meriden, CT) and the results expressed as stimulation index (SI) = {[responder + stimulator (mean counts per minute (CPM))] − [stimulator (CPM)]}/[responder (CPM)] of triplicate measurements.
Expansion of cells
To characterize the function and specificity of the selected T cells, we expanded them in culture for 2 weeks. Selected cells (2 × 104) per well were placed in 24-well plates in the presence of 30Gy-irradiated autologous PBMCs at a responder:stimulator ratio of 1:100 in CTL medium containing 5ng of interleukin (IL)-15 per milliliter. We supplemented the cultures with fresh medium and 5ng/ml IL-15 on days 4, 7, and 10. On day 7, we added 30Gy-irradiated autologous PBMCs at a ratio of 1:10. To compare functionality, unselected cells were expanded in the same manner as the selected cells.
Flow cytometry
Immunophenotyping
We stained the antigen-stimulated T cells before and after selection with monoclonal antibodies to CD3, CD4, CD8, CD14, CD16, CD56 and CD19 (Becton Dickinson, Franklin Lakes, NJ). Cells were washed once with phosphate-buffered saline(PBS)(Sigma, St Louis, MO) with 2% FBS and 0.1% sodium azide (Sigma)), pelleted, and antibodies added in saturating amounts (5μl). After a15-minute incubation at 4°C in the dark, the cells were washed twice and analyzed. Approximately 10,000 live cells from each population were analyzed with a FACS Calibur equipped with Cell Quest software (Becton Dickinson).
Multimer staining
We used soluble HLA-A*0201-GLRYRSMLL (GLR) phycoerythrin (PE)-conjugated tetramer and HLA-B*0702-KPYSGTAYNAL (KPY) and HLA-B*3501-MPNRPNYIAF (MPN) unlabeled pentamer to detect T cells recognizing an epitope from the AdV-hexon antigen. HLA-A*0201-NLVPMVATV (NLV) unlabeled pentamer was used to detect T cells recognizing an epitope from CMV-pp65 antigen and HLA-A*0201-CLGGLLTMV (CLG) and HLA-A*0201-FLYALALLL (FLY) unlabeled pentamer were used to measure cells reactive with the EBV-LMP2 antigen. Tetramers and pentamers (collectively termed multimer) were prepared by the Baylor College of Medicine Tetramer Core Facility and by Proimmune, Bradenton, FL, respectively. For tetramer staining, 106 selected, expanded cells or 106 PBMCs were stained with the PE-labeled tetrameric complex at a 1:100 final dilution, together with anti-CD8 FITC, and anti-CD3 PerCP for 30 minutes at 4°C in the dark. The cells were washed twice, and analyzed immediately.(25) For pentamer staining, we incubated the same number of cells with unlabeled pentamer, followed by Pro5 Flurotag (PE-conjugated) (Proimmune Inc.) according to the manufacturer’s instructions. For each sample, 100,000 live cells were analyzed with a FACS Calibur equipped with Cell Quest software (Becton Dickinson).
Enzyme-linked immunospot (ELISpot) assay
We used Enzyme-linked immunospot (ELISpot) analysis to quantify multivirus-specific T cells. Responder cells were antigen-stimulated, and we compared selected, expanded cells with unselected, expanded cells. The populations were serially diluted from 1 × 105 to 2.5 × 104 cells per well, and we measured the viral-specific activity of responder cells after direct stimulation with1μg/ml each of CMV pp65 protein (CMV), overlapping adenovirus hexon peptides (AdV)(20) or EBV LMP2 protein (EBV). We used PBMCs stimulated with staphylococcal enterotoxin B (1 μg/ml; Sigma-Aldrich) as controls. Each culture condition was run in triplicate. After 20 hours of incubation, plates were developed as previously described.(26;27) After overnight drying at room temperature in the dark, plates were sent to Zellet Consulting, New York, NY. Spot-forming cells and input cell numbers were plotted, and a linear regression calculated after excluding plateau data points. The frequency of T cells specific to each antigen was expressed as specific spot-forming cells (SFCs) after subtracting the background (i.e., the frequency of unstimulated responding cells).
Cytotoxicity assay
We measured the cytotoxic specificity of each T cells population in a standard 4-h chromium-51 release assay, using effector:target ratios of 40:1, 20:1, 10:1, and 5:1.(11) Selected, expanded cells were used as effectors. The target autologous LCLs were either nontransduced or transduced with Ad5f35null at an MOI of 5 PFU per cell or Ad5f35pp65 at an MOI of 100 PFU per cell and used 24 hours after transduction. Alternatively, autologous LCLs were pulsed with overlapping peptides from CMV pp65, overlapping adenovirus hexon peptides (AdV) or overlapping peptides from EBV LMP2 and labeled simultaneously for 1 hour with Cr51, and used as targets. The percent specific lysis was calculated as specific lysis = ([experimental release − spontaneous release]/[maximum release-spontaneous release]) × 100.
Results
Isolation of T cells by IFN-γ capture after stimulation by Ad5f35-CMVpp65
We began by using an adenoviral vector encoding the CMV pp65 gene to simultaneously stimulate T cells specific for AdV and CMV, two pathogens that commonly affect patients after stem cell transplantation.(17;18) We added Ad5f35CMVpp65 vector to the PBMCs of healthy, AdV and CMV seropositive donors immediately following short term (16 to 18 hours) ex vivo culture in a plastic, tissue culture treated 24-well plate. After overnight culture, we isolated T cells that had responded to the AdV and CMV-derived proteins presented by infected cells in the culture, using an IFN-γ capture assay (Miltenyi Biotec)(15;21–23;28) as shown in Figure 1.
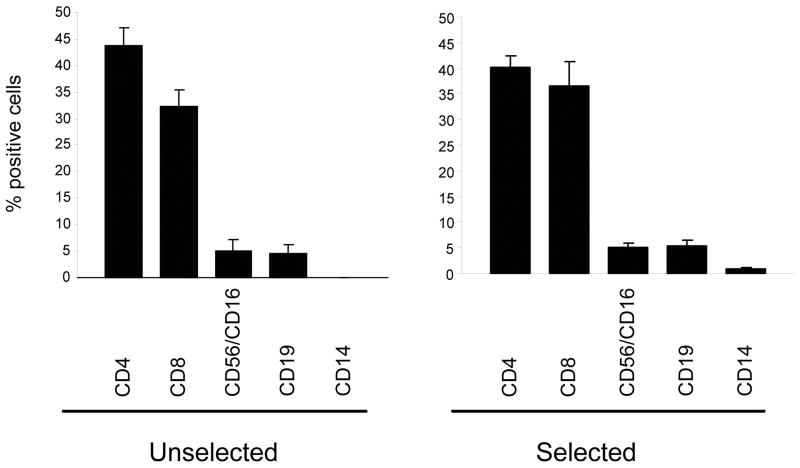
With a starting number of 20 × 106 PBMC, the number of cells after selection ranged from 0.03 × 106 to 0.2 × 106 (mean: 0.11 ± 0.04 × 106; n=6) (Table 1). The phenotype of the IFN-γ-selected population was similar to the bulk, unselected population (Figure 2), containing 40.3% ± 2.0% CD4+ and 36.7% ± 4.6% CD8+ T cells, while the unselected fraction contained 43.7% ± 3.4% CD4+ and 32.4% ± 3.1% CD8+ T cells. Less than <12% of cells had NK cell (CD56/16), B cell (CD19), or monocyte (CD14) markers in either the selected or unselected fractions. Thus the IFN-γ capture assay isolates polyclonal T cells after monocyte activation, Adf35 vector transduction, and overnight culture.
Table 1. Cell numbers before and after adherence and selection.
With a starting number of 20 × 106 PBMC, the number of cells before and after selection, after selection and expansion is shown. About 50% of cells were lost during the overnight adherence step. The number after the expansion is the estimated number started from all the cells obtained after the selection. (Bivirus-specific T cells: n=6, Trivirus-specific T cells: n=5)
| Starting PBMC (×106) | Before selection (×106) | After selection (×106) | After expansion (×106) | |
|---|---|---|---|---|
| Bivirus-specific T cells | 20 ± 5.4 | 8.7 ± 3.2 | 0.11 ± 0.04 | 133 ± 16 |
| Trivirus-specific T cells | 20 ± 4.4 | 11 ± 2.3 | 0.14 ± 0.06 | 155 ± 14 |
Figure 2. Immunophenotype of IFN-γ-selected and unselected T cell populations.
The phenotype of the IFN-γ selected and unselected cells was determined immediately after the selection by staining cells with CD3 PerCP, CD4 PerCP, CD8 FITC, CD56 FITC, CD16 FITC, CD19 FITC and CD14 PerCP. Results are the mean of 4 donors tested.
Expansion of selected, antigen-specific T cells
We could not immediately ascertain the anti-viral specificity of the captured cells: secreted IFN-γ was already captured precluding an immediate ELISpot; T cell antigen receptors are down regulated following antigen engagement; and the numbers of recovered cells are low. We reasoned that if a small fraction of the selected product could be expanded ex vivo, this would leave sufficient cells for infusion and allow subsequent characterization of the infusion product.
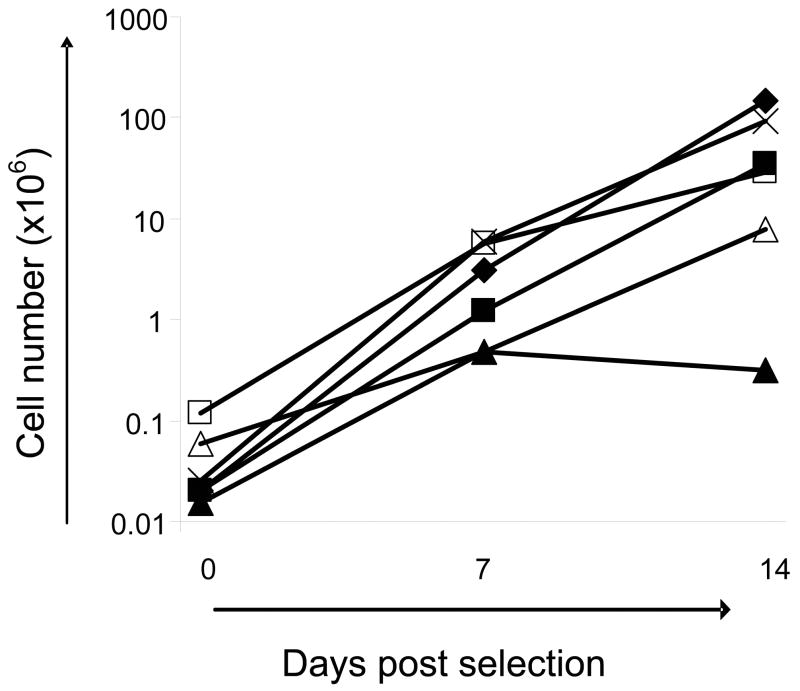
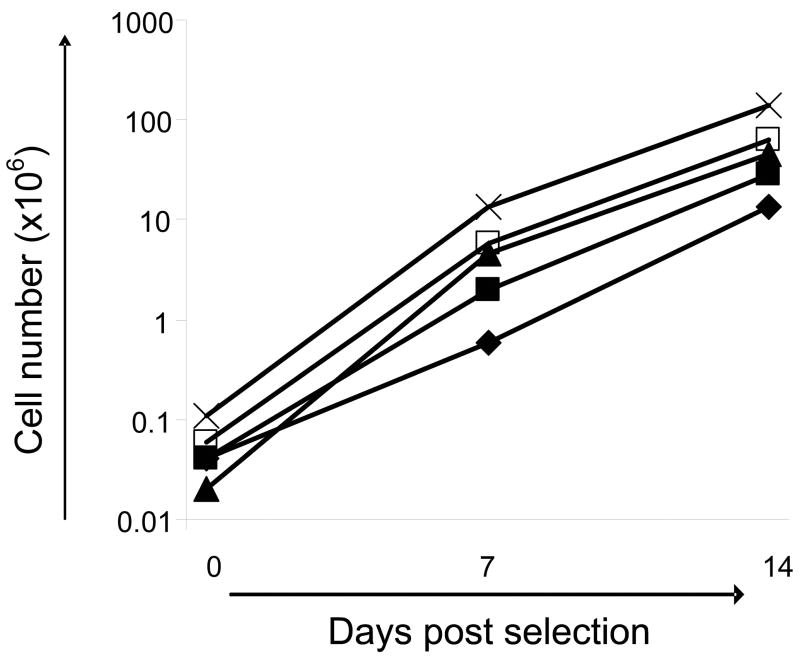
We therefore used autologous, irradiated PBMC as feeder cells at a responder: stimulator ratio of 1:100, with biweekly addition of IL-15 (5ng/ml). The expansion of selected T cells from 6 donors is shown in Figure 3. Overall, we obtained a 2–3 log expansion over 2 weeks, so that 0.04 × 106 cells in our starting population expanded to a mean of 2.8 × 106 cells by day 7 and to 52 × 106 cells by day 14 (range 0.5 × 106–6 × 106 on day 7 and 0.3 × 106–149 × 106 on day 14; n=6) Thus, by 2 weeks we had sufficient T cells to characterize-albeit retrospectively - the infused cell product (see scheme outlined in Figure 1).
Figure 3. Expansion of selected, bivirus-specific T cells.
Selected cells were expanded with autologous, irradiated PBMC as feeder cells (R:S was 1:100) and biweekly addition of IL-15 (5ng/ml). Overall, we obtained a 2–3 log expansion over 2 weeks. Results from 6 donors are shown.
Antigen specificity of the selected and expanded cells
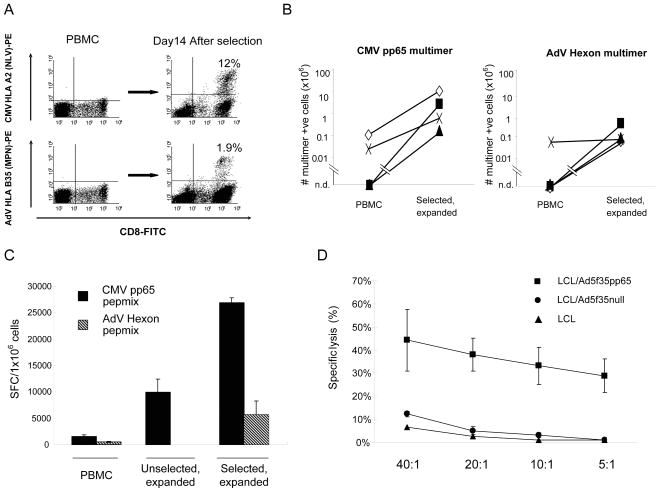
We compared the anti-viral specificity of the patients “input” (unfractionated) T cells and of the selected and expanded T cells. We first used HLA-peptide multimers to evaluate the expansion of virus-specific T cells in the selected and expanded population. For CMV, a mean of 7.4% (range 1.1%–13.0%) (n=4) of the CD8+ T cells were positive for pp65 multimer, NLV and for AdV, a mean of 1.2% (range 0.1%–2.7%) (n=4) CD8+ T cells were positive for the multimers MPN, KPY or GLR. Multimer staining for one representive HLA-A2, B35 donor is shown in Figure 4A, and demonstrates about a 60 fold enrichment in the proportion of T cells that are reactive with the pp65-specific HLA-A2 restricted NLV pentamer following IFN-γ capture assay (top right versus top left). Similarly, HLA-B35-restricted AdV pentamer MPN, reactive T cells increased from undetectable levels in peripheral blood to 1.9% in the selected and expanded fraction (Figure 4A, bottom right versus bottom left). Based on the percentage of multimer positive cells and on cell counts, the absolute number of CMV and AdV- multimer specific T cells was calculated in the starting fraction (PBMC) and after selection/expansion. Multimer-specific T cells could be detected in PBMC in only 2 of 4 for CMV-NLV and only 1 of 4 donors for Adv. Nevertheless, the absolute numbers of NLV-specific CD8+ T cells increased to a mean of 3.7 × 106 T-cells (n=4), and that of AdV-specific CD8+ T cells increased to mean of 2.2 × 105 T-cells (Figure 4B).
Figure 4. Specificity of bivirus-specific T cells following selection and in vitro expansion.
Panel A: After IFN-γ selection and 14 days in vitro expansion, the specificity of the selected and expanded cells was analyzed using multimer staining. Results from one representative donor are shown in panel A. The frequency of T cells specific for an HLA A*0201-restricted CMV pp65 epitope, NLVPMVATV, as well as an HLA B35-restricted AdV Hexon epitope MPNRPNYIAF, was determined by staining T cells with CD3 PerCP, CD8 FITC, and Isotype PE (Control), or NLV- or MPN-multimer PE. The final frequency of NLV- and MPN-specific CTL was 12% and 1.9% in this donor.
Panel B shows the absolute number of CMV pp65 multimer-specific and AdV Hexon multimer-specific T cells in the starting fraction (PBMC) and in the selected and expanded population in four donors tested. The multimer-specific T cells in PBMC were undetectable in 2 of 4 donors for CMV-pp65 and 3 of 4 donors for AdV-Hexon. (n.d. = not detectable.)
Panel C shows the frequency of CMV pp65 and AdV Hexon reactive T cells in PBMC, in the unselected, expanded and in the selected, expanded populations using IFN-γ ELISpot as readout (4 donors screened). Results are expressed as spot forming cells (SFC) per 1×106 input cells.
Panel D:Virus-specific T cells were tested for their ability to lyse autologous virus-expressing targets. Ad5f35pp65-specific CTL killed autologous EBV-LCL transduced with the Ad5f35pp65 vector (EBV/CMV/AdV target). Killing of autologous LCLs transduced with Ad5f35null (EBV/Adv target) was weaker than the killing of CMV pp65 expressing cells. Data are the mean ± s.d. percentage of target lysis by 3 donors at E:T ratios of 40:1, 20:1, 10:1 and 5:1.
To determine whether the antigen-specific, multimer positive cells in the selected and expanded fraction were functional, we measured their ability to specifically produce IFN-γ after stimulation with an overlapping peptide library spanning the entire sequence of CMV pp65 (CMV pepmix), or AdV hexon (hexon pepmix)(20) using the ELISpot assay.(14) There was a mean 17.4 fold increase in CMV-specific IFN-γ-secreting T cells (n=4) and a mean 11.5 fold increase in Adv-specific T cells (n=4) after selection and expansion compared to PBMC. The frequency of virus-specific T cells was greater in selected and expanded CTL than in T cells, which had not been selected but had simply been expanded in vitro after stimulation by Ad5f35-CMVpp65 (unselected, expanded) (Figure 4C). Finally, we measured the cytolytic function of the selected and expanded bivirus-specific, CTL, in a standard chromium release assay. We used target cells (autologous EBV-LCL) transduced with either Ad5f35pp65 or Ad5f35null vectors; controls was autologous EBV-LCL alone. The selected and expanded CTL recognized and killed autologous EBV target cells only when they co-expressed CMV and AdV antigens: the killing of Ad hexon expressing target cells was significantly weaker than the killing of those cells expressing pp65 (Figure 4D).
Depletion of alloreactive cells
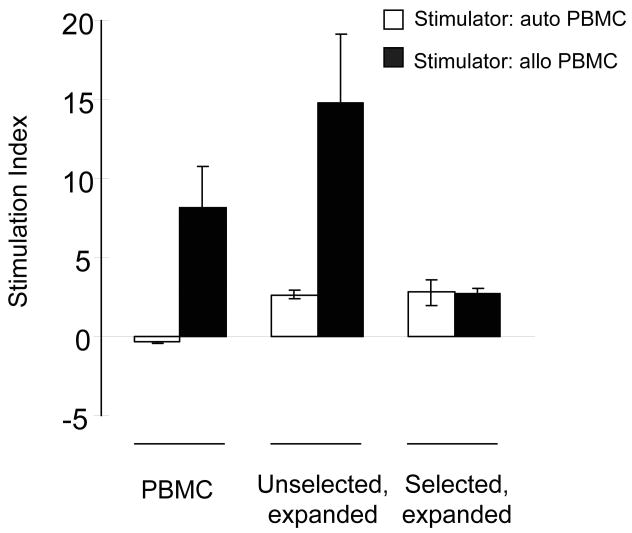
The major purpose of the selection of virus-specific cells is the depletion of alloreactive T cells with GVHD-inducing potential. We therefore measured the alloreactivity of the Ad5f35-CMV-pp65 stimulated and IFN-γ-selected T cells in a mixed lymphocyte reaction (MLR), and compared this with the alloreactivity of bulk PBMC, and of antigen-stimulated but unselected cells from the same donor. We cultured donor PBMCs, stimulated unselected T cells, or stimulated selected T cells with irradiated, HLA-mismatched PBMCs at a stimulator: responder ratio of 1:1 for 3 days, and pulsed them with 3H overnight. Unmanipulated donor PBMC and unselected T cells both showed high levels of alloreactivity against irradiated, allogeneic PBMC (stimulation index (SI) 8.2 ± 2.6 and 14.7 ± 4.4, respectively) (Figure 5). The response of selected T cells to allogeneic PBMC was not greater than to autologous PBMC and although this was greater than background proliferation in the absence of stimulators (SI 2.7 ± 0.3), this activity reflected antigenic stimulation received 48 hours previously. Hence IFN-γ selection of viral antigen-activated T cells enriches for viral antigen specific T cells, and diminishes the alloreactivity of the population.
Figure 5. Depletion of alloreactive cells following IFN-γ selection.
We tested the alloreactive potential of unmanipulated PBMC, as well as unselected and selected T cells using a primary mixed lymphocyte reaction (MLR). Unmanipulated PBMC and unselected population proliferated in response to allogeneic PBMC stimulators (R:S 1:1). However, there was a mean 3.0-fold or mean 5.4-fold decrease in the proliferation of selected population against allogeneic targets, compared to the proliferation of unmanipulated PBMC or unselected population against allogeneic targets respectively. The results are the mean ± s.d. of 4 donors expressed as stimulation index, each assayed in triplicate. Stimulation index = {[responder + stimulator (mean counts per minute (CPM))] − [stimulator (CPM)]}/[responder (CPM)].
Selection and expansion of trivirus-specific CTL
We next extended the IFN-γ capture technology described above to the generation of T cells specific for all three of the viruses (EBV, AdV, CMV) that most frequently cause severe infections after allogeneic stem cell transplantation. EBV has multiple immunodominant and immunogenic antigens, and although it is unclear which of these are most important for providing protective immunity in vivo, healthy donors invariably recognize latent membrane protein (LMP) 2. Moreover, this antigen is expressed by the majority of EBV positive tumors including post-transplant lymphoproliferative disease (PTLD).(11;16) We therefore used an adenoviral vector expressing LMP2 in our system. Because of concerns that antigenic competition between immunodominant pp65 epitopes would overwhelm weaker LMP2 epitopes in the competition for HLA molecules on APCs, we compared two different protocols for the production of AdV-, CMV-, and EBV-enriched T cells. In the first, activated PBMCs were transduced either with Ad5f35pp65 (MOI 10PFU per cell) or Ad5f35-LMP2 (MOI 10PFU per cell) and then combined for the overnight stimulation (Figure 1). In the second, activated PBMCs were transduced with both Ad5f35pp65 and Ad5f35LMP2 in a single step. After overnight culture, IFN-γ-secreting antigen-specific T cells were selected using IFN-γ capture. The selected T cells were subsequently expanded and after 14 days the specificity and function of the generated T cells were compared using multimer staining and ELISpot assay.
In preliminary experiments, T cells generated by separating the activated PBMC into two separate populations prior to transduction consistently contained approximately 2 fold more CMV, AdV, and EBV CD8+ T cells than the double-transduced population as determined by multimer and functional IFN-γ ELISpot assays. This difference was most strongly marked for LMP2, suggesting that antigenic competition was indeed a problem (data not shown).
In all subsequent experiments we separated the activated PBMCs prior to transduction in order to optimize multivirus-specific CTL isolation and then combined the cells for culture and selection to make a single product. Selected, trivirus-specific CTL contained both CD4+ (40.6 ± 4.9%) and CD8+ (33.6 ± 2.9%) T cells. After selection, the number of isolated cells ranged from 0.06 × 106 to 0.36 × 106 cells (mean: 0.14 ± 0.06 × 106; n=5) (Table 1) per 20 × 106 PBMCs. These selected cells could be expanded, using the protocol optimized for bivirus-specific CTL. Selected cells expanded from a mean of 0.05 × 106 to a mean of 5.3 × 106 cells in 7 days (range 0.6 × 106–14 × 106 on day7; n=5) and to a mean of 58 × 106 cells at 14 days (range 14 × 106–141 × 106 on day14; n=5) (Figure 6), which is essentially identical to the expansion of bivirus-specific CTL shown in Figure 3.
Figure 6. Expansion of selected, trivirus-specific T cells.
Trivirus-specific, selected cells were expanded with autologous, irradiated PBMC as feeder cells (R:S was 1:100) and biweekly addition of IL-15 (5ng/ml). Overall, we obtained a 2–3 log expansion over 2 weeks. Results from 5 donors are shown. For the selection of trivirus-specifc T cells, cells transduced with Ad5f35pp65 vector or Ad5f35LMP2 vector separately were harvested, combined into a single culture and processed and selected.
Trivirus-specific CTL contained polyclonal CMV-, AdV-, and EBV-specific T cells
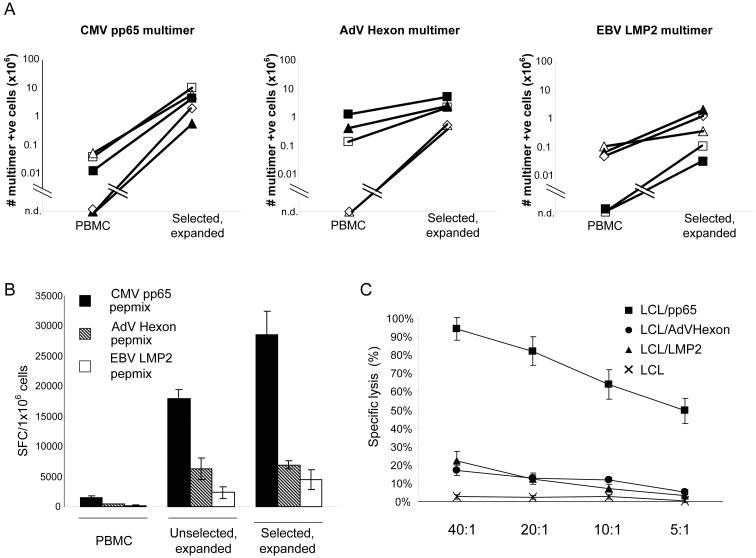
The antigen specificity of the selected and expanded cells was determined using HLA-peptide multimers. For CMV, a mean of 14.9% (range 1.3%–45%; n=5) CD8+ T cells were positive for the pp65-NLV multimer; for Adv, a mean of 6.1% (range 0.9%–18.5%; n=5) of the CD8+ T cells were positive, and for EBV, a mean of 1.7% (range 0.1%–3.9%; n=5) of the CD8+ T cells were positive for the LMP2 multimers, CLG and FLY. The absolute numbers of CMV-, AdV-, and LMP2-multimer-positive T cells were calculated in the starting fraction (PBMC) and after selection and 2 weeks of expansion. Multimer positive T cells were detected in PBMC in only 3 of 5 donors for all three virus multimers. CMV multimer-specific CD8+ T cells increased to a mean of 5.0 × 106 cells (n=5), AdV-specific CD8+ T cells increased to a mean of 2.2 × 106 cells (n=5), and LMP2-specific CD8+ T cells increased to a mean of 6.8 × 105 cells (n=5) from undetectable levels in peripheral blood (Figure 7A).
Figure 7. Multivirus-specificity of selected and expanded T cells. (Trivirus-specific T cells).
After IFN-γ selection and 14 days in vitro expansion, the specificity of the selected and expanded cells was analyzed using multimer staining.
Panel A shows the absolute number of CMV pp65 multimer-specific, AdV Hexon multimer-specific and EBV LMP2 multimer-specific T cells in the starting fraction (PBMC) and in the selected and expanded T cells in five donors tested. The multimer-specific T cells in PBMC were undetectable in 2 of 5 donors for all three virus multimers. (n.d. = not detectable.)
Panel B shows the frequency of CMV pp65, AdV Hexon and EBV LMP2 reactive T cells in PBMC, in the unselected, expanded and in the selected, expanded populations using IFN-γ ELISpot as readout (4 donors screened). Results are expressed as spot forming cells (SFC) per 1×106 input cells.
Panel C: Virus-specific T cells were tested for their ability to lyse autologous virus-expressing targets. Ad5f35pp65 and Ad5f35LMP2-specific CTL killed autologous EBV-LCL pulsed with CMV pp65 protein (EBV/CMV target). The killing of autologous EBV-LCL pulsed with overlapping AdV Hexon peptides (EBV/AdV target) or EBV LMP2 protein (EBV/LMP2 target) was weaker than killing of CMV pp65 expressing cells. Data are the mean ± s.d. percentage of target lysis by 5 donors at E:T ratios of 40:1, 20:1, 10:1 and 5:1.
Selected trivirus-specific CTL produce IFN-γ and kill antigen-expressing target cells post-expansion
Specific IFN-γ release in response to antigenic stimulation was measured by stimulating selected and expanded cells with a CMV, hexon, and LMP2 pepmix in ELISpot assays. There was a mean 18.5 fold increase in IFN-γ secreting T cells responding to CMV-pp65 (n=4), a 14.1 fold increase in the response to AdV-hexon, and a 26.1 fold increase in the response to EBV-LMP2 pepmix compared to the starting fraction (PBMC) (n=4). By comparison in unselected but expanded CTL, there was a mean 1.6 fold increase in IFN-γ secreting T cells responding to CMV-pp65 (n=4), a 1.1 fold increase in the response to AdV-hexon, and a 1.9 fold increase in the response to EBV-LMP2 pepmix (Figure 7B). The selected and expanded cells could also directly kill target cells expressing CMV pp65, AdV hexon, and EBV LMP2 (Figure 7C). Although killing of LMP2 and hexon expressing target cells was lower than killing of CMV pp65 expressing cells, it was nonetheless in the same range as we observed using trivirus-specific CTL generated using conventional expansion protocols.(14)
Discussion
We have developed a strategy to allow the rapid isolation of multivirus-specific T cells for infusion, from small volumes of peripheral blood using the IFN-γ capture assay, while simultaneously expanding a small aliquot of cells for in vitro analysis. Using activated monocytes transduced with chimeric adenoviral vectors expressing CMV-pp65 and EBV-LMP2 we can simultaneously isolate virus-reactive T cells directed against all three viruses.(14) The vector proteins (and perhaps retained DNA backbone) serve as a source of AdV antigens, while the expressed pp65 or LMP2 transgenes reactivate CMV-or EBV-specific T cells, respectively. Importantly, we demonstrate that the selected cells are devoid of alloreactive T cells, indicating that the infused cells should be safe in vivo. By expanding a fraction of the isolated cells ex vivo we confirmed that the selected cells had functional specificity against all the viral antigens used in the initial stimulation. Thus, the process of generating multivirus-specific CTL for infusion can be reduced from 3 months to 3 days without sacrificing the product characterization required to correlate clinical outcome with the phenotype and function of the infused CTLs.
Viral infections after allogeneic HSCT are common, and arise due to lack of endogenous virus-specific T cells particularly in the early post-transplant period.(1) Therefore we and others have developed strategies to reconstitute transplant recipients with virus-reactive cells to provide protection during this high-risk period.(5–10;12–14) Initial attempts to reconstitute antiviral T cell immunity post-HSCT involved the infusion of unmanipulated donor T cells (donor lymphocyte infusions – DLI) that contained a mixed population of virus-reactive cells. However, the frequency of allospecific T cells in PBMC is greater than that of virus-specific T cells, so that severe or fatal GvHD is a common complication of DLI.(3;4) An alternative approach is to expand virus-specific T cells in vitro by repetitive ex vivo stimulation with viral antigens, which can successfully prepare EBV, CMV or AdV specific CTL and potentially prevent or treat the diseases caused by these agents. More recently we have produced multivirus-specific CTL lines containing polyclonal antigen-specific CTL targeting EBV, CMV, and AdV, and showed that small numbers of infused cells (5×106/m2) were able to expand in vivo and were effective and protective against all three viruses.(14) However current methods for the generation of CTL lines involve prolonged culture periods; 4–6 weeks to generate EBV-LCL for use as APCs, followed by 4–6 weeks to produce sufficient CTLs for infusion, sterility testing, and functional analysis. Thus CTL lines must be initiated well in advance of disease, which is impractical for general use.
More recently a number of groups have developed a more rapid and less cumbersome method for the production of virus-specific T cells for infusion. Two methods have been used clinically; tetramer selection and IFN-γ selection. Since there is often a low frequency of circulating T cells that are specific to clinically-relevant viruses, both approaches may require relatively large blood volumes (such as leukapheresis products), which are difficult to collect from unrelated donors. Moreover, tetramer selection is limited to donor-recipient pairs with certain HLA types with known epitope peptide specificities. Suitable tetramers for most HLA alleles have not been identified and so far are limited to HLA class I-restricted CD8+ T cells.(5) The lack of CD4+ T cells in any infused product may compromise the survival of CD8+ T cells.(11) Overall, these limitations are significant. In contrast, the IFN-γ capture assay can be used to capture both CD4+ and CD8+ T cells from any donor, irrespective of HLA type. Feuchtinger and colleagues used the IFN-γ capture assay to specifically isolate AdV-specific T cells for infusion into pediatric patients with active infections.(15) They infused 1.2–50 × 103/kg AdV-specific CTLs into nine pediatric patients and found that the infused cells had dose-independent and sustained in vivo expansion, which was associated with clearance or decrease in viral load in five out of six evaluable patients. Only one patient developed acute exacerbation of chronic GvHD. It is notable that efficacy was independent of T cell dose, implying that small numbers of activated cells can expand in vivo in the presence of antigen. The investigators were unable to confirm this supposition because of the major limitation of the approach; as with tetramer selection the number of isolated T cells is small, precluding any characterization of the product before infusion.
We have built on these initially encouraging results to increase the number of viruses that can be recognized after a single IFN-γ selection procedure, and to characterize the specificity and functionality of the cell product. We have previously shown that AdV-hexon and CMV-pp65-specific T cells can be activated using an Ad5f35-pp65 vector.(14) However, the reactivation of EBV-specific T cells posed a problem, since we have previously relied on EBV-LCL lines to perform this function. EBV-LCL expresses all latent as well as lytic EBV antigens and can simultaneously stimulate effector cells with wide varying precursor frequencies and affinities for different HLA molecules. Establishment of an EBV-LCL line is, however, a lengthy process, requiring as long as 4–6 weeks of in vitro culture. Thus, for our new rapid approach we generated an adenoviral vector expressing the EBV latent protein, LMP2, to stimulate reactive AdV and EBV-LMP2 specific T cells. Although LMP2 is a subdominant antigen, it is broadly immunogenic and both CD4+ and CD8+ epitope peptides derived from this protein are conserved.(29) To prevent antigenic competition for HLA molecules in APC and thus ensure adequate stimulation of weaker antigens, we divided activated monocytes and transduced them separately with either Ad5f35-pp65 or Ad53f5-LMP2, but then recombined responder T cells to simplify and accelerate the selection procedure and to generate a single product to be characterized and infused.
To simultaneously expand a small aliquot of the selected product, we used different combinations of the pro-proliferative cytokines IL-7, IL-12, and IL-15 to determine the optimal expansion protocol. We found that culture in the presence of either IL-15 alone or a combination of IL-7, IL-12, and IL-15 resulted in the best expansion (2–3 logs). In addition, we found no significant difference in the function of cells expanded using these cytokines in terms of their ability to secrete IFN-γ after antigenic stimulation (Supplementary Figure). Since culture with a single cytokine is more cost-effective than multiple cytokines, we chose to proceed with this method for future experiments.
We assessed the specificity and function of the selected and expanded cells against EBV, AdV, and CMV targets, and found them able to secrete IFN-γ and kill target cells after exposure to CMV pp65, AdV hexon, and EBV LMP2 antigens. Although the recognition of LMP2 and hexon was significantly weaker than pp65, it was in the same range as we have previously seen with trivirus-specific CTL generated using traditional expansion protocols.(14)
These results suggest that we can successfully generate trivirus-specific T cells in one, rapid and relatively simple procedure that should readily be adaptable for clinical implementation in reactive, rather than prophylatic studies. Future studies may allow the approach to be extended to provide donor T cells that are protective against other infectious agents that are common in transplant recipients.(1;2)
Supplementary Material
Expansion of bivirus-specific selected and expanded T cells using different combinations of cytokines.
We compared the in vitro expansion of IFN-γ selected T cells using either IL-15 alone (5 ng/ml) or a cocktail of cytokines IL-7(5 ng/ml), IL-12(10 ng/ml), and IL-15 (5 ng/ml). T cells were expanded for 2 weeks with biweekly addition of the appropriate cytokines, and on day 14 the specificity of the expanded cells was assessed by IFN-γ ELISpot assay using pepmixes spanning CMV pp65 and AdV Hexon to stimulate T cells. Results from 3 donors are shown.
Acknowledgments
This work was supported in part by a Specialized Centers for Cell-based Therapy (SCCT) Grant NIH-NHLBI 1 U54 HL1081007, grants from the National Gene Vector Laboratories (NIH-NCRR U42 RR16578) and a Center for Infection & Immunity Research award (to AML and HEH.).
Footnotes
Financial disclosure: The authors have declared there are no financial conflicts of interest in regards to this article.
References
- 1.Bollard CM, Kuehnle I, Leen A, et al. Adoptive immunotherapy for posttransplantation viral infections. Biol Blood Marrow Transplant. 2004;10:143–155. doi: 10.1016/j.bbmt.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Fujita Y, Rooney CM, Heslop HE. Adoptive cellular immunotherapy for viral diseases. Bone Marrow Transplant. 2008;41:193–198. doi: 10.1038/sj.bmt.1705906. [DOI] [PubMed] [Google Scholar]
- 3.Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N Engl J Med. 1994;331:679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- 4.Mackinnon S, Papadopoulos EB, Carabasi MH, et al. Adoptive immunotherapy using donor leukocytes following bone marrow transplantation for chronic myeloid leukemia: is T cell dose important in determining biological response? Bone Marrow Transplant. 1995;15:591–594. [PubMed] [Google Scholar]
- 5.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 7.Gustafsson A, Levitsky V, Zou JZ, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95:807–814. [PubMed] [Google Scholar]
- 8.Heslop HE, Ng CY, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 9.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 10.Riddell SR, Watanabe KS, Goodrich JM, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 11.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 12.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 13.O’Reilly RJ, Doubrovina E, Trivedi D, et al. Adoptive transfer of antigen-specific T-cells of donor type for immunotherapy of viral infections following allogeneic hematopoietic cell transplants. Immunol Res. 2007;38:237–250. doi: 10.1007/s12026-007-0059-2. [DOI] [PubMed] [Google Scholar]
- 14.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 15.Feuchtinger T, Matthes-Martin S, Richard C, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;134:64–76. doi: 10.1111/j.1365-2141.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- 16.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 17.Sili U, Huls MH, Davis AR, et al. Large-scale expansion of dendritic cell-primed polyclonal human cytotoxic T-lymphocyte lines using lymphoblastoid cell lines for adoptive immunotherapy. J Immunother. 2003;26:241–256. doi: 10.1097/00002371-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Yotnda P, Onishi H, Heslop HE, et al. Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Ther. 2001;8:930–937. doi: 10.1038/sj.gt.3301488. [DOI] [PubMed] [Google Scholar]
- 19.Bollard CM, Straathof KC, Huls MH, et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J Immunother. 2004;27:317–327. doi: 10.1097/00002371-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Leen AM, Sili U, Vanin EF, et al. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood. 2004;104:2432–2440. doi: 10.1182/blood-2004-02-0646. [DOI] [PubMed] [Google Scholar]
- 21.Bissinger AL, Rauser G, Hebart H, et al. Isolation and expansion of human cytomegalovirus- specific cytotoxic T lymphocytes using interferon-gamma secretion assay. Exp Hematol. 2002;30:1178–1184. doi: 10.1016/s0301-472x(02)00897-4. [DOI] [PubMed] [Google Scholar]
- 22.Chatziandreou I, Gilmour KC, McNicol AM, et al. Capture and generation of adenovirus specific T cells for adoptive immunotherapy. Br J Haematol. 2007;136:117–126. doi: 10.1111/j.1365-2141.2006.06386.x. [DOI] [PubMed] [Google Scholar]
- 23.Feuchtinger T, Lang P, Hamprecht K, et al. Isolation and expansion of human adenovirus-specific CD4+ and CD8+ T cells according to IFN-gamma secretion for adjuvant immunotherapy. Exp Hematol. 2004;32:282–289. doi: 10.1016/j.exphem.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Yvon ES, Vigouroux S, Rousseau RF, et al. Overexpression of the Notch ligand, Jagged-1, induces alloantigen-specific human regulatory T cells. Blood. 2003;102:3815–3821. doi: 10.1182/blood-2002-12-3826. [DOI] [PubMed] [Google Scholar]
- 25.Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin’s disease. J Exp Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottschalk S, Edwards OL, Sili U, et al. Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies. Blood. 2003;101:1905–1912. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 27.Leen AM, Sili U, Savoldo B, et al. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood. 2004;103:1011–1019. doi: 10.1182/blood-2003-07-2449. [DOI] [PubMed] [Google Scholar]
- 28.Brosterhus H, Brings S, Leyendeckers H, et al. Enrichment and detection of live antigen-specific CD4(+) and CD8(+) T cells based on cytokine secretion. Eur J Immunol. 1999;29:4053–4059. doi: 10.1002/(SICI)1521-4141(199912)29:12<4053::AID-IMMU4053>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expansion of bivirus-specific selected and expanded T cells using different combinations of cytokines.
We compared the in vitro expansion of IFN-γ selected T cells using either IL-15 alone (5 ng/ml) or a cocktail of cytokines IL-7(5 ng/ml), IL-12(10 ng/ml), and IL-15 (5 ng/ml). T cells were expanded for 2 weeks with biweekly addition of the appropriate cytokines, and on day 14 the specificity of the expanded cells was assessed by IFN-γ ELISpot assay using pepmixes spanning CMV pp65 and AdV Hexon to stimulate T cells. Results from 3 donors are shown.