Abstract
Rationale: Urokinase-type plasminogen activator (uPA) receptor (uPAR) is required for the recruitment of neutrophils in response to infection. uPA induces its own expression in lung epithelial cells, which involves its interaction with cell surface uPAR. Regulation of uPAR expression is therefore crucial for uPA-mediated signaling in infectious acute lung injury (ALI).
Objectives: To determine the role of uPA in uPAR expression during ALI caused by sepsis.
Methods: We used Western blot, Northern blot, Northwestern assay, and immunohistochemistry. Phosphate-buffered saline– and lipopolysaccharide (LPS)-treated wild-type and uPA−/− mice were used.
Measurements and Main Results: Biological activities of uPA, including proteolysis, cell adhesion, migration, proliferation, and differentiation, are dependent on its association with uPAR. Bacterial endotoxin (LPS) is a major cause of pulmonary dysfunction and infection-associated mortality. The present study shows that LPS induces uPAR expression both in vitro and in vivo, and that the mechanism involves post-transcriptional stabilization of uPAR mRNA by reciprocal interaction of phosphoglycerate kinase (PGK) and heterogeneous nuclear ribonucleoprotein C (hnRNPC) with uPAR mRNA coding region and 3′ untranslated region determinants, respectively. The process involves tyrosine phosphorylation of PGK and hnRNPC. uPA−/− mice failed to induce uPAR expression after LPS treatment. In these mice, LPS treatment failed to alter the binding of PGK and hnRNPC protein with uPAR mRNA due to lack of tyrosine phosphorylation.
Conclusions: Our study shows that induction of LPS-mediated uPAR expression is mediated through tyrosine phosphorylation of PGK and hnRNPC. This involves expression of uPA as an obligate intermediary.
Keywords: LPS, urokinase-type plasminogen activator, urokinase-type plasminogen activator receptor, tyrosine phosphorylation
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Increased circulating levels of urokinase-type plasminogen activator (uPA) and its receptor (uPAR) are present in endotoxemia and sepsis, conditions associated with the influx of inflammatory cells in the lungs.
What This Study Adds to the Field
This study shows that regulation of lipopolysaccharide-mediated uPAR expression is mediated through tyrosine phosphorylation of phosphoglycerate kinase and heterogeneous nuclear ribonucleoprotein C. The process involves expression of uPA as an obligate intermediary.
Epidemiological studies reveal that infection is the most common cause of acute lung injury (ALI) and acute respiratory distress syndrome, its most severe clinical form (1). Gram-negative organisms account for approximately half of the infections predisposing to ALI (2). Endotoxin (lipopolysaccharide [LPS]) is an important mediator of organ dysfunction and death associated with severe gram-negative infections. In mice, LPS causes ALI, and is characterized by accumulation of neutrophils in the lung, increased expression of proinflammatory cytokines, loss of microvascular and epithelial integrity, and increased interstitial and alveolar edema (3–5).
Urokinase-type plasminogen activator (uPA) is a multifunctional serine protease involved in plasminogen activation (PA) as well as uPA receptor (uPAR)-mediated signal transduction. Previous studies (6–9) have demonstrated rapid increases in circulating PA levels, as well as increased pulmonary concentrations of uPA after endotoxemia or bacterial infections. These studies document a close temporal relationship between the development of LPS-induced ALI and uPA expression. Extending these observations, we recently found that mice that lack uPA (uPA−/− mice) are protected from LPS-induced ALI, and deficiency of uPA prevents both development of pulmonary edema as well as expression of proinflammatory cytokines in the lungs (6).
uPAR also plays a critical role in the pathogenesis of ALI induced by infectious agents in multiple models, as reviewed by Marcel and colleagues (10). Mice deficient in uPAR (uPAR−/−) have impaired clearance of Pseudomonas aeruginosa compared with wild-type (WT) mice. uPAR−/− mice have profoundly diminished neutrophil recruitment in response to P. aeruginosa pneumonia compared with WT mice, indicating that uPAR is required for the recruitment of neutrophils in vivo against a clinically relevant pathogen (11). Many biological activities of uPA, including proteolysis, cell adhesion, migration, proliferation, and differentiation, are dependent on its association with uPAR (12–16). These observations support the hypothesis that uPAR expression plays an important role in LPS-induced ALI. Earlier reports also indicate that the potentiating effects of uPA on LPS-induced responses are sufficiently important to play a major contributory role in the development of ALI (11, 17–19). Bacterial infection and LPS up-regulate uPAR expression in monocytes and neutrophils; such induction is mediated through partial engagement of CD 14 and Toll-like receptor 2 (20).
The stabilities of many mRNAs, including uPAR mRNA, seem to be the major determinant of their abundance, with steady-state mRNA levels correlating directly with persistent mRNA rather than the rapidity of synthesis (21). Increased expression of uPAR mRNA by agents such as cycloheximide D, phorbol myristate acetate (PMA), transforming growth factor (TGF)-β, and tumor necrosis factor (TNF)-α in pleural mesothelial and mesothelioma cells, lung fibroblasts, and airway epithelial cells involves post-transcriptional stabilization of mRNA (12, 22, 23). Mechanisms that regulate uPAR mRNA decay involve interaction of cis elements (51 nt and 110 nt) found in either the coding region (CDR) or 3′ untranslated region (UTR) of mature uPAR mRNA with phosphoglycerate kinase (PGK) and heterogeneous nuclear ribonucleoprotein C (hnRNPC), respectively (13, 21, 23–25). We previously showed that post-transcriptional uPAR mRNA expression in lung epithelial cells in vitro involves a balance between the destabilizing interaction between PGK and a 51-nt uPAR mRNA–CDR determinant, as well as stabilization by hnRNPC binding to 110-nt uPAR mRNA–3′ UTR (13).
The relevance of these findings to the pathogenesis of ALI has been unclear, representing an important gap in our understanding of the contribution of post-transcriptional regulation of uPAR to the pathogenesis of ALI. In the present study, we show, for the first time, that expression of uPAR is enhanced in ALI induced by LPS through stabilization of its mRNA, and that coordinate regulation by PGK and hnRNPC contributes to the response. The regulatory mechanism involves tyrosine phosphorylation of both of these uPAR mRNA binding proteins, resulting in their dissociation from uPAR mRNA, which leads to increased uPAR mRNA stability in the injured lungs.
METHODS
Materials
Culture media, penicillin, and streptomycin were purchased from Gibco BRL laboratory (Grand Island, NY); tissue culture plastics were from Becton Dickinson Labware (Lincoln Park, NJ); bovine serum albumin (BSA), Tris base, aprotinin, phenylmethylsulfonyl fluoride (PMSF), and ammonium persulfate were from Sigma Chemical Co. (St. Louis, MO). Acrylamide, bisacrylamide, and nitrocellulose were from Bio-Rad laboratories (Richmond, CA). Anti-uPAR monoclonal antibody was from American Diagnostica (Greenwich, CT). Anti-phosphotyrosine and anti-β actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Glycine, NP-40, agarose, tetramethylethylenediamine (TEMED), N-2-hydroxyethylpiperazine-N′-ethanesulfonic acid (HEPES), glycerol, methanol, and other reagents were from Fischer Scientific (Pittsburg, PA). In vitro transcription assay kits and 5, 6-dichloro-1-β-D-ribofuranosyl benzamidazole (DRB) were from Ambion (Austin, TX) and Calbiochem (LA Jolla, CA), respectively. Restriction enzymes were from New England Biolabs (Beverly, MA). XAR X-ray film was from Eastman Kodak (Rochester, NY). LPS (Escherichia coli 0111:B4 endotoxin) was from Sigma-Aldrich.
Mice
Transgenic mice with uPA deletion (uPA−/−), as well as control mice on the same genetic background (C57B6/129), have been described previously (6, 18). The mice were kept on a 12:12 hour light:dark cycle, with free access to food and water. All experiments were conducted in accordance with institutional review board–approved protocols.
Model of Endotoxemia-induced Lung Injury
Mice weighing 20–25 g, 8–12 weeks of age, were used for these experiments. ALI was induced by intratracheal injection of LPS at a dose of 25 μg/mouse or phosphate-buffered saline (PBS), as previously described (6, 26, 27). After an incubation period of 24 hours, the mice were killed by giving buthazol intraperitoneally, and blood in the lung vasculature was flushed with 10 ml PBS via right ventricular perfusion, after which the whole lung was harvested, rinsed in PBS, blotted, and stored at −80°C until further use.
Cell Culture
Human bronchial epithelial cells (Beas2B) were obtained from American Type Culture Collection (Manassas, VA). These cells were maintained in LHC-9 medium containing insulin, hydrocortisone, epidermal growth factor, transferrin, T3, retinoic acid, epinephrine, gentamycin, bovine pituitary extracts, and 1% antibiotics, as previously described (28). Cells were grown to subconfluence, and were serum starved overnight in RPMI 1,640 media. The cells were then incubated with PBS or LPS (20 μg/ml) for selected times in serum-free medium.
Preparation of Cytosolic Extract from Beas2B Cells and Western Blotting
Beas2B cells after LPS exposure, as well as cells treated with PBS alone, were collected from culture plates and washed three times in Hanks' balanced salt solution. Cytosolic extracts were prepared by suspending the cell pellets in a buffer containing 25 mM Tris–HCl (pH 7.9), 0.5 mM ethylenediaminetetraacetic acid, and 0.1 mM PMSF. The cells were lysed by four cycles of freezing and thawing, and centrifuged at 12,000 × g for 15 minutes at 4°C. The supernatant was used as the cytosolic extract. The protein content of the cytosolic extract was measured using the Bio-Rad protein assay kit, with serum albumin as the standard. The cytosolic proteins were separated using sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose membrane. The membrane was then blocked with 1% BSA in wash buffer for 1 hour at room temperature, followed by overnight hybridization with PGK polyclonal antibody in the same buffer at 4°C. The membrane was then washed with Tris-HCl buffer (pH 7.4) containing NaCl (0.6%) and Tween-20 (0.05%), and PGK protein was detected by enhanced chemiluminescence (ECL). The membrane was later stripped and subjected to Western blotting for hnRNPC and then for β-actin.
Total Cellular Membrane Extraction and Western Blotting
Beas2B cells were grown to subconfluence in LHC medium and incubated with PBS or LPS in serum-free medium. Cells were washed in PBS, and membrane proteins were isolated as previously described (12, 28). Cell surface uPAR expression was determined by Western blotting using anti-uPAR antibody, as detected by ECL.
Isolation of PGK and hnRNPC
Isolation of PGK and hnRNPC was performed as previously described (29). In brief, the cells were lysed in Western lysis buffer by three freeze–thaw cycles. The PGK and hnRNPC proteins were isolated from the total lysates using specific antibody conjugated with agarose and then separated on 8% SDS-PAGE. The proteins were then transferred to a nitrocellulose membrane and subjected to Northwestern assay using 32P-labeled coding region and 3′ UTR transcripts, respectively, as described previously (29).
Isolation and Culture of Mouse Alveolar Epithelial Cells
Alveolar type (AT) II cells were isolated from uPA−/− and WT mice treated with PBS or LPS (6 μg/20 g) for 16 hours, as described by Bortnick and by Warshamana (30, 31). The cells were plated on Dulbecco's modified Eagle medium containing 10% fetal calf serum at 37°C for 1 hour to remove macrophages and fibroblasts. The nonadherent cells were centrifuged, and cell pellets were used for total cellular membrane protein extraction, as described above.
Preparation of Crude Extract from Mouse Lung Tissue and Western Blotting
Mouse lung tissue extracts were prepared as described previously (32). Briefly, mouse lung tissues were chopped into small pieces with fine scissors and rinsed three times with PBS. The tissues were then homogenized in a 1-ml volume of extraction buffer (25 mM Tris–HCl [pH 7.9], 0.5 mM ethylenediaminetetraacetic acid, and 0.1 mM PMSF), and the homogenate was centrifuged at 12,000 × g for 15 minutes at 4°C. The supernatant was used as crude extract, and the protein content of this extract was measured using a Bio-Rad protein assay kit. Cytosolic proteins were separated using SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was then blocked with 1% BSA in wash buffer for 1 hour at room temperature, followed by overnight hybridization with anti-PGK antibody in the same buffer at 4°C, and the protein was detected by ECL. Membranes were stripped and immunoblotted for hnRNPC and then for β-actin.
Total Cellular Membrane Extraction from Lung Tissue
After the preparation of cytosolic extract, the pellet was resuspended in membrane extraction buffer containing β-D-glucopyranoside, and the suspension was homogenized for 5–10 minutes. The homogenate was allowed to rotate at 4°C for 2 hours (12, 28). The homogenate was then centrifuged at 12,000 × g for 30 minutes. The clarified supernatant was analyzed for uPAR expression by Western blotting using anti-uPAR antibody and detected by ECL.
In vitro Transcription
Linearized plasmid containing a mouse uPAR mRNA transcriptional template of uPAR cDNA was transcribed in vitro using T7 polymerase (Ambion, TX). The uPAR mRNA CDR and 3′ UTR transcripts were synthesized according to the supplier's protocol, except that 50 μCi [32P]UTP was substituted for unlabeled UTP in the reaction mixture, as described previously (23, 24). The mixture was passed over a Sephadex G-25 column to remove unincorporated radioactivity. The specific activity of the transcript was 4.9 × 108 cpm/μg.
Northwestern Assay and Western Blotting
The PGK and hnRNPC proteins were isolated from Beas2B and mouse lung extracts using specific antibodies, as described previously (13, 25, 29). Briefly, isolated proteins were separated on SDS-PAGE and then blotted onto a nitrocellulose membrane. The membrane was blocked with buffer containing 1% BSA and 20 μg of rRNA for 1 hour, after which the membrane was incubated with 32P-labeled uPAR mRNA transcript containing the PGK and/or hnRNPC binding sequence in fresh gel shift buffer for an additional hour at room temperature. The membrane was later washed three times for 10 minutes each with 50 ml of gel shift buffer, air dried, and exposed to X-ray film. The same membrane was later stripped and subjected to Western blotting using anti-phosphotyrosine antibody to determine the tyrosine phosphorylation status of PGK and hnRNPC. Lastly, membranes were stripped and developed with anti-PGK or anti-hnRNPC antibodies to normalize uPAR mRNA binding activities and tyrosine phosphorylation of PGK or hnRNPC proteins with their total expression, as previously described (13, 24, 25, 29).
Random Priming of uPAR/PGK/hnRNPC cDNA
The full-length template of uPAR, PGK, or hnRNPC cDNAs cloned into pcDNA 3.1 was released with Hind III and Xba I, purified on 1% agarose gel, and labeled with [32P]dCTP using a Rediprime labeling kit (GE Healthcare, Piscataway, NJ). Passage through a Sephadex G-25 column was used to remove unincorporated radioactivity. The specific activity of the product was 6 × 107 cpm/μg.
Northern Blotting of uPAR, PGK, and hnRNPC mRNA
Northern blotting assay was used to quantify the expression of uPAR, PGK, and hnRNPC mRNAs. Total RNA from mouse lung and Beas2B cells were isolated using TRI reagent, separated on agarose/formaldehyde gel, and transferred to nitrocellulose membranes. Prehybridization and hybridization were performed at 42°C in polyvinylpyrolidine buffer containing formalin and 100 μg of salmon sperm DNA. Hybridization was performed overnight with the respective cDNA probes (1 ng/ml) labeled to approximately 6 × 108 cpm/μg of DNA. After hybridization, the membranes were washed twice for 20 minutes at 65°C with 2× sodium chloride–sodium citrate (SSC) +1% SDS; 1× SSC +1% SDS, and 0.1× SSC +1% SDS. The membranes were exposed to X-ray film at −70°C overnight. The same membranes were stripped and analyzed for β-actin mRNA. The intensity of the bands was measured by densitometry and normalized against that of β-actin. In separate experiments, Beas2B cells were incubated with PBS or LPS (20 μg/ml) for 12 hours to induce maximum uPAR mRNA expression. DRB (10 μg/ml) was then added to inhibit ongoing transcription. Total RNA was isolated at predetermined time points (0–24 h), and the decay of uPAR mRNA was analyzed by Northern blotting, as described above.
Immunohistochemistry
Mice were killed by an intraperitoneal injection of a lethal dose of Beuthanasia-D (Schering-Plough, Union, NJ). After being killed, lungs from control and LPS-exposed WT or uPA−/− mice were inflated by an intratracheal instillation of 10% formalin in PBS at a constant pressure of 20 cm H2O, and then fixed in 3.7% formalin overnight at room temperature. For immunohistochemistry, 5-μm sections cut from paraffin-embedded lung tissues were deparaffinized with xylene, followed by incubation with 100% and 95% alcohol. After 30 minutes of antigen retrieval with 10 mM sodium citrate buffer (pH 6.0), the slides were incubated with hydrogen peroxide for 30 minutes to quench endogenous peroxidase. The slides were incubated overnight with rabbit anti-mouse uPAR antibody (Santa Cruz Biotechnology), or with rabbit IgG as negative control in PBS containing 0.1% Tween 20, and further processed using the Lab Vision kit (Fremont, CA). The slides were then washed in water, mounted, and examined microscopically (BX 41; Olympus, Santa Valley, PA).
RESULTS
Effect of LPS on uPAR Expression in Beas2B Cells
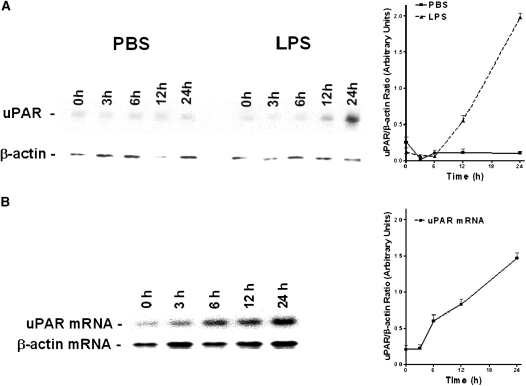
It has been previously reported that PMA, epidermal growth factor, LPS, and TNF-α increase the specific binding capacity of uPA and cell surface expression of uPAR on various cell lines (29, 32–34). To better understand the mechanism by which bacterial endotoxins regulates lung airway epithelial cell uPAR during ALI caused by sepsis, we exposed bronchial epithelial Beas2B cells to higher doses of LPS and analyzed the effect on the expression of uPAR protein and uPAR mRNA. To do so, Bease2B cells were exposed to media containing 20 μg/ml of LPS as well as to PBS for 0–24 hours. The cell lysates were subjected to Western blotting using anti-uPAR antibody. Membranes were later stripped and developed with anti–β-actin antibody to ensure equal loading. LPS induced expression of uPAR in Beas2B cells in a time-dependent manner. Maximal expression of uPAR was observed between 12 and 24 hours after exposure to LPS (Figure 1A). However, there was minimal expression seen in PBS-treated control cells.
Figure 1.
LPS induces urokinase-type plasminogen activator (uPA) receptor (uPAR) expression in lung epithelial cells: (A) Beas2B cells were exposed to phosphate-buffered saline (PBS) or LPS (20 μg/ml) for 0–24 hours in serum-free media. The membrane proteins were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. The membranes were analyzed for uPAR protein using anti-uPAR antibody. The same membranes were stripped and analyzed for β-actin protein to assess equal loading. (B) Total RNA isolated from Beas2B cells exposed to LPS, as in (A) using TRI-reagent, was analyzed for uPAR mRNA expression by Northern blotting using a 32P-labeled uPAR cDNA probe. The same membranes were later stripped and hybridized with a β-actin probe to affirm loading equality. The experiments were repeated three times. In (A) and (B), results from a representative experiment are shown. The graphs show the mean (± SD) density of individual bands from three independent experiments.
We next evaluated the effect of LPS on uPAR mRNA expression after LPS exposure by Northern blot analysis. The results were consistent with that of Western blot analysis. The level of uPAR mRNA was quantified by scanning densitometry and normalized against a β-actin loading control. Resting Beas2B cells express low levels of uPAR mRNA (Figure 1B). LPS induced expression of uPAR mRNA in a time-dependent manner (Figure 1B). Expression uPAR mRNA increased about 10-fold by 24 hours after exposure to LPS.
Effect of LPS on uPAR mRNA Stability in Beas2B Cells
We have previously observed that uPA enhances uPAR expression by post-transcriptional mRNA stabilization (35, 36). Therefore, we next asked if LPS-induced expression of uPAR mRNA in Beas2B cells through this mechanism. To approach this question, Beas2B cells were incubated with PBS or 20 μg/ml of LPS in PBS for 12 hours to maximize uPAR mRNA expression. To block the ongoing transcription, DRB (10 μg/ml) was added to the same media and expression of uPAR mRNA was quantified at various times (0–24 h) after LPS incubation by Northern blot analysis. Expression of uPAR mRNA transcripts in Beas2B cells exposed to LPS was stabilized (t1/2 > 6 h) compared with PBS-treated cells (Figure 2), similar to the effects of uPA reported previously (28).
Figure 2.
Stabilization of urokinase-type plasminogen activator receptor (uPAR) mRNA by LPS. Beas2B cells were incubated with phosphate-buffered saline (PBS) or LPS for 12 hours to induce maximum expression of uPAR mRNA. Ongoing transcription was later blocked by adding 5, 6-dichloro-1-β-D-ribofuranosyl benzamidazole (DRB) (20 μg/ml) to the same media. Total RNA was isolated and uPAR mRNA level was measured at predefined time points after addition of DRB by Northern blot using a 32P-labeled uPAR cDNA probe, followed by hybridization with a β-actin probe to assess for equal loading. The line graph represents the percentage of mRNA decay relative to Time 0 (designated 100%), calculated from the mean values obtained by integrating the densities of individual bands from two independent experiments.
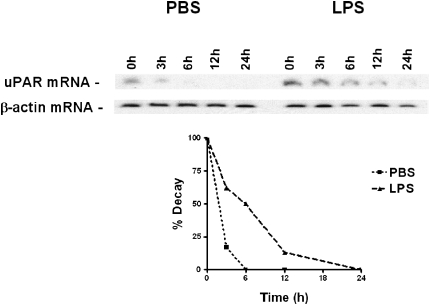
Effect of LPS on PGK-uPAR mRNA CDR Interaction
Previous studies indicated that post-transcriptional stabilization of uPAR mRNA involves active participation of the uPAR mRNA binding proteins, PGK and/or hnRNPC (23–25, 36). Therefore, we next treated the Beas2B cells with PBS or LPS (20 μg/ml) for 0 and 24 hours. The PGK proteins were isolated from the cell lysates (200 μg), and the effect of LPS on PGK binding to uPAR mRNA CDR were then tested by Northwestern assays. LPS treatment decreased PGK–uPAR CDR binding, whereas PBS treatment increased the binding interaction (Figure 3A). However, there was no significant difference in total PGK expression when normalized against β-actin control after Western blotting following PBS or LPS treatment (data not shown). Increased PGK interaction with uPAR mRNA in PBS-treated cells was found with nutrient deprivation. To confirm that LPS inhibits interaction of PGK with uPAR mRNA, we next treated Beas2B cells with LPS for varying time periods. The isolated PGK proteins from the cell lysates at each time point after adding LPS were subjected to Northwestern analysis. As shown in Figure 3B, binding of PGK to uPAR CDR mRNA was decreased in a time-dependent manner by LPS treatment. However, no significant change in total PGK protein expression was found, except for a slight decrease at 3 hours, which appeared to be due to loading differences. Western blotting of LPS-treated total cell lysates showed little change in total expression with time (data not shown).
Figure 3.
Effect of LPS on the interaction of phosphoglycerate kinase (PGK) with urokinase-type plasminogen activator receptor (uPAR) mRNA coding region (CDR) in Beas2B cells. (A) Beas2B cells grown in culture plates to near confluence were incubated with phosphate-buffered saline or LPS (20 μg/ml) for 0 and 24 hours. PGK was isolated from these cells, as described in the methods, separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE), and transferred to nitrocellulose membranes. The membranes were then hybridized with 32P-labeled uPAR CDR mRNA. The same membranes were later stripped and analyzed for PGK protein by Western blot analysis using anti-PGK antibody. The line graph represents the ratio of uPAR CDR mRNA binding activity to PGK protein from three independent experiments shown as the mean ± SD. (B) Beas2B cells grown in culture plates to near confluence were incubated with LPS (20 μg/ml) for 0–24 hours. PGK was isolated from these cells, separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were processed as described above. The line graph presents results from three independent experiments shown as the mean ± SD.
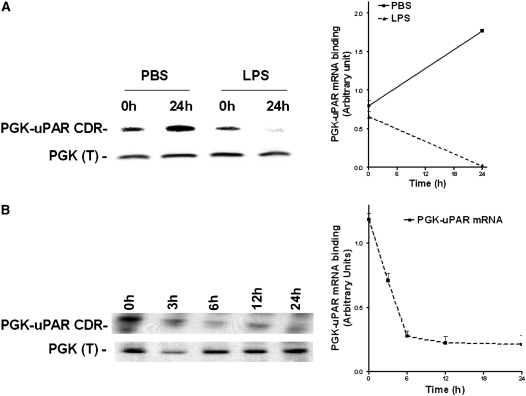
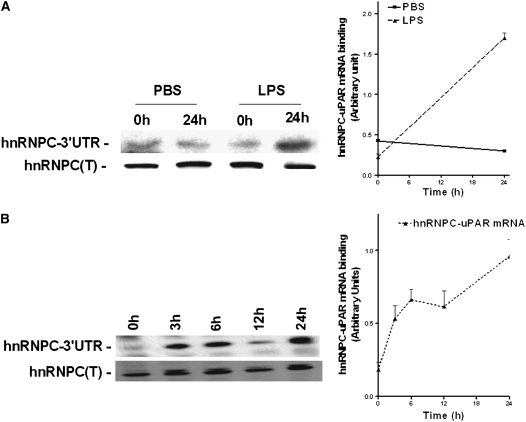
LPS Up-regulates uPAR mRNA 3′ UTR Binding to hnRNPC
Because stabilization of uPAR mRNA is mediated by interrupting the binding of PGK, we next asked if the interaction between hnRNPC and uPAR mRNA 3′ UTR is likewise altered in response to LPS exposure. We isolated hnRNPC from lysates of Beas2B cells incubated with PBS or LPS and assessed their interaction with 32P-labeled uPAR 3′ UTR mRNA. Northwestern analysis indicated that, in contrast to its effect on the interaction between PGK and uPAR mRNA CDR interaction, LPS increased hnRNPC binding to uPAR mRNA 3′ UTR in a time-dependent fashion, with its maximum effect again observed between 12–24 hours after initial exposure. On the other hand, PBS-treated control cells showed no difference in hnRNPC binding in the 0 and 24 hours samples (Figure 4A and 4B). Taken together, the decreased PGK-uPAR mRNA CDR and/or increased hnRNPC–uPAR mRNA 3′ UTR binding profiles are consistent with the observed stabilization and increased expression of uPAR mRNA and protein induced by LPS.
Figure 4.
Effect of LPS on heterogeneous nuclear ribonucleoprotein C (hnRNPC) and urokinase-type plasminogen activator receptor (uPAR) mRNA 3′ UTR binding in Beas2B cells. (A) Beas2B cells grown in culture plates were incubated with phosphate-buffered saline or LPS for 0 and 24 hours, as described in Figure 3. hnRNPC proteins were isolated from these cell lysates, and processed as described above. The line graph represents the mean and SD from three independent experiments. (B) Beas2B cells grown in culture plates were incubated with LPS for 0–24 hours, as described above. hnRNPC proteins were isolated from these cell lysates, separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE), and transferred to nitrocellulose membranes. The membranes were later incubated with [32P]uPAR mRNA 3′ untranslated region. Western blot analysis using anti-hnRNPC antibody was performed on the same membranes after stripping. The line graph represents the mean and SD from three independent experiments.
LPS Enhances uPAR Expression in Mouse Lung Tissue through a uPA-Dependent Mechanism
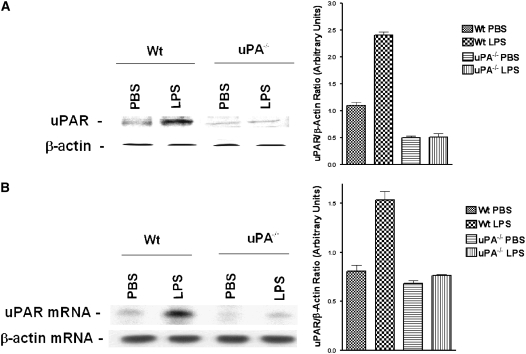
Sepsis increases uPA in the systemic circulation (6). We (28) and others (35, 36) have shown that uPA induces the expression of uPAR in several cell types, including airway epithelial cells. Therefore, we hypothesized that uPA induced during sepsis enhances cell surface expression of uPAR in the lungs of LPS-challenged mice. To determine whether uPA is responsible for increasing cell surface uPAR expression in mice exposed to LPS, uPA gene silenced (uPA−/−) mice were exposed to either saline (PBS) or LPS, and the membrane proteins were analyzed for uPAR. Minimal expression of uPAR was detected in saline-exposed uPA-deficient mice. Of interest, uPAR expression was not induced by LPS in uPA−/− mice in contrast to its effect on WT mice (Figure 5A). We next assessed uPAR expression at the mRNA level by Northern blotting. We found that uPAR mRNA was markedly increased in LPS-challenged WT mice compared with saline (PBS) control animals, whereas uPA-deficient mice failed to increase expression when challenged with LPS (Figure 5B).
Figure 5.
Role of urokinase-type plasminogen activator (uPA) in LPS-induced uPA receptor (uPAR) expression in mouse lungs. (A) Membrane proteins (100 μg/lane) isolated from lung homogenates of phosphate-buffered saline or LPS-challenged wild-type (WT) or uPA−/− mice were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) under nonreducing conditions and transferred to nitrocellulose membranes. The membranes were immunoblotted with anti-uPAR antibody. The same membranes were later stripped and developed with β-actin antibody for equal loading. The bar graph shows the results (mean + SD) from three independent experiments. (B) Total RNA (20 μg) isolated from lung homogenates of the above-treated mice was separated on 1% agarose/formaldehyde gels and subjected to Northern blotting using 32P-labeled uPAR cDNA and β-actin cDNA probes. The results shown are representative of three independent experiments.
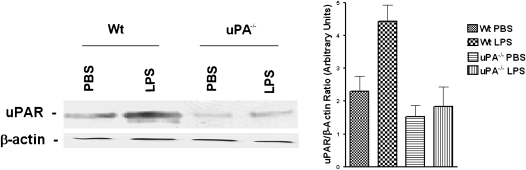
To determine if alveolar epithelial cells contribute to the increased uPAR expression observed in LPS-treated lung extract (Figure 5), we isolated AT II epithelial cells from saline (PBS)- or LPS-treated mouse lungs. The membrane fractions of AT II cells isolated from saline- or LPS-treated mice were analyzed for uPAR expression by Western blotting. As shown in Figure 6, saline-treated AT II cell membrane protein fractions exhibited minimal uPAR antigen, whereas the LPS-treated AT II cells showed pronounced uPAR expression.
Figure 6.
The role of urokinase-type plasminogen activator (uPA) in LPS-induced uPA receptor (uPAR) expression by alveolar epithelial cells isolated from mouse lungs. Membrane proteins (100 μg/lane) extracted from alveolar epithelial cells of phosphate-buffered saline or LPS-challenged WT or uPA−/− mice were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) under nonreducing conditions. The resolved proteins were then electrotransferred to nitrocellulose membranes and immunoblotted with anti-uPAR antibody. The same membranes were then stripped and developed with β-actin antibody to confirm equal loading. The bar graph shows the results (mean + SD) from three independent experiments.
uPAR Expression in Lung Tissue after Exposure to LPS
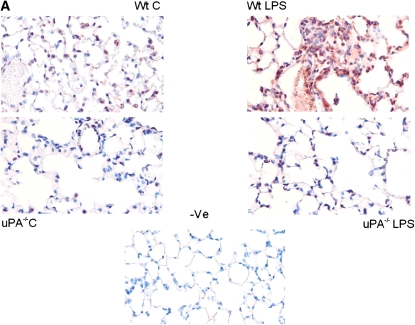
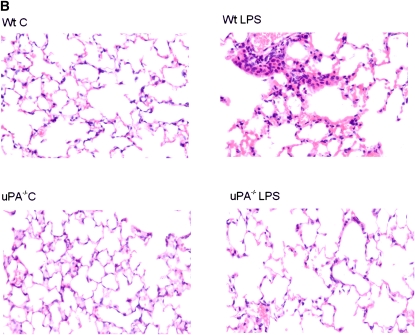
To assess the involvement of uPA in LPS-induced uPAR expression in vivo, and to confirm the cellular source of uPAR expression in situ, paraffin-embedded lung sections (5 μm) of WT and uPA−/− mice exposed to saline (PBS) or LPS were analyzed by immunohistochemistry using anti-uPAR antibody. Microscopic examination by a blinded pulmonary pathologist taking random selection of seven fields from each of the three stained lung sections per mouse from three mice indicated increased uPAR expression (red staining) in both alveolar and airway epithelial cells of WT mice exposed to LPS. However, no significant increase in uPAR staining was observed in uPA−/− mice treated with LPS (Figure 7A). Similarly, sections were also stained with hematoxylin and eosin, and demonstrated that LPS-treated WT mouse lungs had more inflammation and edema when compared with saline (PBS) WT mice as well as saline (PBS)- and LPS-treated uPA−/− mice (Figure 7B). These observations demonstrate that lung epithelial cells contribute to increased uPAR expression by LPS, and that the induction involves uPA expression in vivo.
Figure 7.
Increased urokinase-type plasminogen activator receptor (uPAR) expression in lungs from mice challenged with LPS in vivo. (A) Lung sections (5 μm) from WT and uPA−/− mice challenged with phosphate-buffered saline or LPS were stained with anti-uPAR antibody. Representative fields from one of three mice for each condition are shown at 200× magnification. The negative control (−Ve) shows lung sections from LPS-challenged WT mice incubated with nonimmune rabbit IgG. (B) Lung sections (5 μm) from WT and uPA−/− mice challenged with phosphate-buffered saline (C, control) or LPS were stained with hematoxylin and eosin. Representative fields illustrating the findings of all of the mice for each condition are shown at 200× magnification.
Effect of LPS Response on PGK Expression in Lung Tissue
LPS and uPA (28) induced uPAR expression through mRNA stabilization in lung epithelial cells (Figure 2). In addition, LPS did not induce uPAR expression in uPA-deficient mice. These observations prompted us to determine whether LPS augments uPAR expression via a post-transcriptional stabilization mechanism in mice. As noted above, we previously demonstrated that post-transcriptional regulation of uPAR expression involves the interaction between PGK with a 51-nt CDR determinant (25), as well as hnRNPC binding to a 110-nt uPAR mRNA 3′ UTR determinant in vitro (13). We next sought to determine if these mechanisms are also operative in controlling uPAR expression in the lungs of mice with ALI induced by LPS. To do so, we first determined whether PGK protein and mRNA level in vivo is altered in response to LPS and, if so, is there a difference in the expression of PGK between WT and uPA-deficient mice exposed to LPS. Crude extracts from lung tissues from saline (PBS) control and LPS-challenged WT and uPA−/− mice were analyzed for expression of PGK by Western blotting using an anti-PGK antibody. WT and uPA−/− mice showed robust expression of PGK mRNA and protein (data not shown). Nonetheless, LPS failed to affect total PGK protein or mRNA levels in the lungs of WT or uPA−/− mice.
Effect of LPS on the Interaction of PGK with uPAR mRNA In Vivo
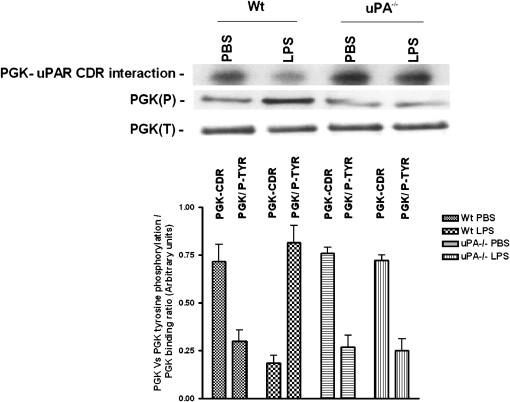
In view of the finding that LPS did not induce PGK protein or mRNA expression in vivo, we hypothesized that LPS stimulates uPAR mRNA and protein expression by reducing the binding affinity of PGK for uPAR mRNA, thereby promoting message stability. To examine this possibility, PGK proteins isolated from the crude lung extracts of saline (PBS)- or LPS-treated WT and uPA−/− mice were assessed for binding to uPAR mRNA CDR by Northwestern assay. Binding of PGK to uPAR CDR mRNA is diminished in WT mice after exposure to LPS compared with PBS control animals.
Because induction of uPAR in cells by PMA, TGF-β, and uPA involves tyrosine phosphorylation (24), we next asked whether changes in tyrosine phosphorylation were responsible for inhibiting PGK binding to uPAR mRNA. Therefore, the same membranes were stripped, and tyrosine phosphorylation of PGK was measured by Western blotting using anti-phosphotyrosine antibody. LPS enhanced tyrosine phosphorylation of PGK in WT mice compared with saline (PBS)-treated control mice (Figure 8). There was no difference in PGK tyrosine phosphorylation between WT and uPA−/− mice exposed to saline. However, LPS failed to augment PGK tyrosine phosphorylation in uPA−/− mice. These observations suggest that LPS stabilizes uPAR mRNA, and induces uPAR expression by inhibiting PGK binding to uPAR mRNA. The process involves phosphorylation of PGK tyrosine residue after LPS exposure. This process is dependent on uPA.
Figure 8.
Effect of LPS on phosphoglycerate kinase (PGK) phosphorylation and its interaction with urokinase-type plasminogen activator receptor (uPAR) mRNA coding region (CDR). PGK isolated from lung homogenates of WT and uPA−/− mice challenged with phosphate-buffered saline (PBS) or LPS, as described in Figure 6, were separated on 8% sodium dodecyl sulfate–polyacrylamide gels under nonreducing conditions. PGK was transferred to nitrocellulose membranes. The membranes were analyzed for PGK-uPAR mRNA CDR interaction by Northwestern assay using a 32P-labeled uPAR mRNA CDR probe. The same membranes were later stripped and analyzed for tyrosine phosphorylation and total PGK expression by Western blotting using anti-phosphotyrosine and anti-PGK antibodies, respectively. The bar graph shows mean and (+ SD) densitometric values from three independent experiments.
hnRNPC Involvement in uPAR mRNA Stabilization after Exposure to LPS
Unlike PGK, hnRNPC binds to a 110-nt uPAR mRNA 3′ UTR sequence that regulates uPAR mRNA decay. Cotransfection of hnRNPC cDNA prevents degradation of β-globin mRNA containing the 110-nt hnRNPC binding uPAR mRNA 3′ UTR determinant. Overexpression of hnRNPC protein increases Beas2B cell surface uPAR by stabilizing uPAR mRNA expression (13). uPA induces uPAR expression through post-transcriptional stabilization of uPAR mRNA (28, 36). Hence, we sought to determine if the hnRNPC–uPAR mRNA 3′ UTR interaction mediates the LPS-induced stabilization of uPAR mRNA and uPAR expression. We initially analyzed crude lung extracts from PBS- or LPS-challenged WT and uPA−/− mice for hnRNPC protein and mRNA expression. Expression of hnRNPC was not altered in these animals after LPS exposure (data not shown). The results indicate that LPS likewise fails to alter hnRNPC mRNA expression. These results exclude the possibility that increased hnRNPC expression causes uPAR mRNA stabilization by LPS.
Effect of LPS on uPAR mRNA Binding to hnRNPC through Tyrosine Phosphorylation
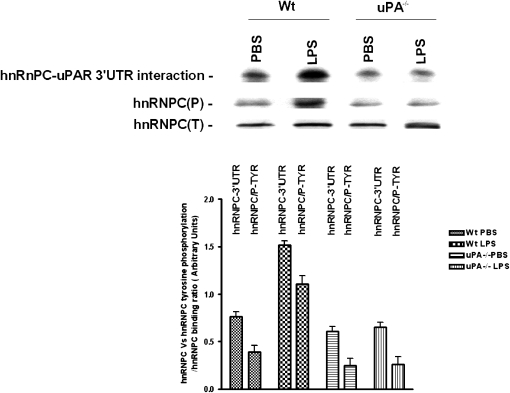
LPS enhanced the interaction of hnRNPC with uPAR mRNA 3′ UTR in Beas2B cells (Figures 4A and 4B). After finding that neither hnRNPC protein nor mRNA was induced in LPS-treated mice, we hypothesized that LPS influenced the binding of hnRNPC protein to uPAR mRNA 3′ UTR without altering its basal expression. To assess this possibility, we studied the interaction of isolated hnRNPC with 32P-labeled uPAR mRNA 3′ UTR. The results from Northwestern analysis indicate that LPS increased hnRNPC binding to uPAR 3′ UTR in LPS-treated WT mice compared with saline (PBS)-treated WT control mice. However, in uPA−/− mice, LPS failed to alter the protein–uPAR mRNA interaction. Moreover, LPS-induced tyrosine phosphorylation of hnRNPC occurs in WT mice, which parallels the LPS-mediated increase in hnRNPC–uPAR mRNA 3′ UTR interaction, but not in uPA−/− mice (Figure 9).
Figure 9.
The role of LPS on hnRNPC binding to urokinase-type plasminogen activator receptor (uPAR) mRNA 3′ untranslated region (UTR) and hnRNPC tyrosine phosphorylation. hnRNPC protein isolated from crude lung extracts of WT and uPA−/− mice challenged with phosphate-buffered saline or LPS were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) under nonreducing conditions and transferred to nitrocellulose membranes. The membranes were later analyzed for uPAR 3′ UTR mRNA interaction by Northwestern assay using a 32P-labeled uPAR mRNA 3′ UTR probe. The same membranes were stripped and analyzed for tyrosine phosphorylation and total hnRNPC expression by Western blotting using anti-phosphotyrosine and anti-hnRNPC antibodies, respectively. The bar graph represents the data (mean + SD) from three independent experiments.
DISCUSSION
Local generation of plasmin by uPA is central to the process by which lung epithelial cells degrade fibrin and extracellular matrices as a consequence of lung injury and repair. Expression of uPA and its cell surface receptor uPAR is germane to a variety of cellular responses involved in the pathogenesis of inflammation. The uPAR focuses uPA-dependent pericellular proteolysis. In addition, interaction of uPA and uPAR facilitates cellular proteolysis, cell proliferation, adhesion, and migration in diverse cell types of many organs, including lung, during infection or injury (12, 32, 37–41).
Bacterial endotoxins are well recognized for their ability to induce pulmonary inflammation in animal models of ALI, but their effect on uPA and uPAR expression is only partially understood (42–46). It has previously been reported that expression of uPA and uPAR, as well as their specific interaction, is enhanced by proinflammatory agents, such as LPS, TGF-β, and TNF-α in various cell lines (12, 32–34, 38–41). These agents intensely and rapidly induce cell surface uPAR expression and the effect that can be traced back to a rapid, antecedent increase in the cellular level of uPAR mRNA.
In the present study, we used LPS to stimulate lung epithelial response in ALI in vitro, and noticed a pronounced effect in uPAR expression on lung epithelial cells. Induction of uPAR was time-dependent, reaching its maximum effect at 24 hours, a phenomenon that is consistent with previously reported findings for PMA in Beas2B and MS-1 cells (23). LPS similarly increased uPAR mRNA by stabilizing uPAR mRNA in Beas2B cells, indicating that post-transcriptional regulation is involved in endotoxin-induced uPAR expression.
Earlier reports have shown that post-transcriptional regulation of uPAR expression is mediated by the binding of PGK with cis elements within a 51-nt sequence of the uPAR mRNA CDR (24, 25, 29). uPAR expression is also increased through mRNA stabilization by hnRNPC binding to a 110-nt uPAR mRNA 3′ UTR determinant. Agents such as PMA, TGF-β, and TNF-α are known to induce uPAR expression via mRNA stabilization and suppress binding of PGK to uPAR mRNA (23, 24, 37). These agents also enhance uPAR mRNA stability by augmenting hnRNPC–uPAR 3′ UTR interaction (13, 29). Therefore, we hypothesized that the induction of uPAR expression by LPS in these cells would be regulated by post-transcriptional mechanisms that involve the interaction between uPAR mRNA and PGK and/or hnRNPC proteins.
LPS alters the interaction of PGK and hnRNPC with uPAR mRNA CDR and 3′ UTR in a coordinated manner that enhances message stability. The mechanism involves tyrosine phosphorylation of both PGK and hnRNPC without altering their basal expression. LPS treatment down-regulated PGK binding to uPAR mRNA CDR in a time-dependent manner, while simultaneously increasing the binding of hnRNPC to the uPAR mRNA 3′ UTR.
Increased uPA and plasminogen activator inhibitor (PAI)-1 are often encountered in severe infections. Bacterial endotoxin and inflammatory cytokines released during infection induce uPA expression (4). Therefore, we asked whether LPS likewise induce uPAR expression in vivo using a murine model of endotoxin injury and whether this up-regulation requires the presence of uPA. AT II cells isolated from LPS-treated WT mice showed increased uPAR expression when compared with those obtained from PBS-treated control mice. No significant difference in uPAR expression was detected in AT II cells from LPS- and PBS-treated uPA−/− mice. uPAR analyses of lung homogenates from LPS-treated WT mice likewise exhibited augmented expression when compared with PBS-treated control mice. These observations demonstrate that LPS induces uPAR expression in the lung, and that AT II cells contribute to the increase. The finding that LPS did not induce uPAR protein or mRNA in uPA-deficient mice further confirms that uPA expression is critical to the process. This is consistent with earlier in vitro observations that uPA induces uPAR expression (28, 36). Increased uPA in the circulation during life-threatening infections (6) and its ability to induce PAI-1 expression in lung epithelial and smooth muscle cells by binding to uPAR via the amino-terminal fragment (47, 48) indicate that both uPA and uPAR are involved in the net increase in the expression of PAI-1 during infection.
uPA−/− mice are protected from endotoxemia-induced development of lung edema, pulmonary neutrophil accumulation, and increase in lung IL-1β, macrophage inflammatory protein 2 and TNF-α cytokine levels (6), and neutrophil migration is inhibited in uPAR−/− mice during lung infection (11). Taken together, these observations strongly suggest the involvement of uPA and uPAR in lung injury caused by infection. Although LPS induces uPA and uPAR expression at the transcriptional and post-transcriptional level (28), the finding that LPS does not induce uPAR in uPA−/− mice, and the ability of uPA to induce uPAR expression only through uPAR mRNA stabilization (28, 36), implicates post-transcriptional regulation in LPS-induced uPAR expression. Stabilization of uPAR mRNA by LPS in Beas2B cells provides additional support for this inference.
These observations lead to the conclusion that increased uPAR mRNA in cells exposed to LPS is due to decreased post-transcriptional turnover. Prior in vitro analyses have shown the involvement of uPAR mRNA binding proteins, PGK and hnRNPC, in post-transcriptional regulation of uPAR mRNA expression (13, 24, 25, 29). Regulation involves tyrosine phosphorylation of both PGK and hnRNPC (24, 29). This was further supported by the fact that LPS strongly induces p38 mitogen-activated protein kinase (MAPK) phosphorylation (6, 49), and inhibition of p38 MAPK blocks uPAR mRNA stabilization (49). Furthermore, PMA, TGF-β, and TNF-α influence the phosphorylation status of PGK, which determines its binding efficiency to uPAR mRNA (23–25, 29, 32).
In the present study, we extended the observations to unravel the regulatory mechanism by which LPS induces uPAR expression in vivo. Our findings show that the mechanism involves the interaction of PGK and hnRNPC with uPAR mRNA at the post-transcriptional level, consistent with in vitro findings. LPS neither induces PGK or hnRNPC protein, nor mRNA expression in either saline (PBS)-treated control or LPS-treated WT mice or uPA−/− mice. However, LPS induces tyrosine phosphorylation of PGK and hnRNPC in LPS-challenged WT mice. LPS-induced tyrosine phosphorylation of PGK prolongs the half-life of uPAR mRNA by down-regulating the binding of PGK protein to uPAR mRNA. LPS also activates hnRNPC via tyrosine phopshorylation, which further stabilizes uPAR mRNA through increased hnRNPC binding to its cognate 3′ UTR determinant. However, additional studies are needed to determine if p38 MAPK activated by LPS is involved in the tyrosine phosphorylation of either PGK or hnRNPC.
We observed greater binding of PGK to uPAR mRNA CDR in saline (PBS)- and LPS -exposed uPA−/− mice compared with LPS-challenged WT mice. In contrast, hnRNPC showed less binding to uPAR mRNA 3′ UTR. LPS failed to induce tyrosine phosphorylation of either PGK or hnRNPC in uPA−/− mice. Overexpression of the protein tyrosine phosphatase, SHP2, dephosphorylates PGK and increases its binding affinity for uPAR mRNA CDR, while inhibiting the binding of hnRNPC to uPAR mRNA 3′ UTR (29), affirming the importance of tyrosine phosphorylation. The opposing effects of PGK and hnRNPC on uPAR mRNA were found to act in concert to enhance uPAR expression.
In conclusion, our study shows, for the first time, that regulation of LPS-mediated uPAR expression is mediated through tyrosine phosphorylation of PGK and hnRNPC. These proteins regulate expression of cell surface uPAR and uPAR mRNA stability in mouse lung tissues. The process involves expression of uPA as an obligate intermediary. This newly recognized mechanism and novel pathway provides opportunities to regulate uPAR-dependent fibrinolysis and signal transduction in LPS-induced ALI.
Acknowledgments
The authors are grateful to M.B. Harish for technical assistance.
Supported by National Heart, Lung, and Blood Institute grants R01-HL071147 and Project 2 of P01HL-62453.
Originally Published in Press as DOI: 10.1164/rccm.200712-1787OC on November 21, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med 1995;332:27–37. [DOI] [PubMed] [Google Scholar]
- 2.Karen EW, Martha SC, Huang CT, Steven GS, Stephen PK, Claude AP. Bacterial priming increases lung injury in gram-negative sepsis. Am J Respir Crit Care Med 1998;158:610–619. [DOI] [PubMed] [Google Scholar]
- 3.Abraham E, Matthay MA, Dinarello CA, Vincent JL, Cohen J, Opal SM, Glauser M, Parsons P, Fisher CJ Jr, Repine JE. Consensus conference definitions for sepsis, septic shock, acute lung injury, and acute respiratory distress syndrome: time for a reevaluation. Crit Care Med 2000;28:232–235. [DOI] [PubMed] [Google Scholar]
- 4.Miller EJ, Cohen AB, Matthay MA. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Crit Care Med 1996;24:1448–1454. [DOI] [PubMed] [Google Scholar]
- 5.Manthous CA. ARDS redux. Clinical Pulmonary Medicine 2006;3:121–127. [Google Scholar]
- 6.Abraham E, Gyetko MR, Kuhn K, Arcaroli J, Strassheim D, Park JS, Shetty S, Idell S. Urokinase-type plasminogen activator potentiates lipopolysaccharide-induced neutrophil activation. J Immunol 2003;170:5644–5651. [DOI] [PubMed] [Google Scholar]
- 7.Braat EA, Nauland U, Dooijewaard G, Rijken DC. A sensitive bioimmunoassay for thrombin-cleaved two-chain urokinase-type plasminogen activator in human body fluids. Thromb Haemost 1996;75:908. [PubMed] [Google Scholar]
- 8.Philippe J, Offner F, Declerck PJ, Leroux-Roels G, Vogelaers D, Baele G, Collen D. Fibrinolysis and coagulation in patients with infectious disease and sepsis. Thromb Haemost 1991;65:291. [PubMed] [Google Scholar]
- 9.Biemond BJ, Levi M, TenCate H. Plasminogen activator and plasminogen activator inhibitor release during experimental endotoxemia in chimpanzees: effect of interventions in the cytokine and coagulation cascades. Clin Sci (Lond) 1995;88:587–594. [DOI] [PubMed] [Google Scholar]
- 10.Levi M, Schultz MJ, Rijneveld AW, van der Poll T. Bronchoalveolar coagulation and fibrinolysis in endotoxemia and pneumonia. Crit Care Med 2003;31:S238–S242. [DOI] [PubMed] [Google Scholar]
- 11.Gyetko MR, Sud S, Kendall T, Fuller JA, Newstead MW, Standiford TJ. Urokinase receptor-deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa infection. J Immunol 2000;165:1513–1519. [DOI] [PubMed] [Google Scholar]
- 12.Shetty S, Kumar A, Johnson AR, Pueblitz S, Idell S. Urokinase receptor in malignant mesothelioma cells: role in tumor cell mitogenesis and proteolysis. Am J Physiol 1995;268(6 pt 1):L972–L982. [DOI] [PubMed] [Google Scholar]
- 13.Shetty S. Regulation of urokinase receptor mRNA stability by hnRNPC in lung epithelial cells. Mol Cell Biochem 2005;272:107–118. [DOI] [PubMed] [Google Scholar]
- 14.Sitrin RG, Shollenberger SB, Strieter RM, Gyetko MR. Endogenously produced urokinase amplifies tumor necrosis factor-α secretion by THP-1 mononuclear phagocytes. J Leukoc Biol 1996;59:302–311. [DOI] [PubMed] [Google Scholar]
- 15.Chapman HA. Plasminogen activators, integrins and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol 1997;9:714–724. [DOI] [PubMed] [Google Scholar]
- 16.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 2002;3:932–943. [DOI] [PubMed] [Google Scholar]
- 17.May AE, Kanse SM, Lund LR, Gisler RH, Imho FBA, Preissner KT. Urokinase receptor (CD87) regulates leukocyte recruitment via β2 integrins in vivo. J Exp Med 1998;188:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyetko MR, Chen GH, McDonald RA, Goodman R, Huffnagle GB, Wilkinson CC, Fuller JA, Toews GB. Urokinase is required for the pulmonary inflammatory response to Cryptococcus neoformans: a murine transgenic model. J Clin Invest 1996;97:1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondino A, Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol 2004;25:450–455. [DOI] [PubMed] [Google Scholar]
- 20.Coleman JL, Benach J. The urokinase receptor can be induced by Borrelia burgdorferi through receptors of the innate immune system. Infect Immun 2003;71:5556–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg ME, Belasco JG. Control of the decay of labile protooncogene and cytokine mRNAs. In: Belasco J, Brewerman G, editors. Control of messenger RNA stability. New York: Academic Press; 1993. pp. 199–218.
- 22.Shetty S, Kumar A, Johnson AR, Pueblitz S, Holiday D, Raghu G, Idell S. Differential expression of the urokinase receptor in fibroblasts from normal and fibrotic human lungs. Am J Respir Cell Mol Biol 1996;15:78–87. [DOI] [PubMed] [Google Scholar]
- 23.Shetty S, Kumar A, Idell S. Posttranscriptional regulation of urokinase receptor mRNA: identification of a novel urokinase receptor mRNA binding protein in human mesothelioma cells. Mol Cell Biol 1997;17:1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shetty S, Idell S. Urokinase receptor mRNA stability involves tyrosine phosphorylation in lung epithelial cells. Am J Respir Cell Mol Biol 2004;30:69–75. [DOI] [PubMed] [Google Scholar]
- 25.Shetty S, Muniyappa H, Halady PK, Idell S. Regulation of urokinase receptor expression by phosphoglycerate kinase. Am J Respir Cell Mol Biol 2004;31:100–106. [DOI] [PubMed] [Google Scholar]
- 26.Parsey MV, Tuder R, Abraham E. Neutrophils are major contributors to intraparenchymal lung IL-1β expression after hemorrhage and endotoxemia. J Immunol 1998;160:1007. [PubMed] [Google Scholar]
- 27.Shenkar R, Abraham E. Mechanisms of lung neutrophil activation after hemorrhage or endotoxemia: roles of reactive oxygen intermediates, NF-κB, and CREB. J Immunol 1999;163:954–962. [PubMed] [Google Scholar]
- 28.Shetty S, Idell S. Urokinase induces expression of its own receptor in Beas2B lung epithelial cells. J Biol Chem 2001;276:24549–24556. [DOI] [PubMed] [Google Scholar]
- 29.Shetty S, Velusamy T, Idell S, Tang H, Shetty P. Regulation of urokinase receptor expression by protein tyrosine phosphatases. Am J Physiol Lung Cell Mol Physiol 2007;292:L414–L421. [DOI] [PubMed] [Google Scholar]
- 30.Bortnick AE, Favari E, Tao JQ, Francone OL, Reilly M, Zhang Y, Rothblat GH, Bates SR. Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 2003;285:L869–L878. [DOI] [PubMed] [Google Scholar]
- 31.Warshamana GS, Corti M, Brody AR. TNF-α, PDGF, and TGF-β(1) expression by primary mouse bronchiolar-alveolar epithelial and mesenchymal cells: TNF-α induces TGF-β(1). Exp Mol Pathol 2001;71:13–33. [DOI] [PubMed] [Google Scholar]
- 32.Shetty S, Idell S. A urokinase receptor mRNA binding protein from rabbit lung fibroblasts and mesothelial cells. Am J Physiol Lung Cell Mol Physiol 1998;274:871–882. [DOI] [PubMed] [Google Scholar]
- 33.Lund LR, Ronne E, Roldan AL, Behrendt N, Romer J, Blasi F, Dano K. Urokinase receptor mRNA level and gene transcription are strongly and rapidly increased by phorbol myristate acetate in human monocyte-like U937 cells. J Biol Chem 1991;263:2358–2363. [PubMed] [Google Scholar]
- 34.Lund LR, Ellis V, Ronne E, Pyke C, Dano K. Transcriptional and post-transcriptional regulation of the receptor for urokinase-type plasminogen activator by cytokines and tumour promoters in the human lung carcinoma cell line A549. Biochem J 1995;310:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazar AP, Henkin J, Goldfarb RH. The urokinase plasminogen activator system in cancer: implications for tumor angiogenesis and metastasis. Angiogenesis 1999;3:15–32. [DOI] [PubMed] [Google Scholar]
- 36.Montuori N, Mattiello A, Mancini A, Santoli M, Taglialatela P, Caputi M, Rossi G, Ragno P. Urokinase type plasminogen activator up regulates the expression of its cellular receptor through post transcriptional mechanism. FEBS Lett 2001;508:379–384. [DOI] [PubMed] [Google Scholar]
- 37.Shetty S, Kumar A, Johnson A, Idell S. Regulation of mesothelial cell mitogenesis by antisense oligonucleotides for the urokinase receptor. Antisense Res Dev 1995;5:307–314. [DOI] [PubMed] [Google Scholar]
- 38.Bhat GJ, Gunaje JJ, Idell S. Urokinase-type plasminogen activator induces tyrosine phosphorylation of a 78-kDa protein in H-157 cells. Am J Physiol 1998;277:L301–L309. [DOI] [PubMed] [Google Scholar]
- 39.Idell S, Kumar A, Zwieb C, Holiday D, Koenig KB, Johnson AR. Effects of TGF-β and TNF-α on procoagulant and fibrinolytic pathways of human tracheal epithelial cells. Am J Physiol 1994;267:L693–L703. [DOI] [PubMed] [Google Scholar]
- 40.Xing RH, Mazar A, Henkin J, Rabbani SA. Prevention of breast cancer growth, invasion, and metastasis by anti-estrogen tamoxifen alone or in combination with urokinase inhibitor B-428. Cancer Res 1997;57:3585–3593. [PubMed] [Google Scholar]
- 41.Wang GJ, Collinge M, Blasi F, Pardi R, Bender JR. Human urokinase receptor expression is inhibited by amiloride and induced by tumor necrosis factor and phorbol ester in colon cancer. Proc Natl Acad Sci USA 1998;95:6296–6301.9600959 [Google Scholar]
- 42.Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med 2001;163:762–769. [DOI] [PubMed] [Google Scholar]
- 43.Matute-Bello G, Winn RK, Martin TR, Liles WC. Sustained lipopolysaccharide-induced lung inflammation in mice is attenuated by functional deficiency of the Fas/Fas ligand system. Clin Diagn Lab Immunol 2004;11:358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol 2005;288:L333–L341. [DOI] [PubMed] [Google Scholar]
- 45.Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 2005;175:3369–3376. [DOI] [PubMed] [Google Scholar]
- 46.Gharib SA, Liles WC, Matute-Bello G, Glenny RW, Martin TR. Computational identification of key biological modules and transcription factors in acute lung injury. Am J Respir Crit Care Med 2006;173:653–658. [DOI] [PubMed] [Google Scholar]
- 47.Shetty S, Khalil B, Cines DB, Idell S. Induction of plasminogen activator inhibitor-1 by urokinase in lung epithelial cells. J Biol Chem 2003;278:18124–18131. [DOI] [PubMed] [Google Scholar]
- 48.Lau HK, Ho J. Regulation of plasminogen activator inhibitor-1 secretion by urokinase and tissue plasminogen activator in rat epithelioid type smooth muscle cells. Br J Haematol 2002;117:151–155. [DOI] [PubMed] [Google Scholar]
- 49.Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem 1993;268:25009–25014. [PubMed] [Google Scholar]