Abstract
Rationale: The mechanisms by which oxidants are sensed by cells and cause inflammation are not well understood.
Objectives: This study aimed to determine how cells “sense” soluble oxidants and how this is translated into an inflammatory reaction.
Methods: Monocytes, macrophages, or HEK293 cells (stably transfected with human Toll-like receptor [TLR]2, TLR2/1, TLR2/6, or TLR4/MD2-CD14) were used. CXC ligand-8 (CXCL8) levels were measured using ELISA. Phosphorylated IL-1 receptor–associated kinase 1 levels were measured using Western blot. TLR2−/− and TLR4−/− mice were challenged with oxidants, and inflammation was measured by monitoring cell infiltration and KC levels.
Measurements and Main Results: Oxidants evoked the release of CXCL8 from monocytes/macrophages; this was abrogated by pretreatment with N-acetylcysteine or binding antibodies to TLR2 and was associated with the rapid phosphorylation of IL-1 receptor–associated kinase 1. Oxidants added to HEK293 cells transfected with TLR2, TLR1/2, or TLR2/6 but not TLR4/MD2-CD14 or control HEK nulls resulted in the release of CXCL8. Oxidant challenge delivered intraperitoneally (2–24 hours) or by inhalation to the lungs (3 days) resulted in a robust inflammation in wild-type mice. TLR2−/− mice did not respond to oxidant challenge in either model. TLR4−/− mice responded as wild-type mice to oxidants at 2 hours but as TLR2−/− mice at later time points.
Conclusions: Oxidant–TLR2 interactions provide a signal that initiates the inflammatory response.
Keywords: Toll-like receptors, inflammation, cigarette smoke, oxidants
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Oxidants play an important role in inflammation and pathology; however, there has been little evidence of how oxidants are sensed and propagate inflammation.
What This Study Adds to the Field
This study shows that exogenous oxidants are sensed by the Toll-like receptor 2, initiating inflammatory processes in the lungs.
It is widely appreciated that pattern recognition receptors are an integral part of the innate immune system (1). This family of receptors includes Toll-like receptors (TLRs), the nuclear-binding oligomerization domain, and retinoic acid–inducible genes (2). The most extensively studied of these are the TLRs. TLRs are firmly established as pathogen receptors; however, evidence suggests they can also sense nonpathogen stimuli, such as hyaluronan degradation products, nutritional lipids, and heat shock proteins, and thereby mediate sterile inflammation (3–5). The emergence of a role for nonpathogen-associated sensing by TLRs has expanded their repertoire, with the consequence that TLRs can be considered as innate immune surveillance receptors for danger signals independent of infection.
Although there is no doubt that oxidants play a part in the resultant damage caused by inflammation, there is considerable debate about how oxidants are sensed and propagate the inflammatory reaction. This is important to elucidate because the inflammation that occurs within a number of life-threatening diseases is associated with a direct increase in oxidant stress (6–9). This point is supported by data from a number of clinical trials demonstrating that antioxidant therapy shows benefits in conditions associated with cardiovascular disease, ageing, and diabetes (10–17). Possibly the most obvious and direct clinical manifestation of oxidant-induced inflammation and disease is that associated with smoking cigarettes. Indeed, the activation of inflammatory responses in vitro by cigarette smoke is entirely mediated by oxidant stress (18). Inflammation induced by oxidant stress has many of the features associated with classical activation of the innate immune system and, as such, can resemble that seen after TLR activation with lipopolysaccharide (LPS), for example. In the current study, we investigated the role of TLRs in the sensing of oxidants by cells and tissues in vitro. Of the genes induced by cigarette smoke and oxidants, CXCL8 is among the most commonly studied (18–25). Moreover, CXCL8 is an established feature of smoking-related inflammation in humans and as such is clinically relevant. We have therefore used CXCL8 as a biomarker of cell activation in this report. We used genetically modified mice to establish the role of TLRs in the sensing of oxidants and the resultant inflammation in vivo in two distinct models, one where oxidants were administered directly into the peritoneal cavity and acute inflammation (2–24 h) monitored (peritonitis model) and a second model where mice inhaled smoke for 3 days (smoke inhalation model).
METHODS
Cell Culture
THP-1 human monocytes, purchased from the European Collection of Cell Culture (Wiltshire, UK), were cultured as previously described (18). HEK 293 cells were stably transfected with pUNO-mcs (Null control), pUNO-hTLR2, pDUO-hTLR1/2, or pDUO-hTLR2/6 pDUO-hTLR4/MD2-CD14 (InvivoGen, San Diego, CA) and maintained according to the manufacturer's instructions. Human peripheral blood mononuclear cells (70% lymphocytes, 30% monocytes, determined by histologic analysis) were isolated from a fresh citrated blood (P/00/062, Royal Brompton Hospital ethics committee) using a Ficoll gradient as previously described (26). Isolated monocytes (70%) were differentiated into macrophages as previously described (18).
Treatment of Cells
Cell treatments were performed as described in the Results section and figure legends. Cigarette smoke extract (CSE) was prepared as previously described (18). In some studies, cells were pretreated for 30 minutes with a mouse monoclonal neutralizing antibody to TLR2 (Clone TLR2.1) or mouse IgG2a isotype control (eBioscience, San Diego, CA) or for 2 hours with N-acetylcysteine.
Assessment of Cell Respiration by MTT Assay
Cell viability was determined using an MTT assay (Sigma, Poole, UK) as previously described (27). None of the treatments described affected cell viability significantly.
Western Blotting
IRAK1 and phosphor-IRAK1 immunoreactivities were measured by Western blot as previously described (28) using specific polyclonal rabbit antibodies (Cell Signaling Technology, Danvers, MA), both at 1:1,000.
ELISA
CXCL8 and KC were measured by ELISA in biological samples according to the manufacturer's instructions (R&D Systems, Oxford, UK).
Measurement of Intracellular Oxidant Stress
Intracellular oxidative stress was measured by oxidative-conversion of dihydrorhodamine 123 to rhodamine 123 (29). Cells were preloaded with dihydrorhodamine 123 (50 μM), and the conversion was monitored by measuring fluorescence at excitation and emission wavelengths of 480 and 536 nm after 30 minutes (LS-5; Perkin-Elmer Co., Norwalk, CT).
Transcription Factor Assay
Phospho-c-Jun (AP-1) levels were measured using a commercially available ELISA (ActiveMotif, Rixensart, Belgium).
Mice and Oxidant-induced Inflammation
TLR2−/− and TLR4−/− mice were backcrossed onto a C57Bl/6J background for nine generations (30). Animal work was performed according to Home Office regulations (Scientific Procedures Act, 1986). Two models of oxidant-induced inflammation were used.
Peritonitis model.
H2O2 (10 mM), CSE (10%), LPS (20 mg/kg), or Pam3CSK4 (2 mg/kg), all diluted in sterile phosphate-buffered saline, were injected intraperitoneally in a volume of 0.5 ml. Peritoneal washes were performed 2, 4, 6, or 24 hours after the onset of inflammation as previously described (31).
Inhaled smoke model.
Mice were placed in a plexiglass chamber (volume of 17 liters) covered by a disposable filter as previously described (32). The smoke produced by cigarette burning was introduced at a rate of 25 ml/min into the chamber with the continuous airflow generated by a mechanical ventilator (Heidolph PD 5101), with no influence on the chamber temperature (< 0.1°C variation). The animals received smoke of two cigarettes (Marlboro Red; Philip Morris USA, Richmond, VA) (filter removed) per exposure, two exposures a day during 3 days. Sixteen hours after exposure, bronchoalveolar lavage (BAL) was performed, cells were counted, and samples were stored for future analysis.
Statistical Analysis
Results are expressed as means ± SEM. For in vitro experiments, each n value represents the mean of one or more wells taken from a single experiment. For in vivo experiments, each n value represents data from an individual animal. The analyses were performed as described in the individual figure legends, and significance levels from control values were assessed using 95% confidence levels.
RESULTS
Oxidant Challenge Is Perceived by Human Monocytic Cells (THP-1) and Results in the Release of CXCL8
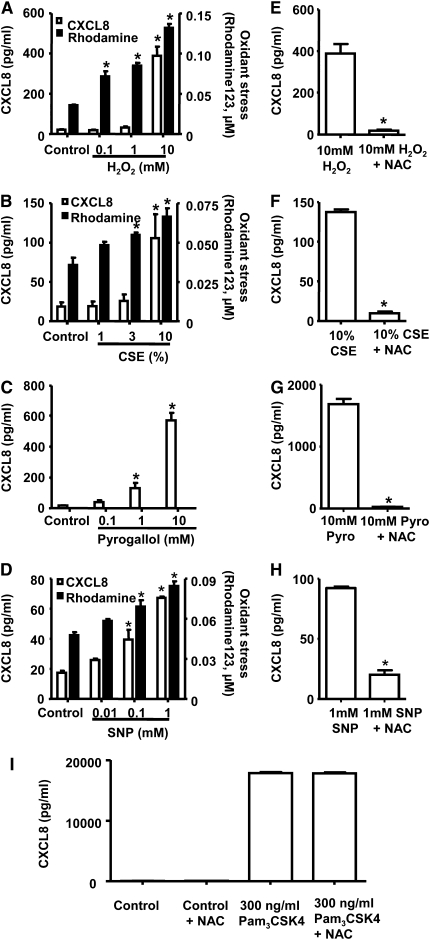
Under controlled culture conditions, THP-1 monocytes released low or undetectable levels of CXCL8. Stimulation with oxidants, including H2O2 (Figure 1A), CSE (Figure 1B), pyrogallol (Figure 1C), or sodium nitroprusside (SNP) (Figure 1D), or with the TLR2/1 PAMP Pam3CSK4 (Figure 1I) for 24 hours led to the release of CXCL8 from THP-1 cells. The presence of extracellular oxidants resulted in a transfer of oxidant tone to the intracellular environment (Figures 1A, 1B, and 1D). It was not possible to show oxidant transfer for pyrogallol because this compound interfered with the fluorescence generated by rhodamine 123. The ability of oxidants (H2O2, CSE, pyrogallol, or SNP) to induce CXCL8 release was blocked by pretreatment of cells with the antioxidant N-acetylcysteine (Figures 1E–1H). By contrast, pretreatment of cells with N-acetylcysteine had no effect on the ability of Pam3CSK4 to increase CXCL8 levels (Figure 1I).
Figure 1.
Oxidant challenge is perceived by THP-1 cells and results in the release of CXCL-8. CXCL-8 levels (after 24 hours) and the conversion of dihydrorhodamine 123 to rhodamine 123 (after 0.5 hours) were measured in THP-1 cells after challenge with increasing concentrations of (A) hydrogen peroxide (H2O2; n = 3), (B) cigarette smoke extract (CSE; n = 3), (C) pyrogallol (n = 3; pyrogallol interferes with the fluorescence generated by rhodamine 123; therefore, no reading is shown) and (D) sodium nitroprusside (SNP) (n = 6). In a separate set of experiments, THP-1 cells were pretreated with N-acetylcyctine (NAC) for 2 hours before challenge with (E) 10 mM H2O2 (n = 3), (F) 10% CSE (n = 3), (G) 10 mM pyrogallol (Pyro; n = 3), and (H) 1 mM SNP (n = 3). (I) Control cells and 300 ng/ml Pam3CSK4. CXCL-8 levels were measured 24 hours after stimulation. For E through H, n = 3 replicates from a representative experiment are shown. Similar results were seen when this protocol was performed on a second, separate occasion. In A through D, *P < 0.05 compared with control values (media alone) using ANOVA followed by a Bonferroni post-test. In E through I, a t test was used to compare cells stimulated with inflammogen and in the presence of NAC.
Oxidant Challenge Induces Inflammatory Cell Recruitment In Vivo via TLR-dependent Mechanisms
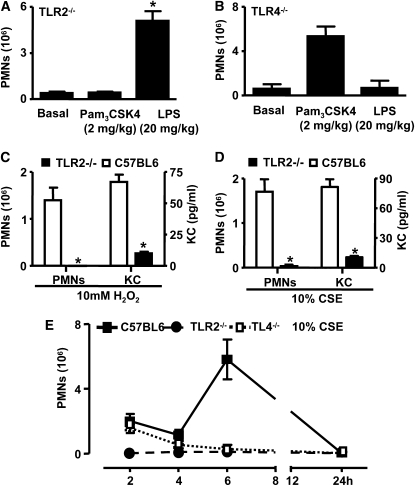
In wild-type mice, injection of the TLR2 ligand Pam3CSK4 or the TLR4 ligand LPS into the peritoneal cavity induced a robust inflammatory response characterized by the influx of polymorphonuclear cells (PMNs) (data not shown). In TLR2−/− mice, Pam3CSK4 was inactive, but LPS induced inflammatory responses comparable to those observed in wild-type mice (Figure 2A). Conversely, in TLR4−/− mice, LPS was inactive, whereas Pam3CSK4 evoked an inflammatory response (Figure 2B). In wild-type mice, intraperitoneal injection of an oxidant challenge (CSE) induced two clear phases of inflammation, which had resolved by 24 hours. There was an initial early phase at 2 hours characterized by the influx of inflammatory cells, predominantly polymorphonuclear cells (PMNs) (75–80% of total cells; Figures 2C–2E) and KC (Figures 2D and 2E), followed by a later phase seen at 6 hours (Figure 2C). The basal levels of PMNs and KC in naive C57BL6 mice were 1.3 ± 0.9 × 106 cells and 5.53 ± 0.75 pg/ml, respectively. The basal levels of PMNs and KC in naive TLR2−/− mice were 0 ± 0 × 106 cells and 7.53 ± 1.56 pg/ml, respectively, with no statistical difference noted between C57BL6 and TLR2−/− mice for PMNs or KC levels. Oxidant-induced peritonitis in vivo had an absolute requirement for TLR2 because responses were absent in TLR2−/− mice (Figures 2C–2E). In contrast to the results seen in TLR2−/− mice, oxidant-induced inflammation seen at 2 hours was intact in TLR4−/− mice. However, the second phase of inflammation, which peaked at 6 hours, was dependent upon functional TLR4 receptors (Figure 2C).
Figure 2.
Impaired inflammatory cell recruitment into the peritoneal cavity (peritonitis) after oxidant challenge in the absence of TLR2 and TLR4. (A) TLR2−/− mice (n = 8) were injected intraperitoneally with phosphate-buffered saline (PBS) (basal levels), Pam3CSK4 (2 mg/kg), and lipopolysaccharide (LPS) (20 mg/kg). Polymorphonuclear cells (PMNs) recruited into the peritoneal cavity were counted 4 hours after administration. (B) TLR4−/− mice (n = 8) were injected intraperitoneally with PBS (basal levels), Pam3CSK4 (2 mg/kg), and LPS (20 mg/kg). PMNs recruited into the peritoneal cavity were counted 4 hours after administration. (C) Wild-type and TLR2−/− mice (n = 6) were injected with 10 mM H2O2. The total number of PMNs was counted, and KC levels were measured in peritoneal lavage fluid 2 hours after induction of inflammation. (D) Wild-type and TLR2−/− mice (n = 9) were injected with 10% (v/v) cigarette smoke extract (CSE). Total numbers of PMNs were counted, and KC levels were measured in peritoneal lavage fluid 2 hours after induction of inflammation. (A–D) *P < 0.05 represents the changes in cell numbers and KC levels compared with wild-type controls using an unpaired nonparametric t test (Mann-Whitney U-Test). Basal levels of PMNs and KC in naive C57BL6 mice were 1.3 ± 0.9 × 106 cells and 5.53 ± 0.75 pg/ml, respectively. Basal levels of PMNs and KC in naive TLR2−/− mice were 0 ± 0 × 106 cells and 7.53 ± 1.56 pg/ml, respectively, with no statistical difference noted between C57BL6 and TLR2−/− mice for PMNs or KC levels. (E) Wild-type, TLR2−/−, and TLR4−/− mice were injected with 10% CSE, and PMNs were recruited into the peritoneal cavity was performed over 24 hours. Data are the mean ± SEM of n = 6 animals. *P < 0.05 represents the changes in cell numbers compared with wild-type controls using ANOVA.
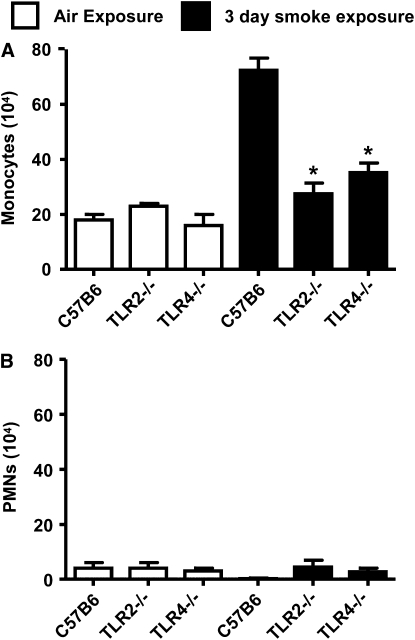
To relate our data obtained using a simple peritonitis model to responses in the lung, where smoke/oxidant-induced inflammation has been studied previously, we performed additional experiments using a standard 3-day smoking model in mice (32). Basal levels of cells in the BAL were similar in all mice tested; C57BL6 was 22 ± 1 × 104 cells, TLR2−/− was 27 ± 1 × 104 cells, and TLR4−/− was 19 ± 5 × 104 cells. Exposure of wild-type mice to cigarette smoke for 3 days produced a robust inflammatory response characterized by increased numbers of monocytes and a smaller number of neutrophils in the BAL (Figure 3A). PMN levels were not increased by smoke challenge in the 3-day inhaled smoke model (Figure 3B). Similar to the peritonitis model, cell recruitment induced by inhaled smoke was ablated in TLR2−/− mice and greatly reduced in the TLR4−/− mice (Figure 3).
Figure 3.
Role of TLR2 and TLR4 in a 3-day smoke-induced model of inflammation. Exposure of wild-type mice to cigarette smoke inhaled twice a day for 3 days resulted in a robust increase in monocytes (A) but not polymorphonuclear cells (B) present in bronchoalveolar lavage. By contrast, monocyte levels were not increased in TLR2−/− or TLR4−/− mice exposed to smoke. The data are the mean ± SEM of n = 5 animals. *P < 0.05 represents the changes in cell numbers and KC levels compared with wild-type controls using an unpaired nonparametric t test (Mann-Whitney U-test). CSE = cigarette smoke extract; LPS = lipopolysaccharide.
Activation of Cells In Vitro by Oxidants Occurs via TLR2-dependent Mechanisms
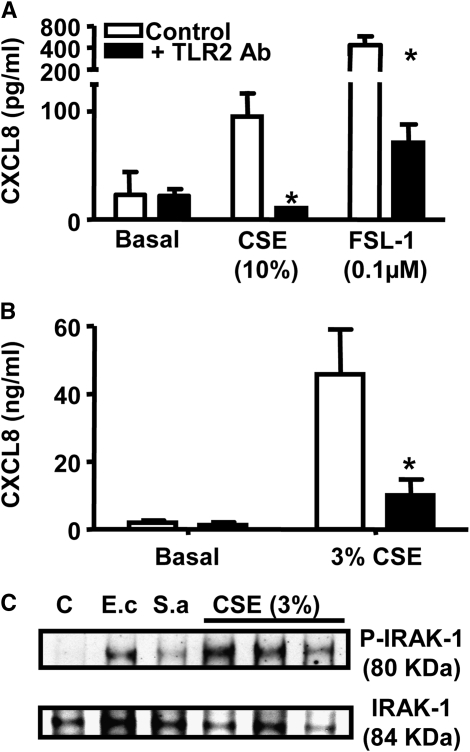
Activation of THP-1 monocytes by oxidant stress induced by CSE or by a “classical” TLR2 ligand, FSL-1, was strongly inhibited by pretreatment with a blocking antibody specific for TLR2 (Figure 4A). Similar to observations made in the THP-1 monocytic cell line, primary cultures of human monocytes were activated by oxidant stress to release CXCL8, which was inhibited by TLR2-specific blocking antibodies (Figure 4B). IRAK1 is the first kinase activated in MyD88-dependent signaling through activation of the TLR2. In human primary monocytes under control culture conditions, IRAK1 was present in the unphosphorylated resting state. However, consistent with the hypothesis that oxidant stress activates cells via TLR2, IRAK1 was phosphorylated in cells treated with CSE for 15 minutes (Figure 4C). As expected, and by way of a control, IRAK1 was phosphorylated in cells stimulated with Escherichia coli or Staphylococcus aureus, which activated TLRs via well characterized PAMPs. To confirm our finding in primary human macrophages and THP-1 cells and to validate whether TLR2 was essential for the sensing of oxidants, we repeated some experiments in HEK 293 cells that contain only specific TLR receptors to exclude the possibility of other direct receptor involvement.
Figure 4.
Oxidant-induced CXCL8 release from human macrophages is via a TLR2-dependent mechanism. (A) THP-1 cells (n = 4) were preincubated with a neutralizing antibody to TLR2 (25 μg/ml) or IgG2a isotype control (25 μg/ml) for 30 minutes before stimulation with 10% cigarette smoke extract (CSE) and the TLR2 ligand FSL-1 (0.1 μM) or (B) blood-derived primary macrophages (i = 3 individual donors) were preincubated with a neutralizing antibody to TLR2 (25 μg/ml) or IgG2a isotype control (25 μg/ml) for 30 minutes before stimulation with 3% CSE. Cells were incubated for 24 hours, and CXCL8 levels were measured by ELISA. *P < 0.05 comparing IgG2a with TLR2 antibody by unpaired t test. (C) Blood-derived primary macrophages were treated for 15 minutes with Escherichia coli (E.c; 108 CFU/ml), Staphylococcus aureus (S.a; 107 CFU/ml), and 3% CSE from three individual donors, cells were extracted in the presence of a phosphatase inhibitor and samples Western blotted for native and phosphorylated forms of IL-1 receptor activated kinase (IRAK1).
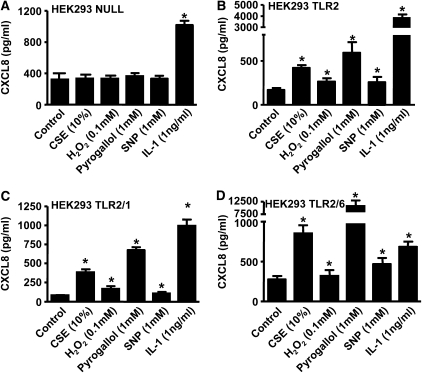
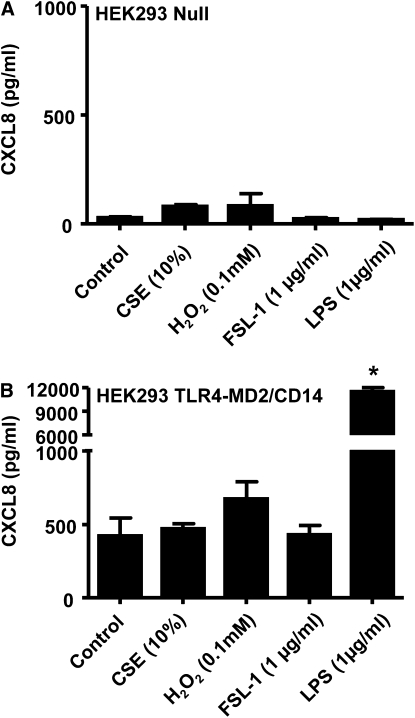
HEK 293 cells do not express TLRs under normal culture conditions. When HEK 293 cells transfected with the control vector (pUNO-mcs vector) were stimulated with our panel of oxidants, the activated cells did not lead to release of CXCL8 (Figure 5A). However, HEK 293 cells responded appropriately to the non-TLR agonist of the MyD88 pathway, IL-1β, by releasing increased levels of CXCL8, showing that the machinery required for TLR signaling was present in these cells (Figure 5A). By contrast, HEK 293 cells transfected to express TLR2, TLR2 and TLR1, or TLR2 and TLR6 sensed oxidant stimulation and released increased levels of CXCL8 (Figures 5B–5D). Oxidant challenge of HEK 293 cells transfected with the TLR4-MD2/CD14 system resulted in no significant elevation in CXCL8 levels; however, these cells produced significant quantities of CXCL8 when stimulated with an authentic TLR4 ligand (LPS). This shows that TLR4, unlike TLR2, is not directly involved in the sensing of oxidants (Figure 6).
Figure 5.
TLR2 is essential for oxidants' activation of CXCL8. CXCL8 levels were measured 24 hours after stimulation with cigarette smoke extract (CSE) (10%), H2O2 (0.1 mM), pyrogallol (1 mM), sodium nitroprusside (SNP) (1 mM), and IL-1β (1 ng/ml) in (A) HEK 293 Null cells (n = 6) stably transfected with the pUNO-mcs vector, (B) HEK 293 TLR2 cells (n = 3) stably transfected with the human TLR2 gene, (C) HEK 293 TLR2/1 (n = 3) cells stably transfected with the human TLR2 and human TLR1 gene, and (D) HEK 293 TLR2/6 cells (n = 3) stably transfected with the human TLR2 and human TLR6 gene. *P < 0.05 compared with control values (media alone) using ANOVA followed by a Bonferroni post-test.
Figure 6.
TLR4 is not required for the initial sensing of oxidants. CXCL8 levels were measured 24 hours after stimulation with cigarette smoke extract (CSE) (10%), H2O2 (0.1 mM), FSL-1 (1 μg/ml), and lipopolysaccharide (LPS) (1 μg/ml) in (A) HEK 293 Null cells (n = 3) stably transfected with the pUNO-mcs vector and (B) HEK 293 TLR4-MD2/CD14 cells (n = 3) stably transfected with the human TLR4, MD2, and CD14 genes. *P < 0.05 compared with control values (media alone) using ANOVA followed by a Bonferroni post-test.
Characterization of the TLR2-independent Sensing of Oxidant Stress by Cells
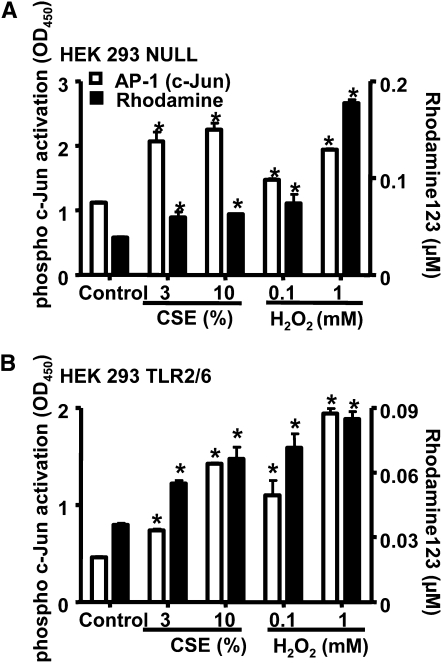
By contrast to CXCL8 release, the transfer of oxidant tone from the extracellular environment to the intracellular environment in HEK 293 cells occurred independently of TLR2 (Figure 7). In addition, activation of the redox-sensitive transcription factor AP-1, in particular c-Jun, was independent of TLR2 (Figure 7).
Figure 7.
Transfer of extracellular oxidants to the intracellular compartment and the subsequent activation of AP-1 is TLR2 independent. Phospho-c-Jun activation (after 2 hours) and the conversion of dihydrorhodamine 123 to rhodamine 123 (after 0.5 hours) were measured after stimulation with cigarette smoke extract (CSE) (3 and 10%) and H2O2 (0.1 and 1 mM) in (A) HEK 293 Null cells (n = 3–6) stably transfected with the pUNO-mcs vector and (B) HEK 293 TLR2/6 cells (n = 3–6) stably transfected with the human TLR2 and human TLR6 gene. *P < 0.05 compared with control values (media alone) using ANOVA followed by a Bonferroni post-test.
DISCUSSION
In the present study, we report that TLR2 is involved in the sensing of oxidants in vitro and in vivo. Using a range of in vitro approaches, we demonstrate that TLR2 alone or in association with TLR1 or TLR6 is required for the induction of CXCL8 release by oxidants. Furthermore, TLR2 was required for oxidant-induced inflammation in vivo. TLR4 also plays a critical role in the inflammation induced by oxidants in vivo, but not in the initial sensing of these inflammogens.
It is well accepted that oxidants cause inflammation. In the current study, we show how a range of oxidants, at subtoxic concentrations, stimulate human monocytic cells to release the neutrophil chemoattractant CXCL8. Oxidant-induced CXCL8 release was blocked by antioxidants and associated with a mild but significant transfer of oxidant stress from the outside to the inside of the cells. Using cell lines or primary cultures, we found that neutralizing antibodies to TLR2 blocked CXCL8 release after oxidant challenge. Similar results have been noted in cardiac myocytes (33). Furthermore, we found that IRAK1 was phosphorylated (an early transduction event in TLR signaling) in cells after stimulation of TLRs with bacteria or oxidants.
Intraperitoneal administration of oxidants, in the form of CSE or H2O2, induced mild but detectable inflammation in vivo that resembled a classic acute response similar to that observed with other TLR2 (zymosan) or TLR4 (LPS) ligands (34). We observed two clear phases of inflammation induced by oxidants. There was an initial early phase at 2 hours followed by a later phase at 6 hours after oxidant challenge. Oxidant-induced inflammation in vivo had an absolute requirement for TLR2 because responses were absent in TLR2−/− mice. As expected, however, TLR2−/− mice responded as wild–type mice when the LPS was administered instead of oxidants. To relate our data obtained using a simple peritonitis model to responses in the lung, where smoke/oxidant-induced inflammation has been studied previously, we performed additional experiments using a 3 day inhaled smoke model as previously described by Doz and colleagues (32). Doz and colleagues used 1R3 Kentucky Research cigarettes; however, because we used Marlborough red for the in vitro studies, we used the latter cigarettes for the 3-day inhaled smoke model. We found that 3 days after inhalation of smoke, inflammation was induced, characterized by predominantly mononuclear cells in the BAL. Consistent with the results obtained in the peritonitis model, inflammatory cell recruitment associated with inhaled cigarette smoke showed an absolute requirement for TLR2. This is in line with a number of recent publications in which mice deficient in a functional TLR2 receptor were protected against pathology associated with “sterile” inflammation, including atherosclerosis, postischemic coronary endothelial dysfunction, and inflammatory bowel disease (35–37), each of which are associated with an increase in oxidative load. These observations are consistent with our data showing that TLR2 is a receptor that senses exogenous oxidants. When this model was used by Doz and colleagues, the inflammation induced by 1R3 Kentucky Research cigarettes was typified by neutrophils rather than monocytes.
A number of publications have implicated TLR4 in oxidant-associated inflammation (38–42). In the current study, we found that, by contrast to results seen with TLR2, TLR4 was not required for the very early phase of inflammation induced by oxidants in the peritonitis model. This was confirmed in a cell-based assay, in which HEK 293 cells transfected with an active TLR4 receptor system and lacking TLR2 showed no direct response to oxidant stimulation with respect to CXCL8. However, inflammation seen in our in vivo models at the 6-hour time point or later depended on a functional TLR4 receptor. These observations show clearly that TLR2, and not TLR4, is needed for the initial sensing of oxidant stress in vivo. In line with our observations at the later time points using the peritonitis model, we found that TLR4 was also required for the inflammation we saw in the 3-day smoke-inhalation model. The apparent role of TLR4 in oxidant-induced inflammation in our model occurring once the initial insult is sensed may be mediated by bacterial components released from the gut (“leaky gut syndrome”) or airways (“leaky lung syndrome”) as a result of local damage (43).
From our in vivo data, it was clear that TLR2, but not TLR4, was essential for the initial early sensing of oxidant insult. We therefore performed additional experiments to better understand the specific role of TLR2 in the cellular sensing of oxidants using specific TLR-expressing cell lines (HEK 293). Using these, we show that oxidant-induced CXCL8 release has an absolute requirement for a functional TLR2 alone or as a heterodimeric complex with TLR1 or TLR6. Although we observed a positive effect of oxidants on CXCL8 release by cells expressing only TLR2, the presence of TLR6 seemed to facilitate the sensing of oxidant-induced cell activation. In control cells that express no TLRs, oxidants did not induce the release of CXCL8. However, control cells did respond by releasing CXCL8 when stimulated with IL-1β, which acts independently of TLRs but via the same adapter protein pathway (MyD88).
Although TLR2 was required for the activation of cells by oxidants to release CXCL8, the absence of TLR2 did not seem to inhibit the cells' ability to transfer an external oxidant stress to the inside of the cell. Thus, TLR2 does not seem to act as an oxidant transducer. In previous studies we (18) and others (44) have shown that oxidant stress induces activation of AP-1. In the current study, we have confirmed these observations, showing that oxidants activate AP-1 in HEK 293 cells. However, in our current study we found that the activation of AP-1 by oxidants occurred independently of TLR2 and CXCL8 release. This suggests that for some genes, oxidants may induce cell activation in some settings (via AP-1 activation) independently of TLR2. The consequence of TLR2-independent AP-1 activation by oxidants requires further investigation. However, our data with CXCL8 are representative of the in vivo situation represented by the two models used in this study.
In conclusion, we show that oxidants activate cells in vitro and in vivo via a TLR2-dependent pathway. TLR4 receptors become critically important after the initial sensing of the oxidant stress and once inflammation has begun. The precise mechanism by which oxidants mediate these effects is not clear but could be due to oxidation of the extracellular portion of the receptor, causing a conformational change, as is seen in other proteins (45, 46). In human disease, oxidant stress is central to inflammation. In the current study, we clearly identify TLR2 as an important sensing receptor for oxidants in the peritoneum and the lungs.
Acknowledgments
The authors thank Emma M. Watson for proofreading this document.
Supported by the British Heart Foundation and British Lung Foundation, UK (M.J.P.-C.) and by the Medical Research Council, UK (S.S.).
Originally Published in Press as DOI: 10.1164/rccm.200707-1019OC on November 14, 2008
Conflict of Interest Statement: M.J.P.-C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.K.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.K.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.F.Q. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.M. has been paid a fee of $1,000 for presenting a seminar at Novartis UK in the area of TLR biology in October 2008. She is in receipt of a research contract/grant funded by Roche (Switzerland) to investigate the mechanisms of antibody-induced cytokine storm; the award is for approximately $750,000 over 3 years beginning in December 2008.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 2.Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol 2006;27:352–357. [DOI] [PubMed] [Google Scholar]
- 3.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant 2006;6:2622–2635. [DOI] [PubMed] [Google Scholar]
- 5.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol 2004;76:514–519. [DOI] [PubMed] [Google Scholar]
- 6.Chan PH. Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann N Y Acad Sci 2005;1042:203–209. [DOI] [PubMed] [Google Scholar]
- 7.Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, Croft KD, Mori TA, Tanous D, Adams MR, et al. Antioxidants protect from atherosclerosis by a HEME oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med 2006;203:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkham P, Rahman I. 2006. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther 2006;111:476–494. [DOI] [PubMed] [Google Scholar]
- 9.Murakami A, Ohigashi H. Cancer-preventive anti-oxidants that attenuate free radical generation by inflammatory cells. Biol Chem 2006;387:387–392. [DOI] [PubMed] [Google Scholar]
- 10.Enstrom JE, Kanim LE, Klein MA. Vitamin C intake and mortality among a sample of the United States population. Epidemiology 1992;3:194–202. [DOI] [PubMed] [Google Scholar]
- 11.Klipstein-Grobusch K, Geleijnse JM, den Breeijen JH, Boeing H, Hofman A, Grobbee DE, Witteman JC. Dietary antioxidants and risk of myocardial infarction in the elderly: the Rotterdam Study. Am J Clin Nutr 1999;69:261–266. [DOI] [PubMed] [Google Scholar]
- 12.Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol 1994;139:1180–1189. [DOI] [PubMed] [Google Scholar]
- 13.Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med 1996;334:1156–1162. [DOI] [PubMed] [Google Scholar]
- 14.Losonczy KG, Harris TB, Havlik RJ. Vitamin E and vitamin C supplement use and risk of all-cause and coronary heart disease mortality in older persons: the Established Populations for Epidemiologic Studies of the Elderly. Am J Clin Nutr 1996;64:190–196. [DOI] [PubMed] [Google Scholar]
- 15.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 1993;328:1450–1456. [DOI] [PubMed] [Google Scholar]
- 16.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med 1993;328:1444–1449. [DOI] [PubMed] [Google Scholar]
- 17.Todd S, Woodward M, Tunstall-Pedoe H, Bolton-Smith C. Dietary antioxidant vitamins and fiber in the etiology of cardiovascular disease and all-causes mortality: results from the Scottish Heart Health Study. Am J Epidemiol 1999;150:1073–1080. [DOI] [PubMed] [Google Scholar]
- 18.Walters MJ, Paul-Clark MJ, McMaster SK, Ito K, Adcock IM, Mitchell JA. Cigarette smoke activates human monocytes by an oxidant-AP-1 signaling pathway: implications for steroid resistance. Mol Pharmacol 2005;68:1343–1353. [DOI] [PubMed] [Google Scholar]
- 19.Mio T, Romberger DJ, Thompson AB, Robbins RA, Heires A, Rennard SI. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am J Respir Crit Care Med 1997;155:1770–1776. [DOI] [PubMed] [Google Scholar]
- 20.Numanami H, Koyama S, Sato E, Haniuda M, Nelson DK, Hoyt JC, Freels JL, Habib MP, Robbins RA. Serine protease inhibitors modulate chemotactic cytokine production by human lung fibroblasts in vitro. Am J Physiol Lung Cell Mol Physiol 2003;284:L882–L890. [DOI] [PubMed] [Google Scholar]
- 21.Robbins RA, Nelson KJ, Gossman GL, Koyama S, Rennard SI. Complement activation by cigarette smoke. Am J Physiol 1991;260:L254–L259. [DOI] [PubMed] [Google Scholar]
- 22.Russell RE, Culpitt SV, DeMatos C, Donnelly L, Smith M, Wiggins J, Barnes PJ. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2002;26:602–609. [DOI] [PubMed] [Google Scholar]
- 23.Birrell MA, Wong S, Catley MC, Belvisi MG. Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages. J Cell Physiol 2008;214:27–37. [DOI] [PubMed] [Google Scholar]
- 24.van der Vaart H, Postma DS, Timens W, ten Hacken NH. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax 2004;59:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metinko AP, Kunkel SL, Standiford TJ, Strieter RM. Anoxia-hyperoxia induces monocyte-derived interleukin-8. J Clin Invest 1992;90:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perretti M, Wheller SK, Flower RJ, Wahid S, Pitzalis C. Modulation of cellular annexin I in human leukocytes infiltrating DTH skin reactions. J Leukoc Biol 1999;65:583–589. [DOI] [PubMed] [Google Scholar]
- 27.Paul-Clark MJ, McMaster SK, Belcher E, Sorrentino R, Anandarajah J, Fleet M, Sriskandan S, Mitchell JA. Differential effects of Gram-positive versus Gram-negative bacteria on NOSII and TNFalpha in macrophages: role of TLRs in synergy between the two. Br J Pharmacol 2006;148:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul-Clark MJ, Gilroy DW, Willis D, Willoughby DA, Tomlinson A. Nitric oxide synthase inhibitors have opposite effects on acute inflammation depending on their route of administration. J Immunol 2001;166:1169–1177. [DOI] [PubMed] [Google Scholar]
- 29.Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med 1994;16:149–156. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez R, Belcher E, Sriskandan S, Lucas R, McMaster S, Vojnovic I, Warner TD, Mitchell JA. Role of Toll-like receptors 2 and 4 in the induction of cyclooxygenase-2 in vascular smooth muscle. Proc Natl Acad Sci USA 2005;102:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul-Clark M, Del Soldato P, Fiorucci S, Flower RJ, Perretti M. 21-NO-prednisolone is a novel nitric oxide-releasing derivative of prednisolone with enhanced anti-inflammatory properties. Br J Pharmacol 2000;131:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol 2008;180:1169–1178. [DOI] [PubMed] [Google Scholar]
- 33.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem 2001;276:5197–5203. [DOI] [PubMed] [Google Scholar]
- 34.Ajuebor MN, Das AM, Virag L, Flower RJ, Szabo C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol 1999;162:1685–1691. [PubMed] [Google Scholar]
- 35.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007;132:1359–1374. [DOI] [PubMed] [Google Scholar]
- 36.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest 2005;115:3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakata Y, Dong JW, Vallejo JG, Huang CH, Baker JS, Tracey KJ, Tacheuchi O, Akira S, Mann DL. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2007;292:H503–H509. [DOI] [PubMed] [Google Scholar]
- 38.Karimi K, Sarir H, Mortaz E, Smit JJ, Hosseini H, De Kimpe SJ, Nijkamp FP, Folkerts G. Toll-like receptor-4 mediates cigarette smoke-induced cytokine production by human macrophages. Respir Res 2006;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powers KA, Szaszi K, Khadaroo RG, Tawadros PS, Marshall JC, Kapus A, Rotstein OD. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med 2006;203:1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, Hong JS, Block ML. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia 2005;52:78–84. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest 2006;116:3050–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Shan P, Qureshi S, Homer R, Medzhitov R, Noble PW, Lee PJ. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol 2005;175:4834–4838. [DOI] [PubMed] [Google Scholar]
- 43.Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology 2005;128:1258–1267. [DOI] [PubMed] [Google Scholar]
- 44.Gensch E, Gallup M, Sucher A, Li D, Gebremichael A, Lemjabbar H, Mengistab A, Dasari V, Hotchkiss J, Harkema J, et al. Tobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNK. J Biol Chem 2004;279:39085–39093. [DOI] [PubMed] [Google Scholar]
- 45.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med 2002;32:797–803. [DOI] [PubMed] [Google Scholar]
- 46.Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med 2002;33:562–571. [DOI] [PubMed] [Google Scholar]