Abstract
Liquid chromatography-mass spectrometry (LC-MS) is now a routine technique with the development of electrospray ionisation (ESI) providing a simple and robust interface. It can be applied to a wide range of biological molecules and the use of tandem MS and stable isotope internal standards allows highly sensitive and accurate assays to be developed although some method optimisation is required to minimise ion suppression effects. Fast scanning speeds allow a high degree of multiplexing and many compounds can be measured in a single analytical run. With the development of more affordable and reliable instruments, LC-MS is starting to play an important role in several areas of clinical biochemistry and compete with conventional liquid chromatography and other techniques such as immunoassay.
Introduction
Coupling of MS to chromatographic techniques has always been desirable due to the sensitive and highly specific nature of MS compared to other chromatographic detectors. The coupling of gas chromatography to MS (GC-MS) was achieved in the 1950s with commercial instruments available from the 1970s. Relatively cheap and reliable GC-MS systems are now a feature of many clinical biochemistry laboratories and are indispensable in several areas where the analysis of complex mixtures and unambiguous identification is required e.g. screening urine samples for inborn errors of metabolism or drugs. The coupling of MS with LC (LC-MS) was an obvious extension but progress in this area was limited for many years due to the relative incompatibility of existing MS ion sources with a continuous liquid stream. Several interfaces were developed but they were cumbersome to use and unreliable, so uptake by clinical laboratories was very limited. This situation changed with the development of the electrospray ion source by Fenn in the 1980s.1 Manufacturers rapidly developed instruments equipped with electrospray sources, which had a great impact on protein and peptide biochemistry. Fenn was awarded the Nobel Prize in 2002 with Koichi Tanaka who developed matrix assisted laser desorption ionisation, another extremely useful MS ionisation technique for the analysis of biological molecules.2
By the mid 1990s, the price and performance of LC-MS instruments had improved to the extent that clinical biochemistry laboratories were able to take advantage of the new technology. Biochemical genetics was one of the first areas to do so, and the analysis of neonatal dried blood spot samples for a range of inborn errors of metabolism was a major early application.3 There are a number of other clinical applications of LC-MS, and the technique is more generally applicable than GC-MS owing to the broader range of biological molecules that can be analysed and the greater use of LC separations in clinical laboratories. The reasons for choosing LC-MS over LC with conventional detectors are essentially the same as with GC-MS, namely high specificity and the ability to handle complex mixtures. Applications of electrospray MS were reviewed in The Clinical Biochemist Reviews in 2003.4 The current review focuses on the principles of LC-MS, practical considerations in setting up LC-MS assays and reviews some of the major applications in clinical biochemistry, concentrating on small molecule applications.
Mass Spectrometry Instrumentation
Mass spectrometers operate by converting the analyte molecules to a charged (ionised) state, with subsequent analysis of the ions and any fragment ions that are produced during the ionisation process, on the basis of their mass to charge ratio (m/z). Several different technologies are available for both ionisation and ion analysis, resulting in many different types of mass spectrometers with different combinations of these two processes. In practice, some configurations are far more versatile than others and the following descriptions focus on the major types of ion sources and mass analysers likely to be used in LC-MS systems within clinical laboratories.
Ion Sources
Electrospray Ionisation Source
Fenn developed ESI into a robust ion source capable of interfacing to LC and demonstrated its application to a number of important classes of biological molecules.1 ESI works well with moderately polar molecules and is thus well suited to the analysis of many metabolites, xenobiotics and peptides. Liquid samples are pumped through a metal capillary maintained at 3 to 5 kV and nebulised at the tip of the capillary to form a fine spray of charged droplets. The capillary is usually orthogonal to, or off-axis from, the entrance to the mass spectrometer in order to minimise contamination. The droplets are rapidly evaporated by the application of heat and dry nitrogen, and the residual electrical charge on the droplets is transferred to the analytes.5 The ionised analytes are then transferred into the high vacuum of the mass spectrometer via a series of small apertures and focusing voltages. The ion source and subsequent ion optics can be operated to detect positive or negative ions, and switching between these two modes within an analytical run can be performed.
Under normal conditions, ESI is considered a “soft” ionisation source, meaning that relatively little energy is imparted to the analyte, and hence little fragmentation occurs. This is in contrast to other MS ion sources, for example the electron impact source commonly used in GC-MS, which causes extensive fragmentation. It is possible to increase ESI “in-source” fragmentation by increasing voltages within the source to increase collisions with nitrogen molecules. This has been used in LC-MS analyses to identify components with common structural features e.g. the glycans in glycopeptides can be fragmented in-source to give 204 m/z reporter ions. This feature has been used to identify glycopeptides in tryptic digests of proteins in order to characterise the structure of the glycans.6 Although useful for some analytes, in-source fragmentation is limited for others, and more consistent fragmentation methods, such as collision induced dissociation (see below), are required to induce extensive fragmentation required for structural studies and tandem MS.
Small molecules (≈ <500 Da) with a single functional group capable of carrying electrical charge give predominantly singly charged ions. This can involve the addition of a proton to the analyte (M+H+) when the ion source is operated in positive ion mode or the loss of a proton (M-H−) when operated in negative ion mode. Adduction of cations (e.g. M+NH4+, M+Na+, M+K+) and anions (e.g. M+formate−, M+acetate−) can occur when salts are present. Larger molecules and molecules with several charge-carrying functional groups such as proteins and peptides can exhibit multiple charging, resulting in ions such as M+2H2+, M+3H3+ etc. For proteins, this results in an envelope of ions with different charge states. This property can be used to accurately determine analytes with high molecular weights including proteins up to 100 kDa on mass spectrometers that scan up to only 4000 m/z. Indeed, it is unusual to detect ions with m/z values above this.
While ESI is the most widely used ion source for biological molecules, neutral and low polarity molecules such as lipids may not be efficiently ionised by this method. Two alternative ionisation methods developed for such analytes are described below.
Atmospheric Pressure Chemical Ionisation Source
In atmospheric pressure chemical ionisation (APCI), as with ESI, liquid is pumped through a capillary and nebulised at the tip. A corona discharge takes place near the tip of the capillary, initially ionising gas and solvent molecules present in the ion source. These ions then react with the analyte and ionise it via charge transfer. The technique is useful for small, thermally stable molecules that are not well ionised by ESI.7,8 For example, free steroids do not ionise well using ESI because they are neutral and relatively non-polar molecules, lacking a functional group capable of carrying charge. APCI has therefore been used to improve the sensitivity of LC-MS analysis of free steroids.9–11 Unlike ESI, multiple charging is not a feature of APCI and singly-charged ions dominate. Single ion sources capable of switching between APCI and ESI are available. The technique has also been applied to lipids and fat soluble vitamins.7,12–16
Atmospheric Pressure Photo-ionisation
Atmospheric pressure photo-ionisation (APPI) uses photons to excite and ionise molecules after nebulisation. The energy of the photons is chosen to minimise concurrent ionisation of solvents and ion source gases. The technique also gives predominantly singly-charged ions and has been used for the analysis of neutral compounds such as steroids17,18 and has been reviewed.19
Mass Analysers
Quadrupole Analysers
The quadrupole analyser consists of a set of four parallel metal rods (Figure 1). A combination of constant and varying (radio frequency) voltages allows the transmission of a narrow band of m/z values along the axis of the rods. By varying the voltages with time it is possible to scan across a range of m/z values, resulting in a mass spectrum. Most quadrupole analysers operate at <4000 m/z and scan speeds up to 1000 m/z per sec or more are common. They usually operate at unit mass resolution meaning that the mass accuracy is seldom better than 0.1 m/z.
Figure 1.
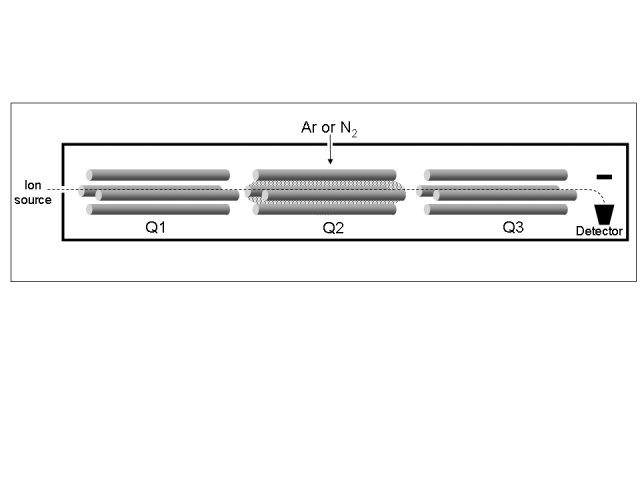
A triple quadrupole mass spectrometer. Q1 and Q3 act as mass filters and can be independently fixed, scanned or stepped. Q2 is a collision cell that contains a low pressure inert gas.
As an alternative to scanning, the quadrupoles can be set to monitor a specific m/z value, then set to monitor another m/z value, and so on. This is achieved by stepping the voltages. This technique is useful in improving the detection limits of targeted analytes because more detector time can be devoted to detecting specific ions instead of scanning across ions that are not produced by the analyte. Stepping can be carried out in a few milliseconds and a panel of m/z values can be stepped through for the detection of several analytes.
Ions can be induced to undergo fragmentation by collisions with an inert gas such as nitrogen or argon, a process called collision induced dissociation. One type of collision cell is a quadrupole that has been designed to maintain the low pressure of the collision gas required for dissociation and transmit most of the fragment ions that are produced. A particularly useful mass spectrometer configuration is obtained by placing a collision cell between two quadrupole mass analysers. This combination is called a triple quadrupole mass spectrometer and is an example of tandem MS in which two or more stages of mass analysis are independently applied (Figure 1). The advantage of tandem MS is the greatly increased specificity of the analysis over single stage mass analysis. For example, 25-hydroxy vitamin D3 produces a major M+H+ ion of 401 m/z during ESI that loses water during collision induced dissociation to produce a major 383 m/z product ion.12 Highly specific detection of 25-hydroxy vitamin D3 is achieved if the first and third quadrupoles are set to transmit ions of only 401 and 383 m/z respectively. This technique is termed single reaction monitoring and the fragmentation is denoted 401>383. Only analytes with this precursor/product ion combination will be detected. In a complex biological sample there may be other components that produce a 401 m/z precursor ion during ESI but there is a low probability that they will also fragment to a 383 m/z product ion.
The first and third quadrupoles can also be simultaneously stepped to different m/z values, and panels of precursor/ product ion pairs can be created to specifically detect a large number of targeted analytes. This process, called multiple reaction monitoring (MRM), is commonly used in LC-MS assays. A number of other useful modes of operation are available for triple quadrupole mass spectrometers and are listed in the Table. Most instruments have the capability of data-dependent acquisition, meaning that it is possible to switch between the different modes of operation within a run based on the results that are being acquired. For example, if a particular MRM signal is detected above a set threshold value, a product ion scan can then be acquired in order to confirm the identity.
Table.
Operational modes of triple quadrupole mass spectrometers.
| Name | First Quadrupole | Third Quadrupole | Applications |
|---|---|---|---|
| Product scan | Fixed | Scanned | Structural studies, identification of unknowns, confirmation by spectral matching with standard |
| Precursor scan | Scanned | Fixed | Detection of structurally related analytes that produce a common fragment ion |
| Neutral loss (or gain) scan | Scanned | Scanned with fixed offset relative to first quadrupole | Detection of structurally related analytes that eliminate (or gain) a common neutral molecule on collision |
| Multiple reaction monitoring | Fixed | Fixed | High sensitivity detection of a panel of targeted analytes |
Product ion scans not only contain structural information about the analyte but they also act as a “finger-print” that can be used to confirm identity with great certainty e.g. if confirmation of an illicit drug is required. This process is similar to that used for GC-MS identifications. However, the appearance of the product ion scan varies significantly between different instruments and, unlike GC-MS, it is more difficult to standardise conditions across instrument platforms.20 Thus, although it is possible to run standards and build an in-house library of product ion spectra for computer based searching, it is difficult to use this library on other instruments. This has hindered the construction of large libraries analogous to those widely available for GC-MS based searching.
Quadrupole analysers, either in the single or triple quadrupole configuration, are widely used in clinical biochemistry LC-MS applications owing to the ease of scanning and the good quality quantitative data obtained. Several other types of analysers are available and some of the more frequently encountered ones are described below.
Time-of-flight Analysers
The time-of-flight (TOF) analyser operates by accelerating ions through a high voltage.21 The velocity of the ions, and hence the time taken to travel down a flight tube to reach the detector, depends on their m/z values. If the initial accelerating voltage is pulsed, the output of the detector as a function of time can be converted into a mass spectrum. The TOF analyser can acquire spectra extremely quickly with high sensitivity. It also has high mass accuracy, which allows molecular formulas to be determined for small molecules.22
Ion Trap Analysers
Ion trap analysers use three hyperbolic electrodes to trap ions in a three-dimensional space using static and radio frequency voltages.23 Ions are then sequentially ejected from the trap on the basis of their m/z values to create a mass spectrum. Alternatively, a specific ion can be isolated in the trap by the application of an exciting voltage while other ions are ejected. An inert gas can also be introduced into the trap to induce fragmentation. An interesting feature of these ion trap analysers is the ability to fragment and isolate ions several times in succession before the final mass spectrum is obtained, resulting in so-called MSn capabilities.24
Hybrid Analysers
Tandem mass spectrometers that use combinations of different mass analysers have also been developed. Two configurations that are particularly useful for LC-MS are described below. The third quadrupole of a triple quadrupole MS can be replaced by a TOF analyser to produce a hybrid quadrupole time-of-flight (QTOF) mass spectrometer.25,26 QTOF instruments have been used extensively in the proteomics field but are more limited in their scanning functions than triple quadrupole instruments. It is also possible to design instruments in which the third quadrupole of a triple quadrupole MS operates in a different mode in which ions are trapped and then sequentially ejected on the basis of their m/z values. This is known as a linear ion trap and the overall configuration is often referred to as a QTrap instrument.27 The end quadrupole can be switched between ion trap mode and conventional quadrupole mode so the instrument combines useful features of both triple quadrupole and ion trap analysers. When used in ion trap mode, sensitivity in product ion scanning is considerably enhanced, and additional fragmentation can be induced within the ion trap allowing an additional stage of fragmentation and mass analysis (MS3).
Ion Suppression
The simultaneous presence of more than one component in the ion source can result in competition in the ionisation process and a subsequent reduction in the MS signals i.e. the signal of a component in a mixture will be less than that of the pure component at the same concentration. This phenomenon is called ion suppression and can be a limitation on the ultimate sensitivity of any LC-MS assay. Salts and other components in plasma, serum and urine samples are a particular limitation in direct injection methods. Buffers and other mobile phase additives are also sources of suppression.
Comparison of the peak area responses for pure standards, calibrators made up in the mobile phase, spiked samples and spiked sample extracts can be used to study ion suppression effects. Variable matrix components (e.g. drugs, physiological changes in endogenous metabolite levels) can cause variation in suppression between different samples, resulting in unreliable quantitative results. Unless stable isotope internal standards are used (see below), sample preparation strategies must be employed to minimise these sources of variable suppression. Causes of ion suppression and strategies to evaluate and minimise its impact on results have been reviewed.28–30
Direct Injection Methods
When highly specific detection such as tandem MS is used, it is often feasible to directly inject samples into the ion source without any chromatographic separation and measure analytes in a complex matrix. This is usually done using multiple reaction monitoring for analytes and internal standards and has the advantage of shortened run times, typically two minutes or less. In its most extreme form, samples can sometimes be simply diluted with internal standards and analysed, so called “dilute and shoot” methods. This approach has been used to analyse urine samples,31 but is more difficult to apply to plasma and other samples with high protein content. Most direct injection analyses benefit from some form of sample preparation that removes matrix components such as proteins and salts. Even after removing these components most biological samples still contain a complex mixture of components, and ion suppression effects generally limit the direct injection approach to analytes with relatively high concentrations. Detection limits are highly dependent on the nature of the analyte, its fragmentation and the sample matrix, but it is generally difficult to detect analytes at <0.1 μmol/L using direct injection methods. Another limitation of direct injection methods is the inability to distinguish isomers e.g. the isomeric amino acids leucine, isoleucine and allo-isoleucine have very similar spectra and cannot be differentiated during neonatal blood spot screening using direct injection tandem MS.32
Liquid Chromatography Considerations
Current ion sources are capable of handling a wide range of flow rates and mobile phase compositions so existing LC separations can often be directly coupled to the mass spectrometer. However, a number of factors can affect the quality of MS data and some modification of the LC separation may be desirable to improve assay performance.
Flow Rate
While standard ESI sources can generally handle flow rates up to 1 mL/min, lower flow rates in the range 0.05 to 0.2 mL/min result in improved sensitivity. Columns with 1.0 or 2.1 mm diameters are therefore well suited to direct coupling with these ion sources. The conversion from 4.6 mm to 1.0 or 2.1 mm columns is generally easily managed and predictable, and the resulting decrease in mobile phase consumption is a useful side benefit. Even higher sensitivities and resolutions can be obtained with capillary columns and sub μL/min flow rates. These separations are generally more demanding as different ion sources and LC pumps are required and care is required in making column connections. These separations are useful in the proteomics area where high sensitivity and resolution are required to identify as many components as possible. However, they are probably less suited for routine clinical laboratory work.
Mobile Phase
Typical solvents used in reverse and normal phase LC (e.g. water, acetonitrile, methanol, ethanol, chloroform) are compatible with ESI. It should noted that a grade of solvent (including water) that is suitable for a conventional LC separation may not always be suitable for an LC-MS based separation e.g. the solvent may be UV transparent to 210 nm but may contain traces of compounds that affect the quality of LC-MS measurements. For example, one study found large differences between commercial grades of methanol.33
The use of buffers containing inorganic ions such as phosphate and sodium acetate should be avoided. They cause significant ion suppression, can create MS adducts of sodium and potassium, and can quickly contaminate the ion source. Substitution of buffers that are more compatible with MS such as those based on ammonium acetate, ammonium formate or ammonium bicarbonate is generally possible. Even so, these buffers still cause ion suppression so the concentration of buffer used should be the minimum required to produce satisfactory chromatography. Ion pairing reagents, such as trifluoroacetic acid and other fluorinated carboxylic acids, also result in ion suppression so their concentrations should also be minimised as much as is practical.
Higher organic content of the mobile phase can result in improvements in ionisation efficiency in ESI, and this may affect the choice of separation mode. For example, polar molecules are poorly retained on reverse phase columns and elute at the beginning of the chromatogram with a low organic content. This will result in relatively poor ionisation efficiency, and polar interferences are more likely to co-elute in this region causing ion suppression. One way this can be overcome is to use hydrophilic interaction chromatography (HILIC) in which polar analytes are retained and elute with higher organic content of the mobile phase.34,35
Resolution and Through-put
Baseline separation of peaks is generally required in conventional LC with UV detection due the non-specific nature of the detector, especially when wavelengths below 250 nm are used. In contrast, if the peaks have independent MS signals, complete chromatographic separation is not required in LC-MS. Therefore, lower resolution, shorter columns are often used in LC-MS assays with consequently shorter run times, although ion suppression effects may be a limitation. Guard columns can sometimes provide sufficient separation from interferences. Another approach used to speed up the analytical process is to use short columns with small particle sizes (<2 μm) and high pressure, dedicated pumping systems (so-called ultra performance LC).36–38 These columns are also capable of generating highly resolved chromatograms with better signal:noise ratios than columns with larger particle sizes.39
Chromatographic resolution also impacts on the scanning speed of the mass spectrometer. To achieve accurate integration it is desirable to have at least 10 scans across the chromatographic peak. It may therefore be necessary to use fast scan speeds with highly resolved peaks, and this may compromise sensitivity when several ions are being monitored. It is possible to minimise this to some extent by dividing the chromatogram into smaller windows and using smaller panels of ions specific for the analytes eluting within these windows.
Quantitation
It is generally difficult to perform quantitative determinations using absolute MS responses. This is because of the large number of factors that influence the absolute MS response such as the cleanliness of the ion source, ion optics and the collision cell, ion suppression, ion source flow rates, collision cell pressure and the ultimate MS vacuum. It is difficult to control all of these factors and, as a consequence, absolute MS responses are subject to significant day-to-day variation. Therefore, internal standards are usually required to achieve reliable and accurate quantitative results. Stable isotope versions of the analyte are ideal internal standards as they have almost identical chemical properties but are easily distinguished during MS. Furthermore, they correct for any losses or inefficiencies in the sample preparation process (Figure 2) and correct for ion suppression. This technique is termed stable isotope dilution and is capable of providing assays that are very accurate and precise. For these reasons, stable isotope dilution LC-MS assays are often suitable as reference methods. Figure 2 implies that quantitation without calibration is possible by knowing the amount of internal standard added. This is done in some assays where producing calibrators is not straight-forward e.g. dried blood spot samples. However, this approach relies on the stable isotope internal standard being 100% pure and having the same molar response as the analyte. For accurate results, calibration curves plotting analyte:internal standard response ratio vs analyte concentration are still required. Considerations in using and selecting stable isotope internal standards have been reviewed.40,41
Figure 2.
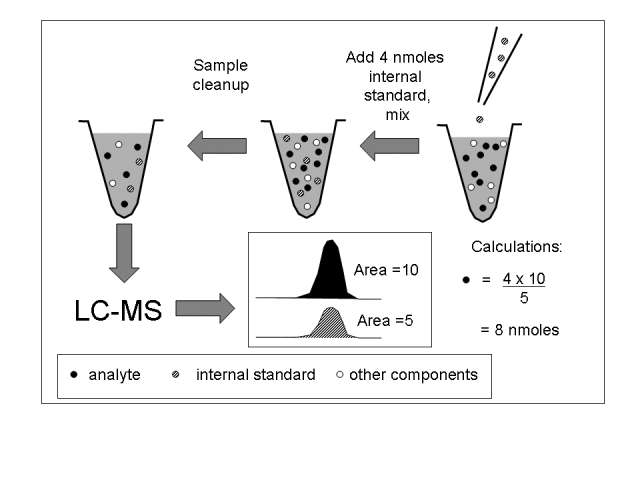
Principle of stable isotope dilution. Four nmoles of internal standard are added at the beginning of the assay, fixing the internal standard:analyte ratio (1:2 in this case). Analyte and internal standard are lost in subsequent processing steps but the ratio remains the same. LC-MS = liquid chromatography-mass spectrometry.
Method Optimisation
In setting up an LC-MS assay a significant number of conditions and parameters need to be taken into account and optimised. The actual conditions are highly dependent on the nature of the analyte and the LC separation, making it difficult to give generic conditions. Each analyte requires individual optimisation. Although published methods are a valuable starting point, assay performance and optimal conditions can vary greatly between different instruments and sample matrices. Sensitivity is highly dependent on the instrument used and the assay conditions. Instrument manufacturers are continually improving the sensitivity of their mass spectrometers and generally offer a range of models with different sensitivities. It is therefore important to assess if an instrument has the necessary sensitivity to achieve the desired limits of detection.
The nature of the analyte will largely determine the optimal ionisation method and polarity used. Detection of some analytes can also be enhanced by derivatisation.42–44 For example, free steroids give relatively weak responses using ESI because they lack a functional group capable of carrying charge. The keto group of keto-steroids such as 17α-hydroxyprogesterone can be derivatised with groups that give a much higher electrospray response, resulting in lower detection limits.45
Ion source parameters and collision energy can be optimised during continuous infusion of a dilute solution of the analyte, preferably in the same mobile phase used for the LC separation. Choice of fragment ion to monitor requires some thought as the most abundant fragment may not always be the best choice. If other significant fragment ions occur, it is advisable to also evaluate them as they may actually provide cleaner chromatograms with superior signal to noise. It is also advisable to monitor a second fragment ion to check for possible interferences. If an interference is present, the ratio of the two fragment ions is different to that of the standard.
Some aspects of method optimisation are illustrated in Figure 3, using the example of methylmalonate measurement in mouse tissues.46,47 Derivatisation to form butyl esters was used to improve the sensitivity. Some ammonium adduction (248 m/z) from the ammonium acetate buffer used for LC separation was apparent with single MS (Figure 3A) but M+H+ (231 m/z) was the most abundant ion and was used for tandem MS. The ion source voltages and collision energy were then optimised for the production of the 119 m/z fragment ion in the product ion spectrum (Figure 3B). For LC-MS, the MRMs 231>119 and the analogous 234>122 (2H3-methylmalonate internal standard) were monitored for methylmalonate quantitation. A second MRM for methylmalonate (231>175) was also monitored as a check for interferences (Figure 3C). The ratio of the 231>119 and 231>175 MRM responses for the methylmalonate peak at 9.46 minutes was similar to the product ion spectrum whereas the same ratio for the succinate peak at 9.23 minutes was quite different.
Figure 3.
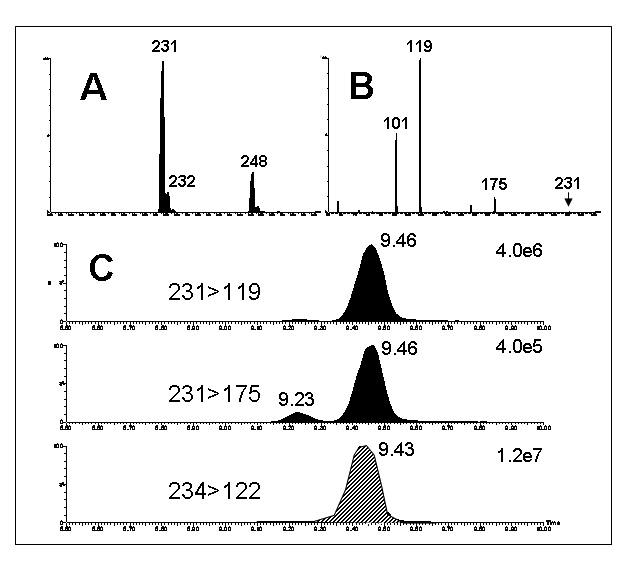
An example of liquid chromatography-mass spectrometry (LC-MS) analysis of methylmalonate in mouse kidney. Samples were derivatised by butylation to improve sensitivity. A :Single MS spectrum of methylmalonate (M+H ammonium acetate buffer. Note the presence of M+NH4+ = 231) in + = 248. B :Tandem mass spectrum of methylmalonate. C: Multiple reaction monitoring (MRM) chromatograms of methlymalonate (231>119 and 231>175) and the internal standard, 2H3-methymalonate (234>122). The small peak at 9.23 min is due to succinate, an isomer of methylmalonate.
The early part of reverse phase chromatograms often contains large amounts of salts and other potential contaminants. Diversion of this part of the chromatogram to waste can be useful in minimising ion source contamination when “dirty” samples are being analysed. When using a highly specific, targeted analysis such as with MRM, the presence of these and other components in the sample is usually not apparent as the chromatograms often contain a single, well-resolved peak with minimal baseline noise. This may cause the analyst to think that the samples are cleaner than they actually are when, in fact, there may be significant amounts of material co-eluting with the analyte of interest, causing ion suppression and poor detection limits. Ion suppression can be evaluated by comparing the absolute responses of the internal standard in standards and samples. If the response is significantly lower in the samples, this indicates significant suppression, and there may be a need to change the sample preparation or chromatography to minimise the co-elution of these suppressants. Although ion suppression can have a detrimental effect on the ultimate detection limit of an assay, it is still possible to obtain reasonable quantitative data provided suitable stable isotope internal standards are used and there is sufficient signal to measure in samples.
Another consideration is whether to use single MS or tandem MS. Tandem MS actually results in a decrease in absolute signal because of losses in the collision cell and the fact that the signal may be spread across several fragments when only one or two are being detected. The advantage of tandem MS is its very high specificity, meaning that chemical noise is greatly reduced and the signal:noise ratio is generally superior to single MS despite the decrease in signal. However, if there is a large decrease in signal because the analyte fragments poorly or produces many, low intensity fragments, the signal: noise ratio of tandem MS may actually be inferior to single MS detection.
Performance Monitoring and Practical Considerations
Many factors can affect the on-going performance of LC-MS systems and it is important to have protocols in place to detect deviations from normal performance. Monitoring the absolute response, peak shape and retention time of internal standards is a simple way of checking the sensitivity of the mass spectrometer and the integrity of the LC system. Applying formal quality control rules to the internal standard response may not be practical due to the large day-to-day variations in absolute MS response, but it is important to visually check plots of the response to detect decreases in sensitivity that can indicate the need to clean the ion source or ion optics. Checking the internal standard response of each sample within a batch is also a useful way of picking up problems with individual samples. Purity of reagents and solvents can also have a significant impact on the quality of results and should be evaluated during method optimisation and when different sources are used. Plastic ware (e.g. primary samples tubes, microtitre plates) used anywhere in the analytical process is also a potential source of interferences.
Although the performance and reliability of LC-MS systems continues to improve, they still require more attention and maintenance than conventional LC systems. Down-times of one day or more occur occasionally, and a second instrument may be needed for applications where turn-around times are critical. Furthermore, operation and maintenance of LC-MS systems requires relatively highly skilled staff.
Applications
Biochemical Screening for Genetic Disorders
The early work of Millington et al. established the potential of screening neonatal dried blood spots for a wide range of inborn errors of metabolism (IEM).48,49 The development of ESI accelerated this process and methods for processing the large number of samples required for testing all newborns were developed.3,50 Australia took a lead in this area and all Australian babies are currently tested using this technique.51 A number of marker amino acids and acyl carnitines are measured in dried blood spots after extraction and derivatisation in microtitre plates using direct injection and MRM or scanning modes. Approximately 20 IEMs can be detected using this technique. Improvements in the sensitivity of recent MS instruments have allowed the omission of the derivatisation stage. MS-based newborn screening strategies have also been developed for galactosaemia,52 sickle cell anaemia53 and lysosomal disorders.54–59 In the case of lysosomal disorders, enzyme activities, rather than metabolites, are measured using specially designed substrates, and the technique can be multiplexed.58
The positive predictive value for some analytes during newborn screening is relatively poor. Therefore, second-tier testing, in which samples with levels above a threshold value are re-analysed using a more specific LC-MS method, has been proposed for these disorders. For example, the levels of tyrosine in tyrosinaemia type 1 may be only slightly elevated and similar to levels in premature or jaundiced babies. Second-tier testing for succinyl acetone, a far more specific marker for tyrosinaemia type 1, in dried blood spots has been developed.60–62 Similar second-tier tests have been developed for maple syrup urine disease32 and methylmalonic acidaemia.63,64 Second-tier LC-MS testing for 17α-hydroxyprogesterone has also been developed to minimise false positive rates from first-tier immunoassays during newborn screening for steroid 21-hydroxylase deficiency.65–68 These second-tier tests add another level of complexity and the need for further resources, so it will be interesting to see how successfully they can be incorporated into newborn screening programs.
High through-put, direct injection techniques have also been developed to screen for a wide range of IEMs in urine samples from symptomatic patients.31,69 Numerous other biochemical genetics applications have been developed including screening for disorders of porphyrin,70,71 purine and pyrimidine,72–74 peroxisomal75–77 and bile acid metabolism.78
Therapeutic Drug Monitoring and Toxicology
Dissatisfaction with the high cost of commercial immunoassays used in therapeutic drug monitoring and their variable cross-reactivity with metabolites has spurred the development of LC-MS assays as alternatives. Assays have been developed for immunosuppressants such as cyclosporin, tacrolimus, sirolimus, everolimus and mycophenolic acid, and this area has been reviewed.79,80 LC-MS assays for aminoglycosides,35 antiretrovirals81–83 and anticancer drugs84 have also been developed. The capacity to multiplex LC-MS assays so that several drugs and metabolites can be measured in one run is a useful feature of these assays, which can simplify laboratory workflows and provide additional pharmacokinetic information.
The use of LC-tandem MS for toxicology screening is attractive because of its potential, relative to GC-MS screening, to provide greater confidence in identifications, detect a wider range of drugs, toxins and their metabolites, and to simplify sample preparation. However, there are some issues that currently limit its widespread adoption as a routine technique. Product ion spectra can vary considerably between instruments and comprehensive spectral libraries are not available.20 Another limitation is that, unlike GC-MS, there is no common set of MS operating conditions that is suitable for most analytes. Different analytes have different optimal parameters (precursor ion, ion source voltages, collision energies, etc.) which usually need to be determined on a case-by-case basis. Most LC-MS toxicology strategies therefore rely on developing a targeted panel containing as many as 301 drugs and metabolites.85 Data-dependent acquisition and rapid product ion scans using a set of different conditions can assist this process but add to the complexity of the analysis. Several LC-MS strategies for clinical and postmortem toxicology screening have been published,85–91 and the field has been reviewed.92–95 Direct analysis of urine samples is possible.96,97 Some potential pitfalls and mis-identifications have been noted but these can be eliminated by carefully matching retention times and mass spectra to a standard and using qualifier ion ratios.98 The sensitivity of current instruments also allows the analysis of oral fluids99 and hair samples.100,101
Vitamins and Related Metabolites
There is considerable interest in using LC-MS for vitamin D measurements due to the variable responses of commercial immunoassays to different forms of vitamin D and its metabolites. Several LC-MS assays for 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in serum and plasma have been described using ESI102,103 and APCI.12,104 Derivatisation has been used to improve the sensitivity for low level metabolites such as 1,25- and 24,25-dihydroxy metabolites,36 salivary metabolites105 and urine metabolites.106 Several comparisons of LC-MS and immunoassays have been published.107–109
LC-MS assays for other fat-soluble vitamins such as various forms of vitamin E13,15 and vitamin K15 have been developed. One assay has multiplexed various forms of vitamins A, D, E and K.110 Methods are also available for water-soluble vitamins such as pyridoxine, riboflavin111 and folate vitamers.112
Total homocysteine is an indicator of risk of cardiovascular disease, and both total homocysteine and methylmalonic acid are important functional indicators of vitamin B12 status. LC-MS assays have been described for plasma methylmalonic acid47,113,114 and plasma total homocysteine.115–119 Assays suitable for dried blood spots have also been developed.120,121 The clinical value of total homocysteine measurement can be improved by combining it with related amino acids such as methione and cysteine122,123 or methylmalonate124 and folate.125
Steroid Hormones
LC-MS analysis is relevant to several areas of steroid biochemistry. Difficulties with the measurement of low testosterone and dihydrotestosterone levels found in women and children using conventional immunoassays has prompted the development of several highly sensitive LC-MS assays capable of providing reliable measurements in these groups.37,126–129 Assays for urine free cortisol,130–133 oestrogens10 and multiplexed assays for various adrenal steroids have also been developed.9,68,134,135
Profiling of urinary steroids by GC-MS is used for the investigation of inborn errors of steroid metabolism and other disturbances of steroid metabolism. These steroids are generally excreted in the form of glucuronide or sulphate conjugates that require enzymatic hydrolysis and subsequent derivatisation for GC-MS. Profiling intact urine conjugates by LC-MS would simplify this process, and methods that demonstrate this are starting to appear.69,136,137
Conclusions
MS using ESI and other ionisation methods can be applied to a much wider range of biological molecules than GC-MS and will thus find greater application in clinical biochemistry. Direct injection methods can determine many analytes with high through-put when highly specific tandem MS is used for detection. LC-MS provides superior specificity and sensitivity compared to direct injection methods. When combined with stable isotope dilution, LC-MS can be used to develop highly accurate and reproducible assays. Modern mass spectrometers are highly sensitive and LC-MS assays are now viable replacements for many immunoassays. Although start-up costs are relatively high, reagent costs are low if prepared in-house, and there may be considerable long-term cost savings when an existing commercial assay can be replaced by LC-MS. LC-MS strategies are also emerging for sensitive, quanitative, multiplexed assays of plasma peptides and proteins.138,139 As the sensitivity of MS continues to improve, LC-MS assays will be of increasing interest as potential replacements for existing immunoassays.
Another advantage of LC-MS assays is the capacity to multiplex several analytes within a single analytical run with minimal incremental cost. This has the potential to simplify laboratory set up (e.g. creation of test panels) and provide additional useful information (e.g. metabolite profiles). Applications which detect hundreds of analytes in a single run have been demonstrated and the multiplexing possibilities are, to a large extent, only limited by the imagination and ingenuity of the analyst. LC-MS assays will probably be most beneficial in the clinical biochemistry laboratory when used for multiplexed and screening type assays where the additional information obtained justifies the costs of instrument purchase and operation.
Footnotes
Competing Interests: None declared.
References
- 1.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 2.The Nobel Prize in Chemistry. [(Accessed 23 November 2008)];2002 http://nobelprize.org/nobel_prizes/chemistry/laureates/2002/
- 3.Rashed MS, Bucknall MP, Little D, Awad A, Jacob M, Alamoudi M, et al. Screening blood spots for inborn errors of metabolism by electrospray tandem mass spectrometry with a microplate batch process and a computer algorithm for automated flagging of abnormal profiles. Clin Chem. 1997;43:1129–41. [PubMed] [Google Scholar]
- 4.Ho CS, Lam CWK, Chan MHM, Cheung RCK, Law LK, Lit LCW, et al. Electrospray ionisation mass spectrometry: principles and clinical applications. Clin Biochem Rev. 2003;24:3–12. [PMC free article] [PubMed] [Google Scholar]
- 5.Kebarle P. A brief overview of the present status of the mechanisms involved in electrospray mass spectrometry. J Mass Spectrom. 2000;35:804–17. doi: 10.1002/1096-9888(200007)35:7<804::AID-JMS22>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Carr SA, Huddleston MJ, Bean MF. Selective identification and differentiation of N- and O-linked oligosaccharides in glycoproteins by liquid chromatography-mass spectrometry. Protein Sci. 1993;2:183–96. doi: 10.1002/pro.5560020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrdwell WC. Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids. Lipids. 2001;36:327–46. doi: 10.1007/s11745-001-0725-5. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg E. The potential of organic (electrospray- and atmospheric pressure chemical ionisation) mass spectrometric techniques coupled to liquid-phase separation for speciation analysis. J Chromatogr A. 2003;1000:841–89. doi: 10.1016/s0021-9673(03)00603-4. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho VM, Nakamura OH, Vieira JGH. Simultaneous quantitation of seven endogenous C-21 adrenal steroids by liquid chromatography tandem mass spectrometry in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872:154–61. doi: 10.1016/j.jchromb.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–84. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 11.Rauh M, Groschl M, Rascher W, Dorr HG. Automated, fast and sensitive quantification of 17 alpha-hydroxy-progesterone, androstenedione and testosterone by tandem mass spectrometry with on-line extraction. Steroids. 2006;71:450–8. doi: 10.1016/j.steroids.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, McCoy LF, Schleicher RL, Pfeiffer CM. Measurement of 25-hydroxyvitamin D3 (25OHD3) and 25-hydroxyvitamin D2 (25OHD2) in human serum using liquid chromatography-tandem mass spectrometry and its comparison to a radioimmunoassay method. Clin Chim Acta. 2008;391:6–12. doi: 10.1016/j.cca.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Andreoli R, Manini P, Poli D, Bergamaschi E, Mutti A, Niessen WMA. Development of a simplified method for the simultaneous determination of retinol, alpha-tocopherol, and beta-carotene in serum by liquid chromatography-tandem mass spectrometry with atmospheric pressure chemical ionization. Anal Bioanal Chem. 2004;378:987–94. doi: 10.1007/s00216-003-2288-0. [DOI] [PubMed] [Google Scholar]
- 14.Lauridsen C, Leonard SW, Griffin DA, Liebler DC, McClure TD, Traber MG. Quantitative analysis by liquid chromatography-tandem mass spectrometry of deuterium-labeled and unlabeled vitamin E in biological samples. Anal Biochem. 2001;289:89–95. doi: 10.1006/abio.2000.4913. [DOI] [PubMed] [Google Scholar]
- 15.Nagy K, Courtet-Compondu M-C, Holst B, Kussmann M. Comprehensive analysis of vitamin E constituents in human plasma by liquid chromatography-mass spectrometry. Anal Chem. 2007;79:7087–96. doi: 10.1021/ac0708689. [DOI] [PubMed] [Google Scholar]
- 16.Suhara Y, Kamao M, Tsugawa N, Okano T. Method for the determination of vitamin K homologues in human plasma using high-performance liquid chromatography-tandem mass spectrometry. Anal Chem. 2005;77:757–63. doi: 10.1021/ac0489667. [DOI] [PubMed] [Google Scholar]
- 17.Guo T, Chan M, Soldin SJ. Steroid profiles using liquid chromatography-tandem mass spectrometry with atmospheric pressure photoionization source. Arch Pathol Lab Med. 2004;128:469–75. doi: 10.5858/2004-128-469-SPULCM. [DOI] [PubMed] [Google Scholar]
- 18.Leinonen A, Kuuranne T, Kostiainen R. Liquid chromatography/mass spectrometry in anabolic steroid analysis--optimization and comparison of three ionization techniques: electrospray ionization, atmospheric pressure chemical ionization and atmospheric pressure photoionization. J Mass Spectrom. 2002;37:693–8. doi: 10.1002/jms.328. [DOI] [PubMed] [Google Scholar]
- 19.Raffaelli A, Saba A. Atmospheric pressure photoionization mass spectrometry. Mass Spectrom Rev. 2003;22:318–31. doi: 10.1002/mas.10060. [DOI] [PubMed] [Google Scholar]
- 20.Jansen R, Lachatre G, Marquet P. LC-MS/MS systematic toxicological analysis: comparison of MS/MS spectra obtained with different instruments and settings. Clin Biochem. 2005;38:362–72. doi: 10.1016/j.clinbiochem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Williamson LN, Bartlett MG. Quantitative liquid chromatography/time-of-flight mass spectrometry. Biomed Chromatogr. 2007;21:567–76. doi: 10.1002/bmc.844. [DOI] [PubMed] [Google Scholar]
- 22.Bristow AWT. Accurate mass measurement for the determination of elemental formula--a tutorial. Mass Spectrom Rev. 2006;25:99–111. doi: 10.1002/mas.20058. [DOI] [PubMed] [Google Scholar]
- 23.Payne AH, Glish GL. Tandem mass spectrometry in quadrupole ion trap and ion cyclotron resonance mass spectrometers. Methods Enzymol. 2005;402:109–48. doi: 10.1016/S0076-6879(05)02004-5. [DOI] [PubMed] [Google Scholar]
- 24.Tozuka Z, Kaneko H, Shiraga T, Mitani Y, Beppu M, Terashita S, et al. Strategy for structural elucidation of drugs and drug metabolites using (MS)n fragmentation in an electrospray ion trap. J Mass Spectrom. 2003;38:793–808. doi: 10.1002/jms.511. [DOI] [PubMed] [Google Scholar]
- 25.Chernushevich IV, Loboda AV, Thomson BA. An introduction to quadrupole-time-of-flight mass spectrometry. J Mass Spectrom. 2001;36:849–65. doi: 10.1002/jms.207. [DOI] [PubMed] [Google Scholar]
- 26.Ens W, Standing KG. Hybrid quadrupole/time-of-flight mass spectrometers for analysis of biomolecules. Methods Enzymol. 2005;402:49–78. doi: 10.1016/S0076-6879(05)02002-1. [DOI] [PubMed] [Google Scholar]
- 27.Hopfgartner G, Varesio E, Tschappat V, Grivet C, Bourgogne E, Leuthold LA. Triple quadrupole linear ion trap mass spectrometer for the analysis of small molecules and macromolecules. J Mass Spectrom. 2004;39:845–55. doi: 10.1002/jms.659. [DOI] [PubMed] [Google Scholar]
- 28.Taylor PJ. Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin Biochem. 2005;38:328–34. doi: 10.1016/j.clinbiochem.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Annesley TM. Ion suppression in mass spectrometry. Clin Chem. 2003;49:1041–4. doi: 10.1373/49.7.1041. [DOI] [PubMed] [Google Scholar]
- 30.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/ MS. Anal Chem. 2003;75:3019–30. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 31.Pitt JJ, Eggington M, Kahler SG. Comprehensive screening of urine samples for inborn errors of metabolism by electrospray tandem mass spectrometry. Clin Chem. 2002;48:1970–80. [PubMed] [Google Scholar]
- 32.Oglesbee D, Sanders KA, Lacey JM, Magera MJ, Casetta B, Strauss KA, et al. Second-tier test for quantification of alloisoleucine and branched-chain amino acids in dried blood spots to improve newborn screening for maple syrup urine disease (MSUD) Clin Chem. 2008;54:542–9. doi: 10.1373/clinchem.2007.098434. [DOI] [PubMed] [Google Scholar]
- 33.Annesley TM. Methanol-associated matrix effects in electrospray ionization tandem mass spectrometry. Clin Chem. 2007;53:1827–34. doi: 10.1373/clinchem.2007.090811. [DOI] [PubMed] [Google Scholar]
- 34.Martens-Lobenhoffer J, Bode-Boger SM. Fast and efficient determination of arginine, symmetric dimethylarginine, and asymmetric dimethylarginine in biological fluids by hydrophilic-interaction liquid chromatography-electrospray tandem mass spectrometry. Clin Chem. 2006;52:488–93. doi: 10.1373/clinchem.2005.060152. [DOI] [PubMed] [Google Scholar]
- 35.Oertel R, Neumeister V, Kirch W. Hydrophilic interaction chromatography combined with tandem-mass spectrometry to determine six aminoglycosides in serum. J Chromatogr A. 2004;1058:197–201. [PubMed] [Google Scholar]
- 36.Aronov PA, Hall LM, Dettmer K, Stephensen CB, Hammock BD. Metabolic profiling of major vitamin D metabolites using Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2008;391:1917–30. doi: 10.1007/s00216-008-2095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Licea-Perez H, Wang S, Szapacs ME, Yang E. Development of a highly sensitive and selective UPLC/ MS/MS method for the simultaneous determination of testosterone and 5alpha-dihydrotestosterone in human serum to support testosterone replacement therapy for hypogonadism. Steroids. 2008;73:601–10. doi: 10.1016/j.steroids.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Ventura R, Roig M, Montfort N, Saez P, Berges R, Segura J. High-throughput and sensitive screening by ultra-performance liquid chromatography tandem mass spectrometry of diuretics and other doping agents. Eur J Mass Spectrom (Chichester, Eng) 2008;14:191–200. doi: 10.1255/ejms.920. [DOI] [PubMed] [Google Scholar]
- 39.Nordstrom A, O’Maille G, Qin C, Siuzdak G. Nonlinear data alignment for UPLC-MS and HPLC-MS based metabolomics: quantitative analysis of endogenous and exogenous metabolites in human serum. Anal Chem. 2006;78:3289–95. doi: 10.1021/ac060245f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duxbury K, Owen L, Gillingwater S, Keevil B. Naturally occurring isotopes of an analyte can interfere with doubly deuterated internal standard measurement. Ann Clin Biochem. 2008;45:210–2. doi: 10.1258/acb.2007.007137. [DOI] [PubMed] [Google Scholar]
- 41.Stokvis E, Rosing H, Beijnen JH. Stable isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: necessity or not? Rapid Commun Mass Spectrom. 2005;19:401–7. doi: 10.1002/rcm.1790. [DOI] [PubMed] [Google Scholar]
- 42.Gao S, Zhang Z-P, Karnes HT. Sensitivity enhancement in liquid chromatography/atmospheric pressure ionization mass spectrometry using derivatization and mobile phase additives. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825:98–110. doi: 10.1016/j.jchromb.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Zaikin VG, Halket JM. Derivatization in mass spectrometry--8. Soft ionization mass spectrometry of small molecules. Eur J Mass Spectrom (Chichester, Eng) 2006;12:79–115. doi: 10.1255/ejms.798. [DOI] [PubMed] [Google Scholar]
- 44.Johnson DW. A modified Girard derivatizing reagent for universal profiling and trace analysis of aldehydes and ketones by electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:2926–32. doi: 10.1002/rcm.3175. [DOI] [PubMed] [Google Scholar]
- 45.Johnson DW. Ketosteroid profiling using Girard T derivatives and electrospray ionization tandem mass spectrometry: direct plasma analysis of androstenedione, 17-hydroxyprogesterone and cortisol. Rapid Commun Mass Spectrom. 2005;19:193–200. doi: 10.1002/rcm.1771. [DOI] [PubMed] [Google Scholar]
- 46.Peters H, Nefedov M, Sarsero J, Pitt J, Fowler KJ, Gazeas S, et al. A knock-out mouse model for methylmalonic aciduria resulting in neonatal lethality. J Biol Chem. 2003;278:52909–13. doi: 10.1074/jbc.M310533200. [DOI] [PubMed] [Google Scholar]
- 47.Magera MJ, Helgeson JK, Matern D, Rinaldo P. Methylmalonic acid measured in plasma and urine by stable-isotope dilution and electrospray tandem mass spectrometry. Clin Chem. 2000;46:1804–10. [PubMed] [Google Scholar]
- 48.Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13:321–4. doi: 10.1007/BF01799385. [DOI] [PubMed] [Google Scholar]
- 49.Millington DS, Norwood DL, Kodo N, Roe CR, Inoue F. Application of fast atom bombardment with tandem mass spectrometry and liquid chromatography/mass spectrometry to the analysis of acylcarnitines in human urine, blood, and tissue. Anal Biochem. 1989;180:331–9. doi: 10.1016/0003-2697(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 50.Ziadeh R, Hoffman EP, Finegold DN, Hoop RC, Brackett JC, Strauss AW, et al. Medium chain acyl-CoA dehydrogenase deficiency in Pennsylvania: neonatal screening shows high incidence and unexpected mutation frequencies. Pediatr Res. 1995;37:675–8. doi: 10.1203/00006450-199505000-00021. [DOI] [PubMed] [Google Scholar]
- 51.Wilcken B, Wiley V, Hammond J, Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003;348:2304–12. doi: 10.1056/NEJMoa025225. [DOI] [PubMed] [Google Scholar]
- 52.Jensen UG, Brandt NJ, Christensen E, Skovby F, Norgaard-Pedersen B, Simonsen H. Neonatal screening for galactosemia by quantitative analysis of hexose monophosphates using tandem mass spectrometry: a retrospective study. Clin Chem. 2001;47:1364–72. [PubMed] [Google Scholar]
- 53.Boemer F, Ketelslegers O, Minon JM, Bours V, Schoos R. Newborn screening for sickle cell disease using tandem mass spectrometry. Clin Chem. 2008;54:2036–41. doi: 10.1373/clinchem.2008.106369. [DOI] [PubMed] [Google Scholar]
- 54.Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis I. Clin Chem. 2008;54:2067–70. doi: 10.1373/clinchem.2008.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clements P. Mass spectrometry as a platform for the diagnosis of lysosomal disorders. Clin Chem. 2004;50:1723–4. doi: 10.1373/clinchem.2004.038133. [DOI] [PubMed] [Google Scholar]
- 56.Dajnoki A, Muhl A, Fekete G, Keutzer J, Orsini J, Dejesus V, et al. Newborn screening for Pompe disease by measuring acid alpha-glucosidase activity using tandem mass spectrometry. Clin Chem. 2008;54:1624–9. doi: 10.1373/clinchem.2008.107722. [DOI] [PubMed] [Google Scholar]
- 57.Wang D, Wood T, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of enzymes in dried blood spots: application to newborn screening for mucopolysaccharidosis II (Hunter disease) Clin Chem. 2007;53:137–40. doi: 10.1373/clinchem.2006.077263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang XK, Elbin CS, Chuang WL, Cooper SK, Marashio CA, Beauregard C, et al. Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin Chem. 2008;54:1725–8. doi: 10.1373/clinchem.2008.104711. [DOI] [PubMed] [Google Scholar]
- 59.Zhou X, Turecek F, Scott CR, Gelb MH. Quantification of cellular acid sphingomyelinase and galactocerebroside beta-galactosidase activities by electrospray ionization mass spectrometry. Clin Chem. 2001;47:874–81. [PubMed] [Google Scholar]
- 60.Magera MJ, Gunawardena ND, Hahn SH, Tortorelli S, Mitchell GA, Goodman SI, et al. Quantitative determination of succinylacetone in dried blood spots for newborn screening of tyrosinemia type I. Mol Genet Metab. 2006;88:16–21. doi: 10.1016/j.ymgme.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Johnson DW, Gerace R, Ranieri E, Trinh M-U, Fingerhut R. Analysis of succinylacetone, as a Girard T derivative, in urine and dried bloodspots by flow injection electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:59–63. doi: 10.1002/rcm.2806. [DOI] [PubMed] [Google Scholar]
- 62.Al-Dirbashi OY, Rashed MS, Brink HJT, Jakobs C, Filimban N, Al-Ahaidib LY, et al. Determination of succinylacetone in dried blood spots and liquid urine as a dansylhydrazone by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:274–80. doi: 10.1016/j.jchromb.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Matern D, Tortorelli S, Oglesbee D, Gavrilov D, Rinaldo P. Reduction of the false-positive rate in newborn screening by implementation of MS/MS-based second-tier tests: the Mayo Clinic experience (2004–2007) J Inherit Metab Dis. 2007;30:585–92. doi: 10.1007/s10545-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 64.la Marca G, Malvagia S, Pasquini E, Innocenti M, Donati MA, Zammarchi E. Rapid 2nd-tier test for measurement of 3-OH-propionic and methylmalonic acids on dried blood spots: reducing the false-positive rate for propionylcarnitine during expanded newborn screening by liquid chromatography-tandem mass spectrometry. Clin Chem. 2007;53:1364–9. doi: 10.1373/clinchem.2007.087775. [DOI] [PubMed] [Google Scholar]
- 65.Minutti CZ, Lacey JM, Magera MJ, Hahn SH, McCann M, Schulze A, et al. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89:3687–93. doi: 10.1210/jc.2003-032235. [DOI] [PubMed] [Google Scholar]
- 66.Lacey JM, Minutti CZ, Magera MJ, Tauscher AL, Casetta B, McCann M, et al. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin Chem. 2004;50:621–5. doi: 10.1373/clinchem.2003.027193. [DOI] [PubMed] [Google Scholar]
- 67.Janzen N, Peter M, Sander S, Steuerwald U, Terhardt M, Holtkamp U, et al. Newborn screening for congenital adrenal hyperplasia: additional steroid profile using liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2007;92:2581–9. doi: 10.1210/jc.2006-2890. [DOI] [PubMed] [Google Scholar]
- 68.Janzen N, Sander S, Terhardt M, Peter M, Sander J. Fast and direct quantification of adrenal steroids by tandem mass spectrometry in serum and dried blood spots. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;861:117–22. doi: 10.1016/j.jchromb.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Pitt JJ. High-throughput urine screening for Smith-Lemli-Opitz syndrome and cerebrotendinous xanthomatosis using negative electrospray tandem mass spectrometry. Clin Chim Acta. 2007;380:81–8. doi: 10.1016/j.cca.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Danton M, Lim CK. Porphyrin profiles in blood, urine and faeces by HPLC/electrospray ionization tandem mass spectrometry. Biomed Chromatogr. 2006;20:612–21. doi: 10.1002/bmc.656. [DOI] [PubMed] [Google Scholar]
- 71.Ford RE, Magera MJ, Kloke KM, Chezick PA, Fauq A, McConnell JP. Quantitative measurement of porphobilinogen in urine by stable-isotope dilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2001;47:1627–32. [PubMed] [Google Scholar]
- 72.van Lenthe H, van Kuilenburg AB, Ito T, Bootsma AH, van Cruchten A, Wada Y, et al. Defects in pyrimidine degradation identified by HPLC-electrospray tandem mass spectrometry of urine specimens or urine-soaked filter paper strips. Clin Chem. 2000;46:1916–22. [PubMed] [Google Scholar]
- 73.Hartmann S, Okun JG, Schmidt C, Langhans CD, Garbade SF, Burgard P, et al. Comprehensive detection of disorders of purine and pyrimidine metabolism by HPLC with electrospray ionization tandem mass spectrometry. Clin Chem. 2006;52:1127–37. doi: 10.1373/clinchem.2005.058842. [DOI] [PubMed] [Google Scholar]
- 74.Ito T, van Kuilenburg AB, Bootsma AH, Haasnoot AJ, van Cruchten A, Wada Y, et al. Rapid screening of high-risk patients for disorders of purine and pyrimidine metabolism using HPLC-electrospray tandem mass spectrometry of liquid urine or urine-soaked filter paper strips. Clin Chem. 2000;46:445–52. [PubMed] [Google Scholar]
- 75.Al-Dirbashi OY, Santa T, Rashed MS, Al-Hassnan Z, Shimozawa N, Chedrawi A, et al. Rapid UPLC-MS/ MS method for routine analysis of plasma pristanic, phytanic, and very long chain fatty acid markers of peroxisomal disorders. J Lipid Res. 2008;49:1855–62. doi: 10.1194/jlr.D800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Johnson DW. A rapid screening procedure for the diagnosis of peroxisomal disorders: quantification of very long-chain fatty acids, as dimethylaminoethyl esters, in plasma and blood spots, by electrospray tandem mass spectrometry. J Inherit Metab Dis. 2000;23:475–86. doi: 10.1023/a:1005612214179. [DOI] [PubMed] [Google Scholar]
- 77.Johnson DW, Trinh M-U, Oe T. Measurement of plasma pristanic, phytanic and very long chain fatty acids by liquid chromatography-electrospray tandem mass spectrometry for the diagnosis of peroxisomal disorders. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;798:159–62. doi: 10.1016/j.jchromb.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 78.Yousef IM, Perwaiz S, Lamireau T, Tuchweber B. Urinary bile acid profile in children with inborn errors of bile acid metabolism and chronic cholestasis; screening technique using electrospray tandem mass-spectrometry (ES/MS/MS) Med Sci Monit. 2003;9:MT21–31. [PubMed] [Google Scholar]
- 79.Taylor PJ. Therapeutic drug monitoring of immunosuppressant drugs by high-performance liquid chromatography-mass spectrometry. Ther Drug Monit. 2004;26:215–9. doi: 10.1097/00007691-200404000-00023. [DOI] [PubMed] [Google Scholar]
- 80.Yang Z, Wang S. Recent development in application of high performance liquid chromatography-tandem mass spectrometry in therapeutic drug monitoring of immunosuppressants. J Immunol Methods. 2008;336:98–103. doi: 10.1016/j.jim.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 81.ter Heine R, Alderden-Los CG, Rosing H, Hillebrand MJX, van Gorp ECM, Huitema ADR, et al. Fast and simultaneous determination of darunavir and eleven other antiretroviral drugs for therapeutic drug monitoring: method development and validation for the determination of all currently approved HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors in human plasma by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:2505–14. doi: 10.1002/rcm.3119. [DOI] [PubMed] [Google Scholar]
- 82.Gehrig A-K, Mikus G, Haefeli WE, Burhenne J. Electrospray tandem mass spectroscopic characterisation of 18 antiretroviral drugs and simultaneous quantification of 12 antiretrovirals in plasma. Rapid Commun Mass Spectrom. 2007;21:2704–16. doi: 10.1002/rcm.3138. [DOI] [PubMed] [Google Scholar]
- 83.Koal T, Sibum M, Koster E, Resch K, Kaever V. Direct and fast determination of antiretroviral drugs by automated online solid-phase extraction-liquid chromatography-tandem mass spectrometry in human plasma. Clin Chem Lab Med. 2006;44:299–305. doi: 10.1515/CCLM.2006.052. [DOI] [PubMed] [Google Scholar]
- 84.Stokvis E, Rosing H, Beijnen JH. Liquid chromatography-mass spectrometry for the quantitative bioanalysis of anticancer drugs. Mass Spectrom Rev. 2005;24:887–917. doi: 10.1002/mas.20046. [DOI] [PubMed] [Google Scholar]
- 85.Mueller CA, Weinmann W, Dresen S, Schreiber A, Gergov M. Development of a multi-target screening analysis for 301 drugs using a QTrap liquid chromatography/tandem mass spectrometry system and automated library searching. Rapid Commun Mass Spectrom. 2005;19:1332–8. doi: 10.1002/rcm.1934. [DOI] [PubMed] [Google Scholar]
- 86.Gergov M, Ojanpera I, Vuori E. Simultaneous screening for 238 drugs in blood by liquid chromatography-ion spray tandem mass spectrometry with multiple-reaction monitoring. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;795:41–53. doi: 10.1016/s1570-0232(03)00498-7. [DOI] [PubMed] [Google Scholar]
- 87.Herrin GL, McCurdy HH, Wall WH. Investigation of an LC-MS-MS (QTrap) method for the rapid screening and identification of drugs in postmortem toxicology whole blood samples. J Anal Toxicol. 2005;29:599–606. doi: 10.1093/jat/29.7.599. [DOI] [PubMed] [Google Scholar]
- 88.Deveaux M, Cheze M, Pepin G. The role of liquid chromatography-tandem mass spectrometry (LC-MS/ MS) to test blood and urine samples for the toxicological investigation of drug-facilitated crimes. Ther Drug Monit. 2008;30:225–8. doi: 10.1097/FTD.0b013e3181676186. [DOI] [PubMed] [Google Scholar]
- 89.Sauvage F-L, Saint-Marcoux F, Duretz B, Deporte D, Lachatre G, Marquet P. Screening of drugs and toxic compounds with liquid chromatography-linear ion trap tandem mass spectrometry. Clin Chem. 2006;52:1735–42. doi: 10.1373/clinchem.2006.067116. [DOI] [PubMed] [Google Scholar]
- 90.Marquet P, Saint-Marcoux F, Gamble TN, Leblanc JCY. Comparison of a preliminary procedure for the general unknown screening of drugs and toxic compounds using a quadrupole-linear ion-trap mass spectrometer with a liquid chromatography-mass spectrometry reference technique. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;789:9–18. doi: 10.1016/s1570-0232(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 91.Pavlic M, Libiseller K, Oberacher H. Combined use of ESI-QqTOF-MS and ESI-QqTOF-MS/MS with mass-spectral library search for qualitative analysis of drugs. Anal Bioanal Chem. 2006;386:69–82. doi: 10.1007/s00216-006-0634-8. [DOI] [PubMed] [Google Scholar]
- 92.Marquet P. Progress of liquid chromatography-mass spectrometry in clinical and forensic toxicology. Ther Drug Monit. 2002;24:255–76. doi: 10.1097/00007691-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 93.Marquet P. Is LC-MS suitable for a comprehensive screening of drugs and poisons in clinical toxicology? Ther Drug Monit. 2002;24:125–33. doi: 10.1097/00007691-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 94.Maurer HH. Multi-analyte procedures for screening for and quantification of drugs in blood, plasma, or serum by liquid chromatography-single stage or tandem mass spectrometry (LC-MS or LC-MS/MS) relevant to clinical and forensic toxicology. Clin Biochem. 2005;38:310–8. doi: 10.1016/j.clinbiochem.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 95.Maurer HH. Current role of liquid chromatography-mass spectrometry in clinical and forensic toxicology. Anal Bioanal Chem. 2007;388:1315–25. doi: 10.1007/s00216-007-1248-5. [DOI] [PubMed] [Google Scholar]
- 96.Fitzgerald RL, Rivera JD, Herold DA. Broad spectrum drug identification directly from urine, using liquid chromatography-tandem mass spectrometry. Clin Chem. 1999;45:1224–34. [PubMed] [Google Scholar]
- 97.Nordgren HK, Beck O. Multicomponent screening for drugs of abuse: direct analysis of urine by LC-MS-MS. Ther Drug Monit. 2004;26:90–7. doi: 10.1097/00007691-200402000-00017. [DOI] [PubMed] [Google Scholar]
- 98.Sauvage F-L, Gaulier J-M, Lachatre G, Marquet P. Pitfalls and prevention strategies for liquid chromatography-tandem mass spectrometry in the selected reaction-monitoring mode for drug analysis. Clin Chem. 2008;54:1519–27. doi: 10.1373/clinchem.2008.105478. [DOI] [PubMed] [Google Scholar]
- 99.Oiestad EL, Johansen U, Christophersen AS. Drug screening of preserved oral fluid by liquid chromatography-tandem mass spectrometry. Clin Chem. 2007;53:300–9. doi: 10.1373/clinchem.2006.074237. [DOI] [PubMed] [Google Scholar]
- 100.Hegstad S, Khiabani HZ, Kristoffersen L, Kunoe N, Lobmaier PP, Christophersen AS. Drug screening of hair by liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2008;32:364–72. doi: 10.1093/jat/32.5.364. [DOI] [PubMed] [Google Scholar]
- 101.Miller EI, Wylie FM, Oliver JS. Simultaneous detection and quantification of amphetamines, diazepam and its metabolites, cocaine and its metabolites, and opiates in hair by LC-ESI-MS-MS using a single extraction method. J Anal Toxicol. 2008;32:457–69. doi: 10.1093/jat/32.7.457. [DOI] [PubMed] [Google Scholar]
- 102.Maunsell Z, Wright DJ, Rainbow SJ. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem. 2005;51:1683–90. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 103.Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SMH. Quantification of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125:914–20. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 104.Tsugawa N, Suhara Y, Kamao M, Okano T. Determination of 25-hydroxyvitamin D in human plasma using high-performance liquid chromatography--tandem mass spectrometry. Anal Chem. 2005;77:3001–7. doi: 10.1021/ac048249c. [DOI] [PubMed] [Google Scholar]
- 105.Higashi T, Shibayama Y, Fuji M, Shimada K. Liquid chromatography-tandem mass spectrometric method for the determination of salivary 25-hydroxyvitamin D3: a noninvasive tool for the assessment of vitamin D status. Anal Bioanal Chem. 2008;391:229–38. doi: 10.1007/s00216-007-1780-3. [DOI] [PubMed] [Google Scholar]
- 106.Higashi T, Homma S, Iwata H, Shimada K. Characterization of urinary metabolites of vitamin D(3) in man under physiological conditions using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2002;29:947–55. doi: 10.1016/s0731-7085(02)00135-8. [DOI] [PubMed] [Google Scholar]
- 107.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–9. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]
- 108.Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays.[see comment] Clin Chem. 2006;52:1120–6. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 109.Terry AH, Sandrock T, Meikle AW. Measurement of 25-hydroxyvitamin D by the Nichols ADVANTAGE, DiaSorin LIAISON, DiaSorin RIA, and liquid chromatography-tandem mass spectrometry. Clin Chem. 2005;51:1565–6. doi: 10.1373/clinchem.2005.054239. [DOI] [PubMed] [Google Scholar]
- 110.Priego Capote F, Jimenez JR, Granados JMM, de Castro MDL. Identification and determination of fat-soluble vitamins and metabolites in human serum by liquid chromatography/triple quadrupole mass spectrometry with multiple reaction monitoring. Rapid Commun Mass Spectrom. 2007;21:1745–54. doi: 10.1002/rcm.3014. [DOI] [PubMed] [Google Scholar]
- 111.Midttun O, Hustad S, Solheim E, Schneede J, Ueland PM. Multianalyte quantification of vitamin B6 and B2 species in the nanomolar range in human plasma by liquid chromatography-tandem mass spectrometry. Clin Chem. 2005;51:1206–16. doi: 10.1373/clinchem.2005.051169. [DOI] [PubMed] [Google Scholar]
- 112.Pfeiffer CM, Fazili Z, McCoy L, Zhang M, Gunter EW. Determination of folate vitamers in human serum by stable-isotope-dilution tandem mass spectrometry and comparison with radioassay and microbiologic assay. Clin Chem. 2004;50:423–32. doi: 10.1373/clinchem.2003.026955. [DOI] [PubMed] [Google Scholar]
- 113.Kushnir MM, Komaromy-Hiller G, Shushan B, Urry FM, Roberts WL. Analysis of dicarboxylic acids by tandem mass spectrometry. High-throughput quantitative measurement of methylmalonic acid in serum, plasma, and urine. Clin Chem. 2001;47:1993–2002. [PubMed] [Google Scholar]
- 114.Schmedes A, Brandslund I. Analysis of methylmalonic acid in plasma by liquid chromatography-tandem mass spectrometry. Clin Chem. 2006;52:754–7. doi: 10.1373/clinchem.2005.058586. [DOI] [PubMed] [Google Scholar]
- 115.Magera MJ, Lacey JM, Casetta B, Rinaldo P. Method for the determination of total homocysteine in plasma and urine by stable isotope dilution and electrospray tandem mass spectrometry. Clin Chem. 1999;45:1517–22. [PubMed] [Google Scholar]
- 116.Kuhn J, Gotting C, Kleesiek K. Rapid micro-scale assay for homocysteine by liquid chromatography-tandem mass spectrometry. Clin Biochem. 2006;39:164–6. doi: 10.1016/j.clinbiochem.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 117.Arndt T, Guessregen B, Hohl A, Heicke B. Total plasma homocysteine measured by liquid chromatography-tandem mass spectrometry with use of 96-well plates. Clin Chem. 2004;50:755–7. doi: 10.1373/clinchem.2003.029686. [DOI] [PubMed] [Google Scholar]
- 118.Tuschl K, Bodamer OA, Erwa W, Muhl A. Rapid analysis of total plasma homocysteine by tandem mass spectrometry. Clin Chim Acta. 2005;351:139–41. doi: 10.1016/j.cccn.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 119.Li S, Jia J, Liu G, Wang W, Cai Y, Wang Y, et al. Improved and simplified LC-ESI-MS/MS method for homocysteine determination in human plasma: application to the study of cardiovascular diseases. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;870:63–7. doi: 10.1016/j.jchromb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 120.McCann SJ, Gillingwater S, Keevil BG, Cooper DP, Morris MR. Measurement of total homocysteine in plasma and blood spots using liquid chromatography-tandem mass spectrometry: comparison with the plasma Abbott IMx method. Ann Clin Biochem. 2003;40:161–5. doi: 10.1258/000456303763046094. [DOI] [PubMed] [Google Scholar]
- 121.Gempel K, Gerbitz KD, Casetta B, Bauer MF. Rapid determination of total homocysteine in blood spots by liquid chromatography-electrospray ionization-tandem mass spectrometry. Clin Chem. 2000;46:122–3. [PubMed] [Google Scholar]
- 122.Weaving G, Rocks BF, Iversen SA, Titheradge MA. Simultaneous quantitation of homocysteine, cysteine and methionine in plasma and urine by liquid chromatography-tandem mass spectrometry. Ann Clin Biochem. 2006;43:474–80. doi: 10.1258/000456306778904605. [DOI] [PubMed] [Google Scholar]
- 123.Rafii M, Elango R, Courtney-Martin G, House JD, Fisher L, Pencharz PB. High-throughput and simultaneous measurement of homocysteine and cysteine in human plasma and urine by liquid chromatography-electrospray tandem mass spectrometry. Anal Biochem. 2007;371:71–81. doi: 10.1016/j.ab.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 124.Hempen C, Wanschers H, van der Sluijs Veer G. A fast liquid chromatographic tandem mass spectrometric method for the simultaneous determination of total homocysteine and methylmalonic acid. Anal Bioanal Chem. 2008;391:263–70. doi: 10.1007/s00216-008-1953-8. [DOI] [PubMed] [Google Scholar]
- 125.Nelson BC, Satterfield MB, Sniegoski LT, Welch MJ. Simultaneous quantification of homocysteine and folate in human serum or plasma using liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77:3586–93. doi: 10.1021/ac050235z. [DOI] [PubMed] [Google Scholar]
- 126.Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Bunker AM, Fitzgerald RL, et al. Performance characteristics of a novel tandem mass spectrometry assay for serum testosterone. Clin Chem. 2006;52:120–8. doi: 10.1373/clinchem.2005.052167. [DOI] [PubMed] [Google Scholar]
- 127.Cawood ML, Field HP, Ford CG, Gillingwater S, Kicman A, Cowan D, et al. Testosterone measurement by isotope-dilution liquid chromatography-tandem mass spectrometry: validation of a method for routine clinical practice. Clin Chem. 2005;51:1472–9. doi: 10.1373/clinchem.2004.044503. [DOI] [PubMed] [Google Scholar]
- 128.Moal V, Mathieu E, Reynier P, Malthiery Y, Gallois Y. Low serum testosterone assayed by liquid chromatography-tandem mass spectrometry. Comparison with five immunoassay techniques. Clin Chim Acta. 2007;386:12–9. doi: 10.1016/j.cca.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 129.Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS, Wang C. Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry. Clim Chem. 2008;54:1855–63. doi: 10.1373/clinchem.2008.103846. [DOI] [PubMed] [Google Scholar]
- 130.Kushnir MM, Rockwood AL, Nelson GJ, Terry AH, Meikle AW. Liquid chromatography-tandem mass spectrometry analysis of urinary free cortisol. Clin Chem. 2003;49:965–7. doi: 10.1373/49.6.965. [DOI] [PubMed] [Google Scholar]
- 131.McCann SJ, Gillingwater S, Keevil BG. Measurement of urinary free cortisol using liquid chromatography-tandem mass spectrometry: comparison with the urine adapted ACS:180 serum cortisol chemiluminescent immunoassay and development of a new reference range. Ann Clin Biochem. 2005;42:112–8. doi: 10.1258/0004563053492775. [DOI] [PubMed] [Google Scholar]
- 132.Taylor RL, Machacek D, Singh RJ. Validation of a high-throughput liquid chromatography-tandem mass spectrometry method for urinary cortisol and cortisone. Clin Chem. 2002;48:1511–9. [PubMed] [Google Scholar]
- 133.Wood L, Ducroq DH, Fraser HL, Gillingwater S, Evans C, Pickett AJ, et al. Measurement of urinary free cortisol by tandem mass spectrometry and comparison with results obtained by gas chromatography-mass spectrometry and two commercial immunoassays. Ann Clin Biochem. 2008;45:380–8. doi: 10.1258/acb.2007.007119. [DOI] [PubMed] [Google Scholar]
- 134.Guo T, Taylor RL, Singh RJ, Soldin SJ. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin Chim Acta. 2006;372:76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 135.Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Owen WE, Bunker AM, et al. Development and performance evaluation of a tandem mass spectrometry assay for 4 adrenal steroids. Clin Chem. 2006;52:1559–67. doi: 10.1373/clinchem.2006.068445. [DOI] [PubMed] [Google Scholar]
- 136.Buiarelli F, Coccioli F, Merolle M, Neri B, Terracciano A. Development of a liquid chromatography tandem mass spectrometry method for the identification of natural androgen steroids and their conjugates in urine samples. Anal Chim Acta. 2004;526:113–20. [Google Scholar]
- 137.Pozo OJ, Van Eenoo P, Van Thuyne W, Deventer K, Delbeke FT. Direct quantification of steroid glucuronides in human urine by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2008;1183:108–18. doi: 10.1016/j.chroma.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 138.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics. 2007;6:2212–29. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hortin GL. A new era in protein quantification in clinical laboratories: application of liquid chromatography-tandem mass spectrometry. Clin Chem. 2007;53:543–4. doi: 10.1373/clinchem.2006.083857. [DOI] [PubMed] [Google Scholar]


