Abstract
Biochemical testing for phaeochromocytoma is performed in diagnostic laboratories using a variety of tests with plasma, serum or 24-hour urine collections. These tests include catecholamines and their methylated metabolites - the metanephrines, either individually or in combination with their sulfated metabolites. High-performance liquid chromatography (HPLC) continues to be the dominant analytical method for biogenic amine quantitation. Chromatographic techniques are changing, with improvements in sample preparation procedures, column technology and more specific analyte detection using tandem mass spectrometry. Enrolments in quality assurance programs indicate that there are still many more laboratories in Australasia analysing urinary catecholamines than metanephrines. Nevertheless, clinical evidence and expert opinion favour metanephrines as the analytes with highest sensitivity for the detection of phaeochromocytoma. Practical issues such as better chemical stability and easier specimen collection also favour metanephrines over catecholamines. For these reasons, it is likely that laboratories increasingly will replace urine catecholamine testing with either plasma or urine metanephrines. However in interpreting positive results, the need remains to consider issues such as pre-test probability and use of potentially interfering medications.
Introduction
For decades, the detection and monitoring of neuroendocrine tumours has been greatly assisted by laboratory measurement of the biogenic amines and their metabolites. With the development of new technologies, biochemical methods for the quantitation of biogenic amines have improved. To select the best diagnostic tests from the many available there is a growing evidence-base of clinical studies. This review focuses on some recent analytical advances for the assay of catecholamines and metanephrines in biological fluids. The biochemical and biological rationale for these assays is discussed along with the application of these methods in clinical studies.
Origins and Clinical Features of Phaeochromocytoma
Phaeochromocytomas are rare neuroendocrine tumours that produce catecholamines. The name phios – dusky, chroma – colour, and cytoma – tumour refers to the colour of the tumour cells when stained with chromium salts. Catecholamine-producing neuroendocrine cells are consequently known as chromaffin cells and are usually found in the adrenal medulla and other ganglia of the sympathetic nervous system.1 Some authors restrict the use of phaeochromocytoma to tumours of the adrenal medulla, while others use the term more broadly to include paragangliomas. The defining characteristic of phaeochromocytoma in clinical practice is the autonomous production of catecholamines.2
Felix Frankel is credited with the first clinical description of phaeochromocytoma.3,4 This case has been made more interesting by a subsequent study tracing descendants of the original patient and undertaking genetic testing.5 The investigators demonstrated a RET gene germ-line mutation in the kindred, thus diagnosing multiple endocrine neoplasia type 2 (MEN-2) 121 years after the original presentation. This study elegantly illustrates the importance of germ-line mutations in patients with phaeochromocytoma and this possibility should always be considered.
Phaeochromocytoma may cause problems for patients in two ways: firstly, due to adverse effects caused by the autonomous production of catecholamines and secondly, due to malignancy and metastatic spread. Case series report 5–20% of phaeochromocytomas as malignant.6–8 The five-year survival of malignant phaeochromocytoma is <50% and first-line treatment is surgery.9–11 Chemotherapy and radionuclide treatments are also used, but data is limited due to the rarity of the condition and the reported experience has been disappointing.10,11 Trials are underway of potentially more effective treatments, particularly using new radioisotope therapies, but currently surgery remains the mainstay of treatment.12
The consequences of catecholamine excess are many and varied. The classical clinical triad of hypertension, headache and sweating is of limited clinical utility, as it is neither sensitive nor specific for phaeochromocytoma.13–16 Patients may have symptoms related to any adrenoreceptor agonist effect or may have no symptoms at all. In the past, patients were most often diagnosed during investigation of hypertension or unexplained spells. More recently, with the widespread use of CT and MRI scanning, phaeochromocytoma is increasingly diagnosed during investigation of adrenal mass lesions identified incidentally by abdominal imaging studies carried out for other reasons.15–20 Patients thus come to attention in three main ways: “dif cult” hypertension, unexplained spells, or by incidental radiological identification of an adrenal mass. A fourth, familial screening, may soon be added to this list.21,22 For patients with hypertension or spells, the presence of additional features suggestive of catecholamine excess should be considered prior to biochemical investigation.23–26 For those with adrenal mass lesions, biochemical investigation is nearly always indicated.15–20,27 Biochemical testing for possible phaeochromocytoma is the primary focus of this review.
Genetic Syndromes of Phaeochromocytoma
The genetic syndromes commonly associated with phaeochromocytoma are shown in Table 1.8,21,22,28–32 These vary in age of onset, location, secretory product and propensity for malignancy. The mean age of diagnosis of phaeochromocytoma in Von Hippel Lindau syndrome is about 20 years, whereas in Neuro bromatosis Type 1 it is about 42 years, similar to sporadic cases.28,29 The incidence of malignancy in phaeochromocytoma associated with succinate dehydrogenase complex, subunit B (SDH-B) mutations is about 1 in 3, whereas with SDH-D they are nearly all benign.30–32 Genetic testing is not considered further in this discussion and for more information readers can refer to recent reviews.33–36
Table 1.
Selected characteristics of genetic syndromes associated with phaeochromocytoma.
| Syndrome/Gene | Locus | Age at diagnosis Mean (range) | Characteristics of phaeochromocytoma |
|---|---|---|---|
| MEN-2/RET | 10q11.2 | 35 (18-50) | bilateral benign adrenergic |
| NF1 | 17111.2 | 40 (25-70) | similar to sporadic |
| VHL | 3p25-25 | 20 (5-50) | bilateral benign noradrenergic |
| SDH-B | 1p36.1-p35 | 25 (10-60) | paragangliomas malignant |
| SDH-D | 11q23 | 30 (5-65) | paragangliomas |
MEN-2 – Multiple Endocrine Neoplasia Type 2
NF1 – Neurofibromatosis Type 1
VHL – Von Hippel-Lindau
SDH – Succinate dehydrogenase
Catecholamine Synthesis and Metabolism
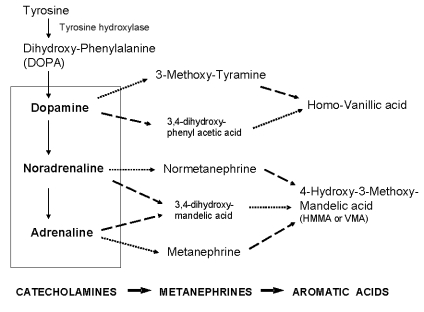
Catecholamines are synthesised from the amino acid tyrosine, which is hydroxylated and decarboxylated to dopamine, as shown in Figure 1. Dopamine is then converted to noradrenaline (also called norepinephrine), which is methylated at the amino group to form adrenaline (also called epinephrine). The rate-limiting step in catecholamine biosynthesis is catalysed by the mono-oxygenase enzyme tyrosine hydroxylase,37 which requires tetrahydrobiopterin as a co-factor in the reaction.38 This knowledge is applied therapeutically when metyrosine, a competitive inhibitor of tyrosine hydroxylase, is used to treat catecholamine excess.
Figure 1.
Biochemical pathways for the metabolism of catecholamines. The dotted line shows reactions catalysed by catechol-O-methyl-transferase, while the dashed line indicates reactions catalysed by monoamine oxidase.
Catecholamines are produced in neurons of the central nervous system, in sympathetic nerves and in chromaffin cells of the adrenal medulla. They are taken up and stored in vesicular granules, where they exist in a highly dynamic state with passive leakage outwards balanced by active transport inwards.39 Monoamines share the storage granule matrix with peptides and proteins, such as chromogranin A.40 When released into the extracellular space, catecholamines are taken up by neuronal and non-neuronal transporters to rapidly terminate signal transmissions and are cleared from the bloodstream with a half-life of less than three minutes.41–43
Catecholamines taken up into cells by transporters can be metabolised by O-methylation to metanephrines (also called metadrenalines) by the enzyme catechol-O-methyl-transferase (COMT), or deaminated by monamine oxidase (MAO) to intermediates such as dihydroxy-phenylacetic acid (DOPAC), dihydroxy-phenylglycol (DHPG) and dihydroxy mandelic acid (Figure 1). These latter intermediates can then be further metabolised by COMT, leading to homovanillic acid (HVA) from dopamine and hydroxy-methoxy-mandelic acid [HMMA, also called VMA (vanillylmandelic acid)] from noradrenaline and adrenaline. As a result of differential expression of the enzymes MAO and COMT, catecholamines produced at neuronal and adrenal medullary locations follow different pathways of metabolism.44 In adrenal chromaffin cells, catecholamines are metabolised by COMT, accounting for more than 90% of circulating metanephrine (also called metadrenaline) and 24% to 40% of circulating normetanephrine (also called normetadrenaline).45 In contrast, most noradrenaline produced by the nervous system is metabolised initially to DHPG in sympathetic nerves and released into the plasma, where it is taken up by the liver and converted to HMMA.39 Except for HMMA, all the catecholamines and their metabolites can be conjugated with sulfate by the sulfotransferase enzyme (SULT1A3).46 The conjugated compounds are water soluble and eliminated by urinary excretion. A more detailed description of catecholamine metabolism is given by Eisenhofer et al. 39
Chromatographic Methods for Catecholamines and Metabolites
The most widely used method in the routine clinical laboratory for the measurement of urinary catecholamines and their metabolites is HPLC. All large pathology laboratories would be expected to have at least one instrument, which can be purchased from a number of commercial suppliers. Original methods relied on fluorescence detection to gain analytical sensitivity,47,48 but the availability of electrochemical detection provided superior specificity and this mode of detection is the most common today.49 However, the use of tandem mass spectrometry is becoming more popular as laboratories realise its advantages, particularly in detecting the low concentrations of metanephrines present in plasma.
Sample Preparation
Prior to chromatography, some pre-analytical clean-up step is required to extract and sometimes concentrate the catecholamines or metanephrines from the biological urine or plasma matrix. For catecholamines, urine is processed directly to isolate only free noradrenaline, adrenaline and dopamine, while for urine metanephrines an acid hydrolysis step is nearly always included to release the conjugated forms, which are the major molecular species present. In an acidified urine, the biogenic amines are protonated as cations, and can be isolated on alumina columns or commercial cartridges packed with ion-exchange supports.47,49–51 To increase processing speed, vacuum manifolds are used to facilitate conditioning, loading, washing and elution steps. Alternatively, fully automated solid-phase extraction (SPE) systems can be purchased to handle larger numbers of samples.49
To extract catecholamines from urine, advantage can be taken of the unique affinity of boronate compounds for the cisdiol structural moiety, to which a covalent linkage is formed at alkaline pH.52 The complexed catecholamines can then be subjected to liquid-liquid extraction53 or direct immobilisation is possible with solid-phase extraction on PBA (phenylboronic acid) cartridges54 followed by elution of the catecholamines under acidic conditions. Talwar et al. found it more convenient to extract the complexed catecholamines from solution with C18-bonded silica cartridges at pH 8.5, and then elute them with acid, as this avoided solvent extraction steps while stabilising the catecholamines from oxidation.55 This approach has been extended to SPE on Bond Elut Plexa, a new hydrophobic-hydrophilic synthetic support with improved binding properties for small molecule extractions from biological fluids, and recovery of biogenic amines in formic acid which is compatible with mass spectrometry.56
Acid-hydrolysed urines are used routinely for total metanephrine measurement, but are often heavily pigmented and require clean-up prior to HPLC separation of normetanephrine and metanephrine. Isolation procedures used in the past involve solvent extraction, or SPE on cation-exchange resins.49 A popular option, which is available commercially, uses dual cation-exchange and anion-exchange columns to separate metanephrines from catecholamines, non-phenolic amines and other interfering hydrophobic compounds.49,57 Recently, an SPE method developed for free catecholamine extraction from urine was found to also recover metanephrines with high yield. This allowed the simultaneous measurement of urinary metanephrines with catecholamines following HPLC separation and detection by tandem mass spectrometry.56
For plasma free metanephrines, direct extraction is possible by SPE on polymer-based supports after the addition of internal standard to correct for extraction efficiency.58 To fully automate the analytical process, an on-line cation-exchange cartridge was used recently in combination with tandem mass spectrometry to produce a run time of 11 minutes per sample.59,60 This allows high throughput of plasma samples but is restricted to large laboratories with sufficient analytical resources to purchase and operate the sample processor as well as the tandem mass spectrometric instrumentation. A recent simple alternative to SPE techniques used isopropanol to precipitate and remove proteins prior to normal phase liquid chromatography with tandem mass spectrometry.61
Chromatography
In the past few years, manufacturers of HPLC columns have released new column technology with smaller particle sizes (sub-2 micron particle diameter) to increase sample throughput in chromatography laboratories.62 These columns have increased resolving power such that the same separations of mixtures of compounds can be achieved in much shorter run times. However, for laboratories to utilise these new columns some upgrade of older HPLC systems may be required to withstand the higher operating pressures. Since analytical methods can be transposed to the new faster HPLC columns without major modifications, this advance is of potential benefit to laboratories that process high workloads.
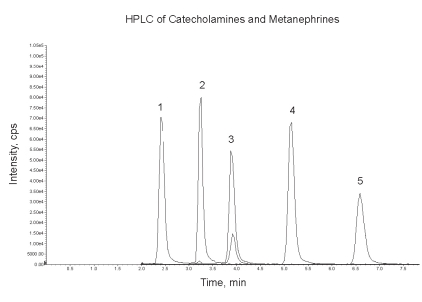
Another recent advance in HPLC is the development of new column phases based on reversed-phase chromatography with modifications to allow retention and separation of polar compounds, such as the biogenic amines, without using ion-pairing reagents. Examples of this type of column packing are the Atlantis dC18 or T3 columns (Waters) and Synergi Hydro or Polar-RP (Phenomenex). Simple acidic mobile phases with pH as low as 2 can be used for separation of catecholamines and metanephrines, as well as other biogenic amines such as HVA, serotonin and 5HIAA. An example of the chromatographic separation of catecholamines and metanephrines obtained on an Atlantis T3 column is shown in Figure 2, with detection by tandem mass spectrometry.
Figure 2.
Separation of five biogenic amines (50 pmol each on-column) on an Atlantis T3 column (150 x 2.1 mm, 3 μm particle size) at 25°C with a flow rate of 200 μL/min of 0.2% formic acid-methanol (98/2, v/v). Peaks were detected by tandem mass spectrometry with multiple reaction monitoring of the ion transition pairs as follows: (1) noradrenaline 170→152, (2) adrenaline 184→166, (3) normetanephrine 166→134, (4) dopamine 154→137 and (5) metanephrine 180→148. The smaller normetanephrine peak is due to the 184→166 ion pair which is common with adrenaline.
Chromatographic Detection
The most widely used method for the detection of catecholamines and metanephrines that have been extracted from urine or plasma and separated by HPLC is electrochemical detection (ECD). This is illustrated by enrolments in the biogenic amines quality assurance program of RCPA Pty Ltd where over 90% of laboratories state their method of analysis as HPLC with ECD. While this detector is very sensitive, it requires a high level of maintenance with frequent cleaning of the electrode and analytical interference from drugs may occur, in particular, paracetamol metabolites and the sympathomimetics.63,64 Issues relating to drug interference are discussed below.
Analytical interference problems are minimised by the higher analytical specificity of tandem mass spectrometry. A summary of published tandem mass spectrometry methods for catecholamines, metanephrines and other metabolites is shown in Table 2.56,58,60,65–68 All methods make use of internal standards labelled with heavy isotopes such as deuterium, as these are essential to correct for ion suppression effects and any losses that occur in the sample preparation procedure. Although the use of HPLC coupled to tandem mass spectrometry still has some way to go for widespread adoption in clinical chemistry laboratories,59,69 for those who have the equipment and operating resources, the technique does improve workflows and the reliability of analytical results for small molecules that are not easily quantified by automated immunoassays. An ELISA kit has been developed commercially and used to measure total urine metanephrines, after their conversion to acyl derivatives,70 but the accuracy of immunoassays with respect to plasma free metanephrines has not been well established.
Table 2.
Features of published methods for the determination of biogenic amines by tandem mass spectrometry.
| Analyte | Fluid/Volume | Clean-Up | LC Column | MRM Ion Pairs m/z | Imprecision Inter-assay CV | Internal Standards | Reference |
|---|---|---|---|---|---|---|---|
| Free catecholamines | Urine/0.3 mL | Liquid-liquid extraction (LLE) | Allure Basix
50 × 2 mm RT 3.5 min |
9 ion pairs | 4 to 7% | Deuterated | Kushnir et al. 65 |
| Total metanephrines | Urine/1 mL | SPE Oasis HLB | RP Amide C16
50 × 4.6 mm RT 3 min |
180 to 148
183 to 151 166 to 134 169 to 137 |
6 to 9% | Tri-Deuterated | Taylor and Singh 66 |
| Free metanephrines | Plasma/1 mL | SPE Oasis HLB | Luna CN
150 × 4.6 mm RT 6 min |
180 to 148
183 to 151 166 to 134 169 to 137 |
6 to 13% | Tri-deuterated | Lagerstedt et al. 58 |
| Free metanephrines | Plasma/0.5 mL | On-line SPE | Atlantis HILIC
50 × 2.1 mm RT 8 min |
9 ion pairs | 2 to 14% | Deuterated | De Jong et al. 60 |
| Combined catecholamines and metanephrines | Urine/0.5 mL | SPE Bond-Elut
Plexa |
Atlantis T3
150 × 2.1 mm RT 5 min |
15 ion pairs | 5 to 7% | Four deuterated, one 13C, 15N | Whiting 56 |
| HMMA (VMA) | Urine/0.6 mL | SPE Oasis HLB
Automated |
RP Amide C16
50 × 4.6 mm RT 3 min |
197 to 137
200 to 140 |
2.5% | D3-VMA | Magera et al.67 |
| HVA | Urine/1.2 mL | SPE Accu-Bond
C18 Automated |
RP Amide C16
50 × 4.6 mm RT 3 min |
181 to 137
189 to 145 |
3 to 5% | 13C-18O-HVA | Magera et al.68 |
Diagnostic Efficacy of Biochemical Tests
Requests for biochemical tests for phaeochromocytoma are most often in the context of a low pre-test probability. These are requested as part of the work-up of patients with spells, dif cult to control hypertension, or incidentally identified adrenal masses. Because of the potentially serious consequences of missing a phaeochromocytoma, test thresholds are set for high sensitivity and consequently have only moderate specificity. The combination of low pre-test probability and moderate specificity results in many false positive tests and dealing with these efficiently is a challenge for both clinicians and laboratory scientists.
Choice of initial test
Plasma free metanephrines or urine total metanephrines are the currently recommended biochemical tests for phaeochromocytoma screening.71 Until recently, the recommended initial test was 24-hour urine free catecholamines (adrenaline and noradrenaline).14,72–75 This recommendation was predicated on three principles. Firstly, catecholamines produced by chromaffin cells have a very short plasma half-life, approximately 80 and 150 seconds for adrenaline and noradrenaline respectively.41–43 Thus, plasma sampling lacks reliability. Secondly, urine free catecholamines represent the 24-hour integral of catecholamine production as the total filtered at the glomerulus.76,77 This second premise is weakened by the effect of renal tubular reabsorption of catecholamine.78,79 Thirdly, the concentrations in the urine could be accurately measured by the available laboratory techniques.80 Most importantly, the empirical data from clinical studies had shown 24-hour urine free catecholamines to better predict phaeochromocytoma than other measures available at the time.14,72
In the early 1990s, specific assays for metanephrine and normetanephrine became more available and some of these assays were sufficiently sensitive to accurately quantitate free metanephrine in plasma.81–84 There are two reasons to hypothesise metanephrines as the preferred analyte in assessing chromaffin cell catecholamine production. Firstly, as outlined previously, metanephrines are the catecholamine metabolites of COMT, thus providing a way of preferentially testing chromaffin cell production of catecholamines with reduced interference from catecholamines produced in the sympathetic nervous system. Secondly, metanephrines have a longer half-life than catecholamines improving the reliability of plasma as a substrate.85 The validity of metanephrines as the preferred analyte has been demonstrated in several large case control studies and a meta-analysis using appropriate statistical methods including ROC curve evaluation.84–95 The first International Symposium of Phaeochromocytoma considered this issue in detail and recommended plasma or urine metanephrine testing as the initial test for investigation of suspected phaeochromoctytoma.71 Catecholamine testing is no longer recommended as first line testing for phaeochromocytoma due to lower sensitivity and consequent false negatives.
Many laboratories in the UK have yet to make a switch from catecholamine to metanephrine testing.96 This is also true in Australia as shown by enrolments in the urine biogenic amine quality assurance programme of the Royal College of Pathologists of Australasia. In 2008, there were 45 laboratories enrolled for catecholamines compared with 15 laboratories for metanephrines.
Plasma or Urine
The choice of plasma or urine as test specimen is more controversial. Both have been demonstrated to have high sensitivity for metanephrines. Proponents of plasma point to its very high sensitivity and the convenience of sample collection.86,91 Proponents of urine point to its greater specificity and consequent reduction in false positives.92,94 The consensus guidelines accept either specimen.71 Published sensitivities and specificities are dependent on choice of patient and control groups, choice of test threshold and analytical precision.97 The sensitivity and specificity achieved in clinical practice will additionally depend on factors such as patient preparation and sampling environment.
Free or Total Metanephrines
Metanephrines are metabolised by sulfation and excreted in the urine predominantly as sulfated metabolites.39 Total metanephrines, in this context, refers to the total of free metanephrine and normetanephrine, and their sulfated metabolites. In urine, total metanephrines have been the preferred analyte for two reasons. Firstly, due to the higher concentrations, earlier less-sensitive assays were able to quantitate total metanephrines. Secondly, as the 24-hour urine test is a measure of 24-hour production by measurement of all metanephrines filtered at the glomerulus, total metanephrine should more closely approximate catecholamine production. Free urine metanephrine excretion has not yet been robustly evaluated in clinical studies.
In contrast, metanephrine concentration in plasma is a function of production and clearance. Measurement of free metanephrine reflects this, whereas total metanephrine concentration also reflects a third variable, clearance of the conjugated metanephrine and thus signal is likely to be lost. The validity of this premise has been demonstrated by empirical data from clinical studies.87 It is for these reasons that plasma free metanephrines or urine total metanephrines are the currently recommended biochemical tests for phaeochromocytoma screening.71
Total or Fractionated Metanephrines
These terms have been widely used and cause confusion. Fractionated metanephrines refers to the separate quantitation and reporting of metanephrine and normetanephrine. Total, in this context, refers to the sum of the two analytes. We believe this term should no longer be used as it is confused with “total” meaning metanephrines plus sulfate metabolites. This is further confused as “total” concentration of a hormone or drug usually refers to the total of free and protein bound concentrations excluding metabolites and other related moieties. Although catecholamines and metanephrines do bind with low affinity to albumin and haemoglobin the clinical relevance of this is unknown and this will not be considered further.98,99 In this review, metanephrines refers to separate measurement and reporting of metanephrine and normetanephrine and total refers to the sum of free metanephrine and conjugated metabolite.100
Interpreting Clinical Studies, Pre-test Probability and False Positives
The most useful studies in evaluating a diagnostic test include large numbers of both cases and controls. For rare conditions such populations are only found at major referral centres which see a selected group of patients, and, by definition, relatively fewer patients without phaeochromocytoma. For biochemical investigation of phaeochromocytoma, the US National Institute of Health (NIH) and the Mayo Clinic have reported their data extensively.86–89,91,94 Large European series have been reported from Vienna, Oxford, Essen and Lille.85,92,93,95 Sawka et al. summarised the published data prior to 2004 in a metanalysis.90 Not being major referral centres, Australasian hospitals are likely to have lower case rates than the large US referral centres and thus a greater proportion of false positives can be expected.
False positive tests cause anxiety, inappropriate use of resources and in some cases patient harm. As phaeochromocytoma is very rare, the pre-test probability of phaeochromocytoma is nearly always low. Thus, most patients with a positive initial test will not have a phaeochromocytoma. The best way of reducing false positives is to reduce inappropriate testing. For example, testing all patients with hypertension is not recommended.71 Appropriate testing is on the basis of pre-test probability and patients will usually fall into one of four groups. These are dif cult hypertension, unexplained spells, adrenal masses identified by abdominal imaging and patients with a personal or family history of a genetic syndrome associated with phaeochromocytoma.
Patients with Spells
Due to the lack of discriminating clinical features, it is hard to offer simple rules to further define pre-test probability in these patients. With the frequency of late diagnosis and “missed” phaeochromocytoma, any clinical suspicion should prompt the clinician to “actively exclude phaeochromocytoma”. Clinical clues include: sweating, headache, tremor, pallor or episodes of non-exertional palpitations. Although the presence of an alternative explanation for these symptoms is often sufficient to exclude phaeochromocytoma, biochemical testing can be useful in the investigation of spells.
Hypertensive Patients
The prevalence of phaeochromocytoma in a general hypertensive population has been estimated at about 0.3%.23–25, 101–103 By selecting patients with a history of paroxysms or of “treatment-resistant hypertension”, this can increase to about 2.5%. In this scenario, for a test with a sensitivity of 99% and a specificity of 87%, 40 patients would be tested with five false positives to identify one true case.
Patients with Incidental Adrenal Masses
In these cases pathologies other than phaeochromocytoma also need to be considered. Large series estimate a prevalence of phaeochromocytoma of about 4%.17–20,27,104,105 The imaging characteristics of these lesions can help to discriminate further, for example, low attenuation lesions on non-contrast CT (<10 Hounsefield units) are unlikely to be phaeochromocytomas.19 If there is any doubt, consultation with a clinical endocrinologist is recommended in addition to the evaluation of the possibility of other hyper-secreting adrenal lesions.
Drugs and Other Test Confounders
Noradrenaline from the sympathetic nervous system is the source of ~66% of circulating normetanephrine.39 Thus, anything that increases noradrenaline production or decreases noradrenaline uptake will increase normetanephrine concentrations and may result in false positive tests.106 Physiological stress increases noradrenaline production whereas supine posture and sleep decrease metanephrine production.
The drugs that most commonly give false positive results are those that inhibit noradrenaline uptake or block sufficient adrenoreceptors to cause increased noradrenaline production. These include tricyclic antidepressants, atypical antipsychotics, alpha-2 receptor blockers and beta blockers, as shown in Table 3.106–110 It is the responsibility of the clinician interpreting the test to carefully consider the potential impact of medications. The magnitude of interference is patient- and dose-dependent. Thus, the most practical method of accounting for interferences is to repeat testing under controlled conditions. Although not widely used, monoamine oxidase inhibitors may increase false positive metanephrines by ‘diverting’ peripheral metabolism of noradrenaline to COMT. Paradoxically, amphetamines and xanthines also increase catecholamine concentrations by induction of tyrosine hydroxylase and competitive antagonism of catecholamine transport. A second group of medications can cause false positive results with electrochemical, but not mass spectrometry detection. These are the sympathomimetics and other similar molecules that cause assay interference.111,112 Importantly, this list includes paracetamol, which is metabolised to catechol compounds with a high incidence of chromatographic interference.64
Table 3.
Medications with potential to cause false positive results.
| Mechanism | Class | Drugs* |
|---|---|---|
| Noradrenaline re-uptake inhibition | Tricyclic antidepressants | Amitriptyline, clomipramine, dothiepin, doxepin, imipramine, maprotiline, mianserin, nortriptyline, trimipramine. |
| Antipsychotics | Clozapine, quetiapine, amisulpride, aripiprazole olanzapine, risperidone, ziprasidone, chlorpromazine, fluphenazine, pericyazine, trifluoperazine. | |
| Noradrenaline re-uptake inhibitors | Bupropion, reboxetine, viloxazine. | |
| Other | Prochlorperazine, reserpine. | |
| Adrenergic receptor blockers | Beta blockers | Carvedilol, labetolol, acebutolol, atenolol, bisolprolol, celiprolol, metoprolol, pindolol, propranolol, sotalol. |
| Alpha-2 blockers | Phenoxybenzamine, phentolamine. | |
| Monoamine oxidase inhibition | Monoamine oxidase inhibitors | Moclobamide, phenelzine, tranylcypromine. |
| Recreational drugs | Amphetamines, xanthines | Cocaine, methamphetamine, caffeine. |
| Structurally related# | Sympathomimetics | Pseudoephedrine, phenylephrine, dobutamine, isoprenaline, salbutamol, terbutaline. |
| Others | Paracetamol, levodopa. |
As insufficient data are available to reliably catalogue interfering drugs, this list is extrapolated from known interfering agents. Drugs were selected from the Australian Medicines Handbook 2008 and the NZ Pharmaceutical Schedule November 2008
Applicable to electrochemical but not mass spectrometry detectors.
Whilst drugs have not been reported to cause false negative results, alpha-2 receptor agonists and dopamine agonists have been reported to lower catecholamine and metanephrine concentrations.113,114 The clinical significance of this effect is currently unknown, although failure of suppression by clonidine, an alpha-2 agonist, is used as a diagnostic test with high specificity for phaeochromocytoma.106
There is limited data on the effect of renal impairment on metanephrines.107 From first principles one would expect plasma free metanephrines to be only modestly affected, whereas plasma total metanephrines would increase due to decreased clearance of the conjugated metabolites. Similarly, one would expect only modest changes in urine total metanephrines, whereas there might be a significant decrease in urine free metanephrines.
Other Analytes
Chromaffin cells also produce molecules other than biogenic amines and these can be used as markers of chromaffin cell mass in the diagnosis of phaeochromocytoma. The best validated of these is chromogranin A. Granins are a family of acid proteins present in the secretory granules of a wide variety of endocrine and neuroendocrine cells, with chromogranin A being specific to chromaffin cells.
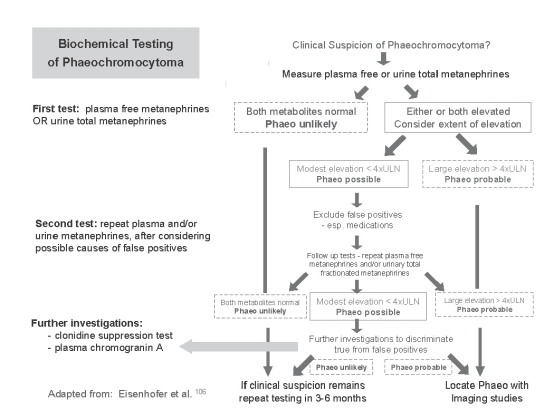
Chromogranin A is highly sensitive but less specific than metanephrines in the diagnosis of phaeochromocytoma.115–120 Importantly, it is biochemically unrelated to catecholamines and thus cleared by different metabolic pathways, which makes it a relatively independent test analyte. Chromogranin A is used in two ways: as a second line test in the diagnostic work-up of patients with raised metanephrines (Figure 3) and as a tumour marker in the follow-up of patients after treatment for chromaffin cell tumours. An important confounder of this test is treatment with proton pump inhibitors, which are widely used and cause marked elevation of chromogranin A.121 Chromogranin A testing is discussed in more detail in recent articles by Grossrubatscher et al.119 and Bilek et al.120
Figure 3.
Suggested algorithm of biogenic amine testing for phaeochromocytoma screening. ULN = upper limit of normal.
An Approach to Biochemical Testing for Suspected Phaeochromocytoma
A suggested algorithm for biochemical testing for phaeochromocytoma is outlined in Figure 3. This is adapted for Australasian practice from an approach published by Eisenhofer et al. in 2003.106 Subsequent published experience, particularly by the Mayo Group, has influenced this adaptation.87–96,106
Before requesting any test, the pre-test probability should be considered. How likely is phaeochromocytoma? Is there an alternative explanation for the symptoms? In most cases, tests for rare conditions should not be part of first line investigations. If testing is clinically indicated, the initial test should be plasma free metanephrines or urine total metanephrines. The choice is dependent on pragmatic issues and local circumstances. However, when the plasma test is available, the convenience is hard to resist. A negative test reliably excludes phaeochromocytoma and alternative explanations for the presenting symptoms should be considered. If the test does not exclude phaeochromocytoma, the options are endocrinology referral or to do a second test.
A (nor)metanephrine concentration above the test threshold is most likely a false positive result, unless it is very high (>4x threshold).94,117 Thus a second test is required and this should seek to minimise false positive results. For this reason, we favour 24-hour urine total metanephrines after stopping medications known or likely to be associated with false positive results (Table 3). The optimal time after stopping medications varies substantially and is predominantly determined by the pharmacokinetics of the individual drug. In most cases five half lives is likely to be sufficient. However, pharmacodynamics may also need to be considered, for example, the effect of phenoxybenzamine, a non-competitive alpha antagonist, will persist until there has been sufficient regeneration of alpha receptors, which may take over a week.122
If the second test is negative, phaeochromocytoma can be excluded. If the test is also positive, one needs to consider both the clinical suspicion (pre-test probability) and the concentration observed (is the result just above the threshold or several times the threshold?). At this stage we recommend referral to a clinical endocrinologist with expertise in this area. Expensive imaging tests should only be undertaken after biochemical diagnosis.
Subsequent Tests
Endocrine investigation of hypersecretory conditions utilises biochemical suppression of production. Most false positive tests are due to production of noradrenaline by the sympathetic nervous system. Clonidine, an alpha-1 receptor agonist, suppresses noradrenaline production by sympathetic nerves, which is the basis of the highly specific clonidine suppression test. The limitation of this test is that it only has about 66% sensitivity, thus suppression of plasma noradrenaline or normetanephrine three hours after clonidine excludes the diagnosis of phaeochromocytoma, but failure to suppress is supportive rather than con rmatory.106 This test should be limited to patients with moderate to high pre-test probability of phaeochromocytoma on the basis of clinical suspicion and metanephrine testing.
Specimen Collection Requirements
Proper sample collection is important for the reliable measurement of catecholamines or metanephrines, whether it be in a 24-hour urine collection or in plasma. Laboratories analysing urine specimens should provide instructions for adult patients on how to collect an accurate 24-hour collection, since interpretative reference ranges are expressed in amount excreted per day. Because of the difficulty in obtaining complete 24-hour urine collections in children, excretions of catecholamines and metanephrines are expressed per mmol creatinine, and age-dependent reference ranges used for biochemical testing.123 With adults the effect of age and gender are small and considered not important for general testing.
Other factors such as drugs and diet can affect assay results. This can be due to direct analytical interferences, such as paracetamol interference in the determination of urinary metanephrines by HPLC with electrochemical detection,64 or, by altering urinary and plasma concentrations of catecholamines or metanephrines. The effects of medications have already been discussed. Diet and recreational stimulants such as caffeine and nicotine are known to influence urinary catecholamines, but are thought to have minimal effect on metanephrines.107 Dietary restrictions are not considered necessary for urine metanephrine testing in the majority of patients. 49,107
The collection of plasma is more convenient than a 24-hour urine for most patients and other biochemical tests can be ordered at the same time in one clinic visit. For plasma free metanephrines, it has been recommended that patients should fast for at least four hours and then rest in the supine position for 30 minutes prior to the collection of EDTA-plasma.87 A change in posture from supine to seated has been shown to increase plasma metanephrines by an average of 30% in one study,124 and to have no significant effect in another.85 If the optimal posture for plasma metanephrine sampling is resting-supine after an overnight fast, this is not usually practical and we therefore suggest a fasting morning EDTA-plasma collected from a quietly seated patient, with interpretation in the context of the sampling conditions.
Catecholamines and metanephrines are known to have different chemical stabilities in urine specimens stored under various conditions. A drawback with urine collections has been the requirement to add concentrated acid to the collection container in order to stabilise catecholamines from oxidation. This also introduces the possibility of deconjugation, artefactually elevating free catecholamine concentrations. Urine metanephrines are more stable than catecholamines, and do not require acid preservative for collection or storage for up to seven days at room temperature.80
In conclusion, metanephrines should be the first line test in the investigation of possible phaeochromocytoma. False positive results remain a common finding complicating the investigative pathway. Effective investigation and management require collaboration between an adequately resourced specialty laboratory and experienced endocrinologists.
Footnotes
Competing Interests: None declared.
References
- 1.Tischler AS. Molecular and cellular biology of pheochromocytomas and extra-adrenal paragangliomas. Endocr Pathol. 2006;17:321–8. doi: 10.1007/s12022-006-0003-3. [DOI] [PubMed] [Google Scholar]
- 2.Manger WM, Gifford RW. Pheochromocytoma. J Clin Hypertens (Greenwich) 2002;4:62–72. doi: 10.1111/j.1524-6175.2002.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manger WM. An overview of pheochromocytoma: history, current concepts vagaries, and diagnostic challenges. Ann N Y Acad Sci. 2006;1073:1–20. doi: 10.1196/annals.1353.001. [DOI] [PubMed] [Google Scholar]
- 4.Fränkel F. Ein Fall von doppelseitigem, völlig latent verlaufenen Nebennierentumor und gleichzeitiger Nephritis mit Veränderungen am Circulationsapparat und Retinitis. Arch Pathol Anat Physiol Klin Med. 1886;103:244–63. [Google Scholar]
- 5.Neumann HP, Vortmeyer A, Schmidt D, Werner M, Erlic Z, Cascon A, et al. Evidence of MEN-2 in the original description of classic pheochromocytoma. N Eng J Med. 2007;357:1311–5. doi: 10.1056/NEJMoa071407. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein RE, O’Neill JA, Jr, Holcomb GW, 3rd, Morgan WM, 3rd, Neblett WW, 3rd, Oates JA, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229:755–66. doi: 10.1097/00000658-199906000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edstrom Elder E, Hjelm Skog AL, Hoog A, Hamberger B. The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur J Surg Oncol. 2003;29:278–83. doi: 10.1053/ejso.2002.1413. [DOI] [PubMed] [Google Scholar]
- 8.Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab. 2006;91:827–36. doi: 10.1210/jc.2005-1862. [DOI] [PubMed] [Google Scholar]
- 9.John H, Ziegler WH, Hauri D, Jaeger P. Pheochromocytomas: can malignant potential be predicted? Urology. 1999;53:679–83. doi: 10.1016/s0090-4295(98)00612-8. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423–36. doi: 10.1677/erc.1.00829. [DOI] [PubMed] [Google Scholar]
- 11.Scholz T, Eisenhofer G, Pacak K, Dralle H, Lehnert H. Clinical review: Current treatment of malignant pheochromocytoma. J Clin Endocrinol Metab. 2007;92:1217–25. doi: 10.1210/jc.2006-1544. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute - Clinical Trials Search Results. [(Accessed 5 November 2008)]; http://www.cancer.gov/search/ResultsClinicalTrials.aspx?protocolsearchid=5393457.
- 13.Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series. Mayo Clin Proc. 1981;56:354–60. [PubMed] [Google Scholar]
- 14.Bravo EL, Gifford RW., Jr Current concepts. Pheochromocytoma: diagnosis, localisation and management. N Engl J Med. 1984;311:1298–303. doi: 10.1056/NEJM198411153112007. [DOI] [PubMed] [Google Scholar]
- 15.Mannelli M, Ianni L, Cilotti A, Conti A. Pheochromocytoma in Italy: a multicentric retrospective study. Eur J Endocrinol. 1999;141:619–24. doi: 10.1530/eje.0.1410619. [DOI] [PubMed] [Google Scholar]
- 16.O’Halloran T, McGreal G, McDermott E, O’Higgins N. 47 years of phaeochromocytomas. Ir Med J. 2001;94:200–3. [PubMed] [Google Scholar]
- 17.Nawar R, Aron D. Adrenal incidentalomas -- a continuing management dilemma. Endocr Relat Cancer. 2005;12:585–98. doi: 10.1677/erc.1.00951. [DOI] [PubMed] [Google Scholar]
- 18.Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16:460–84. doi: 10.1210/edrv-16-4-460. [DOI] [PubMed] [Google Scholar]
- 19.Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309–40. doi: 10.1210/er.2002-0031. [DOI] [PubMed] [Google Scholar]
- 20.Bülow B, Jansson S, Juhlin C, Steen L, Thorén M, Wahrenberg L, et al. Adrenal incidentaloma - follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154:419–23. doi: 10.1530/eje.1.02110. [DOI] [PubMed] [Google Scholar]
- 21.Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–66. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- 22.Castellano M, Mori L, Giacchè M, Agliozzo E, Tosini R, Panarotto A, et al. Genetic mutation screening in an Italian cohort of nonsyndromic pheochromocytoma/ paraganglioma patients. Ann N Y Acad Sci. 2006;1073:156–65. doi: 10.1196/annals.1353.016. [DOI] [PubMed] [Google Scholar]
- 23.Ramsay LE, Williams B, Johnston GD, MacGregor GA, Poston L, Potter JF, et al. British Hypertension Society guidelines for hypertension management 1999: summary. BMJ. 1999;319:630–5. doi: 10.1136/bmj.319.7210.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 25.Australian Heart Foundation Guide to Management of Hypertension. [(Accessed 5 November 2008)];2008 http://www.heartfoundation.org.au/Professional_Information/Clinical_Practice/Hypertension/Pages/default.aspx.
- 26.Sawka AM, Gafni A, Thabane L, Young WF., Jr The economic implications of three biochemical screening algorithms for pheochromocytoma. J Clin Endocrinol Metab. 2004;89:2859–66. doi: 10.1210/jc.2003-031127. [DOI] [PubMed] [Google Scholar]
- 27.Barzon L, Scaroni C, Sonino N, Fallo F, Gregianin M, Macrì C, et al. Incidentally discovered adrenal tumors: endocrine and scintigraphic correlates. J Clin Endocrinol Metab. 1998;83:55–62. doi: 10.1210/jcem.83.1.4501. [DOI] [PubMed] [Google Scholar]
- 28.Zöller ME, Rembeck B, Odén A, Samuelsson M, Angervall L. Malignant and benign tumors in patients with neuro bromatosis type 1 in a defined Swedish population. Cancer. 1997;79:2125–31. [PubMed] [Google Scholar]
- 29.Walther MM, Herring J, Enquist E, Keiser HR, Linehan WM. Von Recklinghausen’s disease and pheochromocytomas. J Urol. 1999;162:1582–6. [PubMed] [Google Scholar]
- 30.Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–51. doi: 10.1001/jama.292.8.943. [Erratum JAMA 2004;292:1686] [DOI] [PubMed] [Google Scholar]
- 31.Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615–21. [PubMed] [Google Scholar]
- 32.Dannenberg H, van Nederveen FH, Abbou M, Verhofstad AA, Komminoth P, de Krijger RR, et al. Clinical characteristics of pheochromocytoma patients with germline mutations in SDHD. J Clin Oncol. 2005;23:1894–901. doi: 10.1200/JCO.2005.07.198. [DOI] [PubMed] [Google Scholar]
- 33.Bornstein SR, Gimenez-Roqueplo AP. Genetic testing in pheochromocytoma: increasing importance for clinical decision making. Ann N Y Acad Sci. 2006;1073:94–103. doi: 10.1196/annals.1353.010. [DOI] [PubMed] [Google Scholar]
- 34.Amar L, Bertherat J, Baudin E, Ajzenberg C, Bressac-de Paillerets B, Chabre O, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005;23:8812–8. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- 35.Gimenez-Roqueplo AP, Lehnert H, Mannelli M, Neumann H, Opocher G, Maher ER, et al. Phaeochromocytoma, new genes and screening strategies. Clin Endocrinol (Oxf) 2006;65:699–705. doi: 10.1111/j.1365-2265.2006.02714.x. [DOI] [PubMed] [Google Scholar]
- 36.Karagiannis A, Mikhailidis DP, Athyros VG, Harsoulis F. Pheochromocytoma: an update on genetics and management. Endocr Relat Cancer. 2007;14:935–56. doi: 10.1677/ERC-07-0142. [DOI] [PubMed] [Google Scholar]
- 37.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–7. [PubMed] [Google Scholar]
- 38.Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Ann Rev Biochem. 1999;68:355–81. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- 39.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–49. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 40.O’Connor DT, Wu H, Gill BM, Rozansky DJ, Tang K, Mahata SK, et al. Hormone storage vesicle proteins. Transcriptional basis of the widespread neuroendocrine expression of chromogranin A, and evidence of its diverse biological actions, intracellular and extracellular. Ann N Y Acad Sci. 1994;733:36–45. doi: 10.1111/j.1749-6632.1994.tb17254.x. [DOI] [PubMed] [Google Scholar]
- 41.Bühler HU, Da Prada M, Haefely W, Picotti GB. Plasma adrenaline, noradrenaline and dopamine in man and different animal species. J Physiol. 1978;276:311–20. doi: 10.1113/jphysiol.1978.sp012235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clutter WE, Bier DM, Shah SD, Cryer PE. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest. 1980;66:94–101. doi: 10.1172/JCI109840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward MM, Mefford IN, Parker SD, Chesney MA, Taylor CB, Keegan DL, et al. Epinephrine and norepinephrine responses in continuously collected human plasma to a series of stressors. Psychosom Med. 1983;45:471–86. doi: 10.1097/00006842-198312000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Eisenhofer G, Huynh TT, Hiroi M, Pacak K. Understanding catecholamine metabolism as a guide to the biochemical diagnosis of pheochromocytoma. Rev Endocr Metab Disord. 2001;2:297–311. doi: 10.1023/a:1011572617314. [DOI] [PubMed] [Google Scholar]
- 45.Eisenhofer G, Rundquist B, Aneman A, Friberg P, Dakak N, Kopin IJ, et al. Regional release and removal of catecholamines and extraneuronal metabolism to metanephrines. J Clin Endocrinol Metab. 1995;80:3009–17. doi: 10.1210/jcem.80.10.7559889. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein DS, Swoboda KJ, Miles JM, Coppack SW, Aneman A, Holmes C, et al. Sources and physiological significance of plasma dopamine sulfate. J Clin Endocrinol Metab. 1999;84:2523–31. doi: 10.1210/jcem.84.7.5864. [DOI] [PubMed] [Google Scholar]
- 47.Jackman GP. Differential assay for urine catecholamines by use of liquid chromatography with fluorescence detection. Clin Chem. 1981;27:1202–4. [PubMed] [Google Scholar]
- 48.Abeling NG, van Gennip AH, Overmars H, Voute PA. Simultaneous determination of catecholamines and metanephrines in urine by HPLC with fluorometric detection. Clin Chim Acta. 1984:211–26. doi: 10.1016/0009-8981(84)90181-5. [DOI] [PubMed] [Google Scholar]
- 49.Peaston RT, Weinkove C. Measurement of catecholamines and their metabolites. Ann Clin Biochem. 2004;41:17–38. doi: 10.1258/000456304322664663. [DOI] [PubMed] [Google Scholar]
- 50.Davidson DF, Fitzpatrick J. A simple, optimised and rapid assay for urinary free catecholamines by HPLC with electrochemical detection. Ann Clin Biochem. 1985;22:297–303. doi: 10.1177/000456328502200313. [DOI] [PubMed] [Google Scholar]
- 51.Huang TH, Wall J, Kabra P. Improved solid-phase extraction and liquid chromatography with electrochemical detection of urinary catecholamines and 5-S-L-cysteinyl-L-dopa. J Chromatogr. 1988;452:409–18. doi: 10.1016/s0021-9673(01)81464-3. [DOI] [PubMed] [Google Scholar]
- 52.De Jong J, Point AJ, Tjaden UR, Beeksma S, Kraak JC. Determination of catecholamines in urine (and plasma) by liquid chromatography after on-line sample treatment on small alumina or dihydroxyborylsilica columns. J Chromatog. 1987;414:285–300. doi: 10.1016/0378-4347(87)80054-3. [DOI] [PubMed] [Google Scholar]
- 53.Smedes F, Kraak JC, Poppe H. Simple and fast solvent extraction system for selective and quantitative isolation of adrenaline, noradrenaline and dopamine from plasma and urine. J Chromatogr. 1982;231:25–39. doi: 10.1016/s0378-4347(00)80506-x. [DOI] [PubMed] [Google Scholar]
- 54.Wu AH, Gornet TG. Preparation of urine samples for liquid-chromatographic determination of catecholamines: bonded-phase phenylboronic acid, cation-exchange resin, and alumina adsorbents compared. Clin Chem. 1985;31:298–302. [PubMed] [Google Scholar]
- 55.Talwar D, Williamson C, McLaughlin A, Gill A, O’Reilly DS. Extraction and separation of urinary catecholamines as their diphenylboronate complexes using C18 solid-phase extraction sorbent and high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;769:341–9. doi: 10.1016/s1570-0232(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 56.Whiting MJ. Simultaneous measurement of urinary metanephrines and catecholamines by liquid chromatography with tandem mass spectrometric detection. Ann Clin Biochem. 2009 doi: 10.1258/acb.2008.008180. in press. [DOI] [PubMed] [Google Scholar]
- 57.Willemsen JJ, Ross HA, Wolthers BG, Sweep CG, Kema IP. Evaluation of specific high-performance liquid-chromatographic determinations of urinary metanephrine and normetanephrine by comparison with isotope dilution mass spectrometry. Ann Clin Biochem. 2001;38:722–30. doi: 10.1258/0004563011900984. [DOI] [PubMed] [Google Scholar]
- 58.Lagerstedt SA, O’Kane DJ, Singh RJ. Measurement of plasma free metanephrine and normetanephrine by liquid chromatographytandem mass spectrometry for diagnosis of pheochromocytoma. Clin Chem. 2004;50:603–11. doi: 10.1373/clinchem.2003.024703. [DOI] [PubMed] [Google Scholar]
- 59.Singh RJ, Eisenhofer G. High-throughput, automated and accurate biochemical screening for pheochromocytoma: are we there yet? Clin Chem. 2007;53:1565–7. doi: 10.1373/clinchem.2007.089128. [DOI] [PubMed] [Google Scholar]
- 60.de Jong WH, Graham KS, van der Molen JC, Links TP, Morris MR, Ross HA, et al. Plasma free metanephrine measurement using automated on-line solid-phase extraction HPLC-tandem mass spectrometry. Clin Chem. 2007;53:1684–93. doi: 10.1373/clinchem.2007.087114. [DOI] [PubMed] [Google Scholar]
- 61.Marney LC, Laha TJ, Baird GS, Rainey PM, Hoofnagle AN. Isopropanol protein precipitation for the analysis of plasma free metanephrines by liquid chromatography-tandem mass spectrometry. Clin Chem. 2008;54:1729 –32. doi: 10.1373/clinchem.2008.104083. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen DT, Guillarme D, Rudaz S, Veuthey JL. Chromatographic behaviour and comparison of column packed with sub-2 micron stationary phases in liquid chromatography. J Chromatogr A. 2006;1128:105–13. doi: 10.1016/j.chroma.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 63.Davidson DF. Urine catecholamine assay by HPLC: in vitro interference by some drugs. Ann Clin Biochem. 1988;25:583–4. doi: 10.1177/000456328802500517. [DOI] [PubMed] [Google Scholar]
- 64.Davidson DF. Paracetamol-associated interference in an HPLC-ECD assay for urinary free metadrenalines and catecholamines. Ann Clin Biochem. 2004;41:316–20. doi: 10.1258/0004563041201626. [DOI] [PubMed] [Google Scholar]
- 65.Kushnir MM, Urry FM, Frank EL, Roberts WL, Shushan B. Analysis of catecholamines in urine by positive-ion electrospray tandem mass spectrometry. Clin Chem. 2002;48:323–31. [PubMed] [Google Scholar]
- 66.Taylor RL, Singh RJ. Validation of liquid chromatography-tandem mass spectrometry method for analysis of urinary conjugated metanephrine and normetanephrine for screening of pheochromocytoma. Clin Chem. 2002;48:533–9. [PubMed] [Google Scholar]
- 67.Magera MJ, Thompson AL, Matern D, Rinaldo P. Liquid chromatography-tandem mass spectrometry method for the determination of vanillylmandelic acid in urine. Clin Chem. 2003;49:825–6. doi: 10.1373/49.5.825. [DOI] [PubMed] [Google Scholar]
- 68.Magera MJ, Stoor AL, Helgeson JK, Matern D, Rinaldo P. Determination of homovanillic acid in urine by stable isotope dilution and electrospray tandem mass spectrometry. Clin Chim Acta. 2001;306:35–41. doi: 10.1016/s0009-8981(01)00397-7. [DOI] [PubMed] [Google Scholar]
- 69.Vogeser M, Seger C. A decade of HPLC-MS/MS in the routine clinical laboratory – goals for further developments. Clin Biochem. 2008;41:649–62. doi: 10.1016/j.clinbiochem.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 70.Wolthers BG, Kema IP, Volmer M, Wesemann R, Westermann J, Manz B. Evaluation of urinary metanephrine and normetanephrine enzyme immunoassay (ELISA) kits by comparison with isotope dilution mass spectrometry. Clin Chem. 1997;43:114–20. [PubMed] [Google Scholar]
- 71.Grossman A, Pacak K, Sawka A, Lenders JW, Harlander D, Peaston RT, et al. Biochemical diagnosis and localisation of pheochromocytoma: can we reach a consensus? Ann N Y Acad Sc. 2006;1073:332–47. doi: 10.1196/annals.1353.038. [DOI] [PubMed] [Google Scholar]
- 72.Bravo EL, Tarazi RC, Gifford RW, Stewart BH. Circulating and urinary catecholamines in pheochromocytoma. Diagnostic and pathophysiologic implications. N Engl J Med. 1979;301:682–6. doi: 10.1056/NEJM197909273011302. [DOI] [PubMed] [Google Scholar]
- 73.Earl JW. The use of HPLC for the estimation of biogenic amines and their metabolites. In: Sampson DC, editor. High performance liquid chromatography in the clinical laboratory. The Clinical Biochemist Monograph; 1986. p. 48. [Google Scholar]
- 74.Bravo EL. Adrenal medullary function. In: Moore WT, Eastman RC, editors. Diagnostic Endocrinology. 2nd ed. St. Louis: Mosby-Year Book, Inc; 1996. pp. 299–309. [Google Scholar]
- 75.Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ. Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Clin Chem. 1993;39:97–103. [PubMed] [Google Scholar]
- 76.Laederach K, Weidmann P. Plasma and urinary catecholamines as related to renal function in man. Kidney Int. 1987;31:107–11. doi: 10.1038/ki.1987.16. [DOI] [PubMed] [Google Scholar]
- 77.Fagerholm U. Prediction of human pharmacokinetics - renal metabolic and excretion clearance. J Pharm Pharmacol. 2007;59:1463–71. doi: 10.1211/jpp.59.11.0002. [DOI] [PubMed] [Google Scholar]
- 78.Launay-Vacher V, Izzedine H, Karie S, Hulot JS, Baumelou A, Deray G. Renal tubular drug transporters. Nephron Physiol. 2006;103:97–106. doi: 10.1159/000092212. [DOI] [PubMed] [Google Scholar]
- 79.Link L, Weidmann P, Probst P, Futterlieb A. Renal handling of norepinephrine and epinephrine in the pig. Pflugers Arch. 1985;405:66–9. doi: 10.1007/BF00591099. [DOI] [PubMed] [Google Scholar]
- 80.Willemsen JJ, Ross HA, Lenders JW, Sweep FC. Stability of urinary fractionated metanephrines and catecholamines during collection, shipment, and storage of samples. Clin Chem. 2007;53:268–72. doi: 10.1373/clinchem.2006.075218. [DOI] [PubMed] [Google Scholar]
- 81.Oishi S, Sasaki M, Ohno M, Sato T. Urinary normetanephrine and metanephrine measured by radio immunoassay for the diagnosis of pheochromocytoma: utility of 24-hour and random 1-hour urine determinations. J Clin Endocrinol Metab. 1988;67:614–8. doi: 10.1210/jcem-67-3-614. [DOI] [PubMed] [Google Scholar]
- 82.Rosano TG, Swift TA, Hayes LW. Advances in catecholamine and metabolite measurements for diagnosis of pheochromocytoma. Clin Chem. 1991;37:1854–67. [PubMed] [Google Scholar]
- 83.Gerlo EA, Sevens C. Urinary and plasma catecholamines and urinary catecholamine metabolites in pheochromocytoma: diagnostic value in 19 cases. Clin Chem. 1994;40:250–6. [PubMed] [Google Scholar]
- 84.Lenders JW, Keiser HR, Goldstein DS, Willemsen JJ, Friberg P, Jacobs MC, et al. Plasma metanephrines in the diagnosis of pheochromocytoma. Ann Intern Med. 1995;123:101–9. doi: 10.7326/0003-4819-123-2-199507150-00004. [DOI] [PubMed] [Google Scholar]
- 85.Raber W, Raffesberg W, Bischof M, Scheuba C, Niederle B, Gasic S, et al. Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch Int Med. 2000;160:2957–63. doi: 10.1001/archinte.160.19.2957. [DOI] [PubMed] [Google Scholar]
- 86.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;20(287):1427–34. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 87.Lenders JW, Pacak K, Eisenhofer G. New advances in the biochemical diagnosis of pheochromocytoma: moving beyond catecholamines. Ann N Y Acad Sci. 2002;970:29–40. doi: 10.1111/j.1749-6632.2002.tb04410.x. [DOI] [PubMed] [Google Scholar]
- 88.Sawka AM, Jaeschke R, Singh RJ, Young WF., Jr A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab. 2003;88:553–8. doi: 10.1210/jc.2002-021251. [DOI] [PubMed] [Google Scholar]
- 89.Kudva YC, Sawka AM, Young WF., Jr The laboratory diagnosis of adrenal pheochromocytoma: the Mayo Clinic experience. J Clin Endocrinol Metab. 2003;88:4533–9. doi: 10.1210/jc.2003-030720. [DOI] [PubMed] [Google Scholar]
- 90.Sawka AM, Prebtani AP, Thabane L, Gafni A, Levine M, Young WF., Jr A systematic review of the literature examining the diagnostic efficacy of measurement of fractionated plasma free metanephrines in the biochemical diagnosis of pheochromocytoma. BMC Endocr Disord. 2004;4:2. doi: 10.1186/1472-6823-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–75. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 92.Brain KL, Kay J, Shine B. Measurement of urinary metanephrines to screen for pheochromocytoma in an unselected hospital referral population. Clin Chem. 2006;52:2060–4. doi: 10.1373/clinchem.2006.070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Unger N, Pitt C, Schmidt IL, Walz MK, Schmid KW, Philipp T, et al. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol. 2006;154:409–17. doi: 10.1530/eje.1.02097. [DOI] [PubMed] [Google Scholar]
- 94.Perry CG, Sawka AM, Singh R, Thabane L, Bajnarek J, Young WF., Jr The diagnostic efficacy of urinary fractionated metanephrines measured by tandem mass spectrometry in detection of pheochromocytoma. Clin Endocrinol (Oxf) 2007;66:703–8. doi: 10.1111/j.1365-2265.2007.02805.x. [DOI] [PubMed] [Google Scholar]
- 95.d’Herbomez M, Forzy G, Bauters C, Tierny C, Pigny P, Carnaille B, et al. An analysis of the biochemical diagnosis of 66 pheochromocytomas. Eur J Endocrinol. 2007;156:569–75. doi: 10.1530/EJE-06-0640. [DOI] [PubMed] [Google Scholar]
- 96.Peaston RT, Ball S. Biochemical detection of phaeochromocytoma: why are we continuing to ignore the evidence? Ann Clin Biochem. 2008;45:6–10. doi: 10.1258/acb.2007.007116. [DOI] [PubMed] [Google Scholar]
- 97.Shultz EK, Aliferis C, Aronsky D. Clinical Evaluation of Methods. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th ed. St Louis: Elsevier Saunders; 2006. pp. 409–24. [Google Scholar]
- 98.Powis G. The binding of catecholamines to human serum proteins. Biochem Pharmacol. 1975;24:707–12. doi: 10.1016/0006-2952(75)90247-6. [DOI] [PubMed] [Google Scholar]
- 99.El-Bahr SM, Kahlbacher H, Patzl M, Palme RG. Binding and clearance of radioactive adrenaline and noradrenaline in sheep blood. Vet Res Commun. 2006;30:423–32. doi: 10.1007/s11259-006-3244-1. [DOI] [PubMed] [Google Scholar]
- 100.Eisenhofer G. Free or total metanephrines for diagnosis of pheochromocytoma: what is the difference? Clin Chem. 2001;47:988–9. [PubMed] [Google Scholar]
- 101.Anderson GH, Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609–15. doi: 10.1097/00004872-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 102.Stenström G, Svärdsudd K. Pheochromocytoma in Sweden 1958–1981. An analysis of the National Cancer Registry Data. Acta Med Scand. 1986;220:225–32. [PubMed] [Google Scholar]
- 103.Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27:193–202. doi: 10.1291/hypres.27.193. [DOI] [PubMed] [Google Scholar]
- 104.Bülow B, Ahrén B Swedish Research Council Study Group of Endocrine Abdominal Tumours. Adrenal incidentaloma--experience of a standardised diagnostic programme in the Swedish prospective study. J Intern Med. 2002;252:239–46. doi: 10.1046/j.1365-2796.2002.01028.x. [DOI] [PubMed] [Google Scholar]
- 105.Kasperlik-Zeluska AA, Rosłonowska E, Słowinska-Srzednicka J, Migdalska B, Jeske W, Makowska A, et al. Incidentally discovered adrenal mass (incidentaloma): investigation and management of 208 patients. Clin Endocrinol (Oxf) 1997;46:29–37. doi: 10.1046/j.1365-2265.1997.d01-1751.x. [DOI] [PubMed] [Google Scholar]
- 106.Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, et al. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab. 2003;88:2656–66. doi: 10.1210/jc.2002-030005. [DOI] [PubMed] [Google Scholar]
- 107.Rosano TG, Eisenhofer G, Whitley RJ. Catecholamines and serotonin. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th ed. St Louis: Elsevier Saunders; 2006. pp. 1033–74. [Google Scholar]
- 108.Krentz AJ, Mikhail S, Cantrell P, Hill GM. Pseudo- phaeochromocytoma syndrome associated with clozapine. BMJ. 2001;322:1213. doi: 10.1136/bmj.322.7296.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Doogue M, Soule S, Hunt P. Another cause of ‘pseudo-phaeochromocytoma’--quetiapine associated with a false positive normetanephrine result. Clin Endocrinol (Oxf) 2007;67:472–3. doi: 10.1111/j.1365-2265.2007.02796.x. [DOI] [PubMed] [Google Scholar]
- 110.Zornitzki T, Knobler H, Schattner A. Reboxetine treatment and pseudopheochromocytoma. QJM. 2007;100:61–2. doi: 10.1093/qjmed/hcl134. [DOI] [PubMed] [Google Scholar]
- 111.Hyams JS, Leichtner AM, Breiner RG, Hill DW, McComb RB, Manger WM. Pseudopheochromocytoma and cardiac arrest associated with phenyl- propanolamine. JAMA. 1985;253:1609–10. [PubMed] [Google Scholar]
- 112.Lurvey A, Yussin A, DeQuattro V. Pseudo- pheochromocytoma after self-administered isoproterenol. J Clin Endocrinol Metab. 1973;36:766–9. doi: 10.1210/jcem-36-4-766. [DOI] [PubMed] [Google Scholar]
- 113.Sjoberg RJ, Simcic KJ, Kidd GS. The clonidine suppression test for pheochromocytoma. A review of its utility and pitfalls. Arch Intern Med. 1992;152:1193–7. [PubMed] [Google Scholar]
- 114.Van Loon GR, Sole MJ, Bain J, Ruse JL. Effects of bromocriptine on plasma catecholamines in normal men. Neuroendocrinology. 1979;28:425–34. doi: 10.1159/000122891. [DOI] [PubMed] [Google Scholar]
- 115.Stridsberg M, Husebye ES. Chromogranin A and chromogranin B are sensitive circulating markers for phaeochromocytoma. Eur J Endocrinol. 1997;136:67–73. doi: 10.1530/eje.0.1360067. [DOI] [PubMed] [Google Scholar]
- 116.Bernini GP, Moretti A, Ferdeghini M, Ricci S, Letizia C, D’Erasmo E, et al. A new human chromogranin ‘A’ immunoradiometric assay for the diagnosis of neuroendocrine tumours. Br J Cancer. 2001;84:636–42. doi: 10.1054/bjoc.2000.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Algeciras-Schimnich A, Preissner CM, Young WF, Jr, Singh RJ, Grebe SK. Plasma chromogranin A or urine fractionated metanephrines follow-up testing improves the diagnostic accuracy of plasma fractionated metanephrines for pheochromocytoma. J Clin Endocrinol Metab. 2008;93:91–5. doi: 10.1210/jc.2007-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Giovanella L, Squin N, Ghelfo A, Ceriani L. Chromogranin A immunoradiometric assay in diagnosis of pheochromocytoma: comparison with plasma metanephrines and 123I-MIBG scan. Q J Nucl Med Mol Imaging. 2006;50:344–7. [PubMed] [Google Scholar]
- 119.Grossrubatscher E, Dalino P, Vignati F, Gambacorta M, Pugliese R, Boniardi M, et al. The role of chromogranin A in the management of patients with phaeochromocytoma. Clin Endocrinol (Oxf) 2006;65:287–93. doi: 10.1111/j.1365-2265.2006.02591.x. [DOI] [PubMed] [Google Scholar]
- 120.Bílek R, Safarík L, Ciprová V, Vlcek P, Lisá L. Chromogranin A, a member of neuroendocrine secretory proteins as a selective marker for laboratory diagnosis of pheochromocytoma. Physiol Res. 2008;57(Suppl 1):S171–9. doi: 10.33549/physiolres.931502. [DOI] [PubMed] [Google Scholar]
- 121.Igaz P, Müllner K, Hargitai B, Igaz I, Tömböl Z, Ràcz K, et al. Marked chromogranin A elevation in a patient with bilateral adrenal incidentalomas, and its rapid normalisation after discontinuation of proton pump inhibitor therapy. Clin Endocrinol (Oxf) 2007;67:805–6. doi: 10.1111/j.1365-2265.2007.02957.x. [DOI] [PubMed] [Google Scholar]
- 122.Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th Ed. New York: McGraw-Hill; 2006. pp. 237–95. [Google Scholar]
- 123.Wu AHB, editor. Tietz Clinical Guide to Laboratory Tests. 4th ed. St. Louis, Missouri: W.B Saunders Co; 2006. pp. 724–30. [Google Scholar]
- 124.Lenders JW, Willemsen JJ, Eisenhofer G, Ross HA, Pacak K, Timmers HJ, et al. Is supine rest necessary before blood sampling for plasma metanephrines? Clin Chem. 2007;53:352–4. doi: 10.1373/clinchem.2006.076489. [DOI] [PubMed] [Google Scholar]