Abstract
Objectives:
To determine prevalence of and risk factors for herpes simplex virus type 2 (HSV-2) and HIV among women being screened for a randomized, controlled trial of HSV suppressive therapy in northwestern Tanzania.
Methods:
Two thousand seven hundred nineteen female facility workers aged 16 to 35 were interviewed and underwent serological testing for HIV and HSV-2. Factors associated with HSV-2 and HIV in women aged 16 to 24 were examined using logistic regression to estimate odds ratios (OR) and 95% confidence intervals (CI).
Results:
HSV-2 seroprevalence was 80%, and HIV seroprevalence was 30%. Among women aged 16 to 24, both infections were significantly and independently associated with older age, being a bar worker, working at a truck stop, and having more lifetime sexual partners. HSV-2 infection was also associated with lower socioeconomic status, increased alcohol intake, younger age at first sex, inconsistent condom use, and vaginal douching. There was a strong association between the 2 infections after adjustment for other factors (OR = 4.22, 95% CI: 2.6 to 6.9).
Conclusions:
Female facility workers in northwestern Tanzania are vulnerable to HSV-2 and HIV infections. Programs designed to increase safer sexual behavior and reduce alcohol use could be effective in reducing HSV-2 incidence and, in turn, HIV infection. This is a suitable population for an HSV suppressive therapy trial.
Keywords: HSV-2, HIV, high risk, Tanzania
Tanzania has been widely affected by HIV since the mid-1980s, and current HIV prevalence among the general adult population in Tanzania is estimated at 6.5%.1 The epidemic is driven by heterosexual transmission, with higher prevalence in groups with higher sexual risk behavior, including women working in bars, restaurants, and guesthouses in trading centers or at major truck stops.
Herpes simplex virus type 2 (HSV-2) infection, a highly prevalent sexually transmitted infection2,3 and the main cause of genital ulcer disease (GUD) worldwide,4,5 is an important cofactor for HIV acquisition, increasing risk of infection by 2- to 3-fold.6,7 Studies in Tanzania have found HSV-2 sero-prevalence of around 25% in women aged 15 to 19,8,9 rising to around 75% in those aged over 25 in rural Mwanza,8,9 with a generally higher prevalence among female bar workers.10,11 The impact of HSV-2 on the spread of HIV was illustrated in a recent cohort study among female bar/hotel workers in Moshi, northern Tanzania.12 HIV incidence in this population was 4.6 per 100 person-years; HSV-2 infection, especially recent infection, was the factor most strongly associated with HIV incidence, causing an estimated 63% of HIV infections.12 Similarly, a nested case-control study of HIV infection in the general population in Mwanza, northwestern Tanzania, estimated that 74% of incident HIV infections in men and 22% in women were attributable to HSV-2.13
Although these observational studies suggest a strong link between HSV-2 seroprevalence and HIV infection, intervention trials are needed to evaluate whether HSV-2 suppressive therapy will reduce the risk of HIV infection. Further, HSV-2 may also increase HIV infectivity, as seen in a randomized placebo-controlled trial in which HSV suppressive therapy significantly reduced frequency and quantity of genital and plasma HIV-1 RNA and genital HSV-2 DNA over a 3-month treatment period.14
In this paper, we report baseline data from female facility workers in northern Tanzania who were screened for enrollment into a randomized controlled trial of the impact of HSV suppressive therapy on HIV incidence and genital shedding. We describe the characteristics of the women screened, report the prevalence of HSV2 and HIV, examine risk factors for these infections, and evaluate the suitability of this study population for randomized controlled trials of herpes suppressive therapy.
METHODS
Study Location
Settings in the Lake Victoria Zone of Tanzania with high transmission of HIV were identified through ongoing work and meetings with regional and district health authorities. These included large truck stops on the roads to Rwanda, Burundi, and Kenya; large trading centers; and communities neighboring gold and diamond mines.
Selection of Trial Population
Women were eligible for screening for enrollment in the trial if they were aged 16 to 35, intended to stay in the study site for the next 2 years, were not pregnant or planning to become pregnant in the next 2 years, and were not breast-feeding. Twenty-one communities were mapped to document the number of potentially eligible women, the number of facilities (such as bars and guesthouses), and likely migration routes for facility workers.
Facility types were defined as bars, guesthouses/hotels, mamalishe (local food-handlers who prepare food on roadsides), kilabu (local brew houses), groceries (small shops that sell beer), disco halls, and nightclubs. Workers in each facility were listed after preparatory meetings. Nineteen study sites were selected based on the number of eligible women, documented mobility patterns, and accessibility. Within each facility, all female workers aged 16 to 35 were invited to attend a screening round at a mobile clinic based in a guesthouse to explain the trial rationale and procedures and to assess eligibility. After mobilization meetings, women from the communities of facility workers and other suitable community members were nominated and recruited to act as community link persons. Owners and/or managers of participating facilities were invited to meetings to explain the study and were asked to support the participation of their female employees in the study.
Study Procedures
Screening was conducted in 3 phases from November 2003 to December 2005. After informed written or finger-printed consent, a 10 mL blood sample was collected, and participants were interviewed to elicit details of sociodemographic factors and behavioral practices. All screened women were given an appointment to return to the mobile clinic in approximately 3 months. All women, including those not eligible for screening, were offered free condoms, risk-reduction counseling, and HIV voluntary counseling and testing (VCT) by a trained on-site counselor. Those who agreed were given precounseling, tested using HIV rapid tests (Capillus HIV-1/HIV-2, Trinity Biotech, Bray, Ireland; and Determine HIV-1/2 test, Abbott Laboratories, Queenborough, UK), and offered immediate posttest counseling. HIV-positive participants identified by VCT were referred to the closest center providing HIV support and care. Antiretroviral therapy (ART) provision started in regional and district hospitals in the Lake Zone during 2004, and women enrolled into the trial who knew their HIV status were referred to the nearest health facilities providing HIV care, including ART. Transport to the HIV clinics was provided where necessary.
Laboratory Methods
The 10 mL blood sample was centrifuged at 1000 g for 10 minutes. Three 2 mL serum aliquots were collected and refrigerated immediately at 2° to 8°C and subsequently frozen at −20°C and transported to the STD Reference Laboratory at the National Institute for Medical Research (NIMR), Mwanza. Sera were tested for HSV-2 using a type-specific IgG enzyme-linked immunosorbent assay (ELISA) (Kalon Biologicals, Surrey, UK) that has a sensitivity of 92.3% and specificity of 97.7% in African sera15 and for HIV using the Murex HIV Ag/Ab Combination ELISA (Murex Biotech, Dartford, UK) and Uni-Form II Ag/Ab micro ELISA system (BioMérieux UK, Basingstoke, UK). Samples discordant or indeterminate on HIV testing were retested. If an ELISA result differed from the original, the sample was tested a third time with this same ELISA. If the ELISA results were unchanged on the first retest or were not resolved on the second retest, the sample was tested by a HIV-1 p24 Ag EIA (Biorad Genetic Systems, Hercules, CA). If positive, then this was taken as the final HIV result. P24 Ag-negative or indeterminate samples were sent to the Institute of Tropical Medicine, Antwerp, for testing by a line immunoassay, INNO-LIA HIV I/II (Innogenetics, Gent, Belgium). The results of the LIA test were taken as the final HIV result.
Statistical Methods
Sociodemographic characteristics of participants were compared across facility type using the χ2 test. Risk factors for HSV-2 infection and HIV infection were analyzed using odds ratios (ORs) and 95% confidence intervals (CIs) obtained by logistic regression. This analysis was restricted to women aged 16 to 24, because infections in this age group are more likely to have occurred recently, which allows a more reliable analysis of risk factors for incident infection from this cross-sectional study design. Potential determinants of each infection were considered in 3 groups: socioeconomic factors, sexual behavioral factors, and biological factors. An initial model included socioeconomic factors only. All factors for which association reached statistical significance at P < 0.1 were included in a multivariate model. Factors that remained independently significantly associated with the outcome (at P < 0.1) were retained. Next, the association between each determinant in the sexual behavior group, and then the biological group, were assessed by adding the single determinant into the multivariate model, including the subset of independently significant socioeconomic factors. A multivariate model was built up that included the subset of socioeconomic factors in the first multivariate model plus any behavioral or biological factors for which the P value after adjusting for the socioeconomic factors was <0.1. The final multivariate logistic regression model was reached by excluding factors 1 at a time until all remaining factors were significant at the P < 0.1 level. History of GUD was not included as a risk factor for HSV-2 because it may have been caused by HSV-2 infection.
RESULTS
Study Population
The mapping activities documented 6212 women working in 2564 facilities. Of these, 2735 (44.0%) women attended the screening visit and 2719 were eligible. Of the 16 women who attended but were not eligible for screening, 7 reported pregnancy or were actively planning to become pregnant in the next 2 years; 1 was moving away, 3 were breast-feeding, and 5 declined to take part.
Overall, 1399 (51%) of the 2719 screened women were local food handlers; 449 (17%) worked in a restaurant, café, or beer shop; 406 (15%) were bar workers; 298 (11%) were guesthouse workers; and 167 (6%) were local brew sellers (Table 1). The regional or district capital towns of Shinyanga, Kahama, Geita, and Nzega had the highest numbers of participants, with 45% of women living in these 4 sites. The highest proportions of bar workers were found in Maganzo, a small-scale diamond mining site (11 of 30; 37%), and Geita, a district capital near a large-scale goldmine (72 of 257; 28%). The mean age of the participants was 26.0 years (standard deviation 5.4 years) and differed significantly by type of facility (P < 0.001; Table 1). Most women (76%) were not currently married or living as married. Bar workers were most likely to be divorced or separated (63%). Overall, 18% of all women and 30% of bar workers had lived in their current site for <1 year and 35% of women had lived in the site for <2 years. Type of facility was also significantly associated with tribe, literacy, and knowledge about HIV transmission (Table 1). Local brew sellers had the lowest literacy level (61%) and were the least likely group to give ≥9 correct responses to questions on HIV knowledge (27%).
TABLE 1. Sociodemographic Characteristics of Study Population by Work Facility Type.
| Local Food Handlers, n (%) |
Guesthouse Workers, n (%) |
Bar Workers, n (%) |
Local Brew Sellers, n (%) |
Restaurant/Café/Beer Shop,* n (%) |
Total, N (%) |
||
|---|---|---|---|---|---|---|---|
| All women | 1399 (51) | 298 (11) | 406 (15) | 167 (6) | 449 (17) | 2719 (100) | |
| Age group, y | P < 0.001 | ||||||
| 16 to 19 | 229 (16) | 31 (10) | 32 (8) | 10 (6) | 80 (18) | 382 (14.1) | |
| 20 to 24 | 370 (26) | 93 (31) | 128 (32) | 30 (18) | 145 (32) | 766 (28.2) | |
| 25 to 29 | 391 (28) | 83 (28) | 152 (37) | 53 (32) | 122 (27) | 801 (29.5) | |
| 30 to 35 | 409 (29) | 91 (31) | 94 (23) | 74 (44) | 102 (23) | 770 (28.3) | |
| Mean age, y | 25.7 | 26.2 | 25.9 | 28.2 | 24.9 | 26.0 | |
| Marital status | P < 0.001 | ||||||
| Unmarried | 400 (29) | 96 (32) | 111 (27) | 26 (16) | 179 (40) | 812 (29.9) | |
| Married/living with partner | 442 (32) | 42 (14) | 27 (7) | 58 (35) | 81 (18) | 650 (23.9) | |
| Divorced/separated | 505 (36) | 140 (47) | 254 (63) | 75 (45) | 170 (38) | 1144 (42.1) | |
| Widowed | 52 (4) | 20 (7) | 14 (3) | 8 (5) | 19 (4) | 113 (4.2) | |
| Length of residence in site, y | P < 0.001 | ||||||
| <1 | 205 (15) | 59 (20) | 121 (30) | 18 (11) | 81 (18) | 484 (17.8) | |
| 1 to 2 | 229 (16) | 50 (17) | 81 (20) | 29 (17) | 92 (21) | 481 (17.7) | |
| 3 to 4 | 172 (12) | 42 (14) | 56 (14) | 24 (14) | 51 (11) | 345 (12.7) | |
| 5 to 9 | 219 (16) | 61 (20) | 59 (14) | 25 (15) | 67 (15) | 431 (15.9) | |
| ≥10 | 289 (21) | 45 (15) | 46 (11) | 31 (19) | 87 (19) | 498 (18.3) | |
| Since birth | 285 (20) | 41 (14) | 43 (10) | 40 (24) | 71 (16) | 480 (17.7) | |
| Tribe† | P < 0.001 | ||||||
| Sukuma | 692 (49) | 105 (35) | 174 (43) | 64 (38) | 191 (43) | 1226 (45.1) | |
| Other | 706 (50) | 193 (65) | 232 (57) | 103 (62) | 258 (57) | 1492 (54.9) | |
| Literacy | P < 0.001 | ||||||
| Yes | 1023 (73) | 239 (80) | 318 (78) | 102 (61) | 365 (81) | 2046 (75.3) | |
| No | 376 (27) | 60 (20) | 88 (22) | 65 (39) | 84 (19) | 673 (24.8) | |
| HIV transmission knowledge, no. correct out of 10 questions |
P < 0.001 | ||||||
| ≤5 | 169 (12) | 28 (9) | 30 (7) | 24 (14) | 39 (9) | 290 (10.7) | |
| 6 to 8 | 753 (54) | 144 (48) | 222 (55) | 98 (59) | 223 (50) | 1440 (53.0) | |
| 9 to 10 | 477 (34) | 126 (42) | 154 (38) | 45 (27) | 187 (42) | 989 (36.4) | |
Includes 1 woman who worked at a nightclub and 1 who was street based.
Excludes 1 woman with missing data.
Behavioral characteristics of participants are shown in Table 2. About half of women (47%) reported fewer than 5 partners in their lifetime, with bar workers generally reporting the highest risk behaviors. Overall, 25% of women reported having at least 10 partners in their lifetime, and 82% reported ever having used a condom. The majority of women (65%) reported no more than 1 partner in the past 3 months. Bar workers were most likely to report at least 3 partners in this period (36% vs. 12% for other facility workers; P < 0.001). Sex in exchange for money or gifts in the past 3 months was relatively common (35% of women overall, 60% among bar workers; Table 2), and 10% of women reported having had sex with a nonpaying casual partner within this period. Consistent condom use was reported by 67% of the women with a paying partner and by 60% of women with a nonpaying casual partner. Only 21% of women reported consistent condom use with regular partners. Overall, 28% of women reported having used a condom every time they had sex in the past 3 months. The main reasons for inconsistent condom use were that the male partner did not like them (49%), they didn't know why they didn't use one (11%), because they trusted their partner (7%), or they had only one partner (6%).
TABLE 2. Behavioral Characteristics of Study Population by Work Facility Type.
| Local Food Handlers, n (%) |
Guesthouse Workers, n (%) |
Bar Workers, n (%) |
Local Brew Sellers, n (%) |
Restaurant/Café/Beer Shop,* n (%) |
Total, N (%) |
||
|---|---|---|---|---|---|---|---|
| Age at first sex,† y | P = 0.001 | ||||||
| <14 | 85 (6) | 25 (8) | 21 (5) | 14 (8) | 33 (7) | 178 (6.6) | |
| 14 to 15 | 419 (30) | 71 (24) | 134 (33) | 53 (32) | 106 (24) | 783 (28.8) | |
| 16 to 17 | 483 (34) | 105 (35) | 123 (30) | 59 (36) | 161 (36) | 931 (34.2) | |
| ≥18 | 370 (26) | 95 (32) | 124 (31) | 40 (24) | 144 (32) | 773 (28.4) | |
| No. partners in lifetime | P < 0.001 | ||||||
| 0 | 31 (2) | 0 (0) | 1 (<1) | 1 (<1) | 3 (1) | 36 (1.3) | |
| 1 | 114 (8) | 9 (3) | 1 (<1) | 10 (6) | 32 (7) | 166 (6.1) | |
| 2 to 4 | 628 (45) | 110 (37) | 94 (23) | 51 (30) | 188 (42) | 1071 (39.4) | |
| 5 to 9 | 383 (27) | 79 (27) | 127 (31) | 58 (35) | 122 (27) | 769 (28.3) | |
| 10 to 24 | 207 (15) | 73 (25) | 125 (31) | 37 (22) | 88 (20) | 530 (19.5) | |
| ≥25 | 35 (3) | 25 (8) | 56 (14) | 10 (6) | 16 (4) | 142 (5.2) | |
| Ever used a condom‡ | P < 0.001 | ||||||
| No | 346 (25) | 20 (7) | 7 (2) | 38 (23) | 66 (15) | 477 (17.5) | |
| Yes | 1053 (75) | 277 (93) | 398 (98) | 129 (77) | 383 (85) | 2240 (82.4) | |
| No. partners in past 3 mo | P < 0.001 | ||||||
| 0 | 198 (14) | 16 (5) | 11 (3) | 12 (7) | 51 (11) | 288 (10.6) | |
| 1 | 830 (59) | 151 (51) | 145 (36) | 108 (65) | 239 (53) | 1473 (54.2) | |
| 2 | 233 (17) | 69 (23) | 105 (26) | 26 (16) | 93 (21) | 526 (19.4) | |
| 3 to 4 | 108 (8) | 41 (14) | 94 (23) | 18 (11) | 53 (12) | 314 (11.6) | |
| ≥5 | 30 (2) | 21 (7) | 51 (13) | 3 (2) | 13 (3) | 118 (4.3) | |
| Sex in exchange for money in past 3 mo |
P < 0.001 | ||||||
| No | 1036 (74) | 297 (66) | 151 (51) | 120 (72) | 158 (39) | 1762 (65) | |
| Yes | 363 (26) | 152 (34) | 146 (49) | 47 (28) | 247 (61) | 955 (35) | |
| Condom use at last sex with regular partner§ |
P < 0.001 | ||||||
| No | 862 (77) | 151 (64) | 177 (55) | 120 (82) | 233 (64) | 1543 (70) | |
| Yes | 262 (23) | 85 (36) | 142 (45) | 27 (18) | 131 (36) | 647 (30) | |
| Condom use at last sex with last client∥ |
P < 0.001 | ||||||
| No | 168 (32) | 23 (13) | 30 (11) | 22 (39) | 49 (24) | 292 (23.8) | |
| Yes | 350 (68) | 148 (87) | 244 (89) | 35 (61) | 157 (76) | 934 (76.2) | |
| Ever used lubricants for sex¶ | P = 0.03 | ||||||
| No | 1258 (92) | 272 (91) | 347 (86) | 146 (88) | 405 (91) | 2428 (89.3) | |
| Yes | 109 (8) | 26 (9) | 58 (14) | 20 (12) | 41 (9) | 254 (9.3) | |
| Ever had dry/tight sex# | P = 0.38 | ||||||
| No | 1340 (96) | 289 (97) | 394 (97) | 157 (94) | 428 (95) | 2608 (96.0) | |
| Yes | 56 (4) | 9 (3) | 12 (3) | 10 (6) | 21 (5) | 108 (4.0) | |
| Ever had anal sex | P = 0.04 | ||||||
| No | 1369 (98) | 291 (98) | 396 (98) | 157 (94) | 441 (98) | 2654 (97.6) | |
| Yes | 30 (2) | 7 (2) | 10 (2) | 10 (6) | 8 (2) | 65 (2.4) | |
| Ever had sex during menstruation |
P < 0.001 | ||||||
| No | 1190 (85) | 239 (80) | 321 (79) | 123 (74) | 378 (84) | 2251 (83) | |
| Yes | 207 (15) | 59 (20) | 85 (21) | 44 (26) | 71 (16) | 466 (17) | |
| Had forced sex in past 3 mo | P = 0.78 | ||||||
| No | 1153 (97) | 382 (96) | 269 (96) | 150 (98) | 375 (97) | 2329 (97) | |
| Yes | 37 (3) | 14 (3) | 11 (4) | 3 (2) | 13 (3) | 78 (3.0) | |
| Frequency of douching per day** |
P < 0.001 | ||||||
| 0 times | 600 (43) | 126 (42) | 121 (30) | 71 (43) | 172 (38) | 1090 (40.2) | |
| 1 to 2 times | 378 (27) | 72 (24) | 119 (29) | 40 (24) | 114 (25) | 723 (26.7) | |
| 3 to 4 times | 398 (29) | 92 (31) | 156 (38) | 54 (32) | 151 (34) | 851 (31.4) | |
| ≥5 times | 17 (1) | 8 (3) | 9 (2) | 2 (1) | 12 (3) | 48 (1.8) | |
| Alcoholic drinks per week | P < 0.001 | ||||||
| 0 | 953 (68) | 132 (44) | 75 (18) | 37 (22) | 238 (53) | 1435 (52.8) | |
| 1 to 4 | 262 (19) | 74 (25) | 104 (26) | 48 (29) | 92 (20) | 581 (21.4) | |
| 5 to 9 | 80 (6) | 42 (14) | 51 (13) | 34 (20) | 45 (10) | 252 (9.3) | |
| 10 to 19 | 57 (4) | 23 (8) | 66 (16) | 21 (13) | 35 (8) | 202 (7.4) | |
| ≥20 | 47 (3) | 26 (9) | 110 (27) | 27 (16) | 39 (9) | 249 (9.2) | |
Includes 1 woman who worked at a nightclub and 1 who was street based.
Excludes 18 women with missing data and the 36 women who had never had sex.
Excludes 2 women with missing data, but includes women who had never had sex.
Based on 2190 women who do not report a current regular partner.
Partner with whom sex was exchanged for money or gifts.
Excludes 1 woman with missing data and the 36 women who had never had sex.
Excludes 3 women with missing data, but includes women who had never had sex.
Excludes 7 women with missing data, but includes women who had never had sex.
Differences in reported sexual practices by facility type showed no clear pattern (Table 2). Overall, 9% of women reported ever using lubrication, and this was most commonly reported among bar workers (14%). Ever using products for dry or tight sex and having ever had anal sex were uncommon (4% and 2% respectively) and were more commonly reported among local brew sellers, as was having had sex during menstruation (26% vs. 17% overall). Of women who had sex within the last 3 months, 3.2% reported being forced to have had sex against their will.
Vaginal cleansing was common and was reported by 60% of all women, and 70% of bar workers. The mean frequency of vaginal cleansing episodes per day was 2.6 (range: 0 to 7). Of the women who reported this practice, the most frequent method was washing inside the vagina with a finger dipped in water and soap (79%). Local brew sellers were most likely to also use other methods, including use of leaves or roots (7%) or a cloth (12%). Almost all women reported hygiene to be the primary reason for vaginal cleansing (99.3%).
HSV/HIV Seroprevalence and Self-Reported Genital Ulceration
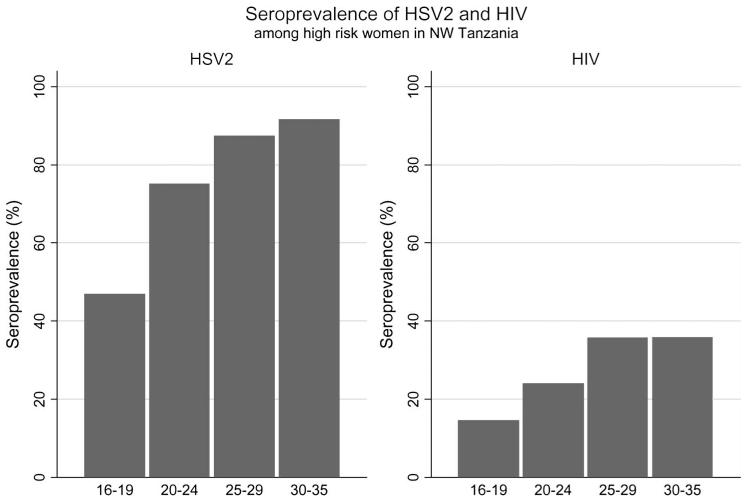
Overall, 80% of women were HSV-2 seropositive, 30% were HIV-1 seropositive, 22% reported ever having had a genital ulcer, and 11% reported an ulcer in the past 3 months. Thirteen women had an indeterminate HSV-2 ELISA result, and 21 had an indeterminate HIV result by INNO-LIA. HSV-2 seroprevalence by site ranged from 69% to 88%, and HIV seroprevalence was from 19% to 36%. Both HSV2 and HIV were significantly less prevalent in trading centers (74% and 24% respectively) than in mining areas and truck stops (82% and 32% respectively; P = 0.001). HSV-2–seropositive women were significantly more likely to report a history of genital ulceration (25% vs. 11%; P < 0.001), and to be HIV-1 seropositive (35% vs. 8%; P < 0.0001). HSV-2 prevalence rose significantly with age, from 47% in women aged 16 to 19 years to 92% in women aged 30 to 35 years (P < 0.001; Figure 1). HIV prevalence was also associated with age, rising from 15% among those aged 16 to 19 to 36% in those aged 30 to 35 (P < 0.001; Figure 1).
FIGURE 1.
Seroprevalence of HSV2 and HIV among high risk women in NW Tanzania, by age group.
Factors Associated With HSV-2 Infection in Women 16 to 24
Factors associated with HSV-2 and HIV serostatus were determined among the 1143 women age 16 to 24 years, in whom infection is most recent. Of these, 66% were HSV-2 seropositive and 21% were HIV seropositive. On univariate analysis, HSV-2 seroprevalence was significantly associated with older age, being currently or previously married, being Sukuma, living in a mining site or a truck stop, shorter length of residence in that site, recent travel, type of work facility, lower levels of education, illiteracy, renting a house, increasing number of dependents, and fewer possessions (Table 3). A multivariate model of socioeconomic factors showed that age, marital status, type of living site, recent travel, work facility type, level of education, and number of dependents were independently associated with HSV-2 serostatus (Table 3). After adjusting for these factors, HSV-2 infection was significantly associated with increasing number of sexual partners (lifetime and in the past 3 months) and younger age at first sex (Table 4). In addition, HSV-2 prevalence was highest among those who used condoms irregularly (OR = 3.45, 95% CI: 1.9 to 6.1 for use “sometimes” compared to always). The prevalence was lowest among women reporting never having used a condom (OR = 0.59, 95% CI: 0.4 to 0.9) compared with consistent users, possibly because these women reported fewer partners and were less likely to be exposed to HSV-2. HSV-2 was also significantly associated with vaginal douching and alcohol use and was inversely associated with ever having received a blood transfusion. Finally, HSV-2 was strongly associated with HIV infection (91% of HIV-1–seropositive women were HSV-2 seropositive, compared with 59% of HIV-1–seronegative women) (Table 4).
TABLE 3. Association of Sociodemographic, Mobility, and Socioeconomic Factors With HSV-2 and HIV Infection Among Female Facility Workers Aged 16 to 24 in Northwestern Tanzania.
| HSV-2, n (%) |
Crude OR* (95% CI) |
Adjusted OR (95% CI) |
HIV, n (%) |
Crude OR (95% CI) |
Adjusted OR‡ (95% CI) |
|||
|---|---|---|---|---|---|---|---|---|
| Sociodemographic | ||||||||
| Age group, y | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | ||||
| 16 to 17 | 54 (39) | 1 | 1 | 13 (9) | 1 | 1 | ||
| 18 to 19 | 125 (52) | 1.71 | 1.59 (1.0 to 2.5) | 42 (18) | 2.09 (1.1 to 4.0) | 1.82 (0.9 to 3.6) | ||
| 20 to 21 | 182 (66) | 2.98 | 2.70 (1.7 to 4.3) | 48 (18) | 2.05 (1.1 to 3.9) | 1.77 (0.9 to 3.5) | ||
| 22 to 24 | 392 (81) | 6.56 | 5.21 (3.2 to 8.4) | 134 (28) | 3.74 (2.0 to 6.9) | 2.99 (1.6 to 5.7) | ||
| Marital status | P < 0.001 | P = 0.09 | P = 0.006 | P = 0.14 | ||||
| Unmarried | 367 (57) | 1 | 1 | 121 (19) | 1 | 1 | ||
| Married/living with partner | 127 (69) | 1.73 (1.2 to 2.5) | 1.11 (0.7 to 1.7) | 30 (16) | 0.84 (0.5 to 1.3) | 0.60 (0.9 to 3.6) | ||
| Divorced/separated | 248 (82) | 3.57 (2.6 to 5.0) | 1.58 (1.1 to 2.3) | 81 (27) | 1.59 (1.2 to 2.2) | 0.98 (0.7 to 1.4) | ||
| Widowed | 11 (92) | 8.39 (1.1 to 65.4) | 2.74 (0.3 to 22.1) | 5 (38) | 2.67 (0.9 to 8.3) | 1.50 (0.5 to 4.9) | ||
| Tribe | 0.02 | P = 0.11 | P = 0.46 | P = 0.65 | ||||
| Non-Sukuma | 380 (63) | 1 | 1 | 131 (22) | 1 | 1 | ||
| Sukuma | 373 (69) | 1.33 (1.0 to 1.7) | 1.25 (1.0 to 1.7) | 106 (20) | 0.90 (0.7 to 1.2) | 0.93 (0.7 to 1.3) | ||
| Religion | P = 0.35 | P = 0.29 | P = 0.06 | P = 0.19 | ||||
| Protestant | 188 (62) | 1 | 1 | 51 (17) | 1 | 1 | ||
| Catholic | 379 (67) | 1.22 (0.9 to 1.6) | 1.26 (0.9 to 1.7) | 116 (21) | 1.25 (0.9 to 1.8) | 1.12 (0.8 to 1.6) | ||
| Muslim | 158 (69) | 1.38 (1.0 to 2.0) | 1.22 (0.8 to 1.8) | 61 (27) | 1.79 (1.2 to 2.7) | 1.59 (1.0 to 2.5) | ||
| Other | 28 (65) | 1.14 (0.6 to 2.3) | 0.71 (0.3 to 1.5) | 9 (20) | 1.25 (0.6 to 2.7) | 1.26 (0.6 to 2.9) | ||
| Type of site | P = 0.005 | P = 0.04 | P = 0.003 | P = 0.005 | ||||
| Trading center | 224 (59) | 1 | 1 | 57 (15) | 1 | 1 | ||
| Mining town | 148 (68) | 1.42 (1.0 to 2.0) | 1.22 (0.8 to 1.8) | 55 (25) | 1.89 (1.2 to 2.9) | 1.60 (1.0 to 2.5) | ||
| Truck stop | 381 (70) | 1.57 (1.2 to 2.1) | 1.49 (1.1 to 2.0) | 125 (23) | 1.67 (1.2 to 2.4) | 1.81 (1.2 to 2.6) | ||
| Mobility | ||||||||
| Duration of residence in site, y | P = 0.02 | P = 0.34 | P = 0.63 | P = 0.53 | ||||
| Since birth | 133 (57) | 1 | 1 | 44 (19) | 1 | 1 | ||
| ≥5 | 181 (67) | 1.48 (1.0 to 2.1) | 0.99 (0.7 to 1.5) | 59 (22) | 1.16 (0.8 to 1.8) | 0.95 (0.6 to 1.5) | ||
| 1 to 4 | 241 (70) | 1.76 (1.2 to 2.5) | 1.34 (0.9 to 2.0) | 66 (20) | 1.02 (0.7 to 1.6) | 0.75 (0.5 to 1.2) | ||
| <1 | 198 (67) | 1.50 (1.1 to 2.1) | 1.07 (0.7 to 1.6) | 68 (23) | 1.27 (0.8 to 1.9) | 0.96 (0.6 to 1.5) | ||
| Recent travel | P < 0.001 | P = 0.0002 | P = 0.01 | P = 0.22 | ||||
| None in past 3 mo | 498 (61) | 1 | 1 | 150 (19) | 1 | 1 | ||
| <1 wk | 61 (79) | 2.42 (1.4 to 4.3) | 2.34 (1.3 to 2.6) | 19 (25) | 1.49 (0.9 to 2.6) | 1.46 (0.8 to 2.6) | ||
| >1 wk | 194 (77) | 2.17 (1.6 to 3.0) | 1.83 (1.3 to 2.6) | 68 (27) | 1.64 (1.2 to 2.3) | 1.29 (0.9 to 1.8) | ||
| No. places lived in past y | P = 0.34 | P = 0.97 | P = 0.01 | P = 0.63 | ||||
| 1 | 388 (63) | 1 | 1 | 125 (20) | 1 | 1 | ||
| 2 | 310 (70) | 1.38 (1.1 to 1.8) | 1.25 (0.9 to 1.7) | 91 (21) | 1.01 (0.7 to 1.4) | 0.89 (0.6 to 1.2) | ||
| ≥3 | 55 (65) | 1.08 (0.7 to 1.7) | 0.67 (0.4 to 1.2) | 21 (26) | 1.34 (0.8 to 2.3) | 0.97 (0.5 to 1.7) | ||
| Socioeconomic | ||||||||
| Facility type | P < 0.001 | P = 0.0004 | P < 0.001 | P < 0.001 | ||||
| Local food seller | 375 (63) | 1 | 1 | 95 (16) | 1 | 1 | ||
| Restaurant/café | 133 (59) | 0.87 (0.6 to 1.2) | 0.92 (0.6 to 1.3) | 46 (21) | 1.36 (0.9 to 2.0) | 1.47 (1.0 to 2.2) | ||
| Guesthouse | 80 (65) | 1.11 (0.7 to 1.7) | 1.00 (0.6 to 1.6) | 25 (20) | 1.31 (0.8 to 2.1) | 1.28 (0.8 to 2.1) | ||
| Local brew seller | 26 (65) | 1.10 (0.6 to 2.2) | 0.81 (0.4 to 1.7) | 11 (29) | 2.12 (1.0 to 4.4) | 2.00 (0.9 to 4.3) | ||
| Bar worker | 139 (88) | 4.35 (2.6 to 7.2) | 2.89 (1.7 to 5.0) | 60 (38) | 3.15 (2.1 to 4.6) | 2.58 (1.7 to 3.9) | ||
| Education | P = 0.001 | P < 0.001 | P = 0.24 | P = 0.15 | ||||
| Secondary | 37 (51) | 1 | 1 | 11 (15) | 1 | 1 | ||
| Primary | 441 (63) | 1.69 (1.0 to 2.7) | 1.94 (1.1 to 3.3) | 139 (20) | 1.38 (0.7 to 2.7) | 1.41 (0.9 to 2.8) | ||
| <Primary | 275 (73) | 2.68 (1.6 to 4.5) | 3.45 (1.9 to 6.1) | 87 (23) | 1.66 (0.8 to 3.3) | 1.80 (0.9 to 3.7) | ||
| Literacy | P < 0.001 | P = 0.15 | P = 0.97 | P = 0.74 | ||||
| Yes | 517 (63) | 1 | 1 | 170 (21) | 1 | 1 | ||
| No | 236 (74) | 1.64 (1.2 to 2.2) | 1.37 (0.9 to 2.1) | 67 (21) | 1.01 (0.7 to 1.4) | 1.06 (0.8 to 1.5) | ||
| Housing | P < 0.001 | P = 0.18 | P = 0.008 | P = 0.32 | ||||
| Own house | 24 (63) | 1 | 1 | 4 (11) | 1 | 1 | ||
| Renting | 341 (76) | 1.86 (0.9 to 3.7) | 1.78 (0.8 to 3.8) | 114 (26) | 2.95 (1.0 to 8.5) | 2.15 (0.7 to 6.5) | ||
| Free room | 344 (60) | 0.87 (0.4 to 1.7) | 1.34 (0.6 to 2.9) | 104 (18) | 1.89 (0.7 to 5.4) | 1.80 (0.6 to 5.4) | ||
| Other | 44 (54) | 0.69 (0.3 to 1.5) | 1.32 (0.5 to 3.2) | 15 (19) | 2.00 (0.6 to 6.5) | 2.44 (0.7 to 8.4) | ||
| Savings | P = 0.11 | P = 0.16 | P = 0.85 | P = 0.61 | ||||
| Some | 323 (69) | 1 | 1 | 98 (21) | 1 | 1 | ||
| None | 430 (64) | 0.81 (0.6 to 1.0) | 0.82 (0.6 to 1.1) | 129 (21) | 0.97 (0.7 to 1.3) | 1.09 (0.8 to 1.5) | ||
| Running water at home | P = 0.46 | P = 0.95 | P = 0.36 | P = 0.51 | ||||
| Yes | 195 (64) | 1 | 1 | 58 (19) | 1 | 1 | ||
| No | 558 (67) | 1.11 (0.8 to 1.5) | 1.01 (0.7 to 1.4) | 179 (22) | 1.16 (0.8 to 1.6) | 1.12 (0.8 to 1.6) | ||
| No. dependents | P < 0.001 | P = 0.01 | P = 0.02 | P = 0.81 | ||||
| 0 | 300 (57) | 1 | 1 | 98 (19) | 1 | 1 | ||
| 1 | 200 (71) | 1.87 (1.4 to 2.5) | 1.23 (0.9 to 1.7) | 56 (20) | 1.09 (0.8 to 1.6) | 0.81 (0.5 to 1.2) | ||
| ≥2 | 251 (76) | 2.47 (1.8 to 3.4) | 1.47 (1.0 to 2.1) | 83 (26) | 1.49 (1.1 to 2.1) | 1.12 (0.8 to 1.6) | ||
| Main source of income | P = 0.34 | P = 0.80 | P = 0.79 | P = 0.59 | ||||
| Non-sex work | 717 (66) | 1 | 1 | 226 (21) | 1 | 1 | ||
| Sex work | 36 (72) | 1.35 (0.7 to 2.5) | 0.91 (0.4 to 1.9) | 11 (22) | 1.10 (0.6 to 2.2) | 0.82 (0.4 to 1.7) | ||
| No. possessions† | P = 0.004 | P = 0.16 | P = 0.003 | P = 0.009 | ||||
| 4 to 6 | 38 (60) | 1 | 1 | 11 (18) | 1 | 1 | ||
| 2 to 3 | 309 (74) | 1.88 (1.1 to 3.3) | 1.85 (1.0 to 3.4) | 115 (28) | 1.79 (0.9 to 3.6) | 1.63 (0.8 to 3.3) | ||
| 0 to 1 | 406 (61) | 1.04 (0.6 to 1.8) | 1.50 (0.8 to 2.7) | 111 (17) | 0.94 (0.5 to 1.9) | 1.00 (0.5 to 2.1) | ||
Adjusted for age, marital status, type of living site, recent travel, work facility type, level of education, and number of dependents.
Number of the following possessions: plot of land, any livestock, bed, radio, television, bicycle.
Adjusted for age, marital status, type and living site, work facility type and number of possessions.
TABLE 4. Association of Behavioral Factors With HSV-2 and HIV Infection Among Female Facility Workers Aged 16 to 24 in Northwestern Tanzania.
| HSV-2, n (%) |
Adjusted OR* (95% CI) |
HIV, n (%) |
Adjusted OR† (95% CI) |
||
|---|---|---|---|---|---|
| No. partners in past 3 mo | P trend < 0.001 | P trend < 0.001 | |||
| 0 | 47 (35) | 1 | 10 (8) | 1 | |
| 1 | 333 (62) | 2.47 (1.6 to 3.9) | 102 (19) | 2.55 (1.3 to 5.1) | |
| 2 | 198 (78) | 4.52 (2.7 to 7.6) | 61 (24) | 2.96 (1.4 to 6.2) | |
| 3 to 4 | 124 (79) | 3.91 (2.2 to 7.1) | 40 (26) | 2.90 (1.3 to 6.3) | |
| ≥5 | 51 (82) | 4.66 (2.0 to 10.7) | 24 (39) | 5.54 (2.3 to 13.2) | |
| No. lifetime partners | P trend < 0.001 | P trend < 0.001 | |||
| 0 | 6 (18) | 0.77 (0.3 to 2.1) | 0 (0) | 0 | |
| 1 | 30 (27) | 1 | 3 (3) | 1 | |
| 2 to 4 | 308 (62) | 3.52 (2.1 to 5.8) | 82 (17) | 5.23 (1.6 to 17.1) | |
| 5 to 9 | 216 (78) | 5.71 (3.3 to 10.0) | 74 (27) | 8.25 (2.5 to 27.3) | |
| 10 to 24 | 142 (86) | 8.76 (4.5 to 17.0) | 53 (33) | 9.81 (2.9 to 33.2) | |
| ≥25 | 48 (89) | 9.23 (3.3 to 25.6) | 23 (43) | 15.5 (4.2 to 57.0) | |
| Age at first sex¶ | P trend = 0.0004 | P trend = 0.008 | |||
| ≥18 | 164 (64) | 1 | 51 (23) | 1 | |
| 16 to 17 | 271 (65) | 1.47 (1.0 to 2.1) | 82 (22) | 1.17 (0.8 to 1.8) | |
| 14 to 15 | 245 (71) | 2.16 (1.4 to 3.3) | 76 (24) | 1.47 (0.9 to 2.3) | |
| <14 | 63 (71) | 2.22 (1.2 to 4.1) | 24 (29) | 2.19 (1.2 to 4.0) | |
| Age difference with partner at first sex¶ | P = 0.93 | P = 0.58 | |||
| Younger or within 2 y | 180 (67) | 1 | 55 (21) | 1 | |
| 3 to 9 y older | 241 (67) | 0.91 (0.6 to 1.3) | 69 (22) | 0.91 (0.6 to 1.4) | |
| >10 y older | 52 (72) | 0.84 (0.4 to 1.6) | 18 (25) | 1.08 (0.6 to 2.1) | |
| Doesn't know partner's age | 274 (67) | 0.91 (0.6 to 1.3) | 92 (23) | 1.18 (0.8 to 1.7) | |
| Condom use‡ | P < 0.001 | P = 0.01 | |||
| Always | 185 (60) | 1 | 73 (24) | 1 | |
| Often | 144 (75) | 1.53 (1.0 to 2.4) | 39 (21) | 0.78 (0.5 to 1.2) | |
| Sometimes | 103 (84) | 3.45 (1.9 to 6.1) | 36 (30) | 1.44 (0.9 to 2.4) | |
| Rarely | 245 (72) | 1.45 (1.0 to 2.1) | 71 (22) | 0.98 (0.7 to 1.5) | |
| Never | 69 (47) | 0.59 (0.4 to 0.9) | 14 (17) | 0.51 (0.3 to 1.0) | |
| Forced sex in past 3 mo | P = 0.33 | P = 0.49 | |||
| No | 702 (66) | 1 | 223 (21) | 1 | |
| Yes | 31 (79) | 1.48 (0.6 to 3.5) | 11 (28) | 1.30 (0.6 to 2.8) | |
| Lubricants for sex¶ | P = 0.28 | P = 0.02 | |||
| No | 667 (67) | 1 | 202 (20) | 1 | |
| Yes | 80 (75) | 1.31 (0.8 to 2.2) | 35 (34) | 1.73 (1.1 to 2.7) | |
| Dry/tight sex¶ | P = 0.18 | P = 0.07 | |||
| No | 720 (65) | 1 | 223 (20) | 1 | |
| Yes | 32 (78) | 1.71 (0.8 to 3.9) | 14 (34) | 1.94 (1.0 to 3.9) | |
| Sex during menstruation | 0.98 | P = 0.23 | |||
| No | 638 (64.8) | 1 | 195 | 1 | |
| Yes | 115 (72.8) | 1.01 (0.7 to 1.5) | 42 | 1.29 (0.9 to 1.9) | |
| Contraceptive method¶ | P = 0.96 | P = 0.11 | |||
| Condom | 97 (63) | 1 | 28 (18) | 1 | |
| Hormonal | 178 (73) | 1.02 (0.6 to 1.7) | 49 (20) | 1.12 (0.6 to 1.9) | |
| IUCD | 3 (75) | 0.76 (0.1 to 9.2) | 2 (50) | 4.47 (0.5 to 40.0) | |
| Tubal ligation | 3 (60) | 0.53 (0.1 to 3.6) | 0 (0) | 0 | |
| Safe/withdrawal/traditional | 29 (74) | 1.33 (0.6 to 3.2) | 11 (28) | 1.83 (0.8 to 4.3) | |
| Nothing | 437 (66) | 1.07 (0.7 to 1.6) | 147 (22) | 1.59 (1.0 to 2.6) | |
| Frequency of douching | P trend = 0.008 | P trend = 0.92 | |||
| Never | 325 (57) | 1 | 97 (17) | 1 | |
| 1 to 2 times a day | 163 (72) | 1.20 (0.8 to 1.7) | 51 (23) | 1.10 (0.7 to 1.6) | |
| ≥3 times a day | 261 (75) | 1.56 (1.2 to 2.2) | 80 (25) | 1.18 (0.8 to 1.7) | |
| HIV transmission knowledge (no. correct out of 10 questions) | P = 0.73 | P = 0.90 | |||
| ≤5 | 80 (62) | 1 | 24 (19) | 1 | |
| 6 to 8 | 399 (67) | 1.20 (0.8 to 1.9) | 125 (21) | 0.93 (0.6 to 1.6) | |
| 9 to 10 | 274 (65) | 1.17 (0.7 to 1.9) | 88 (21) | 1.00 (0.6 to 1.7) | |
| No. alcoholic drinks per wk§ | P trend < 0.001 | P trend = 0.003 | |||
| 0 | 389 (57) | 1 | 103 (15) | 1 | |
| 1 to 4 | 162 (74) | 1.43 (1.0 to 2.1) | 51 (24) | 1.27 (0.9 to 1.9) | |
| 5 to 9 | 67 (85) | 2.71 (1.4 to 5.4) | 29 (37) | 2.37 (1.4 to 4.1) | |
| 10 to 19 | 50 (73) | 1.12 (0.6 to 2.1) | 19 (28) | 1.46 (0.8 to 2.7) | |
| ≥20 | 85 (91) | 4.80 (2.1 to 10.8) | 35 (38) | 1.99 (1.2 to 3.4) | |
| Drug taking∥ | P = 0.06 | P = 0.20 | |||
| No | 678 (64) | 1 | 209 (20) | 1 | |
| Yes | 75 (83) | 1.78 (1.0 to 3.3) | 28 (31) | 1.39 (0.8 to 2.3) | |
| Blood transfusion | P = 0.01 | P = 0.52 | |||
| Never | 688 (66) | 1 | 215 (21) | 1 | |
| Ever | 65 (62) | 0.54 (0.3 to 0.9) | 22 (21) | 0.85 (0.5 to 1.4) | |
| Injections in past 5 y | P = 0.59 | P = 0.08 | |||
| No | 194 (63) | 1 | 72 (23) | 1 | |
| Yes | 559 (67) | 1.09 (0.8 to 1.5) | 165 (20) | 0.74 (0.5 to 1.0) | |
| HIV infection | P < 0.001 | ||||
| No | 527 (59) | 1 | |||
| Yes | 215 (91) | 4.49 (3.1 to 6.6) | |||
| HSV-2 infection | P < 0.001 | ||||
| No | 22 (6) | 1 | |||
| Yes | 215 (29) | 5.44 (3.4 to 8.1) | |||
Adjusted for age, marital status, type of living site, type of work facility, recent travel, level of education, and number of dependents.
Adjusted for age, marital status, type of living site, type of work facility, and number of possessions.
Response to the question “How often do you think you use condoms?” Excludes 36 women who reported never having had sexual intercourse.
Response to the question “How many drinks do you take in a usual week?”
Ever taken marijuana, cocaine, valium, promethazine, pethidine, or heroin.
Excluding the 36 women who reported never having sexual intercourse.
IUCD indicates intrauterine contraceptive device.
Independent risk factors for HSV-2 seroprevalence (Table 5) were older age, lower levels of education, type of work facility (bar workers at highest risk), more dependents, vaginal douching at least 3 times a day, higher risk sexual behavior (increased number of lifetime sexual partners, younger age at first sex, irregular condom use), increased alcohol consumption, and presence of HIV infection. History of genital ulceration was not included as a risk factor for HSV-2 infection because it was likely to be caused by the infection itself, but there was some association between the 2 after adjusting for other variables in Table 5 (OR = 1.38, 95% CI: 0.9 to 2.1).
TABLE 5. Multivariate Model for Factors Associated With HSV-2 and HIV Infection Among Female Facilities Workers Aged 16 to 24 in Northwestern Tanzania.
| HSV-2, n (%) | Adjusted OR* (95% CI) | HIV, n (%) | Adjusted OR† (95% CI) | ||
|---|---|---|---|---|---|
| Age group, y | P trend < 0.001 | P trend = 0.0002 | |||
| 16 to 17 | 54 (39) | 1 | 13 (9) | 1 | |
| 18 to 19 | 125 (52) | 1.22 (0.7 to 2.0) | 42 (18) | 1.44 (0.7 to 2.9) | |
| 20 to 21 | 182 (66) | 2.41 (1.4 to 4.1) | 48 (18) | 1.20 (0.6 to 2.4) | |
| 22 to 24 | 392 (81) | 4.42 (2.6 to 7.6) | 134 (28) | 1.90 (1.0 to 3.7) | |
| Marital status | P = 0.17 | P = 0.22 | |||
| Unmarried | 367 (57) | 1 | 121 (19) | 1 | |
| Married/living with partner | 127 (69) | 1.02 (0.6 to 1.6) | 30 (16) | 0.63 (0.4 to 1.0) | |
| Divorced/separated | 248 (82) | 1.45 (1.0 to 2.2) | 81 (27) | 0.89 (0.6 to 1.3) | |
| Widowed | 11 (92) | 3.58 (0.4 to 30.7) | 5 (38) | 1.65 (0.5 to 5.5) | |
| Type of site | P = 0.18 | P = 0.03 | |||
| Trading center | 224 (59) | 1 | 57 (15) | 1 | |
| Mining town | 148 (68) | 1.10 (0.7 to 1.7) | 55 (25) | 1.50 (1.0 to 2.4) | |
| Truck stop | 381 (70) | 1.36 (1.0 to 1.8) | 125 (23) | 1.64 (1.1 to 2.4) | |
| Education | P trend = 0.005 | P = 0.43 | |||
| Secondary | 37 (51) | 1 | 11 (15) | 1 | |
| Primary | 441 (63) | 2.15 (1.2 to 3.9) | 139 (20) | 1.23 (0.6 to 2.5) | |
| <Primary | 275 (73) | 2.96 (1.5 to 5.6) | 87 (23) | 1.34 (0.6 to 2.8) | |
| Facility type | P = 0.03 | P = 0.04 | |||
| Local food seller | 375 (63) | 1 | 95 (16) | 1 | |
| Restaurant/café | 133 (59) | 0.76 (0.5 to 1.1) | 46 (21) | 1.39 (0.9 to 2.1) | |
| Guesthouse | 80 (65) | 0.80 (0.5 to 1.3) | 25 (20) | 1.10 (0.7 to 1.9) | |
| Local brew seller | 26 (65) | 0.60 (0.3 to 1.4) | 11 (29) | 1.76 (0.8 to 3.8) | |
| Bar worker | 139 (88) | 1.79 (1.0 to 3.2) | 60 (38) | 1.99 (1.2 to 3.1) | |
| No. possessions | P trend = 0.25 | P = 0.04 | |||
| 4 to 6 | 38 (60) | 1 | 11 (18) | 1 | |
| 2 to 3 | 309 (74) | 1.76 (0.9 to 3.4) | 115 (28) | 1.61 (0.8 to 3.3) | |
| 0 to 1 | 406 (61) | 1.66 (0.9 to 3.2) | 111 (17) | 1.09 (0.5 to 2.2) | |
| No. dependents | P = 0.005 | P = 0.40 | |||
| 0 | 300 (57) | 1 | 98 (19) | 1 | |
| 1 | 200 (71) | 1.15 (0.8 to 1.7) | 56 (20) | 0.81 (0.5 to 1.2) | |
| ≥2 | 251 (76) | 1.48 (1.0 to 2.1) | 83 (26) | 1.05 (0.7 to 1.5) | |
| Vaginal douching | P trend = 0.002 | P trend = 0.98 | |||
| Never | 325 (57) | 1 | 97 (17) | 1 | |
| <2 times a day | 163 (72) | 0.96 (0.7 to 1.4) | 51 (23) | 0.95 (0.6 to 1.4) | |
| ≥3 times a day | 246 (76) | 1.48 (1.0 to 2.1) | 80 (25) | 1.02 (0.7 to 1.5) | |
| No. lifetime partners‡ | P trend < 0.001 | P trend < 0.001 | |||
| 0 | 6 (18) | — | 0 (0) | 0 | |
| 1 | 30 (27) | 1 | 3 (3) | 1 | |
| 2 to 4 | 308 (62) | 2.78 (1.6 to 4.7) | 82 (17) | 5.78 (1.8 to 19.2) | |
| 5 to 9 | 216 (78) | 3.99 (2.2 to 7.4) | 74 (27) | 9.01 (2.8 to 30.3) | |
| 10 to 24 | 142 (86) | 5.79 (2.8 to 11.8) | 53 (33) | 11.1 (3.4 to 38.4) | |
| ≥25 | 48 (89) | 5.45 (1.9 to 15.9) | 23 (43) | 17.2 (4.7 to 62.9) | |
| Age at sexual debut‡ | P trend = 0.01 | P trend = 0.21 | |||
| ≥18 | 164 (64) | 1 | 51 (23) | 1 | |
| 16 to 17 | 271 (65) | 1.35 (0.9 to 2.0) | 82 (22) | 0.98 (0.6 to 1.5) | |
| 14 to 15 | 245 (71) | 1.76 (1.1 to 2.8) | 76 (24) | 1.09 (0.7 to 1.7) | |
| <14 | 63 (71) | 1.79 (0.9 to 3.4) | 24 (29) | 1.58 (0.9 to 2.9) | |
| Condom use | P = 0.0002 | P = 0.27 | |||
| Always | 185 (60) | 1 | 73 (24) | 1 | |
| Often | 144 (75) | 1.37 (0.9 to 2.2) | 39 (21) | 0.64 (0.4 to 1.0) | |
| Sometimes | 103 (84) | 2.58 (1.4 to 4.7) | 36 (30) | 1.09 (0.7 to 1.8) | |
| Rarely | 245 (72) | 1.39 (1.0 to 2.1) | 71 (22) | 0.87 (0.6 to 1.3) | |
| Never | 69 (47) | 0.93 (0.6 to 1.5) | 14 (17) | 0.72 (0.4 to 1.4) | |
| No. alcoholic drinks per wk | P trend = 0.006 | P trend = 0.19 | |||
| 0 | 389 (57) | 1 | 103 (15) | 1 | |
| 1 to 4 | 162 (74) | 1.16 (0.8 to 1.7) | 51 (24) | 1.07 (0.7 to 1.6) | |
| 5 to 9 | 67 (85) | 1.81 (0.9 to 3.7) | 29 (37) | 1.84 (1.1 to 3.2) | |
| 10 to 19 | 50 (73) | 0.94 (0.5 to 1.7) | 19 (28) | 1.16 (0.6 to 2.2) | |
| ≥20 | 85 (91) | 3.72 (1.6 to 8.4) | 35 (38) | 1.57 (0.9 to 2.7) | |
| Injections in past 5 y | P = 0.79 | P = 0.02 | |||
| No | 194 (63) | 1 | 72 (23) | 1 | |
| Yes | 559 (67) | 1.05 (0.8 to 1.4) | 165 (20) | 0.68 (0.5 to 1.0) | |
| Ever had genital ulcer | P = 0.14 | P = 0.16 | |||
| No | 594 (63) | 1 | 178 (19) | 1 | |
| Yes | 159 (78) | 1.38 (0.9 to 2.1) | 59 (29) | 1.31 (0.9 to 1.9) | |
| HIV§ | P < 0.001 | ||||
| No | 527 (59) | 1 | |||
| Yes | 215 (91) | 4.07 (2.5 to 6.6) | |||
| HSV-2 | P < 0.0001 | ||||
| No | 22 (6) | 1 | |||
| Yes | 215 (29) | 4.22 (2.6 to 6.9) | |||
Adjusted for age, type of living site, type of work facility, level of education, number of dependents, number of lifetime partners, age at sexual debut, ever used a condom, ever douched, and number of drinks per week.
Adjusted for age, type of living site, type of work facility, number of lifetime partners, number of injections, and number of possessions.
Test for trend excludes those who had never had sexual intercourse.
Excludes 21 women without HIV status.
Factors Associated With HIV Infection in Women 16 to 24
In the univariate analysis, HIV infection was associated with older age, having been previously married, living in a mining town or truck stop, recent travel, number of different places lived in within the past year, work facility type, renting a house, increasing number of dependents, and number of possessions (Table 3). Independent risk factors were age, type of living site, type of work facility, and number of possessions. After adjusting for these factors, HIV was associated with increasing number of partners (both lifetime and in the past 3 months), younger age at first sex, irregular condom use, using lubricants for sex, tight/dry sex, and increasing alcohol intake (Table 4). There was some evidence of an inverse association with a history of having had an injection in the previous 5 years (OR = 0.68, 95% CI: 0.5 to 1.0). In the final multivariate model (Table 5), HIV infection was independently associated with older age, living in a mining town or a truck stop, being a bar worker or local brew seller, and increasing number of lifetime partners. Women who had received an injection within the past 5 years were less likely to be HIV positive. HIV was also independently associated with being HSV-2 seropositive (HIV prevalence 29% among HSV-2 seropositive women vs. 6% among HSV-2 seronegative women; P ≤ 0.001).
DISCUSSION
Women in this population have an extremely high prevalence of HSV-2 infection, with the majority being infected by age 20. One third of women were also infected with HIV, almost double the prevalence seen among women in the general population in these communities.16 The prevalence of HSV-2 (80%) is similar to that observed in a similar occupational cohort in Mbeya Region (87%)10 and among sex workers in other cities in the region such as Kinshasa (82%),17 Nairobi (73%),18 Kisumu (94%),19 and Ndola (87%).19 Somewhat lower prevalence was seen among female bar/hotel workers in Moshi (44% to 56%).11,20
The strong association between HSV-2 and HIV seroprevalence has been seen in many previous studies21-23 and is due to a synergistic relationship between HSV-2 and HIV infections. Both symptomatic GUD and asymptomatic HSV-2 reactivations seem to increase risk of HIV acquisition and transmission.6,7,12,24,25 In addition, susceptibility to HSV-2 infection may be increased among immunocompromised HIV-positive individuals, as seen among high-risk women in Moshi.20
HSV-2 and HIV also share a number of common risk factors including age, occupation, and number of lifetime sexual partners. Women living in truck stops and mining towns and those who worked in bars had the highest risk of both infections. Alcohol is likely to impair judgment and perception of risk from unprotected sex,26,27 which might increase risk of both HSV-2 and HIV. In our study, HSV-2 was also associated with higher levels of alcohol consumption, but the relationship with HIV was less clear. An association between frequency of alcohol intake per week and HSV-2 infection has been seen in men, but not women, in Moshi, although participants in that study were not asked specifically about the amount of alcohol consumed, only frequency.11 A further study in Moshi found that 35% of female bar/hotel workers had a drinking problem,22 as defined by the CAGE scale,28 and that HSV-2 seroincidence was significantly associated with number of drinks on drinking days.20 In our study, there was no evidence of an association of lack of consistent condom use and usual alcohol consumption, although both variables are susceptible to misreporting.
Limitations of our study include the cross-sectional design, which precludes ascertainment of the temporal relationship between most variables, and our reliance on self-reporting for behavioral information and history of GUD. By focusing risk factor analyses on young women, we aimed to evaluate the effect of recent reported behaviors on HSV-2 and HIV infection. In interpreting the results, it is important to note that we have assessed associations using logistic regression to obtain odds ratios, which are statistically robust but do not approximate the prevalence ratio for common outcomes, as seen in this study.
Evaluation of interventions against incident HIV infection and HIV genital shedding require study populations with adequate incidence and prevalence to detect an intervention effect. Women working in bars, hotels, and other facilities have been shown to have a high rate of partner change29 and to constitute a core group for HIV transmission. Furthermore, the study population described here is suitable for an HSV suppressive therapy trial because of the extremely high HSV-2 and HIV prevalence at baseline and ongoing risk for HIV acquisition among those who were HIV uninfected at baseline. Despite a good awareness of HIV risk, demonstrated by the fact that 90% of participants answered at least 6 of 10 HIV knowledge questions correctly, most women had been sexually active within the previous 3 months (with 16% having had at least 3 partners in that period) and consistent condom use was rare. Although mobility is a concern for follow-up of such a cohort, careful mapping of sites and documentation of past and planned migration routes has identified new high-transmission sites in the area and will assist in follow-up.
In a population with such highly prevalent HSV-2 infection, suppressive HSV-2 therapy should reduce the number of subclinical and clinical episodes of HSV-2 reactivation, potentially reducing HIV incidence and HIV genital shedding. The randomized controlled trial of the impact of HSV suppressive therapy on HIV incidence and genital shedding in this population is now completed, and the results will be published separately. These will be instrumental in guiding future health policy on the role of HSV suppressive therapy for HIV prevention.
Acknowledgments
Support provided by the Wellcome Trust and the Department for International Development UK. Salary for Dr. Weiss was provided by the UK Medical Research Council.
Footnotes
Preliminary findings presented at the 16th meeting of the International Society for Sexually Transmitted Disease Research (ISSTDR), Amsterdam, July 11–13, 2005, as “Risk Factors for HIV and HSV in High Risk Women, Tanzania,” abstract #TP016.
REFERENCES
- 1.UNAIDS . Report on the Global AIDS Epidemic 2006. Joint United Nations Programme on HIV/AIDS; Geneva: 2006. UNAIDS/06.20E. [Google Scholar]
- 2.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A–35A. [PubMed] [Google Scholar]
- 3.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(Suppl 1):S3–S28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Herpes Simplex Virus Type 2: Programmatic and Research Priorities in Developing Countries: Report of a WHO/UNAIDS/LSHTM Workshop (London 14–16 February 2001) World Health Organization; Geneva: 2001. WHO/HIV_AIDS/2001.05. [Google Scholar]
- 5.Paz-Bailey G, Ramaswamy M, Hawkes SJ, et al. Herpes simplex virus type 2: epidemiology and management options in developing countries. Sex Transm Infect. 2007;83:16–22. doi: 10.1136/sti.2006.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 7.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 8.Obasi A, Mosha F, Quigley M, et al. Antibody to herpes simplex virus type 2 as a marker of sexual risk behavior in rural Tanzania. J Infect Dis. 1999;179:16–24. doi: 10.1086/314555. [DOI] [PubMed] [Google Scholar]
- 9.Msuya SE, Mbizvo E, Hussain A, et al. Seroprevalence and correlates of herpes simplex virus type 2 among urban Tanzanian women. Sex Transm Dis. 2003;30:588–592. doi: 10.1097/00007435-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Riedner G, Rusizoka M, Hoffmann O, et al. Baseline survey of sexually transmitted infections in a cohort of female bar workers in Mbeya Region, Tanzania. Sex Transm Infect. 2003;79:382–387. doi: 10.1136/sti.79.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapiga SH, Sam NE, Shao JF, et al. Herpes simplex virus type 2 infection among bar and hotel workers in northern Tanzania: prevalence and risk factors. Sex Transm Dis. 2003;30:187–192. doi: 10.1097/00007435-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Kapiga SH, Sam NE, Bang H, et al. The role of herpes simplex virus type 2 and other genital infections in the acquisition of HIV-1 among high-risk women in northern Tanzania. J Infect Dis. 2007;195:1260–1269. doi: 10.1086/513566. [DOI] [PubMed] [Google Scholar]
- 13.del Mar Pujades Rodriguez M, Obasi A, Mosha F, et al. Herpes simplex virus type 2 infection increases HIV incidence: a prospective study in rural Tanzania. AIDS. 2002;16:451–462. doi: 10.1097/00002030-200202150-00018. [DOI] [PubMed] [Google Scholar]
- 14.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 15.van Dyck E, Buve A, Weiss HA, et al. Performance of commercially available enzyme immunoassays for detection of antibodies against herpes simplex virus type 2 in African populations. J Clin Microbiol. 2004;42:2961–2965. doi: 10.1128/JCM.42.7.2961-2965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clift S, Anemona A, Watson-Jones D, et al. Variations of HIV and STI prevalences within communities neighbouring new goldmines in Tanzania: importance for intervention design. Sex Transm Infect. 2003;79:307–312. doi: 10.1136/sti.79.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nzila N, Laga M, Thiam MA, et al. HIV and other sexually transmitted diseases among female prostitutes in Kinshasa. AIDS. 1991;5:715–721. doi: 10.1097/00002030-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Kaul R, Kimani J, Nagelkerke NJ, et al. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: a randomized controlled trial. JAMA. 2004;291:2555–2562. doi: 10.1001/jama.291.21.2555. [DOI] [PubMed] [Google Scholar]
- 19.Morison L, Weiss HA, Buve A, et al. Commercial sex and the spread of HIV in four cities in sub-Saharan Africa. AIDS. 2001;15(Suppl 4):S61–S69. doi: 10.1097/00002030-200108004-00007. [DOI] [PubMed] [Google Scholar]
- 20.Tassiopoulos KK, Seage G, 3rd, Sam N, et al. Predictors of herpes simplex virus type 2 prevalence and incidence among bar and hotel workers in Moshi, Tanzania. J Infect Dis. 2007;195:493–501. doi: 10.1086/510537. [DOI] [PubMed] [Google Scholar]
- 21.Weiss HA, Buve A, Robinson NJ, et al. The epidemiology of HSV-2 infection and its association with HIV infection in four urban African populations. AIDS. 2001;15(Suppl 4):S97–S108. doi: 10.1097/00002030-200108004-00011. [DOI] [PubMed] [Google Scholar]
- 22.Ao TT, Sam NE, Masenga EJ, et al. Human immunodeficiency virus type 1 among bar and hotel workers in northern Tanzania: the role of alcohol, sexual behavior, and herpes simplex virus type 2. Sex Transm Dis. 2006;33:163–169. doi: 10.1097/01.olq.0000187204.57006.b3. [DOI] [PubMed] [Google Scholar]
- 23.Celum C, Levine R, Weaver M, et al. Genital herpes and human immunodeficiency virus: double trouble. Bull World Health Organ. 2004;82:447–453. [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–598. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 26.Weiser SD, Leiter K, Heisler M, et al. A population-based study on alcohol and high-risk sexual behaviors in Botswana. PLoS Med. 2006;3:e392. doi: 10.1371/journal.pmed.0030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mnyika KS, Klepp KI, Kvale G, et al. Determinants of high-risk sexual behaviour and condom use among adults in the Arusha region, Tanzania. Int J STD AIDS. 1997;8:176–183. doi: 10.1258/0956462971919840. [DOI] [PubMed] [Google Scholar]
- 28.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 29.Mgalla Z, Pool R. Sexual relationships, condom use and risk perception among female bar workers in north-west Tanzania. AIDS Care. 1997;9:407–416. doi: 10.1080/713613167. [DOI] [PubMed] [Google Scholar]