Abstract
Background
The role of testosterone in the development of behaviors presaging cannabis use and subsequently cannabis use disorder was investigated in a prospective study of 208 boys. It was theorized that adverse neighborhood correlates with testosterone level that in turn potentiates behaviors predisposing to cannabis consumption and subsequently diagnosis of cannabis use disorder.
Methods
Proportion of boarded-up dwellings in the 1990 census tract and testosterone level were recorded at baseline (age 10–12), followed by assessments of assaultiveness and testosterone level (age 12–14), social dominance/norm-violating behavior (age 16), cannabis use (age 19), and cannabis use disorder (age 22).
Results
Percent of vacant dwellings correlates with testosterone level that in turn predicts assaultive behavior sequentially leading to social dominance/norm-violating behavior, cannabis use, and cannabis use disorder. Externalizing behaviors and cannabis use disorder are not directly predicted by neighborhood quality.
Conclusion
Elevated testosterone level intermediates the association between neighborhood adversity and aggressive socially deviant behaviors presaging cannabis use and cannabis use disorder.
Keywords: substance abuse, cannabis, marijuana, etiology, neighborhood, hormones
Introduction
Emerging findings point to an association between level of androgens and substance abuse. High testosterone level has been documented, for example, in substance abusing youths (1), the early age onset antisocial variant of alcoholism (2), adolescent boys whose fathers qualify for diagnosis of substance use disorder (SUD (3), and adolescent boys who subsequently develop SUD (4). Whereas “leadership, toughness, personalized power and aggressive dominance” have been concluded in a comprehensive review to reflect the psychosocial orientation of males having high testosterone level, albeit within the normal range (5), little is known regarding whether this androgen accounts for significant variance on motivational style predisposing to SUD.
Social potency, evinced as forceful decisive leadership, has significant heritability(6). This trait is more strongly expressed in adolescent offspring of SUD men compared to youths whose fathers do not have SUD (7). Notably, Reynolds et al. (4) observed that testosterone level in 10–12 year old boys predicts social potency 4–6 years later which, in turn, predicts drug use frequency and subsequently SUD diagnosis by young adulthood. In a follow-up study on an expanded sample, Tarter et al. (8) showed that testosterone level in 10–12 year old boys predicts the score on a trait termed social dominance norm-violating behavior at age 16 that, in turn, predicts SUD by age 22. Moreover, sexual maturation, indexed by Tanner stage and testosterone level, predicts sensation seeking behavior predisposing to SUD (9). Considered in aggregate, these findings indicate that elevated testosterone level is associated with behaviors that amplify risk for developing SUD.
Notably, reciprocal prediction has been observed between testosterone level and affiliation with socially deviant peers during adolescence (3). In addition, young males domiciling in an economically disadvantaged neighborhood who have high testosterone level have been observed to evince aggression as a strategy to assert social dominance (10). Significantly, empirical findings in humans (11) and other primates (12) demonstrates that testosterone level is elevated in males who are embedded in an environment featured by diminished resources and concomitantly greater interpersonal competition. Whether significant variance in aggressivity, which has been frequently shown to predispose to substance abuse (13,14), is accounted by androgen level has yet to be investigated.
Youth having elevated testosterone level, and associated motivational style of dominance striving and low adherence to social norms, have recently been shown to be at elevated risk to initiate and habitually use drugs (8). Paralleling these findings, it has also been reported that popularity ratings and approval by peers are higher in substance using youths (15,16,17), although the causal direction underlying this association remains to be clarified. In disadvantaged neighborhoods collective efficacy is low (18) tolerance of social deviancy is high (19), and illegal drugs are more readily available. It has been reported, for example, that cocaine is offered to youths domiciling in poor neighborhoods up to 5.6 times more frequently than in optimum neighborhoods (20).
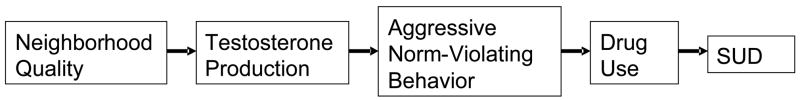
Joining these disparate lines of research, it was hypothesized that physical quality of the neighborhood is correlated with testosterone level that in turn is a predictor of assaultive behavior and social ascendance leading to cannabis use and diagnosis of cannabis use disorder. Figure 1, depicting the theoretical model guiding this investigation, illustrates the interplay of neighborhood context, testosterone level and behavior development culminating in cannabis use disorder. In view of the strong association between disadvantaged neighborhood and high prevalence of illicit drug use and addiction (21,22), this longitudinal investigation sought to clarify whether neighborhood adversity accounts for variance on testosterone level that in turn potentiates behaviors predisposing to cannabis use and ultimately diagnosis of cannabis use disorder. Significantly, cannabis use disorder peaks at approximately 17–18 years of age (23); hence, tracking youths from childhood to young adulthood in this investigation provided the opportunity to delineate the developmental trajectory through the main period of risk.
Figure 1.
Theoretical model tested in this investigation
Methods
2.1 Participants
The boys were ascertained through their fathers (probands) who either qualified for SUD consequent to use of an illicit drug (N = 126) or had no adult psychiatric disorder (N = 194). Previous reports have described the ascertainment criteria and recruitment procedures (3,4). Socioeconomic status of the men was, on average, middle class commensurate with completeing 13.8 (SD = 1.1) years of education.
The boys were enrolled between 1990–1996. Baseline evaluation was conducted when the subjects were 10–12 years of age and follow-up assessments were performed at 12–14, 16, 19 and 22 years of age. By age 22, the prevalence of dependence on marijuana has peaked (23); thus assessing outcome at this age captures the at-risk population. Prior to study, the boys underwent an examination conducted by a registered nurse to determine the presence or history of neurological injury, physical disability, uncorrectable sensory impairment, or fetal alcohol effects. Youths evincing any of these disorders were excluded from study. The boys were required to have a full scale WISC-III-R IQ of at least 80 and ability to read and speak English. At the time of baseline evaluation, 86.1% of the boys lived with both parents, 8.6% with the mother only, 2.4% with the father only, and 2.4% lived part time with each divorced parent.
From a baseline sample of 330, 208 boys underwent all four follow-up evaluations. As can be seen in Table 1, no differences are observed between retained and attrited subjects on socioeconomic status using Hollingshead criteria (24), grade in school, ethnic distribution, and the two predictor variables (neighborhood quality, testosterone level). Full scale IQ is six points lower in the attrited subjects; however, both groups score in the normal range of intelligence.
Table 1.
Comparison of retained and attrited subjects at baseline
| Retained (N =208) | Attrited (N =112) | |||||
|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | F | p | |
| Family socioeconomic status | 41.75 | 14.12 | 39.72 | 12.87 | 1.60 | .21 |
| Grade in school | 4.63 | 1.15 | 4.62 | 1.03 | .02 | .89 |
| WISC-III-R Full Scale IQ | 111.54 | 15.8 | 105.4 | 15.5 | 11.07 | .001 |
| % of Boarded-up vacant dwellings | .01 | .03 | .01 | .02 | .25 | .62 |
| Testosterone level (ng/ml) | 1.46 | 1.9 | 1.39 | 1.8 | .08 | .78 |
| Ethnicity | Chi-square | p-value | ||||
| European American | 159 (76.4%) | 84 (75.0%) | .08 | .77 | ||
| African American | 49 (23.6%) | 28 (25%) | ||||
2.2 Measures
BASELINE (age 10–12)
2.2.1 Neighborhood Quality
The proportion of abandoned and boarded-up dwellings quantified quality of the physical environment. The home address was geocoded and matched to 1990 census tracts using ArcView 9.1 software (25). Because census data are collected every ten years, this information was recorded one time to characterize neighborhood quality during the transition from childhood to adolescence. The number of boarded-up units was recorded and divided by the number of units in the census tract (26). The mean number of vacant dwellings was .01 (SD =.03).
2.2.2 Testosterone
Blood for testosterone determination was collected at 8:30 a.m. The serum was analyzed in triplicate by radioimmunoassay using DSL-4000 Active kits (Diagnostic Systems Laboratories). The mean plasma testosterone concentration in the sample was 1.43 ng/ml (SD= 1.8). The intra-assay and inter-assay coefficient of variation were respectively 6.9% and 8.1%. The assay detection level was 0.08 ng/ml.
FIRST FOLLOW-UP (age 12–14)
2.2.3 Assaultive Behavior (age 12–14)
It was hypothesized that assaultive behavior at age 12–14 portends social dominance/norm-violating behavior at age 16. Accordingly, the Assaultiveness Scale of the Hostility-Guilt Inventory, having superior reliability (alpha = 82) and validity was self-administerd (27). The items comprising this self-administered scale are: 1) I only hit back once in a while, even if someone hits me first; 2) Once in a while I cannot control my wanting to harm others; 3) I cannot think of a good reason for ever hitting anyone; 4) Whoever makes fun of me or my family is asking for a fight; 5) People who are always bugging me are asking for a punch in the nose; and, 6) When I really get mad, I am able to hit someone. Each item is scored 0 or 1. The sample obtained a mean score of 2.54 (SD =1.6).
2.2.4 Testosterone
The same data collection and analysis procedures used at baseline were employed in this assessment. Mean testosterone level of the sample was 3.09 ng/ml (SD = 2.2).
SECOND FOLLOW-UP (age 16)
2.2.5 Social Dominance/Norm-Violating Behavior (SD/NVB)
This trait, previously shown to mediate the association between testosterone level and SUD (8) was derived using three self-report questionnaires: 1) Social Potency scale of the Multidimensional Personality Questionnaire (28); 2) Peer Delinquency Scale (29); and, 3) Perception of Problem Behavior Scale (30). A principal component analysis yielded a one- factor solution accounting for 40% of the variance. The factor loading of each scale was significant beyond the .01 level: social potency =.54; perception of problem behavior =.60; and, peer delinquency =.74. The score on this trait thus reflects a disposition toward forceful leadership in conjunction with low adherence to societal norms of behavior.
THIRD FOLLOW-UP (age 19)
2.2.6 Cannabis Use
The revised Drug Use Screening Inventory (DUSI-R) (31) assessed frequency of cannabis use during the 30 days prior to assessment. Reliability and validity have been previously documented (32,33). Measuring cannabis use provides the opportunity to determine whether consumption mediates the association between SDNVB and cannabis use disorder.
OUTCOME ASSESSMENT (age 22)
Because the fathers were ascertained according to SUD consequent to use of an illegal drug, it is important to focus on the same outcome in their sons. However, apart from cannabis use disorder, the rates of other categories of SUD were too low to conduct statistical analysis. Diagnosis was based on results obtained from an expanded version of the Structured Clinical Interview (SCID)(34) using previously described procedures (35). Current and lifetime diagnoses were consensually formulated by a committee chaired by a psychiatrist, and consisting also of another psychiatrist or psychologist and the clinical associates who conducted the interviews. The rate of cannabis use disorder in the sample was high (27%), consistent with using the high risk family paradigm.
2.3 Procedure
Recruitment was conducted using print and radio advertising, public service announcements and random digit telephone calls. Approximately 20% of the SUD+ men were recruited from treatment facilities. The same procedure was used to recruit SUD− proband fathers except that none were acquired from treatment facilities.
Informed consent was obtained from the parents and assent was obtained from their 10–12 year old son in private sound-attenuating rooms using the procedure approved by the University of Pittsburgh IRB. Informed consent was obtained from the boys at 19 and 22 years of age. All of the subjects enrolled in this project were reviewed semi-annually by CEDAR’s Data Safety and Monitoring Committee, which includes a senior University attorney, to ensure that the protocols were administered within the approved guidelines, and in compliance with requirements to protect the safety and confidentiality of the participants. Privacy was also protected by a Certificate of Confidentiality issued to the Center for Education and Drug Abuse Research (CEDAR) by the National Institute on Drug Abuse. Before initiating the evaluations, the boys underwent a breath alcohol test and urine analysis to screen for drug metabolites. A positive finding required the subject to be rescheduled. Upon completion of the test session, each family member was debriefed and financially compensated.
2.4 Statistical Analysis
Path analysis was utilized to elucidate the relation between neighborhood quality and testosterone level, assaultive behavior, social dominance/norm-violating behavior, cannabis use, and cannabis use disorder. The etiological model was tested with Mplus (36) using the weighted least square parameter estimation method with diagonal weight matrix and robust standard errors. Mediated paths were tested using the method described by Sobel (37). All of the variables used in the path analysis were manifest variables. Significant improvement of model fit was not observed when SES and ethnicity were included in the model; therefore, these latter variables were deleted.
In addition, the temporal sequence of the variables as a causal model was tested using Directed Acyclic Graph (DAG) analysis (38,39). This procedure involved testing the equality of two competing models: a model with direct causal effects only vs. a model with direct and indirect effects. The likelihood ratio test determined model-data fit.
Results
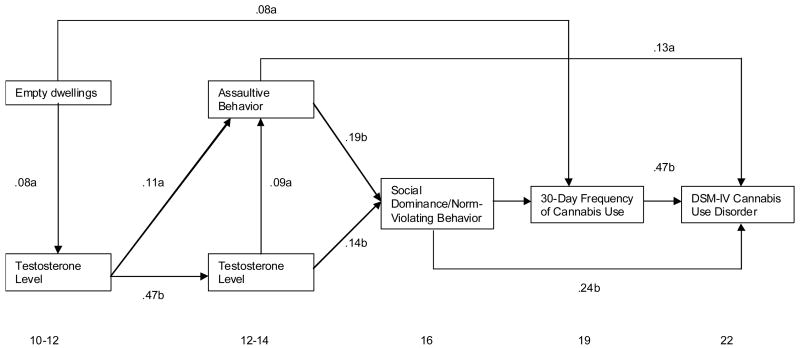
The results, depicted in Figure 2, reveal good model-data fit (χ2 = 7.41, df = 7, p =.23, RMSEA =.01) compared to the null model. As can be seen, there is a direct path between percent of empty dwellings in the subject’s census tract when he was 10–12 years old and cannabis use frequency measured at age 19. In another pathway to cannabis use, empty dwellings was a significant predictor of testosterone level when the boys were 10–12 years of age (baseline) which, in turn, predicted assaultive behavior two years later age 12–14) leading to social dominance/norm-violating behavior (age 16) and subsequently cannabis use (age 19) and cannabis use disorder (age 22). Notably, testosterone level at age 10–12, predicted assaultiveness and testosterone level at age 12–14; this latter variable also correlated with assaultive behavior at age 12–14. In effect, assaultive behavior, a direct predictor of cannabis use disorder 8–10 years later, was predicted by and contemporaneously correlated with testosterone level.
Figure 2.
Developmental trajectory to cannabis use disorder
Assaultive behavior at age 12–14 also predicted social dominance/norm-violating behavior 2–4 years later that in turn predicted cannabis use disorder by age 22. Moreover, assaultive behavior at age 12–14 mediated the association between testosterone level at age 10–12 and social dominance/norm-violating behavior at age 16 (β =.02, z = 2.46, p<.05). The significant relationship between testosterone and social dominance/norm-violating behavior (β =.14, z = 1.99, p =.05) was reduced to a non-significant path (β =.002, z =.06, p.95) in the full model. These results demonstrate that a social motivation style featured by dominance striving in conjunction with low adherence to social norms segues from high testosterone level through assaultive behavior.
Testosterone level at ages 12–14 predicted social dominance/norm-violating behavior at age 16 that, in turn, predicted both cannabis use frequency at age 19 and cannabis use disorder by age 22. Notably, the score on the social dominance/norm-violating trait mediated the relation between assaultiveness at age 12–14 and frequency of cannabis use at age 19 (β =.05, z = 3.07, p<.01). The strength of the relationship between assaultiveness and cannabis use was reduced from β =.17 (z = 3.88, p <.01) to β =.07 (z = 1.86, p =.06) in the full model. Moreover, cannabis use at age 19 mediated the association between social dominance/norm-violating behavior and cannabis use disorder (β =.08, z = 3.80, p <.01). A significant reduction was observed in the magnitude of the path coefficient from β =.36 (z = 5.08, p <.01) to β =.24 (z = 4.37, p <.01) in the full model. These results, in aggregate, demonstrate that high testosterone level concomitant to poor quality neighborhood is a significant predictor of cannabis use disorder mediated by assaultiveness and social dominance/norm-violating behavior.
The results of the likelihood ratio test (χ2 = 6.69, df = 3, p =.08) following the Directed Acyclic Graph (DAG) analysis confirmed the causal linkage of the variables depicted in Figure 1. Cannabis use disorder is caused directly by cannabis use (CU), social dominance/norm-violating behavior (SDNVB), and assaultive behavior (AS), but not by neighborhood quality (NQ) and testosterone level at either 10–12 and 12–14 years of age (T1, T2); that is, Pr(CUD|CU,SN,AB) = Pr(CUD|CU,SN,AB,NQ,T1,T2). Cannabis use was directly caused by neighborhood quality and social dominance/norm-violating behavior but not by testosterone and assaultiveness (χ2 = 3.71, df = 3, p =.29). Commensurate with the main hypothesis of this study, social dominance/norm-violating behavior was directly caused by testosterone at age 12–14 and assaultive behavior, but not neighborhood quality or testosterone at the earlier age 10–12 (χ2 = 1,38, df = 2, p =.50).
Lastly, a post hoc analysis was conducted to determine whether testosterone level and neighborhood quality interact to determine severity of the score on the assaultive behavior scale. Although the theoretical model guiding this study specified a direct temporal pathway in which each variable leads to the next variable in the sequence (see Figure 1), it is informative to also clarify whether the variables have an interactive relationship at the outset of the etiologic trajectory. The results of this analysis yielded, however, no significant interaction (β =.11, SE =.07, t = 1.49, p = NS).
Discussion
Proportion of boarded-up dwellings in the neighborhood is a significant predictor of cannabis use frequency up to nine years later. Moreover, this indicator of disadvantaged neighborhood correlates with testosterone level that, in turn, predicts assaultive behavior presaging cannabis use disorder directly as well as mediated by social dominance/norm-violating behavior. Living in an adverse neighborhood thus predicts cannabis use and biases behavior development to cannabis use disorder via amplified testosterone production. This finding points to an important role of androgenic mechanisms intermediating the previously documented association between disadvantaged neighborhood and substance use (40) and externalizing behavior problems (41).
It is interesting to note that neighborhood quality did not directly predict assaultive behavior, social dominance/norm-violating behavior, or cannabis use disorder. Based on these results, it appears that adverse social environment alone is insufficient to account for these outcomes. These findings do not play down the egregious influence of socioeconomic disadvantage, but rather underscore the need to accommodate individual differences in androgenic mechanisms found in this study that may underlie this relationship. Dwelling in a poor neighborhood is, however, a significant predictor of frequency of cannabis use between ages 10–12 and 19. This is not surprising considering the high availability of illegal drugs and reduced adherence to societal norms in economically depressed communities (17, 18, 19).
Testosterone level influences risk for cannabis use disorder via assaultive behavior and a psychosocial style featured by ascendance striving accompanied by low adherence to social norms. What factors, however, account for the significant association between low neighborhood quality and high testosterone level? A definitive answer to this question is beyond the scope of the present data. Nevertheless, the empirical literature points to at least four plausible mechanisms: First, a diverse array of evidence from research on humans and other animals has documented an elevation of testosterone levels during competition for resources (see 5 for a review of the literature). Second, cannabis use is commonly initiated during adolescence at which time the brain is undergoing extensive reorganization of cortical circuitry. Gonadal steroid hormones are integrally involved in this process of neuromaturation (42). In effect, chronic stress and real and perceived physical threat in disadvantaged neighborhoods result in greater production of gonadal steroids which, in turn, influences neurological development. In addition, androgens, including testosterone, modulate HPA response through inhibitory mechanisms, and are thought to modulate the firing of central arginine vasopressin neurons originating in the extended amygdala which coordinate emotional reactivity and behavioral stress responses (43). Accordingly, socially ascendant norm-violating behavior may be a motivational style resulting from chronic adaptation of biological stress responses systems. Notably, one of the most powerful predictors of adolescent alcohol and drug use is perceived stress (44,45). Several studies have demonstrated that stress can predate adolescent substance use behavior, and that perceived stress can enhance already existing drug experimentation (46,47,48). Lastly, in socioeconomically depressed neighborhoods, masculine honor that is evinced physically in response to threat may potentiate youths with high testosterone levels toward overt aggressive behavior. It has been found, for example, that causes a larger increase in testosterone level among men whose culture places high value on masculine honor. Cohen and colleagues (49) observed a sharper increase in testosterone level following an insult among US southern college students compared to northern students. Associated changes in psychological state included feelings of dominance and anger. Thus, several possible interrelated mechanisms may underlie the association between disadvantaged neighborhoods and elevated testosterone level. In view of the high rates of addiction and antisocial behavior in poor neighborhoods, it can be concluded that research aimed at clarifying the mechanisms underlying the association between androgens and adverse social environment is informative for understanding the etiology of these disorders.
Inasmuch as the sample was ascertained through proband fathers who either qualified for SUD consequent to use of illicit drugs or had no axis I psychiatric disorder, it is important to determine whether the results were related to paternal disorder. Post hoc comparisons of boys having SUD+ and SUD− fathers using one-way ANOVA revealed a higher rate of marijuana use in the former group. However, sons of SUD men were not different from sons of psychiatrically normal fathers on any other variable, namely neighborhood quality, testosterone level, assaultiveneess scale score, and the score on the social dominance/norm-violation trait. Significantly, the absence of a difference between these two groups of children on testosterone level suggests that the genetic factors involved in the risk for SUD cannot account for the variation in concentration of this hormone.
The pathway to cannabis use disorder, involving the interplay of social context, hormonal, and psychological factors underscores the difficult challenge of designing and implementing cost-effective prevention programs. The observation that there is a direct association between neighborhood quality and future cannabis use, complemented by the demonstration that there is an association between adverse environment and amplification of testosterone level that potentiates behaviors predisposing to cannabis use disorder, illustrates the importance of upgrading economically distressed communities for SUD prevention. In view of findings indicating that interpersonal competition promotes testosterone production (5, 11, 12), it is plausible to theorize that increasing economic opportunities would, therefore, attenuate dominance striving concomitant to elevated testosterone level, and accordingly the motivation to consume illicit drugs inasmuch as consumption is associated with enhanced social status (15, 16; 17). Furthermore, the present results demonstrate that effective prevention necessarily requires joining intervention methods directed at both individual and environment components of risk. Whereas interventions directed at enhancing the neighborhood have positive impact on reducing crime and drug use (18), the findings in this study underscore the need to engineer specific changes in the environment that buffer the risk of acquiring behaviors which augment the liability to develop SUD. Related to this issue, it is noteworthy that only four boys admitted to belonging to a gang at the time of baseline assessment, indicating that the results reported herein are not confined to the extreme segment of the population.
Certain limitations of this study are noteworthy. Importantly, the findings are confined to males. It is also noteworthy that neighborhood quality, indexed by proportion of boarded-up and abandoned dwellings, was assessed only at baseline. In addition, the developmental trajectory described in this study does not preclude the importance of other individual and contextual factors which have been shown to impact on risk. Notably, post-hoc analyses of other environment factors documented in the census revealed that only percentage of African-American males in the neighborhood correlated significantly, albeit with low strength (r =.13; p <.01), with testosterone level. Social contextual factors such as percent of households headed by a woman, percent of families below the poverty level, and percent unemployment were not related to testosterone level in the boys. Accordingly, the possibility cannot be entirely discounted that the association between testosterone level and only one variable, namely, vacant dwellings, may be spurious. More likely, the finding is valid considering that easy access to vacant dwellings allows illicit drugs to be consumed alone or with peers, with low risk of apprehension by police. Clearly, additional research is required to elucidate the role of the built environment on the relation between biological mechanisms and overt behavioral manifestations predisposing to substance use disorder. Nevertheless, results reported by Schaal et al. (10) and the findings obtained herein indicate that behaviors biasing the development of externalizing disturbances are presaged by neighborhood adversity and high androgen level. This study additionally observed that a distal outcome a decade or more later is cannabis use disorder.
In summary, proportion of boarded-up dwellings in the neighborhood is a significant predictor of frequency of cannabis use. Disadvantaged neighborhood is also associated with high testosterone level in 10–12 year old boys that in turn predicts as well as correlates with assaultive behavior presaging social dominance norm-violating motivation leading to cannabis use and cannabis use disorder. This heretofore unrecognized etiological pathway illustrates the need for prevention strategies that utilize intervention methods which promote normative biobehavior development in conjunction with interventions directed at ameliorating the contextual contribution to risk. Furthermore, the observation that cannabis use mediates the relation between social dominance/norm-violating behavior and cannabis use disorder indicates that the liability for this latter disorder encompasses the dual features of deviancy proneness and social ascendance.
Acknowledgments
Supported by grants DA05605, K02-DA018701, and K02-DA017822 from the National Institute on Drug Abuse.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Udry JR. Biosocial models of adolescent problem behaviors. Soc Biol. 1990;37:1–10. doi: 10.1080/19485565.1990.9988742. [DOI] [PubMed] [Google Scholar]
- 2.Dabbs JM, Jr, Hopper CH, Jurkovic GJ. Testosterone and personality among college students and military veterans. Pers Ind Diff. 1990;11:1263–1269. [Google Scholar]
- 3.Kirillova G, Vanyukov M, Kirisci L, Reynolds M. Physical maturation, peer environment, and the ontogenesis of substance use disorder. Psychiatry Res. doi: 10.1016/j.psychres.2007.02.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds M, Tarter R, Kirisci L, Kirillova G, Brown S, Clark D, et al. Testosterone level and sexual maturation predict substance use disorders in adolescent boys: A prospective study. Bio Psychiatry. 2007;61:1223–1227. doi: 10.1016/j.biopsych.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci Biobehav Revws. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Tellegen ATJ, Bouchard J, Wilcox KJ, Segal NL, Lykken DT, Rich S. Personality similarity in twins reared apart and together. J Person Soc Psychol. 1988;54(6):1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- 7.Elklins I, McGue M, Malone S, Iacono W. The effect of parental alcohol and drug disorders on adolescent personality. Am J Psychiatry. 2004;161:670–676. doi: 10.1176/appi.ajp.161.4.670. [DOI] [PubMed] [Google Scholar]
- 8.Tarter R, Kirisci L, Kirillova G, Gavaler J, Vanyukov M. Social dominance mediates the association of testosterone and neurobehavior disinhibition on risk for substance use disorder. Psychol Addict Behav. 2007;21:462–468. doi: 10.1037/0893-164X.21.4.462. [DOI] [PubMed] [Google Scholar]
- 9.Kirillova G, Vanyukov M, Gavaler J, Pajer K, Dunn M, Tarter R. Substance abuse in parents and their adolescent offspring. The role of sexual maturation and sensation seeking. J Child Adol Sub Abuse. 2001;10:77–89. [Google Scholar]
- 10.Schaal N, Tremblay RE, Soussignan R, Susman EJ. Male testosterone linked to high social dominance but low physical aggression in early adolescence. J Am Acad Child Adolesc Psychiatry. 1996;34:1322–1330. doi: 10.1097/00004583-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Mazur A, Booth A. Testosterone and dominance in men. Behav Brain Sci. 1998;21:353–363. [PubMed] [Google Scholar]
- 12.Sapolsky R. The physiology of dominance in stable versus unstable social hierarchies. In: Mendoza, Mason W, editors. Primate and Social Conflict. Albany, NY: State University of New York Press; 1993. pp. 171–204. [Google Scholar]
- 13.Stice E, Myers MG, Brown SA. Relations of delinquency to adolescent substance use and problem use. A prospective study. Psychol Addict Behav. 1998;12:136–146. [Google Scholar]
- 14.Windle M. A longitudinal study of antisocial behaviors in early adolescence as predictors of late adolescent substance use: Gender and ethnic group differences. J Abnorm Psychol. 1990;99:86–91. doi: 10.1037//0021-843x.99.1.86. [DOI] [PubMed] [Google Scholar]
- 15.Allen J, Porter M, McFarland F, Marsh P, McElhaney D. The two facets of adolescents’ success with peers: Adolescent popularity, social adaptation and deviant behavior. Child Develop. 2005;76:747–760. doi: 10.1111/j.1467-8624.2005.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halpern C, Kestle C, Halifors D. Perceived physical maturity, age of romantic partner, and adolescent risk behavior. Prev Science. 2007;8:1–10. doi: 10.1007/s11121-006-0046-1. [DOI] [PubMed] [Google Scholar]
- 17.Killeya-Jones L, Nakajima R, Costanzo P. Peer standing and substance use in early-adolescent grade-level networks: A short term longitudinal study. Prev Science. 2007;8:11–23. doi: 10.1007/s11121-006-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampson R, Raudenbush S, Earls F. Neighborhood and violent crime. A multilevel analysis of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 19.Ingoldsby E, Shaw D. Neighborhood contextual factors and early-starting antisocial pathways. Clin Child Fam Psychol Revw. 2002;5:21–55. doi: 10.1023/a:1014521724498. [DOI] [PubMed] [Google Scholar]
- 20.Crum R, Lillie-Blanton M, Anthony J. Neighborhood environment and opportunity to use cocaine and other drugs in late childhood and early adolescence. Drug Alc Depend. 1996;43(3):155–161. doi: 10.1016/s0376-8716(96)01298-7. [DOI] [PubMed] [Google Scholar]
- 21.Hill T, Angel R. Neighborhood disorder, psychological distress, and heavy drinking. Soc Sci Med. 2005;61:965–975. doi: 10.1016/j.socscimed.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Nurco D, Kinlock T, O’Grady K, Lerner M, Hanlon T. Perception of social pathology in the neighborhood and the etiology of narcotic addiction. A retrospective study. J Nerv Ment Dis. 1996;184:35–42. doi: 10.1097/00005053-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Wagner F, Anthony J. Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana and cocaine. Am J Epid. 2002;155:918–925. doi: 10.1093/aje/155.10.918. [DOI] [PubMed] [Google Scholar]
- 24.Hollingshead A. Four factor index of social status. New Haven, CT: Yale University; 1975. Unpublished. [Google Scholar]
- 25.Environmental Systems Research Institute (ESRI), Inc. ArcView (Version 9.1) [Computer Software] Redlands, CA: ESRI, Inc; 2005. [Google Scholar]
- 26.Dailey G. Normalizing census data using ArcMap. ArcUser. 2006:52–53. [Google Scholar]
- 27.Kazdin AE, Rodgers A, Colbus D, Siegel T. Children’s Hostility Inventory: Measurement of aggression and hostility in psychiatric inpatient children. J Clin Child Psychol. 1987;16:320–328. [Google Scholar]
- 28.Tellegen A. Brief Manual for the Differential Personality Questionnaire. 1982. Unpublished manuscript. [Google Scholar]
- 29.Loeber R. Peer Delinquency Scale. Pittsburgh, PA: University of Pittsburgh, Department of Psychiatry; 1989. Unpublished. [Google Scholar]
- 30.Loeber R. Perception of Problem Behaviors Scale. Pittsburgh, PA: University of Pittsburgh, Department of Psychiatry, Pittsburgh Youth Study; 1989. Unpublished. [Google Scholar]
- 31.Tarter R. Evaluation and treatment of adolescent substance abuse: A decision tree method. Am J Drug Alc Abuse. 1990;16:1–46. doi: 10.3109/00952999009001570. [DOI] [PubMed] [Google Scholar]
- 32.Kirisci L, Mezzich A, Tarter R. Norms and sensitivity of the adolescent version of the Drug Use Screening Inventory. Addict Behav. 1995;20:149–157. doi: 10.1016/0306-4603(94)00058-1. [DOI] [PubMed] [Google Scholar]
- 33.Tarter R, Kirisci L. Validity of the Drug Use Screening Inventory for predicting DSM-III-R substance use disorder. J Child Adol Sub Abuse. 2001;10:45–53. [Google Scholar]
- 34.Spitzer R, Williams B, Gibbons M, First M. Users Guide for Structured Clinical Interview for DSM-III-R. New York, NY: New York State Psychiatric Institute; 1990. [Google Scholar]
- 35.Clark DB, Pollock NK, Mezzich A, Cornelius J, Martin C. Diachronic assessment and the emergence of substance use disorders. J Child Adol Sub Abuse. 2001;10:13–22. [Google Scholar]
- 36.Muthen L, Muthen B. Mplus User’s Guide. Los Angeles, CA: Muthen & Muthen; 2001. [Google Scholar]
- 37.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhart S, editor. Sociological Methodology. San Francisco, CA: Jossey-Bass; 1982. [Google Scholar]
- 38.Ghahramani Z. Graphical models: parameter learning. In: Arbib MA, editor. Handbook of Brain Theory and Neural Networks. 2. MIT Press; 2002. [Google Scholar]
- 39.Scheines R. An introduction to causal inference. In: McKim V, Turner S, editors. Causality in Crisis? Univ. of Notre Dame Press; 1997. pp. 185–200. [Google Scholar]
- 40.Luthar SS, D’Avanzo K. Contextual factors in substance use: a study of suburban and inner-city adolescents. Dev Psychopath. 1999;11:845–867. doi: 10.1017/s0954579499002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coie J, Jacobs M. The role of social context in the prevention of conduct disorder. Dev Psychopath. 1992;5:263–275. [Google Scholar]
- 42.Sisk C, Zher J. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrin. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Williamson M, Bingham B, Viau V. Central organization of androgen-sensitive pathways to the hypothalamic-pituitary-adrenal axis: implications for individual differences in responses to homeostatic threat and predisposition to disease. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1239–1248. doi: 10.1016/j.pnpbp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Hansell S, White HR. Adolescent drug use, psychological distress, and physical symptoms. J Health Soc Behav. 1991;32:288–301. [PubMed] [Google Scholar]
- 45.Newcomb MD, Harlow LL. Life events and substance use among adolescents: mediating effects of perceived loss of control and meaninglessness in life. J Pers Soc Psychol. 1986;51:564–577. doi: 10.1037//0022-3514.51.3.564. [DOI] [PubMed] [Google Scholar]
- 46.DeWit DJ, MacDonald K, Offord DR. Childhood stress and symptoms of drug dependence in adolescence and early adulthood: social phobia as a mediator. Am J Orthopsychiatry. 1999;69:61–72. doi: 10.1037/h0080382. [DOI] [PubMed] [Google Scholar]
- 47.Tschann JM, Adler NE, Irwin CE, Jr, Millstein SG, Turner RA, Kegeles SM. Initiation of substance use in early adolescence: the roles of pubertal timing and emotional distress. Health Psychol. 1994;13:326–333. doi: 10.1037//0278-6133.13.4.326. [DOI] [PubMed] [Google Scholar]
- 48.Wills TA. Stress and coping in early adolescence: relationships to substance use in urban school samples. Health Psychol. 1986;5:503–529. doi: 10.1037//0278-6133.5.6.503. [DOI] [PubMed] [Google Scholar]
- 49.Cohen D, Nisbet R, Bowdle B, Schwarg N. Insult, aggression, and the Southern cutlure of honor: An experimental ethnology. J Person Soc Psychol. 1996;70:945–960. doi: 10.1037//0022-3514.70.5.945. [DOI] [PubMed] [Google Scholar]