Abstract
Despite extensive study in many malignancies, maintenance therapy has clinically benefited only two diseases: acute lymphocytic leukemia (ALL) and acute promyelocytic leukemia (APL). ALL maintenance therapy utilizes low-dose 6-mercaptopurine (6MP) and methotrexate (MTX), while maintenance in APL primarily consists of all-trans-retinoic acid (ATRA). 6MP and MTX as used in ALL are also now usually added to maintenance ATRA for APL, based on data suggesting an improved disease-free survival. Although the mechanism of action of MTX and 6MP as maintenance is unknown, low-dose cytotoxic agents are potent inducers of differentiation in vitro. Thus, we studied whether maintenance therapy in ALL, like ATRA in APL, may be inducing terminal differentiation of ALL progenitors. The APL cell line NB4, the ALL cell lines REH and RS4;11, and patients' ALL blasts were incubated with ATRA, 6MP, and MTX in vitro. All three drugs inhibited the clonogenic growth of the APL and ALL cell lines without inducing immediate apoptosis, but associated with induction of phenotypic differentiation. The three drugs similarly upregulated lymphoid antigen expression, while decreasing CD34 expression, on patients' ALL blasts. These data suggest that induction of leukemia progenitor differentiation plays an important role in the mechanism of action of maintenance therapy in ALL.
Keywords: acute lymphocytic leukemia, acute promyelocytic leukemia, maintenance therapy, differentiation
Introduction
The treatment of newly diagnosed acute lymphocytic leukemia (ALL) is divided into two broad phases: remission induction therapy and post-remission therapy. Induction therapy is aimed at eliminating the overt leukemia and its severe toxicities resulting from infiltration of the bone marrow and other tissues. ALL post-remission therapy usually consists of one or more cycles of intermediate-dose chemotherapy, usually termed intensification/consolidation, followed by maintenance therapy. Maintenance therapy, prolonged low-dose cytotoxic chemo-therapy given over 1-2 years, targets the minimal residual disease persisting after intensive treatment. Initial induction therapy now results in complete remissions for more than 90% of both children and adults with ALL; interestingly, although these impressive responses translate into cures for most children, less than one-third of adults with ALL are long-term survivors.1-4
Despite being extensively studied in many malignancies, prolonged low-dose post-remission maintenance therapy has clinically proved beneficial to only two diseases: ALL/lymphoblastic lymphoma and acute promyelocytic leukemia (APL).5-11 Maintenance therapy for ALL consists of the low-dose cytotoxic agents 6-mercaptopurine (6MP) and methotrexate (MTX), and maintenance in APL has primarily been all-trans-retinoic acid (ATRA). Although the mechanisms responsible for the anti-leukemic effect of maintenance therapy in ALL remain unclear, the major mode of action of ATRA in APL appears to be its ability to induce the terminal differentiation of leukemia progenitors.12 In normal tissue, self-renewal potential is lost as cells terminally differentiate;13 likewise, ATRA induced maturation of APL progenitors appears to inhibit their replicative capacity, eventually exhausting the malignant clone.14,15 Low-dose cytotoxic agents, including 6MP and MTX, also have potent leukemia cell differentiating activity in vitro.16-18 Interestingly, the addition of low-dose 6MP and MTX to maintenance ATRA has been recently shown to improve disease-free survival in APL.19 Thus, we hypothesized that maintenance therapy in ALL may also exert an anti-leukemic effect through induction of differentiation of ALL progenitors.
Materials and methods
Patient material
ALL blasts were obtained from five patients with active CD10 + CD34 + B precursor ALL. The median age of the patients was 29 (range 18-59 years), two were newly diagnosed, three were relapsed, four had normal cytogenetics and one showed a deletion of the long arms of both chromosomes 6 and 22. All patients granted informed consent for study of their leukemia cells as approved by the Johns Hopkins Medical Institutes Institutional Review Board. ALL blasts were isolated from freshly harvested bone marrow aspirates or peripheral blood by density centrifugation (density <1.078, Ficoll-Paque; Pharmacia, Piscataway, NJ, USA) followed by two washes with RPMI; the resulting cellular product contained >90% phenotypic blasts. ALL blasts were further isolated using mouse anti-human CD34 antibodies coupled to magnetic microbeads (Miltenyi Biotec, Auburn, CA, USA) followed by magnetic column purification (Miltenyi VarioMACS) according to the manufacturer's instructions.
Cell culture
The human APL cell line NB420 and the human ALL cell lines REH21 and RS4;1122 were maintained in complete media (CM) containing RPMI-1640 medium (GIBCO-BRL, Rockville, MD, USA), supplemented with 10% heat-inactivated fetal bovine serum (FBS; GIBCO-BRL), 50 U/ml penicillin, 50 μg/ml streptomycin and 2 mmol/l L-glutamine. Cells were seeded at a density of 1×105 cells/ml and incubated with ATRA, 6MP and MTX (all three drugs from Sigma, St Louis, MO, USA) for 48-72 h. A 10mM stock solution of ATRA dissolved in ethanol was further diluted in RPMI. Stock solutions of 6MP and MTX (10 mM) in dimethyl sulfoxide (DMSO) were also further diluted in RPMI, such that final concentrations of DMSO were <0.01% or at least 100-fold below differentiating concentrations of DMSO.23,24
Clonogenic assays
Following treatment, NB4, REH and RS4;11 cells were evaluated for clonogenic growth potential as described previously.25 Briefly, cells were washed with CM to remove the drugs, and 2000 cells (NB4 and REH) or 10 000 cells (RS4;11) were placed into 1.2% methylcellulose, 30% FBS, 1% bovine serum albumin (BSA), 10-4 M 2-mercaptoethanol and 2 mM L-glutamine. Samples were plated in quadruplicate onto 35 mm2 tissue culture dishes and incubated in a humidified atmosphere at 371C and 5% CO2. Colonies consisting of >40 cells were counted using an inverted microscope at 7-14 days, and the results are presented as the mean percentage of colonies relative to the untreated media controls.
Flow cytometry
The cell lines and clinical ALL samples were analyzed for phenotypic evidence of differentiation by examining the expression of cell surface antigens as described previously.15,17 Briefly, NB4 cells were washed with phosphate-buffered saline containing 0.2% BSA and stained with the following antibodies for 30 min at 41C: phycoerythrin (PE)-conjugated mouse anti-human CD11b IgG1 or fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD15 IgM antibodies (Beckman Coulter, Fullerteron, CA, USA). REH, RS4;11 and clinical ALL cells were stained with FITC-conjugated mouse anti-human CD19 IgG1 and APC-conjugated mouse anti-human CD38 IgG1 antibodies (Beckman Coulter). Clinical ALL samples were also stained with PE-conjugated mouse anti-human CD34 IgG1 antibodies (Beckman Coulter). Cells were then washed to remove unbound antibody, fixed with 2% paraformaldehyde and evaluated using a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA, USA) with a minimum acquisition of 10 000 events. Non-binding mouse IgG1 PE and FITC antibodies (Beckman Coulter) were used as controls. Results are presented as the relative mean fluorescence intensity compared to the untreated media controls as described previously.25 After the drug incubations, cell death was also quantified by flow cytometry using annexin V-FITC to assess apoptosis and PI staining to assess later death, according to the manufacturer's instructions (BD Pharmingen, San Jose, CA, USA) and as described previously.15 Cells were considered viable if they lacked both annexin V and PI staining.
Statistical analysis
Data are expressed as the mean±s.e.m. Comparisons between treatments were performed using a two-tailed, paired Student's t-test.
Results
6MP and MTX inhibit ALL and APL cell lines clonogenicity without direct cytotoxicity
During maintenance therapy, ALL patients generally receive oral 6MP at 50-75 mg/m2 daily.1-4,26 Peak plasma 6MP concentrations range from 0.01 to 1 µM, and several studies have shown that systemic exposure to 6MP during maintenance predicts the probability of relapse.26-28 The two ALL cell lines were incubated with varying concentrations of 6MP for 72 h and then assayed for apoptosis and clonogenic growth. Following the incubations, the cells were washed to remove the drug to ensure that any loss of colony formation represented the clonogenic potential at the end of incubation rather than a direct antiproliferative effect of drug during the subsequent clonogenic assay. We found that the highest concentrations of 6MP (1 µM) achieved clinically during maintenance therapy had no direct cytotoxic effect on either ALL cell line (Table 1), but nevertheless significantly inhibited their clonogenic capacity (Figure 1). 6MP similarly inhibited the clonogenic growth of the NB4 APL cell line (Figure 2a) without producing immediate apoptosis (Table 1).
Table 1.
Apoptosis of cell lines
| NB4 | REH | RS4;11 | |
|---|---|---|---|
| Abbreviations: ATRA, all-trans retinoic acid; 6MP, 6-mercaptopurine; MTX, methotrexate. | |||
| Results (mean±s.e.m. of three separate experiments) are the percentage of cells positive for annexin V or PI. There was no statistically significant difference in cell viability between the control and each treatment group for any cell line (P>0.05). | |||
| Control | 0 | 0 | 0 |
| ATRA | 0±3.1 | 0.9±0.8 | 2.3±1.9 |
| 6MP | 0±3.2 | 0.3±0.5 | 3.7±1.3 |
| MTX | 2.9±7.5 | 0.2±0.5 | 3.6±3.9 |
Figure 1.
Clonogenic growth of acute lymphocytic leukemia (ALL) cell lines REH and RS4;11 following treatment with all-trans-retinoic acid (ATRA), 6-mercaptopurine (6MP) and methotrexate (MTX). Results represent mean±s.e.m. of five separate experiments with each cell line. P-values are for individual treatment groups compared to control.
Figure 2.
Effects of all-trans-retinoic acid (ATRA), 6-mercaptopurine (6MP) and methotrexate (MTX) on the (a) clonogenic growth of and (b) CD15 expression by NB4 cells. Results represent the mean±s.e.m. of four separate experiments. P-values are for individual treatment groups compared to control.
The dose of MTX during maintenance therapy is usually 20-25 mg/m2 orally once a week.1-4,26 Peak plasma levels during maintenance also range from 0.01-1 μM, although unlike 6MP, most studies have not found a correlation between clinical MTX pharmacokinetics and probability of relapse.26,29 MTX, at 0.01 μM for 72 h, similarly significantly inhibited the clonogenic capacity of the REH, RS4;11 (Figure 1) and NB4 (Figure 2a) cell lines without an immediate effect on apoptosis (Table 1).
6MP, MTX and ATRA induce phenotypic differentiation of APL and ALL cell lines
Loss of clonogenic potential in the absence of direct cytotoxicity is consistent with the loss of cell self-renewal capacity though induction of differentiation.15,17,30 Accordingly, ATRA-mediated induction of NB4 cell terminal differentiation was associated with upregulation of the myeloid antigens CD15 (Figure 2b) and CD11b (data not shown), and inhibition of clonogenic growth (Figure 2a), in the absence of immediate apoptosis (Table 1) as described previously.15 Since 6MP and MTX also inhibited NB4 clonogenicity without inducing immediate apoptosis, we investigated the effects of the agents on NB4 phenotypic differentiation and both agents significantly induced upregulation of CD15 and CD11b as well (Figure 2b).
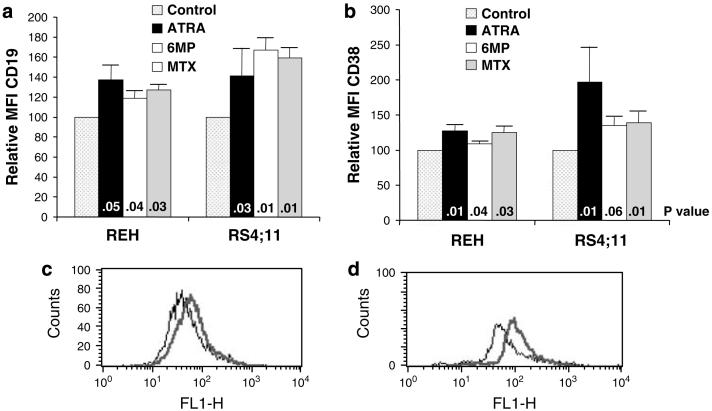
It is now clear that most cases of ALL, like acute myelocytic leukemia (AML), arise from transformed immature hematopoietic stem/progenitor cells with self-renewal and differentiation capacity.31-35 Although not as well defined as myeloid progenitor differentiation, B-cell differentiation is associated with an increase in the expression of both CD19 and CD38.36-39 At the non-cytotoxic doses that inhibited clonogenic growth of the ALL cell lines, 6MP and MTX significantly induced upregulation of CD19 and CD38 in both cell lines (Figure 3). Interestingly, similar to its activity in APL, ATRA also significantly induced phenotypic differentiation of both cell lines (Figure 3).
Figure 3.
Effects of all-trans-retinoic acid (ATRA), 6-mercaptopurine (6MP) and methotrexate (MTX) on (a) CD19 and (b) CD38 expression by acute lymphocytic leukemia (ALL) cell lines. Representative histograms of (c) CD19 expression by REH cells after 6MP treatment and (d) RS4;11 cells after MTX treatment (control — thin line, drug — thick line). Results represent the mean±s.e.m. of five separate experiments. P-values are for individual treatment groups compared to control.
6MP, MTX and ATRA induce phenotypic differentiation of clinical ALL
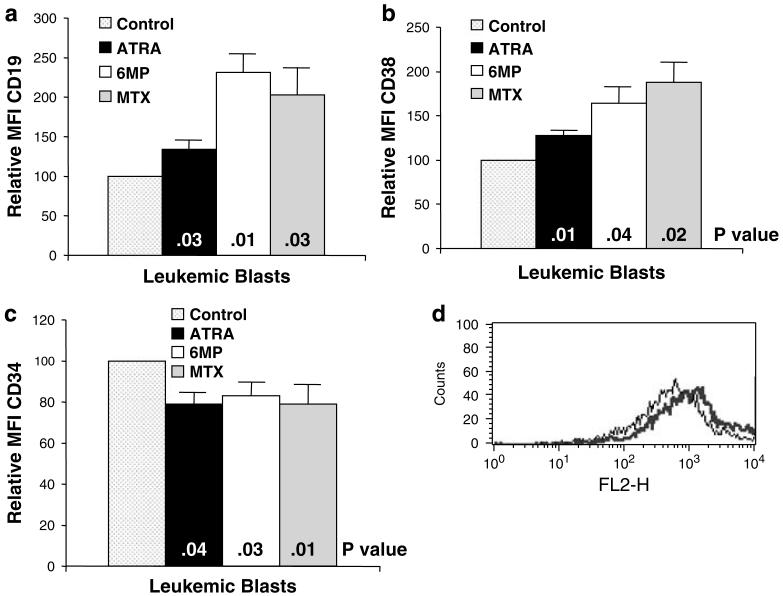
ALL blasts were isolated from the bone marrow of five patients with ALL, and incubated with 6MP, MTX and ATRA using the same conditions as for the cell lines. Similar to the ALL cell lines, all three agents induced significantly increased expression of CD19 and CD38 on the ALL blasts (Figure 4a,b,d). Consistent with induction of differentiation, all three agents also significantly decreased CD34 expression on the blasts (Figure 4c).
Figure 4.
Effects of all-trans-retinoic acid (ATRA), 6-mercaptopurine (6MP) and methotrexate (MTX) on (a) CD19, (b) CD38 and (c) CD34 expression by acute lymphocytic leukemia (ALL) blasts from five patients. (d) Representative histogram of CD38 expression by ALL blasts following treatment with MTX (control — thin line, MTX — thick line). Results represent the mean±s.e.m. P-values are for individual treatment groups compared to control.
Discussion
The failure of prolonged low-dose cytotoxic agents as maintenance therapy in most diseases8-11 is not surprising. The minimal residual disease persisting after initial treatment is generally regarded to be the most drug-resistant population of cancer cells. In fact, in most diseases, post-remission therapy aimed at eliminating minimal residual disease generally maintains initial dose intensity or even intensifies it, as exemplified by consolidation therapy for AML and stem cell transplantation following high-dose therapy for relapsed leukemia or lymphoma. 6MP and MTX are used throughout ALL induction and consolidation, either in combination with other drugs or, in the case of MTX, at high doses with leucovorin rescue as treatment/prophylaxis of the central nervous system and probably other sanctuary sites. In these phases of the ALL treatment paradigm, 6MP and MTX are likely behaving as conventional cytotoxic agents. However, during maintenance, these drugs are used by themselves and at low doses that clinically produce little effect on blood counts, suggesting limited direct cytotoxicity. Thus, the success of maintenance chemotherapy with low-dose 6MP and MTX in ALL remains intriguing and its mechanism of action unclear.
6MP and MTX were empirically added to ATRA maintenance in APL based on two nonrandomized studies that suggested that low-dose ALL-type maintenance could decrease the relapse rate,40,41 and this has been confirmed in recent phase III trials.19 The potent differentiating activity of low-dose cytotoxic agents in vitro,16-18 together with these clinical results,19,40,41 suggested to us that 6MP and MTX may be functioning as differentiating agents clinically. We found that the concentrations of 6MP and MTX achieved clinically during maintenance therapy exhibited little direct cytotoxicity against APL and ALL cells in vitro, but nevertheless inhibited APL and ALL clonogenic growth associated with evidence of phenotypic differentiation. Our in vitro incubations with 6MP and MTX may not fully mirror the prolonged exposures seen with maintenance therapy and thus other mechanisms of action, including direct cytotoxicity, may be operative clinically. Nevertheless, the potent differentiating activity exhibited by low-dose 6MP and MTX against ALL and APL cells in vitro suggests a novel clinical mechanism of action for these agents when used as maintenance therapy in these diseases.
The success of ATRA in APL is generally considered to be the result of a unique sensitivity to differentiation caused by the fusion protein PML-RARα. However, APL cells differentiate in response to a variety of unrelated pharmacologic agents that do not signal through RARα, such as vitamin D,42,43 phorbol esters,44 bryostatin-1,15,42 arsenic trioxide15,45 and even typical cytotoxic anti-cancer agents (Figure 2).18,46 The activity of such unrelated agents in APL suggests that a specific interaction between ATRA and PML-RARα may not be the total story underlying the sensitivity of APL to differentiation induction. Regardless of the mechanism of action, ATRA's success in APL demonstrates the potential of differentiation therapy to extinguish leukemic progenitors. Moreover, non-APL AML progenitors can be induced to undergo terminal differentiation in vitro by a variety of drugs, including retinoids,47 histone deacetylase (HDAC) inhibitors,17,48 demethylating agents49 and also low-dose cytotoxic anti-cancer agents;17 many of these agents are currently being studied clinically in myeloid malignancies.50 Differentiation therapy has not been extensively studied in ALL, possibly because it has been considered a disorder of malignant (differentiated) lymphocytes. However, most cases of ALL arise from transformed immature hematopoietic stem/progenitor cells with self-renewal and differentiation capacity.31-35 Thus, like APL and non-APL AML progenitors, ALL progenitors might also be expected to undergo terminal differentiation in the proper setting.
The excellent initial complete response rates in both children and adults,1-4 suggests that the differentiated lymphoblasts forming the bulk of the leukemia in both age groups are exquisitely sensitive to the agents used during induction therapy. The divergent outcomes in children and adults with ALL may therefore reflect a difference in the effectiveness of maintenance therapy in the two groups.51 It is now clear that ALL can arise from either immature lymphoid (CD34+CD19+ ) progenitors32,35 or more primitive hematopoietic stem cells that are CD34+CD19-.31,33,34 It has even been suggested that ‘pediatric-type’ ALL often arises from the less primitive CD34 +CD19+cells,32 while ‘adult-type’ (that is, poor-risk disease more common in adults) usually arises from the CD34+CD19-cells.34 If true, 6MP and MTX may be more active against CD34+CD19+ lymphoid progenitors than against more primitive CD34+CD19- progenitors. Moreover, agents with activity as inducers of differentiation for malignant myeloid progenitors, such as demethylating agents or HDAC inhibitors, may have more potent differentiating activity against ALL arising from primitive hematopoietic stem cells than 6MP and MTX. If this were the case, such agents might have activity as maintenance therapy in ‘adult-type’ ALL patients.
Acknowledgements
Supported in part by National Institutes of Health grants P01 CA15396 and P01 CA70970. TLL is a recipient of an ASCO Foundation YIA.
References
- 1.Schrappe M, Reiter A, Ludwig W-D, Harbott J, Zimmermann M, Hiddemann W, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. Blood. 2000;95:3310–3322. [PubMed] [Google Scholar]
- 2.Boissel N, Auclerc M-F, Lhéritier V, Perel Y, Thomas X, Leblanc T, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 3.Annino L, Vegna ML, Camera A, Specchia G, Visani G, Fioritoni G, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99:863–871. doi: 10.1182/blood.v99.3.863. [DOI] [PubMed] [Google Scholar]
- 4.Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 5.Cassileth PA, Andersen JW, Bennett JM, Hoagland HC, Mazza JJ, O'Connell MC, et al. Adult acute lymphocytic leukemia: the Eastern Cooperative Oncology Group experience. Leukemia. 1992;6:178–181. [PubMed] [Google Scholar]
- 6.Cuttner J, Mick R, Budman DR, Mayer RJ, Lee EJ, Henderson ES, et al. Phase III trial of brief intensive treatment of adult acute lymphocytic leukemia comparing daunorubicin and mitoxantrone: a CALGB Study. Leukemia. 1991;5:425–431. [PubMed] [Google Scholar]
- 7.Childhood ALL Collaborative Group Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukaemia: overview of 42 trials involving 12 000 randomised children. Lancet. 1996;347:1783–1788. doi: 10.1016/s0140-6736(96)91615-3. [DOI] [PubMed] [Google Scholar]
- 8.Young RC, Canellos GP, Chabner BA, Schein PS, DeVita VT. Maintenance chemotherapy for advanced Hodgkin's disease in remission. Lancet. 1973;1:1339–1343. doi: 10.1016/s0140-6736(73)91672-3. [DOI] [PubMed] [Google Scholar]
- 9.Champlin R, Gale RP. Acute myelogenous leukemia: recent advances in therapy. Blood. 1987;69:1551–1562. [PubMed] [Google Scholar]
- 10.San Miguel JF, Blade Creixenti J, Garcia-Sanz R. Treatment of multiple myeloma. Haematologica. 1999;84:36–58. [PubMed] [Google Scholar]
- 11.DeVita VT., Jr Hodgkin's disease-clinical trials and travails. N Engl J Med. 2003;348:2375–2376. doi: 10.1056/NEJMp030049. [DOI] [PubMed] [Google Scholar]
- 12.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 13.Coffman FD, Studzinski GP. Differentiation-related mechanisms which suppress DNA replication. Exp Cell Res. 1999;248:58–73. doi: 10.1006/excr.1999.4457. [DOI] [PubMed] [Google Scholar]
- 14.Miller WH, Jr, Waxman S. Differentiation induction as a treatment for hematologic malignancies. Oncogene. 2002;21:3496–3506. doi: 10.1038/sj.onc.1205328. [DOI] [PubMed] [Google Scholar]
- 15.Matsui W, Smith BD, Vala M, Beal N, Huff CA, Diehl LF, et al. Requirement for myeloid growth factors in the differentiation of acute promyelocytic leukaemia. Br J Haematol. 2005;128:853–862. doi: 10.1111/j.1365-2141.2005.05395.x. [DOI] [PubMed] [Google Scholar]
- 16.Craig RW, Frankfurt OS, Sakagami H, Takeda K, Bloch A. Macromolecular and cell cycle effects of different classes of agents inducing the maturation of human myeloblastic leukemia (ML-1) cells. Cancer Res. 1984;44:2421–2429. [PubMed] [Google Scholar]
- 17.Matsui WH, Gladstone DE, Vala MS, Barber JP, Brodsky RA, Smith BD, et al. The role of growth factors in the activity of pharmacological differentiation agents. Cell Growth Differ. 2002;13:275–283. [PubMed] [Google Scholar]
- 18.Tsiftsoglou AS, Pappas IS, Vizirianakis IS. Mechanisms involved in the induced differentiation of leukemia cells. Pharmacol Ther. 2003;100:257–290. doi: 10.1016/j.pharmthera.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94:1192–1200. [PubMed] [Google Scholar]
- 20.Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991;77:1080–1086. [PubMed] [Google Scholar]
- 21.Rosenfeld C, Goutner A, Venuat AM, Choquet C, Pico JL, Dore JF, et al. An effect human leukaemic cell line: REH. Eur J Cancer. 1977;13:377–379. doi: 10.1016/0014-2964(77)90085-8. [DOI] [PubMed] [Google Scholar]
- 22.Stong RC, Korsmeyer SJ, Parkin JL, Arthur DC, Kersey JH. Human acute leukemia cell line with the t(4;11) chromosomal rearrangement exhibits B lineage and monocytic characteristics. Blood. 1985;65:21–31. [PubMed] [Google Scholar]
- 23.Friend C, Scher W, Holland JG, Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci USA. 1971;68:378–381. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci USA. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RJ, Barber JP, Vala MS, Collector MI, Kaufmann SH, Ludeman SM, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85:2742–2746. [PubMed] [Google Scholar]
- 26.Balis FM, Holcenberg JS, Poplack DG, Ge J, Sather HN, Murphy RF, et al. Pharmacokinetics and pharmacodynamics of oral methotrexate and mercaptopurine in children with lower risk acute lymphoblastic leukemia: a joint children's cancer group and pediatric oncology branch study. Blood. 1998;92:3569–3577. [PubMed] [Google Scholar]
- 27.Lennard L, Lilleyman JS. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989;7:1816–1823. doi: 10.1200/JCO.1989.7.12.1816. [DOI] [PubMed] [Google Scholar]
- 28.Koren G, Ferrazini G, Sulh H, Langevin AM, Kapelushnik J, Klein J, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. 1990;323:17–21. doi: 10.1056/NEJM199007053230104. [DOI] [PubMed] [Google Scholar]
- 29.Pearson AD, Amineddine HA, Yule M, Mills S, Long DR, Craft AW, et al. The influence of serum methotrexate concentrations and drug dosage on outcome in childhood acute lymphoblastic leukaemia. Br J Cancer. 1991;64:169–173. doi: 10.1038/bjc.1991.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsui W, Huff CA, Vala M, Barber J, Smith BD, Jones RJ. Anti-tumour activity of interferon-alpha in multiple myeloma: role of interleukin 6 and tumor cell differentiation. Br J Haematol. 2003;121:251–258. doi: 10.1046/j.1365-2141.2003.04255.x. [DOI] [PubMed] [Google Scholar]
- 31.George AA, Franklin J, Kerkof K, Shah AJ, Price M, Tsark E, et al. Detection of leukemic cells in the CD34(+)CD38(-) bone marrow progenitor population in children with acute lymphoblastic leukemia. Blood. 2001;97:3925–3930. doi: 10.1182/blood.v97.12.3925. [DOI] [PubMed] [Google Scholar]
- 32.Hotfilder M, Rottgers S, Rosemann A, Jurgens H, Harbott J, Vormoor J. Immature CD34+CD19— progenitor/stem cells in TEL/AML1-positive acute lymphoblastic leukemia are genetically and functionally normal. Blood. 2002;100:640–646. doi: 10.1182/blood.v100.2.640. [DOI] [PubMed] [Google Scholar]
- 33.Cox CV, Evely RS, Oakhill A, Pamphilon DH, Goulden NJ, Blair A. Characterization of acute lymphoblastic leukemia progenitor cells. Blood. 2004;104:2919–2925. doi: 10.1182/blood-2004-03-0901. [DOI] [PubMed] [Google Scholar]
- 34.Hotfilder M, Rottgers S, Rosemann A, Schrauder A, Schrappe M, Pieters R, et al. Leukemic stem cells in childhood high-risk ALL/t(9;22) and t(4;11) are present in primitive lymphoid-restricted CD34+CD19— cells. Cancer Res. 2005;65:1442–1449. doi: 10.1158/0008-5472.CAN-04-1356. [DOI] [PubMed] [Google Scholar]
- 35.Castor A, Nilsson L, Astrand-Grundstrom I, Buitenhuis M, Ramirez C, Anderson K, et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat Med. 2005;11:630–637. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- 36.Stoeckler JD, Stoeckler HA, Kouttab N, Maizel AL. 1alpha, 25-Dihydroxyvitamin D3 modulates CD38 expression on human lymphocytes. J Immunol. 1996;157:4908–4917. [PubMed] [Google Scholar]
- 37.Sato S, Miller AS, Howard MC, Tedder TF. Regulation of B lymphocyte development and activation by the CD19/CD21/CD81/Leu 13 complex requires the cytoplasmic domain of CD19. J Immunol. 1997;159:3278–3287. [PubMed] [Google Scholar]
- 38.Fujimoto M, Poe JC, Hasegawa M, Tedder TF. CD19 regulates intrinsic B lymphocyte signal transduction and activation through a novel mechanism of processive amplification. Immunol Res. 2000;22:281–298. doi: 10.1385/IR:22:2-3:281. [DOI] [PubMed] [Google Scholar]
- 39.Donis-Hernandez FR, Parkhouse RM, Santos-Argumedo L. Ontogeny, distribution and function of CD38-expressing B lymphocytes in mice. Eur J Immunol. 2001;31:1261–1267. doi: 10.1002/1521-4141(200104)31:4<1261::AID-IMMU1261>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marty M, Ganem G, Fischer J, Flandrin G, Berger R, Schaison G, et al. Acute promyelocytic leukemia: retrospective study of 119 patients treated with daunorubicin] Nouv Rev Fr Hematol. 1984;26:371–378. [PubMed] [Google Scholar]
- 41.Kantarjian HM, Keating MJ, Walters RS, Smith TL, McCredie KB, Freireich EJ. Role of maintenance chemotherapy in acute promyelocytic leukemia. Cancer. 1987;59:1258–1263. doi: 10.1002/1097-0142(19870401)59:7<1258::aid-cncr2820590705>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 42.Song XD, Norman AW. Bryostatin-1 and 1alpha, 25-dihydroxyvitamin D3 synergistically stimulate the differentiation of NB4 acute promyelocytic leukemia cells. Leukemia. 1999;13:275–281. doi: 10.1038/sj.leu.2401261. [DOI] [PubMed] [Google Scholar]
- 43.Muto A, Kizaki M, Yamato K, Kawai Y, Kamata-Matsushita M, Ueno H, et al. 1,25-Dihydroxyvitamin D3 induces differentiation of a retinoic acid-resistant acute promyelocytic leukemia cell line (UF-1) associated with expression of p21(WAF1/CIP1) and p27(KIP1) Blood. 1999;93:2225–2233. [PubMed] [Google Scholar]
- 44.Bhatia M, Kirkland JB, Meckling-Gill KA. M-CSF and 1, 25 dihydroxy vitamin D3 synergize with 12-O-tetradecanoylphorbol-13-acetate to induce macrophage differentiation in acute promyelocytic leukemia NB4 cells. Leukemia. 1994;8:1744–1749. [PubMed] [Google Scholar]
- 45.Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 46.Niitsu N, Higashihara M, Honma Y. The catalytic DNA topoisomerase II inhibitor ICRF-193 and all-trans retinoic acid cooperatively induce granulocytic differentiation of acute promyelocytic leukemia cells: candidate drugs for chemo-differentiation therapy against acute promyelocytic leukemia. Exp Hematol. 2002;30:1273–1282. doi: 10.1016/s0301-472x(02)00905-0. [DOI] [PubMed] [Google Scholar]
- 47.Defacque H, Dornand J, Commes T, Cabane S, Sevilla C, Marti J. Different combinations of retinoids and vitamin D3 analogs efficiently promote growth inhibition and differentiation of myelomonocytic leukemia cell lines. J Pharmacol Exp Ther. 1994;271:193–199. [PubMed] [Google Scholar]
- 48.Gozzini A, Rovida E, Dello Sbarba P, Galimberti S, Santini V. Butyrates, as a single drug, induce histone acetylation and granulocytic maturation: possible selectivity on core binding factor-acute myeloid leukemia blasts. Cancer Res. 2003;63:8955–8961. [PubMed] [Google Scholar]
- 49.Pinto A, Attadia V, Fusco A, Ferrara F, Spada OA, Di Fiore PP. 5-Aza-20-deoxycytidine induces terminal differentiation of leukemic blasts from patients with acute myeloid leukemias. Blood. 1984;64:922–929. [PubMed] [Google Scholar]
- 50.Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 51.Copelan EA, McGuire EA. The biology and treatment of acute lymphoblastic leukemia in adults. Blood. 1995;85:1151–1168. [PubMed] [Google Scholar]