Abstract
BACKGROUND:
Strong evidence exists to support the use of statins, acetylsalicylic acid (ASA) and angiotensin-converting enzyme inhibitors (ACEI) in patients at high risk of cardiovascular (CV) events; however, current practice pattern data indicate that a significant care gap exists between evidence and practice.
OBJECTIVES:
To quantify the reduction in CV events that may be obtained with the optimal use of vascular protection therapy in Canadians at high risk of cardiovascular events.
METHODS:
Canadian Community Health Survey data from 2003 were used to estimate the prevalence of heart disease and/or diabetes, which were applied to an age-specific population in Canada to calculate the total number of high-risk patients. The number of events over 10 years was estimated using a state transition model, published risk equations, practice pattern data from Canadian registries and published therapy efficacy from clinical trials.
RESULTS:
Among 2.2 million high-risk Canadians, current care with statin, ASA and ACEI therapy has reduced the estimated occurrence of CV events over the next 10 years by approximately 400,000 from 1.01 million. Universal use of combination statin, ASA and ACEI therapy for high-risk patients, compared with current care, would prevent as many as 143,000 more CV events over the next 10 years.
CONCLUSIONS:
Great advances in the management of CV disease have been made; however, CV disease remains a substantial burden to patients and to the Canadian health care system. Canadian physicians have the opportunity to further reduce this burden through optimal management of high-risk patients based on clinical guidelines.
Keywords: ACE inhibitors, Acetylsalicylic acid, Canadian health care system, Death, Myocardial infarction, Stroke
Abstract
HISTORIQUE :
Des preuves solides appuient l’utilisation des statines, de l’acide acétylsalicylique (AAS) et des inhibiteurs de l’enzyme de conversion de l’angiotensine (IECA) chez les patients exposés à un risque cardiovasculaire (CV) élevé. Or, les données sur les modes de pratique actuels indiquent un important fossé entre ces preuves et la pratique.
OBJECTIFS :
Quantifier la réduction des événements CV qu’il est possible d’obtenir avec une utilisation optimum des traitements vasculoprotecteurs chez les Canadiens exposés à un risque élevé de complications cardiovasculaires.
MÉTHODES :
Les données tirées de l’Enquête sur la santé dans les collectivités canadiennes pour 2003 ont servi à estimer la prévalence de la maladie cardiaque et/ou du diabète qui a été appliquée à la population spécifique à l’âge au Canada afin de calculer le nombre total de patients à risque élevé. Le nombre d’événements sur une période de dix ans a été estimé à l’aide d’un modèle de transition d’état, d’équations de risque publiées, de données sur les modes de pratique provenant de registres canadiens et en tenant compte de l’efficacité des traitements révélée par la publication d’études cliniques.
RÉSULTATS :
Pour 2,2 millions de Canadiens à risque élevé, le traitement actuel par statine, AAS et IECA a ramené l’occurrence estimée des événements CV au cours des dix prochaines années d’environ 1,01 million à 400 000. L’utilisation universelle d’un traitement d’association par statine, AAS et IECA chez les patients à risque élevé, comparativement aux soins actuels, préviendra jusqu’à 143 000 complications CV de plus au cours des dix prochaines années.
CONCLUSION :
La prise en charge de la maladie CV a fait de grands progrès. Par contre, la maladie continue de représenter un fardeau substantiel pour les patients et pour le système de soins de santé canadiene. Les médecins canadiens ont la possibilité d’alléger davantage ce fardeau en observant les directives cliniques pour la prise en charge optimum des patients à risque élevé.
Canadian patients with cardiovascular disease (CVD) are at risk for significant morbidity and mortality related to CV events such as myocardial infarction and stroke. The magnitude of the health problem is substantial, with 419,000 hospitalizations and over 74,000 deaths annually due to disease of the circulatory system (1).
Strong evidence exists to support the use of combination therapy with statins, acetylsalicylic acid (ASA) and angiotensin-converting enzyme inhibitors (ACEI) in patients with CVD and/or diabetes to reduce the risk of CV events. Evidence-based estimates have indicated that the use of all such therapies may result in substantial reduction in the risk associated with CVD (2,3). This evidence has been incorporated into multiple clinical practice guidelines and the importance of optimal treatment has been recognized as part of good clinical practice (4–7). However, current practice patterns indicate a significant and ongoing care gap in the management of patients with CVD by Canadian physicians (8–10).
This care gap exists despite continuing medical education, which is now an integral part of physician licensing to practice. Thus, a call to action is needed with specific quantitative data to energize Canadian physicians toward optimal management of their high-risk patients.
The objective of the present study was to quantify the reduction in CV events that may be obtained with the use of combination statin, ASA and ACEI therapy in Canadians over the age of 50 years who are at high risk of CV events.
METHODS
The present analysis used a state transition cohort model using Canadian population data to predict the incidence of CV events based on patient risk factor profile and treatment. Patients enter the model at a given age and with a given profile of CV risk factors. Each year, a patient may experience a fatal or nonfatal stroke or myocardial infarction (MI), die of other causes or remain disease-free. Probabilities of events were based on Framingham risk equations (further detail provided in step 3, below). The process continued for the 10-year time horizon. Patients were limited to one event per year, but may have multiple events during the model. The analysis compared CV outcomes from three cohorts treated with current usual care, combination statin, ASA and ACEI therapy (optimal therapy), and patients not receiving any statin, ASA or ACEI therapy (no therapy).
Step 1 included the estimation of the prevalence of conditions that placed a patient at high risk of CV events and the calculation of the number of high-risk patients in Canada. Step 2 involved defining current usual care in Canada and the comparator therapies. Step 3 included the calculation of the 10-year CV event risk for patients receiving current usual care in Canada and the expected number of CV events. Step 3 involved defining high-risk patients and current usual care in Canada. Step 4 involved calculating the reduction in the number of CV events with therapy.
Step 1: Number of high-risk Canadians (Table 1)
TABLE 1.
Proportion of patients with one or more of the following conditions: diabetes, heart disease or stroke (based on the Canadian Community Health Survey data set)
| Age (years) | Men (%) | Women (%) |
|---|---|---|
| 50–54 | 12.3 | 9.0 |
| 55–59 | 18.5 | 12.3 |
| 60–64 | 24.7 | 18.1 |
| 65–69 | 30.1 | 22.3 |
| 70–74 | 36.2 | 26.9 |
| 75–79 | 40.3 | 32.7 |
| 80+ | 40.8 | 35.9 |
High-risk patients were defined as those over the age of 50 years with one or more of the following conditions: diabetes, a history of MI or stroke. Although certain patients with multiple risk factors, such as hypertension, smoking or dyslipidemia, can be considered to be at high-risk for CV events, such patients were excluded to be conservative in study estimates. In addition, the analysis does not explicitly include patients with peripheral vascular disease (PVD) due to the lack of data on its prevalence in the absence of diabetes, coronary artery disease (CAD) or cerebrovascular disease (CeVD). Reported rates of PVD could not be used because there is a substantial coprevalence of PVD with diabetes, CAD and CeVD.
Published Canadian and United States CVD prevalence estimates rely on patient self-reported data (11,12). A similar method was used in the present study. The Canadian Community Health Survey (CCHS) cycle 2.1 data for 2003 were analyzed to determine the prevalence of high-risk patients. The CCHS, which has been conducted by Statistics Canada every two years since 2000, provides an estimate of health status and health care system use among household residents across Canada. The 45 min interviewer-administered questionnaire collected self-reported data on a wide range of health issues, including history of diabetes, heart disease or stroke, as well as smoking status, and has been validated against other community surveys. The 2003 dataset included responses from 134,072 people across Canada, 58,895 of whom were older than 50 years of age (13).
Positive responses to questions regarding history of diabetes, heart disease or stroke were used to estimate the prevalence of patients with diabetes alone, a history of MI or stroke but without diabetes, and diabetes and a history of MI or stroke. The total number of patients at high risk was calculated by multiplying the prevalence of patients in each of the three groups, as estimated from the CCHS, with the age-specific population in Canada (14). Growth in the high-risk population was based on Statistics Canada estimates (15) of 29% growth for the Canadian population over 50 years of age from 2006 to 2016 using a medium-growth scenario. Because reliable estimates of change are lacking, this estimate of growth in the high-risk population did not consider possible increases in the prevalence of diabetes, heart disease or stroke.
Step 2: Treatment groups (Table 2)
TABLE 2.
Use of statin, acetylsalicylic acid (ASA) and angiotensin-converting enzyme inhibitor (ACEI) therapy in the Vascular Protection (VP) and Guidelines Oriented Approach in Lipid Lowering (GOALL) registries at baseline, and estimation of weighted relative risk reduction (RRR) for current usual care
| Therapy | Frequency of use (A), % | Estimated RRR for each therapy versus no therapy (B), % | Weighted RRR (C=A×B), % |
|---|---|---|---|
| No therapy (n=519) | 5.42 | 0 | – |
| ASA only (n=777) | 8.11 | 22 | 1.78 |
| Statin only (n=783) | 8.17 | 24 | 1.96 |
| ACEI only (n=327) | 3.41 | 22 | 0.75 |
| ASA and statin (n=2181) | 22.76 | 41* | 9.27 |
| ASA and ACEI (n=735) | 7.67 | 39 | 3.00 |
| Statin and ACEI (n=791) | 8.26 | 41 | 3.36 |
| Statin, ASA and ACEI (n=3468) | 36.2 | 54 | 19.46 |
| Total (n=9581) | 100.0 | – | 39.58 |
Values have been rounded for display.
For example, calculated as RRR=1–(0.78×0.76)
The analysis considered three treatment groups for high-risk patients: optimal therapy consisting of ASA, ACEI and statins, current use of statin, ASA and ACEI therapy, and no therapy. Current usual care with statin, ASA and ACEI therapy was based on patients enrolled in the Vascular Protection (VP) and Guidelines Oriented Approach in Lipid Lowering (GOALL) registries (9,10). The VP registry included patients from 278 family medicine practice and specialty practices across Canada, with data collected from December 2001 to November 2004. All patients in the registry were at high risk for CV events; the majority had clinically overt vascular disease and the remaining patients had diabetes with at least one additional CV risk factor (9). The GOALL registry included patients from 254 family medicine practice and specialty practices across Canada, with data collected from December 2002 to December 2004 (10). Patients in both registries were at high risk for CV events; the majority had clinically overt vascular disease and the remaining patients had diabetes with at least one additional CV risk factor. The majority of patients were receiving at least one of statin (75%), ASA (75%) or ACEI (56%) therapy at baseline; however, combination therapy with all three agents was used by only 36% of patients (Table 2).
It was assumed that these patients are representative of high-risk patients in Canada. The patients in the registry were a median of 66 years old, 65% were male, 35% had a previous MI, 27% had previous unstable angina, 19% had a previous coronary bypass surgery, 8% had congestive heart failure, 18% had a previous stroke or transient ischemic event, and 56% had diabetes mellitus.
Step 3: Number of CV events in patients receiving current usual care
The analysis estimated the expected number of CV events, including MI, stroke and death due to CAD, over the next 10 years in the absence of competing mortality. Ten-year event risks were estimated using Framingham Heart Study risk equations from the report by D’Agostino et al (16) for CAD death and MI in patients with CAD or CeVD, Anderson et al (17) for CAD death and MI in patients without CAD or CeVD, and Wolf et al (18) for stroke in all patients. Stroke risk was divided into fatal and nonfatal stroke, based on a study of stroke survival in the Framingham cohort (19).
The risk equations required estimates of the values for a number of risk factors, such as systolic blood pressure and total cholesterol. The average values for all Canadians at high risk of CV events were not known. Instead, the analysis used risk values from the VP registry as a proxy for risk values for all Canadians at high risk (9). The risk values from the VP Registry included the proportion of patients with atrial fibrillation (3.2%), left ventricular hypertrophy (3.94%) and hypertension requiring medication (70%), as well as mean values for systolic blood pressure (130 mmHg for nondiabetics and 132 mmHg for diabetics), total cholesterol (4.71 mmol/L for nondiabetics and 4.8 mmol/L for diabetics), high-density lipoprotein (1.19 mmol/L for nondiabetics and 1.12 mmol/L for diabetics) and glycosylated hemoglobin (7.3%).
Ten-year event risks were estimated using the following data assumptions. Three age cohorts were run: age of 55 years for patients 50 to 59 years old, age of 65 years for patients 60 to 69 years old, and age of 74 years for patients 70 years or older. A higher average age for people 70 years or older could not be used because the risk equations are not applicable to people older than 74 years. The proportion of patients who smoke was obtained from the CCHS, and was age- and sex-specific. For diabetic patients, the duration of diabetes was assumed to be 10 years. Risk was not adjusted for events occurring during the model, such that a high-risk patient with diabetes who suffered an MI would not face an increased risk of CV events in subsequent years. This assumption is conservative; it underestimates the true number of events.
The 10-year event risks may, in theory, be applied directly to the number of high-risk patients estimated in Step 1 to provide an estimate of the number of CV events, assuming continued current usual care with statin, ASA and ACEI therapy. However, this would overestimate the number of CV events in the population, because some patients die of other causes before they have a CV event. To address this concern, the 10-year risks were combined with non-CV mortality rates within the state transition model.
Each year, the model allowed for nonfatal MI, nonfatal stroke, fatal stroke, fatal CAD events or to death due to non-CV causes. The annual probability of each CV event was calculated from the calculated 10-year risks according to P(t)=1 – exp(–μt), where P(t) is the probability in a given time (t) and μ is the annual hazard rate (20). The annual probability of non-CV death was calculated as: (annual, age- and sex-specific Canadian mortality from all causes) (21) minus (estimated annual probability of CAD death plus estimated annual probability of stroke death). Male and female patients were grouped into four age cohorts (55, 65, 75 and 85 years of age), resulting in a total of eight cohorts considered in the analysis. The number of patients in each cohort was based on the number of men or women at high risk of CV events between the ages of 50 and 59 years, 60 and 69 years, 70 and 79 years, and 80 and 100 years, as estimated in Step 1. That is, the cohort of patients ‘males, 55 years’ was used to approximate the number of events and deaths for all men between 50 and 59 years of age. The number of patients in the cohort ‘males, 55 years’ was equal to the number of high-risk men 50 to 59 years of age estimated in Step 1.
Step 4: Reduction in events with therapy
Each therapy is known to reduce the rate of CV events. The model used relative risk reduction (RRR) for all CV events comparing each therapy with placebo; RRR was 24% (95% CI 19% to 28%) for statins, based on the Heart Protection Study (HPS) (22), 22% (95% CI 18% to 26%) for ASA, based on a published meta-analysis (23), and 22% (95% CI 14% to 30%) for ACEI, based on the Heart Outcomes Prevention Evaluation (HOPE) study (24). It should be noted that the HOPE study included patients with a history of angina, and HPS included patients with a history of angina or hypertension; as such, these patient populations might have been at lower risk than the target population in this analysis.
RRR for each therapy were combined multiplicatively, assuming that the effects were independent, as in previous studies (3,25,26). For example, the RRR of combination statin, ASA or ACEI therapy compared with no statin, ASA or ACEI therapy was estimated to be 54% for all CV events based on combined RRR=1–(0.78×0.78×0.76), where 0.78 represents one minus the RRR with ASA or ACEI alone, and 0.76 represents one minus the RRR with statins alone.
The estimation of the RRR with current usual care involved determining combined RRR for each combination of the three therapies used at the time. These RRR were then combined with the prevalence of use for each therapy combination to calculate a weighted average RRR of 39.6% for current usual care. For example, the RRR for ASA + statin (1–[0.76×0.78]) was weighted by 22.76%, which is the proportion of the VP Registry population using that combination (see Table 2 for the calculations).
Based on the RRR of 39.6% for usual care versus no statin, ASA or ACEI therapy (see Step 4) and the estimated number of events with usual care (see Step 2), the number of events with no therapy was back-calculated. Finally, the number of events with optimal therapy was calculated by applying the RRR with triple therapy compared with no statin, ASA or ACEI therapy (see Step 4) with the calculated number of events with no statin, ASA or ACEI therapy.
Sensitivity analyses
A number of analyses were conducted to test the sensitivity of the results to the uncertainty in the model parameters. Four key areas of uncertainty exist in the analysis: the size of the high-risk population, the current use of statin, ASA and ACEI therapy, the risk of CV events and the RRR with therapy.
For estimates of the population prevalence of diabetes, CAD and CeVD, the CCHS represents a well-documented source of data. However, self-reporting of diabetes is thought to result in under-reporting of true prevalence. The prevalence of diabetes based on health care resource use (laboratory data, hospital admission diagnosis and prescription data), as reported in the Institute for Clinical Evaluative Sciences (ICES) Practice Atlas (27), was used as an alternative. An estimate of CAD or stroke prevalence that was not self-reported could not be found for use in the sensitivity analysis.
Alternative estimates of current statin, ASA and ACEI therapy in Canada with sufficient detail to allow the calculation of a weighted average RRR could not be found in the published literature. This uncertainty is further reviewed in the discussion. The Framingham equations are thought to underestimate event risk in diabetic patients; therefore, the United Kingdom Prospective Diabetes Study (UKPDS) risk equations, which may be more applicable to diabetic patients, were used in a sensitivity analysis to estimate risk in diabetic patients (28–31). A lower and upper range for the event risk was also tested by increasing or decreasing the event risk from the base case analysis by 20%.
Given the possibility of lower-risk patients in the HOPE and HPS studies, the true impact of these therapies on CV event risk is uncertain. The impact of uncertainty in the RRR associated with current usual care and with combination therapy was also tested using the upper and lower 95% CI for ASA, statin and ACEI therapy from their respective trials (22–24).
RESULTS
Number of Canadians at high risk of CV events
Age- and sex-specific prevalence of patients having one or more of diabetes, heart disease and stroke from the analysis of the CCHS is provided in Table 1. The analysis found that diabetes with no history of heart disease or stroke was the main reason for inclusion in the high-risk group at younger ages. For example, 57% of the high-risk male group included patients with only diabetes at 50 to 54 years of age, with the proportion falling to 50% at 65 to 69 years of age and to 34% by 80 years of age or older (data not shown). The proportion of male diabetic patients who also had a history of stroke or heart disease increased with age, to a high of 42.3% of all diabetic patients at age 80 years or older. Similar trends were observed for high-risk female patients. The overall prevalence of one or more of these conditions in patients 50 years or older was 22.4% (data not shown).
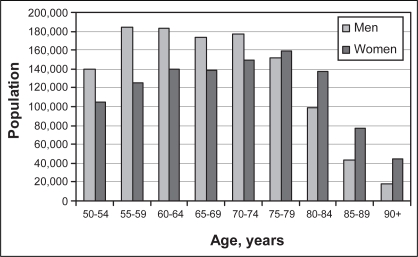
The total number of Canadians at high risk by age and sex is provided in Figure 1. Overall, there are 2.2 million Canadians 50 years of age or older at high risk of future CV events. Of those at high risk, 52% are male; however, in men, the peak prevalence is in the late 50s, while in women, the peak prevalence is in the late 70s. Given the expected growth in the Canadian population older than 50 years of age (15), the number of high-risk Canadians can be expected to increase by approximately 65,000 each year to 2.9 million by 2016. This does not consider the impact of societal changes in lifestyle, which are also expected to independently increase the prevalence of diabetes and heart disease in Canadian people over 50 years of age, or the impact of ongoing immigration of individuals from high CV risk countries into Canada.
Figure 1).
Estimated number of Canadians, by age and sex, who are at high risk of cardiovascular events based on the prevalence of diabetes, heart disease or stroke
Number of CV events within this population
The total numbers of events that are predicted to occur in high-risk patients over the next 10 years are shown in Table 3. It is estimated that 609,805 CV events, including 160,732 deaths due to stroke or CAD, will occur over the next 10 years among the 2.2 million high-risk Canadians over the age of 50 years, assuming treatment patterns remain similar to those observed in the VP Registry.
TABLE 3.
Predicted number of events over 10 years for patients on current usual care or combination statin, acetylsalicylic acid and angiotensin-converting enzyme inhibitor therapy
| Event | No therapy (n) | Current care (n) | Triple combination therapy (n) | Events averted with combination therapy (n) |
|---|---|---|---|---|
| Myocardial infarction | 376,282 | 227,306 | 173,987 | 53,319 |
| Stroke | 367,113 | 221,767 | 169,747 | 52,020 |
| CAD or stroke death | 266,076 | 160,732 | 123,029 | 37,703 |
| Total | 1,009,471 | 609,805 | 466,763 | 143,042 |
CAD Coronary artery disease
Reduction in events with triple combination therapy
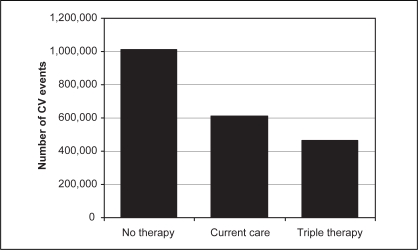
Current usual care with statin, ASA and ACEI therapy has reduced the number of CV events from an estimated 1.01 million to just over 600,000 over the next 10 years, compared with no use of these agents (Figure 2). However, of the 2.2 million high-risk patients, approximately 64% (1.4 million) do not receive triple combination therapy. It is estimated that an additional 143,042 CV events, including 37,703 CV deaths, can be prevented over the next 10 years by treating all high-risk Canadians over the age of 50 years with combination statin, ASA and ACEI therapy from current usual care (Table 3). Both of these estimates of reduction assume that statins, ASA and ACEI have similar effects in clinical practice as their efficacy demonstrated in trials.
Figure 2).
Estimated number of cardiovascular (CV) events among 2.2 million high-risk Canadians over the next 10 years. Current care includes current levels of statin, acetylsalicylic acid and angiotensin-converting enzyme inhibitor use. Triple therapy assumes combination use of statin, acetylsalicylic acid and angiotensin-converting enzyme inhibitor therapy
To close this care gap over the next five years, 1.4 million of the current 2.2 million high-risk patients need to move to triple combination therapy. This represents 286,000 high-risk patients per year. If the expected growth in the high-risk population is considered, the number of Canadians who need to start triple combination therapy would increase to almost 350,000 annually.
Sensitivity analyses
An analysis based on an upper estimate of the prevalence of high-risk patients was not calculated due to data limitations. However, when just the higher diabetes prevalence from ICES was used, the number of high-risk Canadians increased by 200,000 and the number of avoided events with optimal therapy increased by 12,000 (Table 4). The analysis was sensitive to the estimation of event risk. When the UKPDS equations were used for diabetic patients without CAD, the total number of events increased 10.6% and there was a corresponding increase in the incremental benefit with triple combination therapy to approximately 158,231 averted events. When event risk was increased 20%, the incremental benefit of triple combination therapy increased to over 170,000. When event risk was decreased 20%, the incremental benefit declined to approximately 113,172.
TABLE 4.
Sensitivity analysis results
| Analysis | High- risk patients (n) | Events with current usual care (n) | Events with combination therapy (n) | Events averted with combination therapy (n) |
|---|---|---|---|---|
| Base-case | 2,246,005 | 609,805 | 466,763 | 143,042 |
| Population prevalence | ||||
| ICES diabetes prevalence | 2,460,631 | 662,932 | 507,428 | 155,503 |
| Event risk | ||||
| UKPDS equation | 2,246,005 | 674,559 | 516,328 | 158,231 |
| 20% lower event risk | 2,246,005 | 482,467 | 369,295 | 113,172 |
| 20% higher event risk | 2,246,005 | 740,195 | 566,568 | 173,627 |
| Relative risk reduction | ||||
| Lower 95% CI | 2,246,005 | 609,805* | 514,696 | 95,109 |
| Upper 95% CI | 2,246,005 | 609,805* | 399,220 | 210,585 |
The number of events does not change because these were calculated from the risk factor profile using the risk equations. ICES Institute for Clinical Evaluative Sciences; UKPDS United Kingdom Prospective Diabetes Study
Uncertainty in the RRR assumed with treatment also had a significant impact on the estimated benefit of combination therapy. When the upper 95% CI for each therapy’s RRR were used, the incremental benefit of triple combination therapy rose to more than 210,000 avoided events. When the lower 95% CI were used, the incremental benefit decreased to just over 95,000.
DISCUSSION
Canadian physicians have made significant advances in the management of CVD by reducing the estimated 10-year occurrence of CV events by approximately 400,000 from 1.01 million through the current use of statin, ASA and ACEI therapy. However, there remains a considerable care gap in the treatment of high-risk patients. The VP registry, which provided a cross-country practice pattern audit, found that only 36% of high-risk patients are currently on combination statin, ASA and ACEI therapy, in contrast to current guidelines that recommend such therapy for all high-risk patients (4–7,9,10). The present study indicates that this care gap will result in as many as 143,000 preventable CV events over the next 10 years.
Our goal in the coming years should be to close the current care gap by promoting practices consistent with the current guidelines. To close the gap over the next five years requires that approximately 13% of all high-risk patients annually, or 286,000 patients, move to combination statin, ASA and ACEI therapy. If we consider the expected growth in the high-risk population, the number of Canadians who need to start triple combination therapy would increase to almost 350,000 annually.
Previous studies (25,26,32,33) using CVD models have also estimated the reduction in CV event risk with combination therapy in cohorts of high-risk patients in the United States and the developing countries. The strength of the present study for Canadian decision makers is that it used Canadian epidemiological and practice pattern data. In addition, it projected the benefit of combination therapy to the entire Canadian population of high-risk patients to quantify the current care gap.
The analysis was subject to limitations common to all population models, in that it combined data from numerous sources, and required structural and data assumptions. Wherever possible, conservative assumptions were made such that true event rates could be underestimated. Four key areas of uncertainty were explored: the size of the high-risk population, the current use of statin, ASA and ACEI therapy, the risk of CV events and the RRR associated with therapy.
The focus of the study was the estimated 2.2 million Canadians with diabetes or overt CVD. These patients are at highest risk of CV events and their treatment would result in the largest absolute decrease in events compared with other patient populations. However, this patient population may not include all high-risk patients and should not be considered to be inclusive of all patients who may benefit from combination therapy. The analyses did not include high-risk patients younger than 50 years, patients with abdominal aortic disease or individuals with multiple risk factors in the absence of diabetes or established heart disease. The study also excluded patients with PVD; however, some PVD patients would be expected to be captured among patients with diabetes, CAD or stroke. In addition, the prevalence of high-risk patients was derived from the CCHS, which under-reports the prevalence of diabetes compared with resource use-based estimates. The CCHS may also under-report the prevalence of stroke; the questionnaire specifically focuses on existence of disability due to stroke and not stroke occurrence itself. Many strokes and transient ischemic attacks do not result in chronic disability and, hence, might not have been reflected in CCHS estimates.
An analysis based on an upper estimate of the prevalence of high-risk patients was not calculated due to data limitations. However, when just the higher diabetes prevalence from ICES was used, the number of high-risk Canadians increased by 200,000 and the number of avoided events with optimal therapy increased by 12,000.
The analyses assumed that current usual care is reflected by the usage observed in the VP Registry. The VP Registry reported usage of 75%, 75% and 56% for ASA, statin and ACEI, respectively, either alone or in combination regimens (see Table 2). These may, however, be higher than actual practice. Canadian studies have reported ASA and antiplatelet therapy use to be 25% to 37% in patients with a history of CVD and/or diabetes, and as 60% to 64% in patients 90 days after an MI (34,35). Similarly, statin use has been reported to be 25% to 29% in patients with a history of CVD and/or diabetes, and 21% to 43% after an MI (35–38). Finally, use of ACEI has been reported to be 50% to 60% in patients with a history of CVD and/or diabetes, and 36% to 68% after an MI (34–36,38). Overall, the VP Registry appears to have higher use of statin and ASA, while ACEI use is more representative of actual practice. If this is correct, then the analysis has under-represented the current gap in care and substantially fell short of the true benefit of increased use of optimal therapy. The magnitude of this additional benefit is not known; however, the sensitivity analysis using a greater RRR with therapy, which found an increase in avoided events of over 70,000, provides some sense of the added events that can be avoided when the relative benefit of triple therapy is increased.
The analysis considered only the reduction in events possible with increased use of triple combination therapy. It is likely that current usual care also includes suboptimal prescribing of these therapies and that additional benefits can be obtained in currently treated patients by encouraging the use of optimal therapeutic doses. This has not been explored in the analysis but would be expected to increase the relative benefit of triple combination therapy. In addition, the analysis assumes that 100% compliance with triple combination therapy; however, it should be noted that this is not possible due to contraindications and intolerances. If compliance was lower, we would anticipate a smaller reduction in events. However, the estimates of treatment effect are based on clinical trials, in which compliance, while often superior to that observed in ‘real-world’ settings, is still suboptimal and therefore likely underestimates the ‘true’ treatment effect.
The analysis was constrained by a lack of clinical trial data on the efficacy of combination therapy in high-risk patients. The analysis used a simple method of multiplicatively combining relative risks. This method produced more conservative estimates of combined RRR than if an additive method were used. For example, the additive RRR for triple therapy would be 72% compared with 54% for the multiplicative method. Further research is required to validate this assumption. However, there is strong evidence showing that combinations of the agents do convey greater RRR than use of the single agents (2). For example, a meta-analysis by Hennekens et al (39) found a 31% reduction in MI for statin plus ASA versus ASA alone, and 26% for statin plus ASA versus statin alone. The relative reductions for these combinations in the present study are comparable but lower, at 24% and 22%, respectively.
The analysis also assumed that statins, ACEI and ASA have similar effects in clinical practice to their efficacy demonstrated in trials. Effectiveness in actual practice can differ from efficacy in trials due to greater variability in a number of factors. For example, prescribing is conducted by a large group of physicians with varied backgrounds, training, experience and therapeutic preferences. These physicians prescribe the drug to a more heterogeneous group of patients, who undergo less frequent and intensive management than a trial population (40).
The analysis used the best available Canadian data, and in situations of parameter uncertainty, conservative assumptions were used. These conservative assumptions help to balance other factors not included in the combination therapy scenario, such as suboptimal dosing, patient noncompliance and contraindications to the therapies. Overall, the base-case analysis likely provides a conservative estimate of the gains Canadian clinicians have made thus far and the possible additional gains that can be achieved.
By quantifying the current care gap, we have sought to stimulate further dialogue on how greater implementation of the clinical guidelines may be achieved in the Canadian population.
CONCLUSION
Great advances in the management of CVD have been achieved through current monotherapy and combined use of statin, ASA and ACEI therapy for high-risk patients, reducing the estimated occurrence of CV events over the next 10 years by approximately 400,000 from 1.01 million, compared with no use of these agents. However, CVD remains a substantial burden to patients and the Canadian health care system. Canadian physicians have the opportunity to further reduce this burden through more optimal management of high-risk patients based on clinical guidelines. The universal use of combination statin, ASA and ACEI therapy for high-risk patients, compared with current use of 36%, will prevent as many as 143,000 CV events over the next 10 years.
REFERENCES
- 1.Johansen H, Thillaiampalam S, Nguyen D, Sambell C. Diseases of the circulatory system – hospitalization and mortality. Health Rep. 2005;17:49–53. [PubMed] [Google Scholar]
- 2.Hippisley-Cox J, Coupland C.Effect of combinations of drugs on all cause mortality in patients with ischaemic heart disease: Nested case-control analysis BMJ 20053301059–63.(Erratum in 2006;332:912). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusuf S. Two decades of progress in preventing vascular disease. Lancet. 2002;360:2–3. doi: 10.1016/S0140-6736(02)09358-3. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2003;27(Suppl 2) [Google Scholar]
- 5.Genest J, Frohlich J, Fodor G, McPherson R, Working Group on Hypercholesterolemia and Other Dyslipidemias Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: Summary of the 2003 update CMAJ 2003169921–4.(Erratum in 2003;169:1149). [PMC free article] [PubMed] [Google Scholar]
- 6.2005 CHEP Recommendations. < www.stacommunications.com/journals/cardiology/2005/January/PDF/030.pdf> (Version current at March 28, 2008)
- 7.Abramson BL, Huckell V, Anand S, et al. Canadian Cardiovascular Society Canadian Cardiovascular Society Consensus Conference: Peripheral arterial disease – executive summary. Can J Cardiol. 2005;21:997–1006. [PubMed] [Google Scholar]
- 8.Austin PC, Mamdani MM, Juurlink DN, Alter DA, Tu JV. Missed opportunities in the secondary prevention of myocardial infarction: An assessment of the effects of statin underprescribing on mortality. Am Heart J. 2006;151:969–75. doi: 10.1016/j.ahj.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 9.Hackam DG, Tan MK, Honos GN, Leiter LA, Langer A, Goodman SG, Vascular Protection registry investigators How does the prognosis of diabetes compare with that of established vascular disease? Insights from the Canadian Vascular Protection (VP) Registry. Am Heart J. 2004;148:1028–33. doi: 10.1016/j.ahj.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 10.Yan AT, Yan RT, Tan M, et al. Vascular Protection (VP) and Guidelines Oriented Approach to Lipid Lowering (GOALL) Registries Investigators Contemporary management of dyslipidemia in high-risk patients: Targets still not met. Am J Med. 2006;119:676–83. doi: 10.1016/j.amjmed.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Chow CM, Donovan L, Manuel D, Johansen H, Tu JV, Canadian Cardiovascular Outcomes Research Team Regional variation in self-reported heart disease prevalence in Canada. Can J Cardiol. 2005;21:1265–71. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Self-reported heart disease and stroke among adults with and without diabetes –United States, 1999–2001. MMWR Morb Mortal Wkly Rep. 2003;52:1065–70. [PubMed] [Google Scholar]
- 13.Statistics Canada. Canadian Community Health Survey (CCHS) –cycle 2.1 (2003) Ottawa: Statistics Canada; 2004. [Google Scholar]
- 14.Statistics Canada, CANSIM table (for fee) 051–0001. Last modified 2005-10–27.
- 15.Statistics Canada, CANSIM, table (for fee) 052–0004 and Catalogue no. 91–520-X. Last modified 2005-12–21.
- 16.D’Agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal: New results from the Framingham study Am Heart J 2000139272–81.(Erratum in 2002;143:21). [DOI] [PubMed] [Google Scholar]
- 17.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–8. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 18.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profile from the Framingham Study. Stroke. 1991;22:312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 19.Sacco RL, Wolf PA, Kannel WB, McNamara PM. Survival and recurrence following stroke. The Framingham Study. Stroke. 1982;13:290–5. doi: 10.1161/01.str.13.3.290. [DOI] [PubMed] [Google Scholar]
- 20.Kuntz KM, Weinstein MC. Modeling in economic evaluation. In: Drummond M, McGuire A, editors. Economic Evaluation in Health Care: Merging Theory with Practice. Oxford: Oxford University Press; 2001. [Google Scholar]
- 21.Statistics Canada, 2006. Life Tables, Canada, Provinces and Territories 2000–2002. Statistics Canada Catalogue No. 84–537-XIE. Ottawa: [Google Scholar]
- 22.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 23.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients BMJ 200232471–86.(Erratum in 2002;324:141). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G.Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators N Engl J Med 2000342145–53.(Errata in 2000;342:1376, 2000;342:748). [DOI] [PubMed] [Google Scholar]
- 25.Gaziano TA, Opie LH, Weinstein MC. Cardiovascular disease prevention with a multidrug regimen in the developing world: A cost-effectiveness analysis. Lancet. 2006;368:679–86. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: A cost-utility analysis. Ann Intern Med. 2006;144:326–36. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]
- 27.Hux JE, Booth GL, Slaughter PM, Laupacis A. Diabetes in Ontario: An ICES Practice Atlas. Institute for Clinical Evaluative Sciences, 2003.
- 28.Guzder RN, Gatling W, Mullee MA, Mehta RL, Byrne CD. Prognostic value of the Framingham cardiovascular risk equation and the UKPDS risk engine for coronary heart disease in newly diagnosed Type 2 diabetes: Results from a United Kingdom study. Diabet Med. 2005;22:554–62. doi: 10.1111/j.1464-5491.2005.01494.x. [DOI] [PubMed] [Google Scholar]
- 29.UKPDS Risk Engine Version 2.0<www.dtu.ox.ac.uk/index.php?maindoc=/riskengine/history.php> (Version current at March 28, 2008).
- 30.Stevens RJ, Kothari V, Adler AI, Stratton IM, United Kingdom Prospective Diabetes Study (UKPDS) Group The UKPDS risk engine: A model for the risk of coronary heart disease in type II diabetes (UKPDS 56) Clin Sci (Lond) 2001101671–9.(Erratum in 2002;102:679). [PubMed] [Google Scholar]
- 31.Kothari V, Stevens RJ, Adler AI, et al. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke. 2002;33:1776–81. doi: 10.1161/01.str.0000020091.07144.c7. [DOI] [PubMed] [Google Scholar]
- 32.Gaspoz JM, Coxson PG, Goldman PA, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med. 2002;346:1800–6. doi: 10.1056/NEJM200206063462309. [DOI] [PubMed] [Google Scholar]
- 33.Schleinitz MD, Heidenreich PA. A cost-effectiveness analysis of combination antiplatelet therapy for high-risk acute coronary syndromes: Clopidogrel plus aspirin versus aspirin alone. Ann Intern Med. 2005;142:251–9. doi: 10.7326/0003-4819-142-4-200502150-00007. [DOI] [PubMed] [Google Scholar]
- 34.Alter DA, Khaykin Y, Austin PC, Tu JV, Hux JE. Processes and outcomes of care for diabetic acute myocardial infarction patients in Ontario: Do physicians undertreat? Diabetes Care. 2003;26:1427–34. doi: 10.2337/diacare.26.5.1427. [DOI] [PubMed] [Google Scholar]
- 35.Brown LC, Johnson JA, Majumdar SR, Tsuyuki RT, McAlister FA. Evidence of suboptimal management of cardiovascular risk in patients with type 2 diabetes mellitus and symptomatic atherosclerosis. CMAJ. 2004;171:1189–92. doi: 10.1503/cmaj.1031965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson E, Beck C, Richard H, Eisenberg MJ, Pilote L. Drug prescriptions after acute myocardial infarction: Dosage, compliance, and persistence. Am Heart J. 2003;145:438–44. doi: 10.1067/mhj.2003.143. [DOI] [PubMed] [Google Scholar]
- 37.Shah BR, Mamdani M, Jaakkimainen L, Hux JE. Risk modification for diabetic patients. Are other risk factors treated as diligently as glycemia? Can J Clin Pharmacol. 2004;11:e239–44. [PubMed] [Google Scholar]
- 38.Pilote L, Beck CA, Karp I, et al. Canadian Cardiovascular Outcomes Research Team Secondary prevention after acute myocardial infarction in four Canadian provinces, 1997–2000. Can J Cardiol. 2004;20:61–7. [PubMed] [Google Scholar]
- 39.Hennekens CH, Sacks FM, Tonkin A, et al. Additive benefits of pravastatin and aspirin to decrease risks of cardiovascular disease: Randomized and observational comparisons of secondary prevention trials and their meta-analyses. Arch Intern Med. 2004;164:40–4. doi: 10.1001/archinte.164.1.40. [DOI] [PubMed] [Google Scholar]
- 40.ISPOR Real World Task Force Using Real World Data For Coverage And Payment Decisions: The ISPOR Real World Data Task Force Report<www.ispor.org/workpaper/real_world_data.asp> (Version current at March 28, 2008).